Genome-Wide Identification and Characterization of miRNAs and Natural Antisense Transcripts Show the Complexity of Gene Regulatory Networks for Secondary Metabolism in Aristolochia contorta
Abstract
1. Introduction
2. Results and Discussion
2.1. High-Throughput Small RNA Sequencing of A. contorta
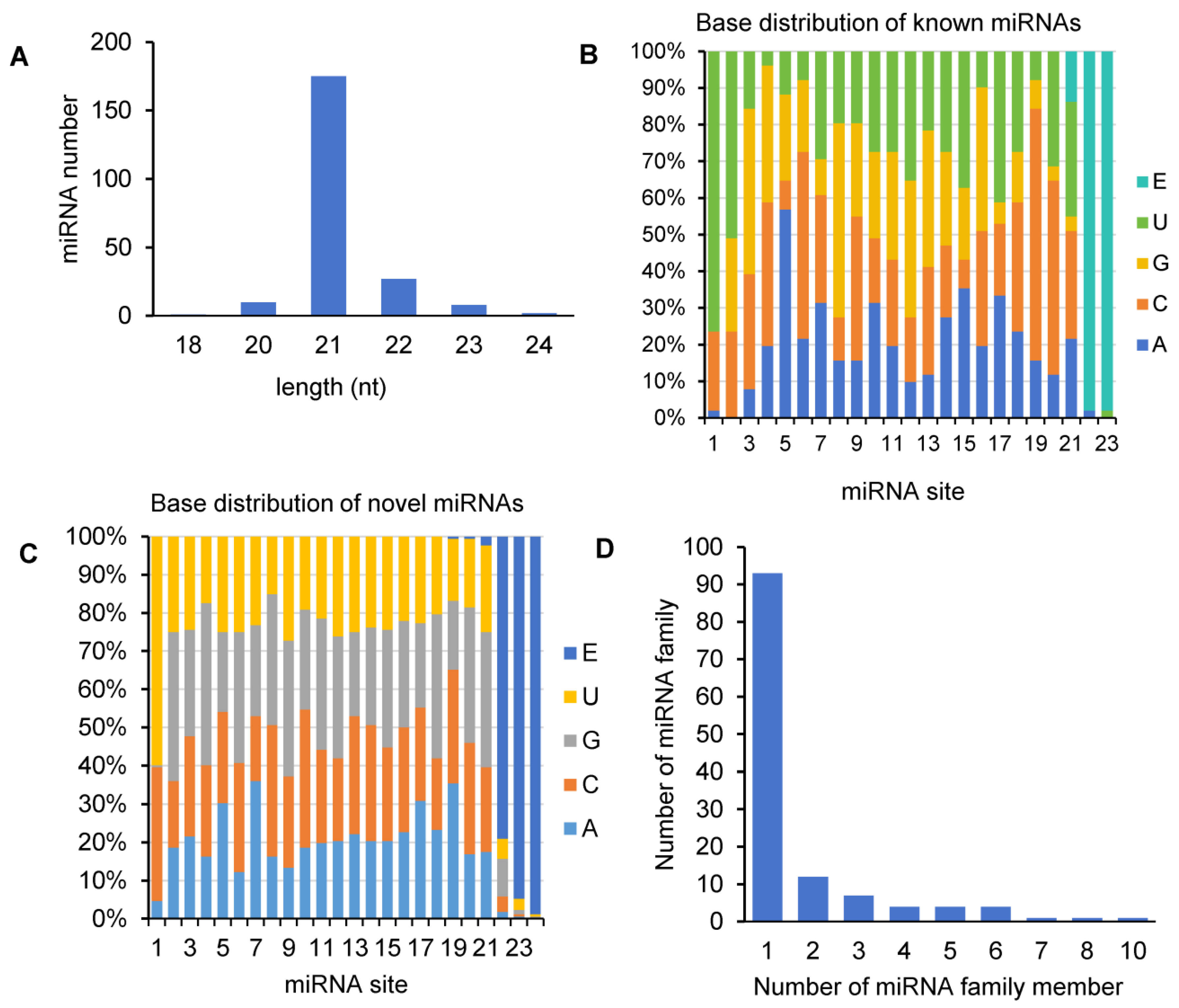
2.2. Identification of 51 Known and 172 Novel miRNAs
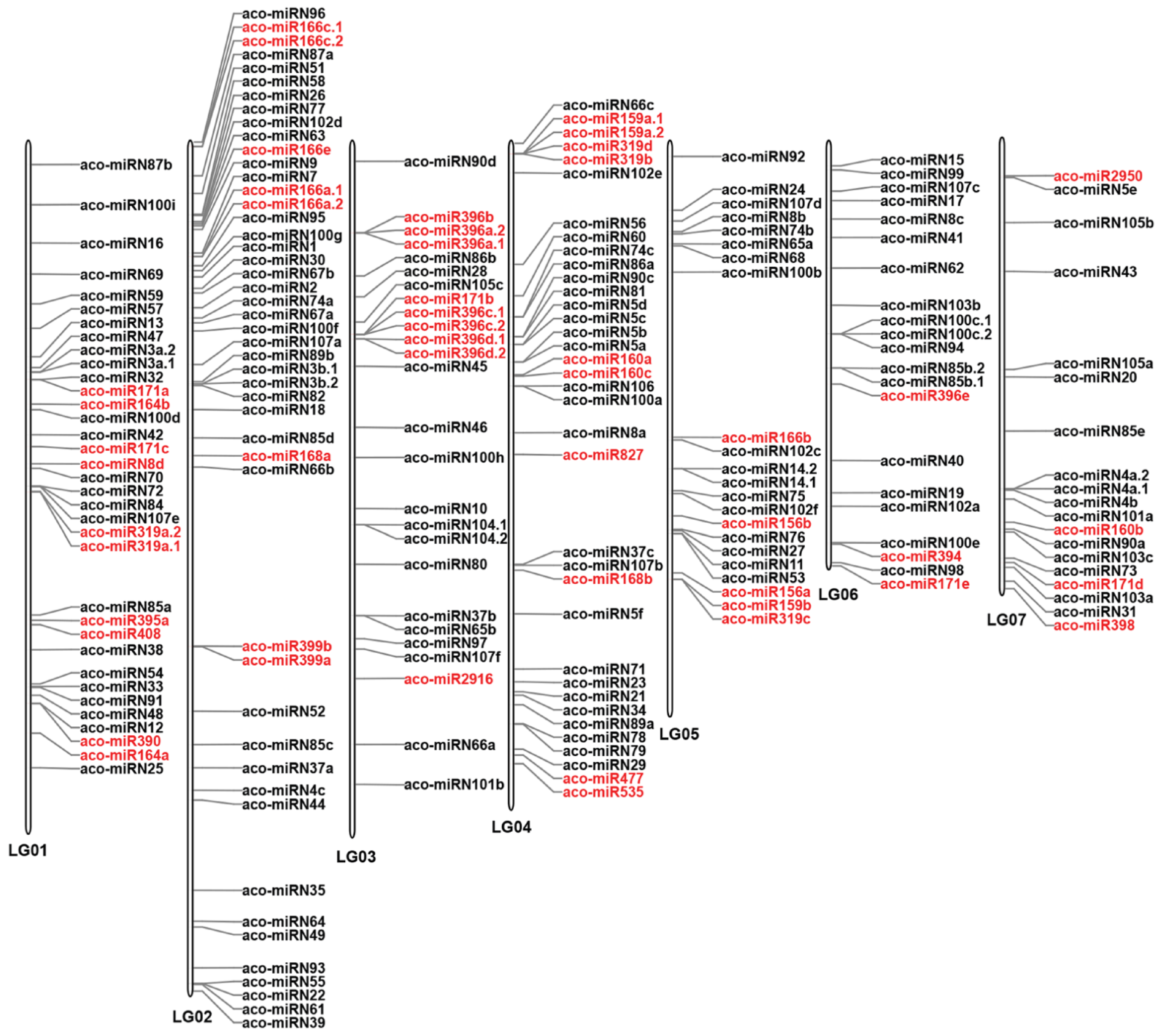
2.3. Characterization and Genome-Wide Distribution of A. contorta miRNAs
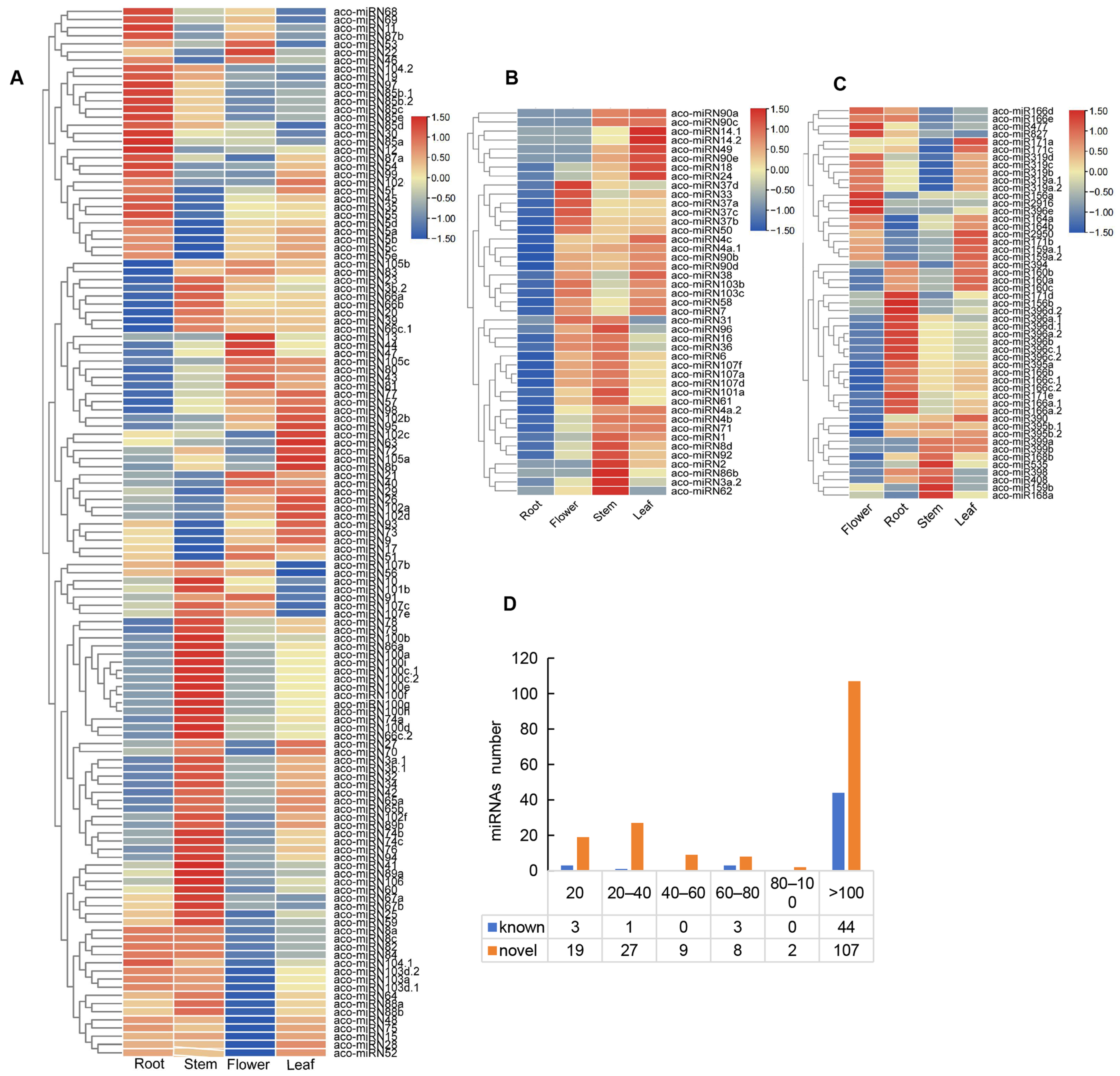
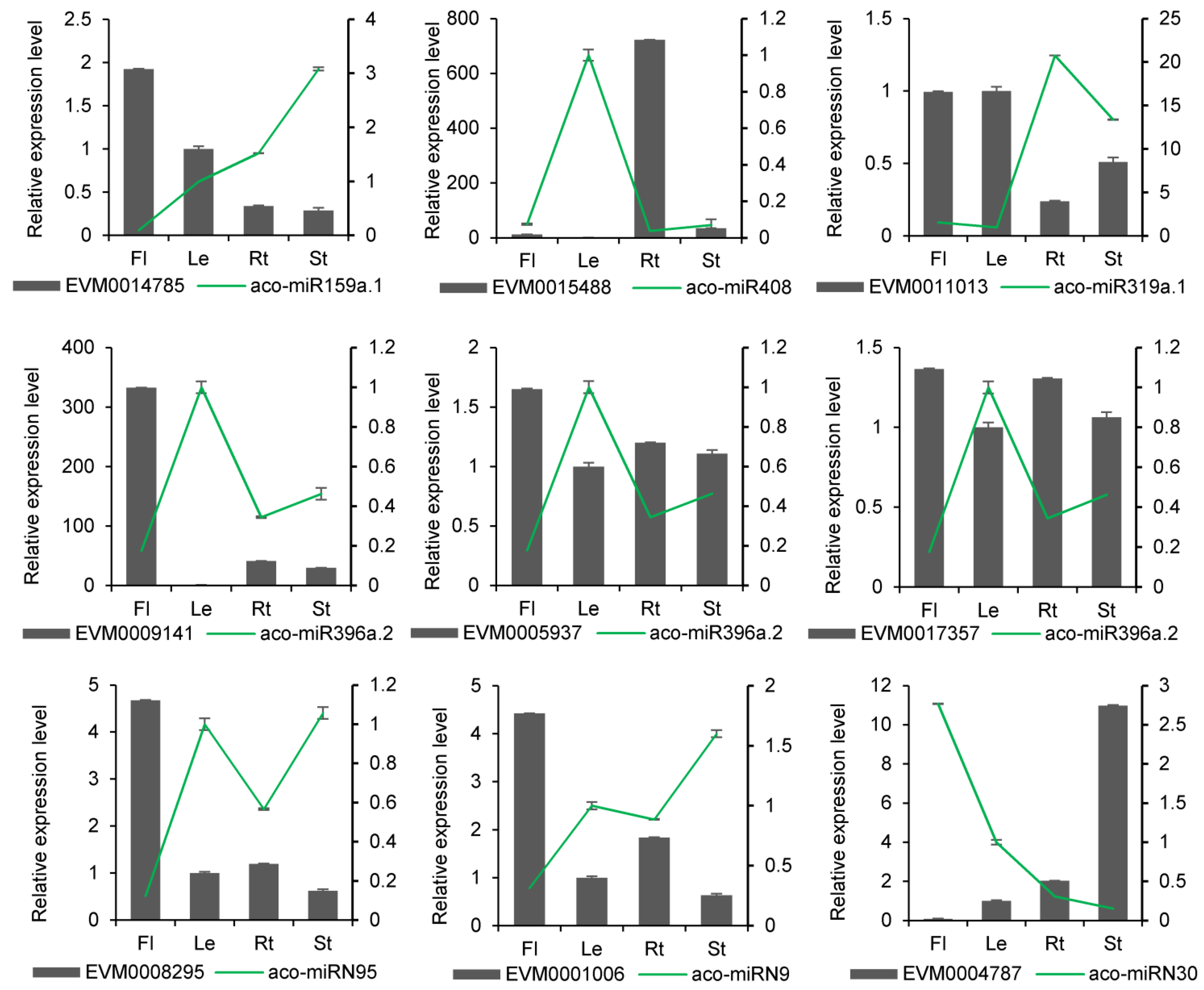
2.4. Differential Expression of miRNAs
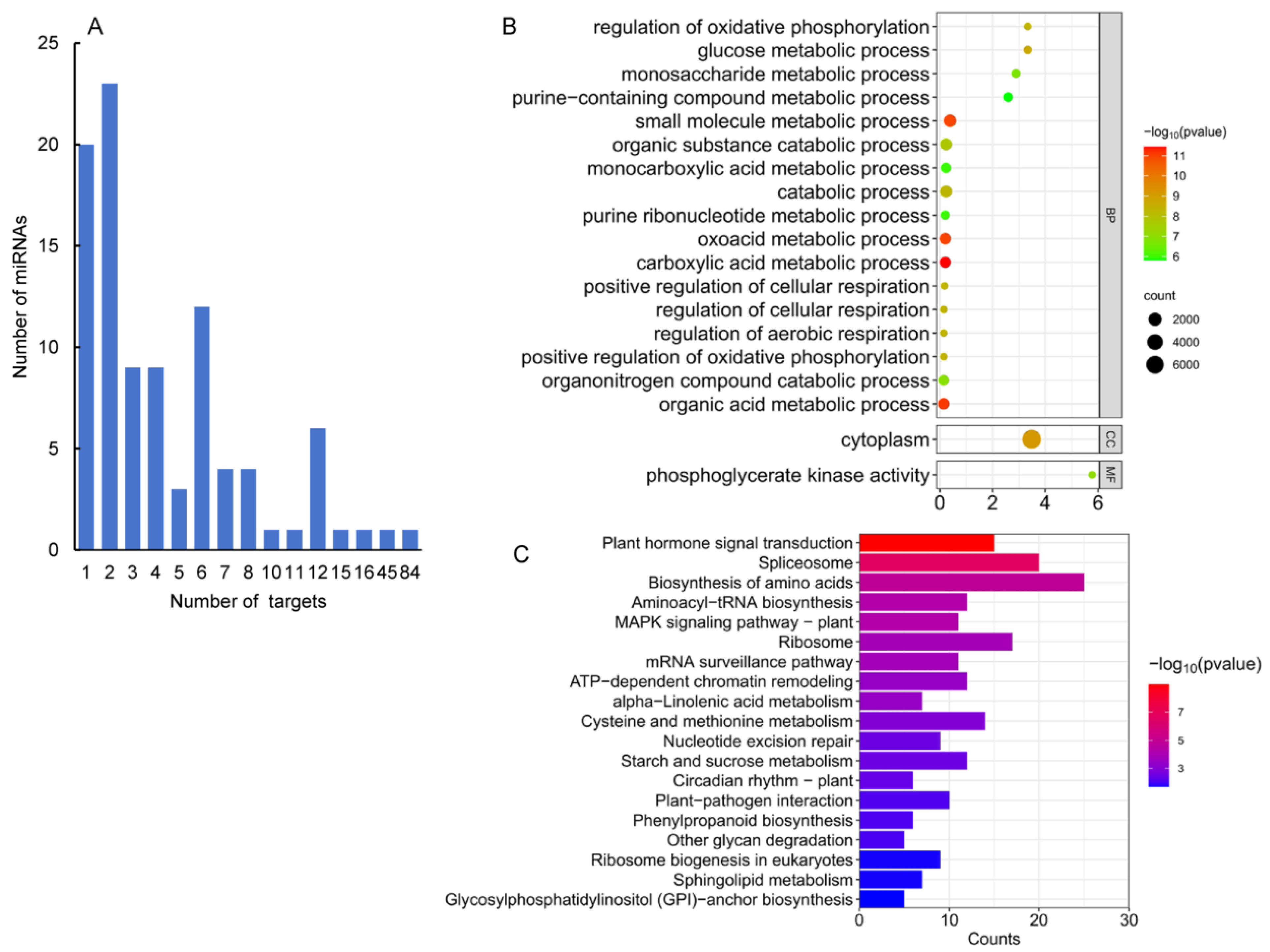
2.5. Computational Prediction of 363 miRNA Targets
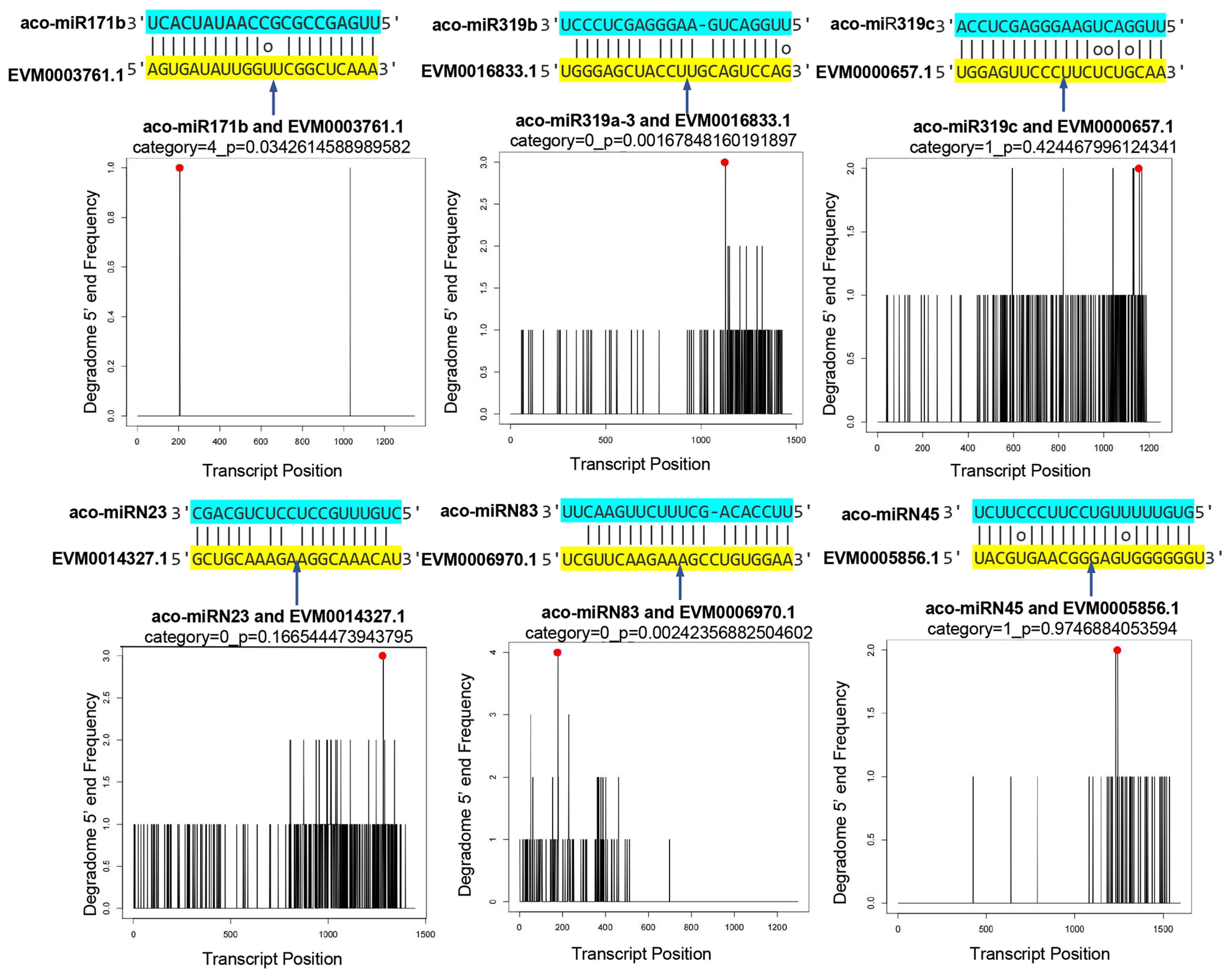
2.6. Validation of miRNA Targets Using Degradome Data
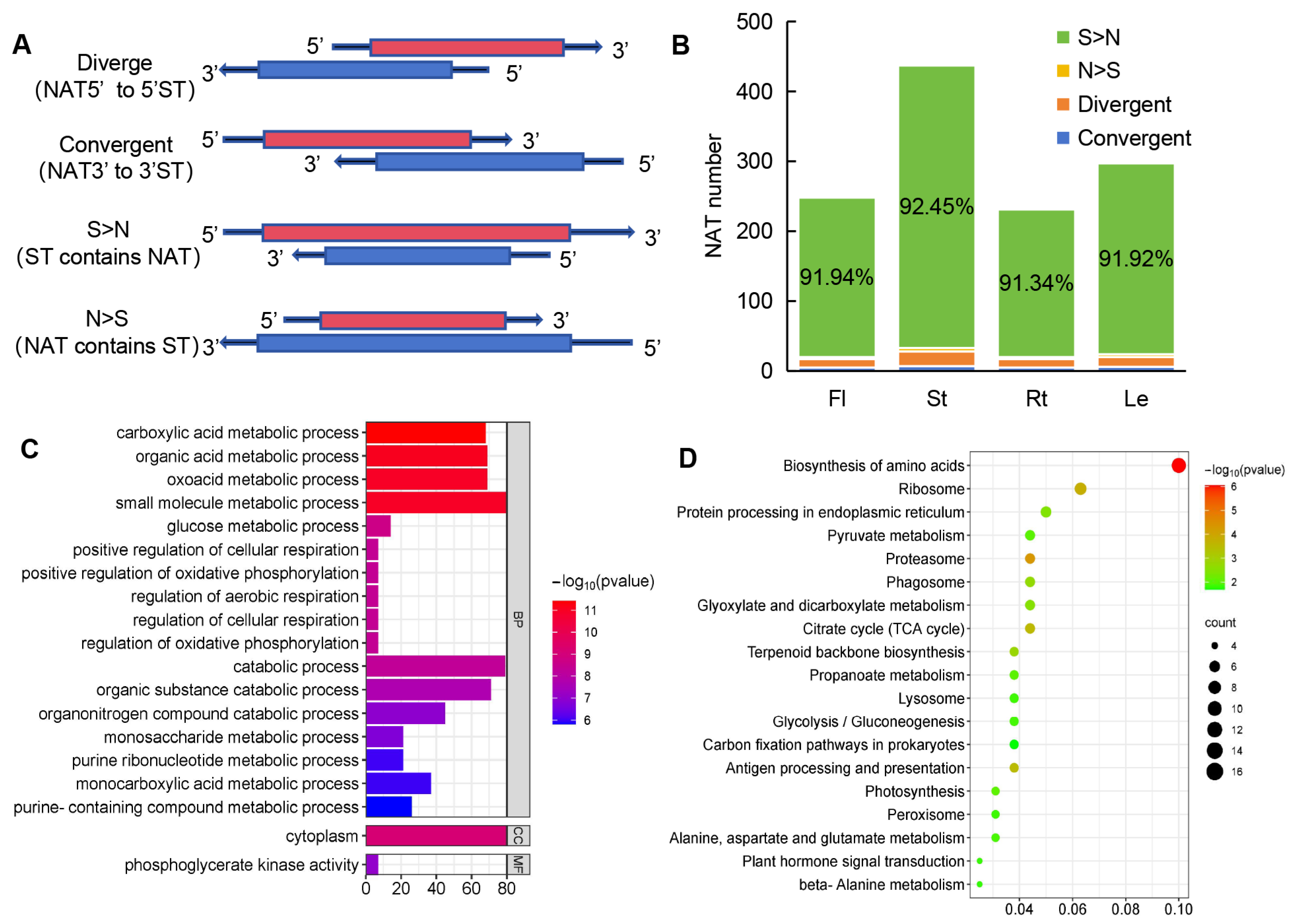
2.7. Identification of 441 NATs and 560 NAT-ST Pairs
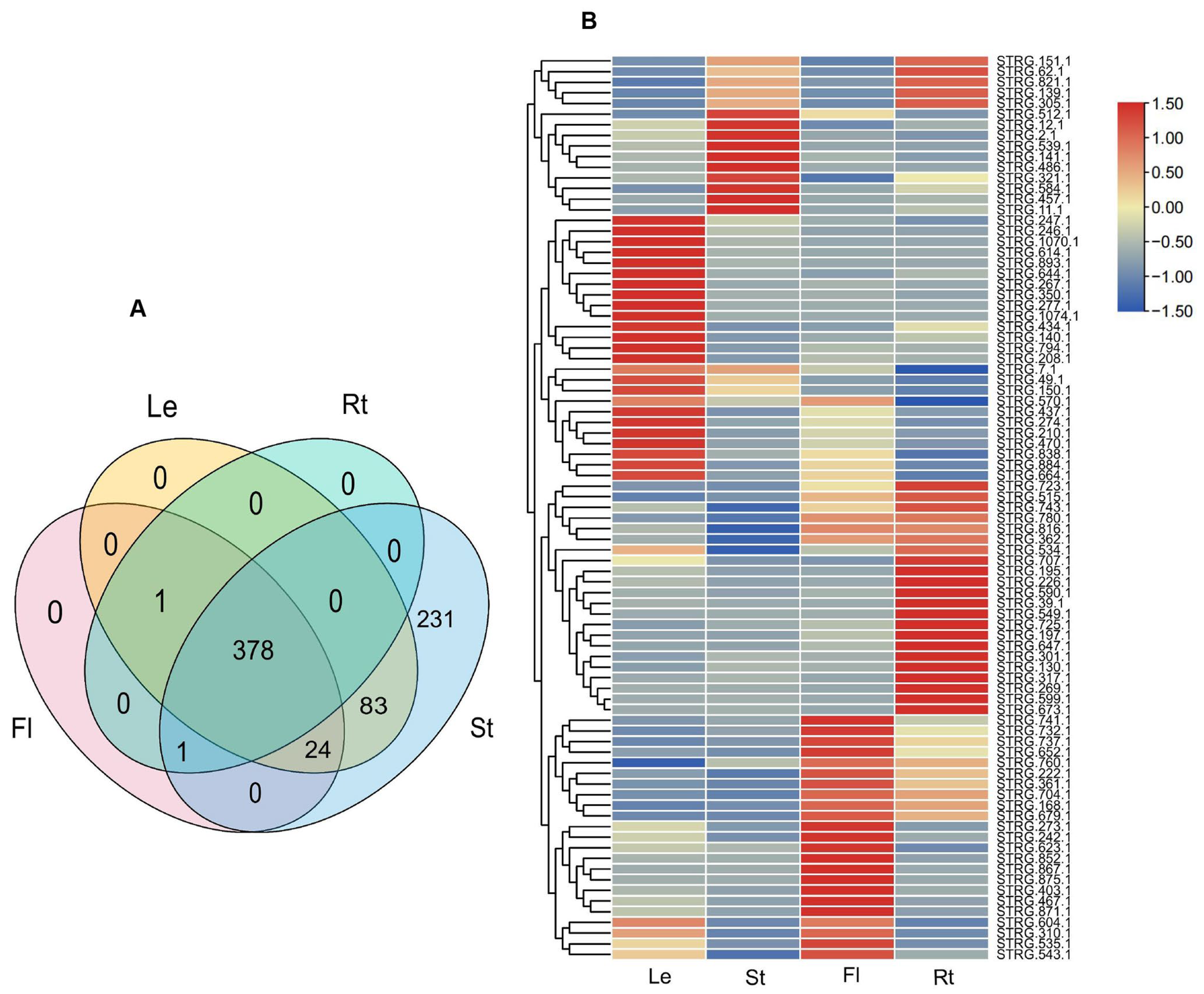
2.8. Expression and Functional Enrichment Analyses of NAT-STs
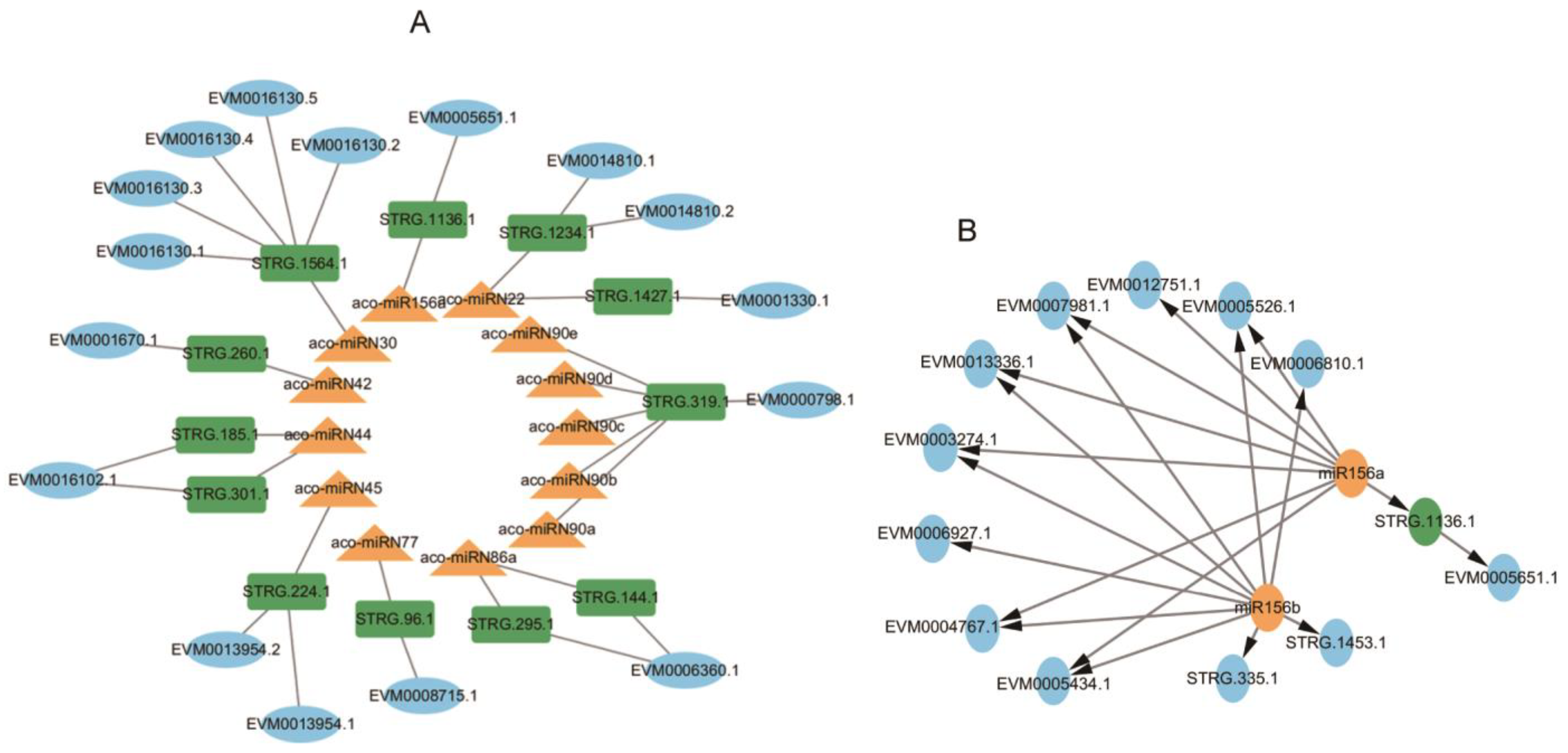
2.9. Identification of 18 Functionally Significant miRNA-NAT-ST Modules
2.10. Gene Regulatory Networks for Secondary Metabolism in A. contorta
3. Materials and Methods
3.1. Plant Materials and Total RNA Extraction
3.2. Small RNA Library Construction and Sequencing
3.3. Identification and Characterization of miRNAs
3.4. Identification and Characterization of lncRNA and NATs
3.5. Gene Ontology and KEGG Pathway Enrichment
3.6. Degradome Library Construction and Analysis
3.7. Quantitative Real-Rime Reverse Transcription-PCR (qRT-PCR)
3.8. WGCNA Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salomé, D.D.C.; Cordeiro, N.M.; Valério, T.S.; Santos, D.A.; Alves, P.B.; Alviano, C.S.; Moreno, D.S.A.; Fernandes, P.D. Aristolochia trilobata: Identification of the anti-inflammatory and antinociceptive effects. Biomedicines 2020, 8, 111. [Google Scholar] [CrossRef]
- Xu, X.L.; Yang, L.J.; Jiang, J.G. Renal toxic ingredients and their toxicology from traditional Chinese medicine. Expert Opin. Drug Metab. Toxicol. 2016, 12, 149–159. [Google Scholar] [CrossRef]
- Cui, X.; Meng, F.; Pan, X.; Qiu, X.; Zhang, S.; Li, C.; Lu, S. Chromosome-level genome assembly of Aristolochia contorta provides insights into the biosynthesis of benzylisoquinoline alkaloids and aristolochic acids. Hortic. Res. 2022, 9, uhac005. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Zhang, S.; Su, J.; Zhu, B.; Pan, X.; Qiu, X.; Cui, X.; Wang, C.; Niu, L.; Li, C.; et al. Characterization of two CYP80 enzymes provides insights into aporphine alkaloid skeleton formation in Aristolochia contorta. Plant J. 2024, 118, 1439–1454. [Google Scholar] [CrossRef]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Li, Y.; Cao, X.; Qi, Y. MicroRNAs and their regulatory roles in plant-environment interactions. Annu. Rev. Plant Biol. 2019, 70, 489–525. [Google Scholar] [CrossRef]
- Lu, S.; Sun, Y.H.; Shi, R.; Clark, C.; Li, L.; Chiang, V.L. Novel and mechanical stress-responsive microRNAs in Populus trichocarpa that are absent from Arabidopsis. Plant Cell 2005, 17, 2186–2203. [Google Scholar] [CrossRef]
- Lu, S.; Sun, Y.H.; Amerson, H.; Chiang, V.L. MicroRNAs in loblolly pine (Pinus taeda L.) and their association with fusiform rust gall development. Plant J. 2007, 51, 1077–1098. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Sun, Y.H.; Chiang, V.L. Stress-responsive microRNAs in Populus trichocarpa. Plant J. 2008, 55, 131–151. [Google Scholar] [CrossRef]
- Li, J.; Li, C.; Deng, Y.; Wei, H.; Lu, S. Characteristics of Salvia miltiorrhiza methylome and the regulatory mechanism of DNA methylation in tanshinone biosynthesis. Hortic. Res. 2023, 10, uhad114. [Google Scholar] [CrossRef]
- Lu, S.; Li, Q.; Wei, H.; Chang, M.J.; Tunlaya-Anukit, S.; Kim, H.; Liu, J.; Song, J.; Sun, Y.H.; Yuan, L.; et al. Ptr-miR397a is a negative regulator of laccase genes affecting lignin content in Populus trichocarpa. Proc. Natl. Acad. Sci. USA 2013, 110, 10848–10853. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Chang, Y.; Li, C.; Qiu, X.; Cui, X.; Meng, F.; Zhang, S.; Li, X.; Lu, S. Chromosome-level genome assembly of Salvia miltiorrhiza with orange roots uncovers the role of Sm2OGD3 in catalyzing 15,16-dehydrogenation of tanshinones. Hortic. Res. 2023, 10, uhad069. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Wang, M.; Pang, Y.; Hu, X.; Sun, C.; Zhou, H.; Deng, Y.; Lu, S. The smi-miR858a-SmMYB module regulates tanshinone and phenolic acid biosynthesis in Salvia miltiorrhiza. Hortic. Res. 2024, 11, uhae047. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Jiang, M.; Li, J.; Xu, Y.; Li, C.; Lu, S. Genome-wide identification and functional analysis of Salvia miltiorrhiza microRNAs reveal the negative regulatory role of smi-miR159a in phenolic acid biosynthesis. Int. J. Mol. Sci. 2024, 25, 5148. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Yang, C.; Chiang, V.L. Conservation and diversity of microRNA-associated copper-regulatory networks in Populus trichocarpa. J Integr. Plant Biol. 2011, 53, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, D.; Li, J.; Shao, F.; Lu, S. Characterization of the polyphenol oxidase gene family reveals a novel microRNA involved in posttranscriptional regulation of PPOs in Salvia miltiorrhiza. Sci. Rep. 2017, 7, 44622. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, C.; Lu, S. Systematic analysis of DEMETER-like DNA glycosylase genes shows lineage-specific Smi-miR7972 involved in SmDML1 regulation in Salvia miltiorrhiza. Sci. Rep. 2018, 8, 7143. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, M.; Qiu, X.; Zhou, H.; Lu, S. Noncoding RNAs in medicinal plants and their regulatory roles in bioactive compound production. Curr. Pharm. Biotechnol. 2021, 22, 341–359. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, Y.; Chen, X.; Chen, Y. Plant noncoding RNAs: Hidden players in development and stress responses. Annu. Rev. Cell Dev. Biol. 2019, 35, 407–431. [Google Scholar] [CrossRef]
- Chen, X.; Meng, L.; He, B.; Qi, W.; Jia, L.; Xu, N.; Hu, F.; Lv, Y.; Song, W. Comprehensive transcriptome analysis uncovers hub long non-coding RNAs regulating potassium use efficiency in Nicotiana tabacum. Front. Plant Sci. 2022, 13, 777308. [Google Scholar] [CrossRef]
- Gawronski, A.R.; Uhl, M.; Zhang, Y.; Lin, Y.Y.; Niknafs, Y.S.; Ramnarine, V.R.; Malik, R.; Feng, F.; Chinnaiyan, A.M.; Collins, C.C.; et al. MechRNA: Prediction of lncRNA mechanisms from RNA-RNA and RNA-protein interactions. Bioinformatics 2018, 34, 3101–3110. [Google Scholar] [CrossRef] [PubMed]
- Santini, L.; Yoshida, L.; De Oliveira, K.D.; Lembke, C.G.; Diniz, A.L.; Cantelli, G.C.; Nishiyama-Junior, M.Y.; Souza, G.M. Antisense transcription in plants: A systematic review and an update on cis-NATs of sugarcane. Int. J. Mol. Sci. 2022, 23, 11603. [Google Scholar] [CrossRef] [PubMed]
- Krappinger, J.C.; Bonstingl, L.; Pansy, K.; Sallinger, K.; Wreglesworth, N.I.; Grinninger, L.; Deutsch, A.; El-Heliebi, A.; Kroneis, T.; Mcfarlane, R.J.; et al. Non-coding natural antisense transcripts: Analysis and application. J. Biotechnol. 2021, 340, 75–101. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Yang, J.; He, Y. Natural antisense transcripts of MIR398 genes suppress microR398 processing and attenuate plant thermotolerance. Nat. Commun. 2020, 11, 5351. [Google Scholar] [CrossRef] [PubMed]
- Wight, M.; Werner, A. The functions of natural antisense transcripts. Essays Biochem. 2013, 54, 91–101. [Google Scholar] [PubMed]
- Cruz de Carvalho, M.H.; Bowler, C. Global identification of a marine diatom long noncoding natural antisense transcripts (NATs) and their response to phosphate fluctuations. Sci. Rep. 2020, 10, 14110. [Google Scholar] [PubMed]
- Fedak, H.; Palusinska, M.; Krzyczmonik, K.; Brzezniak, L.; Yatusevich, R.; Pietras, Z.; Kaczanowski, S.; Swiezewski, S. Control of seed dormancy in Arabidopsis by a cis-acting noncoding antisense transcript. Proc. Natl. Acad. Sci. USA 2016, 113, E7846–E7855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, H.; Zhu, Q.; Gao, Y.; Wang, H.; Zhao, L.; Wang, Y.; Xi, F.; Wang, W.; Yang, Y.; et al. Transcriptome characterization of moso bamboo (Phyllostachys edulis) seedlings in response to exogenous gibberellin applications. BMC Plant Biol. 2018, 18, 125. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Chen, H.; Liu, J.; Du, Q.; Lu, S.; Liu, C. Genome-wide identification and functional characterization of natural antisense transcripts in Salvia miltiorrhiza. Sci. Rep. 2021, 11, 4769. [Google Scholar] [CrossRef]
- Jain, M.; Chevala, V.N.; Garg, R. Genome-wide discovery and differential regulation of conserved and novel microRNAs in chickpea via deep sequencing. J. Exp. Bot. 2014, 65, 5945–5958. [Google Scholar] [CrossRef]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.; Qi, Y. MicroRNAs in a multicellular green alga Volvox carteri. Sci. China Life Sci. 2014, 57, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yan, S.; Zhao, J.; Xiong, H.; An, P.; Wang, J.; Zhang, H.; Zhang, L. Identification of miRNAs and their target genes in Larix olgensis and verified of differential expression miRNAs. BMC Plant Biol. 2019, 19, 247. [Google Scholar] [CrossRef]
- Ding, Y.; Mao, Y.; Cen, Y.; Hu, L.; Su, Y.; Ma, X.; Long, L.; Hu, H.; Hao, C.; Luo, J. Small RNA sequencing reveals various microRNAs involved in piperine biosynthesis in black pepper (Piper nigrum L.). BMC Genom. 2021, 22, 838. [Google Scholar] [CrossRef] [PubMed]
- Lian, C.; Zhang, F.; Yang, H.; Zhang, X.; Lan, J.; Zhang, B.; Liu, X.; Yang, J.; Chen, S. Multi-omics analysis of small RNA, transcriptome, and degradome to identify putative miRNAs linked to MeJA regulated and oridonin biosynthesis in Isodon rubescens. Int. J. Biol. Macromol. 2024, 258 Pt 2, 129123. [Google Scholar] [CrossRef]
- Cui, J.; You, C.; Chen, X. The evolution of microRNAs in plants. Curr. Opin. Plant Biol. 2017, 35, 61–67. [Google Scholar] [CrossRef]
- Cuperus, J.T.; Fahlgren, N.; Carrington, J.C. Evolution and functional diversification of MIRNA genes. Plant Cell 2011, 23, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.J.; Ma, Y.K.; Chen, T.; Wang, M.; Wang, X.J. PsRobot: A web-based plant small RNA meta-analysis toolbox. Nucleic Acids Res. 2012, 40, W22–W28. [Google Scholar] [CrossRef]
- Friedländer, M.R.; Mackowiak, S.D.; Li, N.; Chen, W.; Rajewsky, N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 2012, 40, 37–52. [Google Scholar] [CrossRef]
- Gruber, A.R.; Lorenz, R.; Bernhart, S.H.; Neuböck, R.; Hofacker, I.L. The Vienna RNA websuite. Nucleic Acids Res. 2008, 36, 70–74. [Google Scholar] [CrossRef]
- Devi, K.; Dey, K.K.; Singh, S.; Mishra, S.K.; Modi, M.K.; Sen, P. Identification and validation of plant miRNA from NGS data–an experimental approach. Brief. Funct. Genom. 2019, 18, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Griffiths-Jones, S. miRBase: Annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014, 42, D68–D73. [Google Scholar] [CrossRef] [PubMed]
- Borges, F.; Martienssen, R.A. The expanding world of small RNAs in plants. Nat. Rev. Mol. Cell Biol. 2015, 16, 727–741. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Guo, Z.; Li, L. Evolutionary conservation of microRNA regulatory programs in plant flower development. Dev. Biol. 2013, 380, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Adai, A.; Johnson, C.; Mlotshwa, S.; Archer-Evans, S.; Manocha, V.; Vance, V.; Sundaresan, V. Computational prediction of miRNAs in Arabidopsis thaliana. Genome Res. 2005, 15, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Xu, S.M.; Mu, D.S.; Yang, Z.M. Genomic analysis of rice microRNA promoters and clusters. Gene 2009, 431, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhuang, Z.; Zhao, P.X. psRNATarget: A plant small RNA target analysis server (2017 release). Nucleic Acids Res. 2018, 46, W49–W54. [Google Scholar] [CrossRef] [PubMed]
- Addo-Quaye, C.; Miller, W.; Axtell, M.J. CleaveLand: A pipeline for using degradome data to find cleaved small RNA targets. Bioinformatics 2009, 25, 130–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Czech, B.; Weigel, D. miR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell 2009, 138, 738–749. [Google Scholar] [CrossRef]
- Kato, H.; Nishihama, R.; Weijers, D.; Kohchi, T. Evolution of nuclear auxin signaling: Lessons from genetic studies with basal land plants. J. Exp. Bot. 2018, 69, 291–301. [Google Scholar] [CrossRef]
- Aya, K.; Ueguchi-Tanaka, M.; Kondo, M.; Hamada, K.; Yano, K.; Nishimura, M.; Matsuoka, M. Gibberellin modulates anther development in rice via the transcriptional regulation of GAMYB. Plant Cell 2009, 21, 1453–1472. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, G.; Yang, F.; Liang, Y.; Gao, Q.; Xiang, C.; Li, X.; Yang, R.; Zhang, G.; Jiang, H.; et al. Multilayered regulation of secondary metabolism in medicinal plants. Mol. Hortic. 2023, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, P.; Hole, K.; Pimenta-Marques, A.; Helsens, K.; Vandekerckhove, J.; Martinho, R.G.; Gevaert, K.; Arnesen, T. NatF contributes to an evolutionary shift in protein N-terminal acetylation and is important for normal chromosome segregation. PLoS Genet. 2011, 7, e1002169. [Google Scholar] [CrossRef] [PubMed]
- Morsby, J.J.; Smith, B.D. Advances in optical sensors of N-acetyl- of hexosaminidase (N-acetyl-miniglucosaminidase). Bioconjug. Chem. 2022, 33, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Heim, M.A.; Jakoby, M.; Werber, M.; Martin, C.; Weisshaar, B.; Bailey, P.C. The basic helix-loop-helix transcription factor family in plants: A genome-wide study of protein structure and functional diversity. Mol. Biol. Evol. 2003, 20, 735–747. [Google Scholar] [CrossRef]
- Schluttenhofer, C.; Yuan, L. Regulation of specialized metabolism by WRKY transcription factors. Plant Physiol. 2015, 167, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Hou, X.L.; Xing, G.M.; Liu, J.X.; Duan, A.Q.; Xu, Z.S.; Li, M.Y.; Zhuang, J.; Xiong, A.S. Advances in AP2/ERF super-family transcription factors in plant. Crit. Rev. Biotechnol. 2020, 40, 750–776. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef]
- Sun, L.; Luo, H.; Bu, D.; Zhao, G.; Yu, K.; Zhang, C.; Liu, Y.; Chen, R.; Zhao, Y. Utilizing sequence intrinsic composition to classify protein-coding and long non-coding transcripts. Nucleic Acids Res. 2013, 41, e166. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Yang, D.C.; Kong, L.; Hou, M.; Meng, Y.Q.; Wei, L.; Gao, G. CPC2: A fast and accurate coding potential calculator based on sequence intrinsic features. Nucleic Acids Res. 2017, 45, W12–W16. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Zhang, J.; Zhou, Z. PLEK: A tool for predicting long non-coding RNAs and messenger RNAs based on an improved k-mer scheme. BMC Bioinform. 2014, 15, 311. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Meng, Y.; Zuo, Z.; Xue, J.; Wang, H. NATpipe: An integrative pipeline for systematical discovery of natural antisense transcripts (NATs) and phase-distributed nat-siRNAs from de novo assembled transcriptomes. Sci. Rep. 2016, 6, 21666. [Google Scholar] [CrossRef]
- Deane, R.; Schäfer, W.; Zimmermann, H.P.; Mueller, L.; Görlich, D.; Prehn, S.; Ponstingl, H.; Bischoff, F.R. Ran-binding protein 5 (RanBP5) is related to the nuclear transport factor importin-beta but interacts differently with RanBP1. Mol. Cell Biol. 1997, 17, 5087–5096. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Wei, Y.; Zhang, P.; Liu, X.; Li, X.; Wang, S.; Liang, H.; Zhang, S.H. The bicarbonate transporter (MoAE4) localized on both cytomembrane and tonoplast promotes pathogenesis in Magnaporthe oryzae. J. Fungi 2021, 7, 955. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Cui, J.; Cui, H.; Jiang, N.; Hou, X.; Liu, S.; Gao, P.; Luan, Y.; Meng, J.; Luan, F. Identification of lncRNAs and their regulatory relationships with target genes and corresponding miRNAs in melon response to powdery mildew fungi. Gene 2020, 735, 144403. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.B.; Guo, Q.H.; Liu, P.; Dai, S.; Wu, C.A.; Yang, G.D.; Huang, J.G.; Zhang, S.Z.; Song, J.M.; Zheng, C.C.; et al. A long non-coding RNA functions as a competitive endogenous RNA to modulate TaNAC018 by acting as a decoy for tae-miR6206. Plant Mol. Biol. 2024, 114, 36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shen, J.; Xu, Q.; Dong, J.; Song, L.; Wang, W.; Shen, F. Long noncoding RNA lncRNA354 functions as a competing endogenous RNA of miR160b to regulate ARF genes in response to salt stress in upland cotton. Plant Cell Environ. 2021, 44, 3302–3321. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Wang, L.; Yin, Y.; Jing, X.; Wang, M.; Zhao, M.; Yu, J.; Qiu, Z.; Li, Y.F. Profiling of microRNAs involved in mepiquat chloride-mediated inhibition of internode elongation in cotton (Gossypium hirsutum L.) seedlings. Front. Plant Sci. 2021, 12, 643213. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Deng, Y.; Zhang, G.; Li, J.; Xiao, A.; Zhao, L.; Chen, A.; Tang, H.; Chang, L.; Pan, G.; et al. Comparative transcriptome and proteome analysis provides new insights into the mechanism of protein synthesis in kenaf (Hibiscus cannabinus L.) leaves. Front. Plant Sci. 2022, 13, 879874. [Google Scholar] [CrossRef] [PubMed]
- Conesa, A.; Götz, S.; Garcca-Grcca, J.M.; Terol, J.; Talll, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [PubMed]
- Bu, D.; Luo, H.; Huo, P.; Wang, Z.; Zhang, S.; He, Z.; Wu, Y.; Zhao, L.; Liu, J.; Guo, J.; et al. KOBAS-i: Intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021, 49, W317–W325. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, N.; Yu, Q.; Wang, H. Genome-wide characterization of salt-responsive miRNAs, circRNAs and associated ceRNA networks in tomatoes. Int. J. Mol. Sci. 2021, 22, 12238. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. ClusterProfiler: An R package for comparing biological themes among gene clusters. OMICS J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Gene Ontology Consortium. Gene Ontology Consortium: Going forward. Nucleic Acids Res. 2015, 43, D1049–D1056. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Varkonyi-Gasic, E.; Hellens, R.P. Quantitative stem-loop RT-PCR for detection of microRNAs. Methods Mol. Biol. 2011, 744, 145–157. [Google Scholar]




| Roots | Stems | Leaves | Flowers | |
|---|---|---|---|---|
| Raw reads | 27,955,448 | 22,405,111 | 19,120,948 | 20,509,711 |
| Low quality | 0 | 0 | 0 | 0 |
| Containing ‘N’ reads | 0 | 0 | 0 | 0 |
| Length < 18 | 5,114,294 | 4,310,512 | 2,715,516 | 857,776 |
| Length > 30 | 2,901,226 | 1,225,852 | 1,544,090 | 4,952,904 |
| Clean reads | 19,939,928 | 16,868,747 | 14,861,342 | 14,699,031 |
| Q30 (%) | 95.56 | 96.41 | 96.22 | 95.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, W.; Xu, Y.; Cui, X.; Li, C.; Lu, S. Genome-Wide Identification and Characterization of miRNAs and Natural Antisense Transcripts Show the Complexity of Gene Regulatory Networks for Secondary Metabolism in Aristolochia contorta. Int. J. Mol. Sci. 2024, 25, 6043. https://doi.org/10.3390/ijms25116043
Liang W, Xu Y, Cui X, Li C, Lu S. Genome-Wide Identification and Characterization of miRNAs and Natural Antisense Transcripts Show the Complexity of Gene Regulatory Networks for Secondary Metabolism in Aristolochia contorta. International Journal of Molecular Sciences. 2024; 25(11):6043. https://doi.org/10.3390/ijms25116043
Chicago/Turabian StyleLiang, Wenjing, Yayun Xu, Xinyun Cui, Caili Li, and Shanfa Lu. 2024. "Genome-Wide Identification and Characterization of miRNAs and Natural Antisense Transcripts Show the Complexity of Gene Regulatory Networks for Secondary Metabolism in Aristolochia contorta" International Journal of Molecular Sciences 25, no. 11: 6043. https://doi.org/10.3390/ijms25116043
APA StyleLiang, W., Xu, Y., Cui, X., Li, C., & Lu, S. (2024). Genome-Wide Identification and Characterization of miRNAs and Natural Antisense Transcripts Show the Complexity of Gene Regulatory Networks for Secondary Metabolism in Aristolochia contorta. International Journal of Molecular Sciences, 25(11), 6043. https://doi.org/10.3390/ijms25116043







