Current Insights into Sublethal Effects of Pesticides on Insects
Abstract
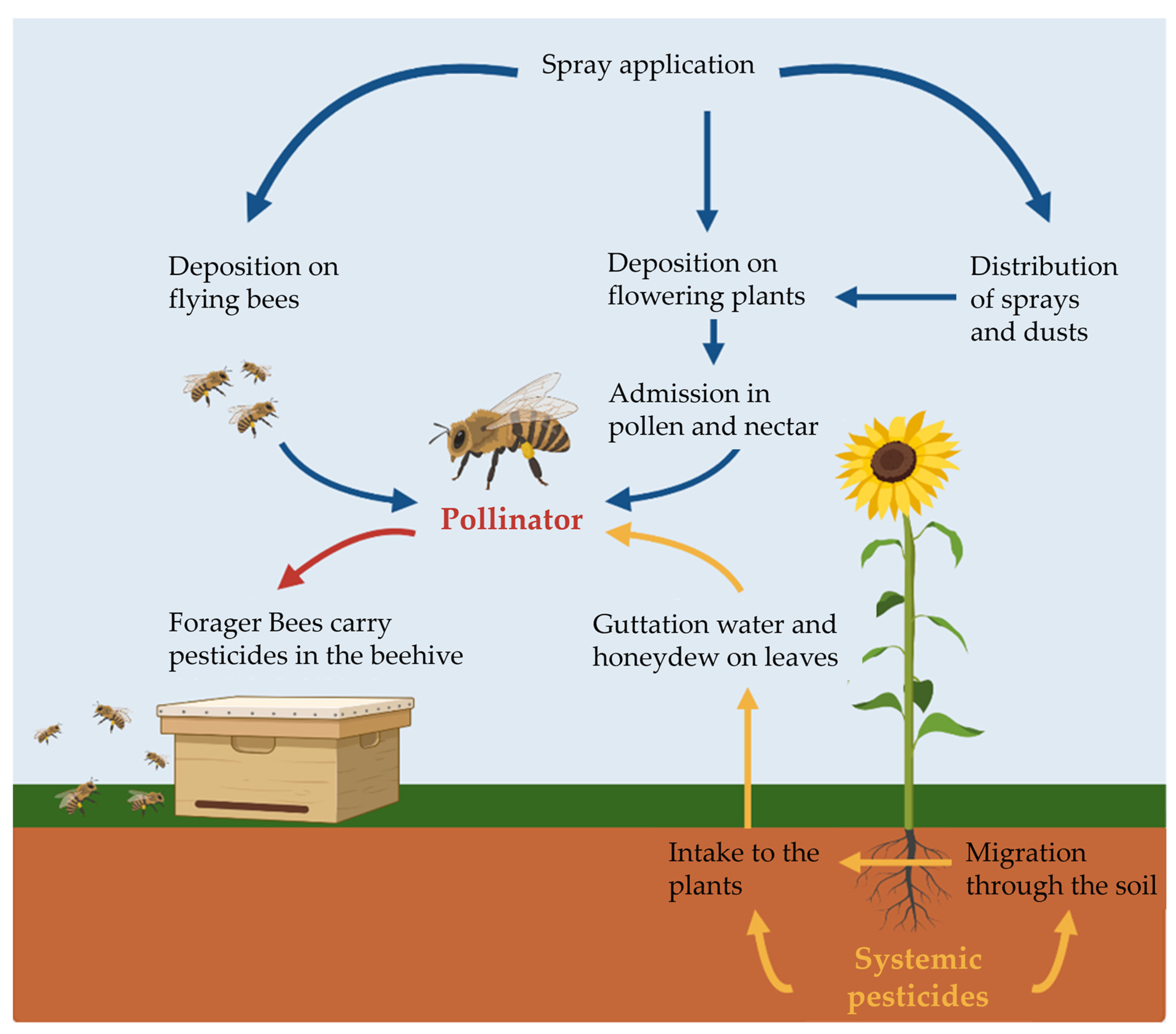
:1. Introduction
2. Physiological Effects
2.1. Biochemistry and Neurochemistry
2.2. Immunology
2.3. Tissues
2.4. Longevity and Development
2.5. Fecundity and Reproduction
2.6. Impact on Populations and Communities
3. Behavioral Effects
3.1. Mobility
3.2. Feeding Behavior
3.3. Oviposition Behavior
3.4. Navigation and Orientation
3.5. Learning Behavior
4. Synergistic Interactions
5. Future Prospects
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vogel, G. Where have all the insects gone? Science 2017, 356, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Leather, S.R. “Ecological Armageddon”—More evidence for the drastic decline in insect numbers. Ann. Appl. Biol. 2018, 172, 1–3. [Google Scholar] [CrossRef]
- Thomas, C.D.; Jones, T.H.; Hartley, S.E. ‘Insectageddon’: A call for more robust data and rigorous analyses. Glob. Chang. Biol. 2019, 25, 1891–1892. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- van Klink, R.; Bowler, D.E.; Gongalsky, K.B.; Swengel, A.B.; Gentile, A.; Chase, J.M. Meta-analysis reveals declines in terrestrial but increases in freshwater insect abundances. Science 2020, 368, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.L. Global insect decline: Comments on Sánchez-Bayo and Wyckhuys (2019). Biol. Conserv. 2019, 233, 332–333. [Google Scholar] [CrossRef]
- Simmons, B.I.; Balmford, A.; Bladon, A.J.; Christie, A.P.; De Palma, A.; Dicks, L.V.; Gallego-Zamorano, J.; Johnston, A.; Martin, P.A.; Purvis, A.; et al. Worldwide insect declines: An important message, but interpret with caution. Ecol. Evol. 2019, 9, 3678–3680. [Google Scholar] [CrossRef] [PubMed]
- Desquilbet, M.; Gaume, L.; Grippa, M.; Céréghino, R.; Humbert, J.F.; Bonmatin, J.M.; Cornillon, P.A.; Maes, D.; Van Dyck, H.; Goulson, D. Comment on ‘Meta-analysis reveals declines in terrestrial but increases in freshwater insect abundances’. Science 2020, 370, eabd8947. [Google Scholar] [CrossRef]
- Rothamsted Research. Available online: https://insectsurvey.com/ (accessed on 4 October 2023).
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef] [PubMed]
- Crossley, M.S.; Meier, A.R.; Baldwin, E.M.; Berry, L.L.; Crenshaw, L.C.; Hartman, G.L.; Lagos-Kutz, D.; Nichols, D.H.; Patel, K.; Varriano, S.; et al. No net insect abundance and diversity declines across US Long Term Ecological Research sites. Nat. Ecol. Evol. 2020, 4, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, F.; Weichert, F.G.; Lozano, V.L.; Groh, K.J.; Bálint, M.; Baumann, L.; Bässler, C.; Brack, W.; Brandl, B.; Curtius, J.; et al. Better integration of chemical pollution research will further our understanding of biodiversity loss. Nat. Ecol. Evol. 2023, 7, 1552–1555. [Google Scholar] [CrossRef] [PubMed]
- Vilcinskas, A. Pathogens associated with invasive or introduced insects threaten the health and diversity of native species. Curr. Opin. Insect Sci. 2019, 33, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Sharma, A.; Shukla, A.; Attri, K.; Kumar, M.; Kumar, P.; Suttee, A.; Singh, G.; Barnwal, R.P.; Singla, N. Global trends in pesticides: A looming threat and viable alternatives. Ecotoxicol. Environ. Saf. 2020, 201, 110812. [Google Scholar] [CrossRef] [PubMed]
- FAO. FAOSTAT Analytical Briefs Series No. 70. Rome. In Pesticides Use and Trade, 1990–2021; FAO: Rome, Italy, 2023. [Google Scholar] [CrossRef]
- Alkassab, A.T.; Kirchner, W.H. Sublethal exposure to neonicotinoids and related side effects on insect pollinators: Honeybees, bumblebees, and solitary bees. J. Plant Dis. Prot. 2017, 124, 1–30. [Google Scholar] [CrossRef]
- Serrão, J.E.; Plata-Rueda, A.; Martínez, L.C.; Zanuncio, J.C. Side-effects of pesticides on non-target insects in agriculture: A mini-review. Naturwissenschaften 2022, 109, 17. [Google Scholar] [CrossRef]
- Hrynko, I.; Kaczyński, P.; Łozowicka, B. A global study of pesticides in bees: QuEChERS as a sample preparation methodology for their analysis—Critical review and perspective. Sci. Total Environ. 2021, 792, 148385. [Google Scholar] [CrossRef] [PubMed]
- Tosi, S.; Sfeir, C.; Carnesecchi, E.; van Engelsdorp, D.; Chauzat, M.P. Lethal, sublethal, and combined effects of pesticides on bees: A meta-analysis and new risk assessment tools. Sci. Total Environ. 2022, 844, 156857. [Google Scholar] [CrossRef] [PubMed]
- Bendahou, N.; Bounias, M.; Fléché, C. Toxicity of cypermethrin and fenitrothion on the hemolymph carbohydrates, head acetylcholinesterase, and thoracic muscle Na+, K+-ATPase of emerging honeybees (Apis mellifera mellifera. L). Ecotoxicol. Environ. Saf. 1999, 44, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Vandame, R.; Belzunces, L.P. Joint actions of deltamethrin and azole fungicides on honey bee thermoregulation. Neurosci. Lett. 1998, 251, 57–60. [Google Scholar] [CrossRef]
- Papaefthimiou, C.; Theophilidis, G. The cardiotoxic action of the pyrethroid insecticide deltamethrin, the azole fungicide prochloraz, and their synergy on the semi-isolated heart of the bee Apis mellifera macedonica. Pestic. Biochem. Phys. 2001, 69, 77–91. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The Sublethal Effects of Pesticides on Beneficial Arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Decourtye, A.; Armengaud, C.; Renou, M.; Devillers, J.; Cluzeau, S.; Gauthier, M.; Pham-Delègue, M.H. Imidacloprid impairs memory and brain metabolism in the honeybee (Apis mellifera L.). Pestic. Biochem. Phys. 2004, 78, 83–92. [Google Scholar] [CrossRef]
- James, R.R.; Xu, J. Mechanisms by which pesticides affect insect immunity. J. Invertebr. Pathol. 2012, 109, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.; Griekscheit, K.; Siede, R.; Grosse, R.; Meixner, M.; Büchler, R. Immunosuppression in Honeybee Queens by the Neonicotinoids Thiacloprid and Clothianidin. Sci. Rep. 2017, 7, 4673. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.; Gorenflo, A.; Siede, R.; Meixner, M.; Büchler, R. The neonicotinoids thiacloprid, imidacloprid, and clothianidin affect the immunocompetence of honey bees (Apis mellifera L.). J. Insect Physiol. 2016, 86, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.; Hohnheiser, B.; Sgolastra, F.; Bosch, J.; Meixner, M.D.; Büchler, R. Immunosuppression response to the neonicotinoid insecticide thiacloprid in females and males of the red mason bee Osmia bicornis L. Sci. Rep. 2020, 10, 4670. [Google Scholar] [CrossRef] [PubMed]
- George, P.J.E.; Ambrose, D.P. Toxic effects of insecticides in the histomorphology of alimentary canal, testis and ovary in a reduviid Rhynocoris kumarii Ambrose and Livingstone (Hemiptera: Reduviidae). J. Adv. Zool. 2004, 25, 46–50. [Google Scholar]
- Delpuech, J.M.; Frey, F.; Carton, Y. Action of insecticides on the cellular immune reaction of Drosophila melanogaster against the parasitoid Leptopilina boulardi. Environ. Toxicol. Chem. 1996, 15, 2267–2271. [Google Scholar] [CrossRef]
- Chmiel, J.A.; Daisley, B.A.; Burton, J.P.; Reid, G. Deleterious Effects of Neonicotinoid Pesticides on Drosophila melanogaster Immune Pathways. Mbio 2019, 10, e01395-19. [Google Scholar] [CrossRef] [PubMed]
- Delpuech, J.M.; Tekinel-Ozalp, P. Epigenetic influences of insecticide on host-parasitoid relations. Redia 1991, 74, 417–424. [Google Scholar]
- Tomé, H.V.V.; Schmehl, D.R.; Wedde, A.E.; Godoy, R.S.M.; Ravaiano, S.V.; Guedes, R.N.C.; Martins, G.F.; Ellis, J.D. Frequently encountered pesticides can cause multiple disorders in developing worker honey bees. Environ. Pollut. 2020, 256, 113420. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, D.E.; Latorre-Estivalis, J.M.; Ons, S.; Farina, W.M. Chronic exposure to glyphosate induces transcriptional changes in honey bee larva: A toxicogenomic study. Environ. Pollut. 2020, 261, 114148. [Google Scholar] [CrossRef] [PubMed]
- Castelli, L.; Balbuena, S.; Branchiccela, B.; Zunino, P.; Liberti, J.; Engel, P.; Antúnez, K. Impact of chronic exposure to sublethal doses of glyphosate on honey bee immunity, gut microbiota and infection by pathogens. Microorganisms 2021, 9, 845. [Google Scholar] [CrossRef] [PubMed]
- Motta, E.V.S.; Powell, J.E.; Moran, N.A. Glyphosate induces immune dysregulation in honey bees. Anim. Microbiome 2022, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Samsel, A.; Seneff, S. Glyphosate’s suppression of cytochrome P450 enzymes and amino acid biosynthesis by the gut microbiome: Pathways to modern diseases. Entropy 2013, 15, 1416–1463. [Google Scholar] [CrossRef]
- Battisti, L.; Pottrich, M.; Lozano, E.R.; Bueno dos Reis Martinez, C.; Sofia, S.H. Review on the sublethal effects of pure and formulated glyphosate on bees: Emphasis on social bees. J. Appl. Entomol. 2022, 147, 1–18. [Google Scholar] [CrossRef]
- Bantz, A.; Camon, J.; Froger, J.A.; Goven, D.; Raymond, V. Exposure to sublethal doses of insecticide and their effects on insects at cellular and physiological levels. Curr. Opin. Insect Sci. 2018, 30, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Castro, G.; Cáceres-Jensen, L.; Guerrero-Bosagna, C.; Villagra, C. Insect Epigenetic Mechanisms Facing Anthropogenic-Derived Contamination, an Overview. Insects 2021, 12, 780. [Google Scholar] [CrossRef]
- Wu, J.Y.; Anelli, C.M.; Sheppard, W.S. Sub-lethal effects of pesticide residues in brood comb on worker honey bee (Apis mellifera) development and longevity. PLoS ONE 2011, 6, e14720. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.; Béguin, M.; Requier, F.; Rollin, O.; Odoux, J.F.; Aupinel, P.; Aptel, J.; Tchamitchian, S.; Decourtye, A. A Common Pesticide Decreases Foraging Success and Survival in Honey Bees. Science 2012, 336, 348–350. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, W.F.; De Meyer, L.; Guedes, R.N.C.; Smagghe, G. Lethal and sublethal effects of azadirachtin on the bumblebee Bombus terrestris (Hymenoptera: Apidae). Ecotoxicology 2015, 24, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Guan, D.; Wei, J.; Ge, H.; Zhou, X.; Zheng, Y.; Qian, K.; Wang, J. Low concentrations of cyantraniliprole negatively affects the development of Spodoptera frugiperda by disruption of ecdysteroid biosynthesis and carbohydrate and lipid metabolism. Pestic. Biochem. Physiol. 2024, 200, 105827. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.C.; Yao, J.; Adamczyk, J.; Luttrell, R. Feeding toxicity and impact of imidacloprid formulation and mixtures with six representative pesticides at residue concentrations on honey bee physiology (Apis mellifera). PLoS ONE 2017, 12, e0178421. [Google Scholar] [CrossRef] [PubMed]
- Fontes, J.; Roja, I.S.; Tavares, J.; Oliveira, L. Lethal and Sublethal Effects of Various Pesticides on Trichogramma achaeae (Hymenoptera: Trichogrammatidae). J. Econ. Entomol. 2018, 111, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Al Naggar, Y.; Baer, B. Consequences of a short time exposure to a sublethal dose of flupyradifurone (Sivanto) pesticide early in life on survival and immunity in the honeybee (Apis mellifera). Sci. Rep. 2019, 9, 19753. [Google Scholar] [CrossRef] [PubMed]
- Taning, C.N.T.; Vanommeslaeghe, A.; Smagghe, G. With or without foraging for food, field-realistic concentrations of sulfoxaflor are equally toxic to bumblebees (Bombus terrestris). Entomol. Gen. 2019, 39, 151–155. [Google Scholar] [CrossRef]
- Siviter, H.; Muth, F. Do novel insecticides pose a threat to beneficial insects? Proc. R. Soc. B 2020, 287, 20201265. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, V.H.; Hranitz, J.M.; McGonigle, M.B.; Manweiler, R.E.; Smith, D.R.; Barthell, J.F. Acute exposure to sublethal doses of neonicotinoid insecticides increases heat tolerance in honey bees. PLoS ONE 2022, 17, e0240950. [Google Scholar] [CrossRef]
- Schneider, M.I.; Smagghe, G.; Pineda, S.; Vinuela, E. Action of insect growth regulator insecticides and spinosad on life history parameters and absorption in third-instar larvae of the endoparasitoid Hyposoter didymator. Biol. Control 2004, 31, 189–198. [Google Scholar] [CrossRef]
- Bortolotti, L.; Sbrenna, A.M.; Sbrenna, G. Action of fenoxycarb on metamorphosis and cocoon spinning in Chrysoperla carnea (Neuroptera: Chrysopidae): Identification of the JHA-sensitive period. Eur. J. Entomol. 2005, 102, 27–32. [Google Scholar] [CrossRef]
- Cônsoli, F.L.; Parra, J.R.P.; Hassan, S.A. Side effects of insecticides used in tomato fields on the egg parasitoid Trichogramma pretiosum Riley (Hym., Trichogrammatidae), a natural enemy of Tuta absoluta (Meyrick) (Lep., Gelechiidae). J. Appl. Entomol. 1998, 122, 43–47. [Google Scholar] [CrossRef]
- Dai, P.L.; Wang, Q.; Sun, J.H.; Liu, F.; Wang, X.; Wu, Y.Y.; Zhou, T. Effects of sublethal concentrations of bifenthrin and deltamethrin on fecundity, growth, and development of the honeybee Apis mellifera ligustica. Environ. Toxicol. Chem. 2010, 29, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.J.; Ramos-Rodriguez, O.; Raine, N.E. Combined pesticide exposure severely affects individual- and colony-level traits in bees. Nature 2012, 491, 105–108. [Google Scholar] [CrossRef]
- Zhao, H.; Li, G.; Guo, D.; Wang, Y.; Liu, Q.; Gao, Z.; Wang, H.; Liu, Z.; Guo, X.; Xu, B. Transcriptomic and metabolomic landscape of the molecular effects of glyphosate commercial formulation on Apis mellifera ligustica and Apis cerana cerana. Sci. Total Environ. 2020, 744, 140819. [Google Scholar] [CrossRef] [PubMed]
- Zanuncio, T.V.; Serrão, J.E.; Zanuncio, J.C.; Guedes, R.N.C. Permethrin-induced hormesis on the predator Supputius cincticeps (Stål,1860) (Heteroptera: Pentatomidae). Crop Prot. 2003, 22, 941–947. [Google Scholar] [CrossRef]
- Sâmia, R.R.; Gontijo, P.C.; Oliveira, R.L.; Carvalho, G.A. Sublethal and transgenerational effects of thiamethoxam applied to cotton seed on Chrysoperla externa and Harmonia axyridis. Pest Manag. Sci. 2019, 75, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Siviter, H.; Folly, A.J.; Brown, M.J.F.; Leadbeater, E. Individual and combined impacts of sulfoxaflor and Nosema bombi on bumblebee (Bombus terrestris) larval growth. Proc. R. Soc. B. 2020, 287, 20200935. [Google Scholar] [CrossRef] [PubMed]
- Taséi, J.N.; Lerin, J.; Ripault, G. Sub-lethal effects of imidacloprid on bumblebees, Bombus terrestris (Hymenoptera: Apidae), during a laboratory feeding test. Pest Manag. Sci. 2000, 56, 784–788. [Google Scholar] [CrossRef]
- Chandel, R.S.; Gupta, P.R. Toxicity of diflubenzuron and penfluron to immature stages of Apis cerana indica F and Apis mellifera L. Apidologie 1992, 23, 465–473. [Google Scholar] [CrossRef]
- Jaycox, E.R.; Skowrone, W.; Guynn, G. Behavioral changes in worker honey bees (Apis mellifera) induced by injections of a juvenile hormone mimic. Ann. Entomol. Soc. Am. 1974, 67, 529–535. [Google Scholar] [CrossRef]
- DeRuijter, A.; VanderSteen, J. A field study on the effect on honeybee brood of insegar (fenoxycarb) applied on blooming apple orchards. Apidologie 1987, 18, 356–357. [Google Scholar]
- Gupta, P.R.; Chandel, R.S. Effects of diflubenzuron and penfluron on workers of Apis cerana indica F. and Apis mellifera L. Apidologie 1995, 26, 3–10. [Google Scholar] [CrossRef]
- Mussen, E.C.; Lopez, J.E.; Peng, C.Y. Effects of selected fungicides on growth and development of larval honey bees, Apis mellifera L. (Hymenoptera: Apidae). Environ. Entomol. 2004, 33, 1151–1154. [Google Scholar] [CrossRef]
- Charleston, D.S.; Kfir, R.; Dicke, M.; Vet, L.E.M. Impact of botanical pesticides derived from Melia azedarach and Azadirachta indica on the biology of two parasitoid species of the diamondback moth. Biol. Control 2005, 33, 131–142. [Google Scholar] [CrossRef]
- Fogel, M.N.; Schneider, M.I.; Desneux, N.; Gonzalez, B.; Ronco, A.E. Impact of the neonicotinoid acetamiprid on immature stages of the predator Eriopis connexa (Coleoptera: Coccinellidae). Ecotoxicology 2013, 22, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Zanuncio, J.C.; Mourão, S.A.; Martínez, L.C.; Wilcken, C.F.; Ramalho, F.S.; Plata-Rueda, A.; Serrão, J.E. Toxic effects of the neem oil (Azadirachta indica) formulation on the stink bug predator, Podisus nigrispinus (Heteroptera: Pentatomidae). Sci. Rep. 2016, 6, 30261. [Google Scholar] [CrossRef] [PubMed]
- Basley, K.; Goulson, D. Effects of Field-Relevant Concentrations of Clothianidin on Larval Development of the Butterfly Polyommatus icarus (Lepidoptera, Lycaenidae). Environ. Sci. Technol. 2018, 52, 3990–3996. [Google Scholar] [CrossRef] [PubMed]
- Dorneles, A.L.; de Souza Rosa-Fontana, A.; Dos Santos, C.F.; Blochtein, B. Larvae of stingless bee Scaptotrigona bipunctata exposed to organophosphorus pesticide develop into lighter, smaller and deformed adult workers. Environ. Pollut. 2021, 272, 116414. [Google Scholar] [CrossRef]
- Simola, D.F.; Graham, R.J.; Brady, C.M.; Enzmann, B.L.; Desplan, C.; Ray, A.; Zwiebel, L.J.; Bonasio, R.; Reinberg, D.; Liebig, J.; et al. Epigenetic (re)programming of caste-specific behavior in the ant Camponotus floridanus. Science 2016, 351, aac6633. [Google Scholar] [CrossRef] [PubMed]
- Morandin, C.; Brendel, V.P.; Sundström, L.; Helanterä, H.; Mikheyev, A.S. Changes in gene DNA methylation and expression networks accompany caste specialization and age-related physiological changes in a social insect. Mol. Ecol. 2019, 28, 1975–1993. [Google Scholar] [CrossRef]
- Lopes, M.P.; Fernandes, K.M.; Tomé, H.V.V.; Gonçalves, W.G.; Miranda, F.R.; Serrão, J.E.; Martins, G.F. Spinosad-mediated effects on the walking ability, midgut, and Malpighian tubules of Africanized honey bee workers. Pest Manag. Sci. 2018, 74, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Castro, B.M.C.; Martínez, L.C.; Plata-Rueda, A.; Soares, M.A.; Wilcken, C.F.; Zanuncio, A.J.V.; Fiaz, M.; Zanuncio, J.C.; Serrão, J.E. Exposure to chlorantraniliprole reduces locomotion, respiration, and causes histological changes in the midgut of velvetbean caterpillar Anticarsia gemmatalis (Lepidoptera: Noctuidae). Chemosphere 2021, 263, 128008. [Google Scholar] [CrossRef] [PubMed]
- Carniero, L.S.; Martínez, L.C.; Gonçalves, W.G.; Santana, L.M.; Serrão, J.E. The fungicide iprodione affects midgut cells of non-target honey bee Apis mellifera workers. Ecotoxicol. Environ. Saf. 2020, 189, 109991. [Google Scholar] [CrossRef] [PubMed]
- Farder-Gomes, C.F.; Fernandes, K.M.; Bernardes, R.C.; Bastos, D.S.S.; Martins, G.F.; Serrão, J.E. Acute exposure to fipronil induces oxidative stress, apoptosis and impairs epithelial homeostasis in the midgut of the stingless bee Partamona helleri Friese (Hymenoptera: Apidae). Sci. Total Environ. 2021, 774, 145679. [Google Scholar] [CrossRef] [PubMed]
- Serra, R.S.; Cossolin, J.F.S.; de Resende, M.T.C.S.; de Castro, M.A.; Oliveira, A.H.; Martínez, L.C.; Serrão, J.E. Spiromesifen induces histopathological and cytotoxic changes in the midgut of the honeybee Apis mellifera (Hymenoptera: Apidae). Chemosphere 2021, 270, 129439. [Google Scholar] [CrossRef] [PubMed]
- Arthidoro de Castro, M.B.; Martínez, L.C.; Serra, R.S.; Cossolin, J.F.S.; Serrão, J.E. Cytotoxic effects on the midgut, hypopharyngeal glands and brain of Apis mellifera honey bee workers exposed to chronic concentrations of lambdacyhalothrin. Chemosphere 2020, 248, 126075. [Google Scholar] [CrossRef] [PubMed]
- Shubin, A.V.; Demidyuk, I.V.; Komissarov, A.A.; Rafieva, L.M.; Kostrov, S.V. Cytoplasmic vacuolization in cell death and survival. Oncotarget 2016, 7, 55863–55889. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.C.; Plata-Rueda, A.; da Silva, N.G.; Gonçalves, W.G.; Zanuncio, J.C.; Bozdoğan, H.; Serrão, J.E. Permethrin induces histological and cytological changes in the midgut of the predatory bug, Podisus nigrispinus. Chemosphere 2018, 212, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.C.; Plata-Rueda, A.; Gonçalves, W.G.; Freire, A.F.P.A.; Zanuncio, J.C.; Bozdoğan, H.; Serrão, J.E. Toxicity and cytotoxicity of the insecticide imidacloprid in the midgut of the predatory bug, Podisus nigrispinus. Ecotoxicol. Environ. Saf. 2019, 167, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Junior, V.C.; Martínez, L.C.; Plata-Rueda, A.; Fernades, F.L.; Tavares, W.S.; Zanuncio, J.C.; Serrão, J.E. Histopathological and cytotoxic changes induced by spinosad on midgut cells of the nontarget predator Podisus nigrispinus Dallas (Heteroptera: Pentatomidae). Chemosphere 2020, 238, 124585. [Google Scholar] [CrossRef] [PubMed]
- De Castro, A.A.; Poderoso, J.C.M.; Ribeiro, R.C.; Legaspi, J.C.; Serrão, J.E.; Zanuncio, J.C. Demographic parameters of the insecticide-exposed predator Podisus nigrispinus: Implications for IPM. Biocontrol 2015, 60, 231–239. [Google Scholar] [CrossRef]
- Milone, J.P.; Tarpy, D.R. Effects of developmental exposure to pesticides in wax and pollen on honey bee (Apis mellifera) queen reproductive phenotypes. Sci. Rep. 2021, 11, 1020. [Google Scholar] [CrossRef]
- Kairo, G.; Provost, B.; Tchamitchian, S.; Abdelkader, F.B.; Bonnet, M.; Cousin, M.; Sénéchal, J.; Benet, P.; Belzunces, L.P.; Brunet, J.L. Drone exposure to the systemic insecticide Fipronil indirectly impairs queen reproductive potential. Sci. Rep. 2016, 6, 31904. [Google Scholar] [CrossRef] [PubMed]
- Bingsohn, L.; Knorr, E.; Vilcinskas, A. The model beetle Tribolium castaneum can be used as an early warning system for transgenerational epigenetic side effects caused by pharmaceuticals. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2016, 185–186, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Rosenheim, J.A.; Hoy, M.A. Sublethal effects of pesticides on the parasitoid Aphytis melinus (Hymenoptera: Aphelinidae). J. Econ. Entomol. 1988, 81, 476–483. [Google Scholar] [CrossRef]
- Krespi, L.; Rabasse, J.M.; Dedryver, C.A.; Nenon, J.P. Effect of three insecticides on the life cycle of Aphidius uzbekistanicus Luz. (Hym, Aphidiidae). J. Appl. Entomol. 1991, 111, 113–119. [Google Scholar] [CrossRef]
- Delpuech, J.M.; Meyet, J. Reduction in the sex ratio of the progeny of a parasitoid wasp (Trichogramma brassicae) surviving the insecticide chlorpyrifos. Arch. Environ. Contam. Toxicol. 2003, 45, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Siefert, P.; Hota, R.; Ramesh, V.; Grünewald, B. Chronic within-hive video recordings detect altered nursing behaviour and retarded larval development of neonicotinoid treated honey bees. Sci. Rep. 2020, 10, 8727. [Google Scholar] [CrossRef] [PubMed]
- Schott, M.; Sandmann, M.; Cresswell, J.E.; Becher, M.A.; Eichner, G.; Brandt, D.T.; Halitschke, R.; Krueger, S.; Morlock, G.; Düring, R.A.; et al. Honeybee colonies compensate for pesticide-induced effects on royal jelly composition and brood survival with increased brood production. Sci. Rep. 2021, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Staub, L.; Villamar-Bouza, L.; Bruckner, S.; Chantawannakul, P.; Kolari, E.; Maitip, J.; Vidondo, B.; Neumann, P.; Williams, G.R. Negative effects of neonicotinoids on male honeybee survival, behaviour and physiology in the field. J. Appl. Ecol. 2021, 58, 2515–2528. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Smagghe, G.; Stark, J.D. Desneux N: Pesticide-induced stress in arthropod pests for optimized integrated pest management programs. Annu. Rev. Entomol. 2016, 61, 43–62. [Google Scholar] [CrossRef] [PubMed]
- Vinken, M. The adverse outcome pathway concept: A pragmatic tool in toxicology. Toxicology 2013, 312, 158–165. [Google Scholar] [CrossRef]
- Groh, K.J.; Carvalho, R.N.; Chipman, J.K.; Denslow, N.D.; Halder, M.; Murphy, C.A.; Roelofs, D.; Rolaki, A.; Schirmer, K.; Watanabe, K.H. Development and application of the adverse outcome pathway framework for understanding and predicting chronic toxicity: I. Challenges and research needs in ecotoxicology. Chemosphere 2015, 120, 764–777. [Google Scholar] [CrossRef] [PubMed]
- Guedes, R.N. Insecticide resistance, control failure likelihood and the First Law of Geography. Pest Manag. Sci. 2017, 73, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Guedes, R.N.; Walse, S.S.; Throne, J.E. Sublethal exposure, insecticide resistance, and community stress. Curr. Opin. Insect Sci. 2018, 21, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.T.; Ågerstrand, M.; Brooks, B.W.; Allen, J.; Bertram, M.J.; Brodin, T.; Dang, Z.; Duquesne, S.; Sahm, R.; Hoffmann, F.; et al. The Role of Behavioral Ecotoxicology in Environmental Protection. Environ. Sci. Tech. 2021, 55, 5620–5628. [Google Scholar] [CrossRef] [PubMed]
- Suchail, S.; Guez, D.; Belzunces, L.P. Discrepancy between acute and chronic toxicity induced by imidacloprid and its metabolites in Apis mellifera. Environ. Toxicol. Chem. 2001, 20, 2482–2486. [Google Scholar] [CrossRef] [PubMed]
- Schott, M.; Bischhoff, G.; Eichner, G.; Vilcinskas, A.; Büchler, R.; Meixner, M.D.; Brandt, A. Temporal dynamics of whole body residues of the neonicotinoid insecticide imidacloprid in live or dead honeybees. Sci. Rep. 2017, 7, 6288. [Google Scholar] [CrossRef] [PubMed]
- Kunkel, B.A.; Held, D.W.; Potter, D.A. Lethal and sublethal effects of bendiocarb, halofenozide, and imidacloprid on Harpalus pennsylvanicus (Coleoptera: Carabidae) following different modes of exposure in turfgrass. J. Econ. Entomol. 2001, 94, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Lunardi, J.S.; Zaluski, R.; Orsi, R.O. Evaluation of motor changes and toxicity of insecticides fipronil and imidacloprid in Africanized honey bees (Hymenoptera: Apidae). Sociobiology 2017, 64, 50–56. [Google Scholar] [CrossRef]
- Delpuech, J.M.; Bardon, C.; Boulétreau, M. Increase of the behavioral response to kairomones by the parasitoid wasp Leptopilina heterotoma surviving insecticides. Arch. Environ. Contam. Toxicol. 2005, 49, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Fiaz, M.; Martínez, L.C.; Plata-Rueda, A.; Gonçalves, W.G.; Souza, D.L.L.; Cossolin, J.F.S.; Carvalho, P.E.G.R.; Martins, G.F.; Serrão, J.E. Pyriproxyfen, a juvenile hormone analog, damages midgut cells and interferes with behaviors of Aedes aegypti larvae. PeerJ 2019, 7, e7489. [Google Scholar] [CrossRef] [PubMed]
- Wiles, J.A.; Jepson, P.C. Sub-lethal effects of deltamethrin residues on the within crop behaviour and distribution of Coccinella septempunctata. Entomol. Exp. Appl. 1994, 72, 33–45. [Google Scholar] [CrossRef]
- Salerno, G.; Colazza, S.; Conti, E. Sub-lethal effects of deltamethrin on walking behaviour and response to host kairomone of the egg parasitoid Trissolcus basalis. Pest Manag. Sci. 2002, 58, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Plata-Rueda, A.; Martínez, L.C.; Da Silva, B.K.R.; Zanuncio, J.C.; Sena Fernandes, M.E.; Serrão, J.E.; Guedes, R.N.C.; Fernandes, F.L. Exposure to cyantraniliprole causes mortality and disturbs behavioral and respiratory response in the coffee berry borer (Hypothenemus hampei). Pest Manag. Sci. 2019, 75, 2236–2241. [Google Scholar] [CrossRef] [PubMed]
- Longley, M.; Jepson, P.C. The influence of insecticide residues on primary parasitoid and hyperparasitoid foraging behaviour in the laboratory. Entomol. Exp. Appl. 1996, 81, 259–269. [Google Scholar] [CrossRef]
- Longley, M.; Jepson, P.C. Effects of honeydew and insecticide residues on the distribution of foraging aphid parasitoids under glasshouse and field conditions. Entomol. Exp. Appl. 1996, 81, 189–198. [Google Scholar] [CrossRef]
- Reingold, S.C.; Camhi, J.M. Abdominal grooming in cockroach: Development of an adult behavior. J. Insect Physiol. 1978, 24, 101–110. [Google Scholar] [CrossRef]
- Rafalimanana, H.; Kaiser, L.; Delpuech, J.M. Stimulating effects of the insecticide chlorpyrifos on host searching and infestation efficacy of a parasitoid wasp. Pest Manag. Sci. 2002, 58, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Kühner, C.; Klingauf, F.; Hassan, S.A. Development of laboratory and semi-field methods to test the side effect of pesticides on Diaeretiella rapae (Hym. Aphidiidae). Med. Fac. Landbouww. Rijksuniv. Gent. 1985, 50, 531–538. [Google Scholar]
- Banken, J.A.O.; Stark, J.D. Multiple routes of pesticide exposure and the risk of pesticides to biological controls: A study of neem and the sevenspotted lady beetle (Coleoptera: Coccinellidae). J. Econ. Entomol. 1998, 91, 1–6. [Google Scholar] [CrossRef]
- Brunner, J.F.; Dunley, J.E.; Doerr, M.D.; Beers, E.H. Effects of pesticides on Colpoclypeus florus (Hymenoptera: Eulophidae) and Trichogramma platneri (Hymenoptera: Trichogrammatidae), parasitoids of leafrollers in Washington. J. Econ. Entomol. 2001, 94, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Desneux, N.; Pham-Delègue, M.H.; Kaiser, L. Effects of sublethal and lethal doses of lambda-cyhalothrin on oviposition experience and host searching behaviour of a parasitic wasp, Aphidius ervi. Pest Manag. Sci. 2004, 60, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.H.; Takagi, M.; Takasu, K. Effects of selective insecticides on host searching and oviposition behavior of Neochrysocharis formosa (Westwood) (Hymenoptera: Eulophidae), a larval parasitoid of the American serpentine leafminer. Appl. Entomol. Zool. 2004, 39, 435–441. [Google Scholar] [CrossRef]
- Stanley, D.A.; Russell, A.L.; Morrison, S.J.; Rogers, C.; Raine, N.E. Investigating the impacts of field-realistic exposure to a neonicotinoid pesticide on bumblebee foraging, homing ability and colony growth. J. Appl. Entomol. 2016, 53, 1440–1449. [Google Scholar] [CrossRef] [PubMed]
- Umoru, P.A.; Powell, W.; Clark, S.J. Effect of pirimicarb on the foraging behaviour of Diaeretiella rapae (Hymenoptera: Braconidae) on host-free and infested oilseed rape plants. Bull. Entomol. Res. 1996, 86, 193–201. [Google Scholar] [CrossRef]
- Komeza, N.; Fouillet, P.; Boulétreau, M.; Delpuech, J.M. Modification, by the insecticide chlorpyrifos, of the behavioral response to kairomones of a Drosophila parasitoid, Leptopilina boulardi. Arch. Environ. Contam. Toxicol. 2001, 41, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Stapel, J.O.; Cortesero, A.M.; Lewis, W.J. Disruptive sublethal effects of insecticides on biological control: Altered foraging ability and life span of a parasitoid after feeding on extrafloral nectar of cotton treated with systemic insecticides. Biol. Control 2000, 17, 243–249. [Google Scholar] [CrossRef]
- Desneux, N.; Rafalimanana, H.; Kaiser, L. Dose-response relationship in lethal and behavioural effects of different insecticides on the parasitic wasp Aphidius ervi. Chemosphere 2004, 54, 619–627. [Google Scholar] [CrossRef]
- Von Frisch, K. The Dance Language and Orientation of Bees; Harvard University Press: Cambridge, MA, USA, 1967; p. 566. [Google Scholar]
- Zhang, S.W.; Lehrer, M.; Srinivasan, M.V. Honeybee memory: Navigation by associative grouping and recall of visual stimuli. Neurobiol. Learn. Mem. 1999, 72, 180–201. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Müller, T.; Spatz, A.K.; Greggers, U.; Grünewald, B.; Menzel, R. Neonicotinoids Interfere with Specific Components of Navigation in Honeybees. PLoS ONE 2014, 9, e91364. [Google Scholar] [CrossRef] [PubMed]
- Menzel, R. Wie Pestizide (Neonicotinoide) die Navigation, die Tanz-Kommunikation und das Lernverhalten von Bienen verändern. In Soziale Insekten in Einer Sich Wandelnden Welt©; Rundgespräche der Kommission für Ökologie; Verlag Dr. Friedrich Pfeil: München, Germany, 2014; Volume 43, pp. 75–83. [Google Scholar]
- Vandame, R.; Meled, M.; Colin, M.E.; Belzunces, L.P. Alteration of the homing-flight in the honey bee Apis mellifera L. exposed to sublethal dose of deltamethrin. Environ. Toxicol. Chem. 1995, 14, 855–860. [Google Scholar] [CrossRef]
- Kenna, D.; Cooley, H.; Pretelli, I.; Ramos Rodrigues, A.; Gill, S.D.; Gill, R.J. Pesticide exposure affects flight dynamics and reduces flight endurance in bumblebees. Ecol. Evol. 2019, 9, 5637–5650. [Google Scholar] [CrossRef] [PubMed]
- Delpuech, J.M.; Froment, B.; Fouillet, P.; Pompanon, F.; Janillon, S.; Boulétreau, M. Inhibition of sex pheromone communications of Trichogramma brassicae (Hymenoptera) by the insecticide chlorpyrifos. Environ. Toxicol. Chem. 1998, 17, 1107–1113. [Google Scholar] [CrossRef]
- Delpuech, J.M.; Gareau, E.; Terrier, O.; Fouillet, P. Sublethal effects of the insecticide chlorpyrifos on the sex pheromonal communication of Trichogramma brassicae. Chemosphere 1998, 36, 1775–1785. [Google Scholar] [CrossRef]
- Delpuech, J.M.; Legallet, B.; Terrier, O.; Fouillet, P. Modifications of the sex pheromonal communication of Trichogramma brassicae by a sublethal dose of deltamethrin. Chemosphere 1999, 38, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, A.A.E.; Newman, A.E.M.; Raine, N.E.; Mitchell, G.W.; Norris, D.R. Effects of early-life exposure to sublethal levels of a common neonicotinoid insecticide on the orientation and migration of monarch butterflies (Danaus plexippus). J. Exp. Biol. 2021, 224, jeb230870. [Google Scholar] [CrossRef] [PubMed]
- Decourtye, A.; Devillers, J.; Cluzeau, S.; Charreton, M.; Pham-Delègue, M.H. Effects of imidacloprid and deltamethrin on associative learning in honeybees under semi-field and laboratory conditions. Ecotoxicol. Environ. Saf. 2004, 57, 410–419. [Google Scholar] [CrossRef]
- El Hassani, A.K.; Dacher, M.; Gauthier, M.; Armengaud, C. Effects of sublethal doses of fipronil on the behavior of the honeybee (Apis mellifera). Pharmacol. Biochem. Behav. 2005, 82, 30–39. [Google Scholar] [CrossRef]
- Aliouane, Y.; El Hassani, A.K.; Gary, V.; Armengaud, C.; Lambin, M.; Gauthier, M. Subchronic exposure of honeybees to sublethal doses of pesticides: Effects on behavior. Environ. Toxicol. Chem. 2009, 28, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Decourtye, A.; Devillers, J.; Genecque, E.; Le Menach, K.; Budzinski, H.; Cluzeau, S.; Pham-Delègue, M.H. Comparative sublethal toxicity of nine pesticides on olfactory learning performances of the honeybee Apis mellifera. Arch. Environ. Contam. Toxicol. 2005, 48, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Weick, J.; Thorn, R.S. Effects of acute sublethal exposure to coumaphos or diazinon on acquisition and discrimination of odor stimuli in the honey bee (Hymenoptera: Apidae). J. Econ. Entomol. 2005, 95, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Guez, D.; Suchail, S.; Gauthier, M.; Maleszka, R.; Belzunces, L.P. Contrasting effects of imidacloprid on habituation in 7- and 8-day-old honeybees (Apis mellifera). Neurobiol. Learn. Mem. 2001, 76, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Mamood, A.; Waller, G. Recovery of learning responses by honeybees following a sublethal exposure to permethrin. Physiol. Entomol. 1990, 15, 55–60. [Google Scholar] [CrossRef]
- Lambin, M.; Armengaud, C.; Raymond, S.; Gauthier, M. Imidacloprid induced facilitation of the proboscis extension reflex habituation in the honeybee. Arch. Insect Biochem. Physiol. 2001, 48, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Bartling, M.T.; Vilcinskas, A.; Lee, K.Z. Sub-Lethal Doses of Clothianidin Inhibit the Conditioning and Biosensory Abilities of the Western Honeybee Apis mellifera. Insects 2019, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Kirkerud, N.H.; Wehmann, H.N.; Galizia, G.; Gustav, D. Apis—A novel approach for conditioning honey bees. Front. Behav. Neurosci. 2013, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Wang, C.; Dong, S.; Li, X.; Nieh, J.C. The pesticide flupyradifurone impairs olfactory learning in Asian honey bees (Apis cerana) exposed as larvae or as adults. Sci. Rep. 2017, 7, 17772. [Google Scholar] [CrossRef] [PubMed]
- Hesselbach, H.; Scheiner, R. Effects of the novel pesticide flupyradifurone (Sivanto) on honeybee taste and cognition. Sci. Rep. 2018, 8, 4954. [Google Scholar] [CrossRef] [PubMed]
- Decourtye, A.; Lacassie, E.; Pham-Delègue, M.H. Learning performances of honeybees (Apis mellifera L.) are differentially affected by imidacloprid according to the season. Pest Manag. Sci. 2003, 59, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Kaila, L.; Léo, D.; Nyckees, D.; Toivonen, M.; Jalli, M.; Loukola, O.J. Chronic oral exposure to Amistar fungicide does not significantly affect colour discrimination but may impact memory retention in bumblebees. Environ. Sci. Eur. 2023, 35, 39. [Google Scholar] [CrossRef]
- Siviter, H.; Koricheva, J.; Brown, M.J.; Leadbeater, E. Quantifying the impact of pesticides on learning and memory in bees. J. Appl. Ecol. 2018, 55, 2812–2821. [Google Scholar] [CrossRef] [PubMed]
- Polonsky, J.; Bhatnagar, S.C.; Griffitsh, D.C.; Pickett, J.A.; Woodcock, C.M. Activity of quassinoids as antifeedants against aphids. J. Chem. Ecol. 1989, 15, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Rieth, J.P.; Levin, M.D. The repellent effect of two pyrethroid insecticides on the honey bee. Physiol. Entomol. 1988, 13, 213–218. [Google Scholar] [CrossRef]
- Calatayud-Vernich, P.; Calatayud, F.; Simó, E.; Picó, Y. Pesticide residues in honey bees, pollen and beeswax: Assessing beehive exposure. Environ. Pollut. 2018, 241, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Mayer, D.F.; Lunden, J.D. Field and laboratory tests of the effects of fipronil on adult female bees of Apis mellifera, Megachile rotundata and Nomia melanderi. J. Apic. Res. 1999, 38, 191–197. [Google Scholar] [CrossRef]
- Colin, M.E.; Bonmatin, J.M.; Moineau, I.; Gaimon, C.; Brun, S.; Vermandere, J.P. A Method to Quantify and Analyze the Foraging Activity of Honey Bees: Relevance to the Sublethal Effects Induced by Systemic Insecticides. Arch. Environ. Contam. Toxicol. 2004, 47, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Haynes, K.F. Sublethal effects of neurotoxic insecticides on insect behavior. Annu. Rev. Entomol. 1988, 33, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Naumann, K.; Currie, R.W.; Isman, M.B. Evaluation of the repellent effects of a neem insecticide on foraging honeybees and other pollinators. Can. Entomol. 1994, 126, 225–230. [Google Scholar] [CrossRef]
- Traynor, K.S.; vanEngelsdorp, D.; Lamas, Z.S. Social disruption: Sublethal pesticides in pollen lead to Apis mellifera queen events and brood loss. Ecotoxicol. Environ. Saf. 2021, 214, 112105. [Google Scholar] [CrossRef] [PubMed]
- Dechaume-Moncharmont, F.X. Butinage Collectif Chez Làbeille Apis mellifera L.: Etude Théorique et Expérimentale. Ph.D. Thesis, Pierre and Marie Curie University, Paris, France, 2003. [Google Scholar]
- Ivanković Tatalović, L. Functional Diversity, Trophic Interaction, Development, and Metabolism of Ground Beetles (Coleoptera: Carabidae) in Perennial Mediterranean Agroecosystems. Ph.D. Thesis, University of Zagreb, Zagreb, Croatia, 2022. [Google Scholar]
- Coulon, M.; Dalmon, A.; Di Prisco, G.; Prado, A.; Arban, F.; Dubois, E.; Ribière-Chabert, M.; Alaux, C.; Thiéry, R.; Le Conte, Y. Interactions between thiamethoxam and deformed wing virus can drastically impair flight behavior of honey bees. Front. Microbiol. 2020, 11, 766. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.E.S.; Alves, F.M.; Pereira, R.C.; Aquino, L.A.; Fernandes, F.L.; Zanuncio, J.C. Lethal and sublethal effects of seven insecticides on three beneficial insects in laboratory assays and field trials. Chemosphere 2016, 156, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.L.; Zheng, Y.; Zhao, J.W.; Desneux, N.; He, Y.X.; Weng, Q.Y. Lethal and sublethal effects of thiamethoxam on the whitefly predator Serangium japonicum (Coleoptera: Coccinellidae) through different exposure routes. Chemosphere 2015, 128, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, C.; Jepson, P.C. The toxic effects of direct pesticide exposure for a nontarget weed-dwelling chrysomelid beetle (Gastrophysa polygoni) in cereals. Environ. Toxicol. Chem. 1995, 14, 993–999. [Google Scholar] [CrossRef]
- Singh, S.R.; Walters, K.F.A.; Port, G.R.; Northing, P. Consumption rate and predatory activity of adult and fourth instar larvae of the seven spot ladybird, Coccinella septempunctata (L.), following contact with dimethoate residue and contaminated prey in laboratory arenas. Biol. Control 2004, 30, 127–133. [Google Scholar] [CrossRef]
- Ahmad, M.; Ossiewatsch, H.R.; Basedow, T. Effects of neem-treated aphids as food/hosts on their predators and parasitoids. J. Appl. Entomol. 2003, 127, 458–464. [Google Scholar] [CrossRef]
- Cabral, S.; Soares, A.O.; Garcia, P. Voracity of Coccinella undecimpunctata: Effects of insecticides when foraging in a prey/plant system. J. Pest Sci. 2011, 84, 373–379. [Google Scholar] [CrossRef]
- Silva, W.M.; Martínez, L.C.; Plata-Rueda, A.; Serrão, J.E.; Zanuncio, J.C. Respiration, predatory behavior and prey consumption by Podisus nigrispinus (Heteroptera: Pentatomidae) nymphs exposed to some insecticides. Chemosphere 2020, 261, 127720. [Google Scholar] [CrossRef] [PubMed]
- Claver, M.A.; Ravichandran, B.; Khan, M.M.; Ambrose, D.P. Impact of cypermethrin on the functional response, predatory and mating behaviour of a non-target potential biological control agent Acanthaspis pedestris (Stål) (Het., Reduviidae). J. Appl. Entomol. 2003, 127, 18–22. [Google Scholar] [CrossRef]
- Gholamzadeh-Chitgar, M.; Hajizadeh, J.; Ghadamyari, M.; Karimi-Malati, A.; Hoda, H. Sublethal effects of diazinon, fenitrothion and chlorpyrifos on the functional response of predatory bug, Andrallus spinidens Fabricius (Hem.: Pentatomidae) in the laboratory conditions. J. King Saudi Univ. Sci. 2014, 24, 113–118. [Google Scholar] [CrossRef]
- Wiles, J.A.; Jepson, P.C. The dietary effects of deltamethrin upon Nebria brevicollis (F.) (Coleoptera: Carabidae). Pestic. Sci. 1993, 38, 329–334. [Google Scholar] [CrossRef]
- Grünwald, S.; Stellzig, J.; Adam, I.V.; Weber, K.; Binger, S.; Boll, M.; Knorr, E.; Twyman, R.M.; Vilcinskas, A.; Wenzel, U. Longevity in the red flour beetle Tribolium castaneum is enhanced by broccoli and depends on nrf-2, jnk-1 and foxo-1 homologous genes. Genes Nutr. 2013, 8, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Cedergreen, N.; Pedersen, K.E.; Fredensborg, B.L. Quantifying synergistic interactions: A meta-analysis of joint effects of chemical and parasitic stressors. Sci. Rep. 2023, 13, 13641. [Google Scholar] [CrossRef] [PubMed]
- Klátyik, S.; Simon, G.; Oláh, M.; Mesnage, R.; Antonioi, M.N.; Zaller, J.G.; Székács, A. Terrestrial ecotoxicity of glyphosate, its formulations, and co-formulants: Evidence from 2010–2023. Environ. Sci. Eur. 2023, 35, 51. [Google Scholar] [CrossRef]
- Backhaus, T.; Blanck, H.; Faust, M. Hazard and Risk Assessment of Chemical Mixtures under REACH: State of the Art, Gaps and Options for Improvement; Report PM/3 2010; Swedish Chemicals Inspectorate: Stockholm, Sweden, 2010. [Google Scholar]
- Cedergreen, N. Quantifying Synergy: A Systematic Review of Mixture Toxicity Studies within Environmental Toxicology. PLoS ONE 2014, 9, e96580. [Google Scholar] [CrossRef] [PubMed]
- Tosi, S.; Nieh, J.C. Lethal and sublethal synergistic effects of a new systemic pesticide, flupyradifurone (Sivanto®), on honeybees. Proc. Biol. Sci. 2019, 286, 20190433. [Google Scholar] [CrossRef] [PubMed]
- Tosi, S.; Nieh, J.C.; Sgolastra, F.; Cabbri, R.; Medrzycki, P. Neonicotinoid pesticides and nutritional stress synergistically reduce survival in honey bees. Proc. R. Soc. 2017, 284, 20171711. [Google Scholar] [CrossRef] [PubMed]
- Alaux, C.; Ducloz, F.; Crauser, D.; Le Conte, Y. Diet effects on honeybee immunocompetence. Biol. Lett. 2010, 6, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Zhu, Y.C.; Adamczyk, J. Responses of honey bees to lethal and sublethal doses of formulated clothianidin alone and mixtures. J. Econ. Entomol. 2018, 20, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Quintela, E.D.; McCoy, C.W. Synergistic effect of imidacloprid two entomopathogenic fungi on the behavior and survival of larvae of Diaprepes abbreviatus (Coleoptera: Curculionidae) in soil. Biol. Microb. Control 1998, 91, 110–122. [Google Scholar] [CrossRef]
- Iwasa, T.; Motoyama, N.; Ambrose, J.T.; Roe, R.M.M. Mechanism for the differential toxicity of neonicotinoid insecticides in the honey bee, Apis mellifera. Crop Prot. 2004, 23, 371–378. [Google Scholar] [CrossRef]
- Di Noi, A.; Caliani, I.; D’Agostino, A.; Cai, G.; Romi, M.; Campani, T.; Ferrante, F.; Baracchi, D.; Casini, S. Assessing the effects of a commercial fungicide and an herbicide, alone and in combination, on Apis mellifera: Insights from biomarkers and cognitive analysis. Chemosphere 2024, 359, 142307. [Google Scholar] [CrossRef] [PubMed]
- Margus, A.; Tikka, S.; Karvanen, J.; Lindström, L. Transgenerational sublethal pyrethroid exposure gives rise to insecticide resistance in a pest insect. Sci. Total Environ. 2024, 908, 168114. [Google Scholar] [CrossRef] [PubMed]
- Sigmund, G.; Ågerstrand, M.; Antonelli, A.; Backhaus, T.; Brodin, T.; Diamond, M.L.; Erdelen, W.R.; Evers, D.C.; Hofmann, T.; Hueffer, T.; et al. Addressing chemical pollution in biodiversity research. Glob. Chang. Biol. 2023, 29, 3240–3255. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartling, M.-T.; Brandt, A.; Hollert, H.; Vilcinskas, A. Current Insights into Sublethal Effects of Pesticides on Insects. Int. J. Mol. Sci. 2024, 25, 6007. https://doi.org/10.3390/ijms25116007
Bartling M-T, Brandt A, Hollert H, Vilcinskas A. Current Insights into Sublethal Effects of Pesticides on Insects. International Journal of Molecular Sciences. 2024; 25(11):6007. https://doi.org/10.3390/ijms25116007
Chicago/Turabian StyleBartling, Merle-Theresa, Annely Brandt, Henner Hollert, and Andreas Vilcinskas. 2024. "Current Insights into Sublethal Effects of Pesticides on Insects" International Journal of Molecular Sciences 25, no. 11: 6007. https://doi.org/10.3390/ijms25116007
APA StyleBartling, M.-T., Brandt, A., Hollert, H., & Vilcinskas, A. (2024). Current Insights into Sublethal Effects of Pesticides on Insects. International Journal of Molecular Sciences, 25(11), 6007. https://doi.org/10.3390/ijms25116007





