In Vitro Cell Culture Model for Osteoclast Activation during Estrogen Withdrawal
Abstract
:1. Introduction
2. Results
2.1. Continuous Estrogen (E2) Treatment during Differentiation Reduced Multinucleated Osteoclast Formation and Resorption That Was Reversed Following E2 Withdrawal (E2-WD)
2.2. The Effects of Estrogen on Osteoclast-Specific Gene Expression In Vitro
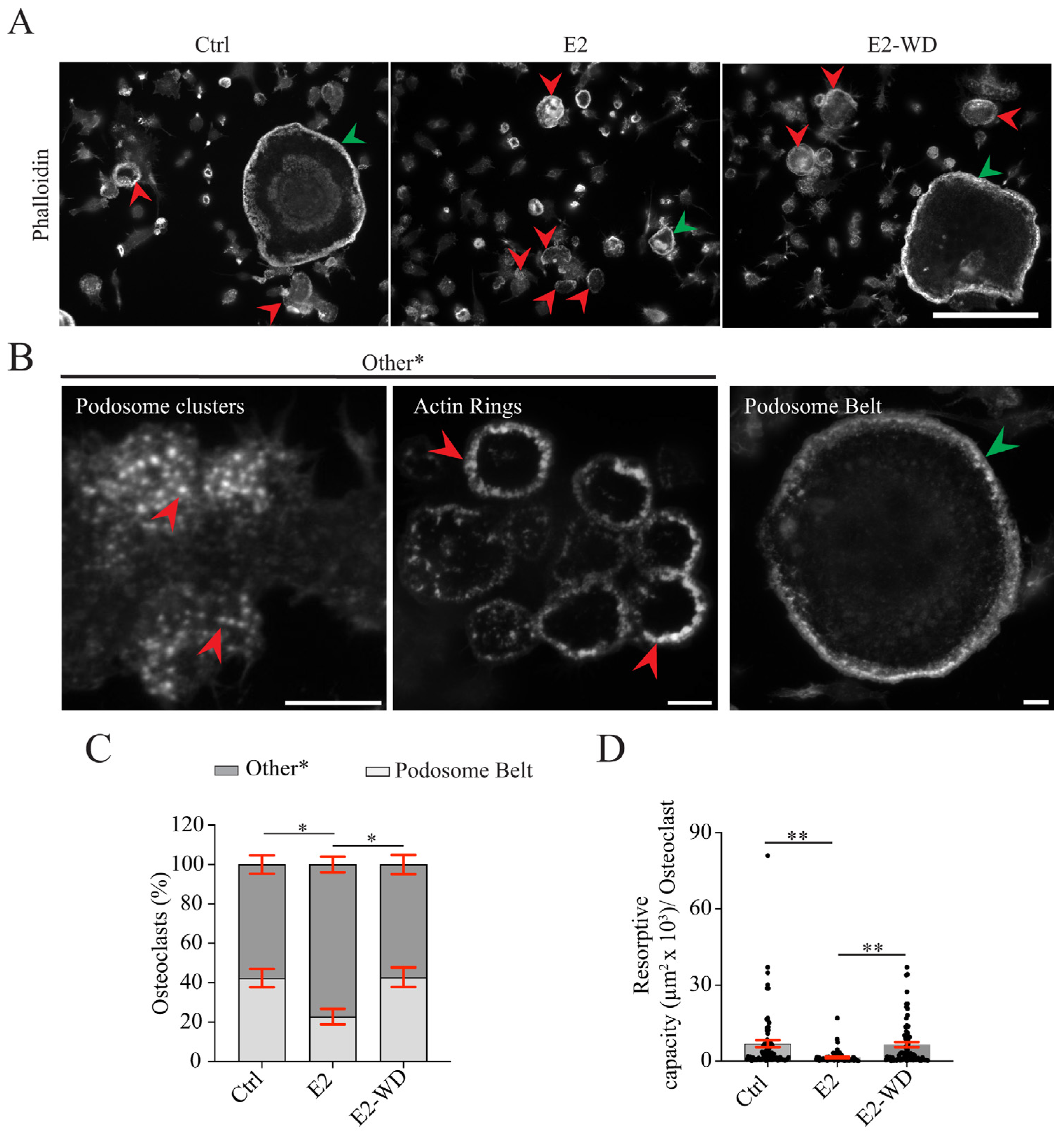
2.3. E2-WD Shortened Podosome Lifespan and Enhanced Podosome Belt Formation/Stability in Osteoclasts
2.4. Osteoclasts under E2-WD Had Lower Levels of Active RhoA Compared to Those Continuously Exposed to E2
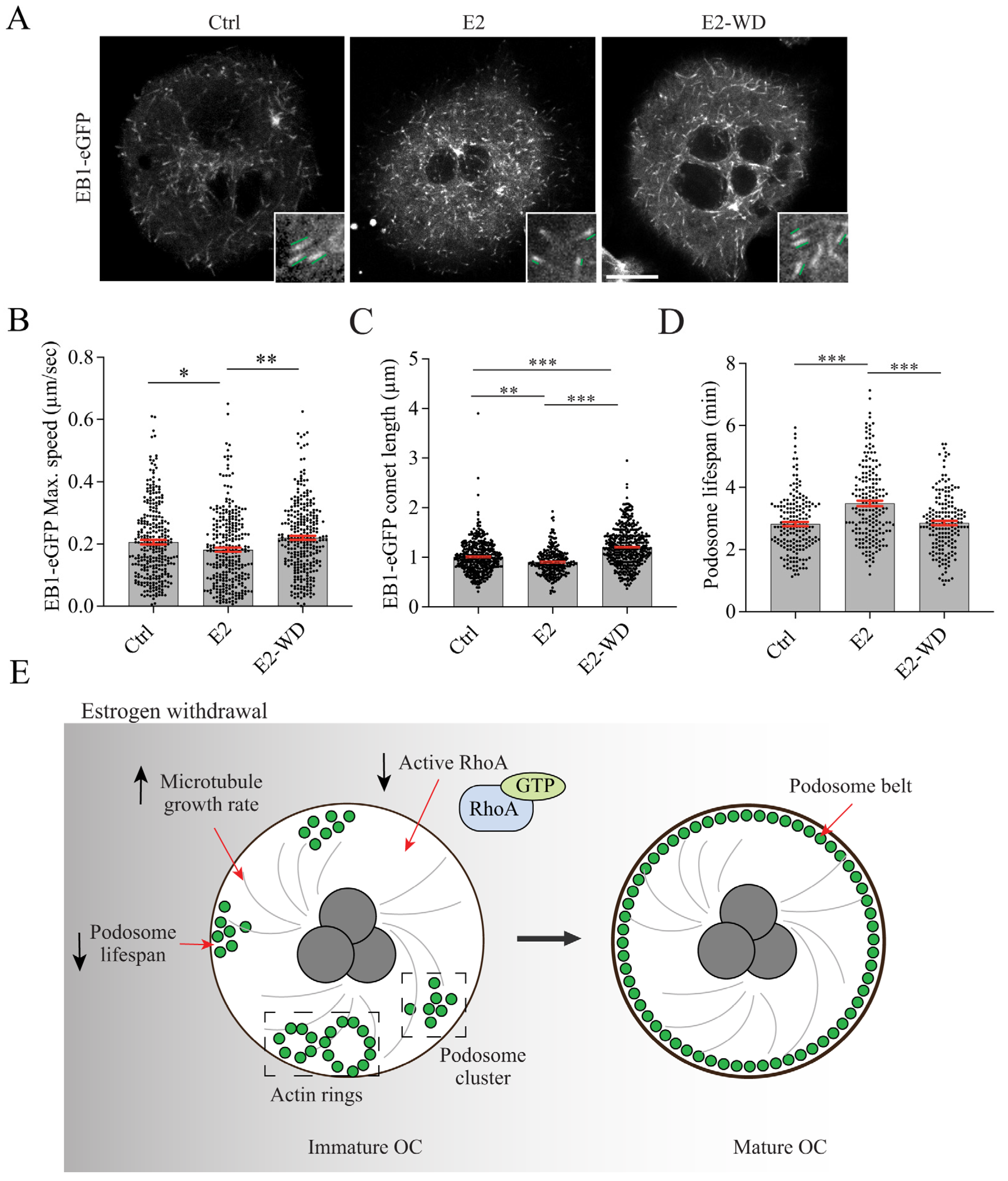
2.5. Effects of Estrogen on Microtubule (MT) Growth and Podosome Lifespan in Osteoclasts
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. RAW 264.7 Cell Culture and Osteoclast Differentiation
4.3. Immunofluorescence and Fixed Cell Imaging
4.4. Biomimetic Calcium Phosphate Substrate, Osteoclast Culture, and Resorption Pit Analysis
4.5. Quantitative RT-PCR
4.6. RhoA Activation Assay
4.7. EB1-GFP Transfection, Live Cell Imaging, and Analysis
4.8. Measuring Podosome Lifespan
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Florencio-Silva, R.; Sasso, G.R.D.S.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Res. Int. 2015, 421746. [Google Scholar] [CrossRef]
- Kenkre, J.S.; Bassett, J.H.D. The bone remodelling cycle. Ann. Clin. Biochem. 2018, 55, 308–327. [Google Scholar] [CrossRef]
- Salari, N.; Ghasemi, H.; Mohammadi, L.; Behzadi, M.H.; Rabieenia, E.; Shohaimi, S.; Mohammadi, M. The global prevalence of osteoporosis in the world: A comprehensive systematic review and meta-analysis. J. Orthop. Surg. Res. 2021, 16, 609. [Google Scholar] [CrossRef]
- Raisz, L.G. Science in medicine Pathogenesis of osteoporosis: Concepts, conflicts, and prospects. J. Clin. Investig. 2005, 115, 3318–3325. [Google Scholar] [CrossRef]
- Garnero, P.; Sornay-Rendu, E.; Chapuy, M.C.; Delmas, P.D. Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J. Bone Miner. Res. 1996, 11, 337–349. [Google Scholar] [CrossRef]
- Cui, J.; Shen, Y.; Li, R. Estrogen synthesis and signaling pathways during aging: From periphery to brain. Trends Mol. Med. 2013, 19, 197–209. [Google Scholar] [CrossRef]
- Simpson, E.; Rubin, G.; Clyne, C.; Robertson, K.; O’Donnell, L.; Jones, M.; Davis, S. The Role of Local Estrogen Biosynthesis in Males and Females. Trends Endocrinol. Metab. 2000, 11, 184–188. [Google Scholar] [CrossRef]
- Purohit, A.; Flanagan, A.M.; Reed, M.J. Estrogen synthesis by osteoblast cell lines. Endocrinology 1992, 131, 2027–2029. [Google Scholar] [CrossRef]
- Janssen, J.M.; Bland, R.; Hewison, M.; Coughtrie, M.W.; Sharp, S.; Arts, J.; Pols, H.A.; van Leeuwen, J.P. Estradiol formation by human osteoblasts via multiple pathways: Relation with osteoblast function. J. Cell. Biochem. 1999, 75, 528–537. [Google Scholar] [CrossRef]
- Eyre, L.J.; Bland, R.; Bujalska, I.J.; Sheppard, M.C.; Stewart, P.M.; Hewison, M. Characterization of aromatase and 17β-hydroxysteroid dehydrogenase expression in rat osteoblastic cells. J. Bone Miner. Res. 1998, 13, 996–1004. [Google Scholar] [CrossRef]
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. In Advances in Protein Chemistry and Structural Biology; Academic Press Inc.: Cambridge, MA, USA, 2019; Volume 116, pp. 135–170. [Google Scholar] [CrossRef]
- Björnström, L.; Sjöberg, M. Mechanisms of Estrogen Receptor Signaling: Convergence of Genomic and Nongenomic Actions on Target Genes. Mol. Endocrinol. 2005, 19, 833–842. [Google Scholar] [CrossRef]
- Safe, S.; Kim, K. Non-Classical Genomic Estrogen Receptor (ER)/Specificity Protein and ER/Activating Protein-1 Signaling Pathways. J. Mol. Endocrinol. 2008, 41, 263–275. [Google Scholar] [CrossRef]
- Parry, T.; Ledee, D.; Willis, M.S.; Portman, M.A. Nuclear Receptors and the Adaptive Response of the Heart. In Endocrinology of the Heart in Health and Disease: Integrated, Cellular, and Molecular Endocrinology of the Heart; Academic Press: Cambridge, MA, USA, 2017; pp. 249–284. [Google Scholar] [CrossRef]
- Feng, Y.; Gregor, P. Cloning of a Novel Member of the G Protein-Coupled Receptor Family Related to Peptide Receptors 1. Biochem. Biophys. Res. Commun. 1997, 231, 651–654. [Google Scholar] [CrossRef]
- Filardo, E.J.; Quinn, J.A.; Bland, K.I.; Frackelton, A.R.; Williams, R. Estrogen-Induced Activation of Erk-1 and Erk-2 Requires the G Protein-Coupled Receptor Homolog, GPR30, and Occurs via Trans-Activation of the Epidermal Growth Factor Receptor through Release of HB-EGF. Mol. Endo-Crinol. 2000, 14, 1649–1660. [Google Scholar] [CrossRef]
- Weitzmann, M.N.; Pacifici, R. Estrogen deficiency and bone loss: An inflammatory tale. J. Clin. Investig. 2006, 116, 1186–1194. [Google Scholar] [CrossRef]
- Nakamura, T.; Imai, Y.; Matsumoto, T.; Sato, S.; Takeuchi, K.; Igarashi, K.; Harada, Y.; Azuma, Y.; Krust, A.; Yamamoto, Y.; et al. Estrogen Prevents Bone Loss via Estrogen Receptor α and Induction of Fas Ligand in Osteoclasts. Cell 2007, 130, 811–823. [Google Scholar] [CrossRef]
- Martin-Millan, M.; Almeida, M.; Ambrogini, E.; Han, L.; Zhao, H.; Weinstein, R.S.; Jilka, R.L.; O’Brien, C.A.; Manolagas, S.C. The estrogen receptor-α in osteoclasts mediates the protective effects of estrogens on cancellous but not cortical bone. Mol. Endocrinol. 2010, 24, 323–334. [Google Scholar] [CrossRef]
- Kim, H.N.; Ponte, F.; Nookaew, I.; Ucer Ozgurel, S.; Marques-Carvalho, A.; Iyer, S.; Warren, A.; Aykin-Burns, N.; Krager, K.; Sardao, V.A.; et al. Estrogens decrease osteoclast number by attenuating mitochondria oxidative phosphorylation and ATP production in early osteoclast precursors. Sci. Rep. 2020, 10, 11933. [Google Scholar] [CrossRef]
- Kameda, T.; Mano, H.; Yuasa, T.; Mori, Y.; Miyazawa, K.; Shiokawa, M.; Nakamaru, Y.; Hiroi, E.; Hiura, K.; Kameda, A.; et al. Estrogen inhibits bone resorption by directly inducing apoptosis of the bone-resorbing osteoclasts. J. Exp. Med. 1997, 186, 489–495. [Google Scholar] [CrossRef]
- Hughes, D.E.; Dai, A.; Tiffee, J.C.; Li, H.H.; Mundy, G.R.; Boyce, B.F. Estrogen promotes apoptosis of murine osteoclasts mediated by TGF–β. Nat. Med. 1996, 2, 1132–1136. [Google Scholar] [CrossRef]
- Shevde, N.K.; Bendixen, A.C.; Dienger, K.M.; Pike, J.W. Estrogens suppress RANK ligand-induced osteoclast differentiation via a stromal cell independent mechanism involving c-Jun repression. Proc. Natl. Acad. Sci. USA 2000, 97, 7829–7834. [Google Scholar] [CrossRef]
- Srivastava, S.; Toraldo, G.; Weitzmann, M.N.; Cenci, S.; Ross, F.P.; Pacifici, R. Estrogen Decreases Osteoclast Formation by Down-regulating Receptor Activator of NF-κB Ligand (RANKL)-induced JNK Activation. J. Biol. Chem. 2001, 276, 8836–8840. [Google Scholar] [CrossRef]
- Palacios, V.G.; Robinson, L.J.; Borysenko, C.W.; Lehmann, T.; Kalla, S.E.; Blair, H.C. Negative regulation of RANKL-induced osteoclastic differentiation in RAW264.7 cells by estrogen and phytoestrogens. J. Biol. Chem. 2005, 280, 13720–13727. [Google Scholar] [CrossRef]
- Saintier, D.; Khanine, V.; Uzan, B.; Ea, H.K.; de Vernejoul, M.C.; Cohen-Solal, M.E. Estradiol inhibits adhesion and promotes apoptosis in murine osteoclasts in vitro. J. Steroid Biochem. Mol. Biol. 2006, 99, 165–173. [Google Scholar] [CrossRef]
- Gavali, S.; Gupta, M.K.; Daswani, B.; Wani, M.R.; Sirdeshmukh, R.; Khatkhatay, M.I. LYN, a key mediator in estrogen-dependent suppression of osteoclast differentiation, survival, and function. Biochim. Et Biophys. Acta-Mol. Basis Dis. 2019, 1865, 547–557. [Google Scholar] [CrossRef]
- Park, H.J.; Gholam-Zadeh, M.; Yoon, S.Y.; Suh, J.H.; Choi, H.S. Estrogen decreases cytoskeletal organization by forming an erα/shp2/c-src complex in osteoclasts to protect against ovariectomy-induced bone loss in mice. Antioxidants 2021, 10, 619. [Google Scholar] [CrossRef]
- Streicher, C.; Heyny, A.; Andrukhova, O.; Haigl, B.; Slavic, S.; Schüler, C.; Kollmann, K.; Kantner, I.; Sexl, V.; Kleiter, M.; et al. Estrogen Regulates Bone Turnover by Targeting RANKL Expression in Bone Lining Cells. Sci. Rep. 2017, 7, 6460. [Google Scholar] [CrossRef]
- Li, J.Y.; Tawfeek, H.; Bedi, B.; Yang, X.; Adams, J.; Gao, K.Y.; Zayzafoon, M.; Weitzmann, M.N.; Pacifici, R. Ovariectomy disregulates osteoblast and osteoclast formation through the T-cell receptor CD40 ligand. Proc. Natl. Acad. Sci. USA 2011, 108, 768–773. [Google Scholar] [CrossRef]
- Geoghegan, I.P.; McNamara, L.M.; Hoey, D.A. Estrogen withdrawal alters cytoskeletal and primary ciliary dynamics resulting in increased Hedgehog and osteoclastogenic paracrine signalling in osteocytes. Sci. Rep. 2021, 11, 9272. [Google Scholar] [CrossRef]
- Brennan, M.; Haugh, M.; O’Brien, F.; McNamara, L. Estrogen Withdrawal from Osteoblasts and Osteocytes Causes Increased Mineralization and Apoptosis. Horm. Metab. Res. 2014, 46, 537–545. [Google Scholar] [CrossRef]
- Hsu, H.; Lacey, D.L.; Dunstan, C.R.; Solovyev, I.; Colombero, A.; Timms, E.; Tan, H.L.; Elliott, G.; Kelley, M.J.; Sarosi, I.; et al. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc. Natl. Acad. Sci. USA 1999, 96, 3540–3545. [Google Scholar] [CrossRef]
- Geoghegan, I.P.; Hoey, D.A.; McNamara, L.M. Estrogen deficiency impairs integrin αvβ3-mediated mechanosensation by osteocytes and alters osteoclastogenic paracrine signalling. Sci. Rep. 2019, 9, 4654. [Google Scholar] [CrossRef]
- Fiorino, C.; Harrison, R.E. E-cadherin is important for cell differentiation during osteoclastogenesis. Bone 2016, 86, 106–118. [Google Scholar] [CrossRef]
- Jeganathan, S.; Fiorino, C.; Naik, U.; Sun, H.S.; Harrison, R.E. Modulation of osteoclastogenesis with macrophage M1- and M2-inducing stimuli. PLoS ONE 2014, 9, e104498. [Google Scholar] [CrossRef]
- Vincent, C.; Kogawa, M.; Findlay, D.M.; Atkins, G.J. The generation of osteoclasts from RAW 264.7 precursors in defined, serum-free conditions. J. Bone Miner. Metab. 2009, 27, 114–119. [Google Scholar] [CrossRef]
- Haisenleder, D.J.; Schoenfelder, A.H.; Marcinko, E.S.; Geddis, L.M.; Marshall, J.C. Estimation of estradiol in mouse serum samples: Evaluation of commercial estradiol immunoassays. Endocrinology 2011, 152, 4443–4447. [Google Scholar] [CrossRef]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef]
- Dougall, W.C.; Glaccum, M.; Charrier, K.; Rohrbach, K.; Brasel, K.; De Smedt, T.; Daro, E.; Smith, J.; Tometsko, M.E.; Maliszewski, C.R.; et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999, 13, 2412–2424. [Google Scholar] [CrossRef]
- Jones, D.H.; Kong, Y.-Y.; Penninger, J.M. Role of RANKL and RANK in bone loss and arthritis. Ann. Rheum. Dis. 2002, 61 (Suppl. 2), 32ii–39ii. [Google Scholar] [CrossRef]
- Zhao, C.; Gao, H.; Liu, Y.; Papoutsi, Z.; Jaffrey, S.; Gustafsson, J.-Å.; Dahlman-Wright, K. Genome-Wide Mapping of Estrogen Receptor-β–Binding Regions Reveals Extensive Cross-Talk with Transcription Factor Activator Protein-1. Cancer Res. 2010, 70, 5174–5183. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, N. Signaling Pathways in Osteoclast Differentiation. Chonnam Med. J. 2016, 52, 12. [Google Scholar] [CrossRef] [PubMed]
- Mensah, K.A.; Ritchlin, C.T.; Schwarz, E.M. RANKL induces heterogeneous DC-STAMP lo and DC-STAMP hi osteoclast precursors of which the DC-STAMP lo precursors are the master fusogens. J. Cell. Physiol. 2010, 223, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Miyamoto, T.; Sawatani, Y.; Iwamoto, K.; Hosogane, N.; Fujita, N.; Morita, K.; Ninomiya, K.; Suzuki, T.; Miyamoto, K.; et al. DC-STAMP is essential for cell–cell fusion in osteoclasts and foreign body giant cells. J. Exp. Med. 2005, 202, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Galson, D.L.; Zhao, C.; Peng, L.; Laplace, C.; Wang, K.Z.Q.; Bachler, M.A.; Amano, H.; Aburatani, H.; Ishikawa, H.; et al. Nuclear Factor of Activated T-Cells (NFAT) Rescues Osteoclastogenesis in Precursors Lacking c-Fos. J. Biol. Chem. 2004, 279, 26475–26480. [Google Scholar] [CrossRef] [PubMed]
- Hayman, A. Tartrate-Resistant Acid Phosphatase (TRAP) and the Osteoclast/Immune Cell Dichotomy. Autoimmunity 2008, 41, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Gu, J.; Liu, X.; Zhu, J.; Wang, Q.; Gao, Q.; Zhang, J.; Cheng, L.; Tong, X.; Qi, X.; et al. Inhibition of osteoclast bone resorption activity through osteoprotegerin-induced damage of the sealing zone. Int. J. Mol. Med. 2014, 34, 856–862. [Google Scholar] [CrossRef]
- Takito, J.; Inoue, S.; Nakamura, M. The Sealing Zone in Osteoclasts: A Self-Organized Structure on the Bone. Int. J. Mol. Sci. 2018, 19, 984. [Google Scholar] [CrossRef]
- Georgess, D.; Machuca-Gayet, I.; Blangy, A.; Jurdic, P. Podosome organization drives osteoclast-mediated bone resorption. Cell Adhes. Migr. 2014, 8, 192–204. [Google Scholar] [CrossRef]
- Schachtner, H.; Calaminus, S.D.J.; Thomas, S.G.; Machesky, L.M. Podosomes in adhesion, migration, mechanosensing and matrix remodeling. Cytoskeleton 2013, 70, 572–589. [Google Scholar] [CrossRef]
- Wang, J.; Xu, C.; Zhang, J.; Bao, Y.; Tang, Y.; Lv, X.; Ma, B.; Wu, X.; Mao, G. RhoA promotes osteoclastogenesis and regulates bone remodeling through mTOR-NFATc1 signaling. Mol. Med. 2023, 29, 49. [Google Scholar] [CrossRef]
- Destaing, O.; Saltel, F.; Gilquin, B.; Chabadel, A.; Khochbin, S.; Ory, S.; Jurdic, P. A novel Rho-mDia2-HDAC6 pathway controls podosome patterning through microtubule acetylation in osteoclasts. J. Cell Sci. 2005, 118, 2901–2911. [Google Scholar] [CrossRef] [PubMed]
- Gil-Henn, H.; Destaing, O.; Sims, N.A.; Aoki, K.; Alles, N.; Neff, L.; Sanjay, A.; Bruzzaniti, A.; De Camilli, P.; Baron, R.; et al. Defective microtubule-dependent podosome organization in osteoclasts leads to increased bone density in Pyk2−/− mice. J. Cell Biol. 2007, 178, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Linder, S.; Hufner, K.; Wintergerst, U.; Aepfelbacher, M. Microtubule-dependent formation of podosomal adhesion structures in primary human macrophages. J. Cell Sci. 2000, 113, 4165–4176. [Google Scholar] [CrossRef]
- Batsir, S.; Geiger, B.; Kam, Z. Dynamics of the sealing zone in cultured osteoclasts. Cytoskeleton 2017, 74, 72–81. [Google Scholar] [CrossRef]
- Ti, Y.; Zhou, L.; Wang, R.; Zhao, J. Inhibition of Microtubule Dynamics Affects Podosome Belt Formation during Osteoclast Induction. Cell Biochem. Biophys. 2015, 71, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Biosse Duplan, M.; Zalli, D.; Stephens, S.; Zenger, S.; Neff, L.; Oelkers, J.M.; Lai, F.P.L.; Horne, W.; Rottner, K.; Baron, R. Microtubule Dynamic Instability Controls Podosome Patterning in Osteoclasts through EB1, Cortactin, and Src. Mol. Cell. Biol. 2014, 34, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Vitre, B.; Coquelle, F.M.; Heichette, C.; Garnier, C.; Chrétien, D.; Arnal, I. EB1 regulates microtubule dynamics and tubulin sheet closure in vitro. Nat. Cell Biol. 2008, 10, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Destaing, O.; Saltel, F.; Géminard, J.C.; Jurdic, P.; Bard, F. Podosomes display actin turnover and dynamic self-organization in osteoclasts expressing actin-green fluorescent protein. Mol. Biol. Cell 2003, 14, 407–416. [Google Scholar] [CrossRef]
- Maurin, J.; Morel, A.; Guérit, D.; Cau, J.; Urbach, S.; Blangy, A.; Bompard, G. The Beta-Tubulin Isotype TUBB6 Controls Microtubule and Actin Dynamics in Osteoclasts. Front. Cell Dev. Biol. 2021, 9, 778887. [Google Scholar] [CrossRef]
- Luxenburg, C.; Addadi, L.; Geiger, B. The molecular dynamics of osteoclast adhesions. Eur. J. Cell Biol. 2006, 85, 203–211. [Google Scholar] [CrossRef]
- Chen, F.; Ouyang, Y.; Ye, T.; Ni, B.; Chen, A. Estrogen inhibits RANKL-induced osteoclastic differentiation by increasing the expression of TRPV5 channel. J. Cell. Biochem. 2014, 115, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, C.; Çolakoğlu, M.; Brun, J.; Thouverey, C.; Bonnet, N.; Ferrari, S.; Trajkovski, M. Primary mouse osteoblast and osteoclast culturing and analysis. STAR Protoc. 2021, 2, 100452. [Google Scholar] [CrossRef] [PubMed]
- Quan, J.; Hou, Y.; Long, W.; Ye, S.; Wang, Z. Characterization of different osteoclast phenotypes in the progression of bone invasion by oral squamous cell carcinoma. Oncol. Rep. 2018, 39, 1043–1051. [Google Scholar] [CrossRef]
- Cuetara, B.L.V.; Crotti, T.N.; O’Donoghue, A.J.; McHugh, K.P. Cloning and characterization of osteoclast precursors from the RAW264.7 cell line. Vitr. Cell. Dev. Biol.-Anim. 2006, 42, 182–188. [Google Scholar] [CrossRef]
- Collin-Osdoby, P.; Osdoby, P. RANKL-mediated osteoclast formation from murine RAW 264.7 cells. Methods Mol. Biol. 2012, 816, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Piao, H.; Chu, X.; Lv, W.; Zhao, Y. Involvement of receptor-interacting protein 140 in estrogen-mediated osteoclasts differentiation, apoptosis, and bone resorption. J. Physiol. Sci. 2017, 67, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, H.; Suzuki, T.; Miyauchi, Y.; Iwasaki, R.; Kobayashi, T.; Sato, Y.; Miyamoto, K.; Hoshi, H.; Hashimoto, K.; Yoshida, S.; et al. Osteoclast stimulatory transmembrane protein and dendritic cell-specific transmembrane protein cooperatively modulate cell-cell fusion to form osteoclasts and foreign body giant cells. J. Bone Miner. Res. 2012, 27, 1289–1297. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, L.; Yang, X.; Liu, Q.; Yang, L.; Zheng, C.; Zhao, Y.; Zhang, Z.; Luo, X. 17β-estradiol attenuates ovariectomy-induced bone deterioration through the suppression of the ephA2/ephrinA2 signaling pathway. Mol. Med. Rep. 2018, 17, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Destaing, O.; Sanjay, A.; Itzstein, C.; Horne, W.C.; Toomre, D.; De Camilli, P.; Baron, R. The tyrosine kinase activity of c-Src regulates actin dynamics and organization of podosomes in osteoclasts. Mol. Biol. Cell 2008, 19, 394–404. [Google Scholar] [CrossRef]
- Luxenburg, C.; Parsons, J.T.; Addadi, L.; Geiger, B. Involvement of the Src-cortactin pathway in podosome formation and turnover during polarization of cultured osteoclasts. J. Cell Sci. 2006, 119, 4878–4888. [Google Scholar] [CrossRef]
- Granot-Attas, S.; Luxenburg, C.; Finkelshtein, E.; Elson, A. Protein tyrosine phosphatase epsilon regulates integrin-mediated podosome stability in osteoclasts by activating Src. Mol. Biol. Cell 2009, 20, 4324–4334. [Google Scholar] [CrossRef] [PubMed]
- Jurdic, P.; Saltel, F.; Chabadel, A.; Destaing, O. Podosome and sealing zone: Specificity of the osteoclast model. Eur. J. Cell Biol. 2006, 85, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Simoncini, T.; Scorticati, C.; Mannella, P.; Fadiel, A.; Giretti, M.S.; Fu, X.D.; Baldacci, C.; Garibaldi, S.; Caruso, A.; Fornari, L.; et al. Estrogen receptor α interacts with Gα13 to drive actin remodeling and endothelial cell migration via the RhoA/Rho kinase/moesin pathway. Mol. Endocrinol. 2006, 20, 1756–1771. [Google Scholar] [CrossRef] [PubMed]
- Kramár, E.A.; Chen, L.Y.; Brandon, N.J.; Rex, C.S.; Liu, F.; Gall, C.M.; Lynch, G. Cytoskeletal changes underlie estrogen’s acute effects on synaptic transmission and plasticity. J. Neurosci. 2009, 29, 12982–12993. [Google Scholar] [CrossRef] [PubMed]
- Oviedo, P.J.; Sobrino, A.; Laguna-Fernandez, A.; Novella, S.; Tarín, J.J.; García-Pérez, M.A.; Sanchís, J.; Cano, A.; Hermenegildo, C. Estradiol induces endothelial cell migration and proliferation through estrogen receptor-enhanced RhoA/ROCK pathway. Mol. Cell. Endocrinol. 2011, 335, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Chellaiah, M.A.; Soga, N.; Swanson, S.; McAllister, S.; Alvarez, U.; Wang, D.; Dowdy, S.F.; Hruska, K.A. Rho-A Is Critical for Osteoclast Podosome Organization, Motility, and Bone Resorption. J. Biol. Chem. 2000, 275, 11993–12002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Udagawa, N.; Nakamura, I.; Murakami, H.; Saito, S.; Yamasaki, K.; Shibasaki, Y.; Morii, N.; Narumiya, S.; Takahashi, N.; et al. The small gtp-binding protein, RHO p21, is involved in bone resorption by regulating cytoskeletal organization in osteoclasts. J. Cell Sci. 1995, 108, 2285–2292. [Google Scholar] [CrossRef] [PubMed]
- Nakano, S.; Inoue, K.; Xu, C.; Deng, Z.; Syrovatkina, V.; Vitone, G.; Zhao, L.; Huang, X.Y.; Zhao, B. G-protein Gα 13 functions as a cytoskeletal and mitochondrial regulator to restrain osteoclast function. Sci. Rep. 2019, 9, 4236. [Google Scholar] [CrossRef] [PubMed]
- Lachowski, D.; Cortes, E.; Matellan, C.; Rice, A.; Lee, D.A.; Thorpe, S.D.; del Río Hernández, A.E. G Protein-Coupled Estrogen Receptor Regulates Actin Cytoskeleton Dynamics to Impair Cell Polarization. Front. Cell Dev. Biol. 2020, 8, 592628. [Google Scholar] [CrossRef]
- Cortes, E.; Lachowski, D.; Rice, A.; Thorpe, S.D.; Robinson, B.; Yeldag, G.; Lee, D.A.; Ghemtio, L.; Rombouts, K.; del Río Hernández, A.E. Tamoxifen mechanically deactivates hepatic stellate cells via the G protein-coupled estrogen receptor. Oncogene 2019, 38, 2910–2922. [Google Scholar] [CrossRef]
- Kipp, J.L.; Ramirez, V.D. Estradiol and testosterone have opposite effects on microtubule polymerization. Neuroendocrinology 2003, 77, 258–272. [Google Scholar] [CrossRef]
- Jurášek, M.; Černohorská, M.; Řehulka, J.; Spiwok, V.; Sulimenko, T.; Dráberová, E.; Darmostuk, M.; Gurská, S.; Frydrych, I.; Buriánová, R.; et al. Estradiol dimer inhibits tubulin polymerization and microtubule dynamics. J. Steroid Biochem. Mol. Biol. 2018, 183, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Urazbaev, A.; Serikbaeva, A.; Tvorogova, A.; Dusenbayev, A.; Kauanova, S.; Vorobjev, I. On the Relationship Between EB-3 Profiles and Microtubules Growth in Cultured Cells. Front. Mol. Biosci. 2021, 8, 745089. [Google Scholar] [CrossRef] [PubMed]
- Fong, K.W.; Au, F.K.C.; Jia, Y.; Yang, S.; Zhou, L.; Qi, R.Z. Microtubule plus-end tracking of end-binding protein 1 (EB1) is regulated by CDK5 regulatory subunit-associated protein 2. J. Biol. Chem. 2017, 292, 7675–7687. [Google Scholar] [CrossRef] [PubMed]
- Bieling, P.; Laan, L.; Schek, H.; Munteanu, E.L.; Sandblad, L.; Dogterom, M.; Brunner, D.; Surrey, T. Reconstitution of a microtubule plus-end tracking system in vitro. Nature 2007, 450, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Dixit, R.; Barnett, B.; Lazarus, J.E.; Tokito, M.; Goldman, Y.E.; Holzbaur, E.L.F. Microtubule plus-end tracking by CLIP-170 requires EB1. Proc. Natl. Acad. Sci. USA 2009, 106, 492. [Google Scholar] [CrossRef] [PubMed]
- Galjart, N.; Perez, F. A plus-end raft to control microtubule dynamics and function. Curr. Opin. Cell Biol. 2003, 15, 48–53. [Google Scholar] [CrossRef]
- Gundersen, G.G.; Gomes, E.R.; Wen, Y. Cortical control of microtubule stability and polarization. Curr. Opin. Cell Biol. 2004, 16, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Ory, S.; Destaing, O.; Jurdic, P. Microtubule dynamics differentially regulates Rho and Rac activity and triggers Rho-independent stress fiber formation in macrophage polykaryons. Eur. J. Cell Biol. 2002, 81, 351–362. [Google Scholar] [CrossRef]
- Kopp, P.; Lammers, R.; Aepfelbacher, M.; Woehlke, G.; Rudel, T.; Machuy, N.; Steffen, W.; Linder, S. The kinesin KIF1C and microtubule plus ends regulate podosome dynamics in macrophages. Mol. Biol. Cell 2006, 17, 2811–2823. [Google Scholar] [CrossRef]
- Maria, S.M.; Prukner, C.; Sheikh, Z.; Mueller, F.; Barralet, J.E.; Komarova, S.V. Reproducible quantification of osteoclastic activity: Characterization of a biomimetic calcium phosphate assay. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2014, 102, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Patntirapong, S.; Habibovic, P.; Hauschka, P.V. Effects of soluble cobalt and cobalt incorporated into calcium phosphate layers on osteoclast differentiation and activation. Biomaterials 2009, 30, 548–555. [Google Scholar] [CrossRef] [PubMed]




| DC-STAMP | F-TACGTGGAGAGAAGCAAGGAA | R-ACACTGAGACGTGGTTTAGGAAT |
| NFATc1 | F-CCCGTCACATTCTGGTCCAT | R-CAAGTAACCGTGTAGCTGCACAA |
| RANK | F-CACAGACAAATGCAAACCTTG | R-GTGTTCTGGAACCATCTTCCTCC |
| TRAP | F-ACGGCTACTTGCGGTTTCA | R-TCCTTGGGAGGCTGGTCTT |
| GAPDH | F-AATGAGCCTTCCTCTGCTCT | R-AACTGGCTATTCAGCTGTGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gandhi, N.; Omer, S.; Harrison, R.E. In Vitro Cell Culture Model for Osteoclast Activation during Estrogen Withdrawal. Int. J. Mol. Sci. 2024, 25, 6134. https://doi.org/10.3390/ijms25116134
Gandhi N, Omer S, Harrison RE. In Vitro Cell Culture Model for Osteoclast Activation during Estrogen Withdrawal. International Journal of Molecular Sciences. 2024; 25(11):6134. https://doi.org/10.3390/ijms25116134
Chicago/Turabian StyleGandhi, Nisha, Safia Omer, and Rene E. Harrison. 2024. "In Vitro Cell Culture Model for Osteoclast Activation during Estrogen Withdrawal" International Journal of Molecular Sciences 25, no. 11: 6134. https://doi.org/10.3390/ijms25116134




