SMYD3 Controls Ciliogenesis by Regulating Distinct Centrosomal Proteins and Intraflagellar Transport Trafficking
Abstract
:1. Introduction
2. Results
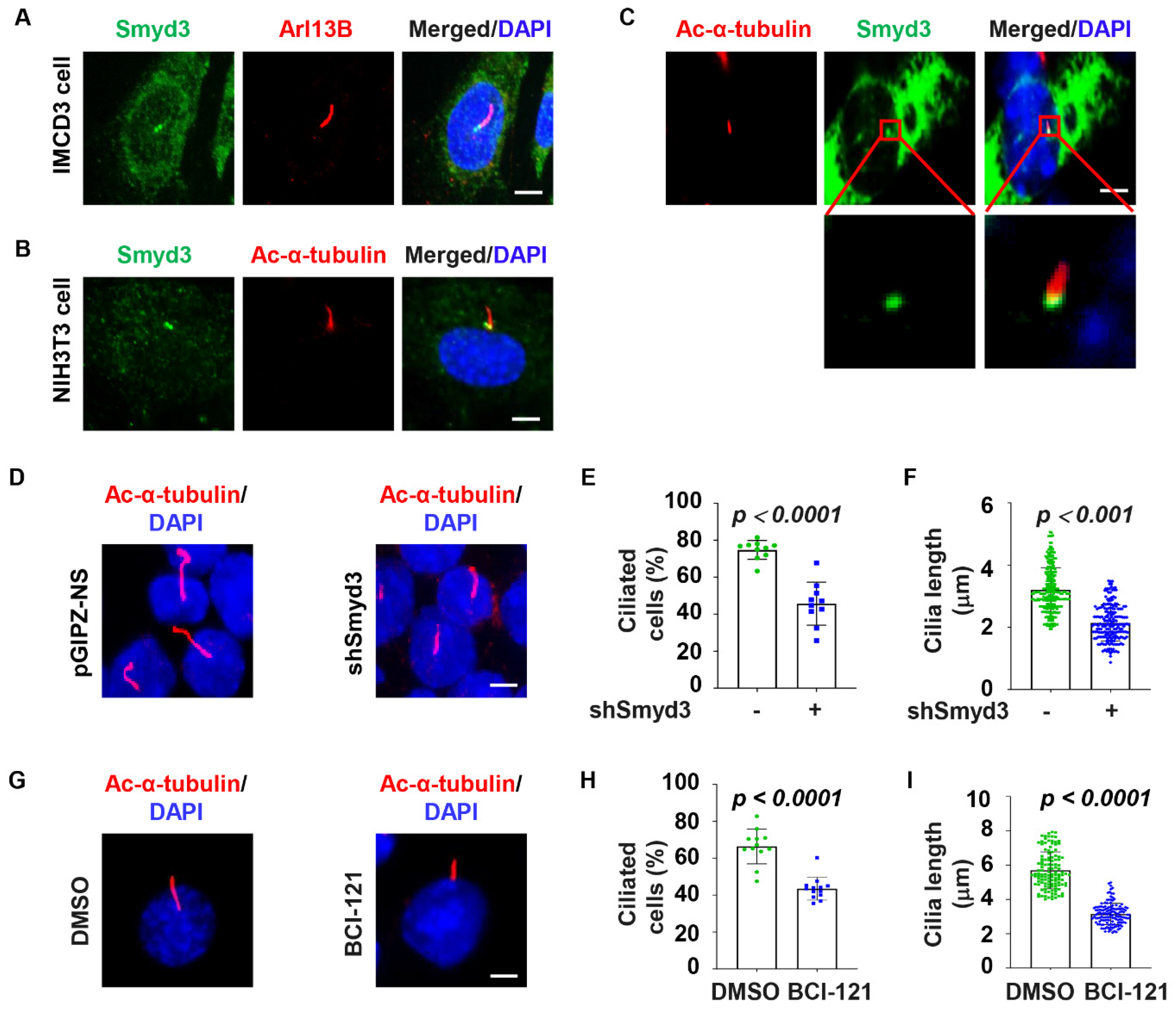
2.1. SMYD3 Is Localized at the Basal Body and Regulates Cilia Biogenesis
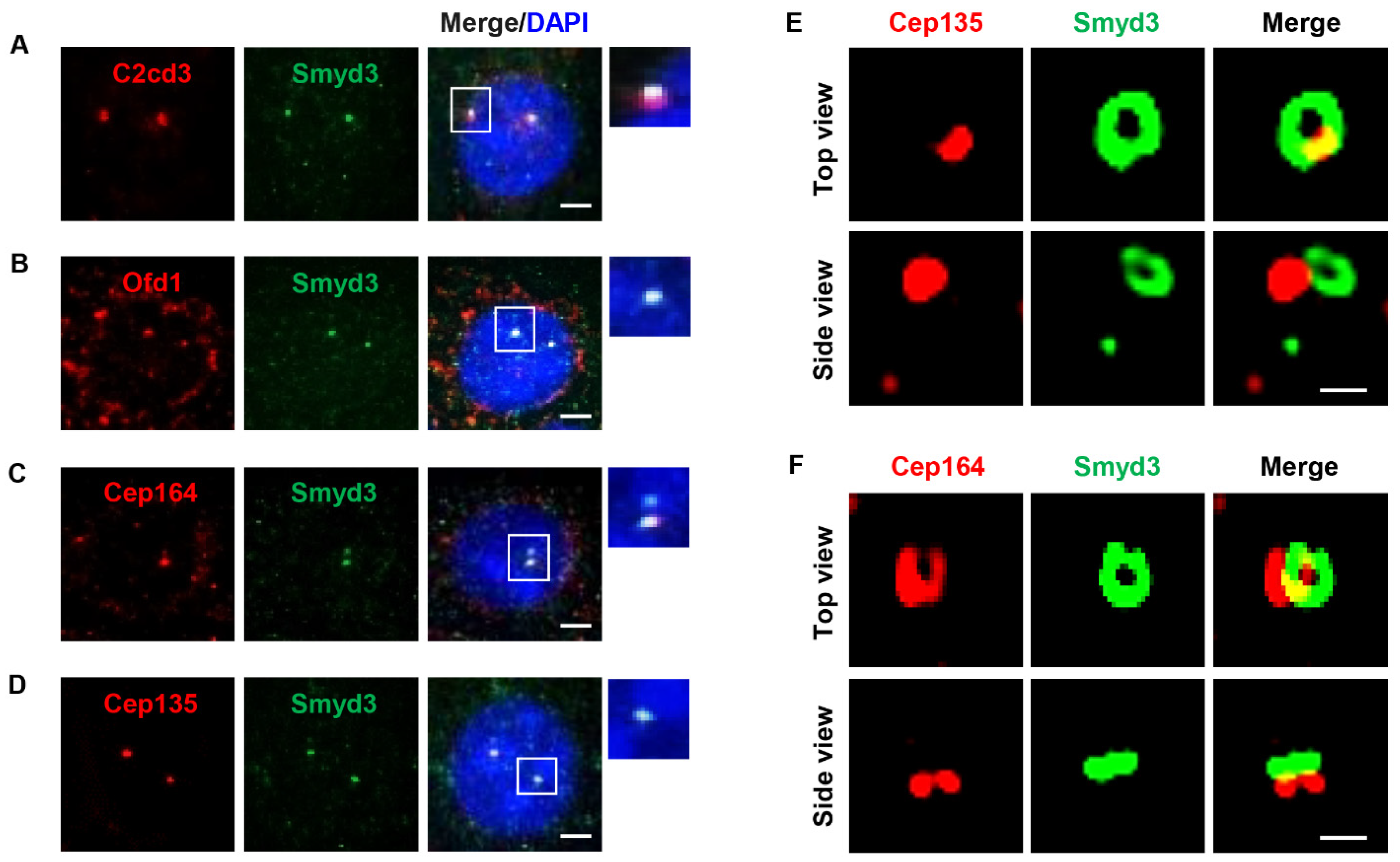
2.2. SMYD3 Is an Appendage Protein
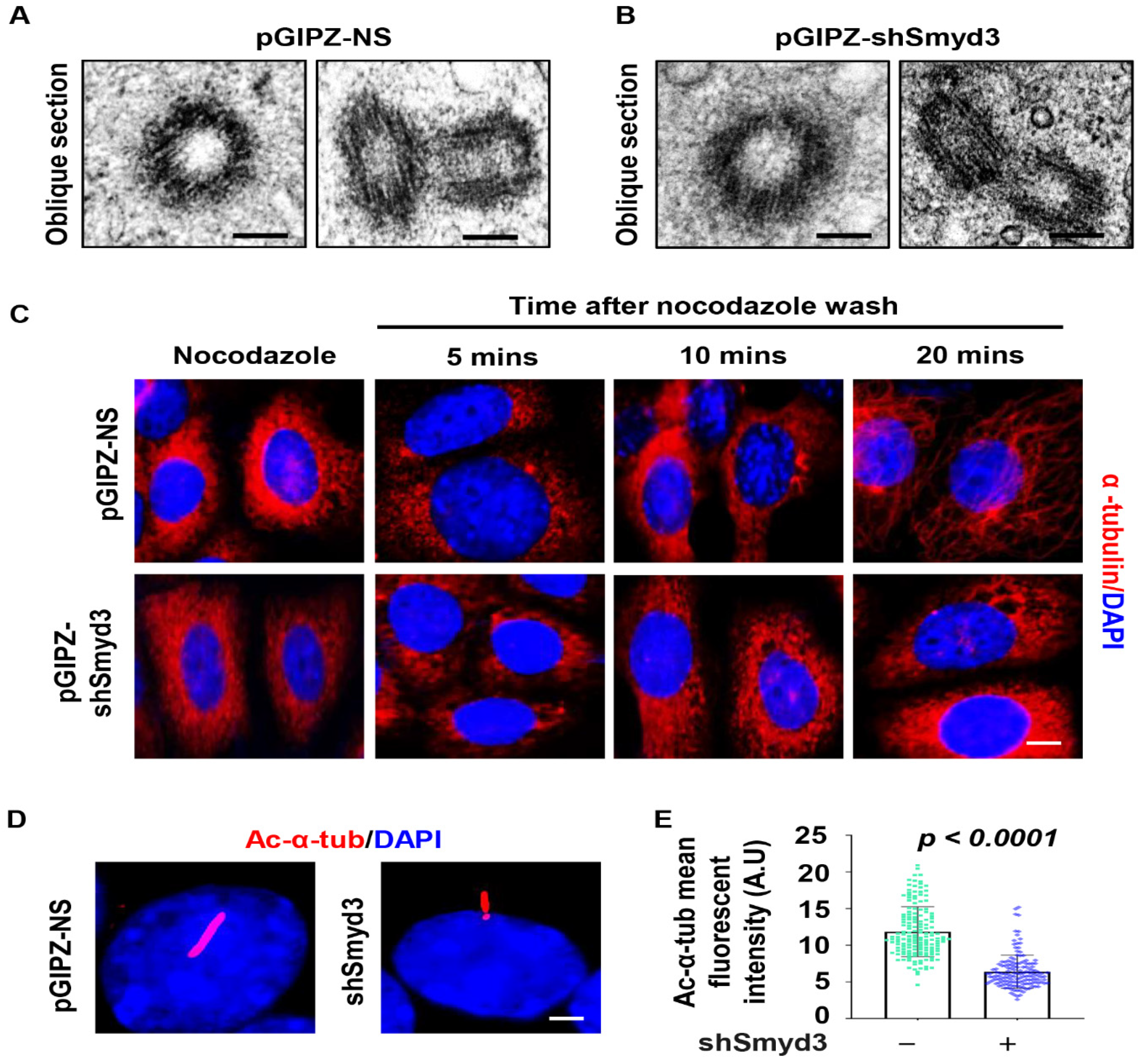
2.3. SMYD3 Modulates Microtubule Stability
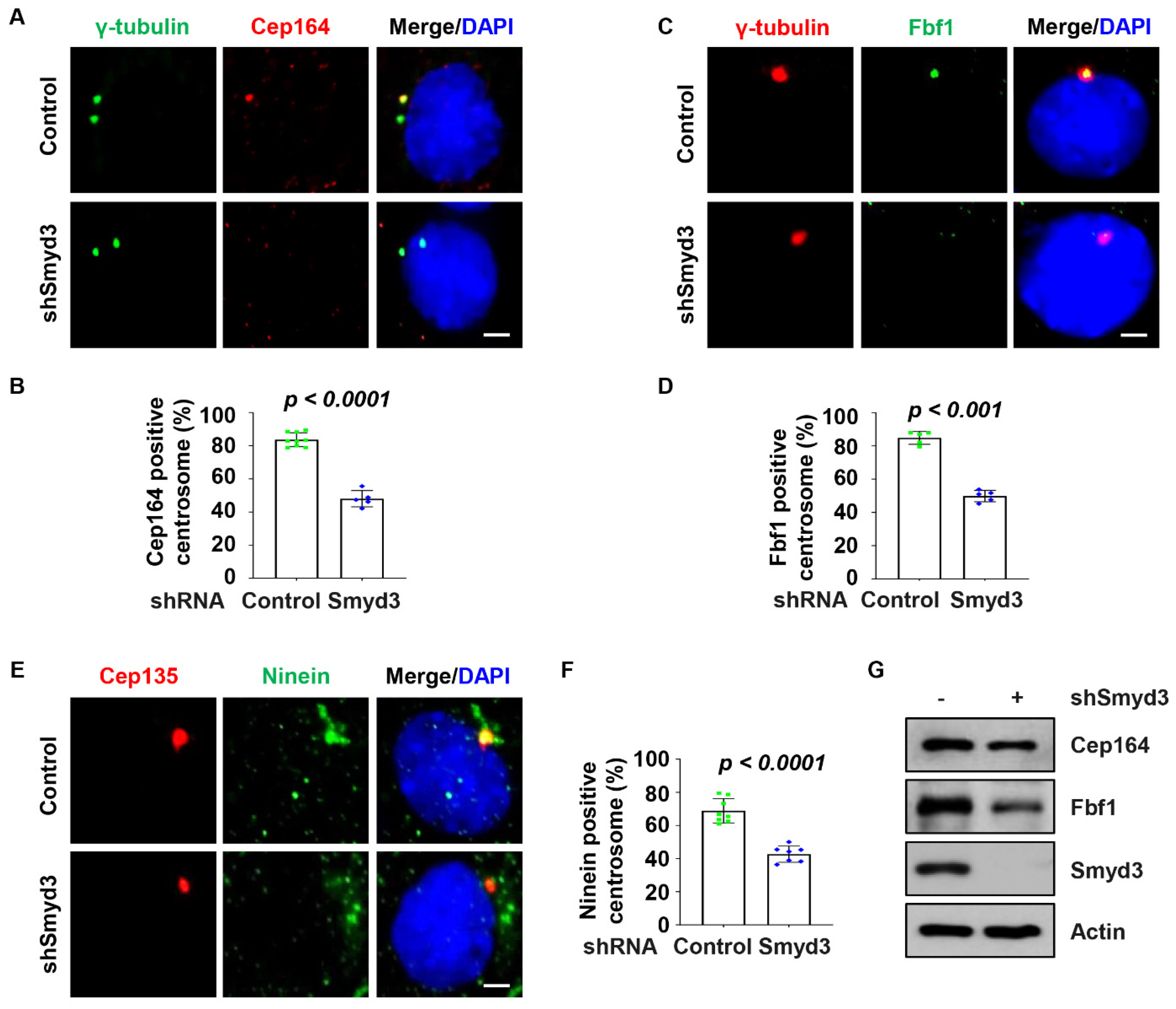
2.4. SMYD3 Regulates Centriolar Appendage Assembly
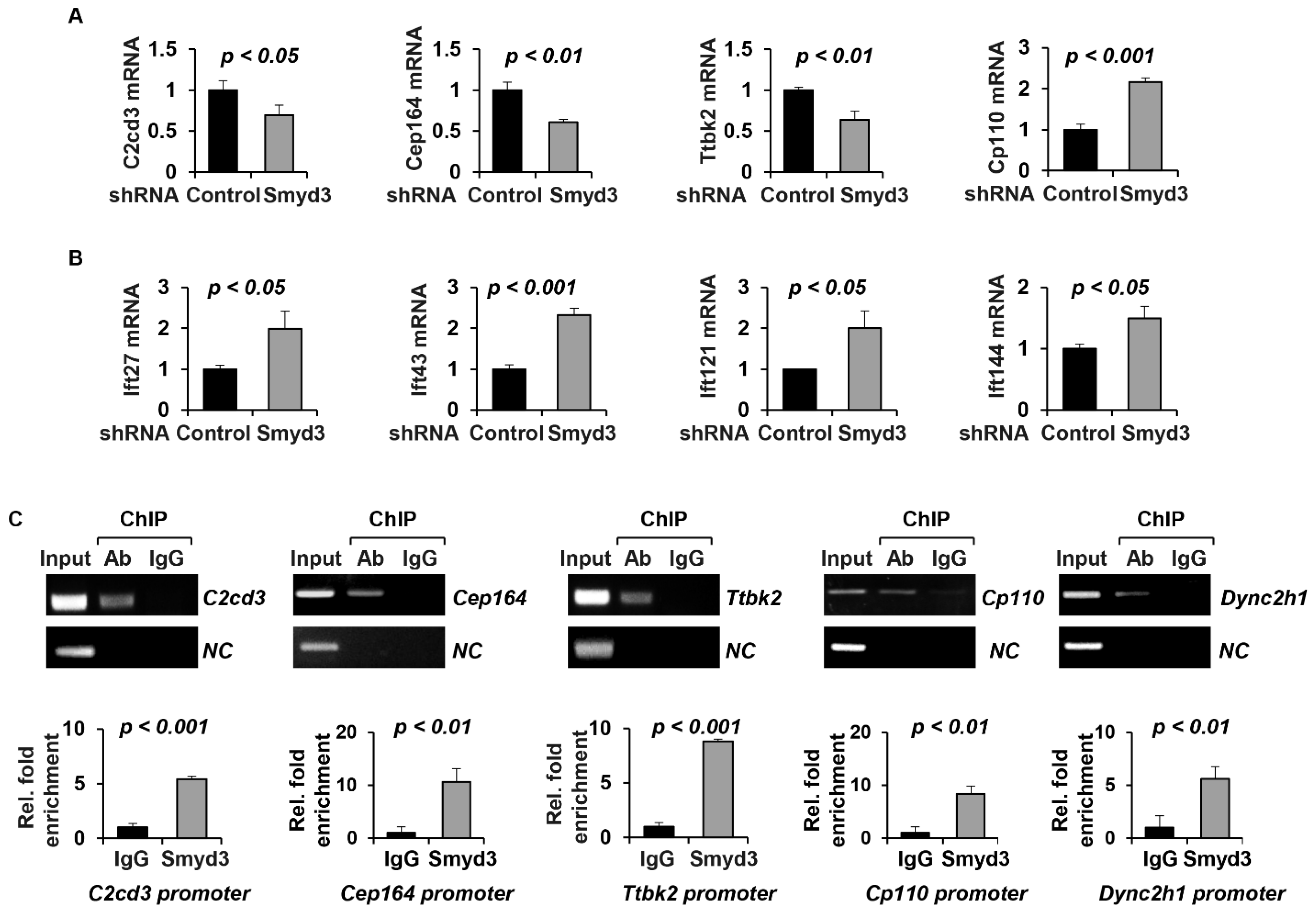
2.5. SMYD3 Modulates TTBK2 and CP110 Basal Body Localization
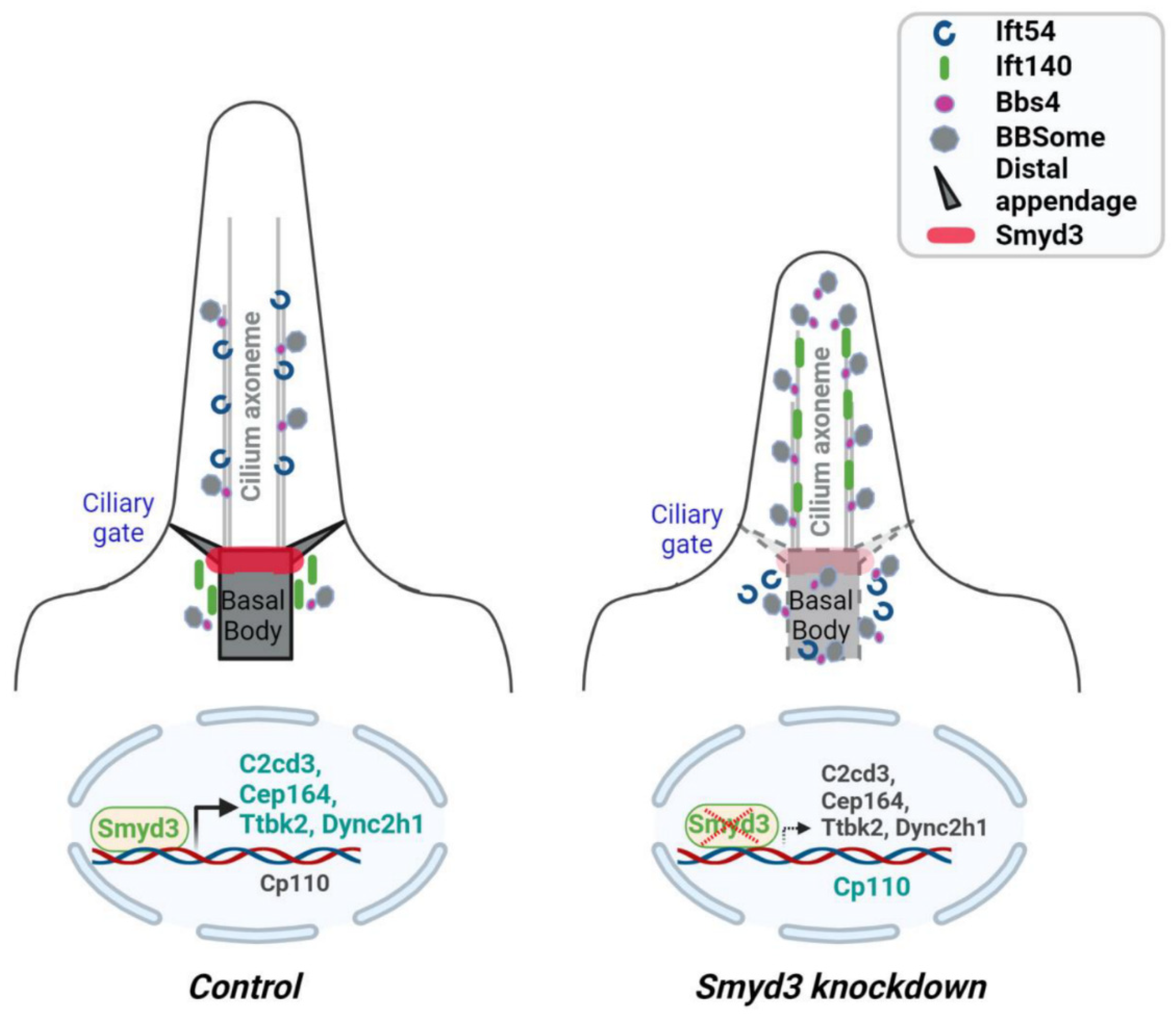
2.6. SMYD3 Depletion Affects Ciliary Trafficking of IFT Proteins
2.7. SMYD3 Regulates the Transcription of Cilia-Related Genes
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Plasmids
4.3. Generating Stable SMYD3 Knockdown IMCD3 Cell Line
4.4. Drug Treatment
4.5. Antibodies and Reagents
4.6. Immunofluorescence Staining
4.7. Western Blot Analysis and Immunoprecipitation
4.8. Microscopy and Imaging
4.9. Transmission Electron Microscopy (TEM)
4.10. Real-Time Quantitative Reverse Transcription PCR (qRT-PCR)
4.11. Chromatin Immunoprecipitation (ChIP) Assay
4.12. RNA Interference
4.13. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moorman, S.J.; Shorr, A.Z. The primary cilium as a gravitational force transducer and a regulator of transcriptional noise. Dev. Dyn. 2008, 237, 1955–1959. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, J.M.; Davis, E.E.; Katsanis, N. The Vertebrate Primary Cilium in Development, Homeostasis, and Disease. Cell 2009, 137, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Nigg, E.A.; Stearns, T. The centrosome cycle: Centriole biogenesis, duplication and inherent asymmetries. Nat. Cell Biol. 2011, 13, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Reiter, J.F.; Blacque, O.E.; Leroux, M.R. The base of the cilium: Roles for transition fibres and the transition zone in ciliary formation, maintenance and compartmentalization. EMBO Rep. 2012, 13, 608–618. [Google Scholar] [CrossRef]
- Bettencourt-Dias, M.; Glover, D.M. Centrosome biogenesis and function: Centrosomics brings new understanding. Nat. Rev. Mol. Cell Biol. 2007, 8, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Marshall, W.F. Ciliogenesis: Building the cell’s antenna. Nat. Rev. Mol. Cell Biol. 2011, 12, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Marshall, W.F. Intraflagellar Transport and Ciliary Dynamics. Cold Spring Harb. Perspect. Biol. 2017, 9, a021998. [Google Scholar] [CrossRef] [PubMed]
- Spellmon, N.; Holcomb, J.; Trescott, L.; Sirinupong, N.; Yang, Z. Structure and Function of SET and MYND Domain-Containing Proteins. Int. J. Mol. Sci. 2015, 16, 1406–1428. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Zhang, S.; Liu, H.M.; Zhang, Y.B.; Blair, C.A.; Mercola, D.; Sassone-Corsi, P.; Zi, X. Histone lysine-specific methyltransferases and demethylases in carcinogenesis: New targets for cancer therapy and prevention. Curr. Cancer Drug Targets 2013, 13, 558–579. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, W.; Gaudet, J.; Cheney, M.D.; Roudaia, L.; Cierpicki, T.; Klet, R.C.; Hartman, K.; Laue, T.M.; Speck, N.A.; et al. Structural basis for recognition of SMRT/N-CoR by the MYND domain and its contribution to AML1/ETO’s activity. Cancer Cell 2007, 11, 483–497. [Google Scholar] [CrossRef]
- Thul, P.J.; Akesson, L.; Wiking, M.; Mahdessian, D.; Geladaki, A.; Ait Blal, H.; Alm, T.; Asplund, A.; Bjork, L.; Breckels, L.M.; et al. A subcellular map of the human proteome. Science 2017, 356, eaal3321. [Google Scholar] [CrossRef] [PubMed]
- Asuthkar, S.; Venkataraman, S.; Avilala, J.; Shishido, K.; Vibhakar, R.; Veo, B.; Purvis, I.J.; Guda, M.R.; Velpula, K.K. SMYD3 Promotes Cell Cycle Progression by Inducing Cyclin D3 Transcription and Stabilizing the Cyclin D1 Protein in Medulloblastoma. Cancers 2022, 14, 1673. [Google Scholar] [CrossRef] [PubMed]
- Proserpio, V.; Fittipaldi, R.; Ryall, J.G.; Sartorelli, V.; Caretti, G. The methyltransferase SMYD3 mediates the recruitment of transcriptional cofactors at the myostatin and c-Met genes and regulates skeletal muscle atrophy. Gene Dev. 2013, 27, 1299–1312. [Google Scholar] [CrossRef] [PubMed]
- Kunizaki, M.; Hamamoto, R.; Silva, F.P.; Yamaguchi, K.; Nagayasu, T.; Shibuya, M.; Nakamura, Y.; Furukawa, Y. The lysine 831 of vascular endothelial growth factor receptor 1 is a novel target of methylation by SMYD3. Cancer Res. 2007, 67, 10759–10765. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.D.; Feldman, J.L. Microtubule-organizing centers: From the centrosome to non-centrosomal sites. Curr. Opin. Cell Biol. 2017, 44, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Tsang, W.Y.; Bossard, C.; Khanna, H.; Peraenen, J.; Swaroop, A.; Malhotra, V.; Dynlacht, B.D. CP110 suppresses primary cilia formation through its interaction with CEP290, a protein deficient in human ciliary disease. Dev. Cell 2008, 15, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Spektor, A.; Tsang, W.Y.; Khoo, D.; Dynlacht, B.D. Cep97 and CP110 suppress a cilia assembly program. Cell 2007, 130, 678–690. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, S.; Katoh, Y.; Terada, M.; Michisaka, S.; Funabashi, T.; Takahashi, S.; Kontani, K.; Nakayama, K. Regulation of ciliary retrograde protein trafficking by Joubert syndrome proteins ARL13B and INPP5E. J. Cell Sci. 2016, 130, 563–576. [Google Scholar] [CrossRef]
- Uytingco, C.R.; Williams, C.L.; Xie, C.; Shively, D.T.; Green, W.W.; Ukhanov, K.; Zhang, L.; Nishimura, D.Y.; Sheffield, V.C.; Martens, J.R. BBS4 is required for intraflagellar transport coordination and basal body number in mammalian olfactory cilia. J. Cell Sci. 2019, 132, jcs222331. [Google Scholar] [CrossRef]
- Pazour, G.J.; Dickert, B.L.; Witman, G.B. The DHC1b (DHC2) isoform of cytoplasmic dynein is required for flagellar assembly. J. Cell Biol. 1999, 144, 473–481. [Google Scholar] [CrossRef]
- Asante, D.; Stevenson, N.L.; Stephens, D.J. Subunit composition of the human cytoplasmic dynein-2 complex. J. Cell Sci. 2014, 127, 4774–4787. [Google Scholar] [CrossRef] [PubMed]
- Hamada, Y.; Tsurumi, Y.; Nozaki, S.; Katoh, Y.; Nakayama, K. Interaction of WDR60 intermediate chain with TCTEX1D2 light chain of the dynein-2 complex is crucial for ciliary protein trafficking. Mol. Biol. Cell 2018, 29, 1628–1639. [Google Scholar] [CrossRef] [PubMed]
- Pazour, G.J.; Wilkerson, C.G.; Witman, G.B. A dynein light chain is essential for the retrograde particle movement of intraflagellar transport (IFT). J. Cell Biol. 1998, 141, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Ocbina, P.J.R.; Anderson, K.V. Intraflagellar Transport, cilia, and mammalian Hedgehog signaling: Analysis in mouse embryonic fibroblasts. Dev. Dyn. 2008, 237, 2030–2038. [Google Scholar] [CrossRef] [PubMed]
- Merrill, A.E.; Merriman, B.; Farrington-Rock, C.; Camacho, N.; Sebald, E.T.; Funari, V.A.; Schibler, M.J.; Firestein, M.H.; Cohn, Z.A.; Priore, M.A.; et al. Ciliary Abnormalities Due to Defects in the Retrograde Transport Protein DYNC2H1 in Short-Rib Polydactyly Syndrome. Am. J. Human. Genet. 2009, 84, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Sarris, M.E.; Moulos, P.; Haroniti, A.; Giakountis, A.; Talianidis, I. Smyd3 Is a Transcriptional Potentiator of Multiple Cancer-Promoting Genes and Required for Liver and Colon Cancer Development. Cancer Cell 2016, 29, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Peserico, A.; Germani, A.; Sanese, P.; Barbosa, A.J.; Di Virgilio, V.; Fittipaldi, R.; Fabini, E.; Bertucci, C.; Varchi, G.; Moyer, M.P.; et al. A SMYD3 Small-Molecule Inhibitor Impairing Cancer Cell Growth. J. Cell Physiol. 2015, 230, 2447–2460. [Google Scholar] [CrossRef] [PubMed]
- Bernard, B.J.; Nigam, N.; Burkitt, K.; Saloura, V. SMYD3: A regulator of epigenetic and signaling pathways in cancer. Clin. Epigenet. 2021, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.T.; Chong, W.M.; Wang, W.J.; Mazo, G.; Tanos, B.; Chen, Z.; Tran, T.M.N.; Chen, Y.D.; Weng, R.R.; Huang, C.E.; et al. Super-resolution architecture of mammalian centriole distal appendages reveals distinct blade and matrix functional components. Nat. Commun. 2018, 9, 2023. [Google Scholar] [CrossRef] [PubMed]
- Cajanek, L.; Nigg, E.A. Cep164 triggers ciliogenesis by recruiting Tau tubulin kinase 2 to the mother centriole. Proc. Natl. Acad. Sci. USA 2014, 111, E2841–E2850. [Google Scholar] [CrossRef]
- Li, L.X.Y.; Zhou, J.X.; Wang, X.D.; Zhang, H.B.; Harris, P.C.; Calvet, J.P.; Li, X.G. Cross-talk between CDK4/6 and SMYD2 regulates gene transcription, tubulin methylation, and ciliogenesis. Sci. Adv. 2020, 6, eabb3154. [Google Scholar] [CrossRef]
- Pazour, G.J.; Dickert, B.L.; Vucica, Y.; Seeley, E.S.; Rosenbaum, J.L.; Witman, G.B.; Cole, D.G. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J. Cell Biol. 2000, 151, 709–718. [Google Scholar] [CrossRef]
- Ocbina, P.J.R.; Eggenschwiler, J.T.; Moskowitz, I.; Anderson, K.V. Complex interactions between genes controlling trafficking in primary cilia. Nat. Genet. 2011, 43, U547–U575. [Google Scholar] [CrossRef]
- Wu, C.Q.; Li, J.; Peterson, A.; Tao, K.X.; Wang, B.L. Loss of dynein-2 intermediate chain Wdr34 results in defects in retrograde ciliary protein trafficking and Hedgehog signaling in the mouse. Human. Mol. Genet. 2017, 26, 2386–2397. [Google Scholar] [CrossRef] [PubMed]
- Gigante, E.D.; Taylor, M.R.; Ivanova, A.A.; Kahn, R.A.; Caspary, T. ARL13B regulates Sonic hedgehog signaling from outside primary cilia. Elife 2020, 9, e50434. [Google Scholar] [CrossRef]
- Deltas, C.C. Mutations of the human polycystic kidney disease 2 (PKD2) gene. Human. Mutat. 2001, 18, 13–24. [Google Scholar] [CrossRef]
- Kim, H.; Heo, K.; Kim, J.H.; Kim, K.; Choi, J.; An, W.J. Requirement of Histone Methyltransferase SMYD3 for Estrogen Receptor-mediated Transcription. J. Biol. Chem. 2009, 284, 19867–19877. [Google Scholar] [CrossRef]
- Cock-Rada, A.M.; Medjkane, S.; Janski, N.; Yousfi, N.; Perichon, M.; Chaussepied, M.; Chluba, J.; Langsley, G.; Weitzman, J.B. SMYD3 Promotes Cancer Invasion by Epigenetic Upregulation of the Metalloproteinase MMP-9. Cancer Res. 2012, 72, 810–820. [Google Scholar] [CrossRef]
- D’Angiolella, V.; Donato, V.; Vijayakumar, S.; Saraf, A.; Florens, L.; Washburn, M.P.; Dynlacht, B.; Pagano, M. SCF(Cyclin F) controls centrosome homeostasis and mitotic fidelity through CP110 degradation. Nature 2010, 466, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.; Wilson, M.D.; Spyrou, C.; Brown, G.D.; Hadfield, J.; Odom, D.T. ChIP-seq: Using high-throughput sequencing to discover protein-DNA interactions. Methods 2009, 48, 240–248. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agborbesong, E.; Zhou, J.X.; Zhang, H.; Li, L.X.; Harris, P.C.; Calvet, J.P.; Li, X. SMYD3 Controls Ciliogenesis by Regulating Distinct Centrosomal Proteins and Intraflagellar Transport Trafficking. Int. J. Mol. Sci. 2024, 25, 6040. https://doi.org/10.3390/ijms25116040
Agborbesong E, Zhou JX, Zhang H, Li LX, Harris PC, Calvet JP, Li X. SMYD3 Controls Ciliogenesis by Regulating Distinct Centrosomal Proteins and Intraflagellar Transport Trafficking. International Journal of Molecular Sciences. 2024; 25(11):6040. https://doi.org/10.3390/ijms25116040
Chicago/Turabian StyleAgborbesong, Ewud, Julie Xia Zhou, Hongbing Zhang, Linda Xiaoyan Li, Peter C. Harris, James P. Calvet, and Xiaogang Li. 2024. "SMYD3 Controls Ciliogenesis by Regulating Distinct Centrosomal Proteins and Intraflagellar Transport Trafficking" International Journal of Molecular Sciences 25, no. 11: 6040. https://doi.org/10.3390/ijms25116040




