The Diagnostic and Prognostic Potentials of Non-Coding RNA in Cholangiocarcinoma
Abstract
1. Introduction
2. lncRNAs as Diagnostic and Prognostic Biomarkers in Cholangiocarcinoma
3. circRNAs as Diagnostic and Prognostic Biomarkers in Cholangiocarcinoma
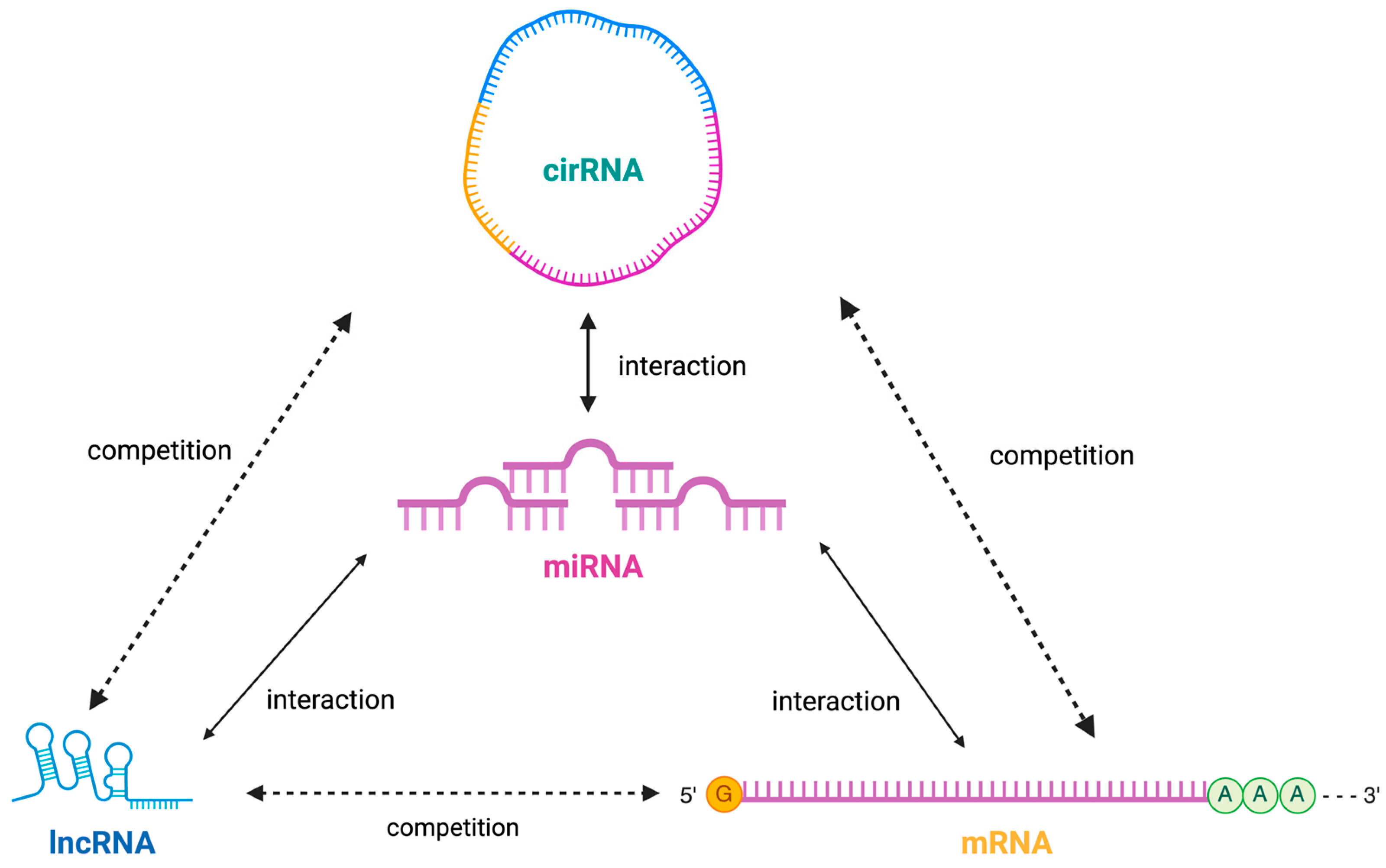
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Razumilava, N.; Gores, G.J. Cholangiocarcinoma. Lancet 2014, 383, 2168–2179. [Google Scholar] [CrossRef] [PubMed]
- Bergquist, A.; von Seth, E. Epidemiology of cholangiocarcinoma. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Tavolari, S.; Brandi, G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019, 39, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Clements, O.; Eliahoo, J.; Kim, J.U.; Taylor-Robinson, S.D.; Khan, S.A. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. J. Hepatol. 2020, 72, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- I Ilyas, S.; Borad, M.J.; Patel, T.; Gores, G.J.; Rizvi, S. Cholangiocarcinoma: Molecular Pathways and Therapeutic Opportunities. Semin. Liver Dis. 2014, 34, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- ENCODE Project Consortium; Birney, E.; Stamatoyannopoulos, J.A.; Dutta, A.; Guigo, R.; Gingeras, T.R.; Margulies, E.H.; Weng, Z.; Snyder, M.; Dermitzakis, E.T.; et al. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature 2007, 447, 799–816. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadou, E.; Jacob, L.S.; Slack, F.J. Non-coding RNA networks in cancer. Nat. Rev. Cancer 2018, 18, 5–18. [Google Scholar] [CrossRef]
- Yan, H.; Bu, P. Non-coding RNA in cancer. Essays Biochem. 2021, 65, 625–639. [Google Scholar] [CrossRef]
- Wangyang, Z.; Daolin, J.; yi, X.; Zhenglong, L.; Lining, H.; Yunfu, C.; Xingming, J. NcRNAs and Cholangiocarcinoma. J. Cancer 2018, 9, 100–107. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, Z.; Zhao, K.; Zhang, G.; Huang, S.; Zhao, Y. Role of noncoding RNAs in cholangiocarcinoma (Review). Int. J. Oncol. 2020, 57, 7–20. [Google Scholar] [CrossRef]
- Shi, T.; Morishita, A.; Kobara, H.; Masaki, T. The Role of microRNAs in Cholangiocarcinoma. Int. J. Mol. Sci. 2021, 22, 7627. [Google Scholar] [CrossRef]
- Kopp, F.; Mendell, J.T. Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell 2018, 172, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Gao, L.; Wang, Y.; Chiu, D.K.Y.; Wang, T.; Deng, Y. Advances in long noncoding RNAs: Identification, structure prediction and function annotation. Brief. Funct. Genom. 2015, 15, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef]
- Wu, Y.; Hayat, K.; Hu, Y.; Yang, J. Long Non-Coding RNAs as Molecular Biomarkers in Cholangiocarcinoma. Front. Cell Dev. Biol. 2022, 10, 890605. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Xiong, J.; Bai, Y.; Mao, J.; Lin, J.; Xu, W.; Zhang, H.; Chen, S.; Zhao, H. Construction and Investigation of a lncRNA-Associated ceRNA Regulatory Network in Cholangiocarcinoma. Front. Oncol. 2019, 9, 649. [Google Scholar] [CrossRef]
- Lapitz, A.; Arbelaiz, A.; O’rourke, C.J.; Lavin, J.L.; La Casta, A.; Ibarra, C.; Jimeno, J.P.; Santos-Laso, A.; Izquierdo-Sanchez, L.; Krawczyk, M.; et al. Patients with Cholangiocarcinoma Present Specific RNA Profiles in Serum and Urine Extracellular Vesicles Mirroring the Tumor Expression: Novel Liquid Biopsy Biomarkers for Disease Diagnosis. Cells 2020, 9, 721. [Google Scholar] [CrossRef]
- Shi, J.; Li, X.; Zhang, F.; Kong, L.; Zhang, X.; Cheng, Y.; Guan, Q.; Cao, X.; Zhu, W.; Ou, K.; et al. The Plasma LncRNA Acting as Fingerprint in Hilar Cholangiocarcinoma. Cell. Physiol. Biochem. 2018, 49, 1694–1702. [Google Scholar] [CrossRef]
- Zhang, F.; Wan, M.; Xu, Y.; Li, Z.; Leng, K.; Kang, P.; Cui, Y.; Jiang, X. Long noncoding RNA PCAT1 regulates extrahepatic cholangiocarcinoma progression via the Wnt/β-catenin-signaling pathway. Biomed. Pharmacother. 2017, 94, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Gong, X.; Wu, J.; Hu, Z.; Zhang, Q.; Gong, J.; Zhu, X. Effect of lncRNA PVT1/miR186/KLF5 Axis on the Occurrence and Progression of Cholangiocarcinoma. BioMed Res. Int. 2021, 2021, 8893652. [Google Scholar] [CrossRef]
- Ge, X.; Wang, Y.; Nie, J.; Li, Q.; Tang, L.; Deng, X.; Wang, F.; Xu, B.; Wu, X.; Zhang, X.; et al. The Diagnostic/Prognostic Potential and Molecular Functions of Long Non-Coding RNAs in the Exosomes Derived from the Bile of Human Cholangiocarcinoma. Oncotarget 2017, 8, 69995–70005. [Google Scholar] [CrossRef]
- Liang, Z.; Zhu, B.; Meng, D.; Shen, X.; Li, X.; Wang, Z.; Li, L. Down-regulation of lncRNA-NEF indicates poor prognosis in intrahepatic cholangiocarcinoma. Biosci. Rep. 2019, 39, BSR20181573. [Google Scholar] [CrossRef]
- Li, J.; Jiang, X.; Xu, Y.; Kang, P.; Huang, P.; Meng, N.; Wang, H.; Zheng, W.; Wang, H.; Wang, Z.; et al. YY1-induced DLEU1/miR-149-5p Promotes Malignant Biological Behavior of Cholangiocarcinoma through Upregulating YAP1/TEAD2/SOX2. Int. J. Biol. Sci. 2022, 18, 4301–4315. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Z.; Jiang, X.; Cui, Y. Overexpression of long noncoding RNA H19 indicates a poor prognosis for cholangiocarcinoma and promotes cell migration and invasion by affecting epithelial-mesenchymal transition. Biomed. Pharmacother. 2017, 92, 17–23. [Google Scholar] [CrossRef]
- Han, B.-W.; Ye, H.; Wei, P.-P.; He, B.; Han, C.; Chen, Z.-H.; Chen, Y.-Q.; Wang, W.-T. Global identification and characterization of lncRNAs that control inflammation in malignant cholangiocytes. BMC Genom. 2018, 19, 735. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.M.; Li, Z.L.; Li, J.L.; Zheng, W.Y.; Li, X.H.; Cui, Y.F.; Sun, D.J. LncRNA CCAT1 as the unfavorable prognostic biomarker for cholangiocarcinoma. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1242–1247. [Google Scholar] [PubMed]
- Bai, J.; Tang, R.; Shang, J.; Qi, S.; Yu, G.; Sun, C. Upregulation of long non-coding RNA CCAT2 indicates a poor prognosis and promotes proliferation and metastasis in intrahepatic cholangiocarcinoma. Mol. Med. Rep. 2018, 17, 5328–5335. [Google Scholar] [CrossRef]
- Jiang, X.; Li, J.; Wang, W.; Hu, Z.; Guan, C.; Zhao, Y.; Li, W.; Cui, Y. AR-induced ZEB1-AS1 represents poor prognosis in cholangiocarcinoma and facilitates tumor stemness, proliferation and invasion through mediating miR-133b/HOXB8. Aging 2020, 12, 1237–1255. [Google Scholar] [CrossRef]
- Angenard, G.; Merdrignac, A.; Louis, C.; Edeline, J.; Coulouarn, C. Expression of long non-coding RNA ANRIL predicts a poor prognosis in intrahepatic cholangiocarcinoma. Dig. Liver Dis. 2019, 51, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.L.; Tang, Y.; Zou, J.; Zhu, B.; Xiao, X.; Wang, J.; Wen, H.; Ye, S. Overexpression of long non-coding RNA CRNDE facilitates epithelial-mesenchymal transition and correlates with poor prognosis in intrahepatic cholangiocarcinoma. Oncol. Lett. 2018, 15, 4105–4112. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Li, J.; Wang, W.; Tian, T.; Wang, G.; Zhao, Y.; Li, W.; Cui, Y. Long Non-coding RNA FOXD2-AS1 Promotes Proliferation, Migration, and Invasion in Cholangiocarcinoma Through Regulating miR-760/E2F3 Axis. Dig. Dis. Sci. 2022, 67, 546–558. [Google Scholar] [CrossRef]
- Wang, X.P.; Song, J.; Liu, G.T.; Wang, J.J.; Guo, H.F. Upregulation of gastric adenocarcinoma predictive long intergenic non coding RNA promotes progression and predicts poor prognosis in perihilar cholangiocarcinoma. Oncol. Lett. 2018, 16, 3964–3972. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Zhang, J.; Xu, J.; Wu, C.; Zeng, Z.; Deng, T.; Wang, X.; Li, X. Long non-coding RNA HOTAIR promotes tumorigenesis and forecasts a poor prognosis in cholangiocarcinoma. Sci. Rep. 2018, 8, 29737. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Chen, S.; Yang, X. HOTTIP Enhances Gemcitabine and Cisplatin Resistance Through Sponging miR-637 in Cholangiocarcinoma. Front. Oncol. 2021, 11, 664916. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wei, B.; Liu, Z.; Chen, Y.; Yang, X.; Zhou, Y.; Song, H. Up-regulated LINC00261 predicts a poor prognosis and promotes a metastasis by EMT process in cholangiocarcinoma. Pathol. Res. Pract. 2020, 216, 152733. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Cao, Y.; Xiao, J.; Zhu, S.; Liang, C.; Zhang, X.; Zhang, C. Long non-coding RNA LINC00665 promotes gemcitabine resistance of Cholangiocarcinoma cells via regulating EMT and stemness properties through miR-424-5p/BCL9L axis. Cell Death Dis. 2021, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Z.; Wang, X.; He, Q.; Tan, J.; Li, C.; Wang, H. Yin Yang 1-induced LINC00667 up-regulates pyruvate dehydrogenase kinase 1 to promote proliferation, migration and invasion of cholangiocarcinoma cells by sponging miR-200c-3p. Hum. Cell 2021, 34, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, Y.; Zhang, P.; Xu, L.; Zhang, L.; Shen, S.; Hu, L. Long noncoding RNA LINC01296 promotes tumor growth and progression by sponging miR-5095 in human cholangiocarcinoma. Int. J. Oncol. 2018, 52, 1777–1786. [Google Scholar] [CrossRef]
- Zhang, B.; Tang, J.; Li, C.; Meng, L.; Ding, B.; Xu, Z.; Guo, Q. Long non-coding RNA LOXL1-AS1 acts as a ceRNA for miR-324-3p to contribute to cholangiocarcinoma progression via modulation of ATP-binding cassette transporter A1. Biochem. Biophys. Res. Commun. 2019, 513, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wang, S.; Zhang, Q.; Zhang, C.; Xu, W.; Wang, Y.; Xu, L. Long non-coding RNA NNT-AS1 functions as an oncogenic gene through modulating MIR-485/BCl9 in cholangiocarcinoma. Cancer Manag. Res. 2019, 11, 7739–7749. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Lu, M.; Li, W.; Wang, P.; Jiang, Y.; Yu, L.; Zhang, L. Long Non-Coding RNA-PAICC Promotes the Tumorigenesis of Human Intrahepatic Cholangiocarcinoma by Increasing YAP1 Transcription. Front. Oncol. 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jiang, X.; Cui, Y. Upregulated long noncoding RNA PANDAR predicts an unfavorable prognosis and promotes tumorigenesis in cholangiocarcinoma. Onco Targets Ther. 2017, 10, 2873–2883. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Li, F.; Liu, L.; Yu, S.; Wang, H.; Gao, X.; Liu, G.; Zhao, Y.; Qiu, G.; Jiang, X. PCAT1 induced by transcription factor YY1 promotes cholangiocarcinoma proliferation, migration and invasion by sponging miR-216a-3p to up-regulate oncogene BCL3. Biol. Chem. 2021, 402, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Ma, D.; Li, F.; Sun, D.; Zeng, Z. Lnc-PKD2-2-3, identified by long non-coding RNA expression profiling, is associated with pejorative tumor features and poor prognosis, enhances cancer stemness and may serve as cancer stem-cell marker in cholangiocarcinoma. Int. J. Oncol. 2019, 55, 45–58. [Google Scholar] [CrossRef]
- Sun, D.; Li, F.; Liu, L.; Yu, S.; Wang, H.; Gao, X.; Liu, G.; Zhao, Y.; Qiu, G.; Jiang, X. PSMA3-AS1 induced by transcription factor PAX5 promotes cholangiocarcinoma proliferation, migration and invasion by sponging miR-376a-3p to up-regulate LAMC1. Aging 2022, 14, 509–525. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Wei, X.; Zhu, H.; Zhu, L.; Li, T.; Li, W. LncRNA-SNHG3 is an Independent Prognostic Biomarker of Intrahepatic Cholangiocarcinoma. Int. J. Clin. Exp. Pathol. 2019. Available online: www.ijcep.com/ (accessed on 29 September 2023).
- Guan, C.; Guo, X.; Zhou, M.; He, X.; Li, F.; Fu, D.; Chen, T. Knockdown of lncRNA SNHG20 Suppressed the Proliferation of Cholangiocarcinoma by Sponging miR-520f-3p. Cancer Biother. Radiopharm. 2020. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Ren, H.; Zhang, Y.; Zuo, D.; Zhao, L.; Zhang, H. Overexpressed long noncoding RNA Sox2ot predicts poor prognosis for cholangiocarcinoma and promotes cell proliferation and invasion. Gene 2018, 645, 131–136. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, X.; Cui, Y.; Li, F.; Wang, H.; Sun, D.; Chen, T.; Liu, L. The Prognostic Potential and Carcinogenesis of Long Non-coding RNA TUG1 in Human Cholangiocarcinoma. Oncotarget 2017. Available online: www.impactjournals.com/oncotarget (accessed on 29 September 2023).
- Xu, Y.; Jiang, X.; Cui, Y.; Li, F.; Wang, H.; Sun, D.; Chen, T.; Liu, L. Long Non-Coding RNA UCA1 Indicates an Unfavorable Prognosis and Promotes Tumorigenesis via Regulating AKT/GSK-3β Signaling Pathway in Cholangiocarcinoma. Oncotarget 2017. Available online: www.impactjournals.com/oncotarget (accessed on 29 September 2023).
- Jiao, M.; Wang, Y.; Zhang, P.; Peng, Z.; Cui, X.; Jiang, G.; Li, W. Long non-coding RNA ZEB1-AS1 predicts a poor prognosis and promotes cancer progression through the miR-200a/ZEB1 signaling pathway in intrahepatic cholangiocarcinoma. Int. J. Oncol. 2020, 56, 1455–1467. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ren, H.; Wang, Z.; Zuo, D.; Li, X.; Zhao, L.; Li, S.; Zhang, H. Up-regulation of ZFAS1 indicates dismal prognosis for cholangiocarcinoma and promotes proliferation and metastasis by modulating USF1 via miR-296-5p. J. Cell Mol. Med. 2019, 23, 8258–8268. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-X.; Chen, L.-L. Circular RNAs: Characterization, cellular roles, and applications. Cell 2022, 185, 2016–2034. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Leng, K.; Yao, Y.; Kang, P.; Liao, G.; Han, Y.; Shi, G.; Ji, D.; Huang, P.; Zheng, W.; et al. A Circular RNA, Cholangiocarcinoma-Associated Circular RNA 1, Contributes to Cholangiocarcinoma Progression, Induces Angiogenesis, and Disrupts Vascular Endothelial Barriers. Hepatology 2021, 73, 1419–1435. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hu, Y.; Lv, X.; Li, B.; Gu, D.; Li, Y.; Sun, Y.; Su, Y. Circ-0000284 arouses malignant phenotype of cholangiocarcinoma cells and regulates the biological functions of peripheral cells through cellular communication. Clin. Sci. 2019, 133, 1935–1953. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, Z.; Li, Z.; Li, S.; Wen, Z.; Cao, L.; Chen, Y.; Xue, P.; Li, H.; Zhang, D. Tumor-associated macrophages promote cholangiocarcinoma progression via exosomal Circ_0020256. Cell Death Dis. 2022, 13, 94. [Google Scholar] [CrossRef]
- Xu, Y.; Gao, P.; Wang, Z.; Su, Z.; Liao, G.; Han, Y.; Cui, Y.; Yao, Y.; Zhong, X. Circ-LAMP1 contributes to the growth and metastasis of cholangiocarcinoma via miR-556-5p and miR-567 mediated YY1 activation. J. Cell. Mol. Med. 2021, 25, 3226–3238. [Google Scholar] [CrossRef]
- Li, H.; Lan, T.; Liu, H.; Liu, C.; Dai, J.; Xu, L.; Cai, Y.; Hou, G.; Xie, K.; Liao, M.; et al. IL-6-induced cGGNBP2 encodes a protein to promote cell growth and metastasis in intrahepatic cholangiocarcinoma. Hepatology 2021, 75, 1402–1419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, Y.; Wang, W.; Yu, S.; Liu, L.; Sun, D.; Li, W.; Jiang, X. Upregulation of circ_0059961 suppresses cholangiocarcinoma development by modulating miR-629-5p/SFRP2 axis. Pathol.-Res. Pract. 2022, 234, 153901. [Google Scholar] [CrossRef]
- Xu, Y.-P.; Dong, Z.-N.; Wang, S.-W.; Zheng, Y.-M.; Zhang, C.; Zhou, Y.-Q.; Zhao, Y.-J.; Zhao, Y.; Wang, F.; Peng, R.; et al. circHMGCS1-016 reshapes immune environment by sponging miR-1236-3p to regulate CD73 and GAL-8 expression in intrahepatic cholangiocarcinoma. J. Exp. Clin. Cancer Res. 2021, 40, 290. [Google Scholar] [CrossRef]
- Weber, S.M.; Ribero, D.; O’Reilly, E.M.; Kokudo, N.; Miyazaki, M.; Pawlik, T.M. Intrahepatic Cholangiocarcinoma: Expert consensus statement. HPB 2015, 17, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Macias, R.I.R.; Kornek, M.; Rodrigues, P.M.; Paiva, N.A.; Castro, R.E.; Urban, S.; Pereira, S.P.; Cadamuro, M.; Rupp, C.; Loosen, S.H.; et al. Diagnostic and prognostic biomarkers in cholangiocarcinoma. Liver Int. 2019, 39, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Tshering, G.; Dorji, P.W.; Chaijaroenkul, W.; Na-Bangchang, K. Biomarkers for the Diagnosis of Cholangiocarcinoma: A Systematic Review. Am. J. Trop. Med. Hyg. 2018, 98, 1788–1797. [Google Scholar] [CrossRef] [PubMed]
| LncRNA | Sample | Expression | Clinical Application | OS | AUC | miRNA Interactions | Ref. |
|---|---|---|---|---|---|---|---|
| ANRIL | Tissue | Up | Tumor size | √ | - | miR-99a, miR-125a | [31] |
| ASAP1-IT1 | Tissue | Up | Lymph node invasion, TNM stage, postoperative recurrence | √ | - | miR-30c, miR-124 | [15] |
| CCAT1 | Tissue | Up | Lymph node invasion, TNM stage | √ | 0.831 | miR-155, miR-143 | [28] |
| CCAT2 | Tissue | Up | Lymph node invasion, TNM stage, microvascular invasion | √ | 0.702 | miR-145, miR-143 | [29] |
| CRNDE | Tissue | Up | Poor differentiation, tumor size, lymph node invasion, TNM stage | √ | - | miR-136, miR-217 | [32] |
| DLEU1 | Tissue | Up | Lymph node invasion, TNM stage | √ | 0.747 | miR-22, miR-181a | [25] |
| FOXD2-AS1 | Tissue | Up | Lymph node invasion, TNM stage | √ | 0.741 | miR-185, miR-124 | [33] |
| GAPLINC | Tissue | Up | Lymph node invasion, TNM stage | √ | 0.713 | miR-211, miR-29b | [34] |
| H19 | Tissue | Up | Tumor size, TNM stage, postoperative recurrence | √ | - | miR-675, miR-200 | [26] |
| HOTAIR | Tissue | Up | Lymph node invasion, TNM stage, postoperative recurrence | √ | - | miR-141, miR-23b | [35] |
| HOTTIP | Tissue | Up | Lymph node invasion, distant metastasis | √ | - | miR-615, miR-148a | [36] |
| HOXD-AS1 | Tissue | Up | Lymph node invasion, TNM stage | √ | 0.786 | miR-125b, miR-130a | [37] |
| LINC00261 | Tissue | Up | Tumor size, lymph node invasion, TNM stage, postoperative recurrence | √ | - | miR-588, miR-34a | [38] |
| LINC00665 | Tissue | Up | Lymph node invasion, distant metastasis, TNM stage | √ | - | miR-138, miR-3194 | [39] |
| LINC00667 | Tissue | Up | Lymph node invasion, TNM stage | √ | 0.830 | miR-361, miR-129 | [40] |
| LINC01296 | Tissue | Up | Lymph node invasion, TNM stage | √ | - | miR-26a, miR-98 | [41] |
| lncRNA-NEF | Plasma | Down | Diagnostic marker, lymph node invasion, TNM stage | √ | 0.864 | miR-155, miR-497 | [24] |
| LOXL1-AS1 | Tissue | Up | Lymph node invasion, TNM stage | √ | - | miR-589, miR-23a | [42] |
| MALAT1 | Tissue, plasma | Up | Tumor size, pathological T stage, perineural invasion | √ | - | miR-145, miR-124 | [20] |
| NNT-AS1 | Tissue | Up | Tumor size, lymph node invasion, TNM stage | √ | - | miR-483, miR-223 | [43] |
| PAICC | Tissue | Up | Tumor number, tumor size, vascular invasion | √ | - | miR-125b, miR-34c | [44] |
| PANDAR | Tissue | Up | Lymph node invasion, TNM stage, postoperative recurrence | √ | - | miR-150, miR-10b | [45] |
| PCAT1 | Tissue | Up | Lymph node invasion, TNM stage | √ | 0.823 | miR-203, miR-21 | [21,46] |
| PKD2-2-3 | Tissue | Up | Poor differentiation, TNM stage | √ | - | miR-125a, miR-192 | [47] |
| PSMA3-AS1 | Tissue | Up | Lymph node invasion, TNM stage | √ | 0.793 | miR-489, miR-214 | [48] |
| SNHG3 | Tissue | Up | Lymph node invasion, distant metastasis, TNM stage | √ | - | miR-338, miR-124 | [49] |
| SNHG20 | Tissue | Up | Lymph node invasion, distant metastasis, TNM stage | √ | 0.748 | miR-140, miR-224 | [50] |
| Sox2ot | Tissue | Up | Lymph node invasion, TNM stage, postoperative recurrence | √ | - | miR-211, miR-134 | [51] |
| TUG1 | Tissue | Up | Tumor stage, intrahepatic metastasis, lymph node metastasis, perineural invasion | √ | - | miR-299, miR-335 | [52] |
| UCA1 | Tissue | Up | Lymph node invasion, TNM stage, postoperative recurrence | √ | - | miR-138, miR-507 | [53] |
| ZEB1-AS1 | Tissue | Up | Lymph node invasion, TNM stage | √ | 0.749 | miR-200b, miR-141 | [54] |
| ZFAS1 | Tissue | Up | Lymph node invasion, TNM stage, postoperative recurrence | √ | - | miR-150, miR-1271 | [55] |
| CircRNA | Sample | Expression | Clinical Application | OS | AUC | miRNA Interactions | Ref. |
|---|---|---|---|---|---|---|---|
| Cdr1as | Tissue | Up | Lymph node infiltration, progressed TNM, postoperative recurrence | √ | - | miR-7 | [56] |
| hsa_circ_102066 (circ-CCAC1) | Bile, Serum EVs | Up | Diagnostic marker, postoperative recurrence | √ | 0.759 | miR-514a-5p, miR-320a | [57] |
| hsa_circ_0000284 (cir-HIPK3) | Plasma exosomes | Up | Biological functions modulation | - | - | miR-124 | [58] |
| hsa_circ_0020256 (cir-NSMCE4A) | Tumor-associated macrophages | Up | Proliferation, migration, invasion | - | - | miR-25-3p, miR-145 | [59] |
| hsa_circ_0043469 (cir-ERBB2) | Bile exosomes | Up | CCA progression, metastasis | - | - | miR-217, miR-331-3p | [60] |
| hsa_circ_0030998 (cir-LAMP1) | Tissue | Up | Postoperative recurrence rates | - | - | miR-198, miR-124-3p | [61] |
| hsa_circ_0003930 (cir-GGNBP2) | Tissue | Up | Shorter OS, shorter RFS after surgical resection | √ | - | miR-200b, miR-145 | [62] |
| hsa_circ_0059961 (cir-ITCH) | Tissue | Down | Positive correlation with survival time | √ | - | miR-7, miR-214-3p | [63] |
| hsa_circ_0008621 (cir-HMGCS1) | Tissue | Up | Shorter survival time, higher recurrence rate | √ | - | miR-140-3p, miR-361-3p | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, R.; Ribeiro, I.P.; Carreira, I.M.; Tralhão, J.G. The Diagnostic and Prognostic Potentials of Non-Coding RNA in Cholangiocarcinoma. Int. J. Mol. Sci. 2024, 25, 6002. https://doi.org/10.3390/ijms25116002
Andrade R, Ribeiro IP, Carreira IM, Tralhão JG. The Diagnostic and Prognostic Potentials of Non-Coding RNA in Cholangiocarcinoma. International Journal of Molecular Sciences. 2024; 25(11):6002. https://doi.org/10.3390/ijms25116002
Chicago/Turabian StyleAndrade, Rita, Ilda Patrícia Ribeiro, Isabel Marques Carreira, and José Guilherme Tralhão. 2024. "The Diagnostic and Prognostic Potentials of Non-Coding RNA in Cholangiocarcinoma" International Journal of Molecular Sciences 25, no. 11: 6002. https://doi.org/10.3390/ijms25116002
APA StyleAndrade, R., Ribeiro, I. P., Carreira, I. M., & Tralhão, J. G. (2024). The Diagnostic and Prognostic Potentials of Non-Coding RNA in Cholangiocarcinoma. International Journal of Molecular Sciences, 25(11), 6002. https://doi.org/10.3390/ijms25116002







