Unique Self-Phosphorylating Polybenzimidazole of the 6F Family for HT-PEM Fuel Cell Application
Abstract
1. Introduction
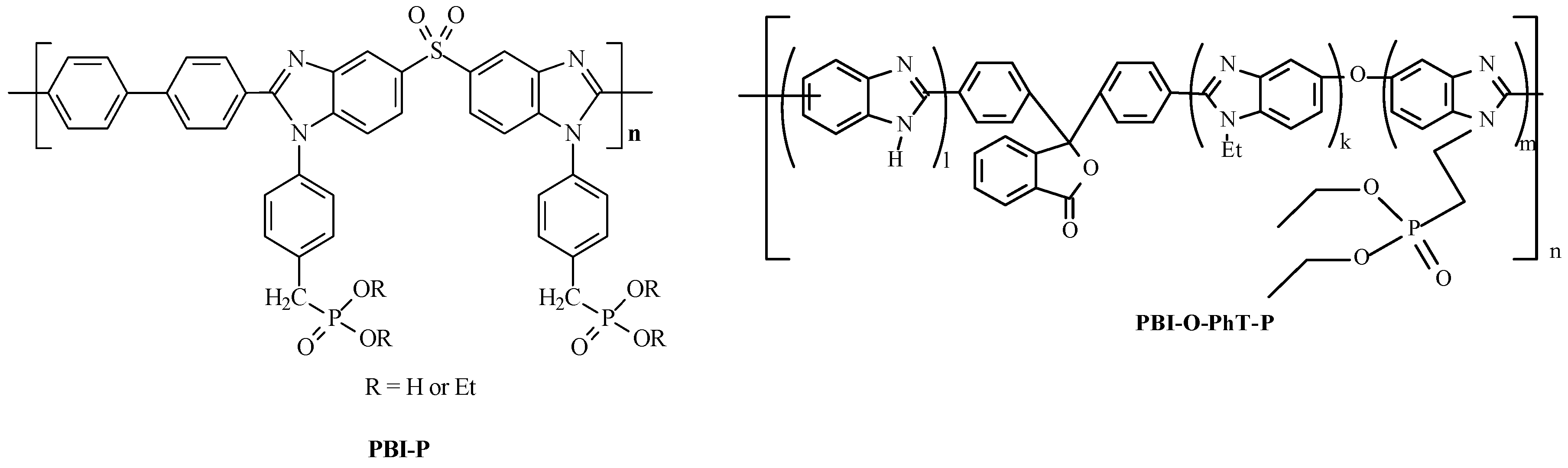
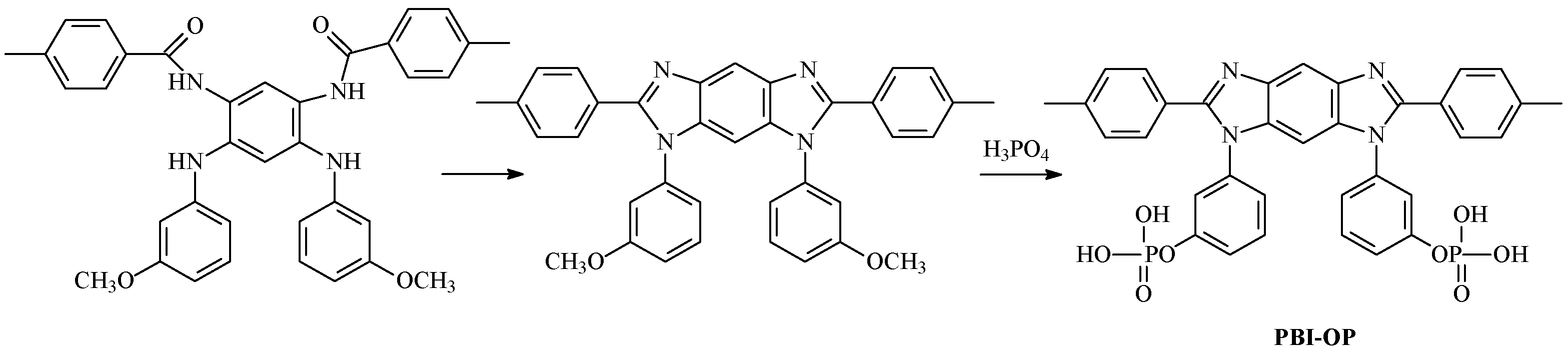
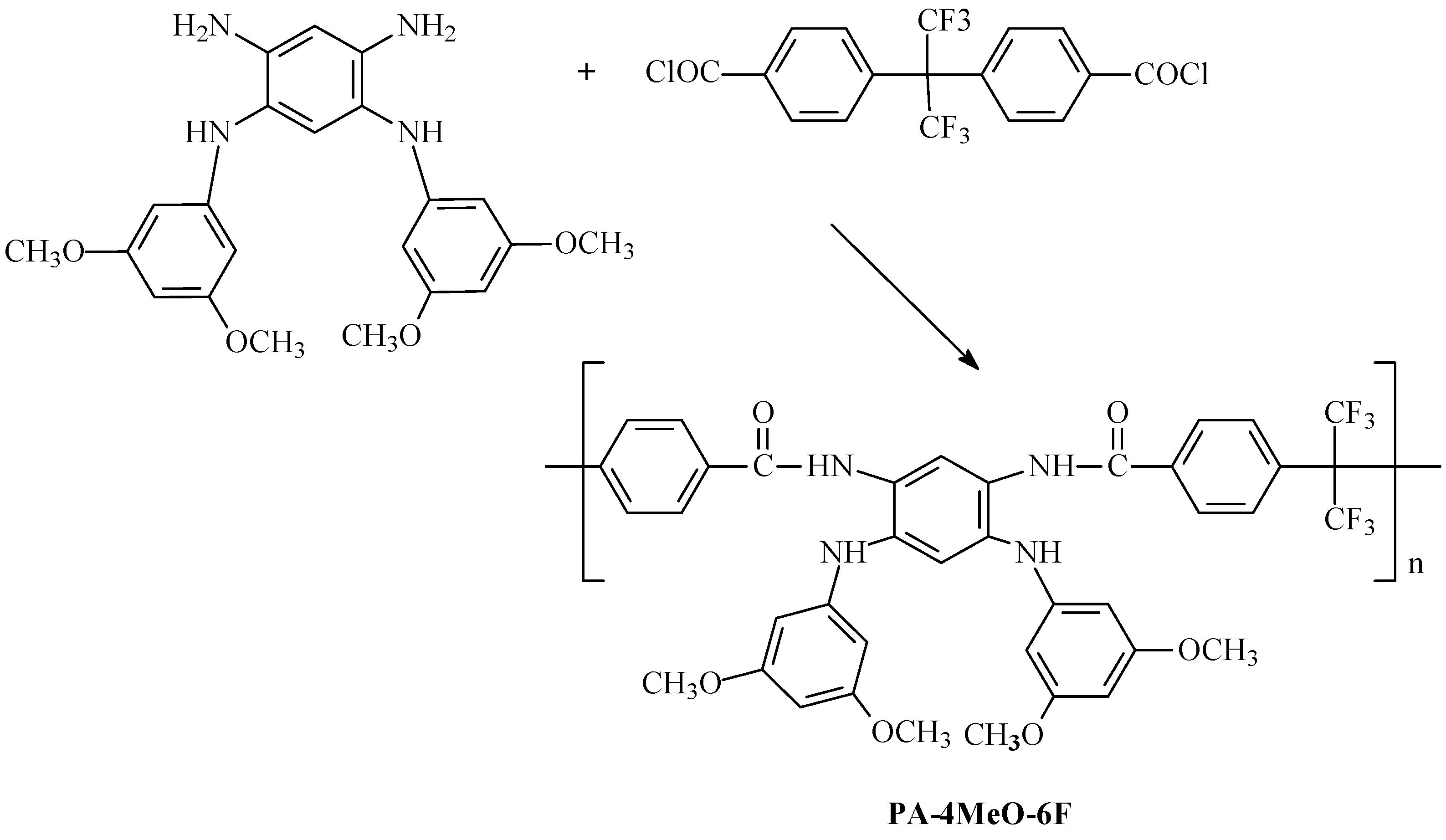
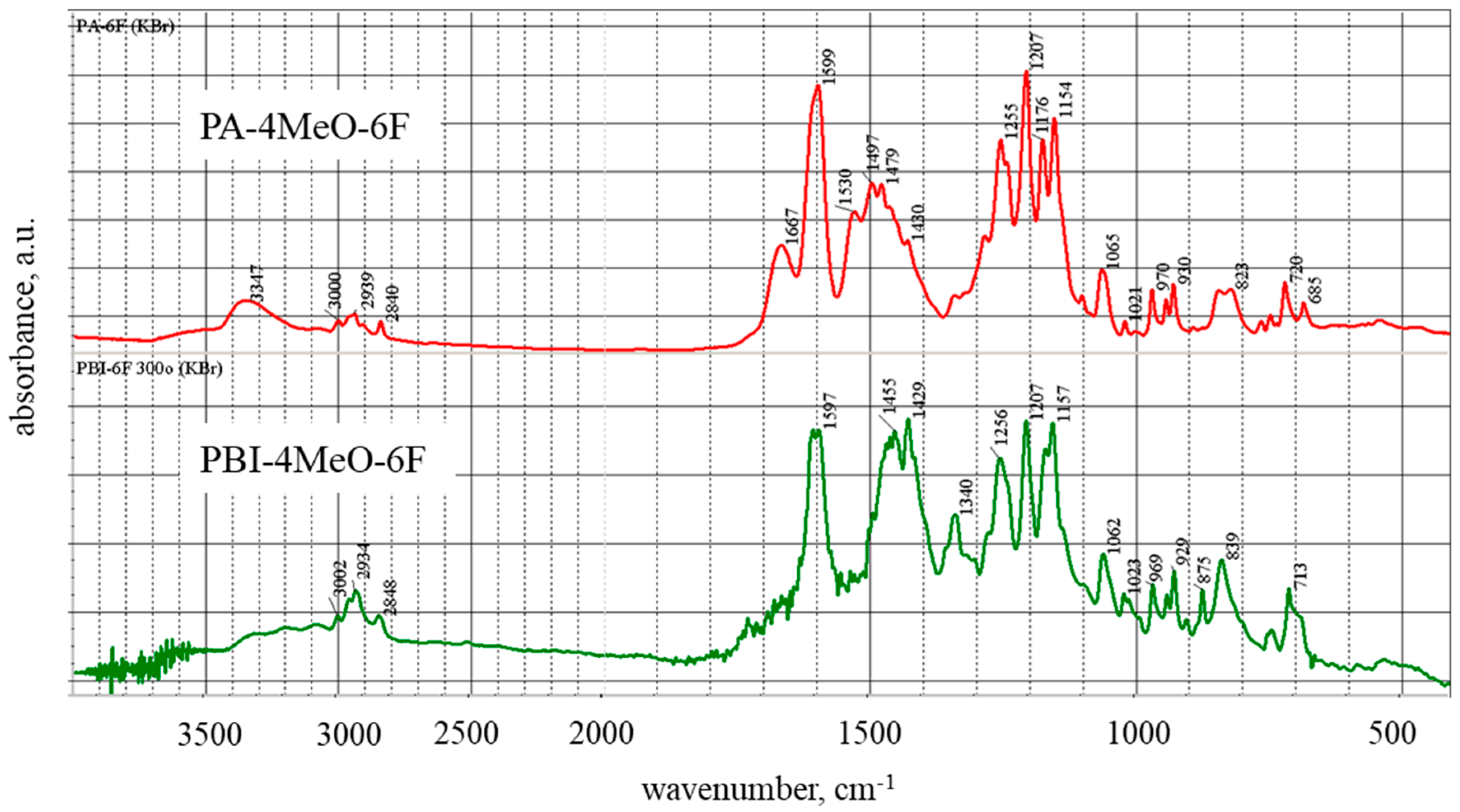
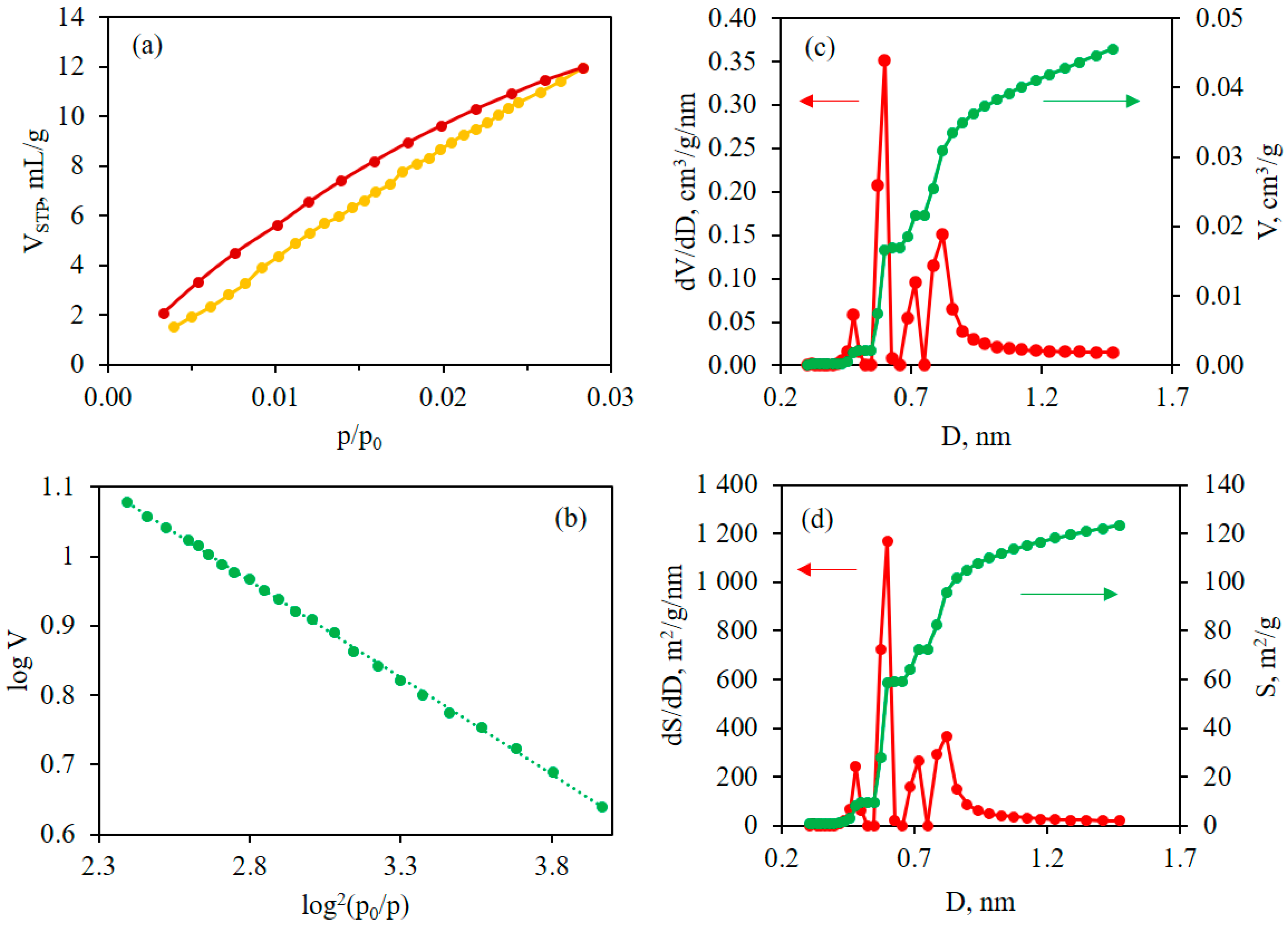
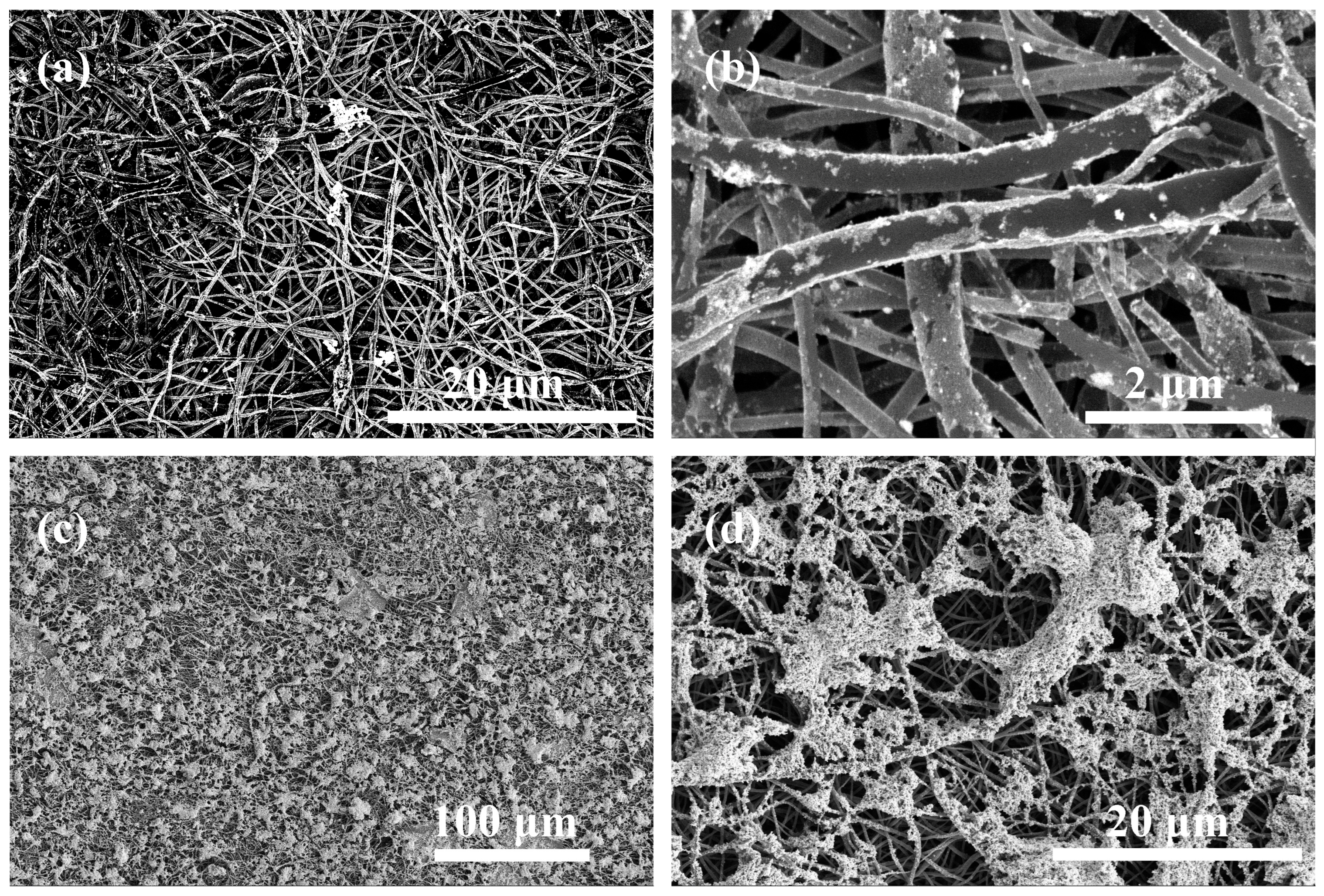
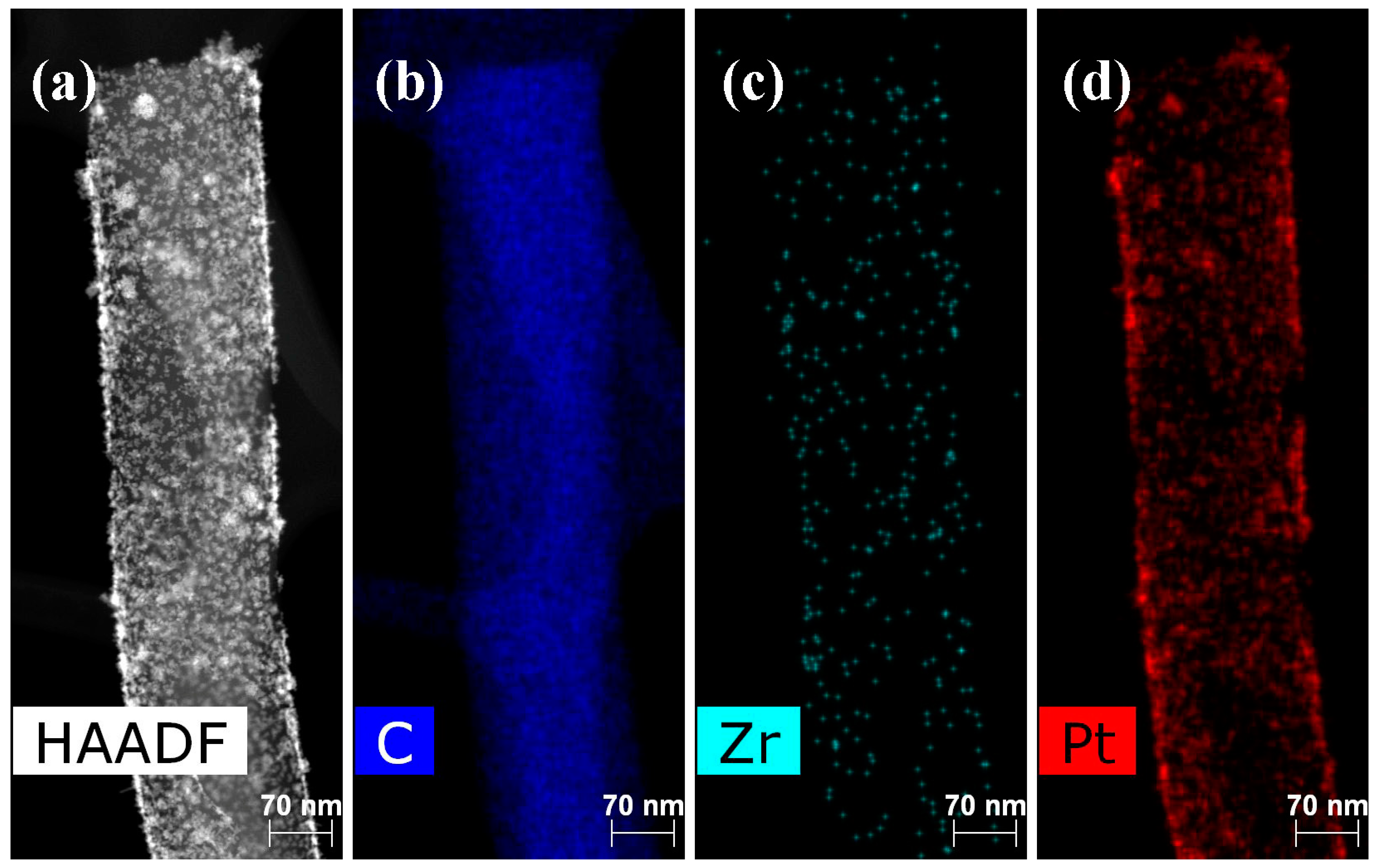
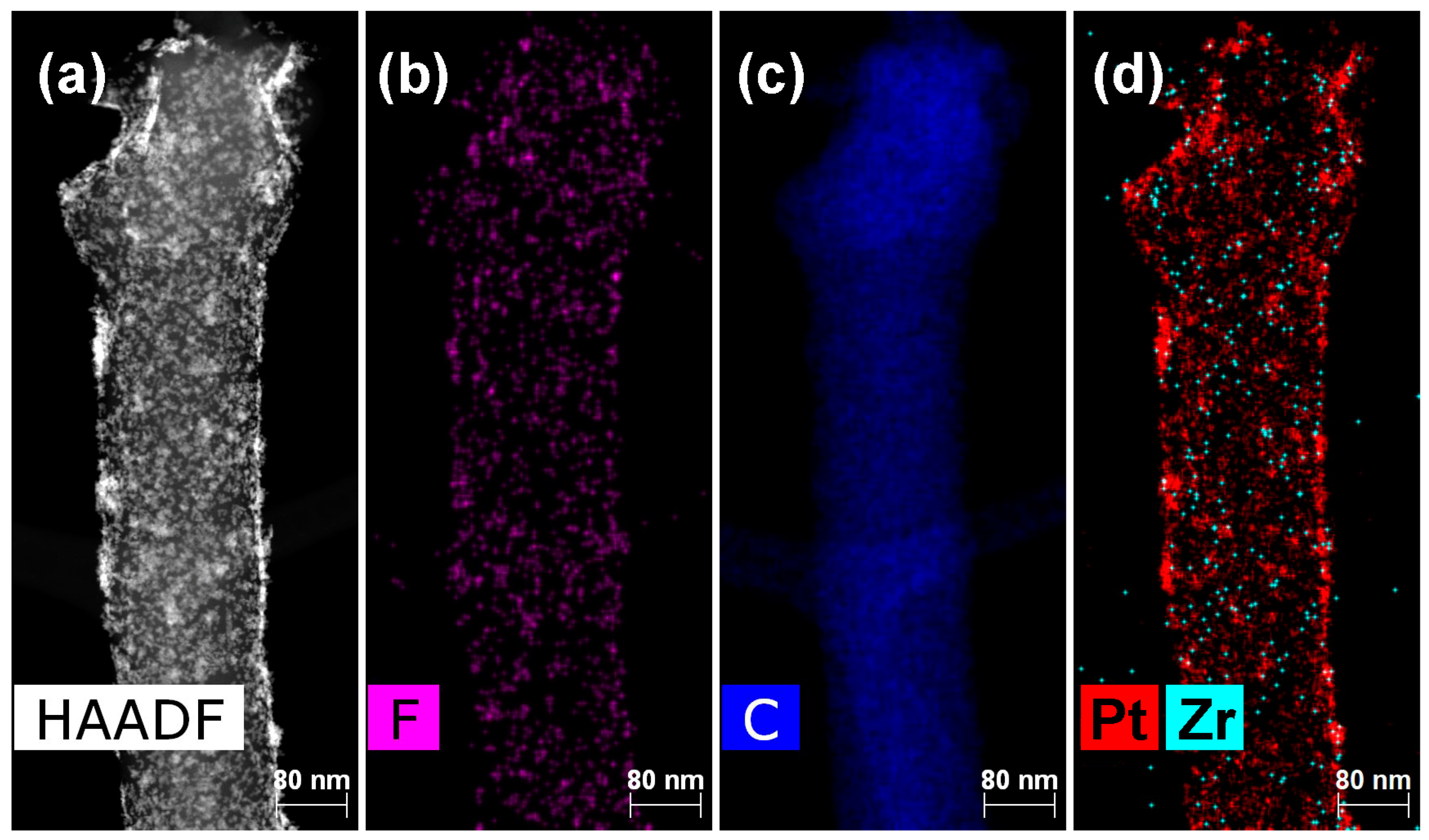
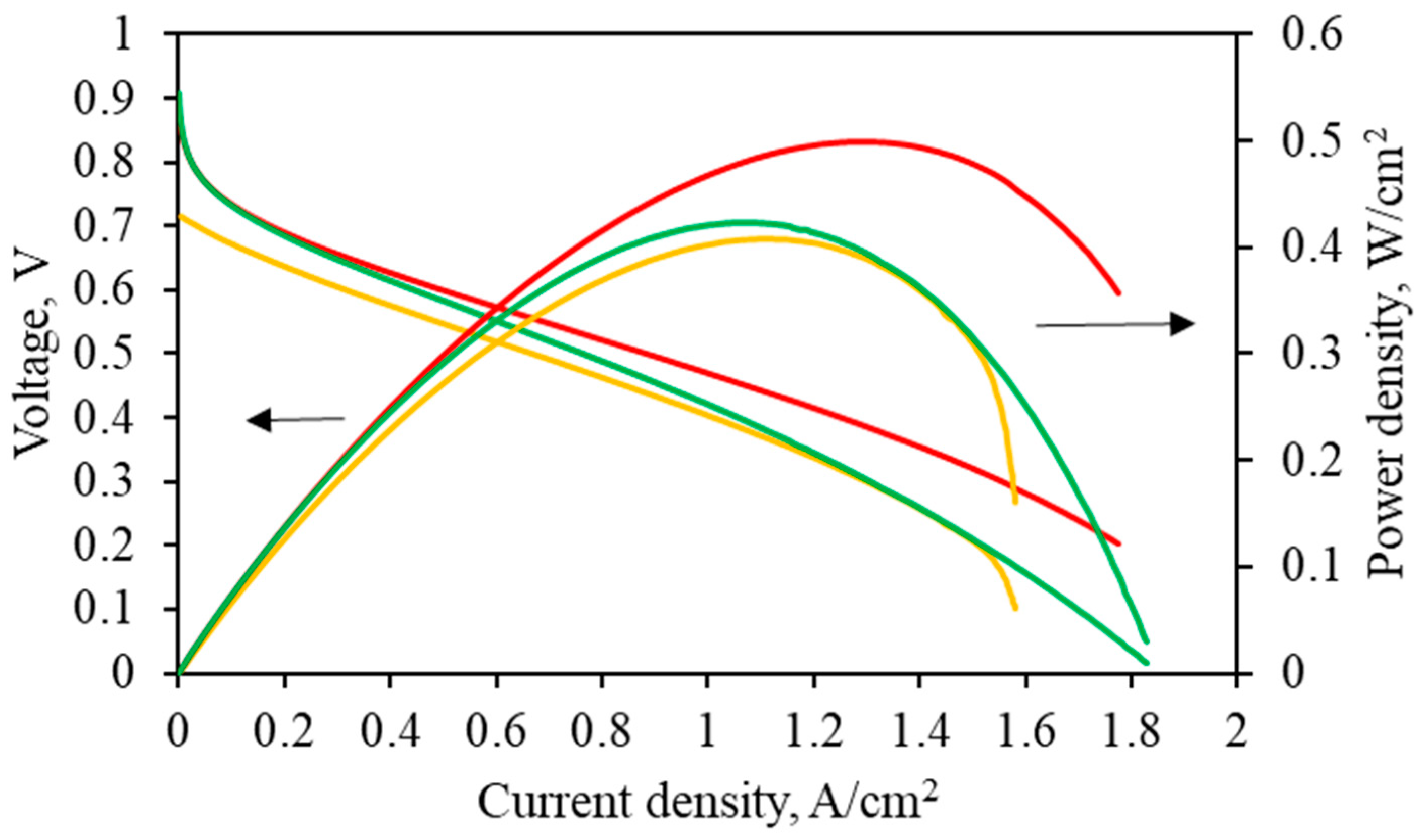
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.2.1. Synthesis of N1,N5-bis(3,5-dimethoxyphenyl)-4,6-dinitro-1,3-benzenediamine
3.2.2. Synthesis of N1,N5-bis(3,5-dimethoxyphenyl)-1,2,4,5-benzenetetramine
3.2.3. Synthesis of Polyamide PA-4MeO-6F and PBI-4MeO-6F
3.3. Physical and Physico-Chemical Methods
3.4. Electrode Preparation
3.4.1. Electrospinning
3.4.2. Stabilization, Zinc Deposition and Pyrolysis
3.4.3. Platinum Deposition
3.4.4. Polymer Deposition
3.5. Gas Permeability
3.6. HT-PEM Fuel Cell Operation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peinemann, K.-V.; Nunes, S.P. Membranes for Energy Conversion; Wiley-VCH: Weinheim, Germany, 2008; Volume 2, p. 304. [Google Scholar] [CrossRef]
- Zhang, J. PEM Fuel Cell Electrocatalysts and Catalyst Layers, Fundamentals and Applications; Springer: London, UK, 2008; p. 1137. [Google Scholar] [CrossRef]
- Aili, D.; Henkensmeier, D.; Martin, S.; Singh, B.; Hu, Y.; Jensen, J.O.; Cleemann, L.N.; Li, Q. Polybenzimidazole-Based High-Temperature Polymer Electrolyte Membrane Fuel Cells: New Insights and Recent Progress. Electrochem. Energy Rev. 2020, 3, 793–845. [Google Scholar] [CrossRef]
- Li, Q.; Aili, D.; Hjuler, H.A.; Jensen, J.O. High Temperature Polymer Electrolyte Membrane Fuel Cells, Approaches, Status and Perspectives; Springer: London, UK, 2016; p. 561. [Google Scholar] [CrossRef]
- Moorthy, S.; Sivasubramanian, G.; Kannaiyan, D.; Deivanayagam, P. Neoteric advancements in polybenzimidazole based polymer electrolytes for high-temperature proton exchange membrane fuel cells—A versatile review. Int. J. Hydrogen Energy 2023, 48, 28103–28118. [Google Scholar] [CrossRef]
- Li, Q.; He, R.; Jensen, J.O.; Bjerrum, N.J. PBI-Based Polymer Membranes for High Temperature Fuel Cells–Preparation, Characterization and Fuel Cell Demonstration. Fuel Cells 2004, 4, 147–159. [Google Scholar] [CrossRef]
- Escorihuela, J.; Olvera-Mancilla, J.; Alexandrova, L.; del Castillo, L.F.; Compañ, V. Recent Progress in the Development of Composite Membranes Based on Polybenzimidazole for High Temperature Proton Exchange Membrane (PEM) Fuel Cell Applications. Polymers 2020, 12, 1861. [Google Scholar] [CrossRef] [PubMed]
- Kalathil, A.; Raghavan, A.; Kandasubramanian, B. Polymer Fuel Cell Based on Polybenzimidazole Membrane: A Review. Polym.-Plast. Technol. Mater. 2019, 58, 465–497. [Google Scholar] [CrossRef]
- Pingitore, A.T.; Molleo, M.; Schmidt, T.J.; Benicewicz, B.C. Polybenzimidazole Fuel Cell Technology: Theory, Performance, and Applications. In Fuel Cells and Hydrogen Production; Encyclopedia of Sustainability Science and Technology Series; Lipman, T., Weber, A., Eds.; Springer: New York, NY, USA, 2019; pp. 477–514. [Google Scholar] [CrossRef]
- Haque, M.A.; Sulong, A.B.; Loh, K.S.; Majlan, E.H.; Husaini, T.; Rosli, R.E. Acid doped polybenzimidazoles based membrane electrode assembly for high temperature proton exchange membrane fuel cell: A review. Int. J. Hydrogen Energy 2017, 42, 9156–9179. [Google Scholar] [CrossRef]
- Quartarone, E.; Angioni, S.; Mustarelli, P. Polymer and Composite Membranes for Proton-Conducting, High-Temperature Fuel Cells: A Critical Review. Materials 2017, 10, 687. [Google Scholar] [CrossRef] [PubMed]
- Myles, T.; Bonville, L.; Maric, R. Catalyst, Membrane, Free Electrolyte Challenges, and Pathways to Resolutions in High Temperature Polymer Electrolyte Membrane Fuel Cells. Catalysts 2017, 7, 16. [Google Scholar] [CrossRef]
- Zeis, R. Materials and characterization techniques for high-temperature polymer electrolyte membrane fuel cells. Beilstein J. Nanotechnol. 2015, 6, 68–83. [Google Scholar] [CrossRef]
- Araya, S.S.; Zhou, F.; Liso, V.; Sahlin, S.L.; Vang, J.R.; Thomas, S.; Gao, X.; Jeppesen, C.; Kaer, S.K. A comprehensive review of PBI-based high temperature PEM fuel cells. Int. J. Hydrogen Energy 2016, 41, 21310–21344. [Google Scholar] [CrossRef]
- Lim, K.H.; Lee, A.S.; Atanasov, V.; Jochen, K.; Park, E.J.; Adhikari, S.; Maurya, S.; Manriquez, L.D.; Jung, J.; Fujimoto, C.; et al. Protonated phosphonic acid electrodes for high power heavy-duty vehicle fuel cells. Nat. Energy 2022, 7, 248–259. [Google Scholar] [CrossRef]
- Vogel, H.; Marvel, C.S. Polybenzimidazoles, new thermally stable polymers. J. Polym. Sci. 1961, 50, 511–539. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Razorenov, D.Y.; Ponomarev, I.I.; Volkova, Y.A.; Skupov, K.M. Synthesis and studies of polybenzimidazoles for high temperature fuel cells. Russ. J. Electrochem. 2014, 50, 694–699. [Google Scholar] [CrossRef]
- Kondratenko, M.S.; Ponomarev, I.I.; Gallyamov, M.O.; Razorenov, D.Y.; Volkova, Y.A.; Kharitonova, E.P.; Khokhlov, A.R. Novel composite Zr/PBI-O-PhT membranes for HT-PEFC applications. Beilstein J. Nanotechnol. 2013, 4, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Ponomarev, I.I.; Skupov, K.M.; Modestov, A.D.; Lysova, A.A.; Ponomarev, I.I.; Vtyurina, E.S. Cardo Polybenzimidazole (PBI-O-PhT) Based Membrane Reinforced with m-polybenzimidazole Electrospun Nanofiber Mat for HT-PEM Fuel Cell Applications. Membranes 2022, 12, 956. [Google Scholar] [CrossRef] [PubMed]
- Ponomarev, I.I.; Goryunov, E.I.; Petrovskii, P.V.; Ponomarev, I.I.; Volkova, Y.A.; Razorenov, D.Y.; Khokhlov, A.R. Synthesis of new monomer 3,3′-diamino-4,4′-bis{p-[(diethoxyphosphoryl)methyl]phenylamino}diphenyl sulfone and polybenzimidazoles on its basis. Dokl. Chem. 2009, 429, 315–320. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Ponomarev, I.I.; Goryunov, E.I.; Volkova, Y.A.; Razorenov, D.Y.; Starikova, Z.A.; Blagodatskikh, I.V.; Buzin, M.I.; Khokhlov, A.R. Chemical modification of cardo poly(benzimidazole) using “click” reaction for membranes of high-temperature hydrogen fuel cells. Dokl. Chem. 2012, 447, 227–232. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Razorenov, D.Y.; Ponomarev, I.I.; Volkova, Y.A.; Skupov, K.M.; Lysova, A.A.; Yaroslavtsev, A.B.; Modestov, A.D.; Buzin, M.I.; Klemenkova, Z.S. Polybenzimidazoles via polyamidation: A more environmentally safe process to proton conducting membrane for hydrogen HT-PEM fuel cell. Eur. Polym. J. 2021, 156, 110613. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Razorenov, D.Y.; Skupov, K.M.; Ponomarev, I.I.; Volkova, Y.A.; Lyssenko, K.A.; Lysova, A.A.; Vtyurina, E.S.; Buzin, M.I.; Klemenkova, Z.S. Self-Phosphorylated Polybenzimidazole: An Environmentally Friendly and Economical Approach for Hydrogen/Air High-Temperature Polymer-Electrolyte Membrane Fuel Cells. Membranes 2023, 13, 552. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Skupov, K.M.; Naumkin, A.V.; Basu, V.G.; Zhigalina, O.M.; Razorenov, D.Y.; Ponomarev, I.I.; Volkova, Y.A. Probing of complex carbon nanofiber paper as gas-diffusion electrode for high temperature polymer electrolyte membrane fuel cell. RSC Adv. 2019, 9, 257–267. [Google Scholar] [CrossRef]
- Skupov, K.M.; Ponomarev, I.I.; Vtyurina, E.S.; Volkova, Y.A.; Ponomarev, I.I.; Zhigalina, O.M.; Khmelenin, D.N.; Cherkovskiy, E.N.; Modestov, A.D. Proton-Conducting Polymer-Coated Carbon Nanofiber Mats for Pt-Anodes of High-Temperature Polymer-Electrolyte Membrane Fuel Cell. Membranes 2023, 13, 479. [Google Scholar] [CrossRef] [PubMed]
- Cetina-Mancilla, E.; González-Díaz, M.O.; Sulub-Sulub, R.; Zolotukhin, M.G.; González-Díaz, A.; Herrera-Kao, W.; Ruiz-Trevino, F.A.; Aguilar-Vega, M. Aging resistant, fluorinated aromatic polymers with ladderized, rigid kink-structured backbones for gas separations. J. Membr. Sci. 2022, 659, 120764. [Google Scholar] [CrossRef]
- Ghanem, B.S.; McKeown, N.B.; Budd, P.M.; Al-Harbi, N.M.; Fritsch, D.; Heinrich, K.; Starannikova, L.; Tokarev, A.; Yampolskii, Y. Synthesis, characterization, and gas permeation properties of a novel group of polymers with intrinsic microporosity: PIM-polyimides. Macromolecules 2009, 42, 7881–7888. [Google Scholar] [CrossRef]
- Han, S.H.; Lee, J.E.; Lee, K.-J.; Park, H.B.; Lee, Y.M. Highly gas permeable and microporous polybenzimidazole membrane by thermal rearrangement. J. Membr. Sci. 2010, 357, 143–151. [Google Scholar] [CrossRef]
- Kumbharkar, S.C.; Karadkar, P.B.; Kharul, U.K. Enhancement of gas permeation properties of polybenzimidazoles by systematic structure architecture. J. Membr. Sci. 2006, 286, 161–169. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2004; 368p. [Google Scholar]
- Lysova, A.A.; Ponomarev, I.I.; Skupov, K.M.; Vtyurina, E.S.; Lysov, K.A.; Yaroslavtsev, A.B. Effect of Organo-Silanes Structure on the Properties of Silane-Crosslinked Membranes Based on Cardo Polybenzimidazole PBI-O-PhT. Membranes 2022, 12, 1078. [Google Scholar] [CrossRef] [PubMed]
- Stern, S.A. The “barrer” permeability unit. J. Polym. Sci. A-2 Polym. Phys. 1968, 6, 1933–1934. [Google Scholar] [CrossRef]
- Lozano-Castello, D.; Cazorla-Amoros, D.; Linares-Solano, A. Usefulness of CO2 adsorption at 273 K for the characterization of porous carbons. Carbon 2004, 42, 1233–1242. [Google Scholar] [CrossRef]
- Ewing, M.B.; Lilley, T.H.; Olofsson, G.M.; Ratzsch, M.T.; Somsen, G. Standard quantities in chemical thermodynamics. Fugacities, activities and equilibrium constants for pure and mixed phases (IUPAC Recommendations 1994). Pure Appl. Chem. 1994, 66, 533–552. [Google Scholar] [CrossRef]
- Skupov, K.M.; Vtyurina, E.S.; Ponomarev, I.I.; Ponomarev, I.I.; Aysin, R.R. Prospective carbon nanofibers based on polymer of intrinsic microporosity (PIM-1): Pore structure regulation for higher carbon sequestration and renewable energy source applications. Polymer 2023, 264, 125546. [Google Scholar] [CrossRef]
- Vtyurina, E.S.; Ponomarev, I.I.; Naumkin, A.V.; Bukalov, S.S.; Aysin, R.R.; Ponomarev, I.I.; Zhigalina, O.M.; Khmelenin, D.N.; Skupov, K.M. Influence of the Polymer Precursor Structure on the Porosity of Carbon Nanofibers: Application as Electrode in High-Temperature Proton Exchange Membrane Fuel Cells. ACS Appl. Nano Mater. 2024, 7, 4313–4323. [Google Scholar] [CrossRef]
- Seselj, N.; Alfaro, S.M.; Bompolaki, E.; Cleemann, L.N.; Torres, T.; Azizi, K. Catalyst Development for High-Temperature Polymer Electrolyte Membrane Fuel Cell (HT-PEMFC) Applications. Adv. Mater. 2023, 35, 2302207. [Google Scholar] [CrossRef] [PubMed]
- Zucconi, A.; Hack, J.; Stocker, R.; Suter, T.A.M.; Rettie, A.J.E.; Brett, D.J.L. Challenges and opportunities for characterisation of high-temperature polymer electrolyte membrane fuel cells: A review. J. Mater. Chem. A 2024, 12, 8014–8064. [Google Scholar] [CrossRef]
- Linares-Solano, A.; Stoeckli, F. Commentary on the paper “On the adsorption affinity coefficient of carbon dioxide in microporous carbons” by E.S. Bickford et al. (Carbon 2004; 42: 1867–71). Carbon 2005, 43, 658–660. [Google Scholar] [CrossRef]
- Mateucci, S.; Yampolskii, Y.; Freeman, B.D.; Pinnau, I. Transport of Gases and Vapors in Glassy and Rubbery Polymers. In Materials Science of Membranes for Gas and Vapor Separation; Freeman, B.D., Yampolskii, Y., Pinnau, I., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 1–45. [Google Scholar] [CrossRef]
- Koros, W.J.; Zimmerman, C.M. Transport and Barrier Properties. In Comprehensive Desk Reference of Polymer Characterization and Analysis; Brady, R.F.J., Ed.; Oxford University Press: Washington, DC, USA, 2003; pp. 680–699. [Google Scholar]
- Ponomarev, I.I.; Skupov, K.M.; Zhigalina, O.M.; Naumkin, A.V.; Modestov, A.D.; Basu, V.G.; Sufiyanova, A.E.; Razorenov, D.Y.; Ponomarev, I.I. New Carbon Nanofiber Composite Materials Containing Lanthanides and Transition Metals Based on Electrospun Polyacrylonitrile for High Temperature Polymer Electrolyte Membrane Fuel Cell Cathodes. Polymers 2020, 12, 1340. [Google Scholar] [CrossRef] [PubMed]
- Skupov, K.M.; Ponomarev, I.I.; Vol’fkovich, Y.M.; Modestov, A.D.; Ponomarev, I.I.; Volkova, Y.A.; Razorenov, D.Y.; Sosenkin, V.E. The Effect of the Stabilization and Carbonization Temperatures on the Properties of Microporous Carbon Nanofiber Cathodes for Fuel Cells on Polybenzimidazole Membrane. Polym. Sci. Ser. C 2020, 62, 231–237. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Baurmeister, J. Properties of high-temperature PEFC Celtec®-P 1000 MEAs in start/stop operation mode. J. Power Sources 2008, 176, 428–434. [Google Scholar] [CrossRef]




| Solvent | C, mol/L | T, °C | Yeild, % | [η], dL/g * | Mw, kg/mol | Mn, kg/mol | Mw/Mn |
|---|---|---|---|---|---|---|---|
| DMA | 1.3 | 25 | 97 | 0.21 | 68 | 31 | 2.19 |
| DMA/Et3N | 1.3 | 25 | 96 | 0.19 | 50 | 21 | 2.38 |
| NMP | 1.0 | −10 | 99 | 0.69 | 78 | 38 | 2.05 |
| Polymer | H2, Barrer | O2, Barrer | N2, Barrer |
|---|---|---|---|
| PBI-4MeO-6F | 41.1 | 7.15 | 1.97 |
| PBI-O-PhT-P | 9.66 | 0.88 | 0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponomarev, I.I.; Volkova, Y.A.; Skupov, K.M.; Vtyurina, E.S.; Ponomarev, I.I.; Ilyin, M.M.; Nikiforov, R.Y.; Alentiev, A.Y.; Zhigalina, O.M.; Khmelenin, D.N.; et al. Unique Self-Phosphorylating Polybenzimidazole of the 6F Family for HT-PEM Fuel Cell Application. Int. J. Mol. Sci. 2024, 25, 6001. https://doi.org/10.3390/ijms25116001
Ponomarev II, Volkova YA, Skupov KM, Vtyurina ES, Ponomarev II, Ilyin MM, Nikiforov RY, Alentiev AY, Zhigalina OM, Khmelenin DN, et al. Unique Self-Phosphorylating Polybenzimidazole of the 6F Family for HT-PEM Fuel Cell Application. International Journal of Molecular Sciences. 2024; 25(11):6001. https://doi.org/10.3390/ijms25116001
Chicago/Turabian StylePonomarev, Igor I., Yulia A. Volkova, Kirill M. Skupov, Elizaveta S. Vtyurina, Ivan I. Ponomarev, Mikhail M. Ilyin, Roman Y. Nikiforov, Alexander Y. Alentiev, Olga M. Zhigalina, Dmitry N. Khmelenin, and et al. 2024. "Unique Self-Phosphorylating Polybenzimidazole of the 6F Family for HT-PEM Fuel Cell Application" International Journal of Molecular Sciences 25, no. 11: 6001. https://doi.org/10.3390/ijms25116001
APA StylePonomarev, I. I., Volkova, Y. A., Skupov, K. M., Vtyurina, E. S., Ponomarev, I. I., Ilyin, M. M., Nikiforov, R. Y., Alentiev, A. Y., Zhigalina, O. M., Khmelenin, D. N., Strelkova, T. V., & Modestov, A. D. (2024). Unique Self-Phosphorylating Polybenzimidazole of the 6F Family for HT-PEM Fuel Cell Application. International Journal of Molecular Sciences, 25(11), 6001. https://doi.org/10.3390/ijms25116001







