New Approach to the Cryopreservation of GV Oocytes and Cumulus Cells through the Lens of Preserving the Intercellular Gap Junctions Based on the Bovine Model
Abstract
1. Introduction
2. Results
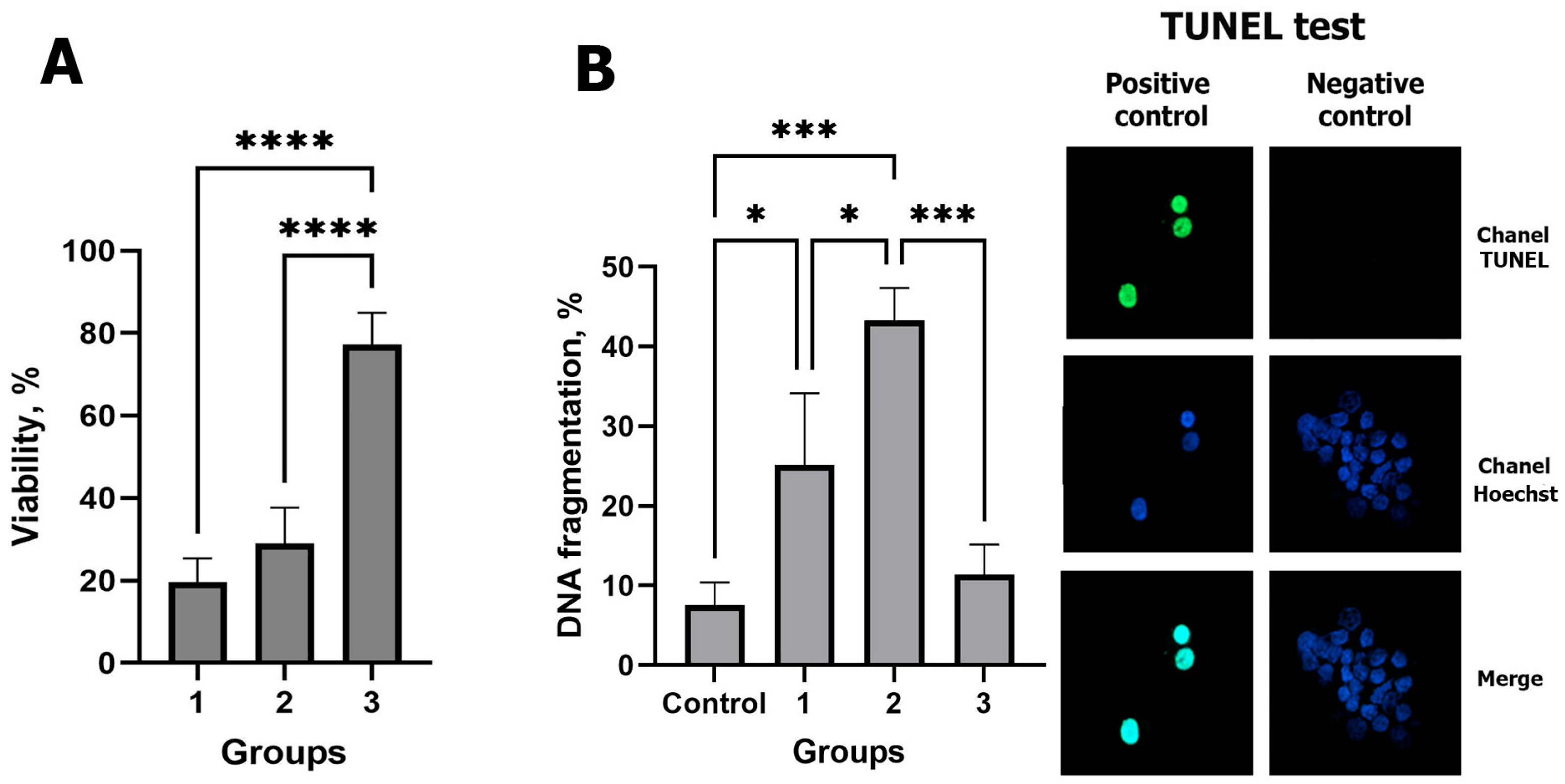
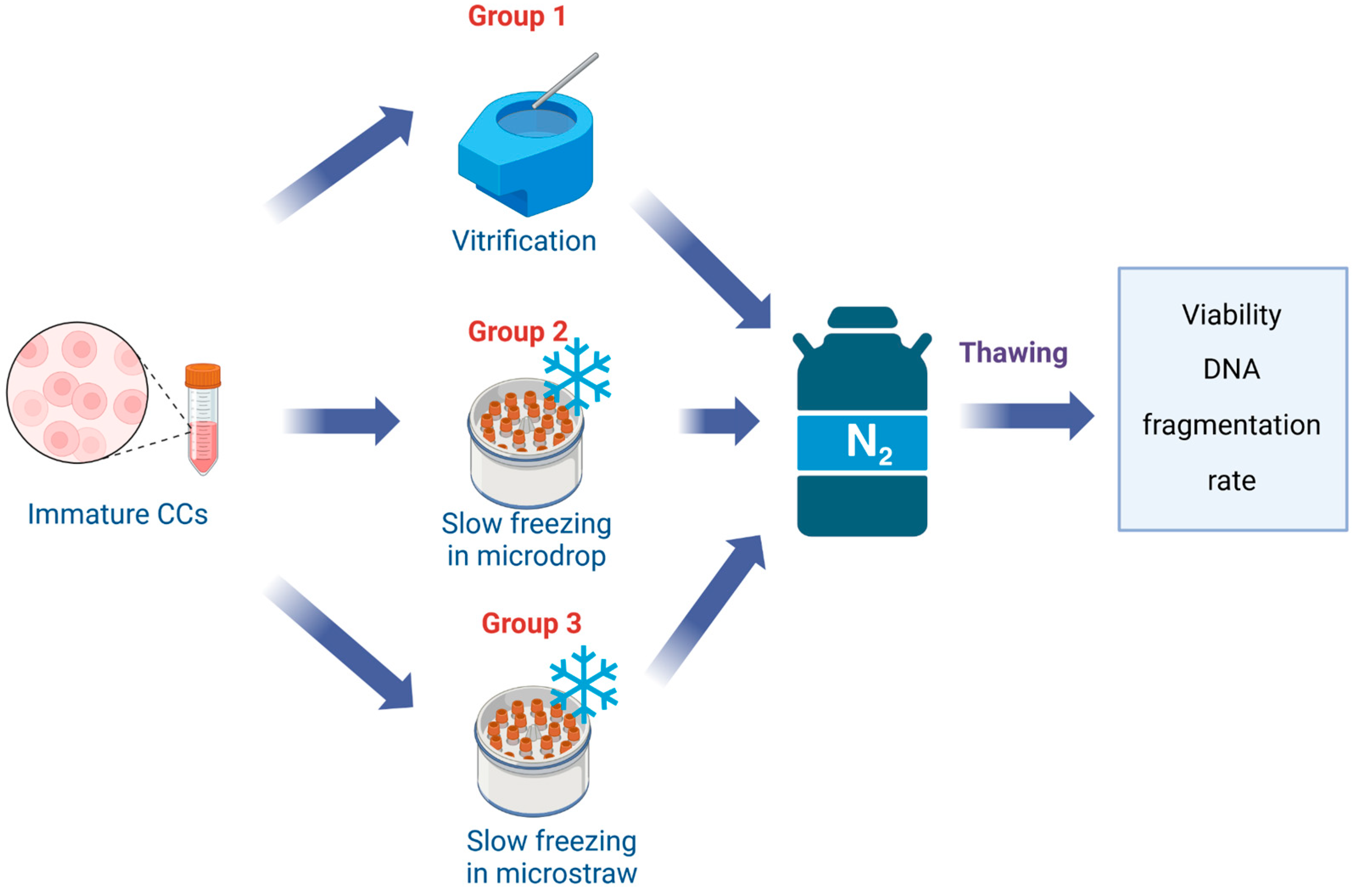
2.1. Effect of the Cryopreservation Method on the Viability and DNA Integrity of CCs
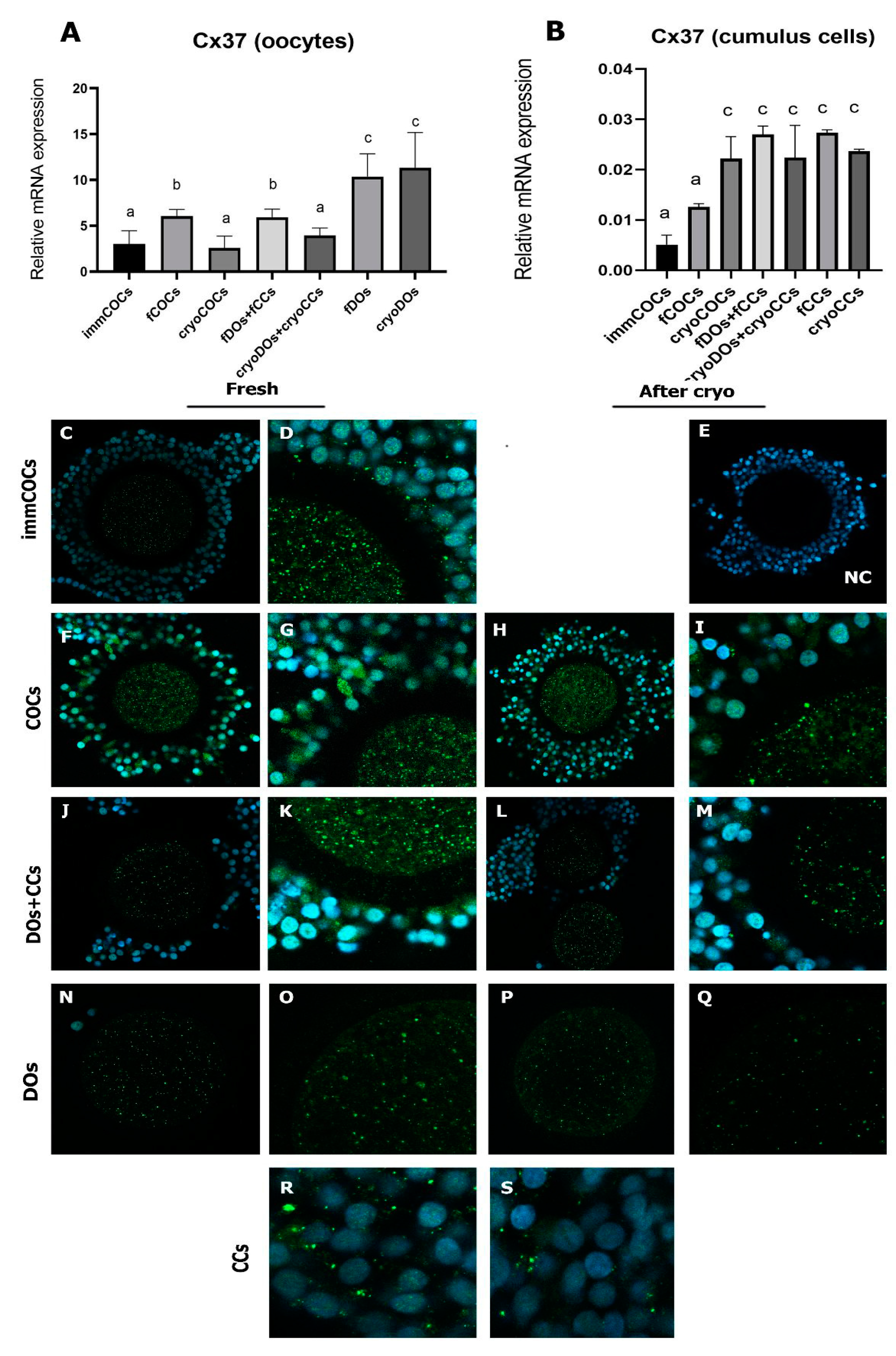
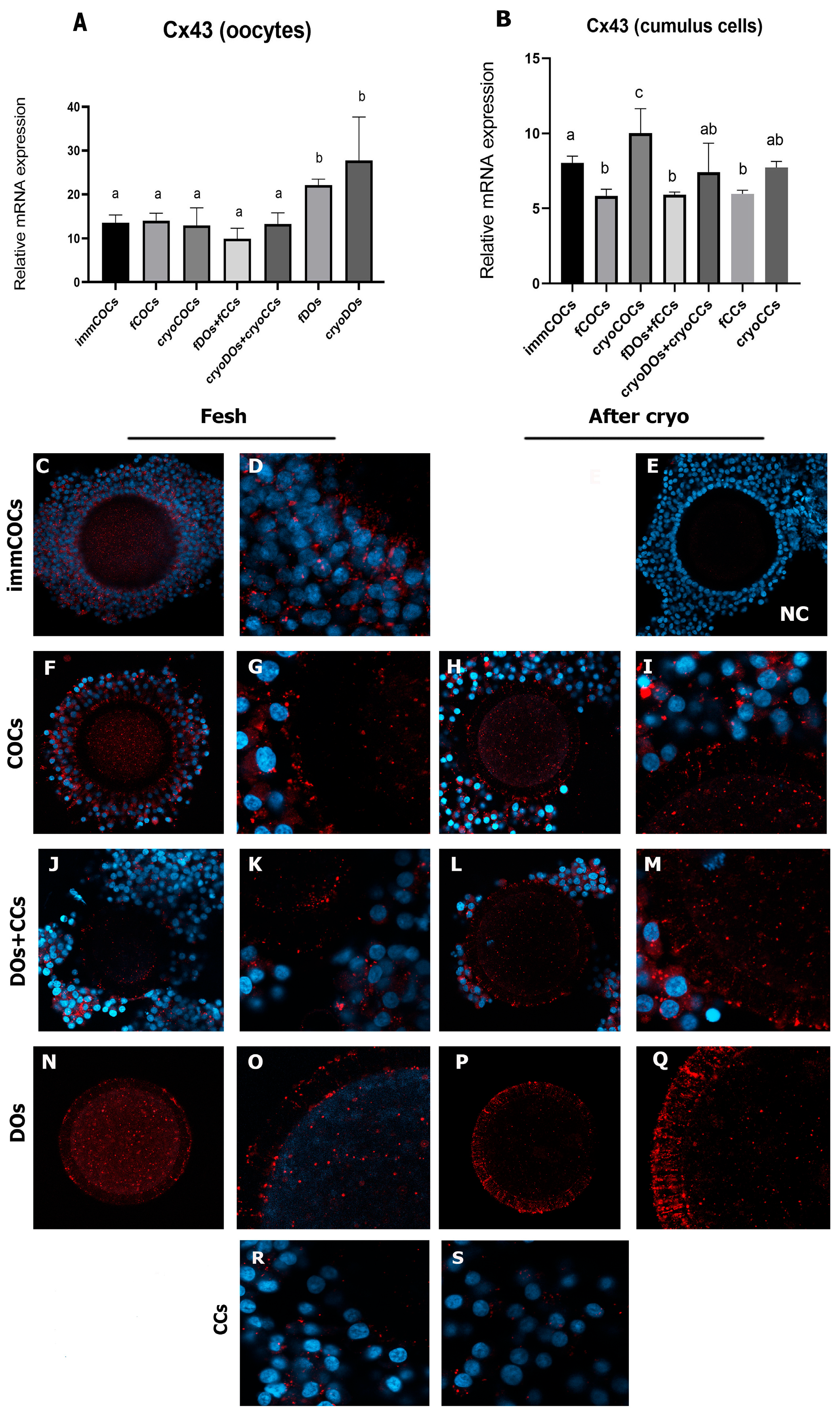
2.2. Effect of Cryopreservation of Oocytes and CCs and IVM Conditions on Intercellular Connections and the Level of mRNA Expression of Cx37 and Cx43
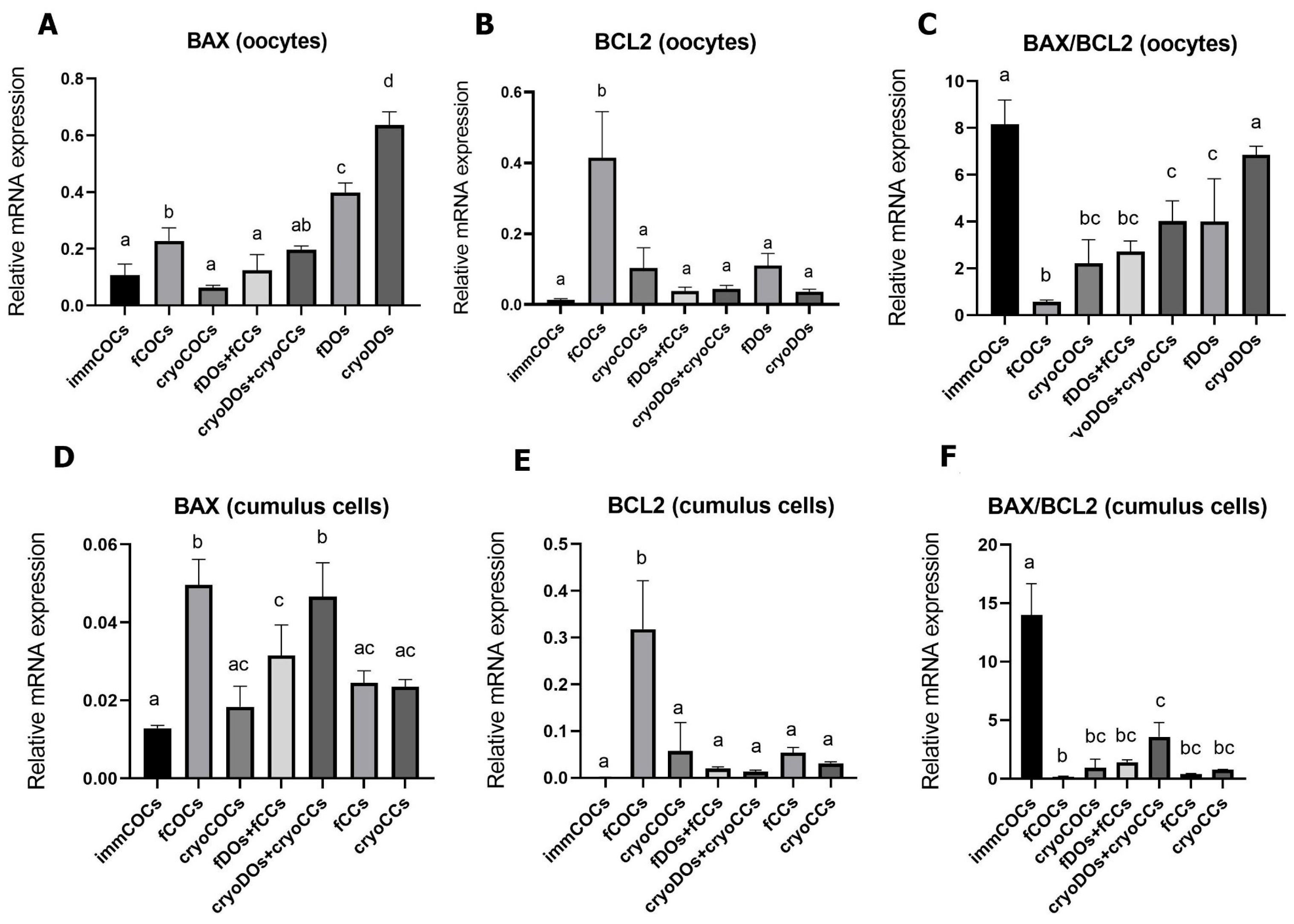
2.3. Effect of Cryopreservation of Oocytes and CCs and IVM Conditions on mRNA Expression Levels of BCL2, BAX, and DNMT3A
2.4. Effect of Cryopreservation of Oocytes and CCs and IVM Conditions on the mRNA Expression of DNMT3A
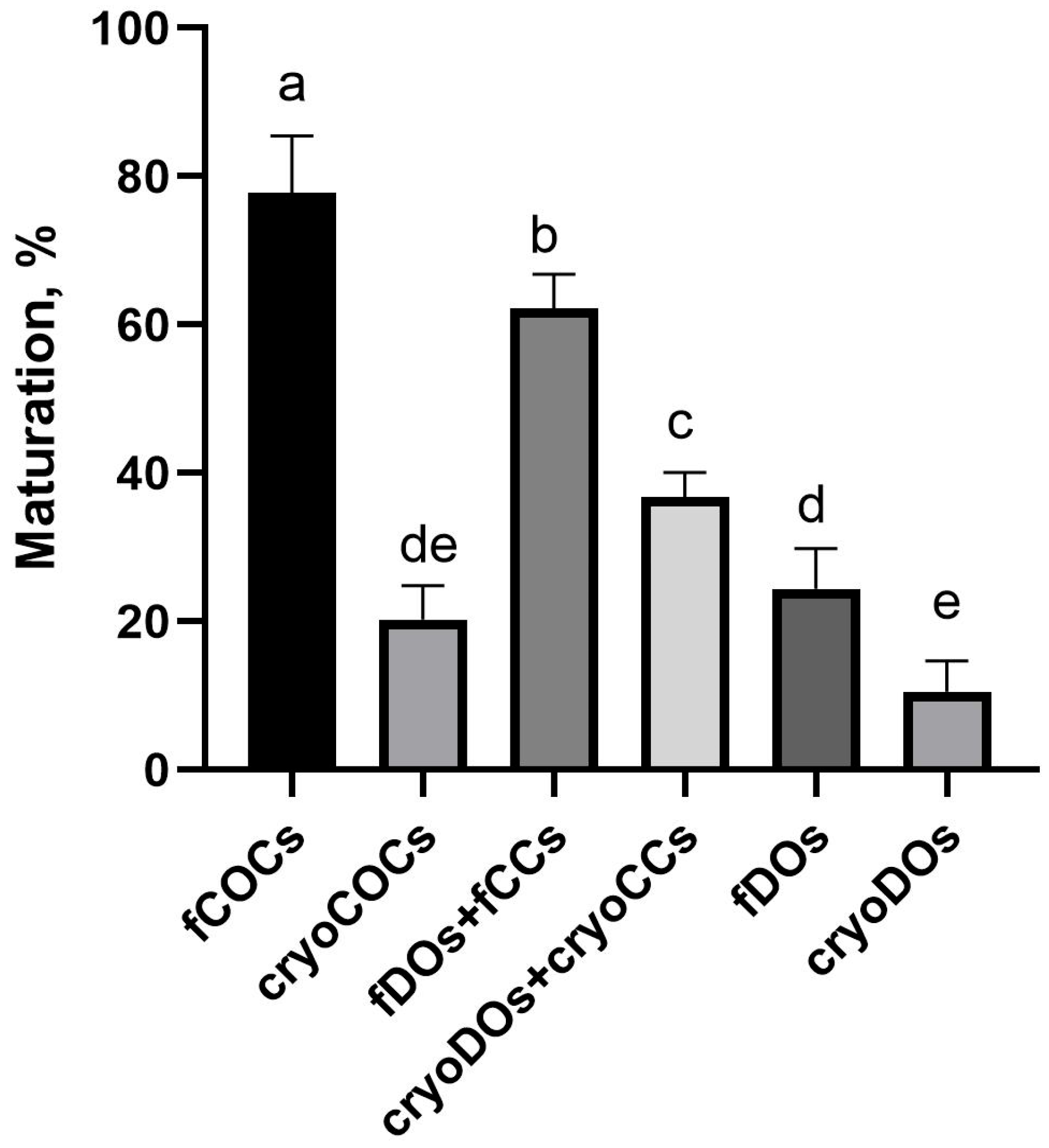
2.5. Effect of Cryopreservation of Oocytes and CCs and Conditions on the Outcomes of IVM
3. Discussion
4. Materials and Methods
4.1. Retrieval of Cumulus–Oocyte Complexes
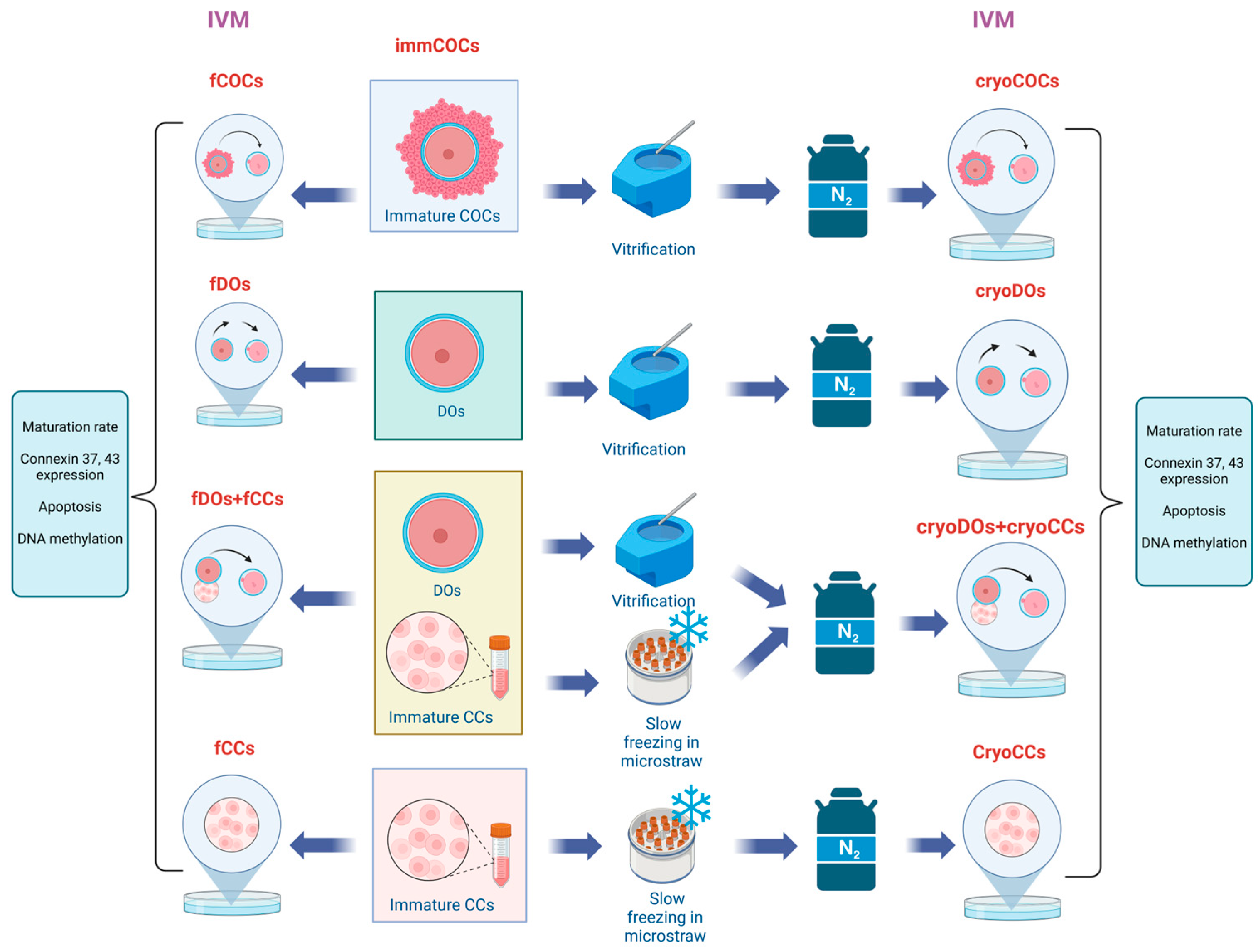
4.2. Experimental Design
4.3. Denudation of Oocytes
4.4. Cryopreservation of COCs and Oocytes
4.5. CCs Suspension’ Cryopreservation Procedure and Their Viability Assessment
4.6. DNA Fragmentation of CCs
4.7. Maturation of COCs and Oocyte–CCs Co-Culture System
4.8. Immunofluorescent Staining
4.9. RNA Isolation, cDNA Synthesis, and Real-Time qPCR
4.10. Statistical Data Processing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yurchuk, T.; Petrushko, M.; Fuller, B. Science of cryopreservation in reproductive medicine—Embryos and oocytes as exemplars. Early Hum. Dev. 2018, 126, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Rienzi, L.; Romano, S.; Albricci, L.; Maggiulli, R.; Capalbo, A.; Baroni, E.; Colamaria, S.; Sapienza, F.; Ubaldi, F. Embryo development of fresh ‘versus’ vitrified metaphase II oocytes after ICSI: A prospective randomized sibling-oocyte study. Hum. Reprod. 2010, 25, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Petrushko, M.; Yurchuk, T.; Todorov, P.; Hristova, E.; Piniaiev, V.; Isachenko, E.; Rahimi, G.; Mallmann, P.; Isachenko, V. New method for cryoprotectant-free freezing of human oligoasthenoteratozoospremic spermatozoa with high-molecular polymer. Cryobiology 2021, 103, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Yurchuk, T.; Petrushko, M. Quarter—Century experience in cryopreservation of human oocytes by vitrification. what has been achieved and what is next? Probl. Cryobiol. Cryomed. 2023, 33, 177–192. [Google Scholar] [CrossRef]
- Pai, H.D.; Baid, R.; Palshetkar, N.P.; Pai, A.; Pai, R.D.; Palshetkar, R. Oocyte Cryopreservation—Current Scenario and Future Perspectives: A Narrative Review. J. Hum. Reprod. Sci. 2021, 14, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.N.; Vagga, A. Cryopreservation: A Review Article. Cureus 2022, 14, e31564. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; Amant, F.; Braat, D.; D’Angelo, A.; Chuva de Sousa Lopes, S.M.; Demeestere, I.; Dwek, S.; Frith, L.; Lambertini, M.; Maslin, C.; et al. ESHRE guideline: Female fertility preservation. Hum. Reprod. Open 2020, 4, hoaa052. [Google Scholar] [CrossRef]
- Lee, J.; Kong, H.S.; Kim, E.J.; Youm, H.W.; Lee, J.R.; Suh, C.S.; Kim, S.H. Ovarian injury during cryopreservation and transplantation in mice: A comparative study between cryoinjury and ischemic injury. Hum. Reprod. 2016, 31, 1827–1837. [Google Scholar] [CrossRef] [PubMed]
- Leonel, E.C.R.; Lucci, C.M.; Amorim, C.A. Cryopreservation of Preantal Follicles. In Cryopreserved Biotechnology in Biomedical and Biological Sciences; Bozkurt, Y., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Yurchuk, T. Cryopreservation of Immature Oocytes at Germinal Vesicle Stage. When Gamete Maturation Performance Seems to Be Most Appropriate? Probl. Cryobiol. Cryomed. 2021, 31, 161–167. [Google Scholar] [CrossRef]
- Kristensen, S.G.; Andersen, C.Y. Cryopreservation of ovarian tissue: Opportunities beyond fertility preservation and a positive view into the future. Front. Endocrinol. 2018, 9, 347. [Google Scholar] [CrossRef]
- Zhou, C.J.; Wu, S.N.; Shen, J.P.; Wang, D.H.; Kong, X.W.; Lu, A.; Li, Y.J.; Zhou, H.X.; Zhao, Y.F.; Liang, C.G. The beneficial effects of cumulus cells and oocyte-cumulus cell gap junctions depends on oocyte maturation and fertilization methods in mice. PeerJ 2016, 4, e1761. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Xu, X.; Liu, S. Intercellular communication in the cumulus–oocyte complex during folliculogenesis: A review. Front. Cell Dev. Biol. 2023, 11, 1087612. [Google Scholar] [CrossRef]
- Virant-Klun, I.; Bauer, C.; Ståhlberg, A.; Kubista, M.; Skutella, T. Human oocyte maturation in vitro is improved by co-culture with cumulus cells from mature oocytes. Reprod. Biomed. Online 2018, 36, 508–523. [Google Scholar] [CrossRef] [PubMed]
- Cecconi, S.; Mauro, A.; Capacchietti, G. Meiotic maturation of incompetent prepubertal sheep oocytes is induced by paracrine factor(s) released by gonadotropin-stimulated oocyte-cumulus cell complexes and involves mitogen-activated protein kinase activation. Endocrinology 2008, 149, 100–107. [Google Scholar] [CrossRef]
- Jia, B.Y.; Xiang, D.C.; Zhang, B.; Quan, G.B.; Shao, Q.Y.; Hong, Q.H.; Wu, G.Q. Quality of vitrified porcine immature oocytes is improved by coculture with fresh oocytes during in vitro maturation. Mol. Reprod. Dev. 2019, 86, 1615–1627. [Google Scholar] [CrossRef]
- Da Broi, M.G.; Giorgi, V.S.I.; Wang, F.; Keefe, D.L.; Albertini, D.; Navarro, P.A. Influence of follicular fluid and cumulus cells on oocyte quality: Clinical implications. J. Assist. Reprod. Genet. 2018, 35, 735–751. [Google Scholar] [CrossRef] [PubMed]
- Jennifer, R.; Prentice, M.A. Cryopreservation of mammalian oocyte for conservation of animal genetics. Vet. Med. Int. 2011, 2011, 146405. [Google Scholar] [CrossRef]
- Ortiz-Escribano, N.; Smits, K.; Piepers, S.; Van den Abbeel, E.; Woelders, H.; Van Soom, A. Role of cumulus cells during vitrification and fertilization of mature bovine oocytes: Effects on survival, fertilization, and blastocyst development. Theriogenology 2016, 86, 635–641. [Google Scholar] [CrossRef] [PubMed]
- López, A.; Betancourt, M.; Ducolomb, Y.; Rodríguez, J.J.; Casas, E.; Bonilla, E.; Bahena, I.; Retana-Márquez, S.; Juárez-Rojas, L.; Casillas, F. DNA damage in cumulus cells generated after the vitrification of in vitro matured porcine oocytes and its impact on fertilization and embryo development. Porcine Health Manag. 2021, 7, 56. [Google Scholar] [CrossRef]
- Casillas, F.; Ducolomb, Y.; López, A.; Betancourt, M. Effect of porcine immature oocyte vitrification on oocyte-cumulus cell gap junctional intercellular communication. Porcine Health Manag. 2020, 6, 37. [Google Scholar] [CrossRef]
- Pereira, B.C.; Ortiz, I.; Dorado, J.; Consuegra, C.; Diaz-Jimenez, M.; Demyda-Peyras, S.; Gosalvez, J.; Hidalgo, M. Evaluation of DNA Damage of Mare Granulosa Cells Before and After Cryopreservation Using a Chromatin Dispersion Test. J. Equine Vet. Sci. 2019, 72, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Fernald, R.D. Comprehensive algorithm for quantitative real-time polymerase chain reaction. J. Comput. Biol. 2005, 12, 1047–1064. [Google Scholar] [CrossRef] [PubMed]
- Baratas, A.; Gosálvez, J.; de la Casa, M.; Camacho, S.; Dorado-Silva, M.; Johnston, S.D.; Roy, R. Cumulus cell DNA damage as an index of human oocyte competence. Reprod. Sci. 2022, 29, 3194–3200. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, I.; Dorado, J.; Pereira, B.; Diaz-Jimenez, M.; Consuegra, C.; Gosalvez, J.; Hidalgo, M. DNA fragmentation of equine cumulus cells from cumulus-oocyte complexes submitted to vitrification and its relationship to the developmental competence of the oocyte. Reprod. Domest. Anim. 2022, 57, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Somfai, T.; Nguyen, H.T.; Nguyen, M.T.; Dang-Nguyen, T.Q.; Kaneko, H.; Noguchi, J.; Kikuchi, K. Vitrification of porcine cumulus-oocyte complexes at the germinal vesicle stage does not trigger apoptosis in oocytes and early embryos, but activates anti-apoptotic Bcl-XL gene expression beyond the 4-cell stage. J. Reprod. Dev. 2020, 66, 115–123. [Google Scholar] [CrossRef]
- Colombo, M.; Zahmel, J.; Jänsch, S.; Jewgenow, K.; Luvoni, G.C. Inhibition of apoptotic pathways improves DNA integrity but not developmental competence of domestic cat immature vitrified oocytes. Front. Vet. Sci. 2020, 7, 588334. [Google Scholar] [CrossRef] [PubMed]
- Hunt, C.J. Cryopreservation: Vitrification and controlled rate cooling. Methods Mol. Biol. 2017, 1590, 41–77. [Google Scholar]
- Murray, K.A.; Gibson, M.I. Chemical approaches to cryopreservation. Nat. Rev. Chem. 2022, 6, 579–593. [Google Scholar] [CrossRef]
- Li, S.-H.; Lin, M.H.; Hwu, Y.M.; Lu, C.H.; Yeh, L.Y.; Chen, Y.K. Correlation of cumulus gene expression, PTX3, and embryo development. Reprod. Biol. Endocrinol. 2015, 13, 93. [Google Scholar] [CrossRef]
- Hasegawa, J.; Yanaihara, A.; Iwasaki, S.; Mitsukawa, K.; Negishi, M.; Okai, T. Reduction of connexin 43 in human cumulus cells yields good embryo competence during ICSI. J. Assist. Reprod. Genet. 2007, 24, 463–466. [Google Scholar] [CrossRef]
- Safrina, A.; Anita, N.; Jusuf, A.A.; Syaidah, R.; Saoemi, H.A. Bax/Bcl2 expression ratio analysis of rat ovaries vitrified with date juice concentrate as a natural extracellular cryoprotectant. J. Hum. Reprod. Sci. 2023, 16, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.Y.; Rajamahendran, R. Expression of Bcl-2 and Bax proteins in relation to quality of bovine oocytes and embryos produced in vitro. Anim. Reprod. Sci. 2002, 70, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Uysal, F.; Sukur, G.; Bozdemir, N.; Cinar, O. Unveiling the impact of DNA methylation machinery: Dnmt1 and Dnmt3a in orchestrating oocyte development and cellular homeostasis. Genesis 2023, 62, e23579. [Google Scholar] [CrossRef] [PubMed]
- Wiltshire, A.; Schaal, R.; Wang, F.; Tsou, T.; McKerrow, W.; Keefe, D. Vitrification with dimethyl sulfoxide induces transcriptomic alteration of gene and transposable element expression in immature human oocytes. Genes 2023, 14, 1232. [Google Scholar] [CrossRef]
- Turathum, B.; Gao, E.M.; Chian, R.C. The function of cumulus cells in oocyte growth and maturation and in subsequent ovulation and fertilization. Cells 2021, 10, 2292. [Google Scholar] [CrossRef]



| Gene Symbol | Gene Name | Accession Numer | TaqMan Assay ID | Product Length (bp) |
|---|---|---|---|---|
| GJA1 (Cx43) | Gap junction protein alpha 1 | NM_174068.2 | Bt03244351_M1 | 67 |
| GJA4 (Cx37) | Gap junction protein alpha 4 | NM_001083738.1 | Bt03257693_g1 | 95 |
| BAX | BCL2 associated X, apoptosis regulator | NM_173894.1 | Bt01016551_g1 | 72 |
| BCL2 | BCL2 apoptosis regulator | NM_001166486.1 | Bt04298952_M1 | 72 |
| DNMT3A | DNA methyltransferase 3 alpha | NM_001206502.2 | Bt01027164_M1 | 65 |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | NM_001034034.2 | Bt03210913_g1 | 66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yurchuk, T.; Likszo, P.; Witek, K.; Petrushko, M.; Skarzynski, D.J. New Approach to the Cryopreservation of GV Oocytes and Cumulus Cells through the Lens of Preserving the Intercellular Gap Junctions Based on the Bovine Model. Int. J. Mol. Sci. 2024, 25, 6074. https://doi.org/10.3390/ijms25116074
Yurchuk T, Likszo P, Witek K, Petrushko M, Skarzynski DJ. New Approach to the Cryopreservation of GV Oocytes and Cumulus Cells through the Lens of Preserving the Intercellular Gap Junctions Based on the Bovine Model. International Journal of Molecular Sciences. 2024; 25(11):6074. https://doi.org/10.3390/ijms25116074
Chicago/Turabian StyleYurchuk, Taisiia, Pawel Likszo, Krzysztof Witek, Maryna Petrushko, and Dariusz J. Skarzynski. 2024. "New Approach to the Cryopreservation of GV Oocytes and Cumulus Cells through the Lens of Preserving the Intercellular Gap Junctions Based on the Bovine Model" International Journal of Molecular Sciences 25, no. 11: 6074. https://doi.org/10.3390/ijms25116074
APA StyleYurchuk, T., Likszo, P., Witek, K., Petrushko, M., & Skarzynski, D. J. (2024). New Approach to the Cryopreservation of GV Oocytes and Cumulus Cells through the Lens of Preserving the Intercellular Gap Junctions Based on the Bovine Model. International Journal of Molecular Sciences, 25(11), 6074. https://doi.org/10.3390/ijms25116074







