Recent Advancements in Bone Tissue Engineering: Integrating Smart Scaffold Technologies and Bio-Responsive Systems for Enhanced Regeneration
Abstract
:1. Introduction
1.1. Bone Anatomy and Physiology
1.2. Bone Remodeling
1.3. Bone Repair
- Hematoma formation (1–5 days)
- 2.
- Fibrocartilaginous callus formation (5–11 days)
- 3.
- Bony callus formation (11–28 days)
- 4.
- Bone remodeling (18 days to years)
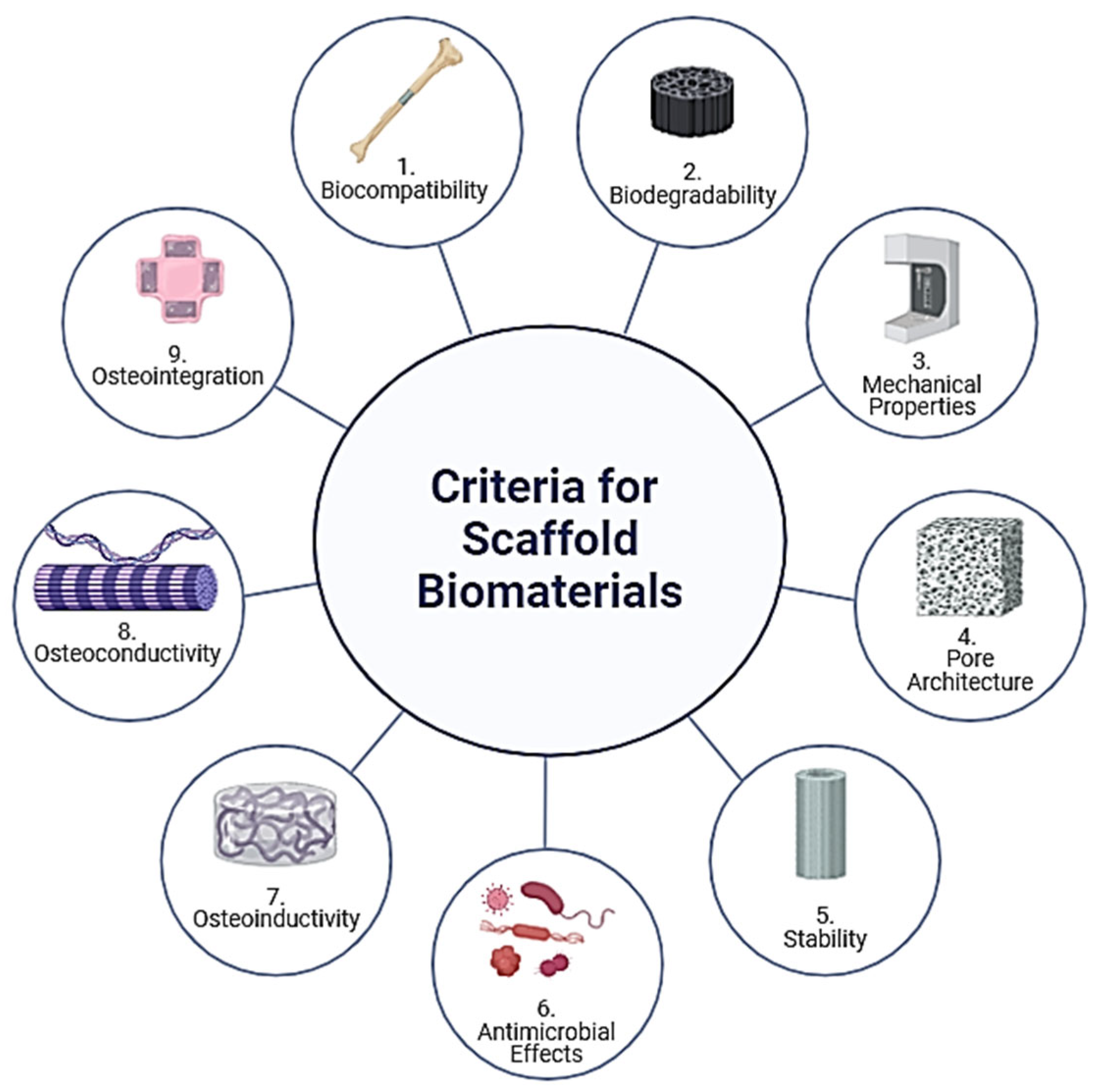
2. Criteria for the Selection of Scaffold Biomaterials
- 1.
- Biocompatibility
- 2.
- Biodegradability
- 3.
- Mechanical Properties
- 4.
- Pore Architecture
- 5.
- Stability
- 6.
- Antimicrobial Effects
2.1. Polymers
2.2. Ceramics
2.3. Metals
2.4. Composites and Hybrid Materials
| Study Ref. | Material Composition | Method Used | Key Findings | Compressive Strength | Bioactivity |
|---|---|---|---|---|---|
| [57] | CS/nano-HAp composites (30/70 weight ratio) | Co-precipitation | High biodegradability and bioactivity; best ratio for mechanical strength and bioactivity | 120 MPa | High in simulated body fluid (SBF) solution |
| [58] | CS hydrogel/HAp | Wet chemical synthesis | Enhanced biocompatibility with MG-63 osteosarcoma cells | - | - |
| [59] | CS/silica/HAp/Ca-GP | Sol–gel | High cell proliferation and growth; promising composite for filling small bone defects | 0.3 to 10 MPa | - |
| [60] | CS/nano-HAp | In situ combination | Improved bioactivity with pre-osteoblasts; nano-HA content positively affects bioactivity | - | Enhanced mineralization |
| [61] | CS/nano-HAp | In situ hybridization | Enhanced mechanical properties; suitable for scaffold applications with homogenous nanoparticle distribution | Acceptable for tissue substitution | Homogeneous integration |
| [23] | CS/HAp/magnetite nanocomposites | Mixed composites | Superior mechanical properties due to magnetite addition | - | In vitro biocompatibility |
| [62] | Hydroxypropyl chitosan (HPCS)/nano-HAp | Genipin crosslinking | Improved mechanical properties and cell mineralization; effective for osteogenic potential and scaffold stability | - | Enhanced alkaline phosphatase (ALP) activity |
| [63] | CS–HAp/PMMA | Freeze drying and radical polymerization | Stable thermal properties; favorable outcomes for cell population and spreading in vitro; addition of PMMA significantly improved mechanical strength | - | Non-toxic to cells |
| [64] | CS/HAp/β-TCP composites | Crosslinking with TPP | Improved mechanical properties and lower biodegradation; ideal for high-load-bearing bone applications | 4 kPa to 17 kPa | 2% lower biodegradation with a higher HAp/β-TCP ratio |
3. Scaffold Fabrication Techniques
3.1. Additive Manufacturing (3D Printing)
3.2. Functionalization of Scaffolds
3.2.1. Cell and Bioactive Molecule-Laden Scaffolds
3.2.2. Surface Modification
4. Bio-Responsive Scaffolds
4.1. Physical Stimuli
4.1.1. Temperature Response
4.1.2. Mechanical and Ultrasound Response
4.1.3. Electrical Response
4.1.4. Magnetic Response
4.2. Chemical Stimuli
4.2.1. pH Response
4.2.2. Redox Response and Reactive Oxygen Species (ROS)
4.2.3. Enzyme Response
5. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, Y.; Yaszemski, M.J.; Mikos, A.G.; Laurencin, C.T. Tissue Engineering of Bone: Material and Matrix Considerations. J. Bone Jt. Surg. 2008, 90, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma 2019, 33, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.; Ghosh, S.; Dasgupta, S.; Dey, A.; Banerjee, R.; Basak, I.; Bhuniya, T. Recent Advances in Tissue Engineering Scaffolds and Commercial Applications. YMER 2022, 21, 865–882. [Google Scholar] [CrossRef]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold Fabrication Technologies and Structure/Function Properties in Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Dixon, D.T.; Gomillion, C.T. Conductive Scaffolds for Bone Tissue Engineering: Current State and Future Outlook. J. Funct. Biomater. 2021, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Zhou, H.; Chen, X.; Zhu, Y. Recent Advances of Responsive Scaffolds in Bone Tissue Engineering. Front. Bioeng. Biotechnol. 2023, 11, 1296881. [Google Scholar] [CrossRef] [PubMed]
- Ntousi, O.; Roumpi, M.; Siogkas, P.; Deligianni, D.; Fotiadis, D.I. Computational Fluid Dynamic Analysis of Customised 3D-Printed Bone Scaffolds with Different Architectures. In Proceedings of the 2023 45th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Sydney, Australia, 24–27 July 2023; Volume 2023, pp. 1–4. [Google Scholar] [CrossRef]
- Kareem, M.M.; Tanner, K.E. Methods of Producing Three Dimensional Electrospun Scaffolds for Bone Tissue Engineering: A Review. Proc. Inst. Mech. Eng. H 2022, 236, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Basic and Applied Bone Biology, 2nd ed.; Burr, D.B.; Allen, M.R. (Eds.) Academic Press: London, UK; San Diego, CA, USA, 2019; ISBN 978-0-12-813259-3. [Google Scholar]
- Kenkre, J.; Bassett, J. The Bone Remodelling Cycle. Ann. Clin. Biochem. 2018, 55, 308–327. [Google Scholar] [CrossRef] [PubMed]
- Bone Tissue Engineering; Hollinger, J.O.; Einhorn, T.A.; Doll, B.; Sfeir, C. (Eds.) CRC Press: Boca Raton, FL, USA, 2004; ISBN 978-0-203-49509-4. [Google Scholar]
- Jahani, M.; Genever, P.G.; Patton, R.J.; Ahwal, F.; Fagan, M.J. The Effect of Osteocyte Apoptosis on Signalling in the Osteocyte and Bone Lining Cell Network: A Computer Simulation. J. Biomech. 2012, 45, 2876–2883. [Google Scholar] [CrossRef]
- Blair, H.C.; Sidonio, R.F.; Friedberg, R.C.; Khan, N.N.; Dong, S.-S. Proteinase Expression during Differentiation of Human Osteoclasts in Vitro. J. Cell. Biochem. 2000, 78, 627–637. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone Grafts and Biomaterials Substitutes for Bone Defect Repair: A Review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Ledda, M.; Merco, M.; Sciortino, A.; Scatena, E.; Convertino, A.; Lisi, A.; Del Gaudio, C. Biological Response to Bioinspired Microporous 3D-Printed Scaffolds for Bone Tissue Engineering. Int. J. Mol. Sci. 2022, 23, 5383. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.H. Autologous Bone Graft: Is It Still the Gold Standard? Injury 2021, 52, S18–S22. [Google Scholar] [CrossRef]
- Paltanea, G.; Manescu (Paltanea), V.; Antoniac, I.; Antoniac, A.; Nemoianu, I.V.; Robu, A.; Dura, H. A Review of Biomimetic and Biodegradable Magnetic Scaffolds for Bone Tissue Engineering and Oncology. Int. J. Mol. Sci. 2023, 24, 4312. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Babensee, J.E. Controlled Delivery of Immunomodulators from a Biomaterial Scaffold Niche to Induce a Tolerogenic Phenotype in Human Dendritic Cells. ACS Biomater. Sci. Eng. 2020, 6, 4062–4076. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, V.; Martín-Moldes, Z.; Deck, A.; Torres-Sanchez, R.; Valat, A.; Cairns, D.; Li, C.; Kaplan, D.L. Functionalized 3D-Printed Silk-Hydroxyapatite Scaffolds for Enhanced Bone Regeneration with Innervation and Vascularization. Biomaterials 2021, 276, 120995. [Google Scholar] [CrossRef]
- Du, B.; Yin, H.; Chen, Y.; Lin, W.; Wang, Y.; Zhao, D.; Wang, G.; He, X.; Li, J.; Li, Z.; et al. A Waterborne Polyurethane 3D Scaffold Containing PLGA with a Controllable Degradation Rate and an Anti-Inflammatory Effect for Potential Applications in Neural Tissue Repair. J. Mater. Chem. B 2020, 8, 4434–4446. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Ren, J.; Jia, X.; Pan, K. The Bone Formation in Vitro and Mandibular Defect Repair Using PLGA Porous Scaffolds. J. Biomed. Mater. Res. 2005, 74A, 562–569. [Google Scholar] [CrossRef]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and Bioactive Porous Polymer/Inorganic Composite Scaffolds for Bone Tissue Engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef]
- Heidari, F.; Razavi, M.; Bahrololoom, M.E.; Bazargan-Lari, R.; Vashaee, D.; Kotturi, H.; Tayebi, L. Mechanical Properties of Natural Chitosan/Hydroxyapatite/Magnetite Nanocomposites for Tissue Engineering Applications. Mater. Sci. Eng. C 2016, 65, 338–344. [Google Scholar] [CrossRef]
- Sabir, M.I.; Xu, X.; Li, L. A Review on Biodegradable Polymeric Materials for Bone Tissue Engineering Applications. J. Mater. Sci. 2009, 44, 5713–5724. [Google Scholar] [CrossRef]
- Babaie, E.; Bhaduri, S.B. Fabrication Aspects of Porous Biomaterials in Orthopedic Applications: A Review. ACS Biomater. Sci. Eng. 2018, 4, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Singh, N. Antibacterial Silk Fibroin Scaffolds with Green Synthesized Silver Nanoparticles for Osteoblast Proliferation and Human Mesenchymal Stem Cell Differentiation. Colloids Surf. B Biointerfaces 2019, 176, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Zhou, X.; Zhang, F.; Diekwisch, T.G.H.; Luan, X.; Yang, J. Triple PLGA/PCL Scaffold Modification Including Silver Impregnation, Collagen Coating, and Electrospinning Significantly Improve Biocompatibility, Antimicrobial, and Osteogenic Properties for Orofacial Tissue Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 37381–37396. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.L.M.S.; Barbosa, L.; Hurtado, C.R.; Ramos, L.D.P.; Montanheiro, T.L.A.; Oliveira, L.D.; Tada, D.B.; Trichês, E.D.S. Bioglass-based Scaffolds Coated with Silver Nanoparticles: Synthesis, Processing and Antimicrobial Activity. J. Biomed. Mater. Res. 2020, 108, 2447–2459. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Ren, Y.; Emmert, S.; Vučković, I.; Stojanovic, S.; Najman, S.; Schnettler, R.; Barbeck, M.; Schenke-Layland, K.; Xiong, X. The Use of Collagen-Based Materials in Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 3744. [Google Scholar] [CrossRef] [PubMed]
- Sukpaita, T.; Chirachanchai, S.; Pimkhaokham, A.; Ampornaramveth, R.S. Chitosan-Based Scaffold for Mineralized Tissues Regeneration. Mar. Drugs 2021, 19, 551. [Google Scholar] [CrossRef] [PubMed]
- Valente, J.F.A.; Valente, T.A.M.; Alves, P.; Ferreira, P.; Silva, A.; Correia, I.J. Alginate Based Scaffolds for Bone Tissue Engineering. Mater. Sci. Eng. C 2012, 32, 2596–2603. [Google Scholar] [CrossRef]
- Li, M.; You, J.; Qin, Q.; Liu, M.; Yang, Y.; Jia, K.; Zhang, Y.; Zhou, Y. A Comprehensive Review on Silk Fibroin as a Persuasive Biomaterial for Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 2660. [Google Scholar] [CrossRef]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as Biomaterial for Bone Scaffolds: Review of Literature. J. Oral Biol. Craniofacial Res. 2020, 10, 381–388. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Noroozi, R.; Sadeghianmaryan, A.; Jalalvand, M.; Hossain, M. Recent Advances in 3D-Printed Polylactide and Polycaprolactone-Based Biomaterials for Tissue Engineering Applications. Int. J. Biol. Macromol. 2022, 218, 930–968. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Peng, X.; Huang, K.; Li, L.; Liu, X.; Chitrakar, C.; Chang, L.; Gu, Z.; Kuang, T. High-Performance Porous PLLA-Based Scaffolds for Bone Tissue Engineering: Preparation, Characterization, and in Vitro and in Vivo Evaluation. Polymer 2019, 180, 121707. [Google Scholar] [CrossRef]
- Jin, S.; Xia, X.; Huang, J.; Yuan, C.; Zuo, Y.; Li, Y.; Li, J. Recent Advances in PLGA-Based Biomaterials for Bone Tissue Regeneration. Acta Biomater. 2021, 127, 56–79. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, Y.; Hirohara, S.; Terada, K.; Ando, T.; Tanihara, M. Collagen-like Polypeptide Poly(Pro-Hyp-Gly) Conjugated with Gly-Arg-Gly-Asp-Ser and Pro-His-Ser-Arg-Asn Peptides Enhances Cell Adhesion, Migration, and Stratification. Biopolymers 2011, 96, 302–315. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.S.P.; Barrows, T.H.; Cartmell, S.H.; Guldberg, R.E. Microarchitectural and Mechanical Characterization of Oriented Porous Polymer Scaffolds. Biomaterials 2003, 24, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D Biomaterial Scaffolds and Osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yong, T.; Liao, S.; Chan, C.K.; Stevens, M.M.; Ramakrishna, S. Distinctive Degradation Behaviors of Electrospun Polyglycolide, Poly(dl-Lactide-Co-Glycolide), and Poly(l-Lactide-Co-ε-Caprolactone) Nanofibers Cultured With/Without Porcine Smooth Muscle Cells. Tissue Eng. Part A 2010, 16, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.A.; Brady, J.M.; Cutright, D.E. Degradation Rates of Oral Resorbable Implants (Polylactates and Polyglycolates): Rate Modification with Changes in PLA/PGA Copolymer Ratios. J. Biomed. Mater. Res. 1977, 11, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, S.; Yildirimer, L.; Zhao, H.; Ding, R.; Wang, H.; Cui, W.; Weitz, D. Injectable Stem Cell-Laden Photocrosslinkable Microspheres Fabricated Using Microfluidics for Rapid Generation of Osteogenic Tissue Constructs. Adv. Funct. Mater. 2016, 26, 2809–2819. [Google Scholar] [CrossRef]
- Bez, M.; Sheyn, D.; Tawackoli, W.; Avalos, P.; Shapiro, G.; Giaconi, J.C.; Da, X.; David, S.B.; Gavrity, J.; Awad, H.A.; et al. In Situ Bone Tissue Engineering via Ultrasound-Mediated Gene Delivery to Endogenous Progenitor Cells in Mini-Pigs. Sci. Transl. Med. 2017, 9, eaal3128. [Google Scholar] [CrossRef]
- Soh, E.; Kolos, E.; Ruys, A.J. Foamed High Porosity Alumina for Use as a Bone Tissue Scaffold. Ceram. Int. 2015, 41, 1031–1047. [Google Scholar] [CrossRef]
- Huang, X.; Yang, H.; Luo, T.; Huang, C.; Tay, F.R.; Niu, L. Hollow Mesoporous Zirconia Delivery System for Biomineralization Precursors. Acta Biomater. 2018, 67, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive Calcium Phosphate Materials and Applications in Bone Regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wu, S.; Liu, X.; Qian, S.; Chu, P.K.; Zheng, Y.; Cheung, K.M.C.; Zhao, Y.; Yeung, K.W.K. A Surface-Engineered Multifunctional TiO2 Based Nano-Layer Simultaneously Elevates the Corrosion Resistance, Osteoconductivity and Antimicrobial Property of a Magnesium Alloy. Acta Biomater. 2019, 99, 495–513. [Google Scholar] [CrossRef] [PubMed]
- Fard, M.G.; Sharifianjazi, F.; Kazemi, S.S.; Rostamani, H.; Bathaei, M.S. Laser-Based Additive Manufacturing of Magnesium Alloys for Bone Tissue Engineering Applications: From Chemistry to Clinic. J. Manuf. Mater. Process. 2022, 6, 158. [Google Scholar] [CrossRef]
- Rahman, M.; Dutta, N.K.; Choudhury, N.R. Microroughness Induced Biomimetic Coating for Biodegradation Control of Magnesium. Mater. Sci. Eng. C 2021, 121, 111811. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Tao, S.; Huang, W.; Khan, Z.S.; Fan, X.; Gu, L.; Wang, Y.; Xu, J.; Cai, X.; Ma, H.; et al. Synthesis and Characterization of in Situ TiC–TiB2 Composite Coatings by Reactive Plasma Spraying on a Magnesium Alloy. Appl. Surf. Sci. 2013, 264, 879–885. [Google Scholar] [CrossRef]
- Lamaka, S.V.; Montemor, M.F.; Galio, A.F.; Zheludkevich, M.L.; Trindade, C.; Dick, L.F.; Ferreira, M.G.S. Novel Hybrid Sol–Gel Coatings for Corrosion Protection of AZ31B Magnesium Alloy. Electrochim. Acta 2008, 53, 4773–4783. [Google Scholar] [CrossRef]
- Pádua, A.S.; Figueiredo, L.; Silva, J.C.; Borges, J.P. Chitosan Scaffolds with Mesoporous Hydroxyapatite and Mesoporous Bioactive Glass. Prog. Biomater. 2023, 12, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.A.; Razak, S.I.A.; Ansari, M.N.M.; Zulkifli, R.M.; Ahmad Zawawi, N.; Arshad, M. Development of Biodegradable Bio-Based Composite for Bone Tissue Engineering: Synthesis, Characterization and In Vitro Biocompatible Evaluation. Polymers 2021, 13, 3611. [Google Scholar] [CrossRef]
- Donate, R.; Monzón, M.; Alemán-Domínguez, M.E.; Ortega, Z. Enzymatic Degradation Study of PLA-Based Composite Scaffolds. Rev. Adv. Mater. Sci. 2020, 59, 170–175. [Google Scholar] [CrossRef]
- Wu, D.-Y.; Wang, S.-S.; Wu, C.-S. A New Composite Fabricated from Hydroxyapatite, Gelatin-MgO Microparticles, and Compatibilized Poly(Butylene Succinate) with Osteogenic Functionality. Biomater. Adv. 2023, 154, 213586. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska-Łańcucka, J.; Gilarska, A.; Buła, A.; Horak, W.; Łatkiewicz, A.; Nowakowska, M. Genipin Crosslinked Bioactive Collagen/Chitosan/Hyaluronic Acid Injectable Hydrogels Structurally Amended via Covalent Attachment of Surface-Modified Silica Particles. Int. J. Biol. Macromol. 2019, 136, 1196–1208. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yubao, L.; Aiping, Y.; Xuelin, P.; Xuejiang, W.; Xiang, Z. Preparation and in Vitro Investigation of Chitosan/Nano-Hydroxyapatite Composite Used as Bone Substitute Materials. J. Mater. Sci. Mater. Med. 2005, 16, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Madhumathi, K.; Shalumon, K.T.; Rani, V.V.D.; Tamura, H.; Furuike, T.; Selvamurugan, N.; Nair, S.V.; Jayakumar, R. Wet Chemical Synthesis of Chitosan Hydrogel–Hydroxyapatite Composite Membranes for Tissue Engineering Applications. Int. J. Biol. Macromol. 2009, 45, 12–15. [Google Scholar] [CrossRef]
- Adamski, R.; Siuta, D. Mechanical, Structural, and Biological Properties of Chitosan/Hydroxyapatite/Silica Composites for Bone Tissue Engineering. Molecules 2021, 26, 1976. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Gao, Y.; Lu, G.; Gong, Y.; Zhao, N.; Zhang, X. A Study on the Bioactivity of Chitosan/Nano-Hydroxyapatite Composite Scaffolds for Bone Tissue Engineering. Eur. Polym. J. 2006, 42, 3171–3179. [Google Scholar] [CrossRef]
- Nikpour, M.R.; Rabiee, S.M.; Jahanshahi, M. Synthesis and Characterization of Hydroxyapatite/Chitosan Nanocomposite Materials for Medical Engineering Applications. Compos. Part B Eng. 2012, 43, 1881–1886. [Google Scholar] [CrossRef]
- Lu, H.-T.; Lu, T.-W.; Chen, C.-H.; Mi, F.-L. Development of Genipin-Crosslinked and Fucoidan-Adsorbed Nano-Hydroxyapatite/Hydroxypropyl Chitosan Composite Scaffolds for Bone Tissue Engineering. Int. J. Biol. Macromol. 2019, 128, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.; Ma, G.; Yang, D.; Nie, J. The Effect of the Prefrozen Process on Properties of a Chitosan/Hydroxyapatite/Poly(Methyl Methacrylate) Composite Prepared by Freeze Drying Method Used for Bone Tissue Engineering. RSC Adv. 2015, 5, 79679–79686. [Google Scholar] [CrossRef]
- Shavandi, A.; Bekhit, A.E.-D.A.; Ali, M.A.; Sun, Z.; Gould, M. Development and Characterization of Hydroxyapatite/β-TCP/Chitosan Composites for Tissue Engineering Applications. Mater. Sci. Eng. C 2015, 56, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dai, H.; Li, J. Immunomodulatory Properties of Mesenchymal Stromal/Stem Cells: The Link with Metabolism. J. Adv. Res. 2023, 45, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; David, B.T.; Trawczynski, M.; Fessler, R.G. Advances in Pluripotent Stem Cells: History, Mechanisms, Technologies, and Applications. Stem Cell Rev. Rep. 2020, 16, 3–32. [Google Scholar] [CrossRef]
- Lewis, P.; Silajdžić, E.; Brison, D.R.; Kimber, S.J. Embryonic Stem Cells. In Cell Engineering and Regeneration; Gimble, J.M., Marolt Presen, D., Oreffo, R.O.C., Wolbank, S., Redl, H., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 315–365. ISBN 978-3-319-08830-3. [Google Scholar]
- Abuwatfa, W.H.; Pitt, W.G.; Husseini, G.A. Scaffold-Based 3D Cell Culture Models in Cancer Research. J. Biomed. Sci. 2024, 31, 7. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Peng, X.; Liu, X.; Mou, X.; Guo, Y.; Yang, L.; Chen, Y.; Zhou, Y.; Shi, Z.; Yang, Z.; et al. Advances in the Application of Bone Morphogenetic Proteins and Their Derived Peptides in Bone Defect Repair. Compos. Part B Eng. 2023, 262, 110805. [Google Scholar] [CrossRef]
- Linkhart, T.A.; Mohan, S.; Baylink, D.J. Growth Factors for Bone Growth and Repair: IGF, TGFβ and BMP. Bone 1996, 19, S1–S12. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J. Current Progress in Growth Factors and Extracellular Vesicles in Tendon Healing. Int. Wound J. 2023, 20, 3871–3883. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Yin, G.; Huang, Z.; Liao, X.; Chen, X.; Yao, Y.; Pu, X. Localized Delivery of Growth Factors for Angiogenesis and Bone Formation in Tissue Engineering. Int. Immunopharmacol. 2013, 16, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Datta, P.; Ayan, B.; Ozbolat, I.T. Bioprinting for Vascular and Vascularized Tissue Biofabrication. Acta Biomater. 2017, 51, 1–20. [Google Scholar] [CrossRef]
- Chen, E.P.; Toksoy, Z.; Davis, B.A.; Geibel, J.P. 3D Bioprinting of Vascularized Tissues for in Vitro and in Vivo Applications. Front. Bioeng. Biotechnol. 2021, 9, 664188. [Google Scholar] [CrossRef]
- Zhang, Y.; Kumar, P.; Lv, S.; Xiong, D.; Zhao, H.; Cai, Z.; Zhao, X. Recent Advances in 3D Bioprinting of Vascularized Tissues. Mater. Des. 2021, 199, 109398. [Google Scholar] [CrossRef]
- Yazdanpanah, Z.; Johnston, J.D.; Cooper, D.M.L.; Chen, X. 3D Bioprinted Scaffolds for Bone Tissue Engineering: State-Of-The-Art and Emerging Technologies. Front. Bioeng. Biotechnol. 2022, 10, 824156. [Google Scholar] [CrossRef] [PubMed]
- Aydogdu, M.O.; Oner, E.T.; Ekren, N.; Erdemir, G.; Kuruca, S.E.; Yuca, E.; Bostan, M.S.; Eroglu, M.S.; Ikram, F.; Uzun, M.; et al. Comparative Characterization of the Hydrogel Added PLA/β-TCP Scaffolds Produced by 3D Bioprinting. Bioprinting 2019, 13, e00046. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Hung, C.-H.; Kuo, C.-N.; Chen, C.-Y.; Peng, Y.-N.; Shie, M.-Y. Improved Bioactivity of 3D Printed Porous Titanium Alloy Scaffold with Chitosan/Magnesium-Calcium Silicate Composite for Orthopaedic Applications. Materials 2019, 12, 203. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Li, L.; Lin, Z.; Yang, W.; Duan, M.; Chen, L.; Xia, Y.; Chen, Z.; Lu, Y.; Zhang, Y. Integrating 3D-Printed PHBV/Calcium Sulfate Hemihydrate Scaffold and Chitosan Hydrogel for Enhanced Osteogenic Property. Carbohydr. Polym. 2018, 202, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Deng, N.; Sun, J.; Li, Y.; Chen, L.; Chen, C.; Wu, Y.; Wang, Z.; Li, L. Experimental Study of rhBMP-2 Chitosan Nano-Sustained Release Carrier-Loaded PLGA/nHA Scaffolds to Construct Mandibular Tissue-Engineered Bone. Arch. Oral Biol. 2019, 102, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Tharakan, S.; Khondkar, S.; Ilyas, A. Bioprinting of Stem Cells in Multimaterial Scaffolds and Their Applications in Bone Tissue Engineering. Sensors 2021, 21, 7477. [Google Scholar] [CrossRef] [PubMed]
- Kumar Parupelli, S.; Saudi, S.; Bhattarai, N.; Desai, S. 3D Printing of PCL-Ceramic Composite Scaffolds for Bone Tissue Engineering Applications. Int. J. Bioprinting 2023, 9, 0196. [Google Scholar] [CrossRef]
- Stoica Oprea, A.E.; Bîrcă, A.C.; Gherasim, O.; Ficai, A.; Grumezescu, A.M.; Oprea, O.-C.; Vasile, B.Ș.; Balta, C.; Andronescu, E.; Hermenean, A.O. Electrospun Fibrous Silica for Bone Tissue Engineering Applications. Pharmaceutics 2023, 15, 1728. [Google Scholar] [CrossRef]
- Sahebalzamani, M.; Ziminska, M.; McCarthy, H.O.; Levingstone, T.J.; Dunne, N.J.; Hamilton, A.R. Advancing Bone Tissue Engineering One Layer at a Time: A Layer-by-Layer Assembly Approach to 3D Bone Scaffold Materials. Biomater. Sci. 2022, 10, 2734–2758. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone Tissue Engineering: Recent Advances and Challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.; Thomas, N.; Varghese, M.; Dalvi, Y.; Joy, S.; Hall, S.; Mathew, A.P. The Design of 3D-Printed Polylactic Acid-Bioglass Composite Scaffold: A Potential Implant Material for Bone Tissue Engineering. Molecules 2022, 27, 7214. [Google Scholar] [CrossRef] [PubMed]
- Aydin, M.S.; Sahin, M.; Dogan, Z.; Kiziltas, G. Microstructural Characterization of PCL-HA Bone Scaffolds Based on Nonsolvent-Induced Phase Separation. ACS Omega 2023, 8, 47595–47605. [Google Scholar] [CrossRef] [PubMed]
- Heo, D.N.; Hospodiuk, M.; Ozbolat, I.T. Synergistic Interplay between Human MSCs and HUVECs in 3D Spheroids Laden in Collagen/Fibrin Hydrogels for Bone Tissue Engineering. Acta Biomater. 2019, 95, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials Design for Bone-Tissue Engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Shekaran, A.; García, A.J. Extracellular Matrix-Mimetic Adhesive Biomaterials for Bone Repair. J. Biomed. Mater. Res. A 2011, 96, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, Z.-C.; Liu, Y.; Chen, Y.-R.; Deng, R.-H.; Zhang, Z.-N.; Yu, J.-K.; Yuan, F.-Z. Function and Mechanism of RGD in Bone and Cartilage Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 773636. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.Y.; Yang, D.H.; You, S.J.; Kim, H.J.; Chun, H.J. In-Situ Forming Injectable GFOGER-Conjugated BMSCs-Laden Hydrogels for Osteochondral Regeneration. NPJ Regen Med. 2023, 8, 2. [Google Scholar] [CrossRef]
- Khan, F.; Tanaka, M. Designing Smart Biomaterials for Tissue Engineering. Int. J. Mol. Sci. 2017, 19, 17. [Google Scholar] [CrossRef]
- Li, Y.; Rodrigues, J.; Tomás, H. Injectable and Biodegradable Hydrogels: Gelation, Biodegradation and Biomedical Applications. Chem. Soc. Rev. 2012, 41, 2193–2221. [Google Scholar] [CrossRef]
- Kim, H.J.; You, S.J.; Yang, D.H.; Chun, H.J.; Kim, M.S. Preparation of Novel RGD-Conjugated Thermosensitive mPEG-PCL Composite Hydrogels and in Vitro Investigation of Their Impacts on Adhesion-Dependent Cellular Behavior. J. Ind. Eng. Chem. 2020, 84, 226–235. [Google Scholar] [CrossRef]
- De Jonge, L.T.; Leeuwenburgh, S.C.G.; Wolke, J.G.C.; Jansen, J.A. Organic–Inorganic Surface Modifications for Titanium Implant Surfaces. Pharm. Res. 2008, 25, 2357–2369. [Google Scholar] [CrossRef]
- Klimek, K.; Ginalska, G. Proteins and Peptides as Important Modifiers of the Polymer Scaffolds for Tissue Engineering Applications-A Review. Polymers 2020, 12, 844. [Google Scholar] [CrossRef] [PubMed]
- Khew, S.T.; Tong, Y.W. The Specific Recognition of a Cell Binding Sequence Derived from Type I Collagen by Hep3B and L929 Cells. Biomacromolecules 2007, 8, 3153–3161. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.J.; Sahoo, J.K.; Ulijn, R.V.; Dalby, M.J. Mesenchymal Stem Cell Fate: Applying Biomaterials for Control of Stem Cell Behavior. Front. Bioeng. Biotechnol. 2016, 4. [Google Scholar] [CrossRef]
- Madl, C.M.; Heilshorn, S.C. Engineering Hydrogel Microenvironments to Recapitulate the Stem Cell Niche. Annu. Rev. Biomed. Eng. 2018, 20, 21–47. [Google Scholar] [CrossRef]
- Wojtowicz, A.M.; Shekaran, A.; Oest, M.E.; Dupont, K.M.; Templeman, K.L.; Hutmacher, D.W.; Guldberg, R.E.; García, A.J. Coating of Biomaterial Scaffolds with the Collagen-Mimetic Peptide GFOGER for Bone Defect Repair. Biomaterials 2010, 31, 2574–2582. [Google Scholar] [CrossRef] [PubMed]
- Mhanna, R.; Öztürk, E.; Vallmajo-Martin, Q.; Millan, C.; Müller, M.; Zenobi-Wong, M. GFOGER-Modified MMP-Sensitive Polyethylene Glycol Hydrogels Induce Chondrogenic Differentiation of Human Mesenchymal Stem Cells. Tissue Eng. Part A 2014, 20, 1165–1174. [Google Scholar] [CrossRef]
- Min, S.; Ko, I.K.; Yoo, J.J. State-of-the-Art Strategies for the Vascularization of Three-Dimensional Engineered Organs. Vasc. Spec. Int. 2019, 35, 77–89. [Google Scholar] [CrossRef]
- Grant, D.S.; Kinsella, J.L.; Fridman, R.; Auerbach, R.; Piasecki, B.A.; Yamada, Y.; Zain, M.; Kleinman, H.K. Interaction of Endothelial Cells with a Laminin A Chain Peptide (SIKVAV) in Vitro and Induction of Angiogenic Behavior in Vivo. J. Cell. Physiol. 1992, 153, 614–625. [Google Scholar] [CrossRef]
- Fittkau, M.H.; Zilla, P.; Bezuidenhout, D.; Lutolf, M.P.; Human, P.; Hubbell, J.A.; Davies, N. The Selective Modulation of Endothelial Cell Mobility on RGD Peptide Containing Surfaces by YIGSR Peptides. Biomaterials 2005, 26, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.S.; Joung, Y.K.; Lee, Y.; Bae, J.W.; Park, H.K.; Park, Y.H.; Park, J.-C.; Park, K.D. Enhanced Patency and Endothelialization of Small-Caliber Vascular Grafts Fabricated by Coimmobilization of Heparin and Cell-Adhesive Peptides. ACS Appl. Mater. Interfaces 2016, 8, 4336–4346. [Google Scholar] [CrossRef]
- Wang, W.; Guo, L.; Yu, Y.; Chen, Z.; Zhou, R.; Yuan, Z. Peptide REDV-modified Polysaccharide Hydrogel with Endothelial Cell Selectivity for the Promotion of Angiogenesis. J. Biomed. Mater. Res. 2015, 103, 1703–1712. [Google Scholar] [CrossRef] [PubMed]
- Gromolak, S.; Krawczenko, A.; Antończyk, A.; Buczak, K.; Kiełbowicz, Z.; Klimczak, A. Biological Characteristics and Osteogenic Differentiation of Ovine Bone Marrow Derived Mesenchymal Stem Cells Stimulated with FGF-2 and BMP-2. Int. J. Mol. Sci. 2020, 21, 9726. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Edwards, J.R.; Ko, S.-Y.; Dong, S.; Liu, H.; Oyajobi, B.O.; Papasian, C.; Deng, H.-W.; Zhao, M. Transcriptional Regulation of BMP2 Expression by the PTH-CREB Signaling Pathway in Osteoblasts. PLoS ONE 2011, 6, e20780. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, H.; Zhang, W.; Huang, E.; Wang, N.; Wu, N.; Wen, S.; Chen, X.; Liao, Z.; Deng, F.; et al. Bone Morphogenetic Protein-9 Effectively Induces Osteo/Odontoblastic Differentiation of the Reversibly Immortalized Stem Cells of Dental Apical Papilla. Stem Cells Dev. 2014, 23, 1405–1416. [Google Scholar] [CrossRef] [PubMed]
- Van Caam, A.; Blaney Davidson, E.; Garcia De Vinuesa, A.; Van Geffen, E.; Van Den Berg, W.; Goumans, M.-J.; Ten Dijke, P.; Van Der Kraan, P. The High Affinity ALK1-Ligand BMP9 Induces a Hypertrophy-like State in Chondrocytes That Is Antagonized by TGFβ1. Osteoarthr. Cartil. 2015, 23, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Luu, H.H.; Song, W.; Luo, X.; Manning, D.; Luo, J.; Deng, Z.; Sharff, K.A.; Montag, A.G.; Haydon, R.C.; He, T. Distinct Roles of Bone Morphogenetic Proteins in Osteogenic Differentiation of Mesenchymal Stem Cells. J. Orthop. Res. 2007, 25, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Luo, Q.; Shu, Y.; Zeng, Z.; Huang, B.; Feng, Y.; Zhang, B.; Wang, X.; Lei, Y.; Ye, Z.; et al. Transcriptomic Landscape Regulated by the 14 Types of Bone Morphogenetic Proteins (BMPs) in Lineage Commitment and Differentiation of Mesenchymal Stem Cells (MSCs). Genes Dis. 2019, 6, 258–275. [Google Scholar] [CrossRef]
- Chen, L.; Zou, X.; Zhang, R.-X.; Pi, C.-J.; Wu, N.; Yin, L.-J.; Deng, Z.-L. IGF1 Potentiates BMP9-Induced Osteogenic Differentiation in Mesenchymal Stem Cells through the Enhancement of BMP/Smad Signaling. BMB Rep. 2016, 49, 122–127. [Google Scholar] [CrossRef]
- Yu, L.; Dawson, L.A.; Yan, M.; Zimmel, K.; Lin, Y.-L.; Dolan, C.P.; Han, M.; Muneoka, K. BMP9 Stimulates Joint Regeneration at Digit Amputation Wounds in Mice. Nat. Commun. 2019, 10, 424. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-W.; Chen, Z.-L.; Piao, Y.-J. Mesenchymal Stem Cells Differentiate into Tenocytes by Bone Morphogenetic Protein (BMP) 12 Gene Transfer. J. Biosci. Bioeng. 2005, 100, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jiang, X.; Lu, A.; Tu, M.; Huang, W.; Huang, P. BMP14 Induces Tenogenic Differentiation of Bone Marrow Mesenchymal Stem Cells In-vitro. Exp. Ther. Med. 2018, 16, 1165–1174. [Google Scholar] [CrossRef]
- Duan, X.; Bradbury, S.R.; Olsen, B.R.; Berendsen, A.D. VEGF Stimulates Intramembranous Bone Formation during Craniofacial Skeletal Development. Matrix Biol. 2016, 52–54, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liu, Y.; Wang, A.; Zhu, Z.; Li, Y.; Zhu, C.; Che, Z.; Liu, T.; Liu, H.; Huang, L. Application of BMP in Bone Tissue Engineering. Front. Bioeng. Biotechnol. 2022, 10, 810880. [Google Scholar] [CrossRef] [PubMed]
- Barati, D.; Shariati, S.R.P.; Moeinzadeh, S.; Melero-Martin, J.M.; Khademhosseini, A.; Jabbari, E. Spatiotemporal Release of BMP-2 and VEGF Enhances Osteogenic and Vasculogenic Differentiation of Human Mesenchymal Stem Cells and Endothelial Colony-Forming Cells Co-Encapsulated in a Patterned Hydrogel. J. Control. Release 2016, 223, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Bouletreau, P.J.; Warren, S.M.; Spector, J.A.; Peled, Z.M.; Gerrets, R.P.; Greenwald, J.A.; Longaker, M.T. Hypoxia and VEGF Up-Regulate BMP-2 mRNA and Protein Expression in Microvascular Endothelial Cells: Implications for Fracture Healing. Plast. Reconstr. Surg. 2002, 109, 2384–2397. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Guo, B.; Xie, J.; Deng, S.; Fu, N.; Lin, S.; Li, G.; Lin, Y.; Cai, X. Crosstalk between Adipose-Derived Stem Cells and Chondrocytes: When Growth Factors Matter. Bone Res. 2016, 4, 15036. [Google Scholar] [CrossRef]
- Chen, M.; Chen, Y.; Wei, C. Nanoparticles Based Composite Coatings with Tunable Vascular Endothelial Growth Factor and Bone Morphogenetic Protein-2 Release for Bone Regeneration. J. Biomed. Mater. Res. 2023, 111, 1044–1053. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Portolés-Gil, N.; López-Periago, A.M.; Domingo, C.; Hosta-Rigau, L. Immobilization of BMP-2 and VEGF within Multilayered Polydopamine-Coated Scaffolds and the Resulting Osteogenic and Angiogenic Synergy of Co-Cultured Human Mesenchymal Stem Cells and Human Endothelial Progenitor Cells. Int. J. Mol. Sci. 2020, 21, 6418. [Google Scholar] [CrossRef]
- Geng, Y.; Duan, H.; Xu, L.; Witman, N.; Yan, B.; Yu, Z.; Wang, H.; Tan, Y.; Lin, L.; Li, D.; et al. BMP-2 and VEGF-A modRNAs in Collagen Scaffold Synergistically Drive Bone Repair through Osteogenic and Angiogenic Pathways. Commun. Biol. 2021, 4, 82. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, X.; Deng, F.; Ye, X.; Shen, Z.; Xia, Y.; Zhang, Y. Integration of BMP-2/PLGA Microspheres with the 3D Printed PLGA/CaSO4 Scaffold Enhances Bone Regeneration. Front. Mater. 2024, 11, 1374409. [Google Scholar] [CrossRef]
- Kim, C.; Lee, J.W.; Heo, J.H.; Park, C.; Kim, D.-H.; Yi, G.S.; Kang, H.C.; Jung, H.S.; Shin, H.; Lee, J.H. Natural Bone-Mimicking Nanopore-Incorporated Hydroxyapatite Scaffolds for Enhanced Bone Tissue Regeneration. Biomater. Res. 2022, 26, 7. [Google Scholar] [CrossRef]
- Crowder, S.W.; Prasai, D.; Rath, R.; Balikov, D.A.; Bae, H.; Bolotin, K.I.; Sung, H.-J. Three-Dimensional Graphene Foams Promote Osteogenic Differentiation of Human Mesenchymal Stem Cells. Nanoscale 2013, 5, 4171. [Google Scholar] [CrossRef]
- Di Luca, A.; Ostrowska, B.; Lorenzo-Moldero, I.; Lepedda, A.; Swieszkowski, W.; Van Blitterswijk, C.; Moroni, L. Gradients in Pore Size Enhance the Osteogenic Differentiation of Human Mesenchymal Stromal Cells in Three-Dimensional Scaffolds. Sci. Rep. 2016, 6, 22898. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Kurashina, K.; De Bruijn, J.D.; Li, Y.; De Groot, K.; Zhang, X. A Preliminary Study on Osteoinduction of Two Kinds of Calcium Phosphate Ceramics. Biomaterials 1999, 20, 1799–1806. [Google Scholar] [CrossRef] [PubMed]
- Schlipf, D.M.; Rankin, S.E.; Knutson, B.L. Pore-Size Dependent Protein Adsorption and Protection from Proteolytic Hydrolysis in Tailored Mesoporous Silica Particles. ACS Appl. Mater. Interfaces 2013, 5, 10111–10117. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Jee, S.E.; Jiao, K.; Tonggu, L.; Li, M.; Wang, L.; Yang, Y.; Bian, J.; Breschi, L.; Jang, S.S.; et al. Collagen Intrafibrillar Mineralization as a Result of the Balance between Osmotic Equilibrium and Electroneutrality. Nat. Mater. 2017, 16, 370–378. [Google Scholar] [CrossRef]
- Chen, Z.; Ni, S.; Han, S.; Crawford, R.; Lu, S.; Wei, F.; Chang, J.; Wu, C.; Xiao, Y. Nanoporous Microstructures Mediate Osteogenesis by Modulating the Osteo-Immune Response of Macrophages. Nanoscale 2017, 9, 706–718. [Google Scholar] [CrossRef]
- Spalazzi, J.P.; Doty, S.B.; Moffat, K.L.; Levine, W.N.; Lu, H.H. Development of Controlled Matrix Heterogeneity on a Triphasic Scaffold for Orthopedic Interface Tissue Engineering. Tissue Eng. 2006, 12, 3497–3508. [Google Scholar] [CrossRef]
- Spalazzi, J.P.; Dagher, E.; Doty, S.B.; Guo, X.E.; Rodeo, S.A.; Lu, H.H. In Vivo Evaluation of a Multiphased Scaffold Designed for Orthopaedic Interface Tissue Engineering and Soft Tissue-to-Bone Integration. J. Biomed. Mater. Res. A 2008, 86, 1–12. [Google Scholar] [CrossRef]
- Grayson, W.L.; Fröhlich, M.; Yeager, K.; Bhumiratana, S.; Chan, M.E.; Cannizzaro, C.; Wan, L.Q.; Liu, X.S.; Guo, X.E.; Vunjak-Novakovic, G. Engineering Anatomically Shaped Human Bone Grafts. Proc. Natl. Acad. Sci. USA 2010, 107, 3299–3304. [Google Scholar] [CrossRef]
- Igwe, J.C.; Mikael, P.E.; Nukavarapu, S.P. Design, Fabrication and in Vitro Evaluation of a Novel Polymer-Hydrogel Hybrid Scaffold for Bone Tissue Engineering: Osteoinductive Hybrid Scaffold for Bone Regeneration. J. Tissue Eng. Regen. Med. 2014, 8, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Durairaj, K.; Balasubramanian, B.; Arumugam, V.A.; Easwaran, M.; Park, S.; Issara, U.; Pushparaj, K.; Al-Dhabi, N.A.; Arasu, M.V.; Liu, W.-C.; et al. Biocompatibility of Veratric Acid–Encapsulated Chitosan/Methylcellulose Hydrogel: Biological Characterization, Osteogenic Efficiency with In Silico Molecular Modeling. Appl. Biochem. Biotechnol. 2023, 195, 4429–4446. [Google Scholar] [CrossRef] [PubMed]
- Woodbury, S.M.; Swanson, W.B.; Douglas, L.; Niemann, D.; Mishina, Y. Temperature-Responsive PCL-PLLA Nanofibrous Tissue Engineering Scaffolds with Memorized Porous Microstructure Recovery. Front. Dent. Med. 2023, 4, 1240397. [Google Scholar] [CrossRef] [PubMed]
- Vejjasilpa, K.; Maqsood, I.; Schulz-Siegmund, M.; Hacker, M.C. Adjustable Thermo-Responsive, Cell-Adhesive Tissue Engineering Scaffolds for Cell Stimulation through Periodic Changes in Culture Temperature. Int. J. Mol. Sci. 2022, 24, 572. [Google Scholar] [CrossRef]
- Ribeiro, C.; Sencadas, V.; Correia, D.M.; Lanceros-Méndez, S. Piezoelectric Polymers as Biomaterials for Tissue Engineering Applications. Colloids Surf. B Biointerfaces 2015, 136, 46–55. [Google Scholar] [CrossRef]
- Hoop, M.; Chen, X.-Z.; Ferrari, A.; Mushtaq, F.; Ghazaryan, G.; Tervoort, T.; Poulikakos, D.; Nelson, B.; Pané, S. Ultrasound-Mediated Piezoelectric Differentiation of Neuron-like PC12 Cells on PVDF Membranes. Sci. Rep. 2017, 7, 4028. [Google Scholar] [CrossRef]
- Kang, S.-W.; La, W.-G.; Kim, B.-S. Open Macroporous Poly(Lactic-Co-Glycolic Acid) Microspheres as an Injectable Scaffold for Cartilage Tissue Engineering. J. Biomater. Sci. Polym. Ed. 2009, 20, 399–409. [Google Scholar] [CrossRef]
- Kapat, K.; Shubhra, Q.T.H.; Zhou, M.; Leeuwenburgh, S. Piezoelectric Nano-Biomaterials for Biomedicine and Tissue Regeneration. Adv. Funct. Mater. 2020, 30, 1909045. [Google Scholar] [CrossRef]
- Zheng, T.; Huang, Y.; Zhang, X.; Cai, Q.; Deng, X.; Yang, X. Mimicking the Electrophysiological Microenvironment of Bone Tissue Using Electroactive Materials to Promote Its Regeneration. J. Mater. Chem. B 2020, 8, 10221–10256. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; More, N.; Kalia, K.; Kapusetti, G. Piezoelectric Smart Biomaterials for Bone and Cartilage Tissue Engineering. Inflamm. Regener. 2018, 38, 2. [Google Scholar] [CrossRef] [PubMed]
- Miszuk, J.; Liang, Z.; Hu, J.; Sanyour, H.; Hong, Z.; Fong, H.; Sun, H. An Elastic Mineralized 3D Electrospun PCL Nanofibrous Scaffold for Drug Release and Bone Tissue Engineering. ACS Appl. Bio Mater. 2021, 4, 3639–3648. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.S.; Hong, R.C.; Chang, W.H.; Chen, L.T.; Lin, F.H.; Liu, H.C. In Vitro Effects of Low-Intensity Ultrasound Stimulation on the Bone Cells. J. Biomed. Mater. Res. 2001, 57, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Claes, L.; Willie, B. The Enhancement of Bone Regeneration by Ultrasound. Prog. Biophys. Mol. Biol. 2007, 93, 384–398. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Schmelz, A.; Seufferlein, T.; Li, Y.; Zhao, J.; Bachem, M.G. Molecular Mechanisms of Low Intensity Pulsed Ultrasound in Human Skin Fibroblasts. J. Biol. Chem. 2004, 279, 54463–54469. [Google Scholar] [CrossRef]
- Yang, M.-H.; Lim, K.-T.; Choung, P.-H.; Cho, C.-S.; Chung, J.H. Application of Ultrasound Stimulation in Bone Tissue Engineering. Int. J. Stem Cells 2010, 3, 74–79. [Google Scholar] [CrossRef]
- He, Y.; Li, F.; Jiang, P.; Cai, F.; Lin, Q.; Zhou, M.; Liu, H.; Yan, F. Remote Control of the Recruitment and Capture of Endogenous Stem Cells by Ultrasound for in Situ Repair of Bone Defects. Bioact. Mater. 2023, 21, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Ambattu, L.A.; Gelmi, A.; Yeo, L.Y. Short-Duration High Frequency MegaHertz-Order Nanomechanostimulation Drives Early and Persistent Osteogenic Differentiation in Mesenchymal Stem Cells. Small 2022, 18, 2106823. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jia, Z.; Dai, M.; Feng, X.; Tang, C.; Liu, L.; Cao, L. Advances in Natural and Synthetic Macromolecules with Stem Cells and Extracellular Vesicles for Orthopedic Disease Treatment. Int. J. Biol. Macromol. 2024, 268, 131874. [Google Scholar] [CrossRef]
- Carina, V.; Costa, V.; Raimondi, L.; Pagani, S.; Sartori, M.; Figallo, E.; Setti, S.; Alessandro, R.; Fini, M.; Giavaresi, G. Effect of Low-Intensity Pulsed Ultrasound on Osteogenic Human Mesenchymal Stem Cells Commitment in a New Bone Scaffold. J. Appl. Biomater. Funct. Mater. 2017, 15, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wang, M.; He, J. A Review of Biomimetic Scaffolds for Bone Regeneration: Toward a Cell-Free Strategy. Bioeng. Transl. Med. 2021, 6, e10206. [Google Scholar] [CrossRef] [PubMed]
- Bassett, C.A.; Pawluk, R.J.; Becker, R.O. Effects of Electric Currents on Bone In Vivo. Nature 1964, 204, 652–654. [Google Scholar] [CrossRef] [PubMed]
- Mobini, S.; Leppik, L.; Thottakkattumana Parameswaran, V.; Barker, J.H. In Vitro Effect of Direct Current Electrical Stimulation on Rat Mesenchymal Stem Cells. PeerJ 2017, 5, e2821. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, H.; Wu, Z.; Wu, N.; Wang, Z.; Chen, X.; Wei, Y.; Zhang, P. Modulation of Osteogenesis in MC3T3-E1 Cells by Different Frequency Electrical Stimulation. PLoS ONE 2016, 11, e0154924. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Watt, C.; Karystinou, A.; Roelofs, A.; McCaig, C.; Gibson, I.; De Bari, C. Directed Migration of Human Bone Marrow Mesenchymal Stem Cells in a Physiological Direct Current Electric Field. Eur. Cell Mater. 2011, 22, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, S. The Electret Effect in Bone and Biopolymers and The Bound-Water Problem. Ann. N. Y. Acad. Sci. 1974, 238, 36–52. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, C.; Lin, Y.; Hu, P.; Shen, Y.; Wang, K.; Meng, S.; Chai, Y.; Dai, X.; Liu, X.; et al. Nanocomposite Membranes Enhance Bone Regeneration Through Restoring Physiological Electric Microenvironment. ACS Nano 2016, 10, 7279–7286. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Su, J.; Li, Y.; Zhang, H.; Shi, Y.; Yan, C.; Lu, J. 3D/4D Printed Bio-Piezoelectric Smart Scaffolds for next-Generation Bone Tissue Engineering. Int. J. Extrem. Manuf. 2023, 5, 032007. [Google Scholar] [CrossRef]
- Jiao, H.; Zhao, K.; Shi, R.; Ma, Y.; Tang, Y.; Bian, T.; Wang, J. An Investigation of the Electrical, Mechanical and Biocompatibility Properties of Barium Titanate/Hydroxyapatite Bulk Ceramics. Mater. Chem. Phys. 2020, 243, 122613. [Google Scholar] [CrossRef]
- Palza, H.; Zapata, P.; Angulo-Pineda, C. Electroactive Smart Polymers for Biomedical Applications. Materials 2019, 12, 277. [Google Scholar] [CrossRef] [PubMed]
- Vaca-González, J.J.; Guevara, J.M.; Moncayo, M.A.; Castro-Abril, H.; Hata, Y.; Garzón-Alvarado, D.A. Biophysical Stimuli: A Review of Electrical and Mechanical Stimulation in Hyaline Cartilage. Cartilage 2019, 10, 157–172. [Google Scholar] [CrossRef]
- Goharkhah, M.; Salarian, A.; Ashjaee, M.; Shahabadi, M. Convective Heat Transfer Characteristics of Magnetite Nanofluid under the Influence of Constant and Alternating Magnetic Field. Powder Technol. 2015, 274, 258–267. [Google Scholar] [CrossRef]
- Fernandes, M.M.; Correia, D.M.; Ribeiro, C.; Castro, N.; Correia, V.; Lanceros-Mendez, S. Bioinspired Three-Dimensional Magnetoactive Scaffolds for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2019, 11, 45265–45275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, S.; Zhu, M.; Zhu, Y.; Zhang, Y.; Liu, Z.; Zhang, C. 3D-Printed Magnetic Fe3O4 /MBG/PCL Composite Scaffolds with Multifunctionality of Bone Regeneration, Local Anticancer Drug Delivery and Hyperthermia. J. Mater. Chem. B 2014, 2, 7583–7595. [Google Scholar] [CrossRef]
- Lanier, O.L.; Ficarrotta, J.M.; Adjei, I.; Wable, D.; Lewis, C.; Nacea, C.; Sharma, B.; Dobson, J.; McFetridge, P. Magnetically Responsive Polymeric Microparticles for the Triggered Delivery of a Complex Mixture of Human Placental Proteins. Macromol. Biosci. 2021, 21, 2000249. [Google Scholar] [CrossRef]
- Geppert, M.; Himly, M. Iron Oxide Nanoparticles in Bioimaging—An Immune Perspective. Front. Immunol. 2021, 12, 688927. [Google Scholar] [CrossRef]
- Gaharwar, U.S.; Kumar, S.; Rajamani, P. Iron Oxide Nanoparticle-Induced Hematopoietic and Immunological Response in Rats. RSC Adv. 2020, 10, 35753–35764. [Google Scholar] [CrossRef] [PubMed]
- Gaharwar, U.S.; Paulraj, R. Iron Oxide Nanoparticles Induced Oxidative Damage in Peripheral Blood Cells of Rat. J. Biomed. Sci. Eng. 2015, 8, 274–286. [Google Scholar] [CrossRef]
- Reddy, U.A.; Prabhakar, P.V.; Mahboob, M. Biomarkers of Oxidative Stress for in Vivo Assessment of Toxicological Effects of Iron Oxide Nanoparticles. Saudi J. Biol. Sci. 2017, 24, 1172–1180. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Qin, C.; Wang, X.-L.; Yang, G.-S.; Shao, K.-Z.; Lan, Y.-Q.; Su, Z.-M.; Huang, P.; Wang, C.-G.; Wang, E.-B. Zeolitic Imidazolate Framework-8 as Efficient pH-Sensitive Drug Delivery Vehicle. Dalton Trans. 2012, 41, 6906. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Xu, W.; Chen, Z.; Chen, M.; Zhang, X.; He, H.; Wu, Y.; Chen, X.; Zhang, T.; Yan, M.; et al. Engineering Hibiscus-Like Riboflavin/ZIF-8 Microsphere Composites to Enhance Transepithelial Corneal Cross-Linking (Adv. Mater. 21/2022). Adv. Mater. 2022, 34, 2270156. [Google Scholar] [CrossRef]
- Doustdar, F.; Ghorbani, M. ZIF-8 Enriched Electrospun Ethyl Cellulose/Polyvinylpyrrolidone Scaffolds: The Key Role of Polyvinylpyrrolidone Molecular Weight. Carbohydr. Polym. 2022, 291, 119620. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shao, Y.; Yang, Y.; Zan, J. Zeolitic Imidazolate Framework-8: A Versatile Nanoplatform for Tissue Regeneration. Front. Bioeng. Biotechnol. 2024, 12, 1386534. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhu, Z.; Zhang, X.; Chen, J.; Yang, X.; Gao, X.; Zhang, S.; Luo, F.; Wang, J.; Zhao, W.; et al. Accelerated Bone Regeneration by MOF Modified Multifunctional Membranes through Enhancement of Osteogenic and Angiogenic Performance. Adv. Healthc. Mater. 2021, 10, 2001369. [Google Scholar] [CrossRef] [PubMed]
- García-González, C.A.; Barros, J.; Rey-Rico, A.; Redondo, P.; Gómez-Amoza, J.L.; Concheiro, A.; Alvarez-Lorenzo, C.; Monteiro, F.J. Antimicrobial Properties and Osteogenicity of Vancomycin-Loaded Synthetic Scaffolds Obtained by Supercritical Foaming. ACS Appl. Mater. Interfaces 2018, 10, 3349–3360. [Google Scholar] [CrossRef] [PubMed]
- Karakeçili, A.; Topuz, B.; Korpayev, S.; Erdek, M. Metal-Organic Frameworks for on-Demand pH Controlled Delivery of Vancomycin from Chitosan Scaffolds. Mater. Sci. Eng. C 2019, 105, 110098. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, H.; Zou, X.; Wang, D.; Fan, Y.; Zhao, X.; Li, M.; Yang, L.; Liang, C. Antibacterial Vancomycin@ZIF-8 Loaded PVA Nanofiber Membrane for Infected Bone Repair. Int. J. Mol. Sci. 2022, 23, 5629. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Zhou, J.; Fang, C.; Rong, C.; Luo, T.; Liu, J.; Zhang, K. Reactive Oxygen Species-Sensitive Materials: A Promising Strategy for Regulating Inflammation and Favoring Tissue Regeneration. Smart Mater. Med. 2023, 4, 427–446. [Google Scholar] [CrossRef]
- Martin, J.R.; Howard, M.T.; Wang, S.; Berger, A.G.; Hammond, P.T. Oxidation-Responsive, Tunable Growth Factor Delivery from Polyelectrolyte-Coated Implants. Adv. Healthc. Mater. 2021, 10, 2001941. [Google Scholar] [CrossRef]
- Lee, P.-C.; Zan, B.-S.; Chen, L.-T.; Chung, T.-W. Multifunctional PLGA-Based Nanoparticles as a Controlled Release Drug Delivery System for Antioxidant and Anticoagulant Therapy. Int. J. Nanomed. 2019, 14, 1533–1549. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, Y.; Fan, W.; Han, P.; Chang, J.; Yuen, J.; Zhang, M.; Xiao, Y. Hypoxia-Mimicking Mesoporous Bioactive Glass Scaffolds with Controllable Cobalt Ion Release for Bone Tissue Engineering. Biomaterials 2012, 33, 2076–2085. [Google Scholar] [CrossRef]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef]
- Schoonraad, S.A.; Trombold, M.L.; Bryant, S.J. The Effects of Stably Tethered BMP-2 on MC3T3-E1 Preosteoblasts Encapsulated in a PEG Hydrogel. Biomacromolecules 2021, 22, 1065–1079. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, W.; Tan, W.; Wu, X.; Dai, Z.; Li, Z.; Yan, Z.; Ji, Y.; Wang, Y.; Su, W.; et al. Injectable MMP1-Sensitive Microspheres with Spatiotemporally Controlled Exosome Release Promote Neovascularized Bone Healing. Acta Biomater. 2023, 157, 321–336. [Google Scholar] [CrossRef]
- Jia, Y.; Liu, J.; Tan, Z.; Liu, J.; Meng, X.; Luo, D.; Fu, X.; Hou, R.; Li, P.; Chen, Y.; et al. Preparation and Characterization of a Biocompatible Glucose-Sensitive Electrospun Nanofibers Scaffolds Containing Dexamethasone with Enhanced Osteogenic Properties in Vitro High Glucose Environment. Biomed. Mater. 2023, 18, 045006. [Google Scholar] [CrossRef]



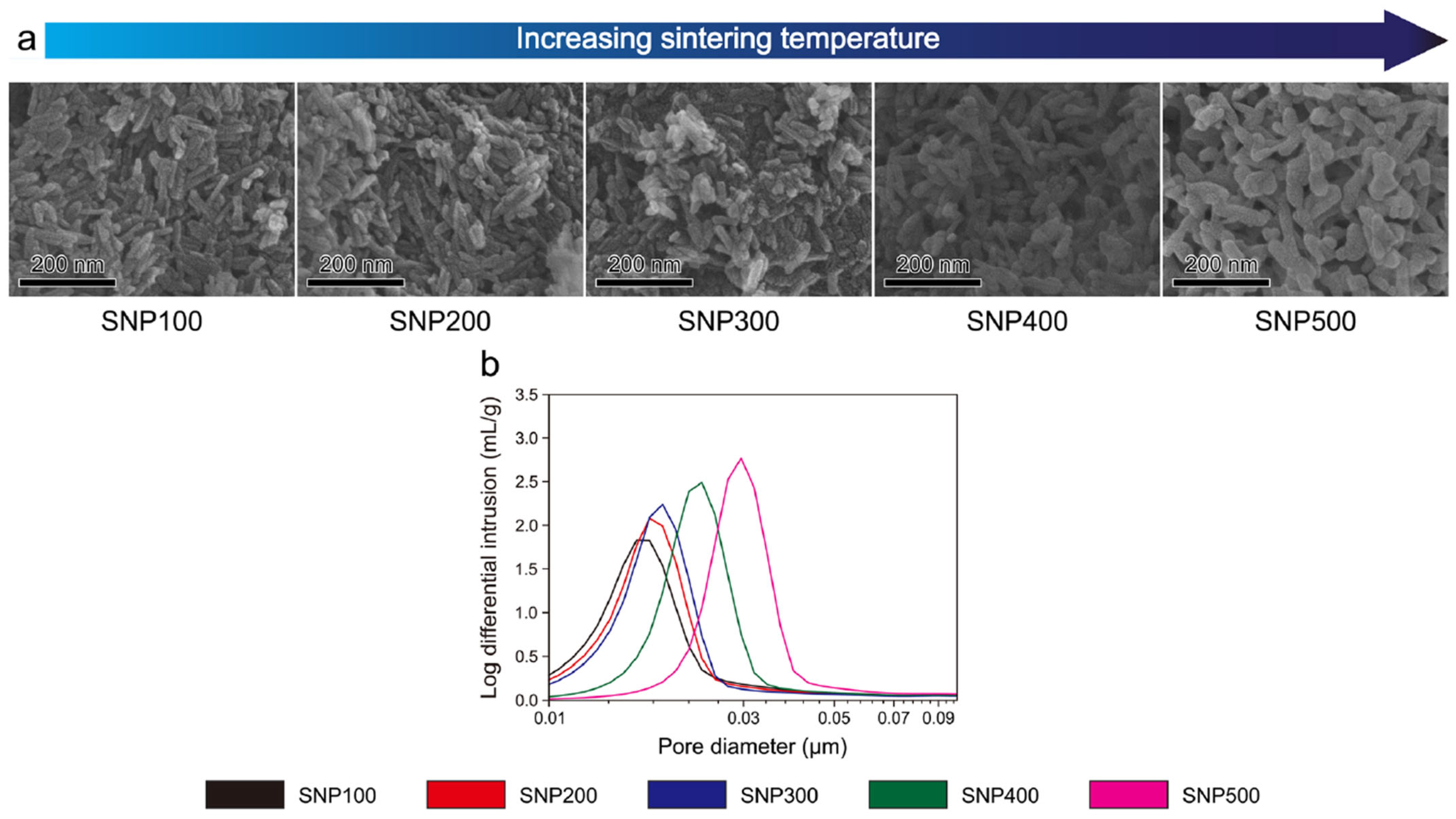
| Study Ref. | Biomaterials Used | Printing Techniques | Key Findings |
|---|---|---|---|
| [77] | PLA/β-TCP/CS with amoxicillin | Bioprinting | LbL rectangular scaffold; mechanical strength: 1.24 ± 0.53 MPa; strong antimicrobial properties, good cell viability, robust mechanical strength, and appropriate porosity were achieved |
| [78] | Mg–CLS/CS-coated Ti–6Al–4V | Selective laser melting | Scaffold height and diameter: 10 mm; mechanical strength: 50.3 ± 1.6 MPa; femoral bone defects in adult rabbits; surface-modified scaffold exhibited improved mechanical strength and enhanced cell adhesion, proliferation, differentiation, and the formation of new bone in a live defect model |
| [79] | PHBV/CaSH/CS | Fused deposition modeling | Scaffold height and diameter: 10 mm; mechanical strength: 16.6 MPa; adult male rats; increased rBMSC osteogenesis by upregulating the expression of osteogenic genes: RUNX2, COL1, OCN, OPN, and BMP2 |
| [80] | PLGA/nHAp/CS with rhBMP2 | Low-temperature deposition manufacturing | Scaffold dimension: 13 × 6 × 4 mm pore size: 431.31 ± 18.40 μm; mandibular bone defect in 13-week-old rabbits; sustained release of rhBMP2, biocompatibility in vitro, and 45.5% new bone formation were observed in vivo |
| [81] | GelMA scaffolds, adipose-derived stem cells | Extrusion bioprinting | Comparable cell viability of printed (79%) and non-printed (80%) scaffolds; co-cultured hydrogels show increased osteogenic differentiation with elevated levels of osteogenic markers; enhanced hydrogel calcification in pre-differentiated hADSCs |
| [82] | PCL, calcium magnesium phosphate | Bioprinting | Tested samples showed high biocompatibility, with over 100% live cells observed on day 3; composite scaffolds improved cell attachment and proliferation, as indicated by LDH release assays; custom-made 3D scaffolds replicated natural bone characteristics and enhanced biomineralization |
| Study Ref. | Material Composition | Method Used | Key Findings |
|---|---|---|---|
| [138] | CS, MC, VA | Sol–gel transition induced by temperature at 37 °C; magnetic stirring | VA enhances osteogenesis; ALP activity significantly increased; hydrogels are non-cytotoxic, stable, and functionally active at 37 °C; 79.65 ± 1.13% VA entrapment efficiency; swelling stable after 1 h; 69% degradation rate over 21 days; 72.5% VA released steadily over 25 days |
| [139] | PCL-PLLA | TIPS of PLLA after in situ polymerization of PCL-diacrylate | Can undergo deformation at a partial-melting temperature of 52 °C; retains its nanofibrous structure upon cooling to 37 °C |
| [140] | TMPTA, NiPAAm, AMO | cDLP | The resin composition allows adjustment of the phase transition temperature of the scaffold, enabling thermally induced mechano-stimulation of cells |
| Study Ref. | Material Composition | Method Used | Key Findings |
|---|---|---|---|
| [147] | Bi-phasic PCL/HAp | Electrospun-based thermally induced self-agglomeration | High elasticity and porosity; incorporated phenamil composite scaffolds showed less burst release and longer lasting sustained release and enhanced osteogenic differentiation of cells in vitro compared to physically surface-adsorbed phenamil |
| [152] | PLA embedded in SDF-1/BMP-2-loaded alginate hydrogels | 3D printing and ionic crosslinking of calcium with guluronic acid chains | Pulsed ultrasound and sinusoidal continuous wave ultrasound promote the recruitment and adhesion of endogenous BMSCs to the scaffold |
| [153] | - | - | Significant upregulation in early osteogenic markers (RUNX2, COL1A1) and sustained increase in late markers (osteocalcin, osteopontin); mechanistic pathways involved piezo channel activation and Rho-associated protein kinase signaling |
| [155] | MgHAp/collagen hybrid composite | - | LIPUS stimulation 20 min/day enhanced cell viability and promoted osteogenic differentiation; improved colonization of the scaffold by human MSCs; activation of the MAPK/ERK pathway and upregulation of osteogenic and angiogenetic genes were observed—enhancing gene expression and protein release due to LIPUS stimuli |
| Study Ref. | Material Composition | Method Used | Key Findings |
|---|---|---|---|
| [162] | BT nanoparticles coated with polydopamine in polyvinylidene fluoridetrifluoroethylene (PVDF-TrFE) matrix | Surface coating; corona poling treatment | Surface potential can be adjusted up to −76.8 mV, closely aligning with the level of endogenous biopotential observed in natural bone, and it retained more than half of its original value even after 12 weeks under bone defect conditions. In vivo, it sustained the electric microenvironment, facilitating rapid bone regeneration and the formation of mature bone structures in vivo |
| [164] | BT/HAp composites | Hydrothermal process | d33 value: 0.61 pC/N and 0.7 pC/N for bulk ceramic composites and dry bone, respectively. Higher BT content increased the piezoelectric coefficient and dielectric constant; improved response to electrical stimuli |
| Study Ref. | Material Composition | Method Used | Key Findings |
|---|---|---|---|
| [168] | CFO in PVDF matrix | Solvent casting, template structuring, magnetic stimulation | A 3D porous structure resembling trabecular bone, with pore sizes ranging from 5 µm to 20 µm; enhanced β-phase crystallization in PVDF, significant osteoprogenitor cell proliferation; induction of proper cellular electro-transduction processes through magnetoelectric responses of the scaffold |
| [169] | MBG/PCL/Fe3O4 | 3D printing | Magnetic heating ability was improved by the addition of Fe3O4 and did not affect the apatite mineralization ability of the scaffolds; greater expression of osteogenesis-related genes (RUNX2, OCN, BSP, BMP-2, and Col-1); ECM mineralization in human BMSCs |
| [170] | PCL/MNPs/placental proteins | Magnetic stimulation | Promoted osteogenic differentiation of umbilical cord MSCs |
| Study Ref. | Material Composition | Method Used | Key Findings |
|---|---|---|---|
| [179] | ZIF-8 in PCL/Col membranes | Electrospinning | Responsive to acidic environments; released Zn2+ concentrations increased significantly under acidic conditions (pH 5.5) |
| [181] | CS/ZIF-8/Van | Wetspinning | 70% of vancomycin released at pH 5.4 over 8 h compared to 55% at pH 7.4; increased VAN release under acidic conditions (pH 5.4) due to higher dissolution of ZIF8 |
| [182] | PVA/ZIF-8/Van | Electrospinning | Enhanced drug release under weak acidic conditions (pH 6.5) typical of infected tissue environments |
| Study Ref. | Material Composition | Method Used | Key Findings |
|---|---|---|---|
| [185] | PEM coatings/thioketal-based polymers, BMP-2 | LbL | Thioketal-based polymers specifically cleaved by physiologic doses of ROS, unlike typical non-specific hydrolysis; enhanced ROS-mediated protein delivery in vitro. A 50% increase in bone regeneration over less sensitive formulations; nearly a threefold extension in BMP-2 delivery half-life compared to conventional hydrolytically sensitive coatings |
| [186] | PGLA/nano-HAp | LbL, surface coating | A responsive H2O2 detection system via the addition of silk fibroin/horse peroxidase; targeted human BMSCs with uptake within 2 h; exocytosis occurring 6 h after cellular uptake; can deliver antioxidants directly to sites of I/R injury and enhance the survival and functionality of hBMSCs in oxidative environments |
| [187] | MBG/Co2+ | - | Co2+ acts as a chemical inducer of HIF-1α, simulating a hypoxic environment that enhances angiogenic and osteogenic responses; enhanced VEGF protein secretion, indicating improved angiogenesis; supported the attachment and proliferation of BMSCs |
| Study Ref. | Material Composition | Method Used | Key Findings |
|---|---|---|---|
| [189] | PEG/MMP-cleavable peptide/BMP-2 | Crosslinking, thiolation of BMP-2, thiol-norbornene click chemistry | Significant increase in expression levels of osteogenic markers Bglap and Ibsp in the presence of tethered BMP-2 and enhanced cell differentiation; confirmed the activation of the BMP canonical signaling pathway (via SMAD 1/5/8 route), which is critical for osteogenesis |
| [190] | GelMA/MMP-1/KLDL-MMP1/BMSC-Exos | Microfluidic chip | Responsive to MMP1 and enabling targeted and controlled release of exosomes; enhanced bone repair in vivo by recruitment of CD90+ stem cells through neovessels |
| [191] | PCL/CS/DEX/GOD | Electrospinning, genipin | Scaffolds effectively promoted osteogenic differentiation of MC3T3-E1 cells in high-glucose conditions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Percival, K.M.; Paul, V.; Husseini, G.A. Recent Advancements in Bone Tissue Engineering: Integrating Smart Scaffold Technologies and Bio-Responsive Systems for Enhanced Regeneration. Int. J. Mol. Sci. 2024, 25, 6012. https://doi.org/10.3390/ijms25116012
Percival KM, Paul V, Husseini GA. Recent Advancements in Bone Tissue Engineering: Integrating Smart Scaffold Technologies and Bio-Responsive Systems for Enhanced Regeneration. International Journal of Molecular Sciences. 2024; 25(11):6012. https://doi.org/10.3390/ijms25116012
Chicago/Turabian StylePercival, Kelly M., Vinod Paul, and Ghaleb A. Husseini. 2024. "Recent Advancements in Bone Tissue Engineering: Integrating Smart Scaffold Technologies and Bio-Responsive Systems for Enhanced Regeneration" International Journal of Molecular Sciences 25, no. 11: 6012. https://doi.org/10.3390/ijms25116012
APA StylePercival, K. M., Paul, V., & Husseini, G. A. (2024). Recent Advancements in Bone Tissue Engineering: Integrating Smart Scaffold Technologies and Bio-Responsive Systems for Enhanced Regeneration. International Journal of Molecular Sciences, 25(11), 6012. https://doi.org/10.3390/ijms25116012






