Transcriptome Analysis of Potato Leaves under Oxidative Stress
Abstract
1. Introduction
2. Results
2.1. Antioxidant Enzyme Activities of Potato under Oxidative Stress
2.2. Overview of RNA Sequencing and Mapping
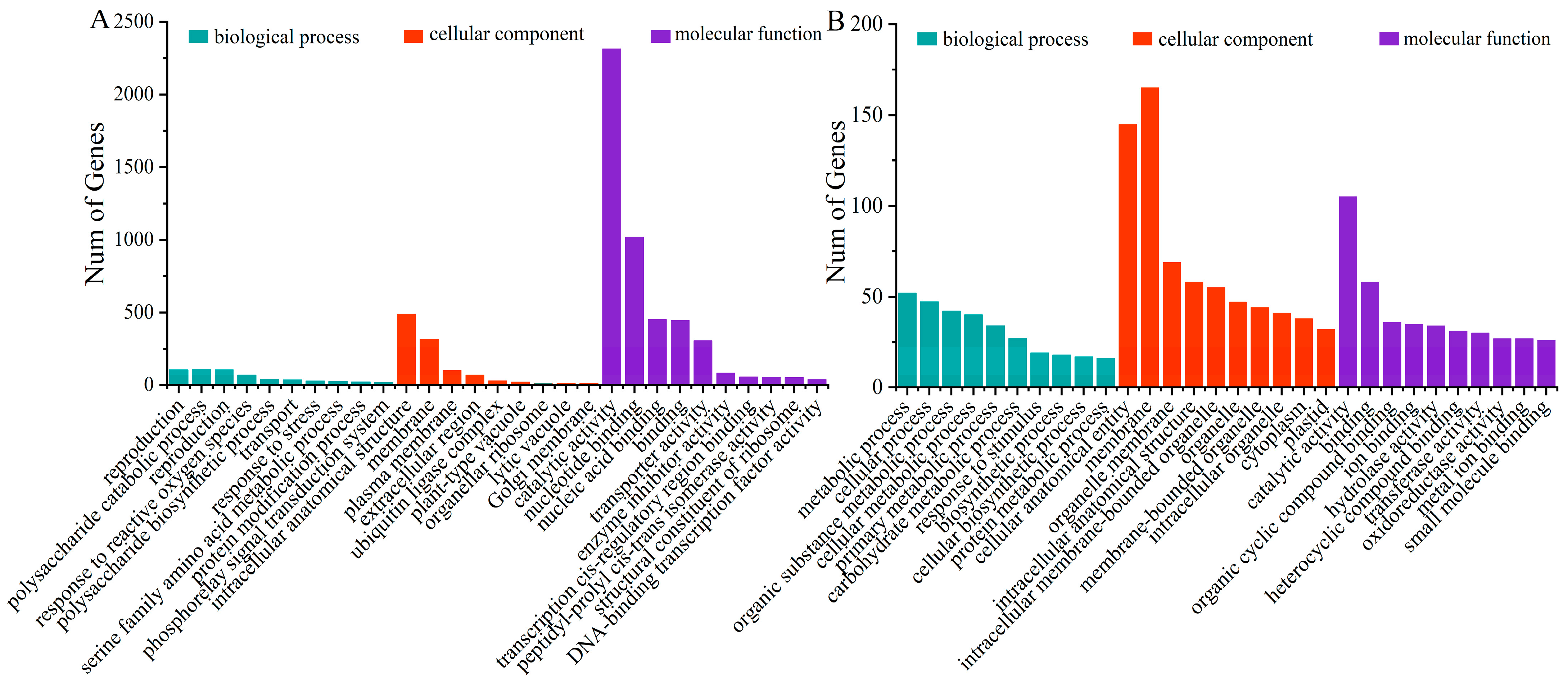
2.3. GO Annotation and Enrichment
2.4. KEGG Analysis of DEGs
2.5. Expression Changes in ROS Scavenging-Related Genes
2.6. Expression Changes in Plant Hormone Signalling
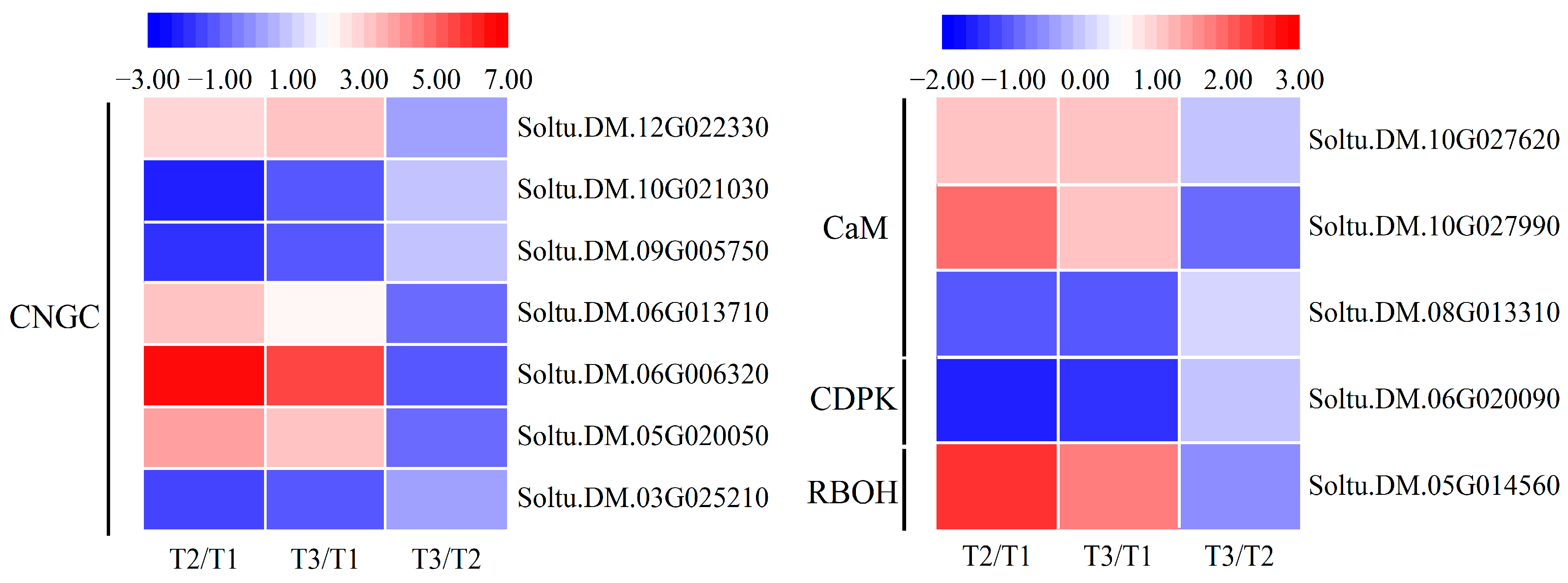
2.7. Genes Involved in Ca2+ Signal Pathway
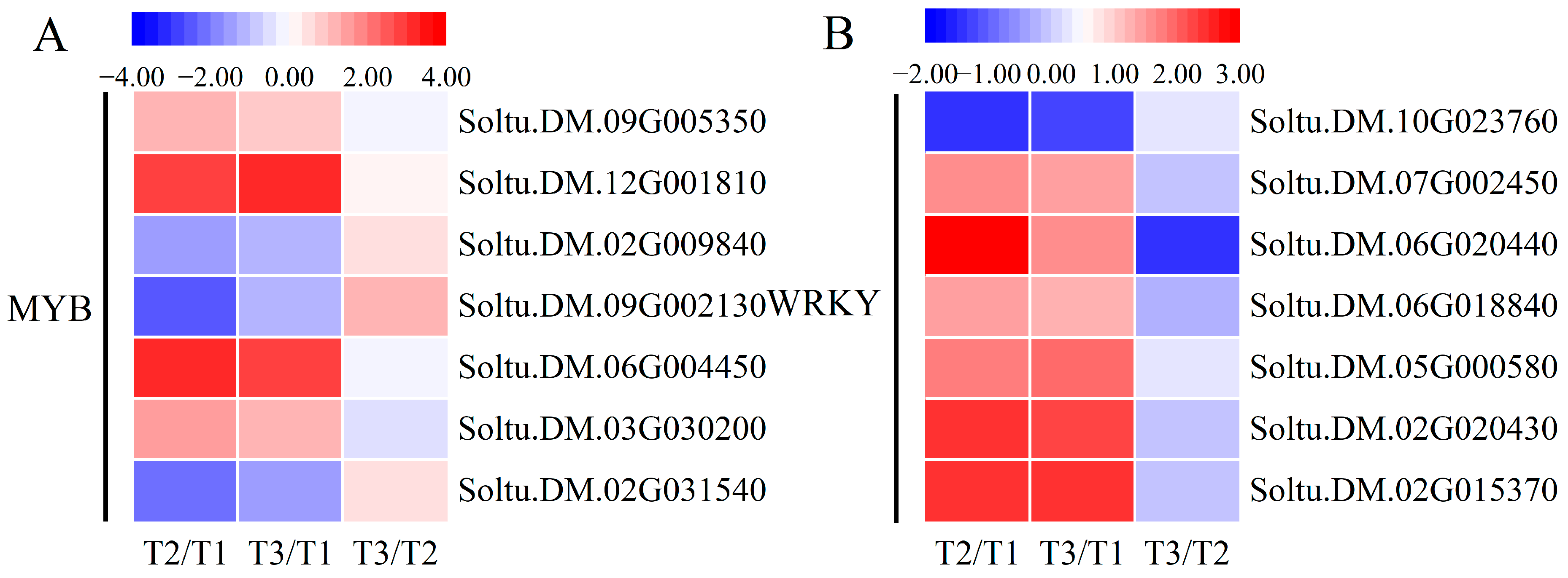
2.8. Expression Changes in Transcription Factors
2.9. Verification of RNA-Seq Data by Quantitative Real-Time PCR (qRT-PCR)
3. Discussion
4. Materials and Methods
4.1. Plant Material and Oxidative Stress Treatment
4.2. GO and KEGG Pathways Enrichment Analysis
4.3. Measurement of Antioxidant Enzyme Activities
4.4. RNA Extraction and RNA-Seq
4.5. Validation of Differentially Expressed Genes by Quantitative Real-Time PCR (qRT-PCR)
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Monneveux, P.; Ramírez, D.A.; Pino, M.T. Drought tolerance in potato (S. tuberosum L.): Can we learn from drought tolerance research in cereals. Plant Sci. 2013, 205, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.A.; Loureiro, M.E.; Oliva, M.A.; Maestri, M. Differential responses of superoxide dismutase in freezing resistant Solanum curtilobum and freezing sensitive Solanum tuberosum subjected to oxidative and water stress. Plant Sci. 2001, 160, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Qin, Y.; Hu, X.; Li, G.; Ding, H.; Xiong, X.; Wang, W. Transcriptome analysis uncovers the gene expression profile of salt-stressed potato (Solanum tuberosum L.). Sci. Rep. 2020, 10, 5411. [Google Scholar] [CrossRef] [PubMed]
- Dalal, A.; Kumar, A.; Yadav, D.; Gudla, T.; Viehhauser, A.; Dietz, K.J.; Kirti, P.B. Alleviation of methyl viologen-mediated oxidative stress by Brassica juncea annexin-3 in transgenic Arabidopsis. Plant Sci. 2014, 219, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Dietz, K.J. Systematic analysis of superoxide-dependent signaling in plant cells: Usefulness and specificity of methyl viologen application. Astron. Astrophys. Suppl. 2009, 136, 179–196. [Google Scholar]
- Zer, H.; Chevion, M.; Goldberg, I. Effect of paraquat on dark-grown Phaseolus vulgaris cells. Weed Sci. 1993, 41, 528–533. [Google Scholar] [CrossRef]
- He, H.; Denecker, J.; Van Der Kelen, K.; Willems, P.; Pottie, R.; Phua, S.Y.; Hannah, M.A.; Vertommen, D.; Van Breusegem, F.; Mhamdi, A. The Arabidopsis mediator complex subunit 8 regulates oxidative stress responses. Plant Cell 2021, 33, 2032–2057. [Google Scholar] [CrossRef] [PubMed]
- Kerchev, P.; Mühlenbock, P.; Denecker, J.; Morreel, K.; Hoeberichts, F.A.; Van Der Kelen, K.; Vandorpe, M.; Nguyen, L.; Audenaert, D.; Van Breusegem, F. Activation of auxin signalling counteracts photorespiratory H2O2-dependent cell death. Plant Cell Environ. 2015, 38, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Kerchev, P.; Waszczak, C.; Lewandowska, A.; Willems, P.; Shapiguzov, A.; Li, Z.; Alseekh, S.; Mühlenbock, P.; Hoeberichts, F.A.; Huang, J.; et al. Lack of GLYCOLATE OXIDASE1, but not GLYCOLATE OXIDASE2, attenuates the photorespiratory phenotype of CATALASE2-deficient Arabidopsis. Plant Physiol. 2016, 171, 1704–1719. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Tripathi, D.K.; Roychoudhury, A. Hydrogen sulphide trapeze: Environmental stress amelioration and phytohormone crosstalk. Plant Physiol. Biochem. 2018, 132, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive oxygen species in plant signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.; Fang, L.; Wang, G.; Li, X.; Huang, S.; Gao, Y.; Zhu, J.; Xiao, L.; Tong, J.; Chen, F.; et al. Wheat methionine sulfoxide reductase A4.1 interacts with heme oxygenase 1 to enhance seedling tolerance to salinity or drought stress. Plant Mol. Biol. 2019, 101, 203–220. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Wang, F.; Ma, L.; Qi, Z.; Liu, S.; Chen, C.; Wu, J.; Wang, P.; Yang, C.; Wu, Y.; et al. Physiological and biochemical mechanisms and cytology of cold tolerance in Brassica napus. Front. Plant Sci. 2020, 11, 1241. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.W.; Kong, X.W.; Wang, N.; Wang, T.T.; Chen, J.; Shi, Z.Q. Thymol confers tolerance to salt stress by activating anti-oxidative defense and modulating Na+ homeostasis in rice root. Ecotoxicol. Environ. Saf. 2020, 188, 109894. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Kim, Y.H.; Kim, S.H.; Kwon, S.Y.; Lee, H.S.; Kim, J.S.; Kwak, S.S. Enhanced tolerance of transgenic sweetpotato plants that express both CuZnSOD and APX in chloroplasts to methyl viologen-mediated oxidative stress and chilling. Mol. Breed. 2007, 19, 227–239. [Google Scholar] [CrossRef]
- Kwon, S.Y.; Jeong, Y.J.; Lee, H.S.; Kim, J.S.; Cho, K.Y.; Allen, R.D.; Kwak, S.S. Enhanced tolerances of transgenic tobacco plants expressing both superoxide dismutase and ascorbate peroxidase in chloroplasts against methyl viologen-mediated oxidative stress. Plant Cell Environ. 2002, 25, 873–882. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Medina, E.; Kim, S.H.; Yun, M.; Choi, W.G. Recapitulation of the function and role of ROS generated in response to heat stress in plants. Plants 2021, 10, 371. [Google Scholar] [CrossRef] [PubMed]
- Zandalinas, S.I.; Mittler, R. ROS-induced ROS release in plant and animal cells. Free Radic. Biol. Med. 2018, 122, 21–27. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Li, X.; Li, X.; Ma, H. Design, synthesis and application of a dual-functional fluorescent probe for reactive oxygen species and viscosity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 246, 119059. [Google Scholar] [CrossRef] [PubMed]
- Kolbert, Z.; Lindermayr, C.; Loake, G.J. The role of nitric oxide in plant biology: Current insights and future perspectives. J. Exp. Bot. 2021, 72, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Foyer, C.H. Intracellular redox compartmentation and ROS-related communication in regulation and signaling. Plant Physiol. 2016, 171, 1581–1592. [Google Scholar] [CrossRef]
- Dietz, K.J.; Turkan, I.; Krieger-Liszkay, A. Redox-and reactive oxygen species-dependent signaling into and out of the photosynthesizing chloroplast. Plant Physiol. 2016, 171, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Chong, L.; Guo, P.; Zhu, Y. Mediator complex: A pivotal regulator of ABA signaling pathway and abiotic stress response in plants. Int. J. Mol. Sci. 2020, 21, 7755. [Google Scholar] [CrossRef]
- Levine, A.; Tenhaken, R.; Dixon, R.; Lamb, C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 1994, 79, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Kiep, V.; Vadassery, J.; Lattke, J.; Maaß, J.P.; Boland, W.; Peiter, E.; Mithöfer, A. Systemic cytosolic Ca2+ elevation is activated upon wounding and herbivory in Arabidopsis. New Phytol. 2015, 207, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Perochon, A.; Aldon, D.; Galaud, J.P.; Ranty, B. Calmodulin and calmodulin-like proteins in plant calcium signaling. Biochimie 2011, 93, 2048–2053. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Chi, Y.; Jiang, Z.; Xu, Y.; Xie, L.; Huang, F.; Wan, D.; Ni, J.; Yuan, F.; Wu, X.; et al. Hydrogen peroxide sensor HPCA1 is an LRR receptor kinase in Arabidopsis. Nature 2020, 578, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Talke, I.N.; Blaudez, D.; Maathuis, F.J.; Sanders, D. CNGCs: Prime targets of plant cyclic nucleotide signalling? Trends Plant Sci. 2003, 8, 286–293. [Google Scholar] [CrossRef] [PubMed]
- DeFalco, T.A.; Bender, K.W.; Snedden, W.A. Breaking the code: Ca2+ sensors in plant signalling. Biochem. J. 2010, 425, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Schulz, P.; Herde, M.; Romeis, T. Calcium-dependent protein kinases: Hubs in plant stress signaling and development. Plant Physiol. 2013, 163, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Toyota, M.; Spencer, D.; Sawai-Toyota, S.; Jiaqi, W.; Zhang, T.; Koo, A.J.; Gilroy, S. Glutamate triggers long-distance, calcium-based plant defense signaling. Science 2018, 361, 1112–1115. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Ren, S.; Han, Y.; Zhang, Q.; Qin, L.; Xing, Y. Identification and analysis of mitogen-activated protein kinase (MAPK) cascades in Fragaria vesca. Int. J. Mol. Sci. 2017, 18, 1766. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Yang, L.; Gai, Y. MAPKKKs in plants: Multidimensional regulators of plant growth and stress responses. Int. J. Mol. Sci. 2023, 24, 4117. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.G.; Toyota, M.; Kim, S.H.; Hilleary, R.; Gilroy, S. Salt stress-induced Ca2+ waves are associated with rapid, long-distance root-to-shoot signaling in plants. Proc. Natl. Acad. Sci. USA 2014, 111, 6497–6502. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Du, Y.; Li, Y.; Ren, D.; Song, C.P. Hydrogen peroxide–mediated activation of MAP kinase 6 modulates nitric oxide biosynthesis and signal transduction in Arabidopsis. Plant Cell 2010, 22, 2981–2998. [Google Scholar] [CrossRef]
- Hoang, M.H.; Nguyen, X.C.; Lee, K.; Kwon, Y.S.; Pham, H.T.; Park, H.C.; Yun, D.J.; Lim, C.O.; Chung, W.S. Phosphorylation by AtMPK6 is required for the biological function of AtMYB41 in Arabidopsis. Biochem. Biophys. Res. Commun. 2012, 422, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Zhao, S.; Yu, X.; Sun, Y.; Li, H.; Ruan, C.; Li, J. Yellowhorn drought-induced transcription factor XsWRKY20 acts as a positive regulator in drought stress through ROS homeostasis and ABA signaling pathway. Plant Physiol. Biochem. 2020, 155, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Lv, F.; Gao, L.; Jiang, S.; Wang, Q.; Li, S.; Wang, P. A WRKY transcription factor CbWRKY27 negatively regulates salt tolerance in Catalpa bungei. Forests 2023, 14, 486. [Google Scholar] [CrossRef]
- Liao, C.; Zheng, Y.; Guo, Y. MYB30 transcription factor regulates oxidative and heat stress responses through ANNEXIN-mediated cytosolic calcium signaling in Arabidopsis. New Phytol. 2017, 216, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; pp. 121–126. Volume 105. [Google Scholar]
- Maehly, A.; Chance, B. Catalases and peroxidases. Methods Biochem Anal 1954, 1, 357–424. [Google Scholar] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]







| Samples | Raw Reads | Clean Reads | Mapped Reads | Mapped Ratio (%) | Q30 (%) | |
|---|---|---|---|---|---|---|
| T1 | T1-1 | 43,684,236 | 42,252,294 | 39,628,427 | 93.79 | 95.28 |
| T1-2 | 43,684,592 | 42,095,838 | 32,081,238 | 76.21 | 95.77 | |
| T1-3 | 43,684,838 | 42,041,882 | 31,926,605 | 75.94 | 95.63 | |
| T2 | T2-1 | 43,683,506 | 42,190,804 | 36,625,837 | 86.81 | 94.81 |
| T2-2 | 43,684,728 | 42,244,960 | 35,097,113 | 83.08 | 95.01 | |
| T2-3 | 45,432,984 | 43,560,802 | 38,298,658 | 87.92 | 95.91 | |
| T3 | T3-1 | 43,676,094 | 42,216,594 | 35,048,217 | 83.02 | 93.89 |
| T3-2 | 45,432,568 | 43,568,446 | 34,568,446 | 79.93 | 95.43 | |
| T3-3 | 43,684,752 | 42,283,118 | 33,957,573 | 80.31 | 95.44 | |
| Gene ID | Description | Log2T2/T1 | Log2T3/T1 |
|---|---|---|---|
| Soltu.DM.02G024700 | mitogen-activated protein kinase 7/14 | 1.99 | 1.88 |
| Soltu.DM.04G025590 | mitogen-activated protein kinase 3 | 1.69 | 1.66 |
| Soltu.DM.11G025980 | mitogen-activated protein kinase 1/3 | 1.56 | 1.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Tang, X.; Zhang, H.; Wei, M.; Zhang, N.; Si, H. Transcriptome Analysis of Potato Leaves under Oxidative Stress. Int. J. Mol. Sci. 2024, 25, 5994. https://doi.org/10.3390/ijms25115994
Liu J, Tang X, Zhang H, Wei M, Zhang N, Si H. Transcriptome Analysis of Potato Leaves under Oxidative Stress. International Journal of Molecular Sciences. 2024; 25(11):5994. https://doi.org/10.3390/ijms25115994
Chicago/Turabian StyleLiu, Juping, Xun Tang, Huanhuan Zhang, Meng Wei, Ning Zhang, and Huaijun Si. 2024. "Transcriptome Analysis of Potato Leaves under Oxidative Stress" International Journal of Molecular Sciences 25, no. 11: 5994. https://doi.org/10.3390/ijms25115994
APA StyleLiu, J., Tang, X., Zhang, H., Wei, M., Zhang, N., & Si, H. (2024). Transcriptome Analysis of Potato Leaves under Oxidative Stress. International Journal of Molecular Sciences, 25(11), 5994. https://doi.org/10.3390/ijms25115994






