hTERT Peptide Fragment GV1001 Prevents the Development of Porphyromonas gingivalis-Induced Periodontal Disease and Systemic Disorders in ApoE-Deficient Mice
Abstract
1. Introduction
2. Results
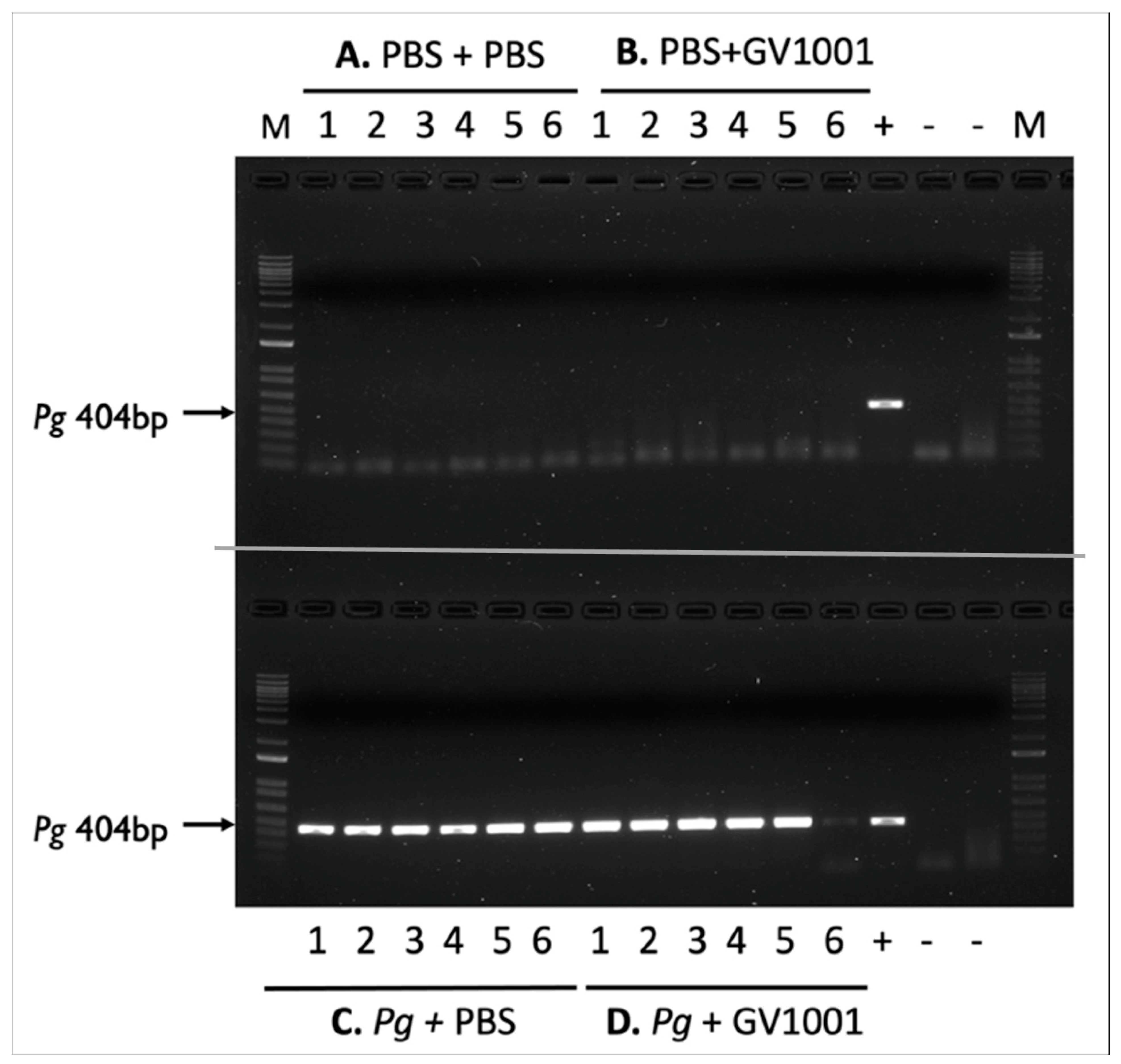
2.1. Topical Application of Pg into the Gingival Pockets Established the Pg Colony Formation in the Pockets, and Systemic Administration Did Not Alter the Colony Formation
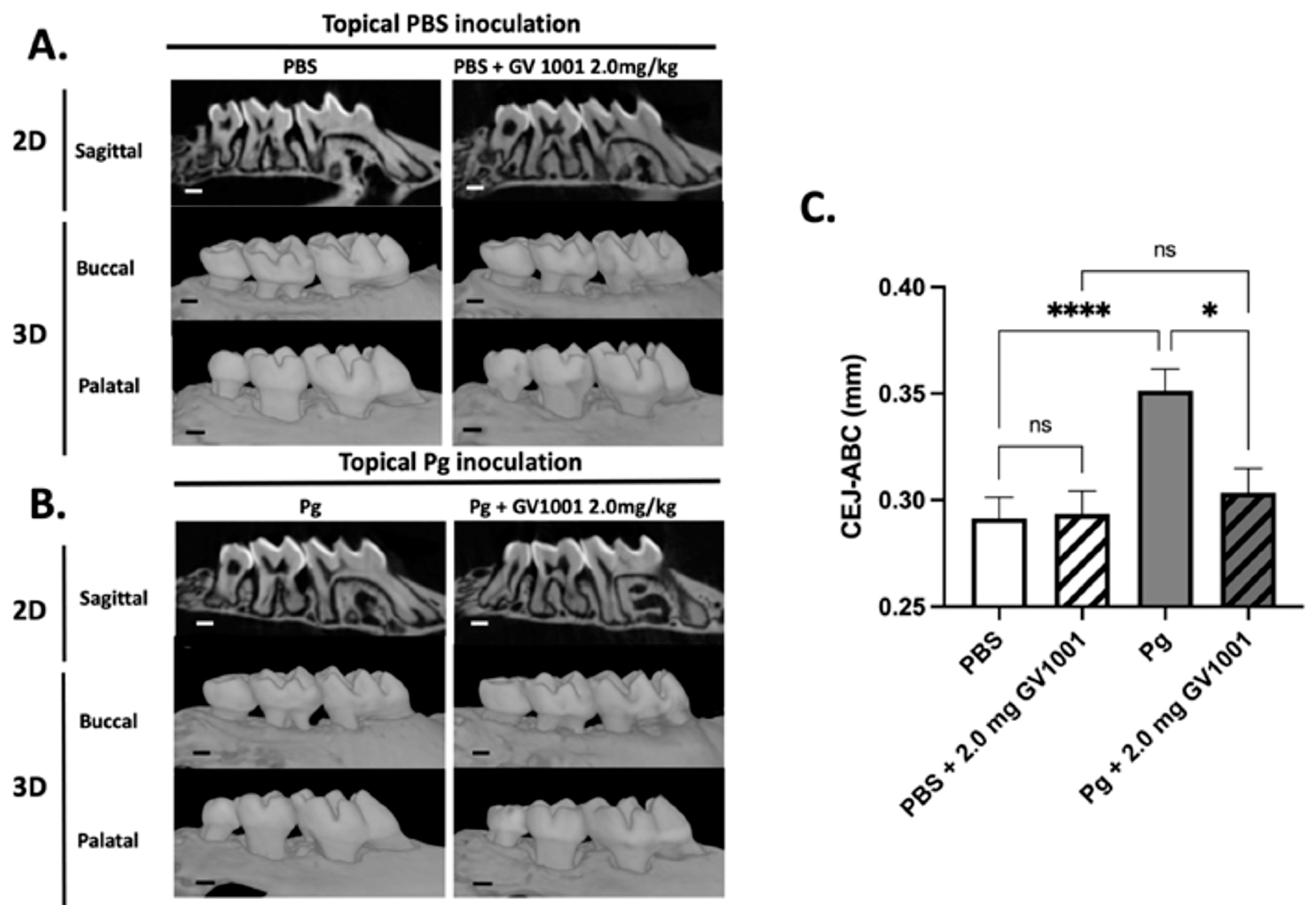
2.2. Systemic GV1001 Prevented the Increased Periodontal Attachment Loss Induced by Pg Inoculation into the Gingival Pocket
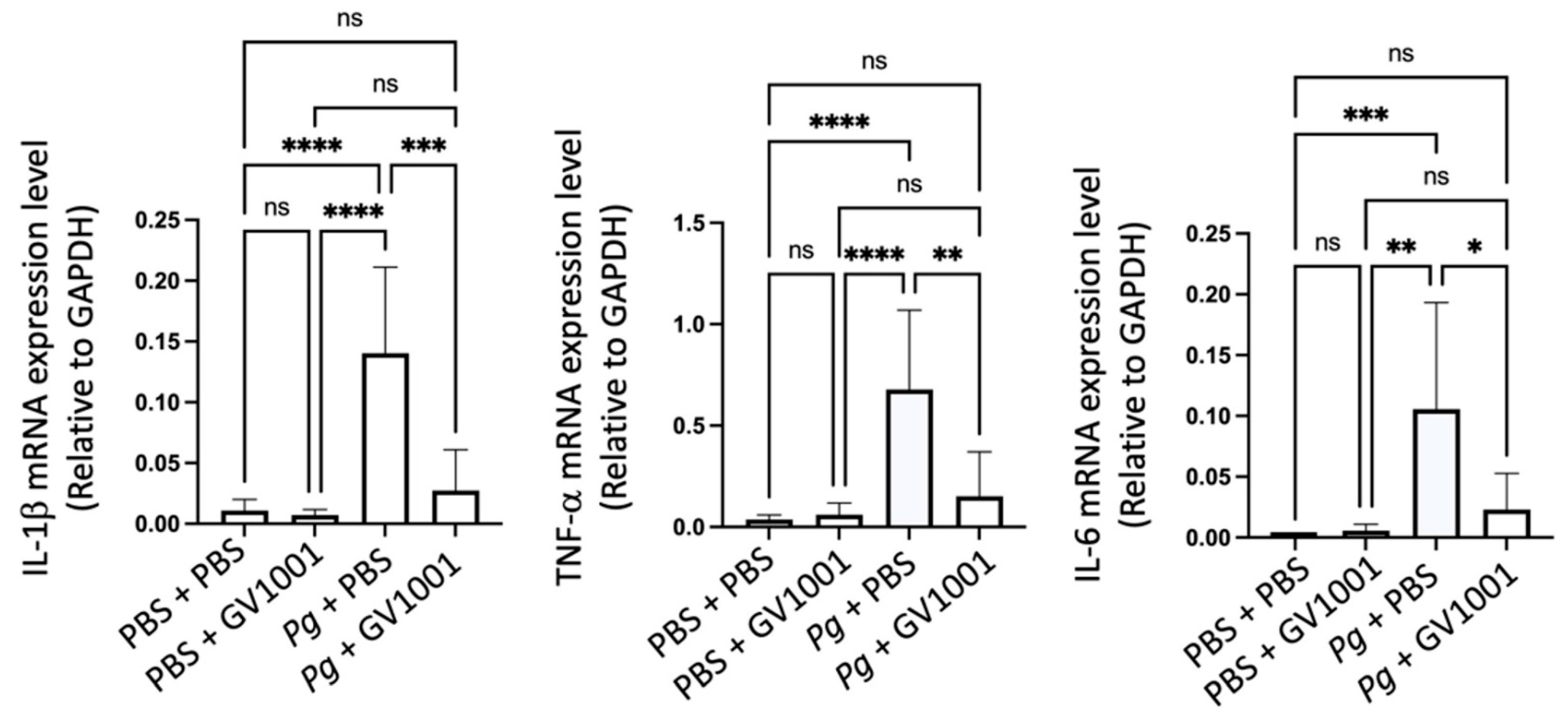
2.3. GV1001 Inhibited Pg-Induced Gingival Inflammation by Suppressing the Expression of Proinflammatory Cytokines in the Gingival Tissue
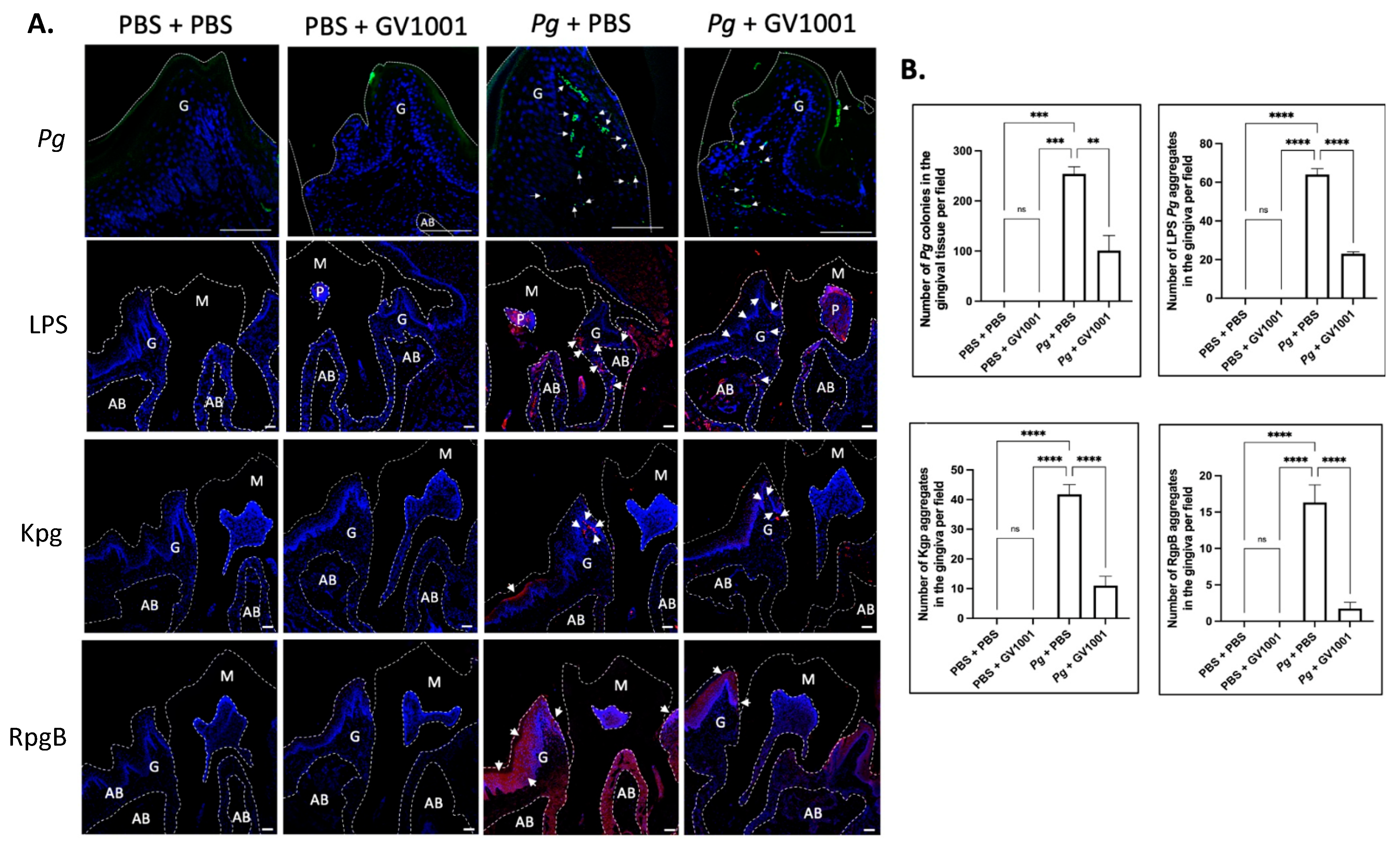
2.4. GV1001 Reduced the Number of Pg DNA Aggregates and the Aggregates of Pg LPS and Gingipains in the Gingival Tissues
2.5. Systemic GV1001 Administration Significantly Inhibited the Alveolar Bone Loss Induced by Topical Pg Inoculation into the Gingival Pocket
2.6. Pg Inoculation Increased the Number of Osteoclasts in the Alveolar Bone, Which GV1001 Significantly Inhibited
2.7. GV1001 Inhibited Osteoclastogenesis In Vitro
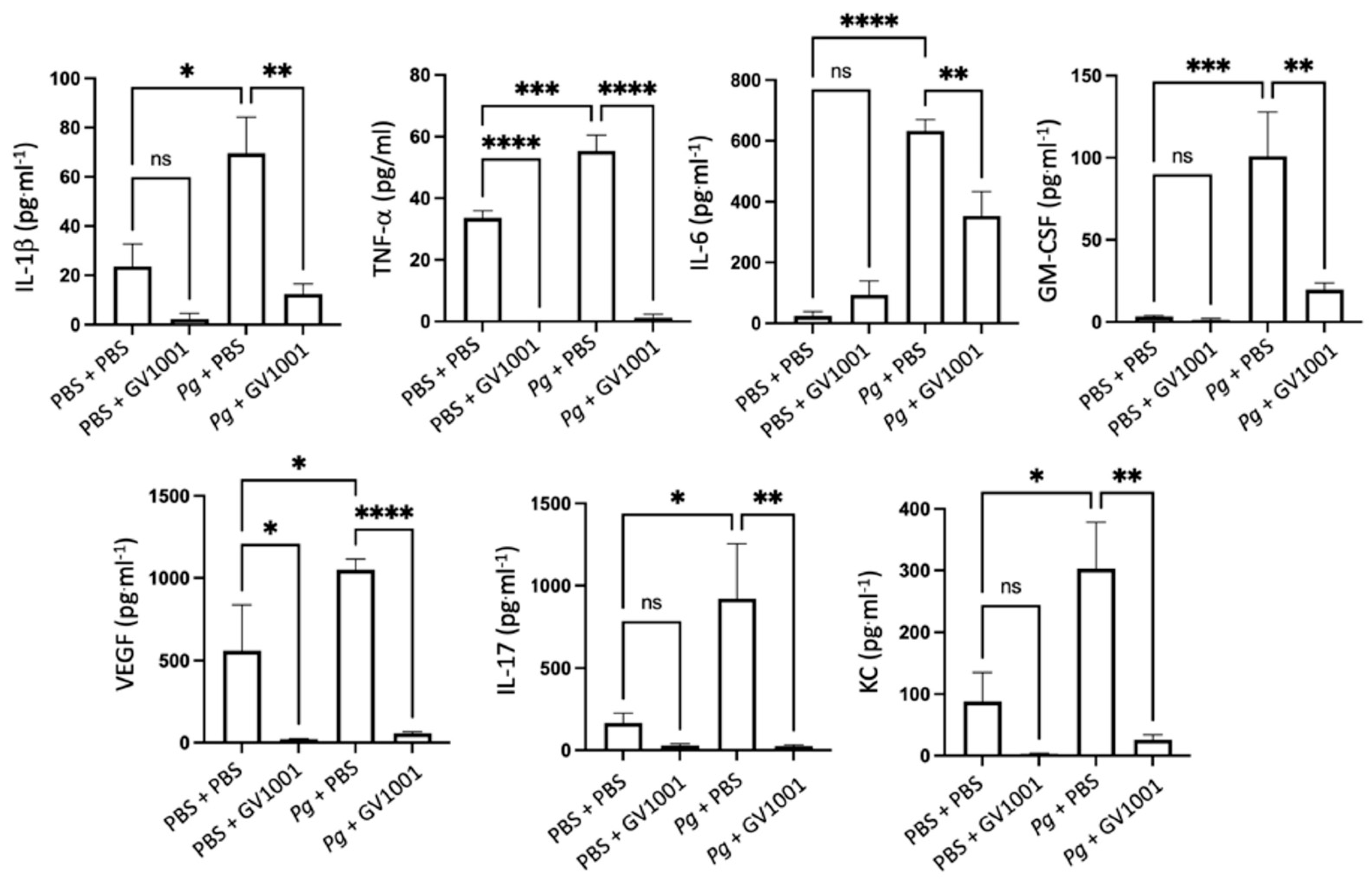
2.8. GV1001 Inhibited Pg-Induced Systemic Inflammation by Suppressing Proinflammatory Cytokine Levels in Serum
2.9. GV1001 Inhibits the Pg-Induced Vascular Inflammation
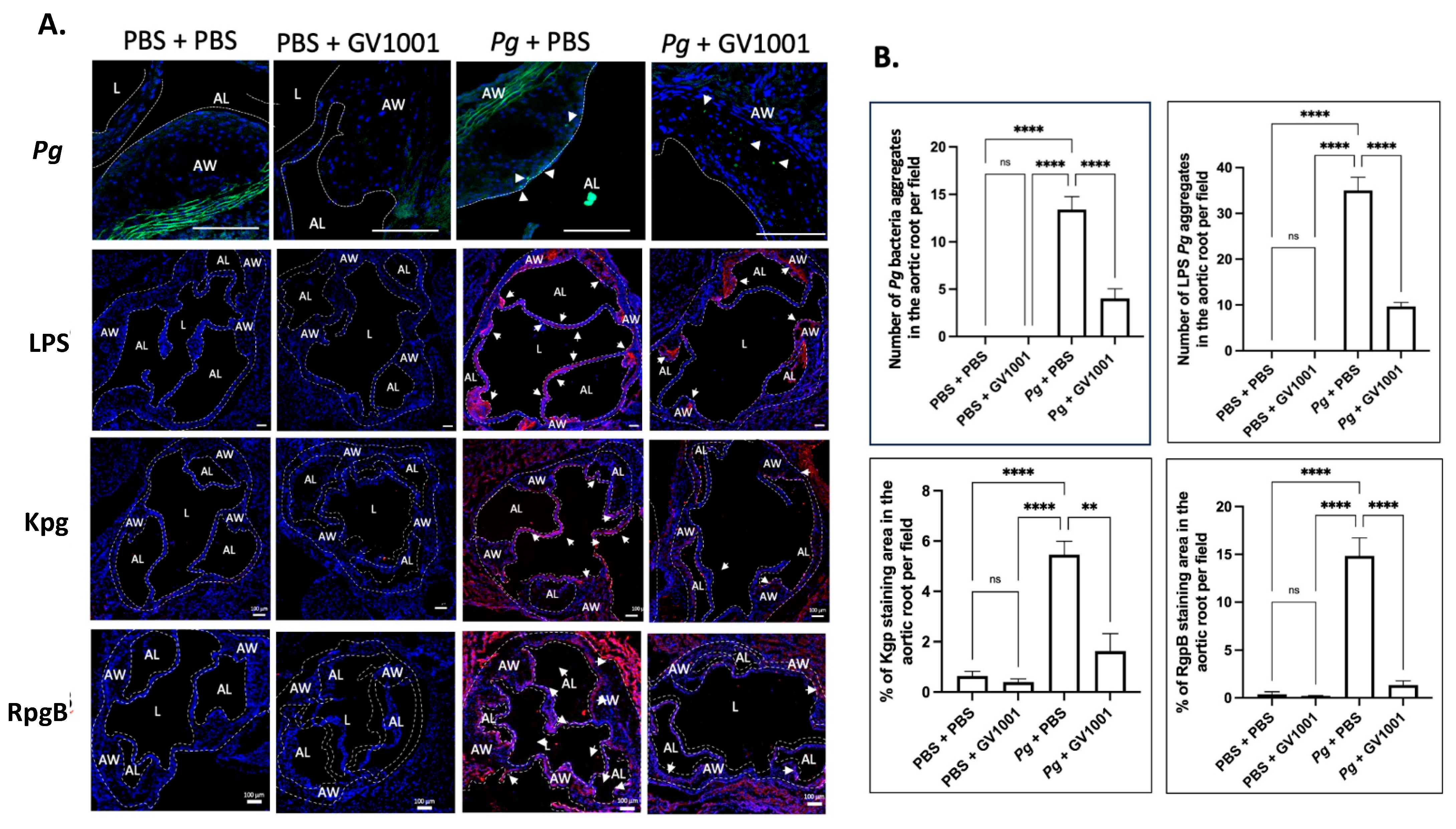
2.10. GV1001 Reduced the Aggregation Number of Pg DNA, Pg LPS, and Gingipains in the Arterial Wall
2.11. GV1001 Altered the Cholesterol Profiles in Serum: Lowering Total Cholesterol (TC) and Low-Density Lipoprotein (LDL) Levels
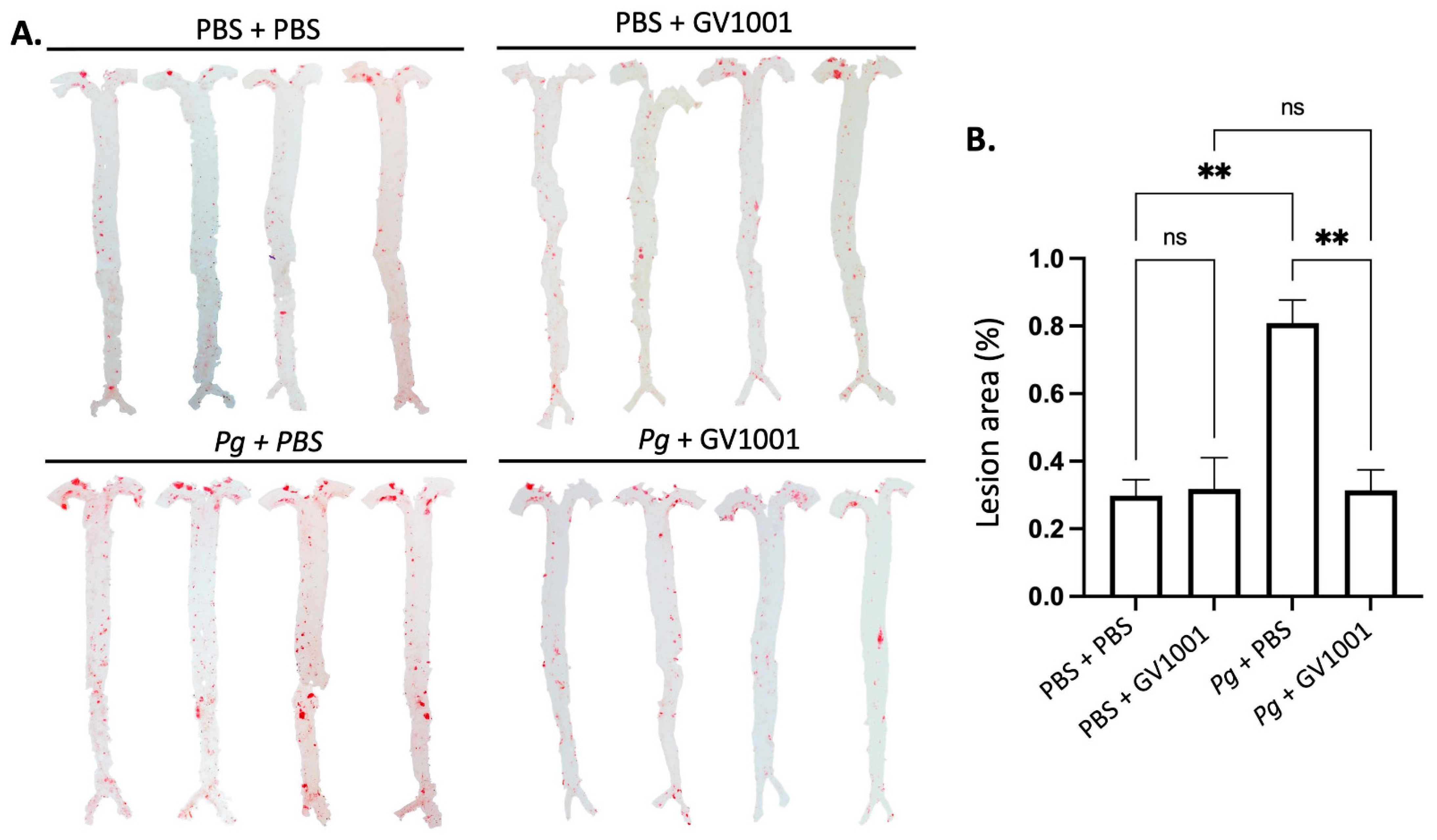
2.12. GV1001 Completely Blocked the Pg-Induced Enhanced Lipid Deposition (Atherosclerosis) in the Arterial Wall
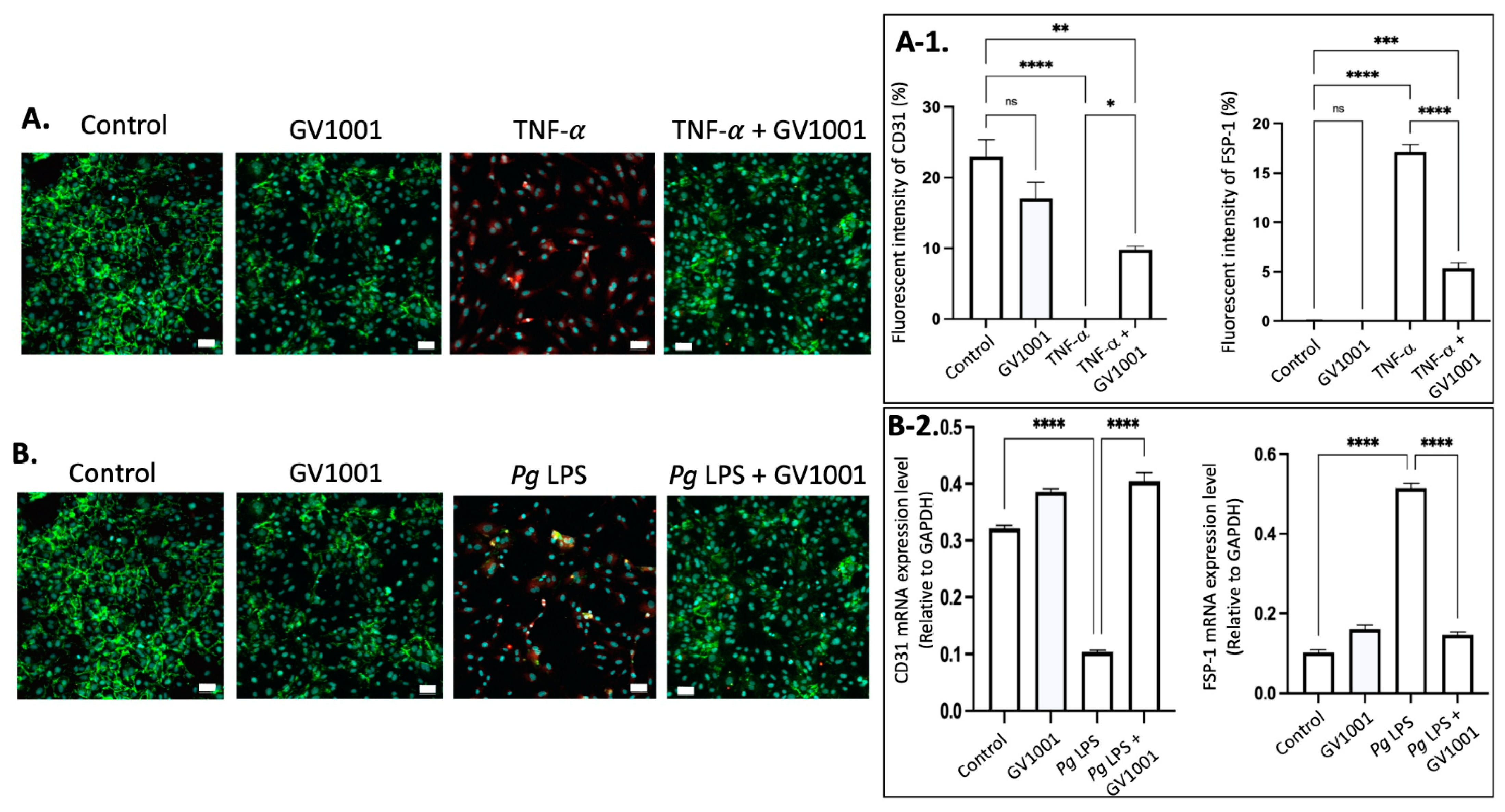
2.13. GV1001 Inhibited the Endothelial–Mesenchymal Transition (EndMT) Induced by TNF- and Pg LPS
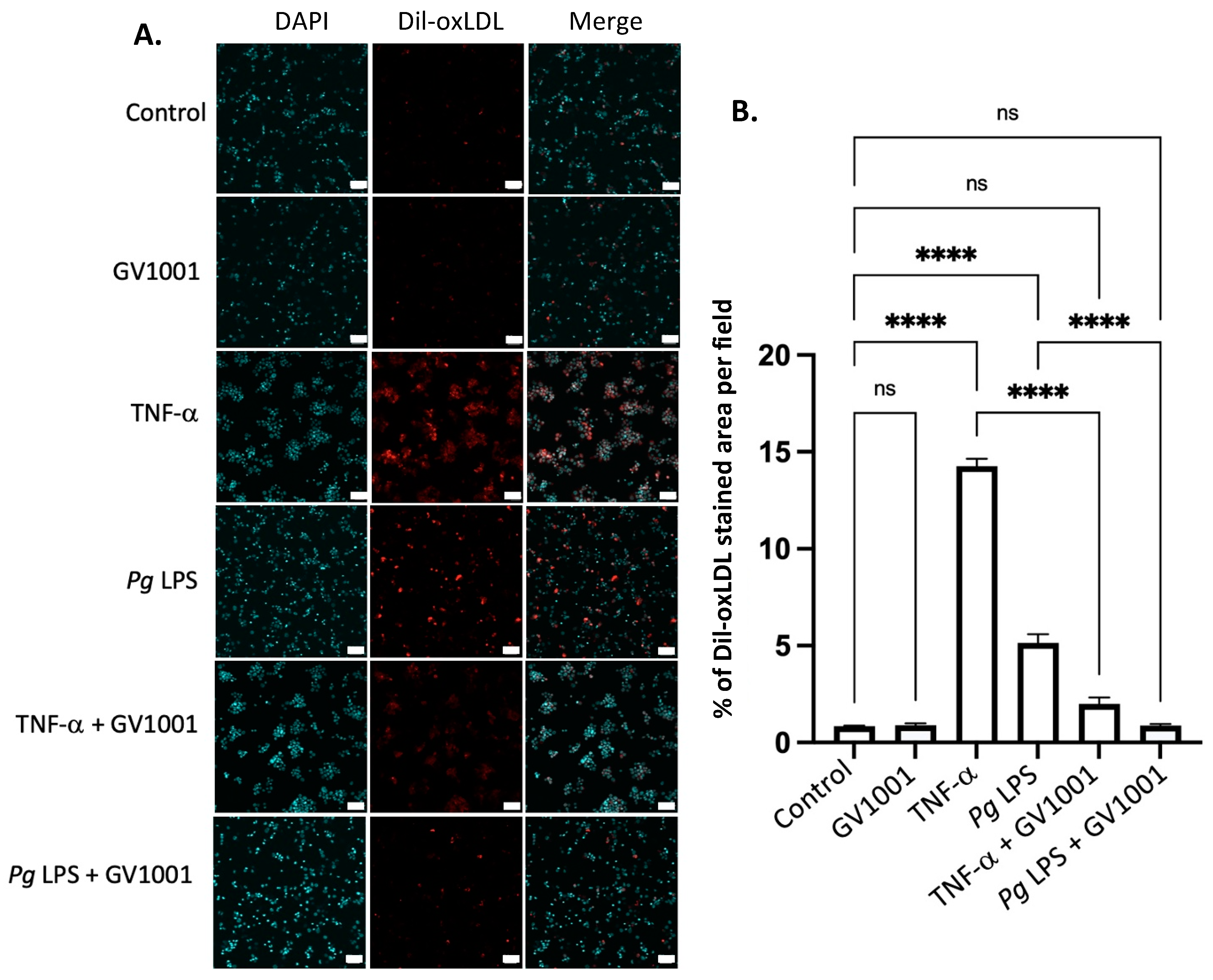
2.14. GV1001 Inhibited the Uptake of Dil-Ox-LDL by THP-1 Macrophages
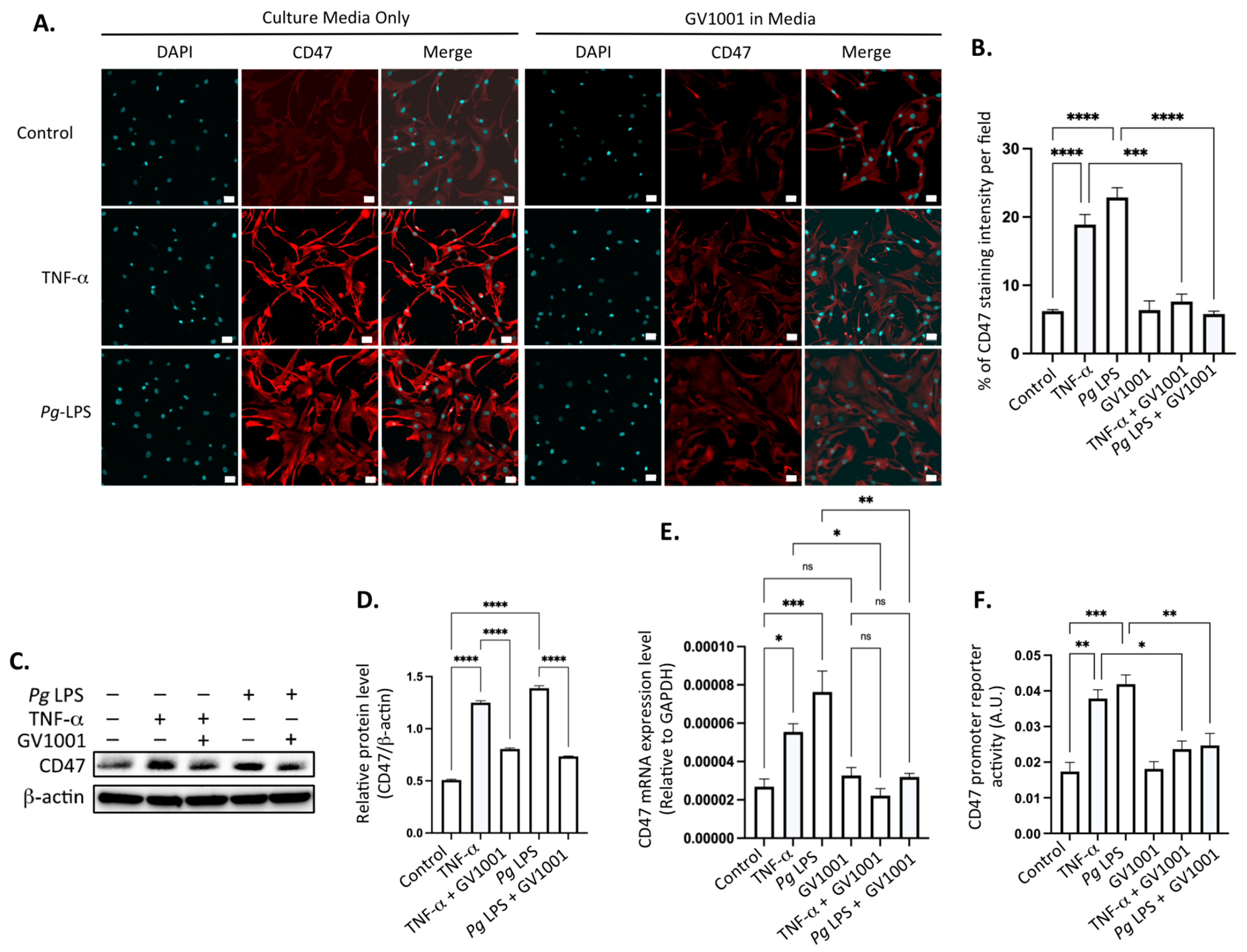
2.15. Pg-Induced Periodontal Disease Increased the Level of the Cluster of Differentiation 47 (CD47) in Atherosclerotic Plaques, and GV1001 Reversed the Increase
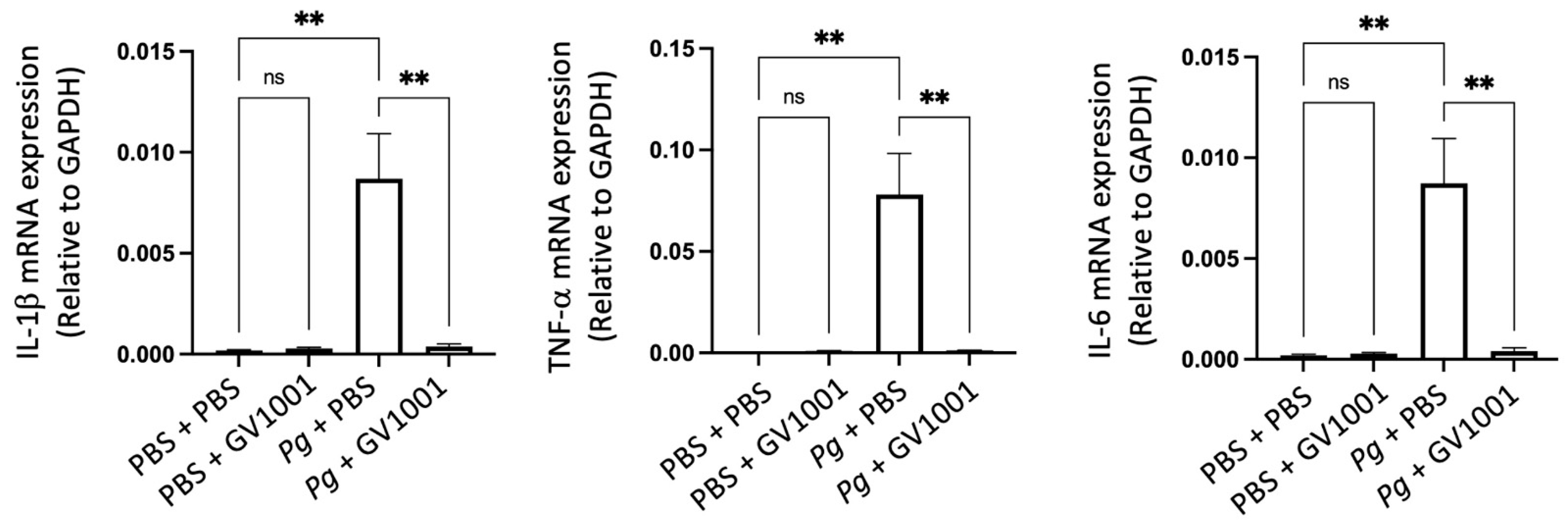
2.16. Pg-Induced Periodontal Disease Induced Neuroinflammation by Increasing the Expression of Proinflammation Cytokines
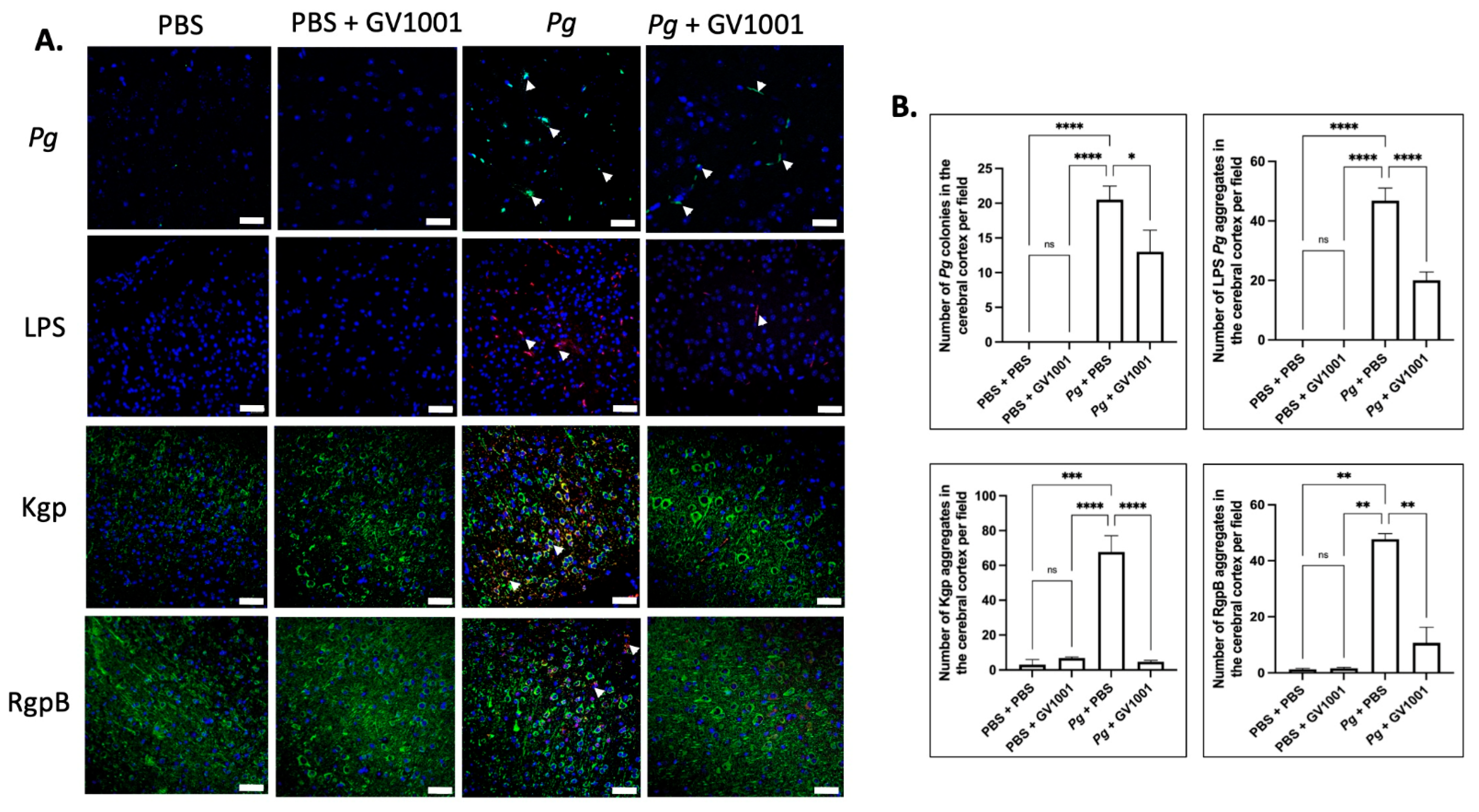
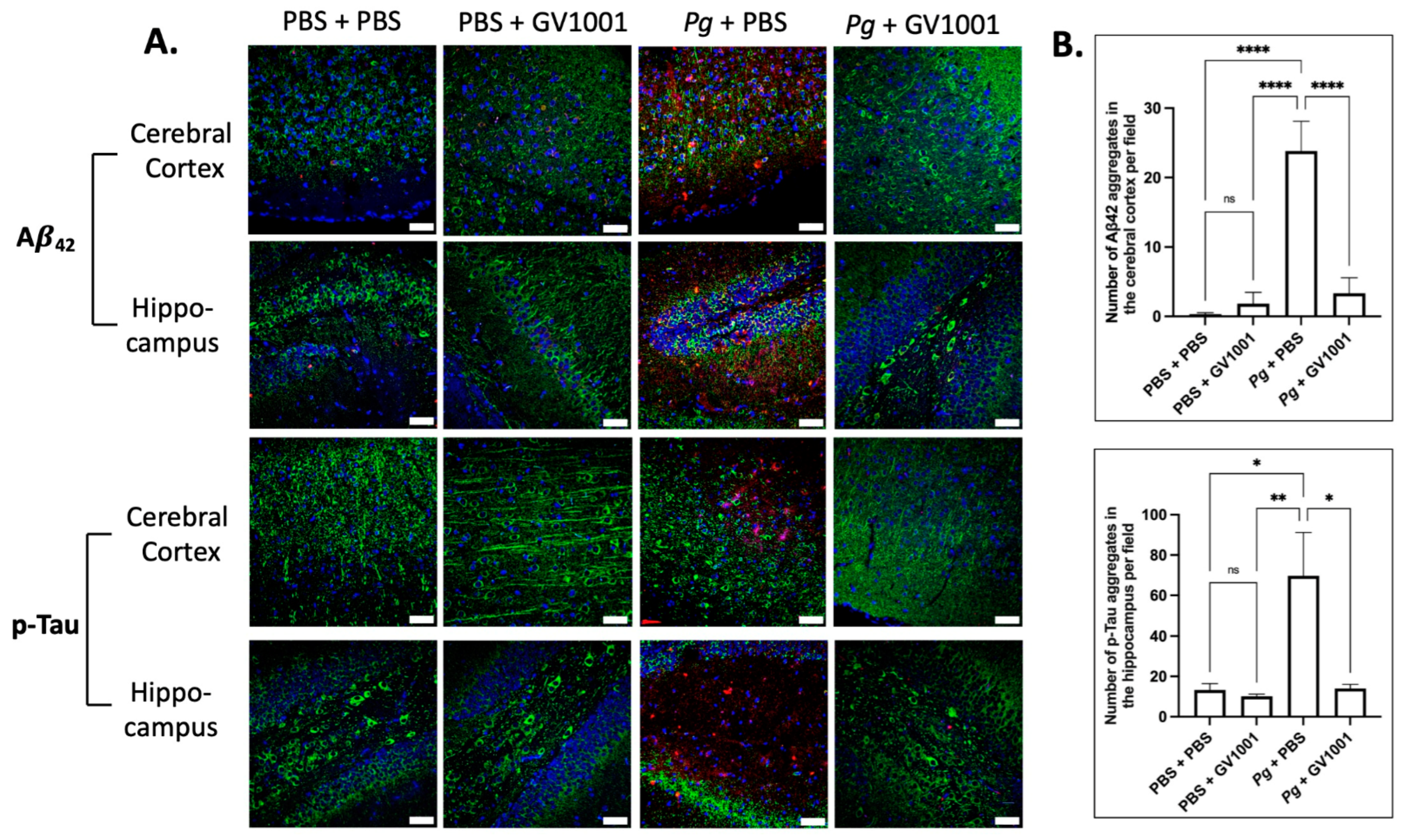
2.17. Pg-Induced Periodontal Disease Induced an Accumulation of Pg DNA, Pg LPS, and Gingipain Aggregates in the Cerebral Cortex and Hippocampus of Mice, Which Were Notably Reduced by Systemic GV1001
2.18. Pg-Induced Periodontal Disease Induced an Accumulation of A42 and p-Tau in the Brain, and GV1001 Notably Decreased the Accumulation
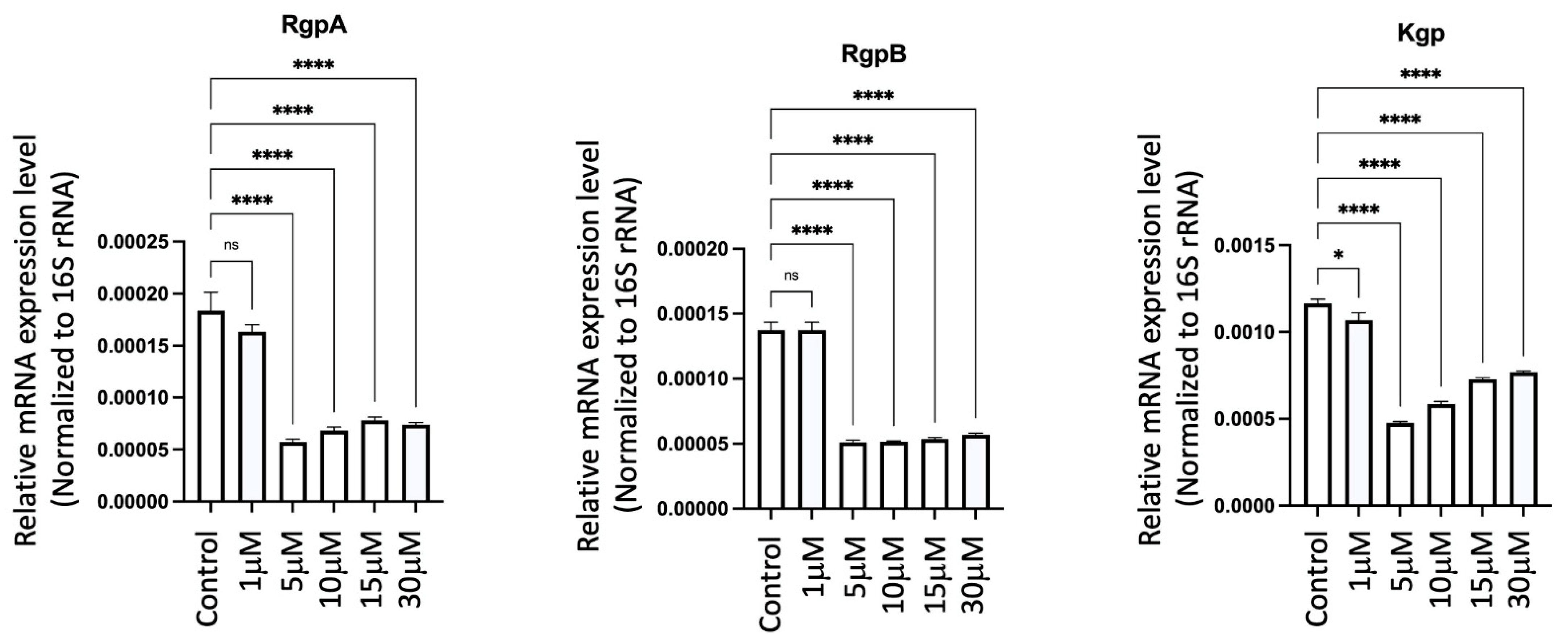
2.19. GV1001 Significantly Inhibited the Expression of Pg Gingipains
3. Discussion
4. Materials and Methods
4.1. Overall Experimental Design and Its Rationale for Evaluating the Preventive Effect of GV1001 on the Development of Pg-Induced Periodontal Disease in ApoE-Deficient Mice
4.2. Animals and Animal Welfare
4.3. Creation of Gingival Pocket to Retain Topically Applied PBS or Bacteria in the Pocket and Groups of Animals
4.4. Preparation of PBS Containing 1% Methyl Cellulose, Culture of Pg and Topical Inoculation of PBS or Pg Directly into the Gingival Pocket, and the sc Injection of PBS or GV1001
4.5. Sample and Tissue Collections
4.6. Frozen Sectioning
4.7. Detection of Pg DNA Aggregates from the Gingival Pocket
4.8. Micro-Computed Tomography (μCT) and Histological Analysis of Maxillae
4.9. Histological and Immunofluorescence Analysis
4.10. Osteoclastogenesis Assay
4.11. Antibody Production for Pg Kgp and RgpB
4.12. Serum Lipid and Proinflammatory Cytokines Measurement in Mouse Serum
4.13. Quantitative Real-Time Polymerase Chain Reaction
4.14. Fluorescence In Situ Hybridization (FISH) Analysis for the Detection of Pg DNA Aggregates in the Gingival, Aortic Wall, and Brain Tissue
4.15. En Face Analysis
4.16. Cell Culture and Reagents
4.17. Induction of EndMT
4.18. Western Blotting
4.19. CD47 Promoter Activity Assay
4.20. Oil O Red Staining
4.21. Determination of the Effect of GV1001 on the Gingipain Expression in Pg
4.22. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Kim, S.; Kim, B.J.; Kim, I.; Kim, J.H.; Kim, H.K.; Ryu, H.; Choi, D.R.; Hwang, I.G.; Song, H.; Kwon, J.H.; et al. A phase II study of chemotherapy in combination with telomerase peptide vaccine (GV1001) as second-line treatment in patients with metastatic colorectal cancer. J. Cancer 2022, 13, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tian, Y.; Sun, F.; Lei, G.; Cheng, J.; Tian, C.; Yu, H.; Deng, Z.; Lu, S.; Wang, L.; et al. A Recombinant Oncolytic Influenza Virus Carrying GV1001 Triggers an Antitumor Immune Response. Hum. Gene Ther. 2024, 35, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Inderberg-Suso, E.M.; Trachsel, S.; Lislerud, K.; Rasmussen, A.M.; Gaudernack, G. Widespread CD4+ T-cell reactivity to novel hTERT epitopes following vaccination of cancer patients with a single hTERT peptide GV1001. Oncoimmunology 2012, 1, 670–686. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Seo, E.H.; Lee, S.H.; Kim, B.J. The Telomerase-Derived Anticancer Peptide Vaccine GV1001 as an Extracellular Heat Shock Protein-Mediated Cell-Penetrating Peptide. Int. J. Mol. Sci. 2016, 17, 2054. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Shin, K.H.; Kim, S.; Shon, W.J.; Kim, R.H.; Park, N.H.; Kang, M.K. hTERT peptide fragment GV1001 demonstrates radioprotective and antifibrotic effects through suppression of TGF-beta signaling. Int. J. Mol. Med. 2018, 41, 3211–3220. [Google Scholar] [PubMed]
- Lee, S.A.; Kim, J.; Sim, J.; Kim, S.G.; Kook, Y.H.; Park, C.G.; Kim, H.R.; Kim, B.J. A telomerase-derived peptide regulates reactive oxygen species and hepatitis C virus RNA replication in HCV-infected cells via heat shock protein 90. Biochem. Biophys. Res. Commun. 2016, 471, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.Y.; Yan, J.J.; Yang, J. Protective effect of peptide GV1001 against renal ischemia-reperfusion injury in mice. Transplant. Proc. 2014, 46, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.E.; Kim, H.J.; Jheon, S.; Lim, C. Protective effects of GV1001 on myocardial ischemia-reperfusion injury. Mol. Med. Rep. 2017, 16, 7315–7320. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.J.; Kwon, K.Y.; Kum, K.Y.; Lee, W.C.; Baek, S.H.; Kang, M.K.; Shon, W.J. The Anti-Inflammatory Effect of Human Telomerase-Derived Peptide on P. gingivalis Lipopolysaccharide-Induced Inflammatory Cytokine Production and Its Mechanism in Human Dental Pulp Cells. Mediat. Inflamm. 2015, 2015, 385127. [Google Scholar] [CrossRef]
- Choi, J.; Kim, H.; Kim, Y.; Jang, M.; Jeon, J.; Hwang, Y.I.; Shon, W.J.; Song, Y.W.; Kang, J.S.; Lee, W.J. The Anti-inflammatory Effect of GV1001 Mediated by the Downregulation of ENO1-induced Pro-inflammatory Cytokine Production. Immune Netw. 2015, 15, 291–303. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, Y.J.; Kim, S.; Momeni, M.; Lee, A.; Treanor, A.; Kim, S.; Kim, R.H.; Park, N.H. GV1001 Inhibits the Severity of the Ligature-Induced Periodontitis and the Vascular Lipid Deposition Associated with the Periodontitis in Mice. Int. J. Mol. Sci. 2023, 24, 12566. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kwon, H.S.; Lee, K.-Y.; Kim, Y.E.; Son, J.-W.; Choi, N.-Y.; Lee, E.J.; Han, M.-H.; Park, D.W.; Kim, S.; et al. GV1001 modulates neuroinflammation and improves memory behavior theorugh the activation of gonodotropin-releasing hormone receptors in a triple trnasgenic Alzheimer’s disease mouse model. Brain Behav. Immun. 2024, 115, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.H.; Kwon, H.S.; Choi, S.H.; Jeong, J.H.; Na, H.R.; Lee, C.N.; Yang, Y.; Lee, A.Y.; Lee, J.H.; Park, K.W.; et al. Efficacy and safety of GV1001 in patients with moderate-to-severe Alzheimer’s disease already receiving donepezil: A phase 2 randomized, double-blind, placebo-controlled, multicenter clinical trial. Alzheimers Res. Ther. 2021, 13, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Fi, C.; Wo, W. Periodontal disease and systemic diseases: An overview on recent progresses. J. Biol. Regul. Homeost. Agents 2021, 35, 1–9. [Google Scholar] [PubMed]
- Shahoumi, L.A.; Saleh, M.H.A.; Meghil, M.M. Virulence Factors of the Periodontal Pathogens: Tools to Evade the Host Immune Response and Promote Carcinogenesis. Microorganisms 2023, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Shazam, H.; Shaikh, F.; Hussain, Z. Bone Turnover Markers in Chronic Periodontitis: A Literature Review. Cureus 2020, 12, e6699. [Google Scholar] [CrossRef]
- Sanz, M.; Marco Del Castillo, A.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and cardiovascular diseases: Consensus report. J. Clin. Periodontol. 2020, 47, 268–288. [Google Scholar] [CrossRef]
- Nijakowski, K.; Gruszczynski, D.; Kolasinska, J.; Kopala, D.; Surdacka, A. Periodontal Disease in Patients with Psoriasis: A Systematic Review. Int. J. Environ. Res. Public. Health 2022, 19, 11302. [Google Scholar] [CrossRef]
- Ciurea, A.; Rednic, N.V.; Soanca, A.; Micu, I.C.; Stanomir, A.; Onet, D.; Surlin, P.; Filipescu, I.; Roman, A.; Stratul, S.I.; et al. Current Perspectives on Periodontitis in Systemic Sclerosis: Associative Relationships, Pathogenic Links, and Best Practices. Diagnostics 2023, 13, 841–855. [Google Scholar] [CrossRef]
- Tiensripojamarn, N.; Lertpimonchai, A.; Tavedhikul, K.; Udomsak, A.; Vathesatogkit, P.; Sritara, P.; Charatkulangkun, O. Periodontitis is associated with cardiovascular diseases: A 13-year study. J. Clin. Periodontol. 2021, 48, 348–356. [Google Scholar] [CrossRef]
- Wereszczynski, M.; Smigiel, A.; Tomaszewska, I.; Niedzwienska, A. Investigating the relationship between periodontitis and specific memory processes in the search for cognitive markers of Alzheimer’s disease risk. Sci. Rep. 2023, 13, 11555–11568. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.S.; Kim, S.Y.J.; Lee, S.H.; Kim, R.H.; Park, N.H. Hyperlipidemia is necessary for the initiation and progression of atherosclerosis by severe periodontitis in mice. Mol. Med. Rep. 2022, 26, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.S.; Kim, S.; Bostrom, K.I.; Wang, C.Y.; Kim, R.H.; Park, N.H. Periodontitis-induced systemic inflammation exacerbates atherosclerosis partly via endothelial-mesenchymal transition in mice. Int. J. Oral Sci. 2019, 11, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Bando, Y.; Chang, C.; Kwon, J.; Tarverti, B.; Kim, D.; Lee, S.H.; Ton-That, H.; Kim, R.; Nara, P.L.; et al. Topical application of Porphyromonas gingivalis into the gingival pocket in mice leads to chronic-active infection, periodontitis and systemic inflammation. Int. J. Mol. Med. 2022, 50, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Darveau, R.P. Periodontitis: A polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 2010, 8, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Wang, M.; Liang, S. Induction of distinct TLR2-mediated proinflammatory and proadhesive signaling pathways in response to Porphyromonas gingivalis fimbriae. J. Immunol. 2009, 182, 6690–6696. [Google Scholar] [CrossRef] [PubMed]
- Takii, R.; Kadowaki, T.; Baba, A.; Tsukuba, T.; Yamamoto, K. A functional virulence complex composed of gingipains, adhesins, and lipopolysaccharide shows high affinity to host cells and matrix proteins and escapes recognition by host immune systems. Infect. Immun. 2005, 73, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Hiyari, S.; Atti, E.; Camargo, P.M.; Eskin, E.; Lusis, A.J.; Tetradis, S.; Pirih, F.Q. Heritability of periodontal bone loss in mice. J. Periodontal Res. 2015, 50, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Desta, T.; Raptis, M.; Darveau, R.P.; Graves, D.T. P. gingivalis and E. coli lipopolysaccharides exhibit different systemic but similar local induction of inflammatory markers. J. Periodontol. 2008, 79, 1241–1247. [Google Scholar] [CrossRef]
- Suh, J.S.; Lee, S.H.; Fouladian, Z.; Lee, J.Y.; Kim, T.; Kang, M.K.; Lusis, A.J.; Bostrom, K.I.; Kim, R.H.; Park, N.H. Rosuvastatin Prevents the Exacerbation of Atherosclerosis in Ligature-Induced Periodontal Disease Mouse Model. Sci. Rep. 2020, 10, 6383–6399. [Google Scholar] [CrossRef]
- O’Brien-Simpson, N.M.; Paolini, R.A.; Hoffmann, B.; Slakeski, N.; Dashper, S.G.; Reynolds, E.C. Role of RgpA, RgpB, and Kgp proteinases in virulence of Porphyromonas gingivalis W50 in a murine lesion model. Infect. Immun. 2001, 69, 7527–7534. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T. The role of gingipains in the pathogenesis of periodontal disease. J. Periodontol. 2003, 74, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, R.E.; Wijeyewickrema, L.C.; Pike, R.N. The gingipains: Scissors and glue of the periodontal pathogen, Porphyromonas gingivalis. Future Microbiol. 2009, 4, 471–487. [Google Scholar] [CrossRef]
- Grenier, D.; Roy, S.; Chandad, F.; Plamondon, P.; Yoshioka, M.; Nakayama, K.; Mayrand, D. Effect of inactivation of the Arg- and/or Lys-gingipain gene on selected virulence and physiological properties of Porphyromonas gingivalis. Infect. Immun. 2003, 71, 4742–4748. [Google Scholar] [CrossRef] [PubMed]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Diaz, P.I. Porphyromonas gingivalis: Immune subversion activities and role in periodontal dysbiosis. Curr. Oral Health Rep. 2020, 7, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.; Cui, Z.Y.; Huang, X.F.; Zhang, D.D.; Guo, R.J.; Han, M. Inflammation and atherosclerosis: Signaling pathways and therapeutic intervention. Signal Transduct. Target. Ther. 2022, 7, 131–153. [Google Scholar] [CrossRef]
- Sanchez-Duffhues, G.; Garcia de Vinuesa, A.; van de Pol, V.; Geerts, M.E.; de Vries, M.R.; Janson, S.G.; van Dam, H.; Lindeman, J.H.; Goumans, M.J.; Ten Dijke, P. Inflammation induces endothelial-to-mesenchymal transition and promotes vascular calcification through downregulation of BMPR2. J. Pathol. 2019, 247, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Xie, S.; Hong, D.; Ding, Y. An invitro model of foam cell formation induced by a stretchable microfluidic device. Sci. Rep. 2019, 9, 7461–7471. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, H. Targeting the Cancer Biomarker CD47: A Review on the Diverse Mechanisms of the CD47 Pathway in Cancer Treatment. Anticancer Agents Med. Chem. 2016, 16, 658–667. [Google Scholar] [CrossRef]
- Matlung, H.L.; Szilagyi, K.; Barclay, N.A.; van den Berg, T.K. The CD47-SIRPalpha signaling axis as an innate immune checkpoint in cancer. Immunol. Rev. 2017, 276, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Grootaert, M.O.J.; Bennett, M.R. Vascular smooth muscle cells in atherosclerosis: Time for a re-assessment. Cardiovasc. Res. 2021, 117, 2326–2339. [Google Scholar] [CrossRef] [PubMed]
- Jonasson, L.; Holm, J.; Skalli, O.; Bondjers, G.; Hansson, G.K. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis 1986, 6, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [PubMed]
- Kanagasingam, S.; Chukkapalli, S.S.; Welbury, R.; Singhrao, S.K. Porphyromonas gingivalis is a Strong Risk Factor for Alzheimer’s Disease. J. Alzheimers Dis. Rep. 2020, 4, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Hocevar, K.; Vizovisek, M.; Wong, A.; Koziel, J.; Fonovic, M.; Potempa, B.; Lamont, R.J.; Potempa, J.; Turk, B. Proteolysis of Gingival Keratinocyte Cell Surface Proteins by Gingipains Secreted From Porphyromonas gingivalis—Proteomic Insights Into Mechanisms Behind Tissue Damage in the Diseased Gingiva. Front. Microbiol. 2020, 11, 722–734. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Shiotsu, N.; Uchida-Fukuhara, Y.; Guo, J.; Weng, Y.; Ikegame, M.; Wang, Z.; Ono, K.; Kamioka, H.; Torii, Y.; et al. Outer membrane vesicles derived from Porphyromonas gingivalis induced cell death with disruption of tight junctions in human lung epithelial cells. Arch. Oral Biol. 2020, 118, 104841–104847. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Choi, Y.S.; Choi, Y. Bacterial invasion and persistence: Critical events in the pathogenesis of periodontitis? J. Periodontal Res. 2015, 50, 570–585. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef]
- Xie, H.; Qin, Z.; Ling, Z.; Ge, X.; Zhang, H.; Guo, S.; Liu, L.; Zheng, K.; Jiang, H.; Xu, R. Oral pathogen aggravates atherosclerosis by inducing smooth muscle cell apoptosis and repressing macrophage efferocytosis. Int. J. Oral Sci. 2023, 15, 26–40. [Google Scholar] [CrossRef]
- Kojima, Y.; Volkmer, J.P.; McKenna, K.; Civelek, M.; Lusis, A.J.; Miller, C.L.; Direnzo, D.; Nanda, V.; Ye, J.; Connolly, A.J.; et al. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature 2016, 536, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.; Thoudam, B.D.; Thokchom, N.; Khumukcham, S.; Sajjan, A.; Ponnan, S. Comparative evaluation of serum high-density lipoprotein and low-density lipoprotein levels and glycated hemoglobin levels and periodontal status in type 2 diabetic patients: A pilot project. J. Oral Maxillofac. Pathol. 2022, 26, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Vicol, C.; Buculei, I.; Melinte, O.E.; Dobrin, M.E.; Stavarache, E.I.; Gavrilescu, C.M.; Postolache, P.; Matei, D.; Trofor, A. The Lipid Profile and Biochemical Parameters of COPD Patients in Relation to Smoking Status. Biomedicines 2022, 10, 2936. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Treatment of Alzheimer’s Disease and Blood-Brain Barrier Drug Delivery. Pharmaceuticals 2020, 13, 394. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, S.; Kadowaki, T.; Nakanishi, H. Secreted gingipains from Porphyromonas gingivalis increase permeability in human cerebral microvascular endothelial cells through intracellular degradation of tight junction proteins. Neurochem. Int. 2022, 154, 105282–105291. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.M.; Hadjichrysanthou, C.; Evans, S.; Wong, M.M. Why do so many clinical trials of therapies for Alzheimer’s disease fail? Lancet 2017, 390, 2327–2329. [Google Scholar] [CrossRef] [PubMed]
- Schott, J.M.; Aisen, P.S.; Cummings, J.L.; Howard, R.J.; Fox, N.C. Unsuccessful trials of therapies for Alzheimer’s disease. Lancet 2019, 393, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Park, H.H.; Lee, K.Y.; Park, D.W.; Choi, N.Y.; Lee, Y.J.; Son, J.W.; Kim, S.; Moon, C.; Kim, H.W.; Rhyu, I.J.; et al. Tracking and protection of transplanted stem cells using a ferrocenecarboxylic acid-conjugated peptide that mimics hTERT. Biomaterials 2018, 155, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Binder, T.A.; Goodson, J.M.; Socransky, S.S. Gingival fluid levels of acid and alkaline phosphatase. J. Periodontal Res. 1987, 22, 14–19. [Google Scholar] [CrossRef]
- Lalla, E.; Lamster, I.B.; Feit, M.; Huang, L.; Schmidt, A.M. A murine model of accelerated periodontal disease in diabetes. J. Periodontal Res. 1998, 33, 387–399. [Google Scholar] [CrossRef]
- Graves, D.T.; Kang, J.; Andriankaja, O.; Wada, K.; Rossa, C., Jr. Animal models to study host-bacteria interactions involved in periodontitis. Front. Oral Biol. 2012, 15, 117–132. [Google Scholar] [PubMed]
- Baker, P.J.; Evans, R.T.; Roopenian, D.C. Oral infection with Porphyromonas gingivalis and induced alveolar bone loss in immunocompetent and severe combined immunodeficient mice. Arch. Oral Biol. 1994, 39, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Bainbridge, B.; Verma, R.K.; Eastman, C.; Yehia, B.; Rivera, M.; Moffatt, C.; Bhattacharyya, I.; Lamont, R.J.; Kesavalu, L. Role of Porphyromonas gingivalis phosphoserine phosphatase enzyme SerB in inflammation, immune response, and induction of alveolar bone resorption in rats. Infect. Immun. 2010, 78, 4560–4569. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.J.; Dixon, M.; Evans, R.T.; Roopenian, D.C. Heterogeneity of Porphyromonas gingivalis strains in the induction of alveolar bone loss in mice. Oral Microbiol. Immunol. 2000, 15, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, A.L.; Abd-El-Aleem, S.; Morales-Aza, B.; Donaldson, L.F. A model of periodontitis in the rat: Effect of lipopolysaccharide on bone resorption, osteoclast activity, and local peptidergic innervation. J. Clin. Periodontol. 2004, 31, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Nishida, E.; Hara, Y.; Kaneko, T.; Ikeda, Y.; Ukai, T.; Kato, I. Bone resorption and local interleukin-1alpha and interleukin-1beta synthesis induced by Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis lipopolysaccharide. J. Periodontal Res. 2001, 36, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, J.; Girnary, M.S.; Jing, L.; Miao, M.Z.; Zhang, S.; Sun, L.; Morelli, T.; Schoenfisch, M.H.; Inohara, N.; Offenbacher, S.; et al. An experimental murine model to study periodontitis. Nat. Protoc. 2018, 13, 2247–2267. [Google Scholar] [CrossRef]
- Kimura, S.; Nagai, A.; Onitsuka, T.; Koga, T.; Fujiwara, T.; Kaya, H.; Hamada, S. Induction of experimental periodontitis in mice with Porphyromonas gingivalis-adhered ligatures. J. Periodontol. 2000, 71, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Velsko, I.M.; Chukkapalli, S.S.; Rivera, M.F.; Lee, J.Y.; Chen, H.; Zheng, D.; Bhattacharyya, I.; Gangula, P.R.; Lucas, A.R.; Kesavalu, L. Active invasion of oral and aortic tissues by Porphyromonas gingivalis in mice causally links periodontitis and atherosclerosis. PLoS ONE 2014, 9, e97811. [Google Scholar] [CrossRef]
- Rivera, M.F.; Lee, J.Y.; Aneja, M.; Goswami, V.; Liu, L.; Velsko, I.M.; Chukkapalli, S.S.; Bhattacharyya, I.; Chen, H.; Lucas, A.R.; et al. Polymicrobial infection with major periodontal pathogens induced periodontal disease and aortic atherosclerosis in hyperlipidemic ApoE(null) mice. PLoS ONE 2013, 8, e57178. [Google Scholar] [CrossRef]
- Sunde, P.T.; Olsen, I.; Gobel, U.B.; Theegarten, D.; Winter, S.; Debelian, G.J.; Tronstad, L.; Moter, A. Fluorescence in situ hybridization (FISH) for direct visualization of bacteria in periapical lesions of asymptomatic root-filled teeth. Microbiology 2003, 149, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Alshaikh, A.; Kim, S.; Kim, J.; Chun, C.; Mehrazarin, S.; Lee, J.; Lux, R.; Kim, R.H.; Shin, K.H.; et al. Porphyromonas gingivalis Impairs Oral Epithelial Barrier through Targeting GRHL2. J. Dent. Res. 2019, 98, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Tangirala, R.K.; Rubin, E.M.; Palinski, W. Quantitation of atherosclerosis in murine models: Correlation between lesions in the aortic origin and in the entire aorta, and differences in the extent of lesions between sexes in LDL receptor-deficient and apolipoprotein E-deficient mice. J. Lipid Res. 1995, 36, 2320–2328. [Google Scholar] [CrossRef]
- Zhang, F.; Xia, X.; Chai, R.; Xu, R.; Xu, Q.; Liu, M.; Chen, X.; Liu, B.; Liu, S.; Liu, N. Inhibition of USP14 suppresses the formation of foam cell by promoting CD36 degradation. J. Cell Mol. Med. 2020, 24, 3292–3302. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Kim, S.Y.; Lee, A.; Kim, Y.-J.; Chang, C.; Ton-That, H.; Kim, R.; Kim, S.; Park, N.-H. hTERT Peptide Fragment GV1001 Prevents the Development of Porphyromonas gingivalis-Induced Periodontal Disease and Systemic Disorders in ApoE-Deficient Mice. Int. J. Mol. Sci. 2024, 25, 6126. https://doi.org/10.3390/ijms25116126
Chen W, Kim SY, Lee A, Kim Y-J, Chang C, Ton-That H, Kim R, Kim S, Park N-H. hTERT Peptide Fragment GV1001 Prevents the Development of Porphyromonas gingivalis-Induced Periodontal Disease and Systemic Disorders in ApoE-Deficient Mice. International Journal of Molecular Sciences. 2024; 25(11):6126. https://doi.org/10.3390/ijms25116126
Chicago/Turabian StyleChen, Wei, Sharon Y. Kim, Alicia Lee, Yun-Jeong Kim, Chungyu Chang, Hung Ton-That, Reuben Kim, Sangjae Kim, and No-Hee Park. 2024. "hTERT Peptide Fragment GV1001 Prevents the Development of Porphyromonas gingivalis-Induced Periodontal Disease and Systemic Disorders in ApoE-Deficient Mice" International Journal of Molecular Sciences 25, no. 11: 6126. https://doi.org/10.3390/ijms25116126
APA StyleChen, W., Kim, S. Y., Lee, A., Kim, Y.-J., Chang, C., Ton-That, H., Kim, R., Kim, S., & Park, N.-H. (2024). hTERT Peptide Fragment GV1001 Prevents the Development of Porphyromonas gingivalis-Induced Periodontal Disease and Systemic Disorders in ApoE-Deficient Mice. International Journal of Molecular Sciences, 25(11), 6126. https://doi.org/10.3390/ijms25116126





