Biomineral-Based Composite Materials in Regenerative Medicine
Abstract
:1. Introduction
2. Biominerals
2.1. Definition and Types of Biominerals
2.2. Natural Occurrence and Formation of Biominerals
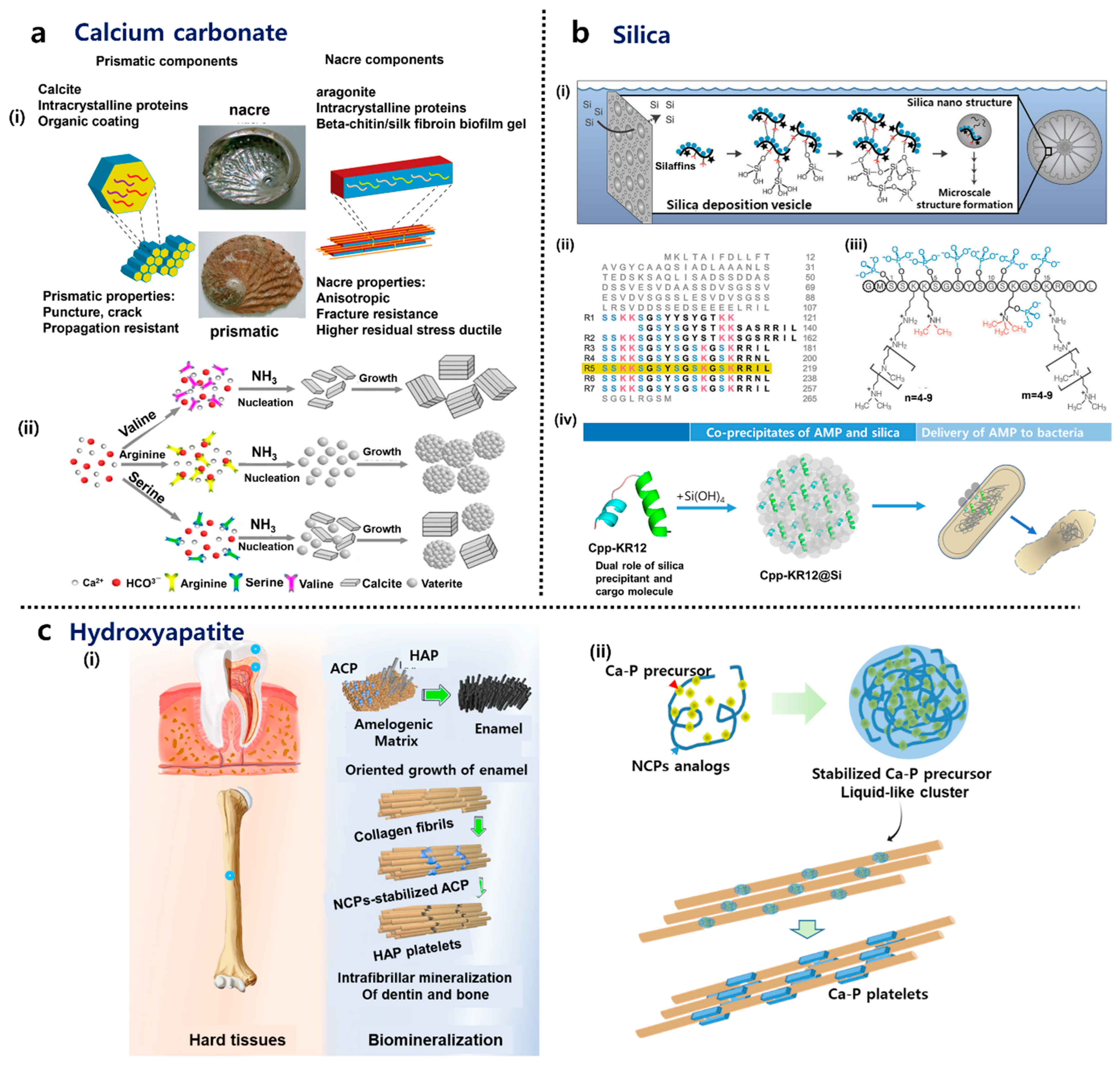
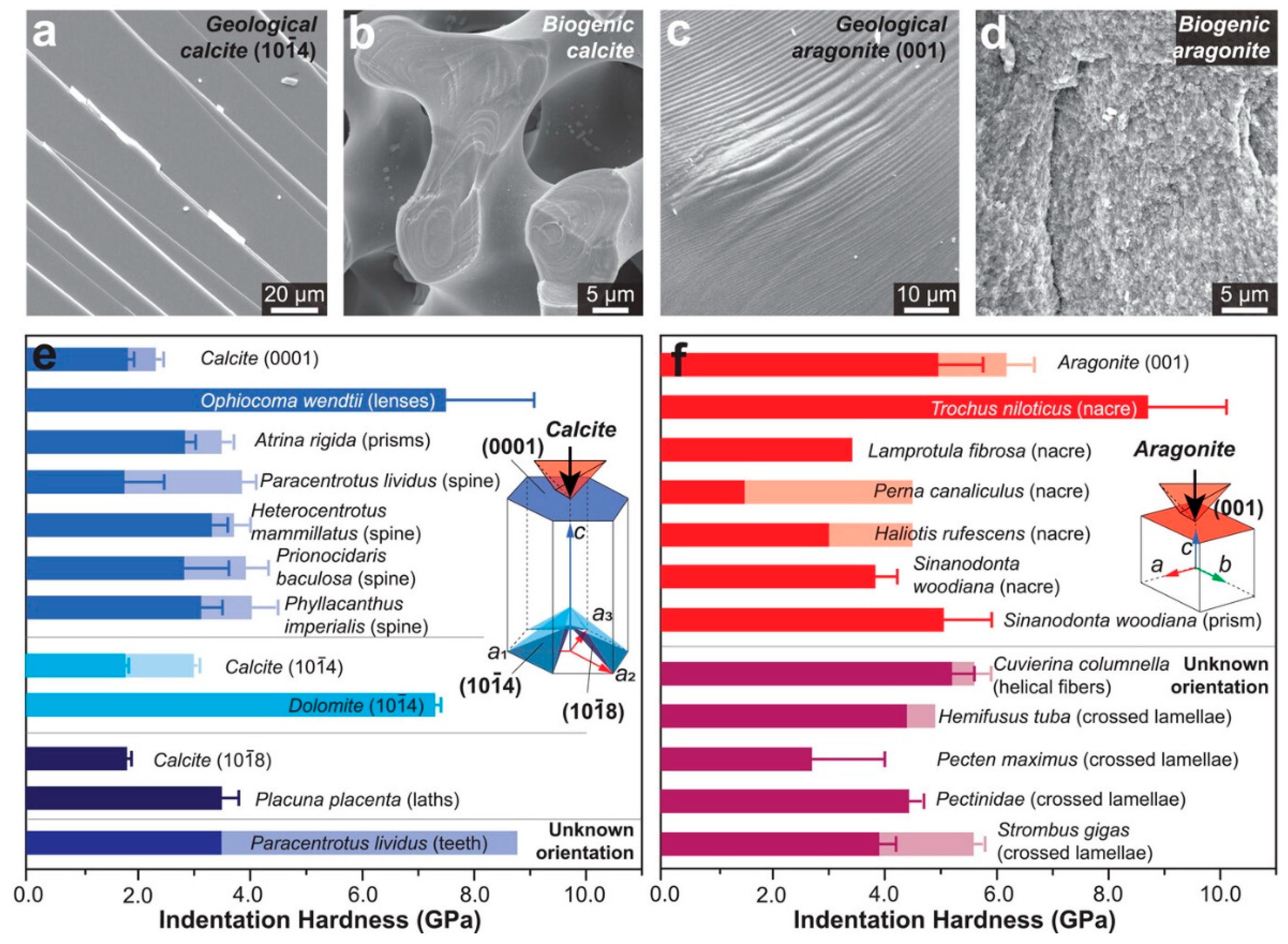
2.2.1. Calcium Carbonate
2.2.2. Hydroxyapatite (HAP)
2.2.3. Silica
3. Classification of Biomaterials Used in Regenerative Medicine
3.1. Polymer Materials
3.2. Metallic Materials
3.3. Biomineral Materials
3.4. Composite Materials
3.5. Role of Biominerals in Enhancing the Properties of Composite Materials
4. Biominerals and Composite Materials in Regenerative Medicine
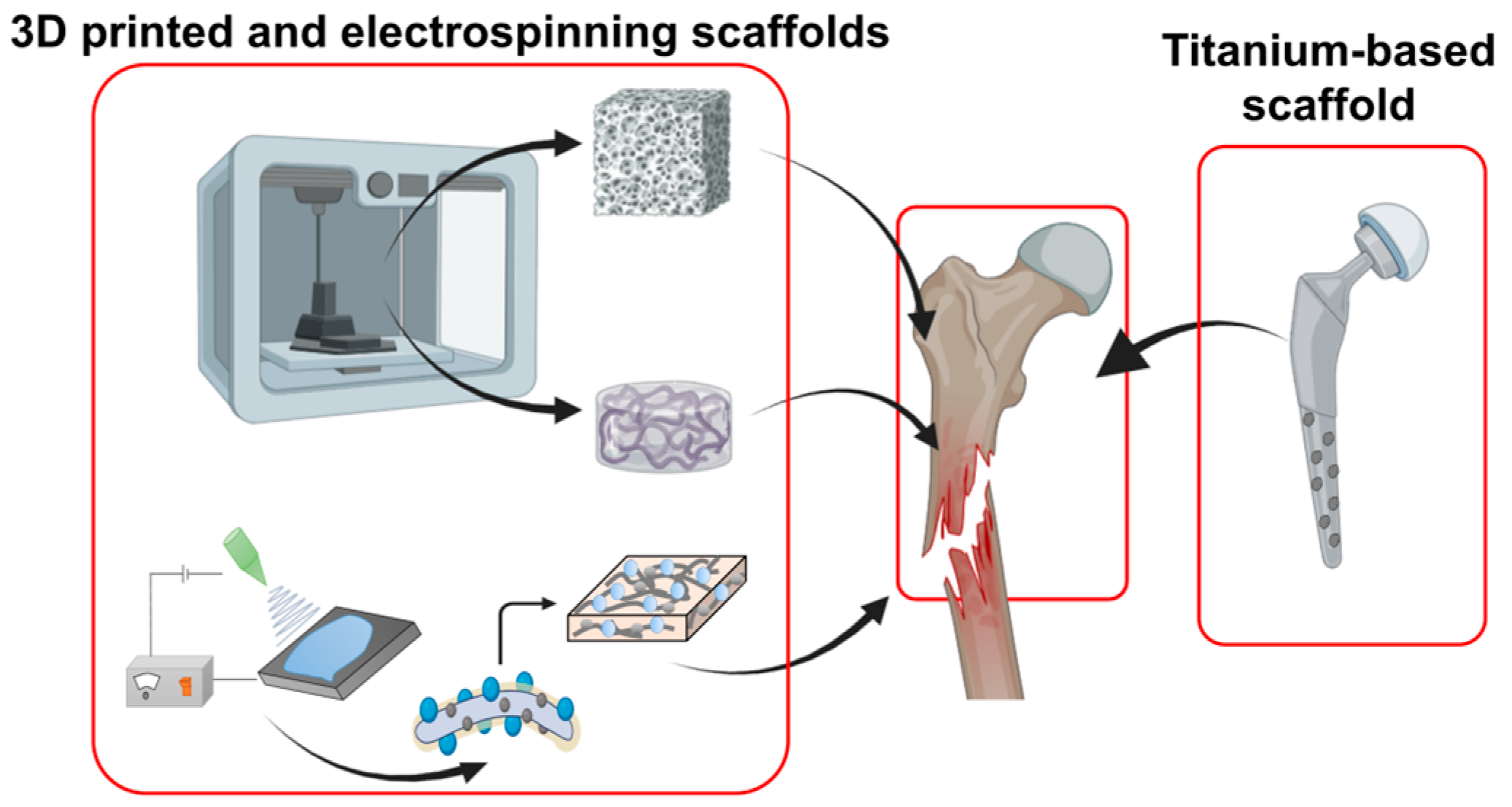
4.1. Bone Regeneration
4.2. Dental Applications
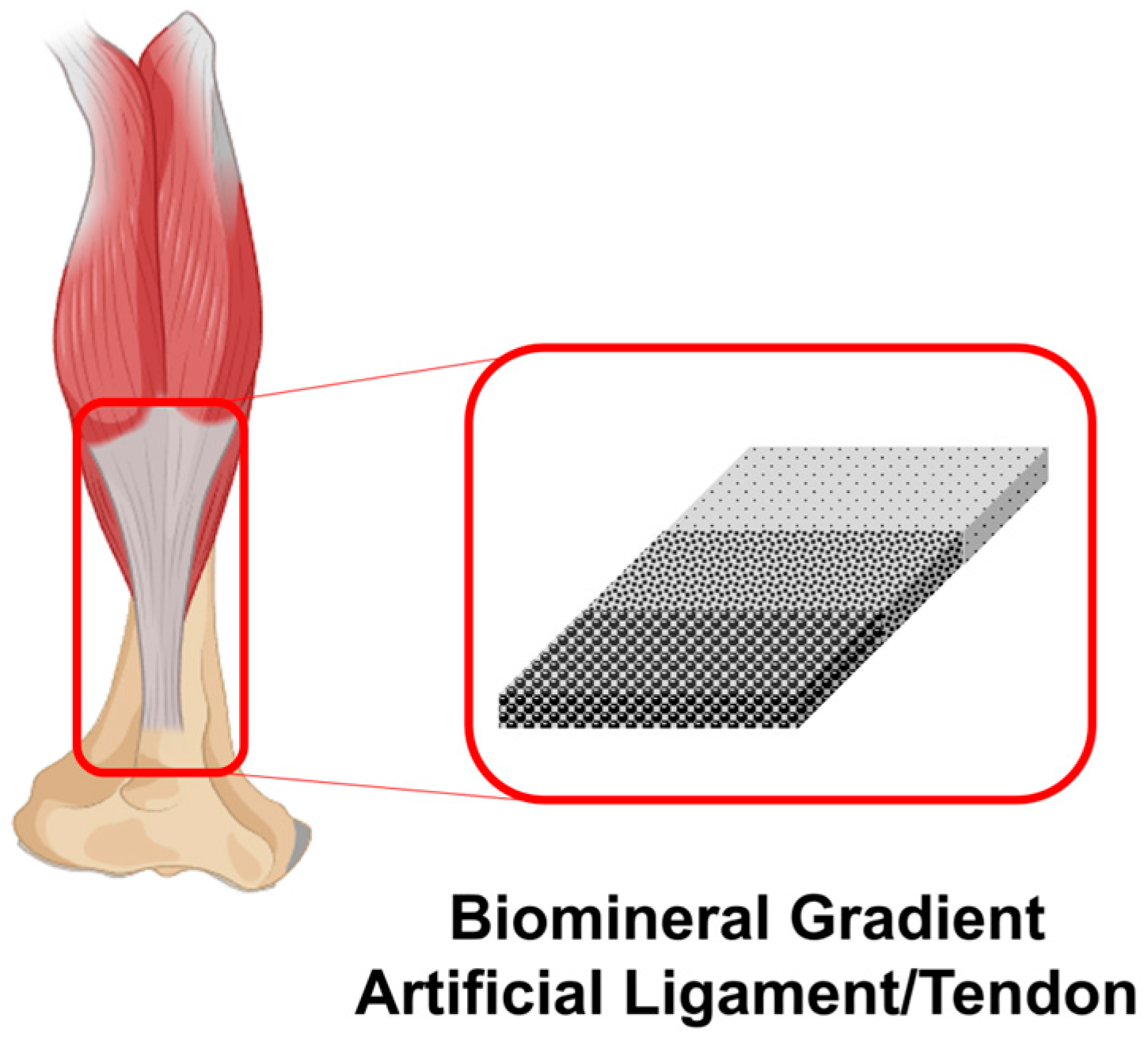
4.3. Artificial Ligament/Tendon Application
4.4. Wound-Healing Application
4.5. Drug Delivery Application
5. Challenges and Future Directions
5.1. Current Challenges in the Use of Biomineral Composite Materials in Regenerative Medicine
5.2. Future Research Directions
6. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walden, G.; Liao, X.; Donell, S.; Raxworthy, M.J.; Riley, G.P.; Saeed, A. A clinical, biological, and biomaterials perspective into tendon injuries and regeneration. Tissue Eng. Part B Rev. 2017, 23, 44–58. [Google Scholar] [CrossRef]
- Ameer, G.A.; Mahmood, T.A.; Langer, R. A biodegradable composite scaffold for cell transplantation. J. Orthopaed Res. 2002, 20, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Mao, A.S.; Mooney, D.J. Regenerative medicine: Current therapies and future directions. Prac. Natl. Acad. Sci. USA 2015, 112, 14452–14459. [Google Scholar] [CrossRef] [PubMed]
- Ogueri, K.S.; Jafari, T.; Ivirico, J.L.E.; Laurencin, C.T. Polymeric Biomaterials for Scaffold-Based Bone Regenerative Engineering. Regen. Eng. Transl. Med. 2019, 5, 128–154. [Google Scholar] [CrossRef] [PubMed]
- Mohaghegh, N.; Endo-Kimura, M.; Wang, K.; Wei, Z.; Hassani Najafabadi, A.; Zehtabi, F.; Hosseinzadeh Kouchehbaghi, N.; Sharma, S.; Markowska-Szczupak, A.; Kowalska, E. Apatite-coated Ag/AgBr/TiO2 nanocomposites: Insights into the antimicrobial mechanism in the dark and under visible-light irradiation. Appl. Surf. Sci. 2023, 617, 156574. [Google Scholar] [CrossRef]
- Dzobo, K.; Thomford, N.E.; Senthebane, D.A.; Shipanga, H.; Rowe, A.; Dandara, C.; Pillay, M.; Motaung, K. Advances in Regenerative Medicine and Tissue Engineering: Innovation and Transformation of Medicine. Stem Cells Int. 2018, 2018, 2495848. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.A.; Won, J.-E.; Knowles, J.C.; Kim, H.-W. Naturally and synthetic smart composite biomaterials for tissue regeneration. Adv. Drug Deliv. Rev. 2013, 65, 471–496. [Google Scholar] [CrossRef] [PubMed]
- Aslam Khan, M.U.; Abd Razak, S.I.; Al Arjan, W.S.; Nazir, S.; Sahaya Anand, T.J.; Mehboob, H.; Amin, R. Recent Advances in Biopolymeric Composite Materials for Tissue Engineering and Regenerative Medicines: A Review. Molecules 2021, 26, 619. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.P.; Ushida, T.; Tateishi, T. Scaffold design for tissue engineering. Macromol. Biosci. 2002, 2, 67–77. [Google Scholar] [CrossRef]
- Kumbuloglu, O.; Lassila, L.V.; User, A.; Vallittu, P.K. A study of the physical and chemical properties of four resin composite luting cements. Int. J. Prosthodont. 2004, 17, 357. [Google Scholar]
- Jemeljanova, M.; Ozola, R.; Klavins, M. Physical-chemical properties and possible applications of clay minerals and humic acid composite materials. Agron. Res. 2019, 17, 1023–1032. [Google Scholar]
- Bedian, L.; Villalba-Rodriguez, A.M.; Hernandez-Vargas, G.; Parra-Saldivar, R.; Iqbal, H.M.N. Bio-based materials with novel characteristics for tissue engineering applications—A review. Int. J. Biol. Macromol. 2017, 98, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Ciulla, M.G.; Massironi, A.; Sugni, M.; Ensign, M.A.; Marzorati, S.; Forouharshad, M. Recent Advances in the Development of Biomimetic Materials. Gels 2023, 9, 833. [Google Scholar] [CrossRef] [PubMed]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic-inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef]
- Engel, E.; Michiardi, A.; Navarro, M.; Lacroix, D.; Planell, J.A. Nanotechnology in regenerative medicine: The materials side. Trends Biotechnol. 2008, 26, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Addadi, L.; Weiner, S. Biomineralization: Mineral formation by organisms. Phys. Scr. 2014, 89, 098003. [Google Scholar] [CrossRef]
- Hardy, J.G.; Torres-Rendon, J.G.; Leal-Egana, A.; Walther, A.; Schlaad, H.; Colfen, H.; Scheibel, T.R. Biomineralization of Engineered Spider Silk Protein-Based Composite Materials for Bone Tissue Engineering. Materials 2016, 9, 560. [Google Scholar] [CrossRef]
- Upadhyay, R.K. Role of Calcium Bio-Minerals in Regenerative Medicine and Tissue Engineering. J. Stem Cell Res. Ther. 2017, 2, 166–175. [Google Scholar] [CrossRef]
- Haftek, M.; Abdayem, R.; Guyonnet-Debersac, P. Skin Minerals: Key Roles of Inorganic Elements in Skin Physiological Functions. Int. J. Mol. Sci. 2022, 23, 6267. [Google Scholar] [CrossRef]
- Su, Y.; Cappock, M.; Dobres, S.; Kucine, A.J.; Waltzer, W.C.; Zhu, D. Supplemental mineral ions for bone regeneration and osteoporosis treatment. Eng. Regen. 2023, 4, 170–182. [Google Scholar] [CrossRef]
- Luz, G.M.; Mano, J.F. Mineralized structures in nature: Examples and inspirations for the design of new composite materials and biomaterials. Compos. Sci. Technol. 2010, 70, 1777–1788. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Zheng, X.; Jin, H.J.; Li, X. Biomineralization: An opportunity and challenge of nanoparticle drug delivery systems for cancer therapy. Adv. Healthc. Mater. 2020, 9, 2001117. [Google Scholar] [CrossRef] [PubMed]
- Jantschke, A. Non-silicate Minerals (Carbonates, Oxides, Phosphates, Sulfur-Containing, Oxalates, and Other Organic Crystals) Induced by Microorganisms. In Mineral Formation by Microorganisms; Berenjian, A., Seifan, M., Eds.; Springer: Cham, Switzerland, 2022; Volume 36, pp. 161–241. [Google Scholar]
- Deng, Z.; Jia, Z.; Li, L. Biomineralized Materials as Model Systems for Structural Composites: Intracrystalline Structural Features and Their Strengthening and Toughening Mechanisms. Adv. Sci. 2022, 9, e2103524. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, E.A.; Ritchie, R.O. Bone as a Structural Material. Adv. Healthc. Mater. 2015, 4, 1287–1304. [Google Scholar] [CrossRef] [PubMed]
- Beniash, E.; Stifler, C.A.; Sun, C.Y.; Jung, G.S.; Qin, Z.; Buehler, M.J.; Gilbert, P. The hidden structure of human enamel. Nat. Commun. 2019, 10, 4383. [Google Scholar] [CrossRef]
- Perry, C.C. An overview of silica in biology: Its chemistry and recent technological advances. Prog. Mol. Subcell. Biol. 2009, 47, 295–313. [Google Scholar]
- Evans, J.S. “Tuning in” to mollusk shell nacre- and prismatic-associated protein terminal sequences. Implications for biomineralization and the construction of high performance inorganic-organic composites. Chem. Rev. 2008, 108, 4455–4462. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, F.; Zhang, J.; Yang, L.; Shi, X.; Fang, Q.; Ma, X. Biomimetic synthesis of calcium carbonate with different morphologies under the direction of different amino acids. Res. Chem. Intermed. 2012, 39, 2407–2415. [Google Scholar] [CrossRef]
- Niu, Y.-Q.; Liu, J.-H.; Aymonier, C.; Fermani, S.; Kralj, D.; Falini, G.; Zhou, C.-H. Calcium carbonate: Controlled synthesis, surface functionalization, and nanostructured materials. Chem. Soc. Rev. 2022, 51, 7883–7943. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.K.; Chanut, N.; Voigt, C.A. Silica Nanostructures Produced Using Diatom Peptides with Designed Post-Translational Modifications. Adv. Funct. Mater. 2020, 30, 2000849. [Google Scholar] [CrossRef]
- Ki, M.-R.; Kim, S.H.; Park, T.I.; Pack, S.P. Self-Entrapment of Antimicrobial Peptides in Silica Particles for Stable and Effective Antimicrobial Peptide Delivery System. Int. J. Mol. Sci. 2023, 24, 16423. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Dong, Z.; Ke, X.; Luo, J.; Li, J. Advances in biomineralization-inspired materials for hard tissue repair. Int. J. Oral Sci. 2021, 13, 42. [Google Scholar] [CrossRef]
- Grasby, S.E. Naturally precipitating vaterite (μ-CaCO3) spheres: Unusual carbonates formed in an extreme environment. Geochim. Cosmochim. Acta 2003, 67, 1659–1666. [Google Scholar] [CrossRef]
- Raz, S.; Hamilton, P.C.; Wilt, F.H.; Weiner, S.; Addadi, L. The Transient Phase of Amorphous Calcium Carbonate in Sea Urchin Larval Spicules: The Involvement of Proteins and Magnesium Ions in Its Formation and Stabilization. Adv. Funct. Mater. 2003, 13, 480–486. [Google Scholar] [CrossRef]
- Loste, E.; Wilson, R.M.; Seshadri, R.; Meldrum, F.C. The role of magnesium in stabilising amorphous calcium carbonate and controlling calcite morphologies. J. Cryst. Growth 2003, 254, 206–218. [Google Scholar] [CrossRef]
- Suzuki, M.; Saruwatari, K.; Kogure, T.; Yamamoto, Y.; Nishimura, T.; Kato, T.; Nagasawa, H. An acidic matrix protein, Pif, is a key macromolecule for nacre formation. Science 2009, 325, 1388–1390. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sommerdijk, N. Aragonite formation in confinements: A step toward understanding polymorph control. Proc. Natl. Acad. Sci. USA 2018, 115, 8469–8471. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, K.; Ni, Y.; He, L. Anomalous inapplicability of nacre-like architectures as impact-resistant templates in a wide range of impact velocities. Nat. Commun. 2022, 13, 7719. [Google Scholar] [CrossRef]
- Sroga, G.E.; Vashishth, D. Effects of Bone Matrix Proteins on Fracture and Fragility in Osteoporosis. Curr. Osteoporos. Rep. 2012, 10, 141–150. [Google Scholar] [CrossRef]
- Elsharkawy, S.; Al-Jawad, M.; Pantano, M.F.; Tejeda-Montes, E.; Mehta, K.; Jamal, H.; Agarwal, S.; Shuturminska, K.; Rice, A.; Tarakina, N.V.; et al. Protein disorder-order interplay to guide the growth of hierarchical mineralized structures. Nat. Commun. 2018, 9, 2145. [Google Scholar] [CrossRef]
- Olszta, M.J.; Cheng, X.; Jee, S.S.; Kumar, R.; Kim, Y.-Y.; Kaufman, M.J.; Douglas, E.P.; Gower, L.B. Bone structure and formation: A new perspective. Mater. Sci. Eng. R. Rep. 2007, 58, 77–116. [Google Scholar] [CrossRef]
- Yang, W.; Meyers, M.A.; Ritchie, R.O. Structural architectures with toughening mechanisms in Nature: A review of the materials science of Type-I collagenous materials. Prog. Mater. Sci. 2019, 103, 425–483. [Google Scholar] [CrossRef]
- Nair, A.K.; Gautieri, A.; Chang, S.-W.; Buehler, M.J. Molecular mechanics of mineralized collagen fibrils in bone. Nat. Commun. 2013, 4, 1724. [Google Scholar] [CrossRef]
- El Gezawi, M.; Wolfle, U.C.; Haridy, R.; Fliefel, R.; Kaisarly, D. Remineralization, Regeneration, and Repair of Natural Tooth Structure: Influences on the Future of Restorative Dentistry Practice. ACS Biomater. Sci. Eng. 2019, 5, 4899–4919. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.N.; Zhang, W.; Pashley, D.H.; Breschi, L.; Mao, J.; Chen, J.H.; Tay, F.R. Biomimetic remineralization of dentin. Dent. Mater. 2014, 30, 77–96. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.; Butler, W.T.; Qin, C. Dentin sialophosphoprotein in biomineralization. Connect. Tissue Res. 2010, 51, 404–417. [Google Scholar] [CrossRef]
- Wang, H.; Tannukit, S.; Zhu, D.; Snead, M.L.; Paine, M.L. Enamel Matrix Protein Interactions. J. Bone Miner. Res. 2005, 20, 1032–1040. [Google Scholar] [CrossRef]
- Sharma, V.; Srinivasan, A.; Nikolajeff, F.; Kumar, S. Biomineralization process in hard tissues: The interaction complexity within protein and inorganic counterparts. Acta Biomater. 2021, 120, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Yu, Z.; Ackerman, L.; Zhang, Y.; Bonde, J.; Li, W.; Cheng, Y.; Habelitz, S. Protein nanoribbons template enamel mineralization. Proc. Natl. Acad. Sci. USA 2020, 117, 19201–19208. [Google Scholar] [CrossRef] [PubMed]
- Gil-Bona, A.; Bidlack, F.B. Tooth Enamel and its Dynamic Protein Matrix. Int. J. Mol. Sci. 2020, 21, 4458. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.; Tao, J.; Ruan, Q.; De Yoreo, J.J.; Moradian-Oldak, J. Matrix metalloproteinase-20 mediates dental enamel biomineralization by preventing protein occlusion inside apatite crystals. Biomaterials 2016, 75, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hu, Z.; Ge, M.; Jin, W.; Tang, R.; Li, Q.; Xu, W.; Shi, J.; Xie, Z. Intraosseous Injection of Calcium Phosphate Polymer-Induced Liquid Precursor Increases Bone Density and Improves Early Implant Osseointegration in Ovariectomized Rats. Int. J. Nanomed. 2021, 16, 6217–6229. [Google Scholar] [CrossRef] [PubMed]
- Patoine, K.; Ta, K.; Gilbert, A.; Percuoco, M.; Gerdon, A.E. Equilibrium interactions of biomimetic DNA aptamers produce intrafibrillar calcium phosphate mineralization of collagen. Acta Biomater. 2024, 179, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Kröger, N. Biomolecules Involved in Frustule Biogenesis and Function. In The Molecular Life of Diatoms; Falciatore, A., Mock, T., Eds.; Springer: Cham, Switzerland, 2022; pp. 313–343. [Google Scholar]
- Hildebrand, M.; Lerch, S.J.L.; Shrestha, R.P. Understanding Diatom Cell Wall Silicification—Moving Forward. Front. Mar. Sci. 2018, 5, 125. [Google Scholar] [CrossRef]
- Martin-Jézéquel, V.; Hildebrand, M.; Brzezinski, M.A. Silicon Metabolism in Diatoms: Implications for Growth. J. Phycol. 2003, 36, 821–840. [Google Scholar] [CrossRef]
- Kroger, N.; Deutzmann, R.; Sumper, M. Polycationic peptides from diatom biosilica that direct silica nanosphere formation. Science 1999, 286, 1129–1132. [Google Scholar] [CrossRef]
- Kroger, N.; Deutzmann, R.; Bergsdorf, C.; Sumper, M. Species-specific polyamines from diatoms control silica morphology. Proc. Natl. Acad. Sci. USA 2000, 97, 14133–14138. [Google Scholar] [CrossRef]
- Kotzsch, A.; Gröger, P.; Pawolski, D.; Bomans, P.H.H.; Sommerdijk, N.A.J.M.; Schlierf, M.; Kröger, N. Silicanin-1 is a conserved diatom membrane protein involved in silica biomineralization. BMC Biol. 2017, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Cha, J.; Stucky, G.D.; Morse, D.E. Silicatein alpha: Cathepsin L-like protein in sponge biosilica. Proc. Natl. Acad. Sci. USA 1998, 95, 6234–6238. [Google Scholar] [CrossRef]
- Shimizu, K.; Morse, D.E. Silicatein: A Unique Silica-Synthesizing Catalytic Triad Hydrolase from Marine Sponge Skeletons and Its Multiple Applications. Methods Enzymol. 2018, 605, 429–455. [Google Scholar] [PubMed]
- Schloßmacher, U.; Wiens, M.; Schröder, H.C.; Wang, X.; Jochum, K.P.; Müller, W.E.G. Silintaphin-1—interaction with silicatein during structure-guiding bio-silica formation. FEBS J. 2011, 278, 1145–1155. [Google Scholar] [CrossRef]
- Casey, W.H.; Kinrade, S.D.; Knight, C.T.G.; Rains, D.W.; Epstein, E. Aqueous silicate complexes in wheat, Triticum aestivum L. Plant Cell Environ. 2003, 27, 51–54. [Google Scholar] [CrossRef]
- Mitani, N. Uptake system of silicon in different plant species. J. Exp. Bot. 2005, 56, 1255–1261. [Google Scholar] [CrossRef]
- Perry, C.C.; Mann, S. Aspects of Biological Silicification. In Origin, Evolution, and Modern Aspects of Biomineralization in Plants and Animals; Springer: Boston, MA, USA, 1989; pp. 419–431. [Google Scholar]
- Harrison, C.C. Evidence for intramineral macromolecules containing protein from plant silicas. Phytochemistry 1996, 41, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Soukup, M.; Martinka, M.; Bosnić, D.; Čaplovičová, M.; Elbaum, R.; Lux, A. Formation of silica aggregates in sorghum root endodermis is predetermined by cell wall architecture and development. Ann. Bot. 2017, 120, 739–753. [Google Scholar] [CrossRef]
- Kumar, S.; Adiram-Filiba, N.; Blum, S.; Sanchez-Lopez, J.A.; Tzfadia, O.; Omid, A.; Volpin, H.; Heifetz, Y.; Goobes, G.; Elbaum, R.; et al. Siliplant1 protein precipitates silica in sorghum silica cells. J. Exp. Bot. 2020, 71, 6830–6843. [Google Scholar] [CrossRef]
- Epstein, E. Silicon: Its manifold roles in plants. Ann. Appl. Biol. 2009, 155, 155–160. [Google Scholar] [CrossRef]
- Lechner, C.C.; Becker, C.F. Silaffins in Silica Biomineralization and Biomimetic Silica Precipitation. Mar. Drugs 2015, 13, 5297–5333. [Google Scholar] [CrossRef] [PubMed]
- Ki, M.R.; Nguyen, T.K.M.; Park, T.I.; Park, H.M.; Pack, S.P. Biomimetic Silica Particles with Self-Loading BMP-2 Knuckle Epitope Peptide and Its Delivery for Bone Regeneration. Pharmaceutics 2023, 15, 1061. [Google Scholar] [CrossRef]
- Ki, M.R.; Kim, S.H.; Nguyen, T.K.M.; Son, R.G.; Jun, S.H.; Pack, S.P. BMP2-Mediated Silica Deposition: An Effective Strategy for Bone Mineralization. ACS Biomater. Sci. Eng. 2023, 9, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.K.M.; Ki, M.R.; Lee, C.S.; Pack, S.P. Nanosized and tunable design of biosilica particles using novel silica-forming peptide-modified chimeric ferritin templates. J. Ind. Eng. Chem. 2019, 73, 198–204. [Google Scholar] [CrossRef]
- Abdelhamid, M.A.A.; Pack, S.P. Biomimetic and bioinspired silicifications: Recent advances for biomaterial design and applications. Acta Biomater. 2021, 120, 38–56. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.X.; Choi, J.W. Biodegradable polymer scaffolds with well-defined interconnected spherical pore network. Tissue Eng. 2001, 7, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.; Chen, H.; Zhang, Y.; Li, P.; Wu, W. Nanocellulose: A promising nanomaterial for fabricating fluorescent composites. Cellulose 2022, 29, 7011–7035. [Google Scholar] [CrossRef]
- Heise, K.; Kontturi, E.; Allahverdiyeva, Y.; Tammelin, T.; Linder, M.B.; Nonappa; Ikkala, O. Nanocellulose: Recent Fundamental Advances and Emerging Biological and Biomimicking Applications. Adv. Mater. 2020, 33, e2004349. [Google Scholar] [CrossRef]
- Gelaw, B.B.; Kasaew, E.; Belayneh, A.; Tesfaw, D.; Tesfaye, T. Review of the sources, synthesis, and applications of nanocellulose materials. Polym. Bull. 2023, 81, 7713–7735. [Google Scholar] [CrossRef]
- Sabir, M.I.; Xu, X.X.; Li, L. A review on biodegradable polymeric materials for bone tissue engineering applications. J. Mater. Sci. 2009, 44, 5713–5724. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, J.; Li, J.; Liang, X.; Zhou, W.; Peng, S. Strategies and techniques for improving heat resistance and mechanical performances of poly(lactic acid) (PLA) biodegradable materials. Int. J. Biol. Macromol. 2022, 218, 115–134. [Google Scholar] [CrossRef]
- Budak, K.; Sogut, O.; Aydemir Sezer, U. A review on synthesis and biomedical applications of polyglycolic acid. J. Polym. Res. 2020, 27, 208. [Google Scholar] [CrossRef]
- Siddiqui, N.; Asawa, S.; Birru, B.; Baadhe, R.; Rao, S. PCL-Based Composite Scaffold Matrices for Tissue Engineering Applications. Mol. Biotechnol. 2018, 60, 506–532. [Google Scholar] [CrossRef]
- Swider, E.; Koshkina, O.; Tel, J.; Cruz, L.J.; de Vries, I.J.M.; Srinivas, M. Customizing poly(lactic-co-glycolic acid) particles for biomedical applications. Acta Biomater. 2018, 73, 38–51. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob. Cardiol. Sci. Pract. 2013, 2013, 316–342. [Google Scholar] [CrossRef] [PubMed]
- Satchanska, G.; Davidova, S.; Petrov, P.D. Natural and Synthetic Polymers for Biomedical and Environmental Applications. Polymers 2024, 16, 1159. [Google Scholar] [CrossRef]
- Epps, T.H.; Korley, L.T.J.; Yan, T.; Beers, K.L.; Burt, T.M. Sustainability of Synthetic Plastics: Considerations in Materials Life-Cycle Management. JACS Au 2021, 2, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Arifvianto, B.; Zhou, J. Fabrication of Metallic Biomedical Scaffolds with the Space Holder Method: A Review. Materials 2014, 7, 3588–3622. [Google Scholar] [CrossRef]
- Ghassemi, T.; Shahroodi, A.; Ebrahimzadeh, M.H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current Concepts in Scaffolding for Bone Tissue Engineering. Arch. Bone Jt. Surg. 2018, 6, 90–99. [Google Scholar]
- Deng, F.; Liu, L.; Li, Z.; Liu, J. 3D printed Ti6Al4V bone scaffolds with different pore structure effects on bone ingrowth. J. Biol. Eng. 2021, 15, 4. [Google Scholar] [CrossRef]
- Zuo, W.; Yu, L.; Lin, J.; Yang, Y.; Fei, Q. Properties improvement of titanium alloys scaffolds in bone tissue engineering: A literature review. Ann. Transl. Med. 2021, 9, 1259. [Google Scholar] [CrossRef] [PubMed]
- ElBatal, H.A.; Azooz, M.A.; Khalil, E.M.A.; Monem, A.S.; Hamdy, Y.M. Characterization of some bioglass-ceramics. Mater. Chem. Phys. 2003, 80, 599–609. [Google Scholar] [CrossRef]
- Dorozhkin, S.V.; Epple, M. Biological and medical significance of calcium phosphates. Angew. Chem. Int. Edit 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Suzuki, O. Octacalcium phosphate: Osteoconductivity and crystal chemistry. Acta Biomater. 2010, 6, 3379–3387. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Abdallah, M.N.; Hanafi, A.A.; Misbahuddin, S.; Rashid, H.; Glogauer, M. Mechanisms of In Vivo Degradation and Resorption of Calcium Phosphate Based Biomaterials. Materials 2015, 8, 7913–7925. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, P.; Tong, Y.; Guo, S.; Zhao, G.; Zhang, M.; Yu, H.; Zhao, X.; Tang, Q.; Liu, Y. Photolithographic High-Conductivity Transparent Conformal rGO/PEDOT:PSS Electrodes for Flexible Skin-like All Solution-Processed Organic Transistors. Adv. Mater. Technol. 2022, 7, 2200660. [Google Scholar] [CrossRef]
- Saffarian Tousi, N.; Velten, M.F.; Bishop, T.J.; Leong, K.K.; Barkhordar, N.S.; Marshall, G.W.; Loomer, P.M.; Aswath, P.B.; Varanasi, V.G. Combinatorial effect of Si4+, Ca2+, and Mg2+ released from bioactive glasses on osteoblast osteocalcin expression and biomineralization. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 2757–2765. [Google Scholar] [CrossRef] [PubMed]
- Allouche, J.; Boissiere, M.; Helary, C.; Livage, J.; Coradin, T. Biomimetic core-shell gelatine/silica nanoparticles: A new example of biopolymer-based nanocomposites. J. Mater. Chem. 2006, 16, 3120–3125. [Google Scholar] [CrossRef]
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J.E. Scaffold Design for Bone Regeneration. J. Nanosci. Nanotechno. 2014, 14, 15–56. [Google Scholar] [CrossRef]
- Agotegaray, M.A.; Lassalle, V.L. Silica: Chemical Properties and Biological Features. In Silica-Coated Magnetic Nanoparticles; Springer: Berlin/Heidelberg, Germany, 2017; pp. 27–37. [Google Scholar]
- Beck, G.R.; Ha, S.W.; Camalier, C.E.; Yamaguchi, M.; Li, Y.; Lee, J.K.; Weitzmann, M.N. Bioactive silica-based nanoparticles stimulate bone-forming osteoblasts, suppress bone-resorbing osteoclasts, and enhance bone mineral density in vivo. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 793–803. [Google Scholar] [CrossRef]
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 2004, 5, 117–141. [Google Scholar] [CrossRef]
- Motameni, A.; Çardaklı, İ.S.; Gürbüz, R.; Alshemary, A.Z.; Razavi, M.; Farukoğlu, Ö.C. Bioglass-polymer composite scaffolds for bone tissue regeneration: A review of current trends. Int. J. Polym. Mater. Polym. Biomater. 2023, 73, 600–619. [Google Scholar] [CrossRef]
- Görgen, S.; Benzerara, K.; Skouri-Panet, F.; Gugger, M.; Chauvat, F.; Cassier-Chauvat, C. The diversity of molecular mechanisms of carbonate biomineralization by bacteria. Discov. Mater. 2021, 1, 2. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, Y.; Deveaux, J.G.; Masoud, M.A.; Chandra, F.S.; Chen, H.; Zhang, D.; Feng, L. Biomineralization Forming Process and Bio-inspired Nanomaterials for Biomedical Application: A Review. Minerals 2019, 9, 68. [Google Scholar] [CrossRef]
- Li, S.; Huang, J.; Liu, C.; Liu, Y.; Zheng, G.; Xie, L.; Zhang, R. Interactive Effects of Seawater Acidification and Elevated Temperature on the Transcriptome and Biomineralization in the Pearl Oyster Pinctada fucata. Environ. Sci. Technol. 2016, 50, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, H.; Guo, S.; Qi, J.; Zhang, R.; Liu, X.; Sun, L.; Zong, M.; Cheng, H.; Wu, X.; et al. Applications of Bacterial Cellulose-Based Composite Materials in Hard Tissue Regenerative Medicine. Tissue Eng. Regen. Med. 2023, 20, 1017–1039. [Google Scholar] [CrossRef]
- Xue, N.; Ding, X.; Huang, R.; Jiang, R.; Huang, H.; Pan, X.; Min, W.; Chen, J.; Duan, J.A.; Liu, P.; et al. Bone Tissue Engineering in the Treatment of Bone Defects. Pharmaceuticals 2022, 15, 879. [Google Scholar] [CrossRef]
- Kim, H.; Che, L.; Ha, Y.; Ryu, W. Mechanically-reinforced electrospun composite silk fibroin nanofibers containing hydroxyapatite nanoparticles. Mat. Sci. Eng. C-Mater. 2014, 40, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef]
- Kolodziejska, B.; Kaflak, A.; Kolmas, J. Biologically Inspired Collagen/Apatite Composite Biomaterials for Potential Use in Bone Tissue Regeneration-A Review. Materials 2020, 13, 1748. [Google Scholar] [CrossRef]
- Krishnakumar, G.S.; Gostynska, N.; Dapporto, M.; Campodoni, E.; Montesi, M.; Panseri, S.; Tampieri, A.; Kon, E.; Marcacci, M.; Sprio, S.; et al. Evaluation of different crosslinking agents on hybrid biomimetic collagen-hydroxyapatite composites for regenerative medicine. Int. J. Biol. Macromol. 2018, 106, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Bahraminasab, M. Challenges on optimization of 3D-printed bone scaffolds. Biomed. Eng. Online 2020, 19, 69. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.-D. Introduction to Composite Materials. In Composite and Nanocomposite Materials—From Knowledge to Industrial Applications; IntechOpen Limited: London, UK, 2020. [Google Scholar] [CrossRef]
- Beaumont, P.W.R. The Structural Integrity of Composite Materials and Long-Life Implementation of Composite Structures. Appl. Compos. Mater. 2020, 27, 449–478. [Google Scholar] [CrossRef]
- Wegst, U.G.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired structural materials. Nat. Mater. 2015, 14, 23–36. [Google Scholar] [CrossRef]
- Yao, H.B.; Ge, J.; Mao, L.B.; Yan, Y.X.; Yu, S.H. 25th anniversary article: Artificial carbonate nanocrystals and layered structural nanocomposites inspired by nacre: Synthesis, fabrication and applications. Adv. Mater. 2014, 26, 163–187. [Google Scholar] [CrossRef]
- Zou, L.; Zhang, Y.; Liu, X.; Chen, J.; Zhang, Q. Biomimetic mineralization on natural and synthetic polymers to prepare hybrid scaffolds for bone tissue engineering. Colloids Surf. B Biointerfaces 2019, 178, 222–229. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, Y.; Wang, Y.; Duan, R.; Liu, L. Gelatin/Hyaluronic Acid Photocrosslinked Double Network Hydrogel with Nano-Hydroxyapatite Composite for Potential Application in Bone Repair. Gels 2023, 9, 742. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Lu, Z.; Lu, C.; Sun, X.; Ni, B.; Cölfen, H.; Xiong, R. Bioinspired Stabilization of Amorphous Calcium Carbonate by Carboxylated Nanocellulose Enables Mechanically Robust, Healable, and Sensing Biocomposites. ACS Nano 2023, 17, 6664–6674. [Google Scholar] [CrossRef]
- Fratzl, P.; Weinkamer, R. Nature’s hierarchical materials. Prog. Mater. Sci. 2007, 52, 1263–1334. [Google Scholar] [CrossRef]
- Aitken, Z.H.; Luo, S.; Reynolds, S.N.; Thaulow, C.; Greer, J.R. Microstructure provides insights into evolutionary design and resilience of Coscinodiscus sp. frustule. Proc. Natl. Acad. Sci. USA 2016, 113, 2017–2022. [Google Scholar] [CrossRef]
- Meza, L.R.; Das, S.; Greer, J.R. Strong, lightweight, and recoverable three-dimensional ceramic nanolattices. Science 2014, 345, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Y.; Ding, B.; Li, X. Design, Fabrication, and Mechanics of 3D Micro-/Nanolattices. Small 2020, 16, e1902842. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Meza, L.R.; Schaedler, T.A.; Schwaiger, R.; Zheng, X.; Valdevit, L. Nanolattices: An Emerging Class of Mechanical Metamaterials. Adv. Mater. 2017, 29, 1701850. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Ren, H.; Meng, Z.; Chen, X.; Li, Z.; Wang, L.; Chen, W.; Wang, Y.; Du, J. Ultrastiff metamaterials generated through a multilayer strategy and topology optimization. Nat. Commun. 2024, 15, 2984. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jia, Y.; Duan, K.; Xiao, R.; Qiao, J.; Liang, S.; Wang, S.; Chen, J.; Wu, H.; Lu, Y.; et al. One-photon three-dimensional printed fused silica glass with sub-micron features. Nat. Commun. 2024, 15, 2689. [Google Scholar] [CrossRef] [PubMed]
- Lavania, S.; Mehta, J.; Bhardwaj, P.; Tripathi, A.; Gupta, N.; Gupta, P. Biocomposites: Prospects and Manifold Applications for Human and Environmental Sustainability. ECS J. Solid State Sci. Technol. 2023, 12, 037002. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef]
- Feng, Y.; Wu, D.; Knaus, J.; Kessler, S.; Ni, B.; Chen, Z.; Avaro, J.; Xiong, R.; Colfen, H.; Wang, Z. A Bioinspired Gelatin-Amorphous Calcium Phosphate Coating on Titanium Implant for Bone Regeneration. Adv. Healthc. Mater. 2023, 12, e2203411. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Chen, H.; Yuan, B.; Zhou, Y.; Min, L.; Xiao, Z.; Zhu, X.; Tu, C.; Zhang, X. Electrochemical Deposition of Nanostructured Hydroxyapatite Coating on Titanium with Enhanced Early Stage Osteogenic Activity and Osseointegration. Int. J. Nanomed. 2020, 15, 6605–6618. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, L.; Zhou, Z.; Luo, X.; Wang, T.; Zhao, X.; Lu, B.; Chen, F.; Zheng, L. Calcium Phosphate-Based Biomaterials for Bone Repair. J. Funct. Biomater. 2022, 13, 187. [Google Scholar] [CrossRef]
- Nakayama, M.; Kato, T. Biomineral-Inspired Colloidal Liquid Crystals: From Assembly of Hybrids Comprising Inorganic Nanocrystals and Organic Polymer Components to Their Functionalization. Acc. Chem. Res. 2022, 55, 1796–1808. [Google Scholar] [CrossRef]
- Campodoni, E.; Montanari, M.; Artusi, C.; Bergamini, L.; Bassi, G.; Destro, E.; Fenoglio, I.; Panseri, S.; Tampieri, A.; Sanson, A.; et al. Biomineralization: A new tool for developing eco-sustainable Ti-doped hydroxyapatite-based hybrid UV filters. Biomater. Adv. 2023, 151, 213474. [Google Scholar] [CrossRef] [PubMed]
- John, L. Selected developments and medical applications of organic-inorganic hybrid biomaterials based on functionalized spherosilicates. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 88, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Lyons, J.G.; Plantz, M.A.; Hsu, W.K.; Hsu, E.L.; Minardi, S. Nanostructured Biomaterials for Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 922. [Google Scholar] [CrossRef]
- Li, J.; Qin, L.; Yang, K.; Ma, Z.; Wang, Y.; Cheng, L.; Zhao, D. Materials evolution of bone plates for internal fixation of bone fractures: A review. J. Mater. Sci. Technol. 2020, 36, 190–208. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, B.; Sehgal, R.; Wani, M.; Kumar, D.; Sharma, M.D.; Singh, V.; Sehgal, R.; Kumar, V. Advantages and disadvantages of metal nanoparticles. In Nanoparticles Reinforced Metal Nanocomposites: Mechanical Performance and Durability; Springer: Berlin/Heidelberg, Germany, 2023; pp. 209–235. [Google Scholar]
- Fathi, A.M.; Ahmed, M.K.; Afifi, M.; Menazea, A.A.; Uskokovic, V. Taking Hydroxyapatite-Coated Titanium Implants Two Steps Forward: Surface Modification Using Graphene Mesolayers and a Hydroxyapatite-Reinforced Polymeric Scaffold. ACS Biomater. Sci. Eng. 2021, 7, 360–372. [Google Scholar] [CrossRef]
- Alves, H.L.; Dos Santos, L.A.; Bergmann, C.P. Injectability evaluation of tricalcium phosphate bone cement. J. Mater. Sci. Mater. Med. 2008, 19, 2241–2246. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Thi Minh Le, P.; Ito, M.; Shintani, S.A.; Takadama, H. Tri-functional calcium-deficient calcium titanate coating on titanium metal by chemical and heat treatment. Coatings 2019, 9, 561. [Google Scholar] [CrossRef]
- Feng, B.; Weng, J.; Yang, B.; Qu, S.; Zhang, X. Characterization of titanium surfaces with calcium and phosphate and osteoblast adhesion. Biomaterials 2004, 25, 3421–3428. [Google Scholar] [CrossRef]
- Krishani, M.; Shin, W.Y.; Suhaimi, H.; Sambudi, N.S. Development of Scaffolds from Bio-Based Natural Materials for Tissue Regeneration Applications: A Review. Gels 2023, 9, 100. [Google Scholar] [CrossRef]
- Tajvar, S.; Hadjizadeh, A.; Samandari, S.S. Scaffold degradation in bone tissue engineering: An overview. Int. Biodeterior. Biodegrad. 2023, 180, 105599. [Google Scholar] [CrossRef]
- Kim, Y.; Lim, J.Y.; Yang, G.H.; Seo, J.-H.; Ryu, H.-S.; Kim, G. 3D-printed PCL/bioglass (BGS-7) composite scaffolds with high toughness and cell-responses for bone tissue regeneration. J. Ind. Eng. Chem. 2019, 79, 163–171. [Google Scholar] [CrossRef]
- Kargozar, S.; Hamzehlou, S.; Baino, F. Potential of Bioactive Glasses for Cardiac and Pulmonary Tissue Engineering. Materials 2017, 10, 1429. [Google Scholar] [CrossRef]
- Yu, X.; Tang, X.; Gohil, S.V.; Laurencin, C.T. Biomaterials for Bone Regenerative Engineering. Adv. Healthc. Mater. 2015, 4, 1268–1285. [Google Scholar] [CrossRef]
- Kim, S.H.; Ki, M.R.; Park, K.S.; Yeo, K.B.; Pack, S.P. Chimeric protein-mediated dual mineral formation on biopolymer: Non-segregated and well-distributed deposition of CaCO(3) and silica particles. Colloids Surf. B Biointerfaces 2022, 219, 112808. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, S.; Liu, Y.; Christensen, R.; Raina, D.B.; Tagil, M.; Lidgren, L. Antibiotic containing bone cement in prevention of hip and knee prosthetic joint infections: A systematic review and meta-analysis. J. Orthop. Translat 2020, 23, 53–60. [Google Scholar] [CrossRef]
- Radha, G.; Manjubaashini, N.; Balakumar, S. Nano-hydroxyapatite/natural polymer composite scaffolds for bone tissue engineering: A brief review of recent trend. Vitr. Models 2023, 2, 125–151. [Google Scholar] [CrossRef]
- Subash, A.; Basanth, A.; Kandasubramanian, B. Biodegradable polyphosphazene—Hydroxyapatite composites for bone tissue engineering. Int. J. Polym. Mater. Polym. Biomater. 2022, 72, 1093–1111. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, C.; Bi, F.; Li, P.; Tian, K. The hydroxyapatite modified 3D printed poly L-lactic acid porous screw in reconstruction of anterior cruciate ligament of rabbit knee joint: A histological and biomechanical study. BMC Musculoskelet. Disord. 2023, 24, 151. [Google Scholar] [CrossRef]
- Shin, K.; Acri, T.; Geary, S.; Salem, A.K. Biomimetic Mineralization of Biomaterials Using Simulated Body Fluids for Bone Tissue Engineering and Regenerative Medicine. Tissue Eng. Part. A 2017, 23, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; Ki, M.R.; Kim, E.H.; Park, C.J.; Ryu, J.J.; Jang, H.S.; Pack, S.P.; Jo, Y.K.; Jun, S.H. Biosilicated collagen/beta-tricalcium phosphate composites as a BMP-2-delivering bone-graft substitute for accelerated craniofacial bone regeneration. Biomater. Res. 2021, 25, 13. [Google Scholar] [CrossRef] [PubMed]
- Ki, M.-R.; Kim, S.H.; Rho, S.; Kim, J.K.; Min, K.H.; Yeo, K.B.; Lee, J.; Lee, G.; Jun, S.-H.; Pack, S.P. Use of biosilica to improve loading and delivery of bone morphogenic protein 2. Int. J. Biol. Macromol. 2024, 254, 127876. [Google Scholar] [CrossRef] [PubMed]
- Yactayo-Alburquerque, M.T.; Alen-Mendez, M.L.; Azanedo, D.; Comande, D.; Hernandez-Vasquez, A. Impact of oral diseases on oral health-related quality of life: A systematic review of studies conducted in Latin America and the Caribbean. PLoS ONE 2021, 16, e0252578. [Google Scholar] [CrossRef] [PubMed]
- El-Kalla, I.H.; Shalan, H.M.; Bakr, R.A. Impact of Dental Trauma on Quality of Life among 11–14 Years Schoolchildren. Contemp. Clin. Dent. 2017, 8, 538–544. [Google Scholar] [PubMed]
- Xu, J.; Shi, H.; Luo, J.; Yao, H.; Wang, P.; Li, Z.; Wei, J. Advanced materials for enamel remineralization. Front. Bioeng. Biotechnol. 2022, 10, 985881. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Pan, H.; Tao, J.; Xu, X.; Mao, C.; Gu, X.; Tang, R. Repair of enamel by using hydroxyapatite nanoparticles as the building blocks. J. Mater. Chem. 2008, 18, 4079–4084. [Google Scholar] [CrossRef]
- Shao, C.; Jin, B.; Mu, Z.; Lu, H.; Zhao, Y.; Wu, Z.; Yan, L.; Zhang, Z.; Zhou, Y.; Pan, H.; et al. Repair of tooth enamel by a biomimetic mineralization frontier ensuring epitaxial growth. Sci. Adv. 2019, 5, eaaw9569. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shao, C.; Jin, B.; Zhang, Z.; Zhao, Y.; Xu, X.; Tang, R. Crosslinking ionic oligomers as conformable precursors to calcium carbonate. Nature 2019, 574, 394–398. [Google Scholar] [CrossRef]
- Tsuchida, S.; Nakayama, T. Recent Clinical Treatment and Basic Research on the Alveolar Bone. Biomedicines 2023, 11, 843. [Google Scholar] [CrossRef]
- Liu, B.; Yin, N.B.; Xiao, R.; Li, B.H.; Li, H.D.; Chen, S.X.; Li, S.L.; Wang, Y.Q. Evaluating the efficacy of recombinant human bone morphogenic protein-2 in the treatment of alveolar clefts with autologous bone grafting using computer-aided engineering techniques. Br. J. Oral Maxillofac. Surg. 2021, 59, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Moraschini, V.; de Almeida, D.C.F.; Calasans-Maia, M.D.; Kischinhevsky, I.C.C.; Louro, R.S.; Granjeiro, J.M. Immunological response of allogeneic bone grafting: A systematic review of prospective studies. J. Oral Pathol. Med. 2020, 49, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yi, G.S.; Lee, J.W.; Kim, D.J. Physicochemical characterization of porcine bone-derived grafting material and comparison with bovine xenografts for dental applications. J. Periodontal Implant. Sci. 2017, 47, 388–401. [Google Scholar] [CrossRef] [PubMed]
- Taschieri, S.; Moses, O.; Dellavia, C.; Canciani, E.; Nemcovsky, C.; Francetti, L.; Corbella, S. Comparative Study of Deproteinized Bovine Bone Mineral and Bovine Bone Mineral Enriched with a Polymer and Gelatin in Maxillary Sinus Floor Elevation Procedures. Int. J. Periodontics Restor. Dent. 2021, 41, 579–586. [Google Scholar] [CrossRef]
- Tomas, M.; Candrlic, M.; Juzbasic, M.; Ivanisevic, Z.; Matijevic, N.; Vcev, A.; Cvijanovic Peloza, O.; Matijevic, M.; Peric Kacarevic, Z. Synthetic Injectable Biomaterials for Alveolar Bone Regeneration in Animal and Human Studies. Materials 2021, 14, 2858. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Schaller, B.; Zhang, Y.; Pippenger, B.E.; Miron, R.J. In vitro evaluation of an injectable biphasic calcium phosphate (BCP) carrier system combined with recombinant human bone morphogenetic protein (rhBMP)-9. Bio-Med. Mater. Eng. 2017, 28, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Cheon, E.J.; Kim, S.H.; Lee, D.K.; Jo, Y.K.; Ki, M.R.; Park, C.J.; Jang, H.S.; Ahn, J.S.; Pack, S.P.; Jun, S.H. Osteostimulating Ability of beta-tricalcium Phosphate/collagen Composite as a Practical Bone-grafting Substitute: In vitro and in vivo Comparison Study with Commercial One. Biotechnol. Bioproc. E 2021, 26, 923–932. [Google Scholar] [CrossRef]
- Venkatasubbu, G.D.; Ramasamy, S.; Ramakrishnan, V.; Kumar, J. Hydroxyapatite-alginate nanocomposite as drug delivery matrix for sustained release of ciprofloxacin. J. Biomed. Nanotechnol. 2011, 7, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, K.; Kotaki, M.; Ramakrishna, S. Guided bone regeneration membrane made of polycaprolactone/calcium carbonate composite nano-fibers. Biomaterials 2005, 26, 4139–4147. [Google Scholar] [CrossRef]
- Castro, A.G.B.; Diba, M.; Kersten, M.; Jansen, J.A.; van den Beucken, J.J.J.P.; Yang, F. Development of a PCL-silica nanoparticles composite membrane for Guided Bone Regeneration. Mat. Sci. Eng. C-Mater. 2018, 85, 154–161. [Google Scholar] [CrossRef]
- Donos, N.; Akcali, A.; Padhye, N.; Sculean, A.; Calciolari, E. Bone regeneration in implant dentistry: Which are the factors affecting the clinical outcome? Periodontology 2000 2023, 93, 26–55. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.C.; Rolim, W.R.; Viana, M.M.; Souza, T.R.; Gonçalves, F.; Tanaka, C.J.; Bueno-Silva, B.; Seabra, A.B. Biogenic synthesis and antimicrobial activity of silica-coated silver nanoparticles for esthetic dental applications. J. Dent. 2020, 96, 103327. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Song, Y.; Xuan, X.; Chen, S.; Wu, X.; Jiang, H.B.; Lee, E.-S.; Wang, X. Characterization of a bioresorbable magnesium-reinforced PLA-integrated GTR/GBR membrane as dental applications. Scanning 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wyss, U.P.; Pichora, D.; Goosen, M.F. An investigation of poly (lactic acid) degradation. J. Bioact. Compat. Polym. 1994, 9, 80–100. [Google Scholar] [CrossRef]
- Lim, J.; Jun, S.H.; Tallarico, M.; Park, J.B.; Park, D.H.; Hwang, K.G.; Park, C.J. A Randomized Controlled Trial of Guided Bone Regeneration for Peri-Implant Dehiscence Defects with Two Anorganic Bovine Bone Materials Covered by Titanium Meshes. Materials 2022, 15. [Google Scholar] [CrossRef] [PubMed]
- Eftekhar Ashtiani, R.; Alam, M.; Tavakolizadeh, S.; Abbasi, K.; Yazdanian, M. The Role of Biomaterials and Biocompatible Materials in Implant-Supported Dental Prosthesis. Evid.-Based Complement. Altern. Med. 2021, 2021, 3349433. [Google Scholar] [CrossRef] [PubMed]
- Dureja, A.; Acharya, S.R.; Kini, S.; Mayya, A.; Hedge, V. Biocompatibility and Performance of Dental Composite Restorations: A Narrative Review on Free Monomer Release, Concerns and Solutions. Eng. Proc. 2024, 59, 160. [Google Scholar] [CrossRef]
- Singer, L.; Fouda, A.; Bourauel, C. Biomimetic approaches and materials in restorative and regenerative dentistry: Review article. BMC Oral Health 2023, 23, 105. [Google Scholar] [CrossRef]
- Furko, M.; Horváth, Z.E.; Czömpöly, O.; Balázsi, K.; Balázsi, C. Biominerals Added Bioresorbable Calcium Phosphate Loaded Biopolymer Composites. Int. J. Mol. Sci. 2022, 23, 15737. [Google Scholar] [CrossRef]
- Wen, B.; Dai, Y.; Han, X.; Huo, F.; Xie, L.; Yu, M.; Wang, Y.; An, N.; Li, Z.; Guo, W. Biomineralization-inspired mineralized hydrogel promotes the repair and regeneration of dentin/bone hard tissue. NPJ Regen. Med. 2023, 8, 11. [Google Scholar] [CrossRef]
- Liang, J.; Lu, X.; Zheng, X.; Li, Y.R.; Geng, X.; Sun, K.; Cai, H.; Jia, Q.; Jiang, H.B.; Liu, K. Modification of titanium orthopedic implants with bioactive glass: A systematic review of in vivo and in vitro studies. Front. Bioeng. Biotechnol. 2023, 11, 1269223. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhao, X.; Zhao, J.; Zhao, Z.; Wang, Q.; Zhang, C. Biologically Modified Polyether Ether Ketone as Dental Implant Material. Front. Bioeng. Biotechnol. 2020, 8, 620537. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Liu, P.; Zhang, X.; Jingguo, x.; Yongjie, w.; Zou, X.; Mei, X.; Zhang, S.; Zhang, S. Strategies to improve bioactive and antibacterial properties of polyetheretherketone (PEEK) for use as orthopedic implants. Mater. Today Bio 2022, 16, 100402. [Google Scholar] [CrossRef] [PubMed]
- Gultan, T.; Yurtsever, M.C.; Gumusderelioglu, M. NaOH-etched/boron-doped nanohydroxyapatite-coated PEEK implants enhance the proliferation and differentiation of osteogenic cells. Biomed. Mater. 2020, 15, 035019. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhou, W.; Jia, Z.; Xiong, P.; Li, Y.; Wang, P.; Li, Q.; Cheng, Y.; Zheng, Y. Endowing polyetheretherketone with synergistic bactericidal effects and improved osteogenic ability. Acta Biomater. 2018, 79, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Xia, D.; Zhou, W.; Li, Y.; Xiong, P.; Li, Q.; Wang, P.; Li, M.; Zheng, Y.; Cheng, Y. pH-responsive silk fibroin-based CuO/Ag micro/nano coating endows polyetheretherketone with synergistic antibacterial ability, osteogenesis, and angiogenesis. Acta Biomater. 2020, 115, 220–234. [Google Scholar] [CrossRef] [PubMed]
- Asahara, H.; Inui, M.; Lotz, M.K. Tendons and Ligaments: Connecting Developmental Biology to Musculoskeletal Disease Pathogenesis. J. Bone Miner. Res. 2017, 32, 1773–1782. [Google Scholar] [CrossRef] [PubMed]
- Leong, N.L.; Kator, J.L.; Clemens, T.L.; James, A.; Enamoto-Iwamoto, M.; Jiang, J. Tendon and Ligament Healing and Current Approaches to Tendon and Ligament Regeneration. J. Orthop. Res. 2020, 38, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Shiroud Heidari, B.; Ruan, R.; Vahabli, E.; Chen, P.; De-Juan-Pardo, E.M.; Zheng, M.; Doyle, B. Natural, synthetic and commercially-available biopolymers used to regenerate tendons and ligaments. Bioact. Mater. 2023, 19, 179–197. [Google Scholar] [CrossRef]
- Silva, M.; Ferreira, F.N.; Alves, N.M.; Paiva, M.C. Biodegradable polymer nanocomposites for ligament/tendon tissue engineering. J. Nanobiotechnol. 2020, 18, 23. [Google Scholar] [CrossRef]
- Zhu, C.; Qiu, J.; Thomopoulos, S.; Xia, Y. Augmenting Tendon-to-Bone Repair with Functionally Graded Scaffolds. Adv. Healthc. Mater. 2021, 10, e2002269. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.; Zhang, T.; Ju, W.; Chen, X.; Heng, B.C.; Shen, W.; Yin, Z. Biomimetic strategies for tendon/ligament-to-bone interface regeneration. Bioact. Mater. 2021, 6, 2491–2510. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, X.; Han, J.; Yu, Y.; Chen, F.; Yao, J. Boost Tendon/Ligament Repair with Biomimetic and Smart Cellular Constructs. Front. Bioeng. Biotechnol. 2021, 9, 726041. [Google Scholar] [CrossRef]
- Wu, G.; Deng, X.; Song, J.; Chen, F. Enhanced biological properties of biomimetic apatite fabricated polycaprolactone/chitosan nanofibrous bio-composite for tendon and ligament regeneration. J. Photochem. Photobiol. B Biol. 2018, 178, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Li, L.; Dong, L.; Wang, S.; Huang, Z.; Qian, Y.; Wang, C.; Liu, W.; Yang, L. Gradient Biomineralized Silk Fibroin Nanofibrous Scaffold with Osteochondral Inductivity for Integration of Tendon to Bone. ACS Biomater. Sci. Eng. 2020, 7, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Lagier, R.; Baud, C.A. Magnesium whitlockite, a calcium phosphate crystal of special interest in pathology. Pathol. Res. Pract. 2003, 199, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Zhao, Y.; Li, J.; Chen, X.; Lu, Z.; Li, L.; Guo, J. Citrate-based mussel-inspired magnesium whitlockite composite adhesives augmented bone-to-tendon healing. J. Mater. Chem. B 2021, 9, 8202–8210. [Google Scholar] [CrossRef]
- Palmer, L.C.; Newcomb, C.J.; Kaltz, S.R.; Spoerke, E.D.; Stupp, S.I. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem. Rev. 2008, 108, 4754–4783. [Google Scholar] [CrossRef]
- Richardson, M. Understanding the structure and function of the skin. Nurs. Times 2003, 99, 46–48. [Google Scholar]
- Landen, N.X.; Li, D.; Stahle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef]
- Ye, H.; De, S. Thermal injury of skin and subcutaneous tissues: A review of experimental approaches and numerical models. Burns 2017, 43, 909–932. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Jan, A.; Wajid, M.A.; Tariq, S. Management of Chronic Non-Healing Wounds by Hirudotherapy. World J. Plast. Surg. 2017, 6, 9–17. [Google Scholar] [PubMed]
- Zhang, H.; Lin, X.; Cao, X.; Wang, Y.; Wang, J.; Zhao, Y. Developing natural polymers for skin wound healing. Bioact. Mater. 2024, 33, 355–376. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.M.; Ngoc Le, T.T.; Nguyen, A.T.; Thien Le, H.N.; Pham, T.T. Biomedical materials for wound dressing: Recent advances and applications. RSC Adv. 2023, 13, 5509–5528. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Liang, Y.; Zhang, H.; Guo, B. Antibacterial biomaterials for skin wound dressing. Asian J. Pharm. Sci. 2022, 17, 353–384. [Google Scholar] [CrossRef] [PubMed]
- Hodge, J.G.; Zamierowski, D.S.; Robinson, J.L.; Mellott, A.J. Evaluating polymeric biomaterials to improve next generation wound dressing design. Biomater. Res. 2022, 26, 50. [Google Scholar] [CrossRef]
- Oliveira, C.; Sousa, D.; Teixeira, J.A.; Ferreira-Santos, P.; Botelho, C.M. Polymeric biomaterials for wound healing. Front. Bioeng. Biotechnol. 2023, 11, 1136077. [Google Scholar] [CrossRef] [PubMed]
- Alven, S.; Peter, S.; Mbese, Z.; Aderibigbe, B.A. Polymer-Based Wound Dressing Materials Loaded with Bioactive Agents: Potential Materials for the Treatment of Diabetic Wounds. Polymers 2022, 14, 724. [Google Scholar] [CrossRef]
- Rozan, H.E.; Wu, G.; Zhou, Z.; Li, Q.; Sharaf, M.; Chen, X. The complex hydrogel based on diatom biosilica and hydroxybutyl chitosan for wound healing. Colloids Surf. B Biointerfaces 2022, 216, 112523. [Google Scholar] [CrossRef]
- Ehterami, A.; Salehi, M.; Farzamfar, S.; Samadian, H.; Vaez, A.; Sahrapeyma, H.; Ghorbani, S. A promising wound dressing based on alginate hydrogels containing vitamin D3 cross-linked by calcium carbonate/d-glucono-delta-lactone. Biomed. Eng. Lett. 2020, 10, 309–319. [Google Scholar] [CrossRef]
- He, W.; Huang, X.; Zhang, J.; Zhu, Y.; Liu, Y.; Liu, B.; Wang, Q.; Huang, X.; He, D. CaCO(3)-Chitosan Composites Granules for Instant Hemostasis and Wound Healing. Materials 2021, 14, 3350. [Google Scholar] [CrossRef] [PubMed]
- Min, K.H.; Kim, D.H.; Youn, S.; Pack, S.P. Biomimetic Diatom Biosilica and Its Potential for Biomedical Applications and Prospects: A Review. Int. J. Mol. Sci. 2024, 25, 2023. [Google Scholar] [CrossRef]
- Li, Q.; Hu, E.; Yu, K.; Xie, R.; Lu, F.; Lu, B.; Bao, R.; Zhao, T.; Dai, F.; Lan, G. Self-Propelling Janus Particles for Hemostasis in Perforating and Irregular Wounds with Massive Hemorrhage. Adv. Funct. Mater. 2020, 30, 2004153. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, Y.; Li, Z.; Chen, J.; Luo, J.; Bai, L.; Huang, H.; Cao, E.; Yin, Z.; Han, Y.; et al. Injectable Self-Expanding/Self-Propelling Hydrogel Adhesive with Procoagulant Activity and Rapid Gelation for Lethal Massive Hemorrhage Management. Adv. Mater. 2024, 36, 2308701. [Google Scholar] [CrossRef] [PubMed]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Seo, Y.; Kwon, D.; Kang, S.; Yu, J.; Park, H.; Lee, S.D.; Lee, T. Recent Progress in Diatom Biosilica: A Natural Nanoporous Silica Material as Sustained Release Carrier. Pharmaceutics 2023, 15, 2434. [Google Scholar] [CrossRef] [PubMed]
- Delalat, B.; Sheppard, V.C.; Rasi Ghaemi, S.; Rao, S.; Prestidge, C.A.; McPhee, G.; Rogers, M.-L.; Donoghue, J.F.; Pillay, V.; Johns, T.G.; et al. Targeted drug delivery using genetically engineered diatom biosilica. Nat. Commun. 2015, 6, 8791. [Google Scholar] [CrossRef] [PubMed]
- Sasirekha, R.; Sheena, T.S.; Sathiya Deepika, M.; Santhanam, P.; Townley, H.E.; Jeganathan, K.; Dinesh Kumar, S.; Premkumar, K. Surface engineered Amphora subtropica frustules using chitosan as a drug delivery platform for anticancer therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 94, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.; Nazeer, N.; Bissessur, R.; Ahmed, M. Diatoms embedded, self-assembled carriers for dual delivery of chemotherapeutics in cancer cell lines. Int. J. Pharm. 2020, 573, 118887. [Google Scholar] [CrossRef]
- Vasani, R.B.; Losic, D.; Cavallaro, A.; Voelcker, N.H. Fabrication of stimulus-responsive diatom biosilica microcapsules for antibiotic drug delivery. J. Mater. Chem. B 2015, 3, 4325–4329. [Google Scholar] [CrossRef]
- Min, K.H.; Shin, J.W.; Ki, M.-R.; Pack, S.P. Green synthesis of silver nanoparticles on biosilica diatomite: Well-dispersed particle formation and reusability. Process Biochem. 2023, 125, 232–238. [Google Scholar] [CrossRef]
- Li, M.; Wu, J.; Lin, D.; Yang, J.; Jiao, N.; Wang, Y.; Liu, L. A diatom-based biohybrid microrobot with a high drug-loading capacity and pH-sensitive drug release for target therapy. Acta Biomater. 2022, 154, 443–453. [Google Scholar] [CrossRef]
- Arosio, P.; Ingrassia, R.; Cavadini, P. Ferritins: A family of molecules for iron storage, antioxidation and more. Biochimica Biophysica Acta (BBA)—General. Subjects 2009, 1790, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Truffi, M.; Fiandra, L.; Sorrentino, L.; Monieri, M.; Corsi, F.; Mazzucchelli, S. Ferritin nanocages: A biological platform for drug delivery, imaging and theranostics in cancer. Pharmacol. Res. 2016, 107, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Ki, M.-R.; Nguyen, T.K.M.; Jun, H.S.; Pack, S.P. Biosilica-enveloped ferritin cage for more efficient drug deliveries. Process Biochem. 2018, 68, 182–189. [Google Scholar] [CrossRef]
- Ki, M.-R.; Kim, J.K.; Kim, S.H.; Nguyen, T.K.M.; Kim, K.H.; Pack, S.P. Compartment-restricted and rate-controlled dual drug delivery system using a biosilica-enveloped ferritin cage. J. Ind. Eng. Chem. 2020, 81, 367–374. [Google Scholar] [CrossRef]
- Chifiriuc, M.C.; Filip, R.; Constantin, M.; Pircalabioru, G.G.; Bleotu, C.; Burlibasa, L.; Ionica, E.; Corcionivoschi, N.; Mihaescu, G. Common themes in antimicrobial and anticancer drug resistance. Front. Microbiol. 2022, 13, 960693. [Google Scholar] [CrossRef] [PubMed]
- Ugalde-Arbizu, M.; Aguilera-Correa, J.J.; Garcia-Almodovar, V.; Ovejero-Paredes, K.; Diaz-Garcia, D.; Esteban, J.; Paez, P.L.; Prashar, S.; San Sebastian, E.; Filice, M.; et al. Dual Anticancer and Antibacterial Properties of Silica-Based Theranostic Nanomaterials Functionalized with Coumarin343, Folic Acid and a Cytotoxic Organotin(IV) Metallodrug. Pharmaceutics 2023, 15, 560. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Chen, X.; Xu, X.; Zhang, Y.; Zhang, C.; Mo, R. Tumor-Specific Self-Degradable Nanogels as Potential Carriers for Systemic Delivery of Anticancer Proteins. Adv. Funct. Mater. 2018, 28, 1707371. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, F.; Du, Y.; Cui, W.; Dou, Y.; Lin, Y.; Zhao, Z.; Ma, X. Effect of tetrahedral DNA nanostructures on LPS-induced neuroinflammation in mice. Chin. Chem. Lett. 2022, 33, 1901–1906. [Google Scholar] [CrossRef]
- Wang, X.; Shen, X.; Li, J.; Ge, X.; Ouyang, J.; Na, N. Biomineralization of DNA Nanoframeworks for Intracellular Delivery, On-Demand Diagnosis, and Synergistic Cancer Treatments. Anal. Chem. 2022, 94, 16803–16812. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lian, B. Application of Calcium Carbonate as a Controlled Release Carrier for Therapeutic Drugs. Minerals 2023, 13, 1136. [Google Scholar] [CrossRef]
- Kost, J.; Huwyler, J.; Puchkov, M. Calcium Phosphate Microcapsules as Multifunctional Drug Delivery Devices. Adv. Funct. Mater. 2023, 33, 2303333. [Google Scholar] [CrossRef]
- Kurkuri, M.; Losic, D.; Uthappa, U.T.; Jung, H.-Y. Advanced Porous Biomaterials for Drug Delivery Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2022; p. 468. [Google Scholar]
- Miyamaru, C.; Koide, M.; Kato, N.; Matsubara, S.; Higuchi, M. Fabrication of CaCO3-Coated Vesicles by Biomineralization and Their Application as Carriers of Drug Delivery Systems. Int. J. Mol. Sci. 2022, 23, 789. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Ao, Y.; Feng, J.; Wang, C.; Zhang, J. Biomineralization inspired synthesis of CaCO3-based DDS for pH-responsive release of anticancer drug. Mater. Today Commun. 2021, 27, 102256. [Google Scholar] [CrossRef]
- Min, K.H.; Kim, D.H.; Pack, S.P. Size Control of Biomimetic Curved-Edge Vaterite with Chiral Toroid Morphology via Sonochemical Synthesis. Biomimetics 2024, 9, 174. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, A.; Iqbal, M.Z.; Kong, X. Calcium Carbonate-Based Nanoparticles for Gene Delivery. In Gene Delivery; Springer: Singapore, 2022; pp. 481–503. [Google Scholar]
- Liu, Y.; Jiang, Z.; Tong, S.; Sun, Y.; Zhang, Y.; Zhang, J.; Zhao, D.; Su, Y.; Ding, J.; Chen, X. Acidity-Triggered Transformable Polypeptide Self-Assembly to Initiate Tumor-Specific Biomineralization. Adv. Mater. 2023, 35, 220329. [Google Scholar] [CrossRef]
- Lee, S.S.; Du, X.; Kim, I.; Ferguson, S.J. Scaffolds for bone-tissue engineering. Matter 2022, 5, 2722–2759. [Google Scholar] [CrossRef]
- Gardner, A.B.; Lee, S.K.; Woods, E.C.; Acharya, A.P. Biomaterials-based modulation of the immune system. Biomed. Res. Int. 2013, 2013, 732182. [Google Scholar] [CrossRef]
- Dubey, A.; Vahabi, H.; Kumaravel, V. Antimicrobial and Biodegradable 3D Printed Scaffolds for Orthopedic Infections. ACS Biomater. Sci. Eng. 2023, 9, 4020–4044. [Google Scholar] [CrossRef]
- Diao, J.; OuYang, J.; Deng, T.; Liu, X.; Feng, Y.; Zhao, N.; Mao, C.; Wang, Y. 3D-Plotted Beta-Tricalcium Phosphate Scaffolds with Smaller Pore Sizes Improve In Vivo Bone Regeneration and Biomechanical Properties in a Critical-Sized Calvarial Defect Rat Model. Adv. Healthc. Mater. 2018, 7, e1800441. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ye, W.; Ma, Z.; Xie, W.; Zhong, L.; Wang, Y.; Rong, Q. 3D printed PCL/β-TCP cross-scale scaffold with high-precision fiber for providing cell growth and forming bones in the pores. Mater. Sci. Eng. C 2021, 127, 112197. [Google Scholar] [CrossRef]
- Li, M.; Peng, B.; Lyu, Q.; Chen, X.; Hu, Z.; Zhang, X.; Xiong, B.; Zhang, L.; Zhu, J. Scalable production of structurally colored composite films by shearing supramolecular composites of polymers and colloids. Nat. Commun. 2024, 15, 1874. [Google Scholar] [CrossRef] [PubMed]
- Cuneo, T.; Gao, H. Recent advances on synthesis and biomaterials applications of hyperbranched polymers. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1640. [Google Scholar] [CrossRef] [PubMed]
- Qiu, T.; Hanna, E.; Dabbous, M.; Borislav, B.; Toumi, M. Regenerative medicine regulatory policies: A systematic review and international comparison. Health Policy 2020, 124, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Kepplinger, E.E. FDA’s Expedited Approval Mechanisms for New Drug Products. Biotechnol. Law. Rep. 2015, 34, 15–37. [Google Scholar] [CrossRef]
- Abdelhamid, M.A.A.; Ki, M.-R.; Pack, S.P. Biominerals and Bioinspired Materials in Biosensing: Recent Advancements and Applications. Int. J. Mol. Sci. 2024, 25, 4678. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhang, B.; Wang, Z.; Su, W.; Niu, S.; Han, Z.; Ren, L. Bioinspired Strategies for Excellent Mechanical Properties of Composites. J. Bionic Eng. 2022, 19, 1203–1228. [Google Scholar] [CrossRef]
- Xie, J.; Ping, H.; Tan, T.; Lei, L.; Xie, H.; Yang, X.-Y.; Fu, Z. Bioprocess-inspired fabrication of materials with new structures and functions. Prog. Mater. Sci. 2019, 105, 100571. [Google Scholar] [CrossRef]
- Parra-Torrejón, B.; Jayawarna, V.; Rodrigo-Navarro, A.; Gonzalez-Valdivieso, J.; Dobre, O.; Ramírez-Rodríguez, G.B.; Salmeron-Sanchez, M.; Delgado-López, J.M. Bioinspired mineralization of engineered living materials to promote osteogenic differentiation. Biomater. Adv. 2023, 154, 213587. [Google Scholar] [CrossRef]
- Zuo, L.; Yang, Y.; Zhang, H.; Ma, Z.; Xin, Q.; Ding, C.; Li, J. Bioinspired Multiscale Mineralization: From Fundamentals to Potential Applications. Macromol. Biosci. 2023, 24, e2300348. [Google Scholar] [CrossRef] [PubMed]
- Dutour Sikiric, M. Special Issue: Biomimetic Organic-Inorganic Composites. Materials 2022, 15, 3074. [Google Scholar] [CrossRef] [PubMed]
- Gu, G.X.; Chen, C.-T.; Richmond, D.J.; Buehler, M.J. Bioinspired hierarchical composite design using machine learning: Simulation, additive manufacturing, and experiment. Mater. Horiz. 2018, 5, 939–945. [Google Scholar] [CrossRef]
- Choi, W.; Advincula, R.C.; Wu, H.F.; Jiang, Y. Artificial intelligence and machine learning in the design and additive manufacturing of responsive composites. MRS Commun. 2023, 13, 714–724. [Google Scholar] [CrossRef]
- Yu, C.H.; Tseng, B.Y.; Yang, Z.; Tung, C.C.; Zhao, E.; Ren, Z.F.; Yu, S.S.; Chen, P.Y.; Chen, C.S.; Buehler, M.J. Hierarchical Multiresolution Design of Bioinspired Structural Composites Using Progressive Reinforcement Learning. Adv. Theory Simul. 2022, 5, 2200459. [Google Scholar] [CrossRef]
- Park, K.; Song, C.; Park, J.; Ryu, S. Multi-objective Bayesian optimization for the design of nacre-inspired composites: Optimizing and understanding biomimetics through AI. Mater. Horiz. 2023, 10, 4329–4343. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Wanigasekara, C.; Rajan, G.; Swain, A.; Prusty, B.G. An approach for process optimisation of the Automated Fibre Placement (AFP) based thermoplastic composites manufacturing using Machine Learning, photonic sensing and thermo-mechanics modelling. Manuf. Lett. 2022, 32, 10–14. [Google Scholar] [CrossRef]
- Chiu, Y.-H.; Liao, Y.-H.; Juang, J.-Y. Designing Bioinspired Composite Structures via Genetic Algorithm and Conditional Variational Autoencoder. Polymers 2023, 15, 281. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, Y.; Yang, C.; Park, K.; Gu, G.X.; Ryu, S. Deep learning framework for material design space exploration using active transfer learning and data augmentation. Npj Comput. Mater. 2021, 7, 140. [Google Scholar] [CrossRef]
- Sharma, A.; Mukhopadhyay, T.; Rangappa, S.M.; Siengchin, S.; Kushvaha, V. Advances in Computational Intelligence of Polymer Composite Materials: Machine Learning Assisted Modeling, Analysis and Design. Arch. Comput. Methods Eng. 2022, 29, 3341–3385. [Google Scholar] [CrossRef]
- Chen, G.; Liang, X.; Zhang, P.; Lin, S.; Cai, C.; Yu, Z.; Liu, J. Bioinspired 3D Printing of Functional Materials by Harnessing Enzyme-Induced Biomineralization. Adv. Funct. Mater. 2022, 32, 2113262. [Google Scholar] [CrossRef]
- Jia, Z.; Deng, Z.; Li, L. Biomineralized Materials as Model Systems for Structural Composites: 3D Architecture. Adv. Mater. 2022, 34, 2106259. [Google Scholar] [CrossRef] [PubMed]
- Jagadeesh, P.; Puttegowda, M.; Rangappa, S.M.; Alexey, K.; Gorbatyuk, S.; Khan, A.; Doddamani, M.; Siengchin, S. A comprehensive review on 3D printing advancements in polymer composites: Technologies, materials, and applications. Int. J. Adv. Manuf. Technol. 2022, 121, 127–169. [Google Scholar] [CrossRef]
- Zhang, B.; Gui, X.; Song, P.; Xu, X.; Guo, L.; Han, Y.; Wang, L.; Zhou, C.; Fan, Y.; Zhang, X. Three-Dimensional Printing of Large-Scale, High-Resolution Bioceramics with Micronano Inner Porosity and Customized Surface Characterization Design for Bone Regeneration. ACS Appl. Mater. Interfaces 2022, 14, 8804–8815. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Luo, D.; Liu, Y. Effect of the nano/microscale structure of biomaterial scaffolds on bone regeneration. Int. J. Oral Sci. 2020, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Di Marzio, N.; Eglin, D.; Serra, T.; Moroni, L. Bio-Fabrication: Convergence of 3D Bioprinting and Nano-Biomaterials in Tissue Engineering and Regenerative Medicine. Front. Bioeng. Biotechnol. 2020, 8, 326. [Google Scholar] [CrossRef]
- Bhatti, S.S.; Singh, J. 3D printing of biomaterials for biomedical applications: A review. Int. J. Interact. Des. Manuf. (IJIDeM) 2023. [Google Scholar] [CrossRef]



| Base Material | Biomineral for Composite | Structural Composite/ Fabrication | Potential Advantages | Ref. | ||
|---|---|---|---|---|---|---|
| Bone | Non-corrosion metal | Electrospun PCL nanofibers@titanium | Carbonated HAP nanoparticles | Coating | Improve cell adhesion, corrosion resistance, and overall implant properties | [143] |
| Synthetic polymer | PCL | BG | Doping | High cell-response values and toughness | [149] | |
| Polyphosphazene | HAP | Solvent casting, melt blending, or in situ polymerization | Enhanced bioactivity, biocompatibility, and osteoconductivity | [155] | ||
| PLLA | HAP | HAP absorbed on porous, 3D-printed PLLA screw | Increase the inductivity of bone, promote bone growth in the bone tunnel, and promote bone integration at the tendon–bone interface | [156] | ||
| Natural polymer | Gelatin | Biosilica, CaCO3 | Immobilization on electrospun fiber | Improve cell attachment and bone differentiation | [152] | |
| Chitosan, collagen, silk fibroin, hyaluronic acid, and gelatin | Nano-HAP | Freeze-drying | Enhanced cellular attachment, survival, and osteogenic differentiation Improved mechanical properties | [154] | ||
| Collagen | Biosilica/β-TCP | Embedded in collagen | Improve bone regeneration | [158] | ||
| Dental | Ceramic granule | HAP | Biosilica | Coating | Enhanced BMP2 delivery and bone regeneration | [159] |
| HAP TEA and ethanol | Amorphous calcium phosphate Calcium phosphate ion clusters | Epitaxial growth of enamel apatite crystals | Similar morphological texture and mechanical strength between the repaired layer and native enamel | [164] | ||
| BMP2 | Autologous bone | Adsorption | Enhanced bone growth | [167] | ||
| Polymer and gelatin | Bovine bone mineral | Xenograft enriched with gelatin and a polymer | Higher proportion of lamellar bone and osteoid | [170] | ||
| Calcium phosphate PCL | Calcium phosphate Mg, Zn, Sr | Spin coating | Faster dissolution rate | [185] | ||
| Nanoparticles | Silver nanoparticles | Silica-coated silver nanoparticles | Coating | Biocompatible and antimicrobial | [178] | |
| Polymer | Collagen | Magnesium-doped hydroxyapatite | Embedded | Strong cell–material interaction | [114] | |
| Polyacrylic acid, carboxymethyl chitosan, and dentin matrix | Calcium phosphates | Embedded in hydrogel | Self-repairing ability, injectability, and the promotion of odontogenesis and osteogenic differentiation | [186] | ||
| Implants | Ti implants | BGs | Coating | Excellent cell compatibility, antibacterial and anti-inflammatory properties, and higher levels of osseointegration and osteogenesis | [187] | |
| PEEK implants | Nano-HAP | Coating | Enhanced proliferation and differentiation of osteogenic cells | [190] | ||
| Tendon/ Ligament | Synthetic polymer | PCL/chitosan | Nano-HAP | Embedded via an in situ sol-gel process | Enhancement of morphological, mechanical, and biological properties in favor of tendon and ligament regeneration | [200] |
| Citrate-based, mussel-inspired adhesive Prepolymer (PEG-PPG-PEG) | Magnesium whitlockite | Embedded in injectable adhesive | Hemostatic ability, osteoconductivity, and osteo-inductivity Promote a conducive environment for bone-tendon healing | [203] | ||
| Natural polymer | Silk fibroin | SBF | Gradient coating | Bone marrow mesenchymal stem cell growth and differentiation Improved osseointegration | [201] | |
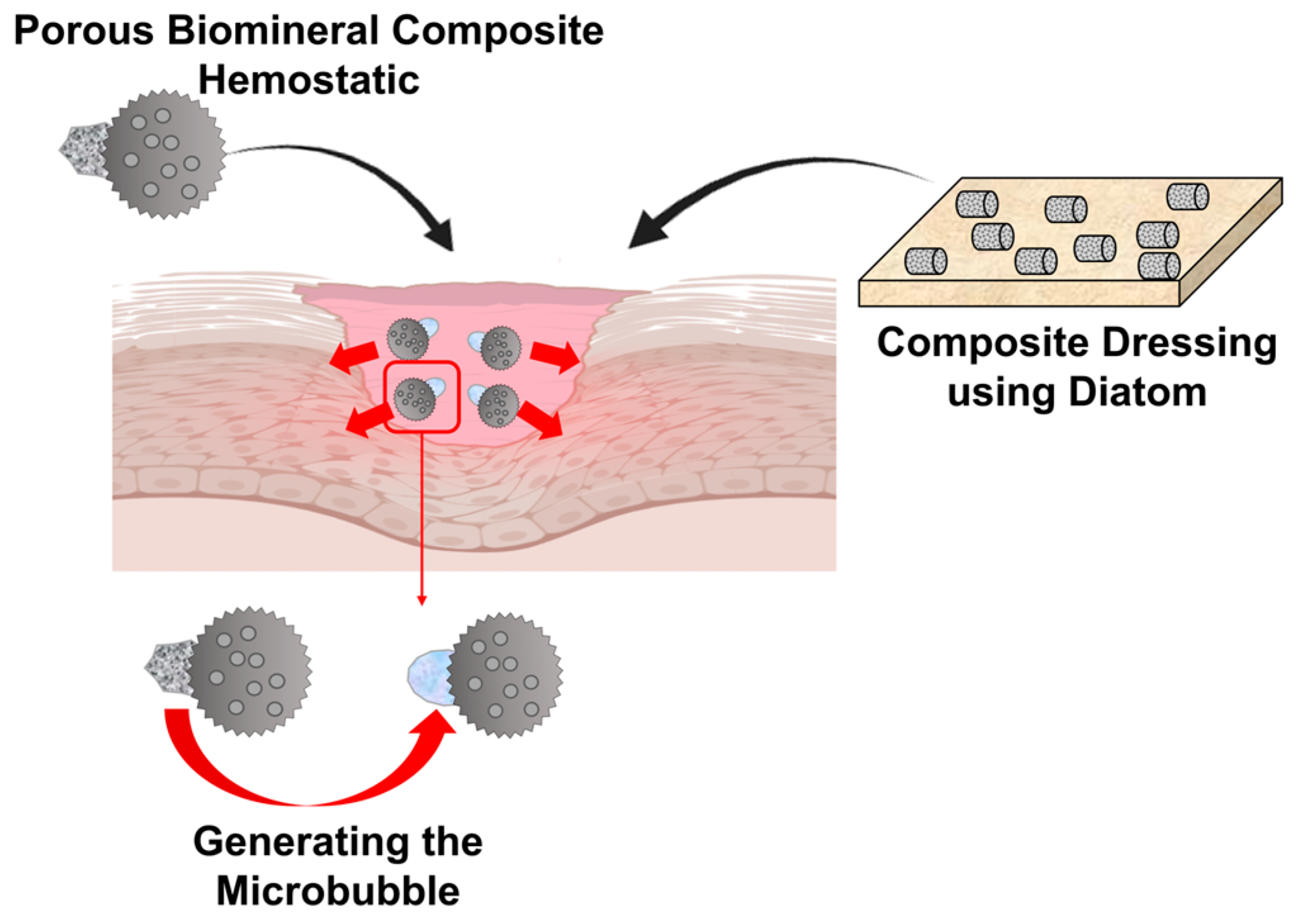
| Wound healing | Natural polymer Synthetic polymer Protein | Hydroxybutyl chitosan | Diatom biosilica loaded with doxycycline | Coating | Improve hemostasis and hemorrhage High loading capacity and sustained release of doxycycline Antimicrobial activity | [215] |
| Chitosan | CaCO3 | Embedded | Instant hemostasis accelerated wound healing | [217] | ||
| Negatively modified, microporous starch | CaCO3 | Flower-shaped calcium carbonate crystals uniaxially grown on microporous starch | Rapid hemorrhage control of deep bleeding sites | [219] | ||
| Oxidized dextran Quaternized chitosan | CaCO3 | Embedded in hydrogel in the form of oxidized detran/CaCO3 mixture | Hemostatic CO2 forming | [220] | ||
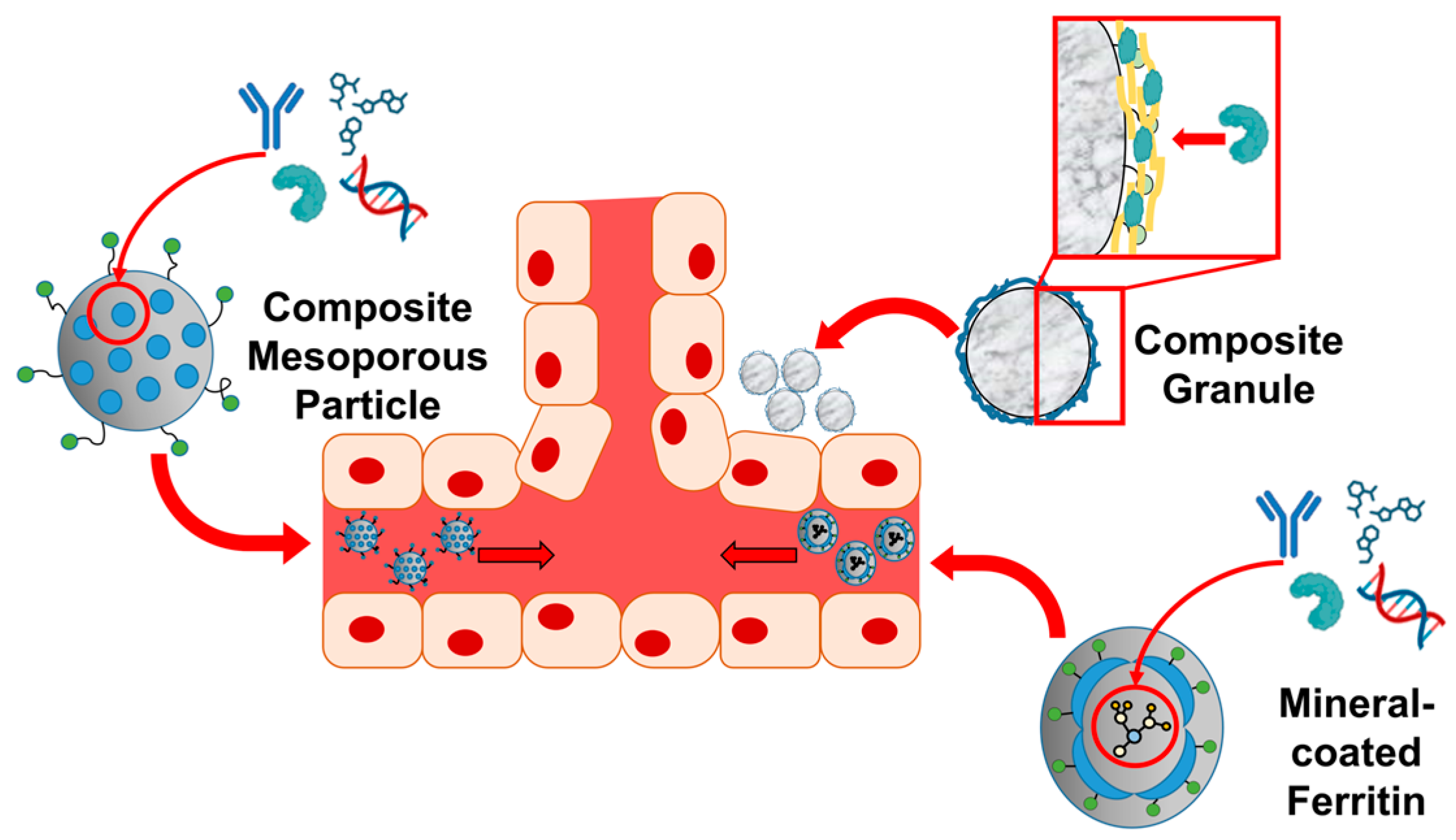
| Drug delivery | Mineral | BMP2 | Biosilica | Coprecipitates | Enhanced BMP2 delivery and osteogenesis | [75,76] |
| Diatom | Diatom biosilica | Fe3O4 magnetic nanoparticle | Attachment to diatom biosilica | Controllable with magnets | [228] | |
| Cage protein | Ferritin | Silica | Silica coating | Controllable drug delivery | [77,231,232] | |
| DNA | DNA nanoframework | Silica | Silica coating | Effectively prevent degradations and leakages of loaded siRNA and doxorubicin | [237] | |
| Vesicles | Vesicles embedded with the peptide lipids | CaCO3 | CaCO3-coated vesicles | pH-controlled release | [241] | |
| Amino acid | L-Lysine | CaCO3 | l-lysine-mediated CaCO3 synthesis | Significant differences in drug-loading rate, loading capacity, and pH sensitivity due to differences in crystal form and morphology | [242] | |
| L-aspartic acid, D-aspartic acid | CaCO3 | Chiral-curved CaCO3 | Control of morphology and size of CaCO3, | [243] | ||
| Peptide | CPP-KR12 | Biosilica | Coprecipitates | Reduced cytotoxicity Enhanced antimicrobial peptide delivery | [35] | |
| Dodecylamine-poly((γ dodecyl-l- glutamate)- co-(l-histidine))-block-poly(l-glutamate-graft-alendronate) | Calcium phosphate | Coprecipitates via ionic interaction | Blockade therapy for osteosarcoma and inhibition of pulmonary metastases | [245] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.H.; Ki, M.-R.; Han, Y.; Pack, S.P. Biomineral-Based Composite Materials in Regenerative Medicine. Int. J. Mol. Sci. 2024, 25, 6147. https://doi.org/10.3390/ijms25116147
Kim SH, Ki M-R, Han Y, Pack SP. Biomineral-Based Composite Materials in Regenerative Medicine. International Journal of Molecular Sciences. 2024; 25(11):6147. https://doi.org/10.3390/ijms25116147
Chicago/Turabian StyleKim, Sung Ho, Mi-Ran Ki, Youngji Han, and Seung Pil Pack. 2024. "Biomineral-Based Composite Materials in Regenerative Medicine" International Journal of Molecular Sciences 25, no. 11: 6147. https://doi.org/10.3390/ijms25116147





