Evolving Strategies for Extracellular Vesicles as Future Cardiac Therapeutics: From Macro- to Nano-Applications
Abstract
1. The Burden of Cardiovascular Disease: From Cell Therapy to Paracrine Strategies
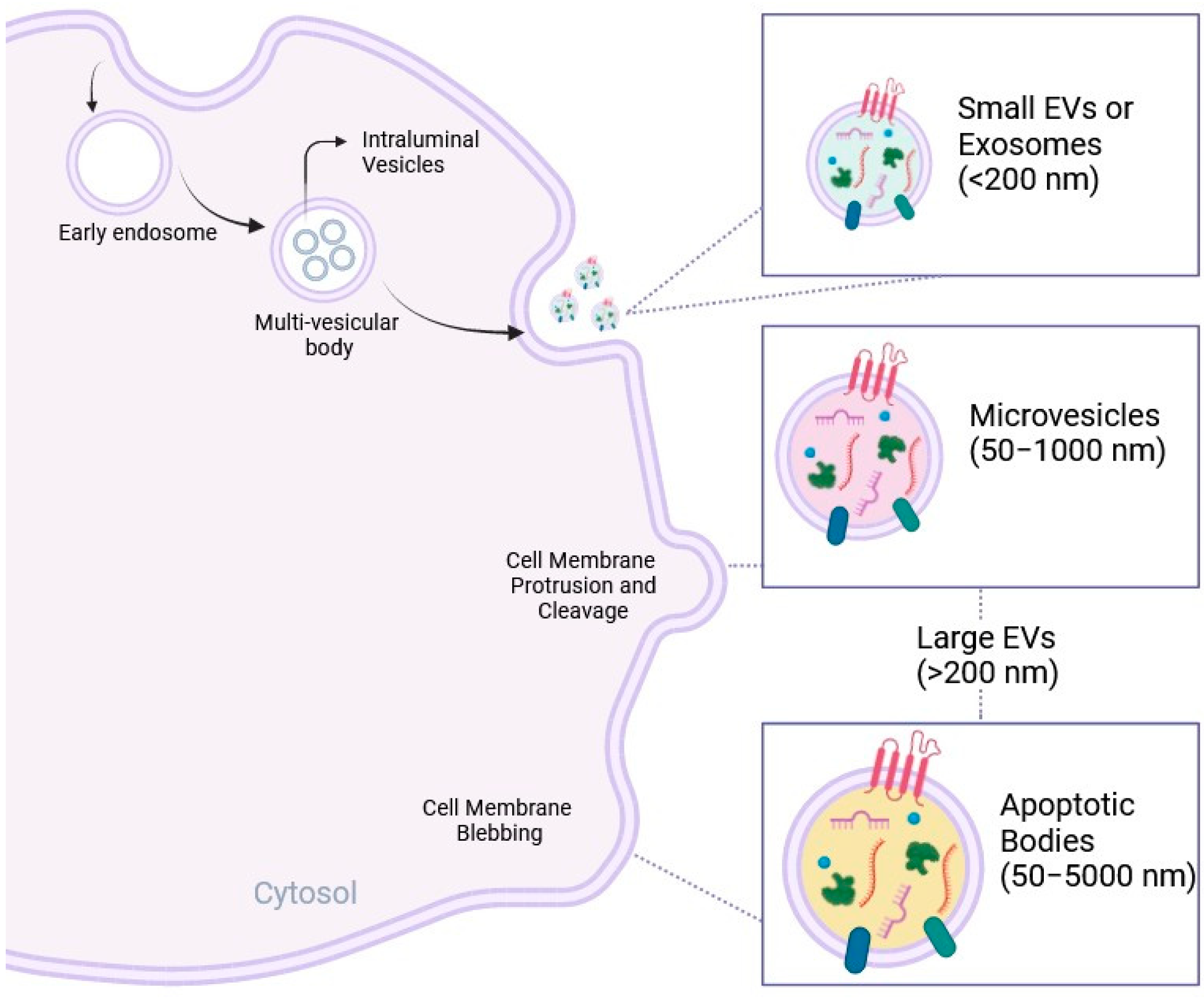
2. EVs as Biological Conveyors of Cell Communication
3. Exploiting EVs in Cardiovascular Disease
4. Defining the Optimal Source of EVs for Future Cardiac Paracrine Therapy
5. Optimization of Cardiac Delivery: From Macro- to Nano-Applications
6. Translational Challenges
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Truby, L.K.; Rogers, J.G. Advanced Heart Failure: Epidemiology, Diagnosis, and Therapeutic Approaches. JACC Heart Fail. 2020, 8, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Roger, V.L. Epidemiology of Heart Failure. Circ. Res. 2013, 113, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Segers, V.F.M.; Lee, R.T. Stem-Cell Therapy for Cardiac Disease. Nature 2008, 451, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, O.; Bhardwaj, R.D.; Bernard, S.; Zdunek, S.; Barnabé-Heide, F.; Walsh, S.; Zupicich, J.; Alkass, K.; Buchholz, B.A.; Druid, H.; et al. Evidence for Cardiomyocyte Renewal in Humans. Science 2009, 324, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Ferrini, A.; Stevens, M.M.; Sattler, S.; Rosenthal, N. Toward Regeneration of the Heart: Bioengineering Strategies for Immunomodulation. Front. Cardiovasc. Med. 2019, 6, 26. [Google Scholar] [CrossRef]
- Lundy, S.D.; Gantz, J.A.; Pagan, C.M.; Filice, D.; Laflamme, M.A. Pluripotent Stem Cell Derived Cardiomyocytes for Cardiac Repair. Curr. Treat. Options Cardiovasc. Med. 2014, 16, 319. [Google Scholar] [CrossRef]
- Paoletti, C.; Divieto, C.; Chiono, V. Impact of Biomaterials on Differentiation and Reprogramming Approaches for the Generation of Functional Cardiomyocytes. Cells 2018, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- Madonna, R.; Van Laake, L.W.; Davidson, S.M.; Engel, F.B.; Hausenloy, D.J.; Lecour, S.; Leor, J.; Perrino, C.; Schulz, R.; Ytrehus, K.; et al. Position Paper of the European Society of Cardiology Working Group Cellular Biology of the Heart: Cell-Based Therapies for Myocardial Repair and Regeneration in Ischemic Heart Disease and Heart Failure. Eur. Heart J. 2016, 37, 1789–1798. [Google Scholar] [CrossRef]
- Volkova, M.; Raymond Russell, I. Anthracycline Cardiotoxicity: Prevalence, Pathogenesis and Treatment. Curr. Cardiol. Rev. 2011, 7, 214. [Google Scholar] [CrossRef]
- Guha, A.; Caraballo, C.; Jain, P.; Miller, P.E.; Owusu-Guha, J.; Clark, K.A.A.; Velazquez, E.J.; Ahmad, T.; Baldassarre, L.A.; Addison, D.; et al. Outcomes in Patients with Anthracycline-induced Cardiomyopathy Undergoing Left Ventricular Assist Devices Implantation. ESC Heart Fail. 2021, 8, 2866. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; Dainiak, N.; Berger, H.J.; Goldman, L.; Johnstone, D.; Reduto, L.; Duffy, T.; Schwartz, P.; Gottschalk, A.; Zaret, B.L.; et al. Serial Assessment of Doxorubicin Cardiotoxicity with Quantitative Radionuclide Angiocardiography. N. Engl. J. Med. 2010, 300, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Broughton, K.M.; Wang, B.J.; Firouzi, F.; Khalafalla, F.; Dimmeler, S.; Fernandez-Aviles, F.; Sussman, M.A. Mechanisms of Cardiac Repair and Regeneration. Circ. Res. 2018, 122, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Sid-Otmane, C.; Perrault, L.P.; Ly, H.Q. Mesenchymal Stem Cell Mediates Cardiac Repair through Autocrine, Paracrine and Endocrine Axes. J. Transl. Med. 2020, 18, 336. [Google Scholar] [CrossRef]
- Menasché, P. Cell Therapy Trials for Heart Regeneration—Lessons Learned and Future Directions. Nat. Rev. Cardiol. 2018, 15, 659–671. [Google Scholar] [CrossRef]
- Gnecchi, M.; He, H.; Noiseux, N.; Liang, O.D.; Zhang, L.; Morello, F.; Mu, H.; Melo, L.G.; Pratt, R.E.; Ingwall, J.S.; et al. Evidence Supporting Paracrine Hypothesis for Akt-Modified Mesenchymal Stem Cell-Mediated Cardiac Protection and Functional Improvement. FASEB J. 2006, 20, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal Information for Studies of Extracellular Vesicles (MISEV2023): From Basic to Advanced Approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Lei, Y.; Zheng, J.; Peng, J.; Li, Y.; Yu, L.; Chen, Y. Identification of Markers for Migrasome Detection. Cell Discov. 2019, 5, 27. [Google Scholar] [CrossRef]
- Kakarla, R.; Hur, J.; Kim, Y.J.; Kim, J.; Chwae, Y.J. Apoptotic Cell-Derived Exosomes: Messages from Dying Cells. Exp. Mol. Med. 2020, 52, 1–6. [Google Scholar] [CrossRef]
- Stȩpień, E.; Rzaca, C.; Moskal, P. Novel Biomarker and Drug Delivery Systems for Theranostics—Extracellular Vesicles. Bio-Algorithms Med-Syst. 2021, 17, 301–309. [Google Scholar] [CrossRef]
- Vilaça-Faria, H.; Salgado, A.J.; Teixeira, F.G. Mesenchymal Stem Cells-Derived Exosomes: A New Possible Therapeutic Strategy for Parkinson’s Disease? Cells 2019, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Li, Y.; Peng, J.; Wu, D.; Zhao, X.; Cui, Y.; Chen, L.; Yan, X.; Du, Y.; Yu, L. Discovery of the Migrasome, an Organelle Mediating Release of Cytoplasmic Contents during Cell Migration. Cell Res. 2014, 25, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Klibi, J.; Niki, T.; Riedel, A.; Pioche-Durieu, C.; Souquere, S.; Rubinstein, E.; Moulec, S.L.E.; Guigay, J.; Hirashima, M.; Guemira, F.; et al. Blood Diffusion and Th1-Suppressive Effects of Galectin-9-Containing Exosomes Released by Epstein-Barr Virus-Infected Nasopharyngeal Carcinoma Cells. Blood 2009, 113, 1957–1966. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and Mechanisms of Extracellular Vesicle Uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef] [PubMed]
- Costa Verdera, H.; Gitz-Francois, J.J.; Schiffelers, R.M.; Vader, P. Cellular Uptake of Extracellular Vesicles Is Mediated by Clathrin-Independent Endocytosis and Macropinocytosis. J. Control Release 2017, 266, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Popēna, I.; Ābols, A.; Saulīte, L.; Pleiko, K.; Zandberga, E.; Jēkabsons, K.; Endzeliņš, E.; Llorente, A.; Linē, A.; Riekstiņa, U. Effect of Colorectal Cancer-Derived Extracellular Vesicles on the Immunophenotype and Cytokine Secretion Profile of Monocytes and Macrophages. Cell Commun. Signal. 2018, 16, 17. [Google Scholar] [CrossRef] [PubMed]
- Pužar Dominkuš, P.; Stenovec, M.; Sitar, S.; Lasič, E.; Zorec, R.; Plemenitaš, A.; Žagar, E.; Kreft, M.; Lenassi, M. PKH26 Labeling of Extracellular Vesicles: Characterization and Cellular Internalization of Contaminating PKH26 Nanoparticles. Biochim. Biophys. Acta Biomembr. 2018, 1860, 1350–1361. [Google Scholar] [CrossRef] [PubMed]
- Joshi, B.S.; de Beer, M.A.; Giepmans, B.N.G.; Zuhorn, I.S. Endocytosis of Extracellular Vesicles and Release of Their Cargo from Endosomes. ACS Nano 2020, 14, 4444–4455. [Google Scholar] [CrossRef]
- Klyachko, N.L.; Arzt, C.J.; Li, S.M.; Gololobova, O.A.; Batrakova, E.V. Extracellular Vesicle-Based Therapeutics: Preclinical and Clinical Investigations. Pharmaceutics 2020, 12, 1171. [Google Scholar] [CrossRef]
- Minciacchi, V.R.; Freeman, M.R.; Di Vizio, D. Extracellular Vesicles in Cancer: Exosomes, Microvesicles and the Emerging Role of Large Oncosomes. Semin. Cell Dev. Biol. 2015, 40, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Bebelman, M.P.; Smit, M.J.; Pegtel, D.M.; Baglio, S.R. Biogenesis and Function of Extracellular Vesicles in Cancer. Pharmacol. Ther. 2018, 188, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bao, Q.; Huang, Q.; Chen, Y.; Wang, Q.; Sang, R.; Wang, L.; Xie, Y.; Chen, W. Tumor-Derived Extracellular Vesicles Regulate Cancer Progression in the Tumor Microenvironment. Front. Mol. Biosci. 2022, 8, 796385. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Miyaki, S.; Ishitobi, H.; Matsuyama, S.; Nakasa, T.; Kamei, N.; Akimoto, T.; Higashi, Y.; Ochi, M. Mesenchymal-Stem-Cell-Derived Exosomes Accelerate Skeletal Muscle Regeneration. FEBS Lett. 2015, 589, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yu, Y.; Hu, S.; Chen, Y.; Shen, Z. The Therapeutic Potential of Mesenchymal Stem Cells for Cardiovascular Diseases. Cell Death Dis. 2020, 11, 349. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.T.; White, A.J.; Matsushita, S.; Malliaras, K.; Steenbergen, C.; Zhang, Y.; Li, T.S.; Terrovitis, J.; Yee, K.; Simsir, S.; et al. Intramyocardial Injection of Autologous Cardiospheres or Cardiosphere-Derived Cells Preserves Function and Minimizes Adverse Ventricular Remodeling in Pigs With Heart Failure Post-Myocardial Infarction. J. Am. Coll. Cardiol. 2011, 57, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Gallet, R.; Dawkins, J.; Valle, J.; Simsolo, E.; De Couto, G.; Middleton, R.; Tseliou, E.; Luthringer, D.; Kreke, M.; Smith, R.R.; et al. Exosomes Secreted by Cardiosphere-Derived Cells Reduce Scarring, Attenuate Adverse Remodelling, and Improve Function in Acute and Chronic Porcine Myocardial Infarction. Eur. Heart J. 2017, 38, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Peng, Y.; Feng, Y.; Xu, Z.; Feng, P.; Cao, J.; Chen, Y.; Chen, X.; Cao, X.; Yang, Y.; et al. Immune Cell-Derived Extracellular Vesicles—New Strategies in Cancer Immunotherapy. Front. Immunol. 2021, 12, 771551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, C.; Hu, B.; Niu, X.; Liu, X.; Zhang, G.; Zhang, C.; Li, Q.; Wang, Y. Exosomes Derived from Human Endothelial Progenitor Cells Accelerate Cutaneous Wound Healing by Promoting Angiogenesis Through Erk1/2 Signaling. Int. J. Biol. Sci. 2016, 12, 1472. [Google Scholar] [CrossRef] [PubMed]
- Gebara, N.; Scheel, J.; Skovronova, R.; Grange, C.; Marozio, L.; Gupta, S.; Giorgione, V.; Caicci, F.; Benedetto, C.; Khalil, A.; et al. Single Extracellular Vesicle Analysis in Human Amniotic Fluid Shows Evidence of Phenotype Alterations in Preeclampsia. J. Extracell. Vesicles 2022, 11, 12217. [Google Scholar] [CrossRef]
- Alić, V.K.; Malenica, M.; Biberić, M.; Zrna, S.; Valenčić, L.; Šuput, A.; Fabris, L.K.; Wechtersbach, K.; Kojc, N.; Kurtjak, M.; et al. Extracellular Vesicles from Human Cerebrospinal Fluid Are Effectively Separated by Sepharose CL-6B—Comparison of Four Gravity-Flow Size Exclusion Chromatography Methods. Biomedicines 2022, 10, 785. [Google Scholar] [CrossRef] [PubMed]
- Hinzman, C.P.; Jayatilake, M.; Bansal, S.; Fish, B.L.; Li, Y.; Zhang, Y.; Bansal, S.; Girgis, M.; Iliuk, A.; Xu, X.; et al. An Optimized Method for the Isolation of Urinary Extracellular Vesicles for Molecular Phenotyping: Detection of Biomarkers for Radiation Exposure. J. Transl. Med. 2022, 20, 199. [Google Scholar] [CrossRef] [PubMed]
- Buntsma, N.; van der Pol, E.; Nieuwland, R.; Gąsecka, A. Extracellular Vesicles in Coronary Artery Disease. Adv. Exp. Med. Biol. 2023, 1418, 81–103. [Google Scholar] [CrossRef] [PubMed]
- Balbi, C.; Vassalli, G. Exosomes: Beyond Stem Cells for Cardiac Protection and Repair. Stem Cells 2020, 38, 1387–1399. [Google Scholar] [CrossRef]
- Saheera, S.; Jani, V.P.; Witwer, K.W.; Kutty, S. Extracellular Vesicle Interplay in Cardiovascular Pathophysiology. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H1749–H1761. [Google Scholar] [CrossRef] [PubMed]
- Hutcheson, J.D.; Aikawa, E. Extracellular Vesicles in Cardiovascular Homeostasis and Disease. Curr. Opin. Cardiol. 2018, 33, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Venkat, P.; Seyfried, D.; Chopp, M.; Yan, T.; Chen, J. Brain-Heart Interaction: Cardiac Complications After Stroke. Circ. Res. 2017, 121, 451–468. [Google Scholar] [CrossRef] [PubMed]
- Akhmerov, A.; Parimon, T. Extracellular Vesicles, Inflammation, and Cardiovascular Disease. Cells 2022, 11, 2229. [Google Scholar] [CrossRef] [PubMed]
- Schiano, C.; Balbi, C.; de Nigris, F.; Napoli, C. Basic Pathogenic Mechanisms and Epigenetic Players Promoted by Extracellular Vesicles in Vascular Damage. Int. J. Mol. Sci. 2023, 24, 7509. [Google Scholar] [CrossRef] [PubMed]
- Burrello, J.; Caporali, E.; Gauthier, L.G.; Pianezzi, E.; Balbi, C.; Rigamonti, E.; Bolis, S.; Lazzarini, E.; Biemmi, V.; Burrello, A.; et al. Risk Stratification of Patients with SARS-CoV-2 by Tissue Factor Expression in Circulating Extracellular Vesicles. Vasc. Pharmacol. 2022, 145, 106999. [Google Scholar] [CrossRef] [PubMed]
- Balbi, C.; Burrello, J.; Bolis, S.; Lazzarini, E.; Biemmi, V.; Pianezzi, E.; Burrello, A.; Caporali, E.; Grazioli, L.G.; Martinetti, G.; et al. Circulating Extracellular Vesicles Are Endowed with Enhanced Procoagulant Activity in SARS-CoV-2 Infection. EBioMedicine 2021, 67, 103369. [Google Scholar] [CrossRef]
- Castellani, C.; Burrello, J.; Fedrigo, M.; Burrello, A.; Bolis, S.; Di Silvestre, D.; Tona, F.; Bottio, T.; Biemmi, V.; Toscano, G.; et al. Circulating Extracellular Vesicles as Non-Invasive Biomarker of Rejection in Heart Transplant. J. Heart Lung Transplant. 2020, 39, 1136–1148. [Google Scholar] [CrossRef] [PubMed]
- Burrello, J.; Burrello, A.; Vacchi, E.; Bianco, G.; Caporali, E.; Amongero, M.; Airale, L.; Bolis, S.; Vassalli, G.; Cereda, C.W.; et al. Supervised and Unsupervised Learning to Define the Cardiovascular Risk of Patients According to an Extracellular Vesicle Molecular Signature. Transl. Res. 2022, 244, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Burrello, J.; Biemmi, V.; Dei Cas, M.; Amongero, M.; Bolis, S.; Lazzarini, E.; Bollini, S.; Vassalli, G.; Paroni, R.; Barile, L. Sphingolipid Composition of Circulating Extracellular Vesicles after Myocardial Ischemia. Sci. Rep. 2020, 10, 16182. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Just, J.; Ankerlund, R.; Ryun, K. Extracellular Vesicles in Acute Stroke Diagnostics. Biomedicines 2020, 8, 248. [Google Scholar] [CrossRef]
- Otero-Ortega, L.; Alonso-López, E.; Pérez-Mato, M.; Laso-García, F.; Gómez-De Frutos, M.C.; Diekhorst, L.; García-Bermejo, M.L.; Conde-Moreno, E.; Fuentes, B.; de Leciñana, M.A.; et al. Circulating Extracellular Vesicle Proteins and MicroRNA Profiles in Subcortical and Cortical-Subcortical Ischaemic Stroke. Biomedicines 2021, 9, 786. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh-Esfehani, R.; Soudyab, M.; Parizadeh, S.M.; Jaripoor, M.E.; Nejad, P.S.; Shariati, M.; Nabavi, A.S. Circulating Exosomes and Their Role in Stroke. Curr. Drug Targets 2020, 21, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Biemmi, V.; Milano, G.; Ciullo, A.; Cervio, E.; Burrello, J.; Cas, M.D.; Paroni, R.; Tallone, T.; Moccetti, T.; Pedrazzini, G.; et al. Inflammatory Extracellular Vesicles Prompt Heart Dysfunction via TRL4-Dependent NF-ΚB Activation. Theranostics 2020, 10, 2773–2790. [Google Scholar] [CrossRef] [PubMed]
- Silva-Palacios, A.; Arroyo-Campuzano, M.; Flores-García, M.; Patlán, M.; Hernández-Díazcouder, A.; Alcántara, D.; Ramírez-Camacho, I.; Arana-Hidalgo, D.; Soria-Castro, E.; Sánchez, F.; et al. Citicoline Modifies the Expression of Specific MiRNAs Related to Cardioprotection in Patients with ST-Segment Elevation Myocardial Infarction Subjected to Coronary Angioplasty. Pharmaceuticals 2022, 15, 925. [Google Scholar] [CrossRef]
- Sanz-Ros, J.; Mas-Bargues, C.; Romero-García, N.; Huete-Acevedo, J.; Dromant, M.; Borrás, C. Extracellular Vesicles as Therapeutic Resources in the Clinical Environment. Int. J. Mol. Sci. 2023, 24, 2344. [Google Scholar] [CrossRef]
- Murphy, D.E.; de Jong, O.G.; Brouwer, M.; Wood, M.J.; Lavieu, G.; Schiffelers, R.M.; Vader, P. Extracellular Vesicle-Based Therapeutics: Natural versus Engineered Targeting and Trafficking. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Guan, Y.; Xie, A.; Yan, Z.; Gao, S.; Li, W.; Rao, L.; Chen, X.; Chen, T. Extracellular Vesicles: A Rising Star for Therapeutics and Drug Delivery. J. Nanobiotechnol. 2023, 21, 231. [Google Scholar] [CrossRef]
- Sadeghi, S.; Tehrani, F.R.; Tahmasebi, S.; Shafiee, A.; Hashemi, S.M. Exosome Engineering in Cell Therapy and Drug Delivery. Inflammopharmacology 2023, 31, 145–169. [Google Scholar] [CrossRef]
- Tenchov, R.; Sasso, J.M.; Wang, X.; Liaw, W.S.; Chen, C.A.; Zhou, Q.A. Exosomes—Nature’s Lipid Nanoparticles, a Rising Star in Drug Delivery and Diagnostics. ACS Nano 2022, 16, 17802–17846. [Google Scholar] [CrossRef]
- Sluijter, J.P.G.; Davidson, S.M.; Boulanger, C.M.; Buzás, E.I.; De Kleijn, D.P.V.; Engel, F.B.; Giricz, Z.; Hausenloy, D.J.; Kishore, R.; Lecour, S.; et al. Extracellular Vesicles in Diagnostics and Therapy of the Ischaemic Heart: Position Paper from the Working Group on Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc. Res. 2018, 114, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Varderidou-Minasian, S.; Lorenowicz, M.J. Mesenchymal Stromal/Stem Cell-Derived Extracellular Vesicles in Tissue Repair: Challenges and Opportunities. Theranostics 2020, 10, 5979–5997. [Google Scholar] [CrossRef] [PubMed]
- Nazari-Shafti, T.Z.; Neuber, S.; Garcia Duran, A.; Xu, Z.; Beltsios, E.; Seifert, M.; Falk, V.; Stamm, C. Human Mesenchymal Stromal Cells and Derived Extracellular Vesicles: Translational Strategies to Increase Their Proangiogenic Potential for the Treatment of Cardiovascular Disease. Stem Cells Transl. Med. 2020, 9, 1558–1569. [Google Scholar] [CrossRef] [PubMed]
- Bollini, S.; Smits, A.M.; Balbi, C.; Lazzarini, E.; Ameri, P. Triggering Endogenous Cardiac Repair and Regeneration via Extracellular Vesicle-Mediated Communication. Front. Physiol. 2018, 9, 401851. [Google Scholar] [CrossRef] [PubMed]
- Balbi, C.; Costa, A.; Barile, L.; Bollini, S. Message in a Bottle: Upgrading Cardiac Repair into Rejuvenation. Cells 2020, 9, 724. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.M.; Andreadou, I.; Barile, L.; Birnbaum, Y.; Cabrera-Fuentes, H.A.; Cohen, M.V.; Downey, J.M.; Girao, H.; Pagliaro, P.; Penna, C.; et al. Circulating Blood Cells and Extracellular Vesicles in Acute Cardioprotection. Cardiovasc. Res. 2019, 115, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Firoozi, S.; Pahlavan, S.; Ghanian, M.H.; Rabbani, S.; Barekat, M.; Nazari, A.; Pakzad, M.; Shekari, F.; Hassani, S.N.; Moslem, F.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Alone or in Conjunction with a SDKP-Conjugated Self-Assembling Peptide Improve a Rat Model of Myocardial Infarction. Biochem. Biophys. Res. Commun. 2020, 524, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Constantin, A.; Comarița, I.K.; Alexandru, N.; Filippi, A.; Bojin, F.; Gherghiceanu, M.; Vîlcu, A.; Nemecz, M.; Niculescu, L.S.; Păunescu, V.; et al. Stem Cell-derived Extracellular Vesicles Reduce the Expression of Molecules Involved in Cardiac Hypertrophy—In a Model of Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Front. Pharmacol. 2022, 13, 1003684. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wu, Y.; He, W.; Tian, Y.; Zhao, X. Exosomes Secreted from Bone Marrow Mesenchymal Stem Cells Suppress Hypertrophy through Hippo-YAP Pathway in Heart. Genet. Mol. Biol. 2023, 46, e20220221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, J.; Gao, S.; Lin, W.; Gao, P.; Gao, K.; Zhang, Y.; Du, K.; Yang, X.; Wang, W.; et al. Antiapoptosis and Antifibrosis Effects of Qishen Granules on Heart Failure Rats via Hippo Pathway. Biomed. Res. Int. 2019, 2019, 1642575. [Google Scholar] [CrossRef]
- Barile, L.; Lionetti, V.; Cervio, E.; Matteucci, M.; Gherghiceanu, M.; Popescu, L.M.; Torre, T.; Siclari, F.; Moccetti, T.; Vassalli, G. Extracellular Vesicles Fromhuman Cardiac Progenitor Cells Inhibit Cardiomyocyte Apoptosis and Improve Cardiac Function Aftermyocardial Infarction. Cardiovasc. Res. 2014, 103, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Milano, G.; Biemmi, V.; Lazzarini, E.; Balbi, C.; Ciullo, A.; Bolis, S.; Ameri, P.; Di Silvestre, D.; Mauri, P.; Barile, L.; et al. Intravenous Administration of Cardiac Progenitor Cell-Derived Exosomes Protects against Doxorubicin/Trastuzumab-Induced Cardiac Toxicity. Cardiovasc. Res. 2020, 116, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Zhou, X.; Ge, Z.; Song, Y.; Wang, H.; Liu, X.; Zhang, D. Exosomes from Adipose-Derived Mesenchymal Stem Cells Ameliorate Cardiac Damage after Myocardial Infarction by Activating S1P/SK1/S1PR1 Signaling and Promoting Macrophage M2 Polarization. Int. J. Biochem. Cell Biol. 2019, 114, 105564. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, T.; Zhang, X.; Zhang, H.; Yan, N.; Zhang, G.; Yan, R.; Li, Y.; Yu, J.; He, J.; et al. Exosomes Derived from Human Placental Mesenchymal Stem Cells Ameliorate Myocardial Infarction via Anti-Inflammation and Restoring Gut Dysbiosis. BMC Cardiovasc. Disord. 2022, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- De Couto, G.; Gallet, R.; Cambier, L.; Jaghatspanyan, E.; Makkar, N.; Dawkins, J.F.; Berman, B.P.; Marbán, E. Exosomal MicroRNA Transfer Into Macrophages Mediates Cellular Postconditioning. Circulation 2017, 136, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Lima Correa, B.; El Harane, N.; Gomez, I.; Hocine, R.; Vilar, J.; Desgres, M.; Bellamy, V.; Keirththana, K.; Guillas, C.; Perotto, M. Extracellular Vesicles from Human Cardiovascular Progenitors Trigger a Reparative Immune Response in Infarcted Hearts. Cardiovasc. Res. 2021, 117, 292–307. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.; Zhang, L.; Duan, L.; Wang, X.; Min, Y.; Yu, H. Extracellular Vesicles Derived from Human Bone Marrow Mesenchymal Stem Cells Promote Angiogenesis in a Rat Myocardial Infarction Model. J. Mol. Med. 2014, 92, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Balbi, C.; Lodder, K.; Costa, A.; Moimas, S.; Moccia, F.; van Herwaarden, T.; Rosti, V.; Campagnoli, F.; Palmeri, A.; De Biasio, P.; et al. Reactivating Endogenous Mechanisms of Cardiac Regeneration via Paracrine Boosting Using the Human Amniotic Fluid Stem Cell Secretome. Int. J. Cardiol. 2019, 287, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Balbi, C.; Garbati, P.; Palamà, M.E.F.; Reverberi, D.; De Palma, A.; Rossi, R.; Paladini, D.; Coviello, D.; De Biasio, P.; et al. Investigating the Paracrine Role of Perinatal Derivatives: Human Amniotic Fluid Stem Cell-Extracellular Vesicles Show Promising Transient Potential for Cardiomyocyte Renewal. Front. Bioeng. Biotechnol. 2022, 10, 902038. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, C.V.; Liaw, N.Y.; Gunadasa-Rohling, M.; Matthaei, M.; Braga, L.; Kennedy, T.; Salinas, G.; Voigt, N.; Giacca, M.; Zimmermann, W.H.; et al. Regenerative Potential of Epicardium-Derived Extracellular Vesicles Mediated by Conserved MiRNA Transfer. Cardiovasc. Res. 2022, 118, 597–611. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, J.J.; Wang, S.S.; Li, L. Mechanism of Adipose-Derived Mesenchymal Stem Cell Exosomes in the Treatment of Heart Failure. World J. Stem Cells 2023, 15, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Takov, K.; He, Z.; Johnston, H.E.; Timms, J.F.; Guillot, P.V.; Yellon, D.M.; Davidson, S.M. Small Extracellular Vesicles Secreted from Human Amniotic Fluid Mesenchymal Stromal Cells Possess Cardioprotective and Promigratory Potential. Basic. Res. Cardiol. 2020, 115, 26. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, X.; Li, P.; Lu, X.; Yan, J.; Tan, H.; Zhang, C. Exosomes Derived from Human Amniotic Fluid Mesenchymal Stem Cells Alleviate Cardiac Fibrosis via Enhancing Angiogenesis in Vivo and in Vitro. Cardiovasc. Diagn. Ther. 2021, 11, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, X.; Cao, W.; Ma, J.; Sun, L.; Qian, H.; Zhu, W.; Xu, W. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Relieve Acute Myocardial Ischemic Injury. Stem Cells Int. 2015, 2015, 761643. [Google Scholar] [CrossRef] [PubMed]
- Streef, T.J.; Smits, A.M. Epicardial Contribution to the Developing and Injured Heart: Exploring the Cellular Composition of the Epicardium. Front. Cardiovasc. Med. 2021, 8, 750243. [Google Scholar] [CrossRef] [PubMed]
- Anto Michel, N.; Ljubojevic-Holzer, S.; Bugger, H.; Zirlik, A. Cellular Heterogeneity of the Heart. Front. Cardiovasc. Med. 2022, 9, 868466. [Google Scholar] [CrossRef] [PubMed]
- Litviňuková, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Worth, C.L.; Lindberg, E.L.; Kanda, M.; Polanski, K.; Heinig, M.; Lee, M.; et al. Cells of the Adult Human Heart. Nature 2020, 588, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Amini, H.; Rezaie, J.; Vosoughi, A.; Rahbarghazi, R.; Nouri, M. Cardiac Progenitor Cells Application in Cardiovascular Disease. J. Cardiovasc. Thorac. Res. 2017, 9, 127. [Google Scholar] [CrossRef] [PubMed]
- Shouman, S.; Zaher, A.; Abdelhameed, A.; Elshaboury, S.; Sakr, S.; Fouda, B.E.; Mohamed, H.; El-Badri, N. Cardiac Progenitor Cells. Adv. Exp. Med. Biol. 2021, 1312, 51–73. [Google Scholar] [CrossRef] [PubMed]
- Emmert, M.Y.; Burrello, J.; Wolint, P.; Hilbe, M.; Andriolo, G.; Balbi, C.; Provasi, E.; Turchetto, L.; Radrizzani, M.; Nazari-Shafti, T.Z.; et al. Intracoronary Delivery of Extracellular Vesicles from Human Cardiac Progenitor Cells Reduces Infarct Size in Porcine Acute Myocardial Infarction. Eur. Heart J. 2024, 45, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Barile, L.; Marbán, E. Injury Minimization after Myocardial Infarction: Focus on Extracellular Vesicles. Eur. Heart J. 2024, 45, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.G.E.; Cheng, K.; Marbán, E. Exosomes as Critical Agents of Cardiac Regeneration Triggered by Cell Therapy. Stem Cell Rep. 2014, 2, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Desgres, M.; Lima Correa, B.; Petrusca, L.; Autret, G.; Pezzana, C.; Marigny, C.; Guillas, C.; Bellamy, V.; Vilar, J.; Perier, M.C.; et al. Therapeutic Potential of Extracellular Vesicles Derived from Cardiac Progenitor Cells in Rodent Models of Chemotherapy-Induced Cardiomyopathy. Front. Cardiovasc. Med. 2023, 10, 1206279. [Google Scholar] [CrossRef] [PubMed]
- Kervadec, A.; Bellamy, V.; El Harane, N.; Arakélian, L.; Vanneaux, V.; Cacciapuoti, I.; Nemetalla, H.; Périer, M.C.; Toeg, H.D.; Richart, A.; et al. Cardiovascular Progenitor-Derived Extracellular Vesicles Recapitulate the Beneficial Effects of Their Parent Cells in the Treatment of Chronic Heart Failure. J. Heart Lung Transplant. 2016, 35, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Adamiak, M.; Cheng, G.; Bobis-Wozowicz, S.; Zhao, L.; Kedracka-Krok, S.; Samanta, A.; Karnas, E.; Xuan, Y.T.; Skupien-Rabian, B.; Chen, X.; et al. Induced Pluripotent Stem Cell (IPSC)-Derived Extracellular Vesicles Are Safer and More Effective for Cardiac Repair Than IPSCs. Circ. Res. 2018, 122, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Mesquita, T.; Cho, J.H.; Li, C.; Sanchez, L.; Holm, K.; Akhmerov, A.; Liu, W.; Li, Y.; Ibrahim, A.G.; et al. Systemic Delivery of Extracellular Vesicles Attenuates Atrial Fibrillation in Heart Failure With Preserved Ejection Fraction. JACC Clin. Electrophysiol. 2023, 9, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, H.; Ma, X.; Adila, A.; Wang, B.; Liu, F.; Chen, B.; Wang, C.; Ma, Y. Preservation of the Cardiac Function in Infarcted Rat Hearts by the Transplantation of Adipose-Derived Stem Cells with Injectable Fibrin Scaffolds. Exp. Biol. Med. 2010, 235, 1505–1515. [Google Scholar] [CrossRef] [PubMed]
- Riaud, M.; Martinez, M.C.; Montero-Menei, C.N. Scaffolds and Extracellular Vesicles as a Promising Approach for Cardiac Regeneration after Myocardial Infarction. Pharmaceutics 2020, 12, 1195. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Liu, S.; Qin, S.; Yang, J.; Yue, T.; Ye, B.; Tang, Y.; Feng, J.; Hou, J.; Danzeng, D. Injectable Hydrogel-Based Combination Therapy for Myocardial Infarction: A Systematic Review and Meta-Analysis of Preclinical Trials. BMC Cardiovasc. Disord. 2024, 24, 119. [Google Scholar] [CrossRef] [PubMed]
- Lv, K.; Li, Q.; Zhang, L.; Wang, Y.; Zhong, Z.; Zhao, J.; Lin, X.; Wang, J.; Zhu, K.; Xiao, C.; et al. Incorporation of Small Extracellular Vesicles in Sodium Alginate Hydrogel as a Novel Therapeutic Strategy for Myocardial Infarction. Theranostics 2019, 9, 7403–7416. [Google Scholar] [CrossRef] [PubMed]
- Pezzana, C.; Cras, A.; Simelière, F.; Guesdon, R.; Desgres, M.; Correa, B.L.; Peuffier, A.; Bellamy, V.; Gouarderes, S.; Alberdi, A.; et al. Biomaterial-Embedded Extracellular Vesicles Improve Recovery of the Dysfunctional Myocardium. Biomaterials 2022, 291, 121877. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Li, Z.; Huang, K.; Caranasos, T.G.; Rossi, J.S.; Cheng, K. Minimally Invasive Delivery of Therapeutic Agents by Hydrogel Injection into the Pericardial Cavity for Cardiac Repair. Nat. Commun. 2021, 12, 1412. [Google Scholar] [CrossRef] [PubMed]
- Monguió-Tortajada, M.; Prat-Vidal, C.; Moron-Font, M.; Clos-Sansalvador, M.; Calle, A.; Gastelurrutia, P.; Cserkoova, A.; Morancho, A.; Ramírez, M.Á.; Rosell, A.; et al. Local Administration of Porcine Immunomodulatory, Chemotactic and Angiogenic Extracellular Vesicles Using Engineered Cardiac Scaffolds for Myocardial Infarction. Bioact. Mater. 2021, 6, 3314–3327. [Google Scholar] [CrossRef] [PubMed]
- Monguió-Tortajada, M.; Prat-Vidal, C.; Martínez-Falguera, D.; Teis, A.; Soler-Botija, C.; Courageux, Y.; Munizaga-Larroudé, M.; Moron-Font, M.; Bayes-Genis, A.; Borràs, F.E.; et al. Acellular Cardiac Scaffolds Enriched with MSC-Derived Extracellular Vesicles Limit Ventricular Remodelling and Exert Local and Systemic Immunomodulation in a Myocardial Infarction Porcine Model. Theranostics 2022, 12, 4656–4670. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Cui, X.; Zhang, Z.; Xu, Y.; Guo, J.; Soliman, B.G.; Lu, Y.; Qin, Z.; Wang, Q.; Zhang, H.; et al. Injection-Free Delivery of MSC-Derived Extracellular Vesicles for Myocardial Infarction Therapeutics. Adv. Healthc. Mater. 2022, 11, 2100312. [Google Scholar] [CrossRef] [PubMed]
- De Abreu, R.C.; Fernandes, H.; da Costa Martins, P.A.; Sahoo, S.; Emanueli, C.; Ferreira, L. Native and Bioengineered Extracellular Vesicles for Cardiovascular Therapeutics. Nat. Rev. Cardiol. 2020, 17, 685–697. [Google Scholar] [CrossRef]
- Kanki, S.; Jaalouk, D.E.; Lee, S.; Yu, A.Y.C.; Gannon, J.; Lee, R.T. Identification of Targeting Peptides for Ischemic Myocardium by in Vivo Phage Display. J. Mol. Cell Cardiol. 2011, 50, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Zahid, M.; Phillips, B.E.; Albers, S.M.; Giannoukakis, N.; Watkins, S.C.; Robbins, P.D. Identification of a Cardiac Specific Protein Transduction Domain by in Vivo Biopanning Using a M13 Phage Peptide Display Library in Mice. PLoS ONE 2010, 5, e12252. [Google Scholar] [CrossRef] [PubMed]
- McGuire, M.J.; Samli, K.N.; Johnston, S.A.; Brown, K.C. In Vitro Selection of a Peptide with High Selectivity for Cardiomyocytes in Vivo. J. Mol. Biol. 2004, 342, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Y.; Zhao, Z.; Meng, Q.; Yu, Y.; Sun, J.; Yang, Z.; Chen, Y.; Li, J.; Ma, T.; et al. Engineered Exosomes With Ischemic Myocardium-Targeting Peptide for Targeted Therapy in Myocardial Infarction. J. Am. Heart Assoc. 2018, 7, e008737. [Google Scholar] [CrossRef] [PubMed]
- Mentkowski, K.I.; Lang, J.K. Exosomes Engineered to Express a Cardiomyocyte Binding Peptide Demonstrate Improved Cardiac Retention in Vivo. Sci. Rep. 2019, 9, 10041. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yun, N.; Mun, D.; Kang, J.Y.; Lee, S.H.; Park, H.; Park, H.; Joung, B. Cardiac-Specific Delivery by Cardiac Tissue-Targeting Peptide-Expressing Exosomes. Biochem. Biophys. Res. Commun. 2018, 499, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ding, N.; Guan, G.; Liu, G.; Huo, D.; Li, Y.; Wei, K.; Yang, J.; Cheng, P.; Zhu, C. Rapid Delivery of Hsa-MiR-590-3p Using Targeted Exosomes to Treat Acute Myocardial Infarction Through Regulation of the Cell Cycle. J. Biomed. Nanotechnol. 2018, 14, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Antes, T.J.; Middleton, R.C.; Luther, K.M.; Ijichi, T.; Peck, K.A.; Liu, W.J.; Valle, J.; Echavez, A.K.; Marbán, E. Targeting extracellular vesicles to injured tissue using membrane cloaking and surface display. J. Nanobiotechnol. 2018, 16, 61. [Google Scholar] [CrossRef] [PubMed]
- Taiariol, L.; Chaix, C.; Farre, C.; Moreau, E. Click and Bioorthogonal Chemistry: The Future of Active Targeting of Nanoparticles for Nanomedicines? Chem. Rev. 2022, 122, 340–384. [Google Scholar] [CrossRef] [PubMed]
- Takayama, Y.; Kusamori, K.; Nishikawa, M. Click Chemistry as a Tool for Cell Engineering and Drug Delivery. Molecules 2019, 24, 172. [Google Scholar] [CrossRef]
- Zhu, L.P.; Tian, T.; Wang, J.Y.; He, J.N.; Chen, T.; Pan, M.; Xu, L.; Zhang, H.X.; Qiu, X.T.; Li, C.C.; et al. Hypoxia-Elicited Mesenchymal Stem Cell-Derived Exosomes Facilitates Cardiac Repair through MiR-125b-Mediated Prevention of Cell Death in Myocardial Infarction. Theranostics 2018, 8, 6163–6177. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.Y.; Mun, D.; Chun, Y.; Park, D.S.; Kim, H.; Yun, N.; Joung, B. Engineered Small Extracellular Vesicle-Mediated NOX4 SiRNA Delivery for Targeted Therapy of Cardiac Hypertrophy. J. Extracell. Vesicles 2023, 12, 12371. [Google Scholar] [CrossRef] [PubMed]
- Vandergriff, A.; Huang, K.; Shen, D.; Hu, S.; Hensley, M.T.; Caranasos, T.G.; Qian, L.; Cheng, K. Targeting Regenerative Exosomes to Myocardial Infarction Using Cardiac Homing Peptide. Theranostics 2018, 8, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Pan, Y.; Ning, X.; Shi, X.; Zhong, J.; Fan, X.; Li, W.; Teng, Y.; Liu, X.; Yu, B.; et al. Targeted Heart Repair by Tβ4-Loaded Cardiac-Resident Macrophage-Derived Extracellular Vesicles Modified with Monocyte Membranes. Acta Biomater. 2023, 169, 372–386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Song, Y.; Huang, Z.; Chen, J.; Tan, H.; Yang, H.; Fan, M.; Li, Q.; Wang, Q.; Gao, J.; et al. Monocyte Mimics Improve Mesenchymal Stem Cell-Derived Extracellular Vesicle Homing in a Mouse MI/RI Model. Biomaterials 2020, 255, 120168. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Huang, K.; Ma, H.; Liang, H.; Dinh, P.U.; Chen, J.; Shen, D.; Allen, T.A.; Qiao, L.; Li, Z.; et al. Platelet-Inspired Nanocells for Targeted Heart Repair After Ischemia/Reperfusion Injury. Adv. Funct. Mater. 2019, 29, 1803567. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Ni, L.; Zhang, X.; Wang, H.; Liu, L.; Wei, M.; Li, G.; Bei, Y. Platelet Membrane-Fused Circulating Extracellular Vesicles Protect the Heart from Ischemia/Reperfusion Injury. Adv. Healthc. Mater. 2023, 12, 2300052. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Song, Y.; Wang, Q.; Chen, J.; Gao, J.; Tan, H.; Li, S.; Wu, Y.; Yang, H.; Huang, H.; et al. Engineering Extracellular Vesicles with Platelet Membranes Fusion Enhanced Targeted Therapeutic Angiogenesis in a Mouse Model of Myocardial Ischemia Reperfusion. Theranostics 2021, 11, 3916–3931. [Google Scholar] [CrossRef] [PubMed]
- Ciullo, A.; Biemmi, V.; Milano, G.; Bolis, S.; Cervio, E.; Fertig, E.T.; Gherghiceanu, M.; Moccetti, T.; Camici, G.G.; Vassalli, G.; et al. Exosomal Expression of CXCR4 Targets Cardioprotective Vesicles to Myocardial Infarction and Improves Outcome after Systemic Administration. Int. J. Mol. Sci. 2019, 20, 468. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Quarto, R.; Bollini, S. Small Extracellular Vesicles from Human Amniotic Fluid Samples as Promising Theranostics. Int. J. Mol. Sci. 2022, 23, 590. [Google Scholar] [CrossRef] [PubMed]
- Akbar, A.; Malekian, F.; Baghban, N.; Kodam, S.P.; Ullah, M. Methodologies to Isolate and Purify Clinical Grade Extracellular Vesicles for Medical Applications. Cells 2022, 11, 186. [Google Scholar] [CrossRef] [PubMed]
- Brennan, K.; Martin, K.; FitzGerald, S.P.; O’Sullivan, J.; Wu, Y.; Blanco, A.; Richardson, C.; Mc Gee, M.M. A Comparison of Methods for the Isolation and Separation of Extracellular Vesicles from Protein and Lipid Particles in Human Serum. Sci. Rep. 2020, 10, 1039. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wong, D.K.; Hong, K.Y.; Raffai, R.L. Cushioned–Density Gradient Ultracentrifugation (C–DGUC): A Refined and High Performance Method for the Isolation, Characterization & Use of Exosomes. Methods Mol. Biol. 2018, 1740, 69. [Google Scholar] [CrossRef] [PubMed]
- Vanderboom, P.M.; Dasari, S.; Ruegsegger, G.N.; Pataky, M.W.; Lucien, F.; Heppelmann, C.J.; Lanza, I.R.; Nair, K.S. A Size-Exclusion-Based Approach for Purifying Extracellular Vesicles from Human Plasma. Cell Rep. Methods 2021, 1, 100055. [Google Scholar] [CrossRef] [PubMed]
- Gámez-Valero, A.; Monguió-Tortajada, M.; Carreras-Planella, L.; Franquesa, M.; Beyer, K.; Borràs, F.E. Size-Exclusion Chromatography-Based Isolation Minimally Alters Extracellular Vesicles’ Characteristics Compared to Precipitating Agents. Sci. Rep. 2016, 6, 33641. [Google Scholar] [CrossRef] [PubMed]
- Contreras, H.; Alarcón-Zapata, P.; Nova-Lamperti, E.; Ormazabal, V.; Varas-Godoy, M.; Salomon, C.; Zuniga, F.A. Comparative Study of Size Exclusion Chromatography for Isolation of Small Extracellular Vesicle from Cell-Conditioned Media, Plasma, Urine, and Saliva. Front. Nanotechnol. 2023, 5, 1146772. [Google Scholar] [CrossRef]
- Chen, J.; Li, P.; Zhang, T.; Xu, Z.; Huang, X.; Wang, R.; Du, L. Review on Strategies and Technologies for Exosome Isolation and Purification. Front. Bioeng. Biotechnol. 2022, 9, 811971. [Google Scholar] [CrossRef] [PubMed]
- Haraszti, R.A.; Miller, R.; Stoppato, M.; Sere, Y.Y.; Coles, A.; Didiot, M.C.; Wollacott, R.; Sapp, E.; Dubuke, M.L.; Li, X.; et al. Exosomes Produced from 3D Cultures of MSCs by Tangential Flow Filtration Show Higher Yield and Improved Activity. Mol. Ther. 2018, 26, 2838. [Google Scholar] [CrossRef] [PubMed]
- Busatto, S.; Vilanilam, G.; Ticer, T.; Lin, W.L.; Dickson, D.W.; Shapiro, S.; Bergese, P.; Wolfram, J. Tangential Flow Filtration for Highly Efficient Concentration of Extracellular Vesicles from Large Volumes of Fluid. Cells 2018, 7, 273. [Google Scholar] [CrossRef]
- Franco, C.; Ghirardello, A.; Bertazza, L.; Gasparotto, M.; Zanatta, E.; Iaccarino, L.; Valadi, H.; Doria, A.; Gatto, M. Size-Exclusion Chromatography Combined with Ultrafiltration Efficiently Isolates Extracellular Vesicles from Human Blood Samples in Health and Disease. Int. J. Mol. Sci. 2023, 24, 3663. [Google Scholar] [CrossRef] [PubMed]
- Nordin, J.Z.; Lee, Y.; Vader, P.; Mäger, I.; Johansson, H.J.; Heusermann, W.; Wiklander, O.P.B.; Hällbrink, M.; Seow, Y.; Bultema, J.J.; et al. Ultrafiltration with Size-Exclusion Liquid Chromatography for High Yield Isolation of Extracellular Vesicles Preserving Intact Biophysical and Functional Properties. Nanomedicine 2015, 11, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.; Kim, H.J.; Park, J.; Lee, H.; Lee, K.N.; Kim, K.; Lee, J.; Yoon, S.J.; Kim, T.; Jeong, S.; et al. Isolation of Bovine Milk Exosome Using Electrophoretic Oscillation Assisted Tangential Flow Filtration with Antifouling of Micro-Ultrafiltration Membrane Filters. ACS Appl. Mater. Interfaces 2023, 15, 26069–26080. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.B.; Lee, G.B.; Moon, M.H. Size Separation of Exosomes and Microvesicles Using Flow Field-Flow Fractionation/Multiangle Light Scattering and Lipidomic Comparison. Anal. Chem. 2022, 94, 8958–8965. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lyden, D. Asymmetric-Flow Field-Flow Fractionation Technology for Exomere and Small Extracellular Vesicle Separation and Characterization. Nat. Protoc. 2019, 14, 1027–1053. [Google Scholar] [CrossRef] [PubMed]
- Casajuana Ester, M.; Day, R.M. Production and Utility of Extracellular Vesicles with 3D Culture Methods. Pharmaceutics 2023, 15, 663. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Cuscino, N.; Contino, F.; Bulati, M.; Pampalone, M.; Amico, G.; Zito, G.; Carcione, C.; Centi, C.; Bertani, A.; et al. Changes in the Transcriptome Profiles of Human Amnion-Derived Mesenchymal Stromal/Stem Cells Induced by Three-Dimensional Culture: A Potential Priming Strategy to Improve Their Properties. Int. J. Mol. Sci. 2022, 23, 863. [Google Scholar] [CrossRef] [PubMed]


| Cell Source | EVs | In Vitro Outcome | In Vivo Outcome |
|---|---|---|---|
| Adult MSCs | hBM-MSC-EVs | Pro-angiogenic effect on HUVEC [81] Model of myocardial oxidative stress: ⬇ mNVCM Apoptosis [71] | Rat model of AMI: ⬇ Infarct Size Neovascularization ⬆ Cardiac Function [81] |
| Rat BM-MSC-EVs | Model of cardiac hypertrophy on H9c2 cells: ⬇ Bax, Caspase-3 ⬆ Bcl-2 ⬇ BNP, TNF-α ⬇ IL-1β, IL-4, IL-6 [73,74] | ||
| Human AD-MSC-EVs | Model of cardiac hypertrophy with iPSC-CM: ⬇ ANF, COL1A1, IL-6 [77] | ||
| Murine AD-MSC-EVs | Rat model of Doxorubicin-induced HF: ⬆ ATP content, EF, FS ⬇ ANP, Bax, Caspase-3, p53 [85] | ||
| Rat AD-MSC-EVs | Rat model of AMI: ⬆ Cardiac function M2 macrophage transition ⬇ IL-6, IL-1β, IFN-γ, TNF-α [77] | ||
| Fetal/ Perinatal MSCs | Human AFSC-EVs | Rat model of AMI: ⬆ Cardiac function Cell-cycle re-entry Endogenous regenerative processes [73] Rat model of ischemia/reperfusion: ⬇ Infarct size [86] Rat model of ISO-induced fibrosis: ⬇ Collagen1, α-SMA ⬆ Angiogenesis [87] Mouse model of AMI: ⬆ Cell-cycle progression [83] | |
| Human UC-MSC-EVs | Rat model of AMI: ⬇ Cardiac fibrosis ⬆ Cardiac function [88] | ||
| Human PMSC-EVs | Mouse model of AMI: ⬇ AST, BNP ⬇ IL-1β, IL-6 and TNF-α [78] | ||
| Cardiac stromal cells | Murine EPDCs-EVs | ⬆ mNVCM proliferation EHM cryoinjury model ⬇ Contractile function [84] | Mouse model of AMI: Cell-cycle re-entry [84] |
| Human CPC-EVs | HL-1 serum deprivation: ⬇ Cell death HUVEC tube formation [75] | Rat and porcine model of AMI: ⬇ Cell apoptosis ⬆ Angiogenesis [75,94,95] Murine model of doxorubicin/trastuzumab-induced cardiotoxicity: ⬇ ROS levels ⬇ Collagen1 deposition ⬇ Inflammation, reducing CD68+ macrophages [76] | |
| iPSCs | Murine iPS-EVs | Mouse model of AMI: ⬆ Cardiac Function [99] | |
| Human iPSC-Pg-EVs | Mouse model of chronic HF: preserved LV function [100] Mouse model of AMI: ⬇ M1 macrophage ⬆ M2 macrophage ⬇ IL-1α, IL-2, IL-6 ⬆ IL-10 [80] Rodent model of chemotherapy-induced cardiomyopathy: ⬇ Maladaptive remodeling ⬇ Myh6/Myh7 [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerricchio, L.; Barile, L.; Bollini, S. Evolving Strategies for Extracellular Vesicles as Future Cardiac Therapeutics: From Macro- to Nano-Applications. Int. J. Mol. Sci. 2024, 25, 6187. https://doi.org/10.3390/ijms25116187
Guerricchio L, Barile L, Bollini S. Evolving Strategies for Extracellular Vesicles as Future Cardiac Therapeutics: From Macro- to Nano-Applications. International Journal of Molecular Sciences. 2024; 25(11):6187. https://doi.org/10.3390/ijms25116187
Chicago/Turabian StyleGuerricchio, Laura, Lucio Barile, and Sveva Bollini. 2024. "Evolving Strategies for Extracellular Vesicles as Future Cardiac Therapeutics: From Macro- to Nano-Applications" International Journal of Molecular Sciences 25, no. 11: 6187. https://doi.org/10.3390/ijms25116187
APA StyleGuerricchio, L., Barile, L., & Bollini, S. (2024). Evolving Strategies for Extracellular Vesicles as Future Cardiac Therapeutics: From Macro- to Nano-Applications. International Journal of Molecular Sciences, 25(11), 6187. https://doi.org/10.3390/ijms25116187








