The Therapeutic Potential of Intra-Articular Injection of Synthetic Deer Antler Peptides in a Rat Model of Knee Osteoarthritis
Abstract
1. Introduction
2. Results
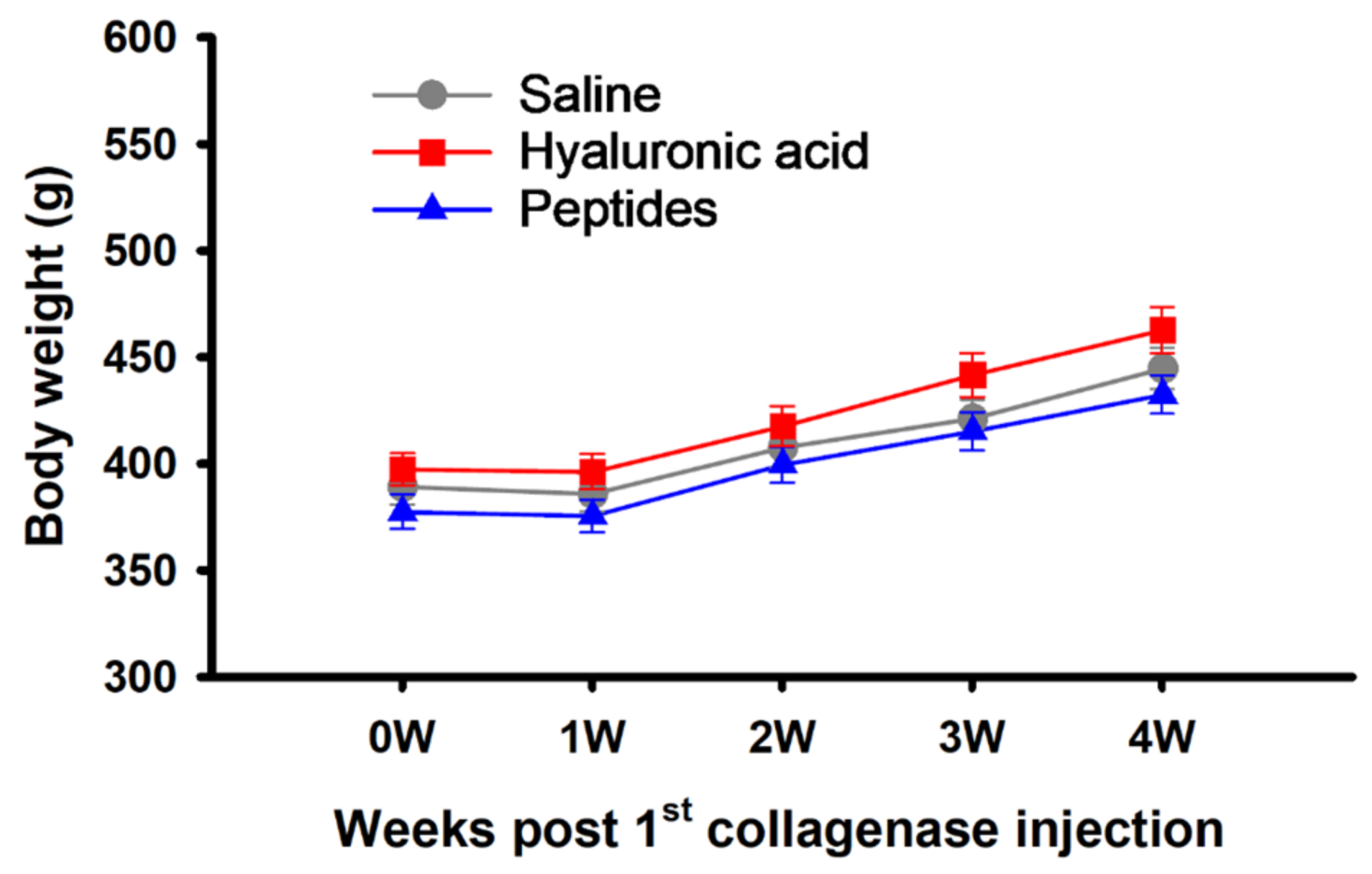
2.1. Body Weight
2.2. Knee Diameter Changes
2.3. Weight-Bearing
2.4. Mechanical Allodynia
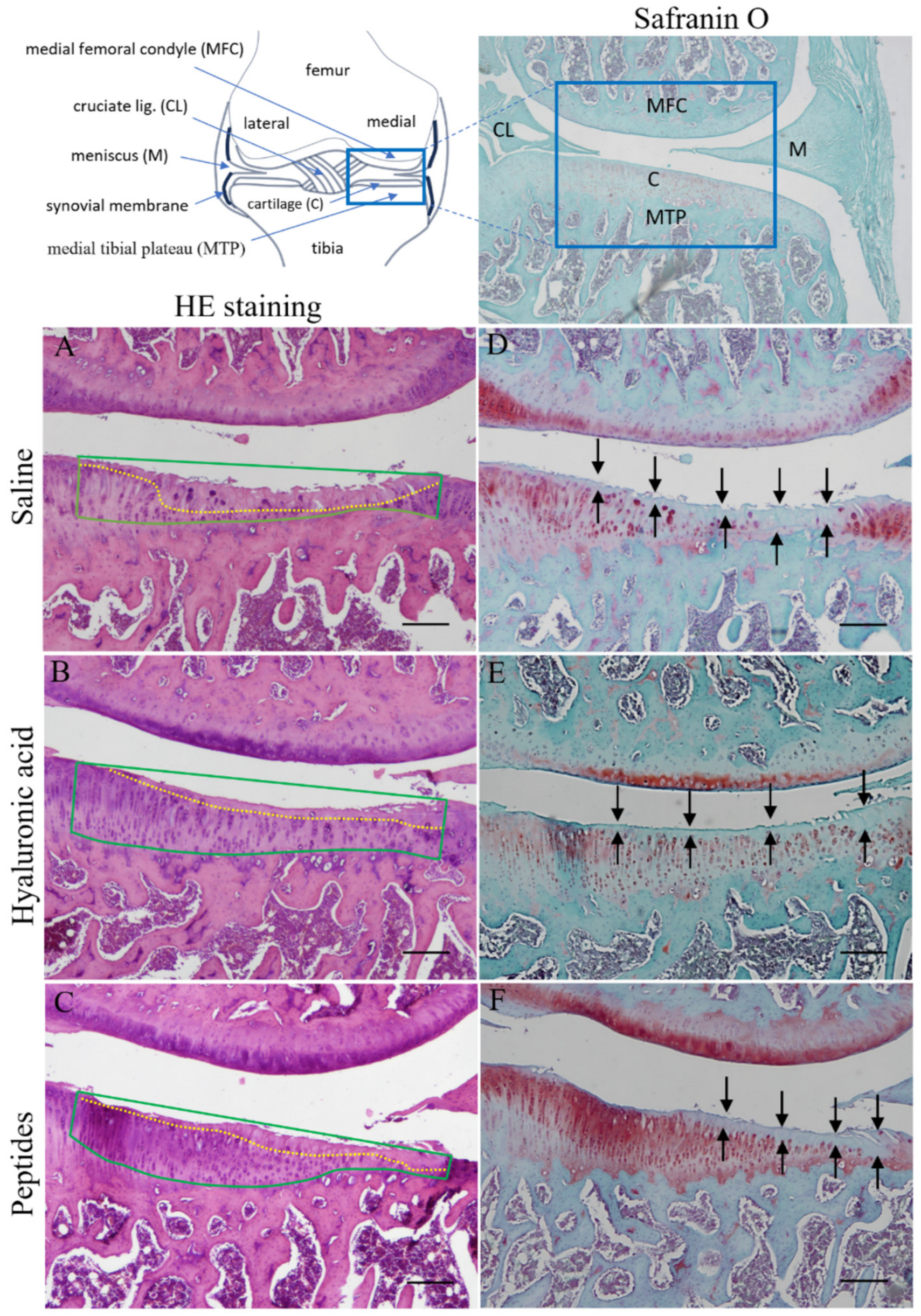
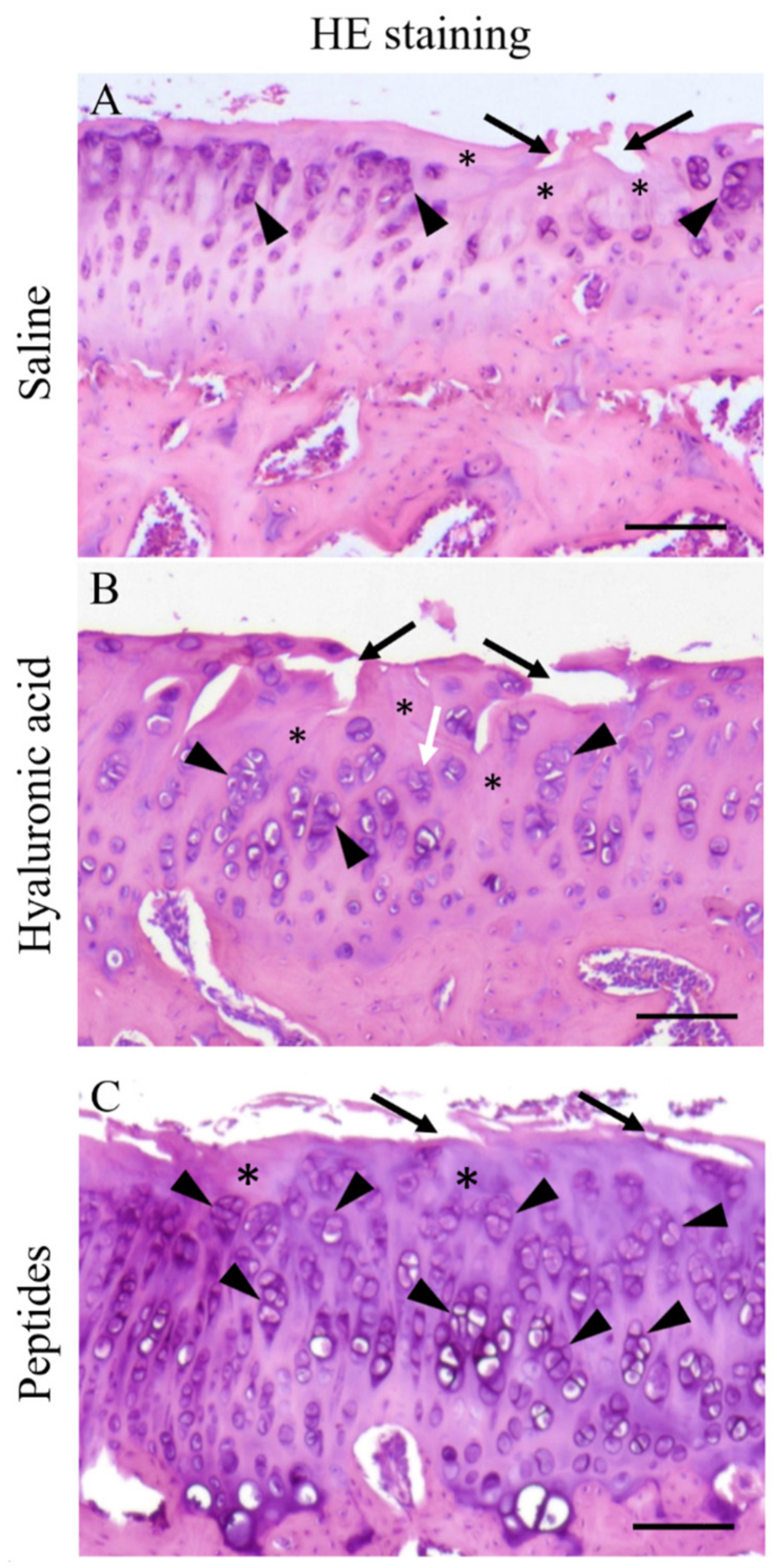
2.5. Histopathology and Histopathological Scoring
3. Discussion
4. Materials and Methods
4.1. Animals
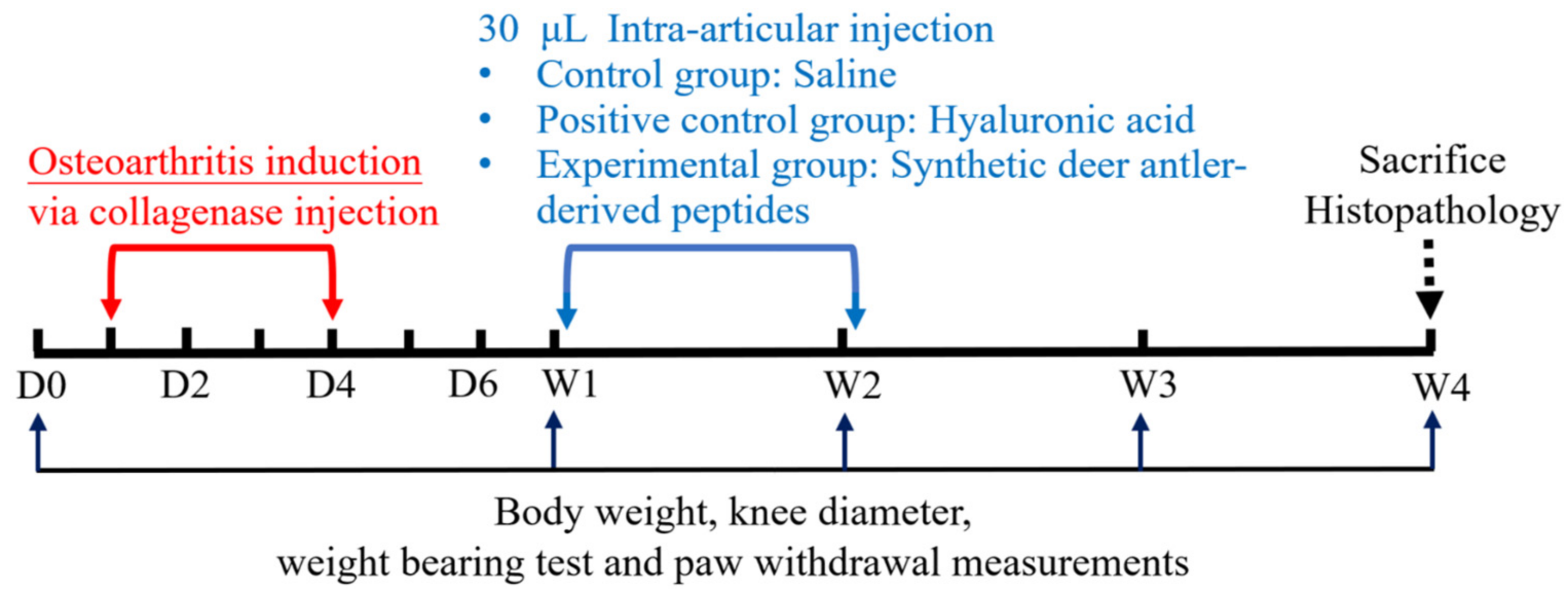
4.2. Study Design
4.3. Induction of OA and Subsequent Treatments
4.4. Joint Swelling Evaluation
4.5. Arthritic Pain Evaluation
4.6. Histopathological Examination
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA 2021, 325, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.P. Osteoarthritis. Nat. Rev. Dis. Primers 2016, 2, 16072. [Google Scholar] [CrossRef] [PubMed]
- Charlier, E.; Relic, B.; Deroyer, C.; Malaise, O.; Neuville, S.; Collée, J.; Malaise, M.G.; De Seny, D. Insights on Molecular Mechanisms of Chondrocytes Death in Osteoarthritis. Int. J. Mol. Sci. 2016, 17, 2146. [Google Scholar] [CrossRef] [PubMed]
- Pritzker, K.P.; Aigner, T. Terminology of osteoarthritis cartilage and bone histopathology—A proposal for a consensus. Osteoarthr. Cartil. 2010, 18 (Suppl. S3), S7–S9. [Google Scholar] [CrossRef] [PubMed]
- Roos, E.M.; Arden, N.K. Strategies for the prevention of knee osteoarthritis. Nat Rev Rheumatol 2016, 12, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L. Osteoarthritis of the Knee. N. Engl. J. Med. 2021, 384, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Primorac, D.; Molnar, V.; Rod, E.; Jeleč, Ž.; Čukelj, F.; Matišić, V.; Vrdoljak, T.; Hudetz, D.; Hajsok, H.; Borić, I. Knee Osteoarthritis: A Review of Pathogenesis and State-Of-The-Art Non-Operative Therapeutic Considerations. Genes 2020, 11, 854. [Google Scholar] [CrossRef]
- Safiri, S.; Kolahi, A.A.; Smith, E.; Hill, C.; Bettampadi, D.; Mansournia, M.A.; Hoy, D.; Ashrafi-Asgarabad, A.; Sepidarkish, M.; Almasi-Hashiani, A.; et al. Global, regional and national burden of osteoarthritis 1990-2017: A systematic analysis of the Global Burden of Disease Study 2017. Ann. Rheum. Dis. 2020, 79, 819–828. [Google Scholar] [CrossRef]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [PubMed]
- Coaccioli, S.; Sarzi-Puttini, P.; Zis, P.; Rinonapoli, G.; Varrassi, G. Osteoarthritis: New Insight on Its Pathophysiology. J. Clin. Med. 2022, 11, 6013. [Google Scholar] [CrossRef] [PubMed]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Rheumatol. 2020, 72, 220–233. [Google Scholar] [CrossRef]
- McAlindon, T.E.; Bannuru, R.R.; Sullivan, M.C.; Arden, N.K.; Berenbaum, F.; Bierma-Zeinstra, S.M.; Hawker, G.A.; Henrotin, Y.; Hunter, D.J.; Kawaguchi, H.; et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr. Cartil. 2014, 22, 363–388. [Google Scholar] [CrossRef] [PubMed]
- Arden, N.K.; Perry, T.A.; Bannuru, R.R.; Bruyère, O.; Cooper, C.; Haugen, I.K.; Hochberg, M.C.; McAlindon, T.E.; Mobasheri, A.; Reginster, J.Y. Non-surgical management of knee osteoarthritis: Comparison of ESCEO and OARSI 2019 guidelines. Nat. Rev. Rheumatol. 2021, 17, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.V.; Jüni, P.; Saadat, P.; Xing, D.; Yao, L.; Bobos, P.; Agarwal, A.; Hincapié, C.A.; da Costa, B.R. Viscosupplementation for knee osteoarthritis: Systematic review and meta-analysis. BMJ 2022, 378, e069722. [Google Scholar] [CrossRef] [PubMed]
- Fuggle, N.R.; Cooper, C.; Oreffo, R.O.C.; Price, A.J.; Kaux, J.F.; Maheu, E.; Cutolo, M.; Honvo, G.; Conaghan, P.G.; Berenbaum, F.; et al. Alternative and complementary therapies in osteoarthritis and cartilage repair. Aging Clin. Exp. Res. 2020, 32, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, H.; Cao, P.; Ma, T.; Zhao, Y.; Xie, F.; Yao, C.; Zhang, X. Complementary and alternative therapies for knee osteoarthritis: A protocol for systematic review and network meta-analysis. Medicine 2020, 99, e23035. [Google Scholar] [CrossRef] [PubMed]
- Nirmal, P.; Koppikar, S.; Bhondave, P.; Narkhede, A.; Nagarkar, B.; Kulkarni, V.; Wagh, N.; Kulkarni, O.; Harsulkar, A.; Jagtap, S. Influence of six medicinal herbs on collagenase-induced osteoarthritis in rats. Am. J. Chin. Med. 2013, 41, 1407–1425. [Google Scholar] [CrossRef] [PubMed]
- Nirmal, P.S.; Jagtap, S.D.; Narkhede, A.N.; Nagarkar, B.E.; Harsulkar, A.M. New herbal composition (OA-F2) protects cartilage degeneration in a rat model of collagenase induced osteoarthritis. BMC Complement. Altern. Med. 2017, 17, 6. [Google Scholar] [CrossRef] [PubMed]
- Widyowati, R.; Suciati, S.; Hariyadi, D.M.; Chang, H.I.; Ipg Suryawan, N.; Tarigan, N.; Sholikhah, I.; Ardianto, C.; Nurhan, A.D.; Sagitaras, I.B. The pro-inflammatory cytokine IL-Iβ alteration by deer (Rusa unicolor) antler extract on osteoarthritis rat model. Saudi Pharm. J. 2023, 31, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Pan, D.; Zhang, M.; Leng, X.; Yao, B. Deer antler extract potentially facilitates xiphoid cartilage growth and regeneration and prevents inflammatory susceptibility by regulating multiple functional genes. J. Orthop. Surg. Res. 2021, 16, 208. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Zhou, Z.; Zhang, M.; Leng, X.; Zhao, D. Investigating the molecular control of deer antler extract on articular cartilage. J. Orthop. Surg. Res. 2021, 16, 8. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.Q.; Zhao, Y.J.; Li, F.; Shu, B.; Lin, S.R.; Sun, L.; Wang, Y.J.; Zheng, H.X. Velvet antler polypeptide partially rescue facet joint osteoarthritis-like phenotype in adult β-catenin conditional activation mice. BMC Complement. Altern. Med. 2019, 19, 191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, X.; Duan, L.; Li, X.; Zhang, Y.; Zhou, Q. The effects of velvet antler polypeptides on the phenotype and related biological indicators of osteoarthritic rabbit chondrocytes. Acta Biochim. Pol. 2011, 58, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Moreau, M.; Dupuis, J.; Bonneau, N.H.; Lécuyer, M. Clinical evaluation of a powder of quality elk velvet antler for the treatment of osteoarthrosis in dogs. Can. Vet. J. 2004, 45, 133–139. [Google Scholar] [PubMed]
- Lien, C.Y.; Lu, C.W.; Lin, Y.H.; Wu, W.J.; Hsu, C.H.; Chuang, T.Y.; Lin, K.F.; Chuang, W.C.; Lee, M.C.; Wu, C.H. Chinese Herbal Medicine, Guilu Erxian Glue, as Alternative Medicine for Adverse Side Effects of Chemotherapy in Doxorubicin-Treated Cell and Mouse Models. Evid. Based Complement. Altern. Med. 2021, 2021, 5548968. [Google Scholar] [CrossRef]
- Lai, Y.X.; Tseng, C.Y.; Yang, H.Y.; Yang, S.H.; Lee, P.W.; Yang, T.H. Effect assessment of traditional Chinese medicine, Guilu Erxian Jiao, in patients with osteoporosis: A case-control study of the Chang gung memorial hospital. Explore (NY) 2024. [Google Scholar] [CrossRef]
- Tsai, C.C.; Chou, Y.Y.; Chen, Y.M.; Tang, Y.J.; Ho, H.C.; Chen, D.Y. Effect of the herbal drug guilu erxian jiao on muscle strength, articular pain, and disability in elderly men with knee osteoarthritis. Evid. Based Complement. Altern. Med. 2014, 2014, 297458. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.A.; Yeh, Y.C.; Chang, Z.Y. The efficacy and safety of traditional Chinese medicine Guilu Erxian Jiao in the treatment of knee osteoarthritis: A systematic review and meta-analysis. Complement. Ther. Clin. Pract. 2021, 46, 101515. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.J.; Lin, J.H.; Lin, S.Z.; Tsai, W.T.; Wu, J.R.; Chen, H.P. Isolation, Identification, and Characterization of Bioactive Peptides in Human Bone Cells from Tortoiseshell and Deer Antler Gelatin. Int. J. Mol. Sci. 2023, 24, 1759. [Google Scholar] [CrossRef]
- Takagi, H.; Shiomi, H.; Ueda, H.; Amano, H. A novel analgesic dipeptide from bovine brain is a possible Met-enkephalin releaser. Nature 1979, 282, 410–412. [Google Scholar] [CrossRef] [PubMed]
- Kiso, Y.; Kitagawa, K.; Nobuyuki, K.; Akita, T.; Takagi, H.; Amano, H.; Fukui, K. Neo-kyotorphin (Thr—Ser—Lys—Tyr—Arg), a new analgesic peptide. FEBS Lett. 1983, 155, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Yoshihara, Y.; Misawa, H.; Fukushima, N.; Katada, T.; Ui, M.; Takagi, H.; Satoh, M. The kyotorphin (tyrosine-arginine) receptor and a selective reconstitution with purified Gi, measured with GTPase and phospholipase C assays. J. Biol. Chem. 1989, 264, 3732–3741. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, Y.; Ueda, H.; Fujii, N.; Shide, A.; Yajima, H.; Satoh, M. Purification of a novel type of calcium-activated neutral protease from rat brain. Possible involvement in production of the neuropeptide kyotorphin from calpastatin fragments. J. Biol. Chem. 1990, 265, 5809–5815. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-J.; Tsai, W.-T.; Wu, J.-R.; Chen, H.-P. Biological Activities of Deer Antler-Derived Peptides on Human Chondrocyte and Bone Metabolism. Pharmaceuticals 2024, 17, 434. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.J.; Chuu, J.J.; Peng, Y.J.; Cheng, Y.H.; Chang, C.H.; Chang, C.M.; Liu, H.W. The potent anti-inflammatory effect of Guilu Erxian Glue extracts remedy joint pain and ameliorate the progression of osteoarthritis in mice. J. Orthop. Surg. Res. 2018, 13, 259. [Google Scholar] [CrossRef] [PubMed]
- Akkiraju, H.; Nohe, A. Role of Chondrocytes in Cartilage Formation, Progression of Osteoarthritis and Cartilage Regeneration. J. Dev. Biol. 2015, 3, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.N., Jr.; Myers, S.L.; Brandt, K.D.; Mickler, E.A. Effect of intraarticular hyaluronan injection in experimental canine osteoarthritis. Arthritis Rheumatol. 1998, 41, 976–985. [Google Scholar] [CrossRef]
- Ghosh, P.; Read, R.; Armstrong, S.; Wilson, D.; Marshall, R.; McNair, P. The effects of intraarticular administration of hyaluronan in a model of early osteoarthritis in sheep. I. Gait analysis and radiological and morphological studies. Semin. Arthritis Rheum. 1993, 22 (Suppl. S1), 18–30. [Google Scholar] [CrossRef] [PubMed]
- Adaes, S.; Mendonca, M.; Santos, T.N.; Castro-Lopes, J.M.; Ferreira-Gomes, J.; Neto, F.L. Intra-articular injection of collagenase in the knee of rats as an alternative model to study nociception associated with osteoarthritis. Arthritis Res. Ther. 2014, 16, R10. [Google Scholar] [CrossRef] [PubMed]
- Cucchiarini, M.; de Girolamo, L.; Filardo, G.; Oliveira, J.M.; Orth, P.; Pape, D.; Reboul, P. Basic science of osteoarthritis. J. Exp. Orthop. 2016, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, P.; Zhu, S.; Bi, R. Comparison of early-stage changes of osteoarthritis in cartilage and subchondral bone between two different rat models. PeerJ 2020, 8, e8934. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Wen, Z.H.; Chang, Y.C.; Huang, S.Y.; Tang, C.C.; Chen, W.F.; Hsieh, S.P.; Hsieh, C.S.; Jean, Y.H. Intra-articular magnesium sulfate (MgSO4) reduces experimental osteoarthritis and nociception: Association with attenuation of N-methyl-D-aspartate (NMDA) receptor subunit 1 phosphorylation and apoptosis in rat chondrocytes. Osteoarthr. Cartil. 2009, 17, 1485–1493. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, M.; Izumi, M.; Aso, K.; Sugimura, N.; Kato, T.; Tani, T. Effects of intra-articular hyaluronic acid injection on immunohistochemical characterization of joint afferents in a rat model of knee osteoarthritis. Eur. J. Pain 2015, 19, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Yamada, E.F.; Salgueiro, A.F.; Goulart, A.D.S.; Mendes, V.P.; Anjos, B.L.; Folmer, V.; da Silva, M.D. Evaluation of monosodium iodoacetate dosage to induce knee osteoarthritis: Relation with oxidative stress and pain. Int. J. Rheum. Dis. 2019, 22, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.R.; Carlson, C.S.; Rendahl, A.K.; Loeser, R.F. Optimization of histologic grading schemes in spontaneous and surgically-induced murine models of osteoarthritis. Osteoarthr. Cartil. 2021, 29, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Gerwin, N.; Bendele, A.M.; Glasson, S.; Carlson, C.S. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the rat. Osteoarthr. Cartil. 2010, 18 (Suppl. S3), S24–S34. [Google Scholar] [CrossRef] [PubMed]
- Pauli, C.; Whiteside, R.; Heras, F.L.; Nesic, D.; Koziol, J.; Grogan, S.P.; Matyas, J.; Pritzker, K.P.; D’Lima, D.D.; Lotz, M.K. Comparison of cartilage histopathology assessment systems on human knee joints at all stages of osteoarthritis development. Osteoarthr. Cartil. 2012, 20, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Camplejohn, K.L.; Allard, S.A. Limitations of safranin ‘O’ staining in proteoglycan-depleted cartilage demonstrated with monoclonal antibodies. Histochemistry 1988, 89, 185–188. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, Y.-C.; Chen, L.-J.; Wang, J.-H.; Ho, T.-J.; Tseng, G.-F.; Chen, H.-P. The Therapeutic Potential of Intra-Articular Injection of Synthetic Deer Antler Peptides in a Rat Model of Knee Osteoarthritis. Int. J. Mol. Sci. 2024, 25, 6041. https://doi.org/10.3390/ijms25116041
Hung Y-C, Chen L-J, Wang J-H, Ho T-J, Tseng G-F, Chen H-P. The Therapeutic Potential of Intra-Articular Injection of Synthetic Deer Antler Peptides in a Rat Model of Knee Osteoarthritis. International Journal of Molecular Sciences. 2024; 25(11):6041. https://doi.org/10.3390/ijms25116041
Chicago/Turabian StyleHung, Yu-Chou, Li-Jin Chen, Jen-Hung Wang, Tsung-Jung Ho, Guo-Fang Tseng, and Hao-Ping Chen. 2024. "The Therapeutic Potential of Intra-Articular Injection of Synthetic Deer Antler Peptides in a Rat Model of Knee Osteoarthritis" International Journal of Molecular Sciences 25, no. 11: 6041. https://doi.org/10.3390/ijms25116041
APA StyleHung, Y.-C., Chen, L.-J., Wang, J.-H., Ho, T.-J., Tseng, G.-F., & Chen, H.-P. (2024). The Therapeutic Potential of Intra-Articular Injection of Synthetic Deer Antler Peptides in a Rat Model of Knee Osteoarthritis. International Journal of Molecular Sciences, 25(11), 6041. https://doi.org/10.3390/ijms25116041







