Melatonin Delays Arthritis Inflammation and Reduces Cartilage Matrix Degradation through the SIRT1-Mediated NF-κB/Nrf2/TGF-β/BMPs Pathway
Abstract
:1. Introduction
2. Results
2.1. In Vivo Experimental Results
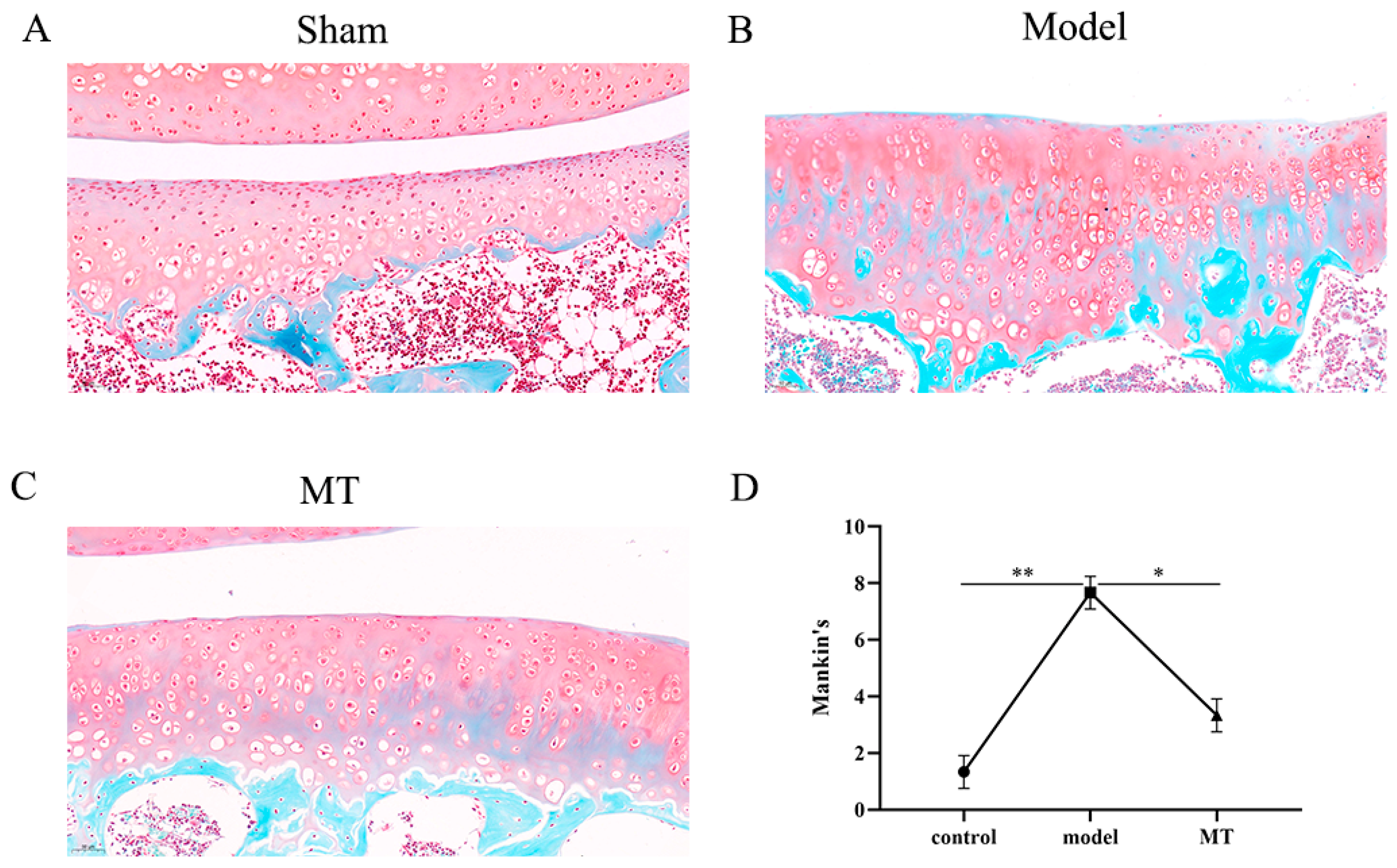
2.1.1. MT Alleviated Articular Cartilage Pathology
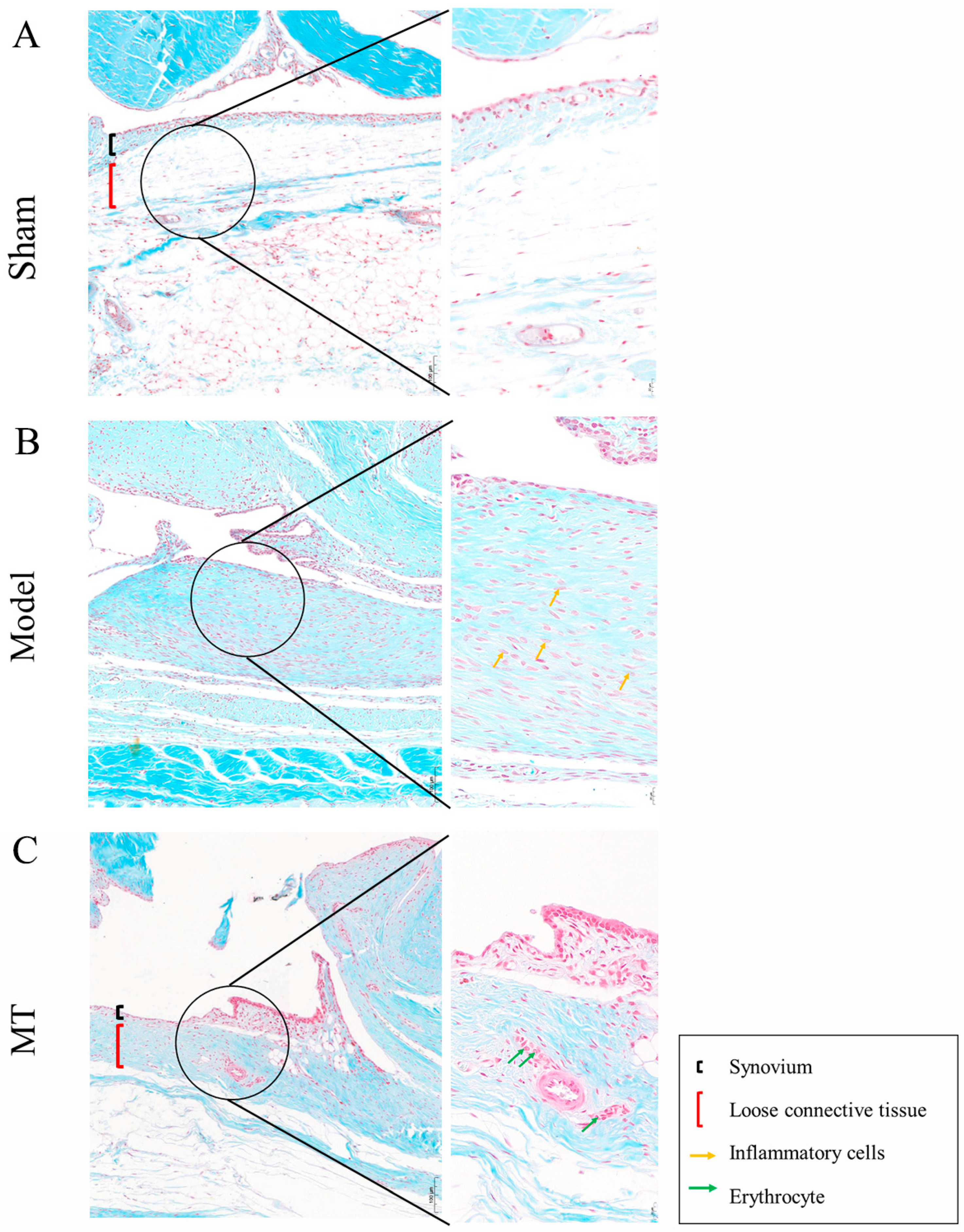
2.1.2. MT Reduced Synovial Tissue Inflammation
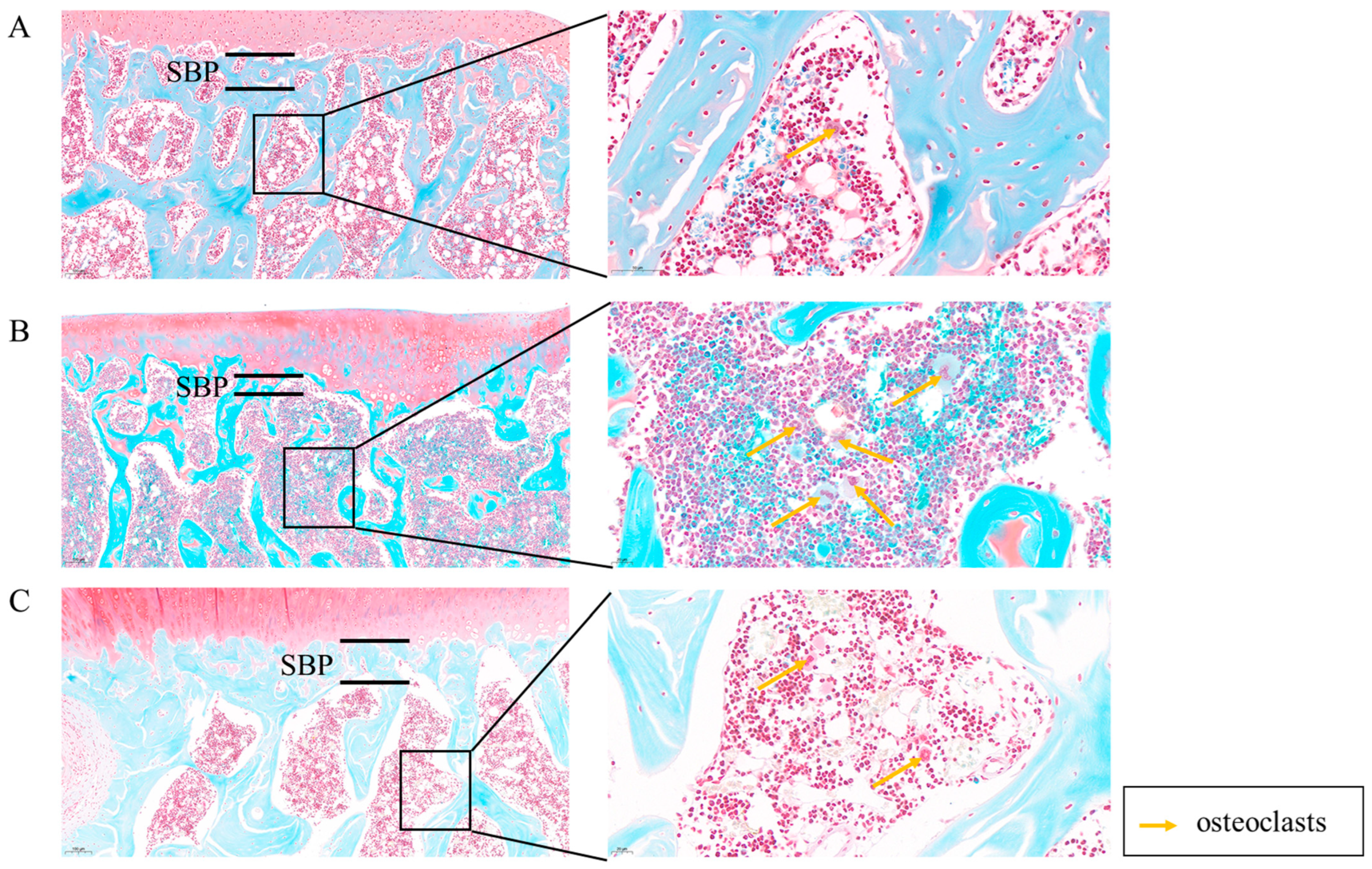
2.1.3. MT Improves Subchondral Bone Lesions
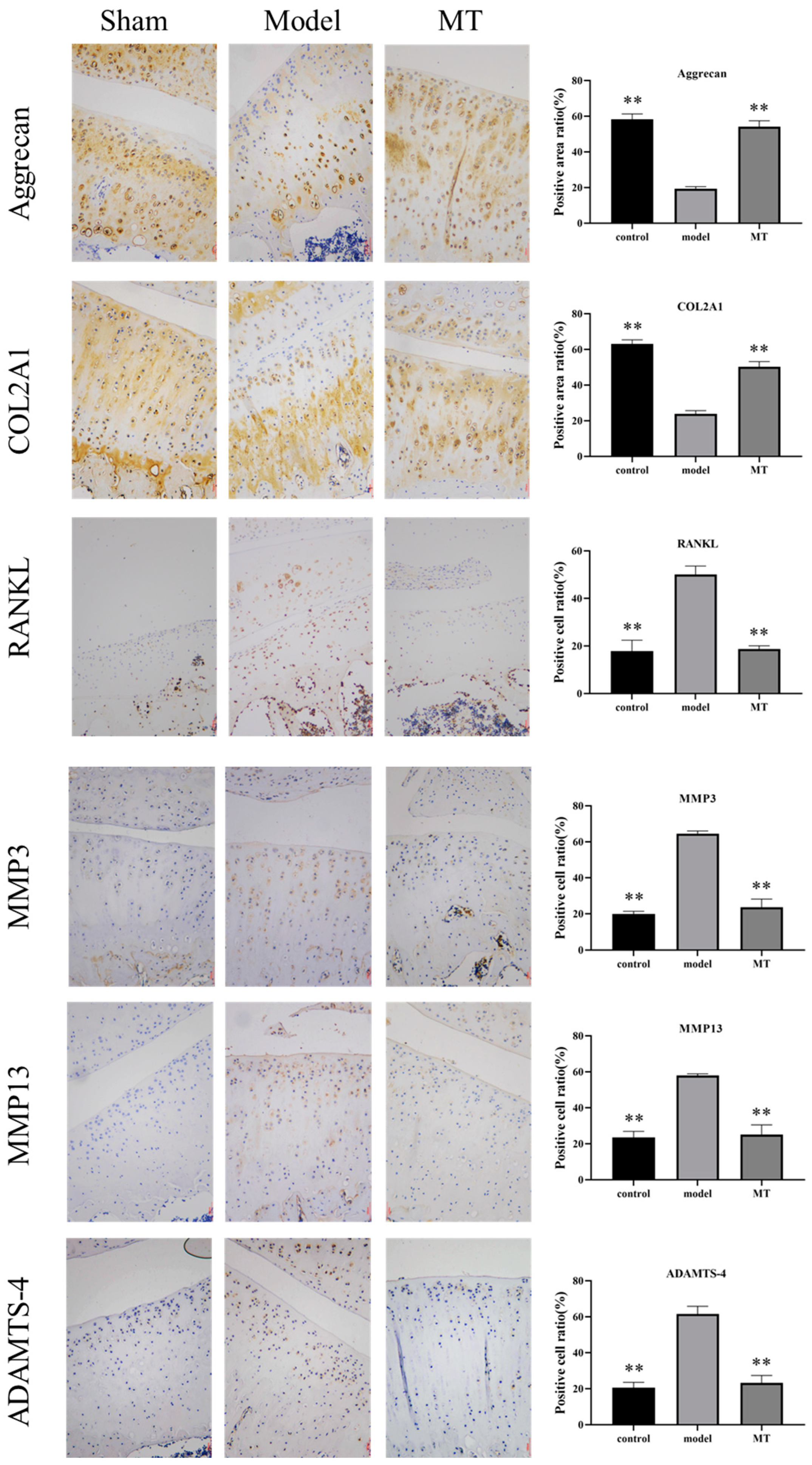
2.1.4. The Effects of MT on the Expression of Cartilage Metabolism-Related Proteins
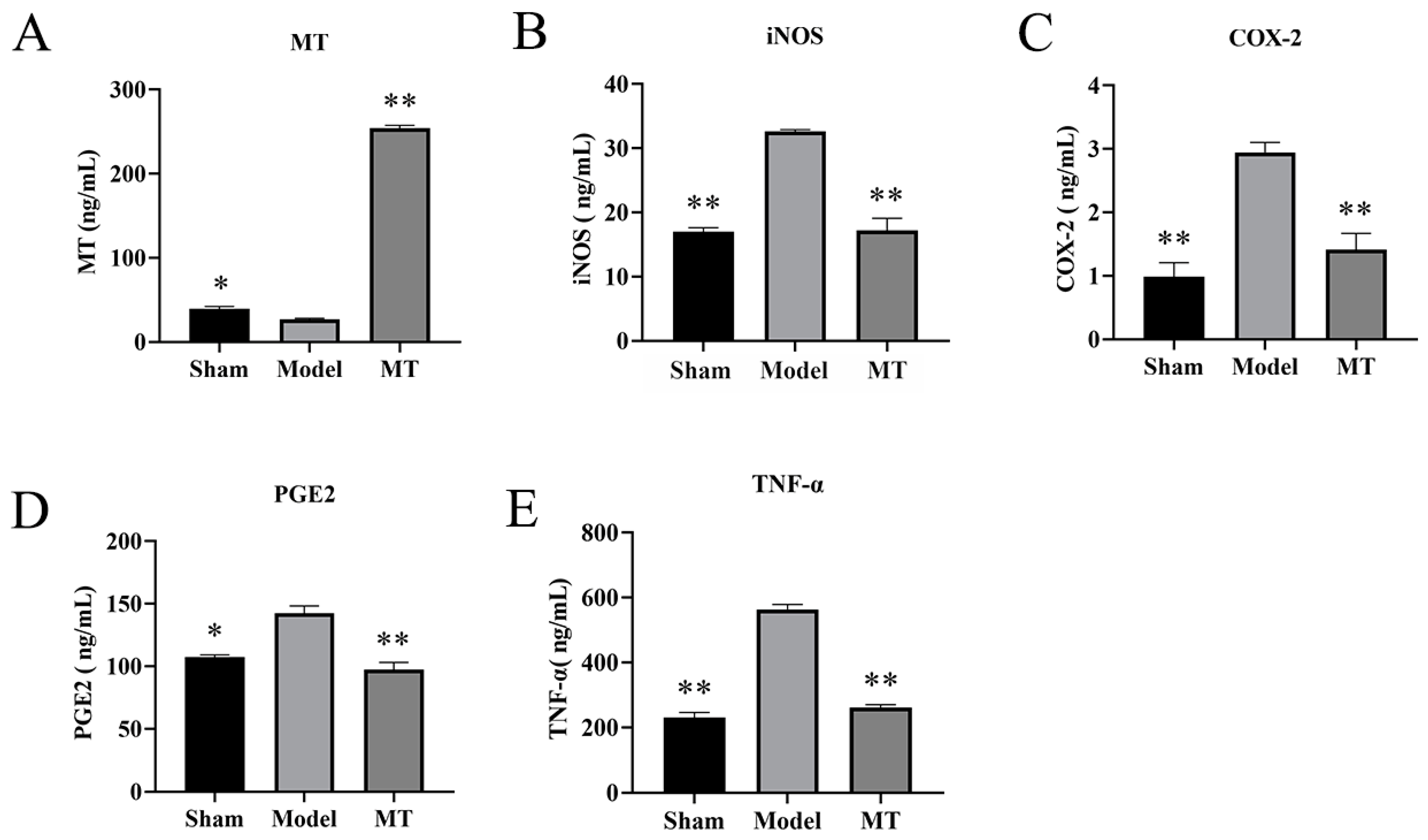
2.1.5. MT Reduces the Levels of TNF-α, COX-2, PGE2, and iNOS in Rat Serum
2.2. In Vitro Experimental Results
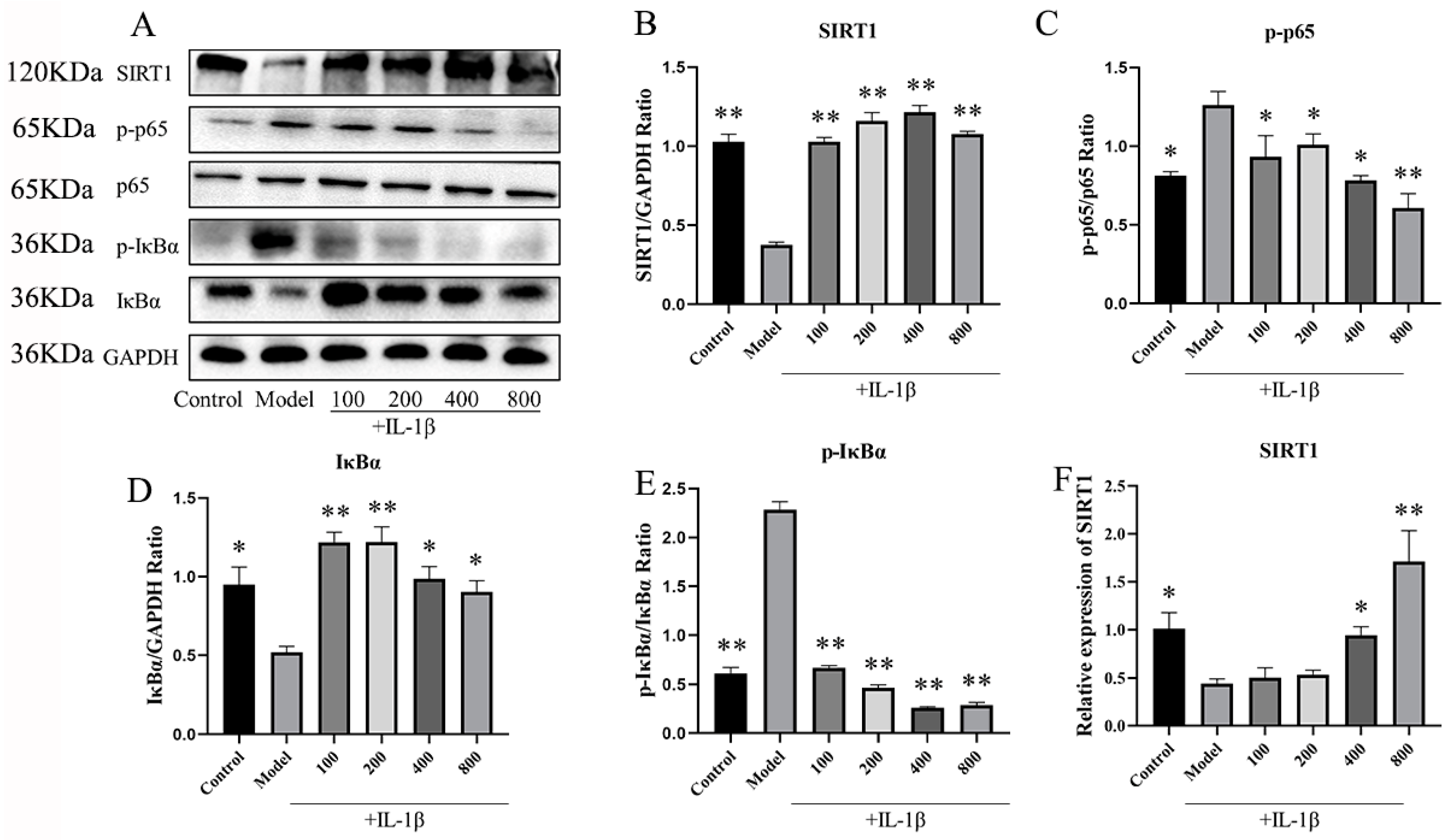
2.2.1. MT Activates SIRT1 and Inhibits the NF-κB Pathway in IL-1β-Induced Chondrocytes
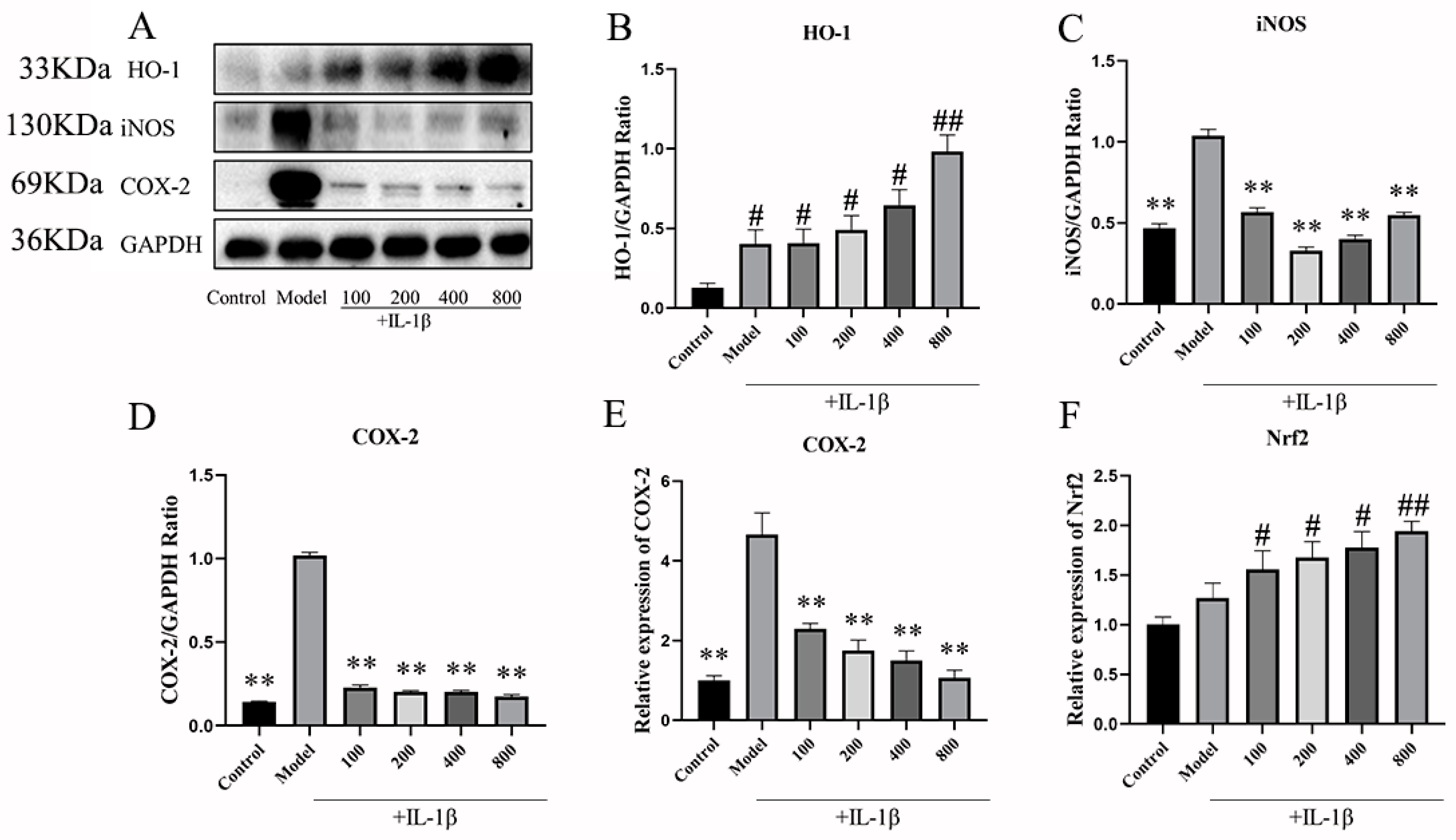
2.2.2. The Effects of MT on the Expression of Nrf1, HO-1, iNOS, and COX-2 in Chondrocytes
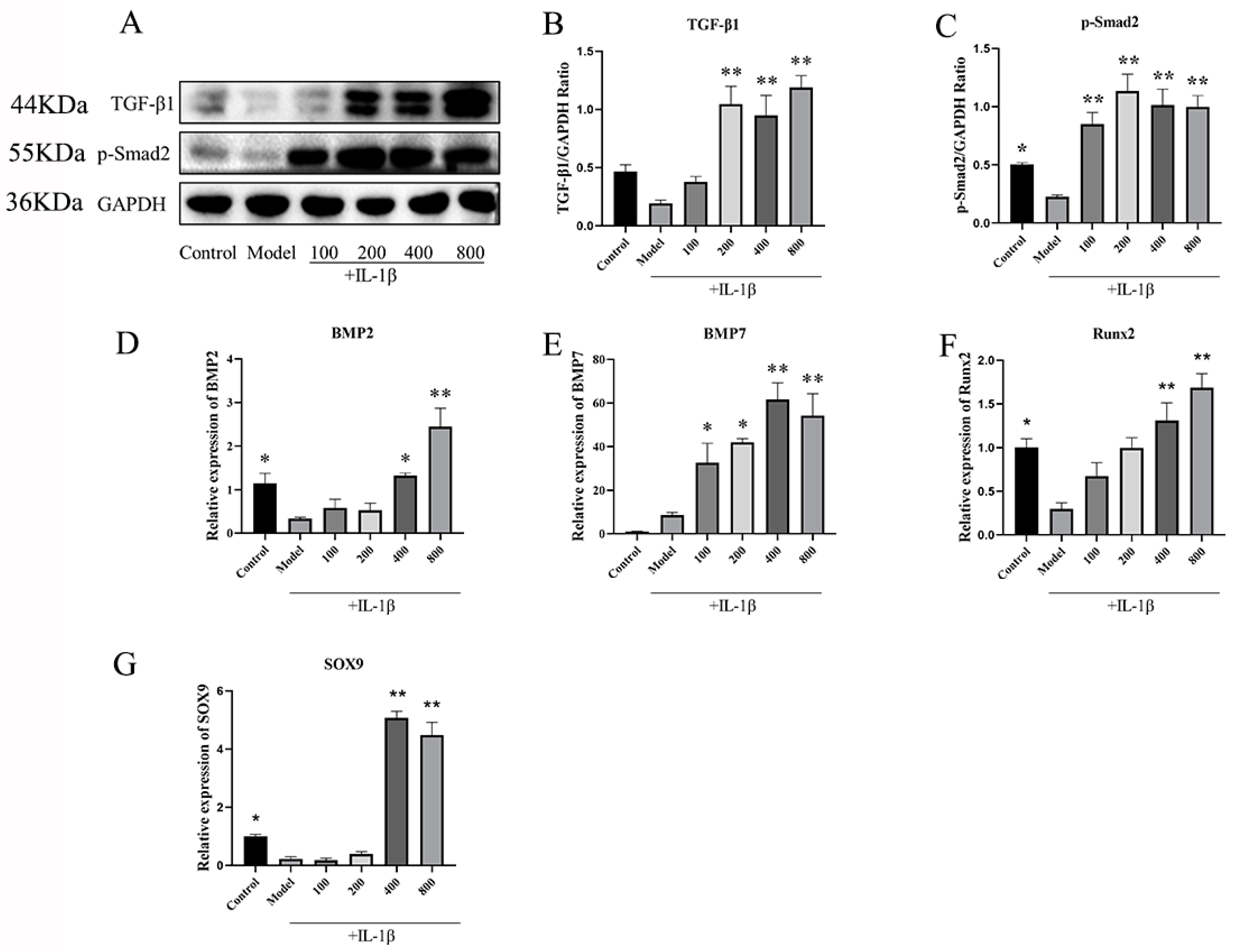
2.2.3. MT Promotes the Expression of TGF-β1/p-Smad2/BMPs in Chondrocytes
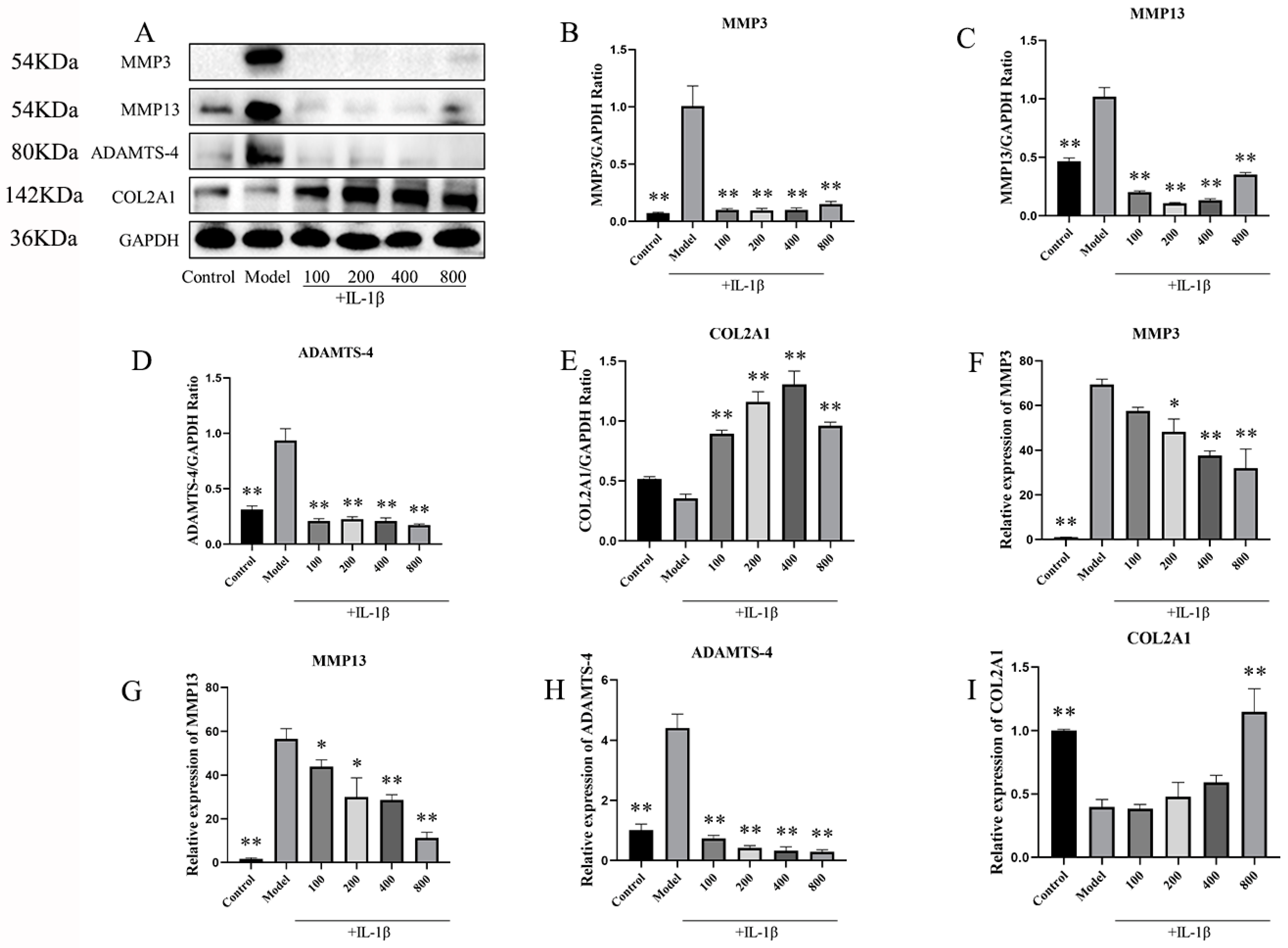
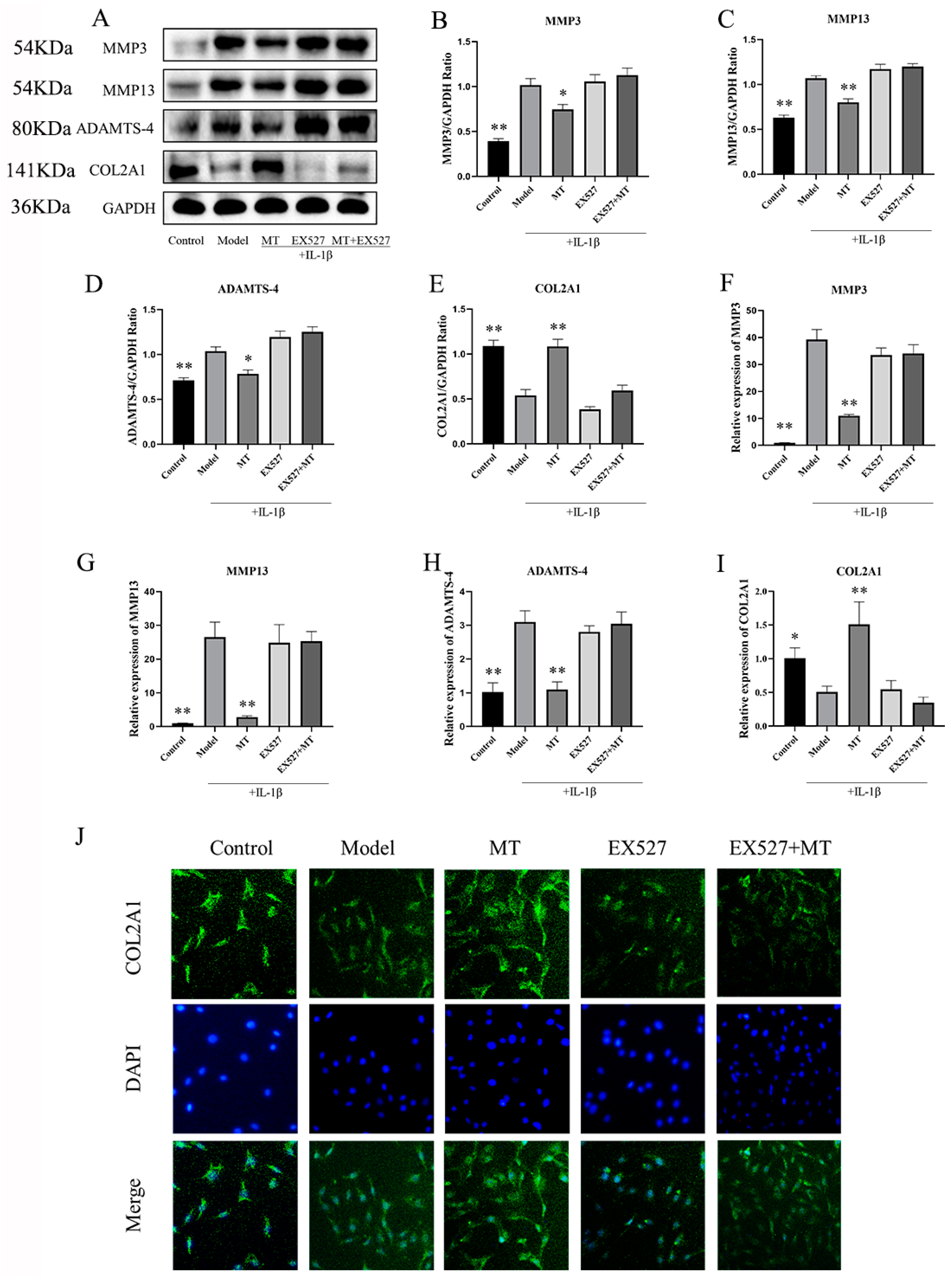
2.2.4. In IL-1β-Induced Chondrocytes, MT Decreased MMP3, MMP13, and ADAMTS-4 and Increased COL2A1
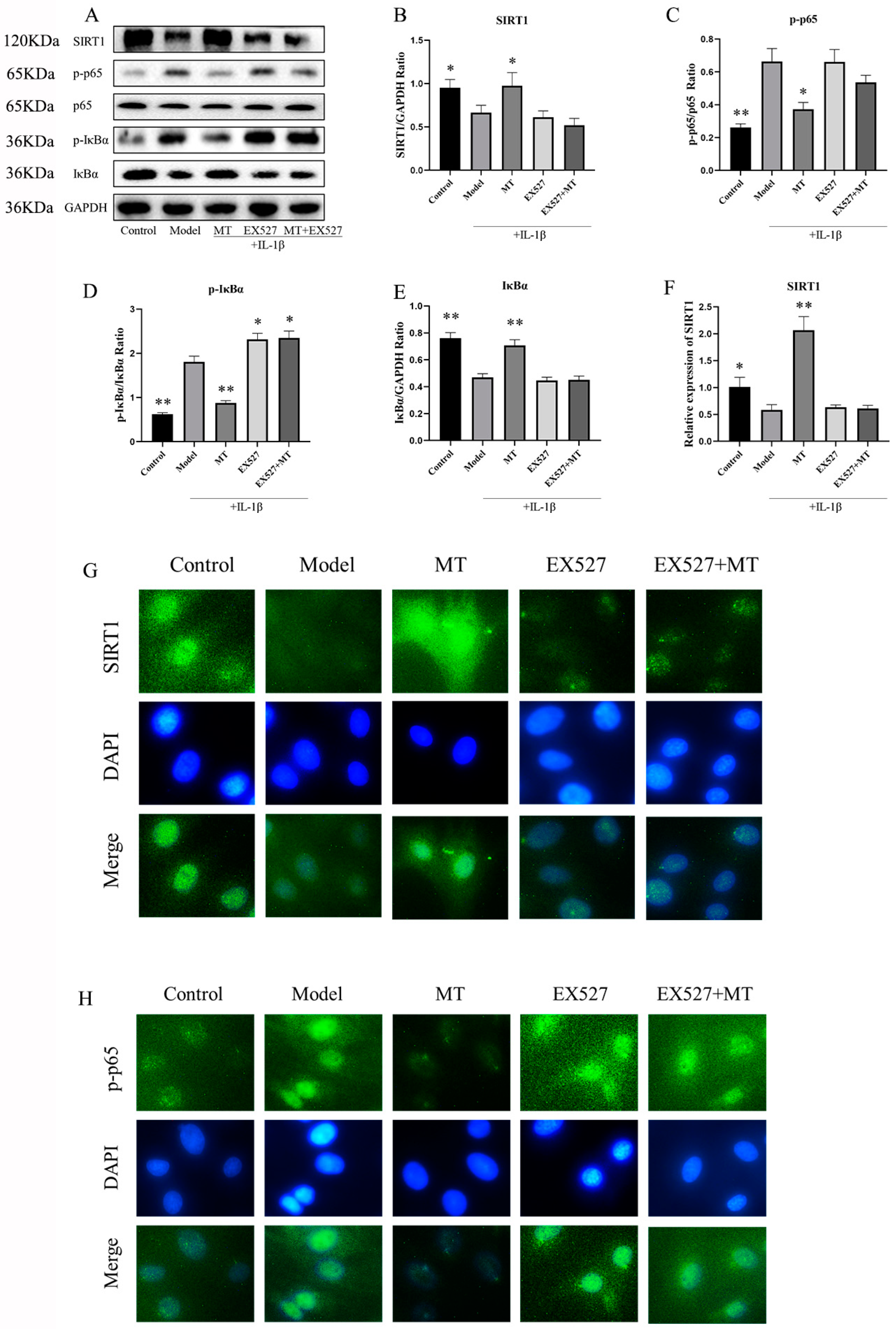
2.2.5. The Effect of the SIRT1 Inhibitor EX537 on Melatonin in Chondrocytes
EX527 Reverses the Effect of MT on the SIRT1 and NF-κB Pathways in IL-1β-Treated Chondrocytes
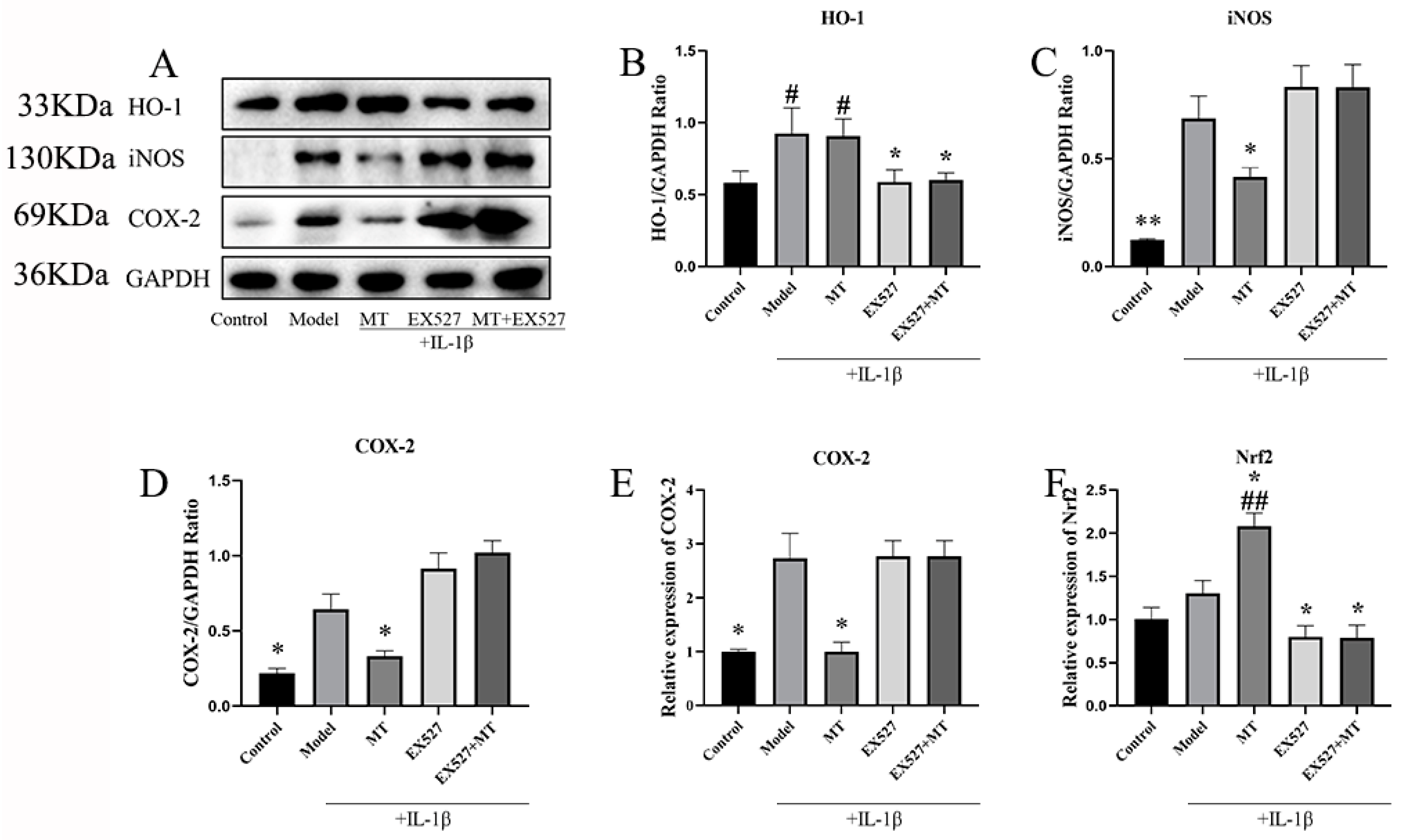
The Effects of EX527 on HO-1, iNOS, COX-2, and Nrf2 in MT-Induced Chondrocytes
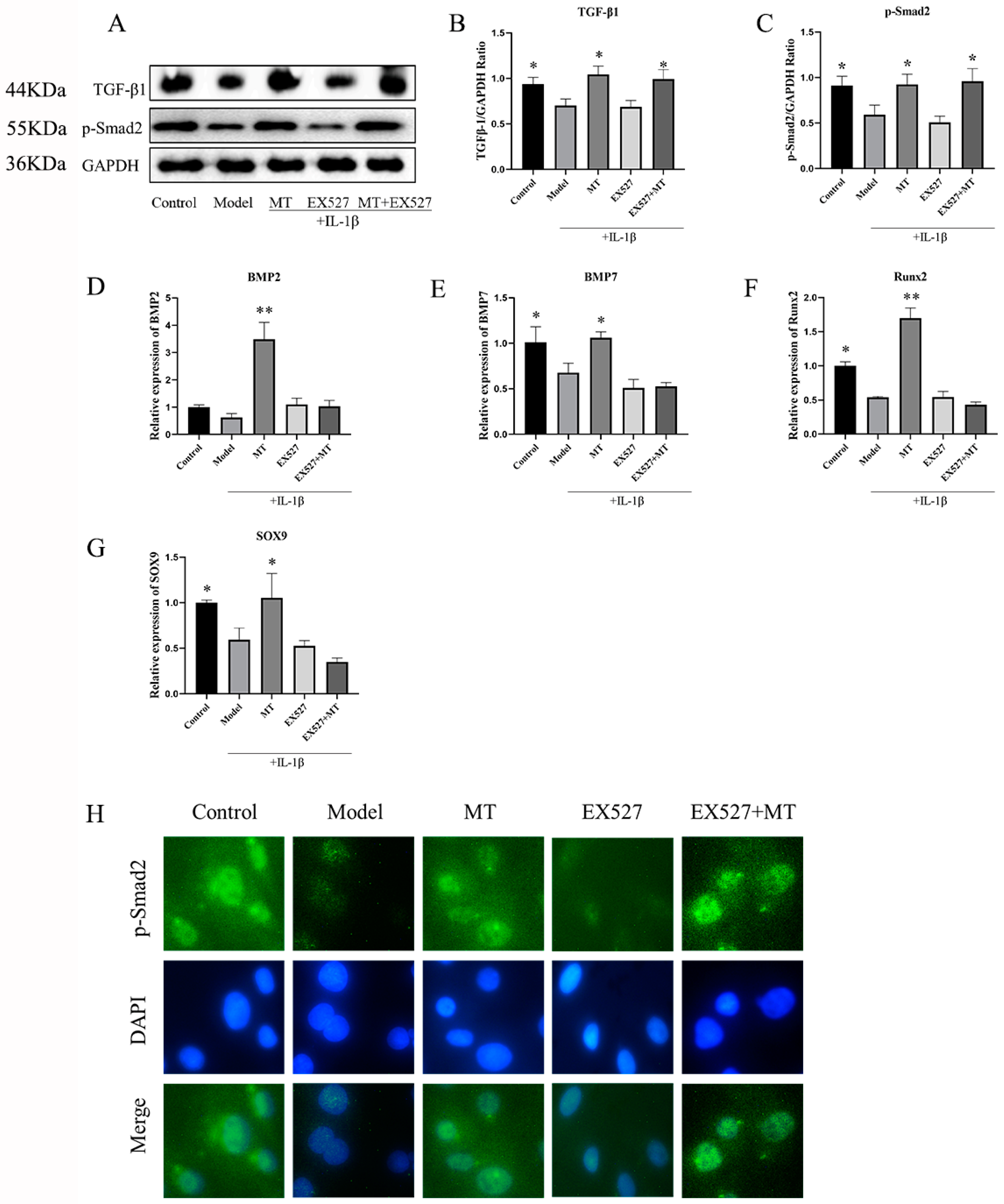
The Effects of EX527 on the TGF-β1/Smad2/BMPs Pathway in MT-Treated Chondrocytes
The Effects of EX527 on the Expression of MMP3, MMP13, ADAMTS-4, and COL2A1 in MT-Treated Chondrocytes
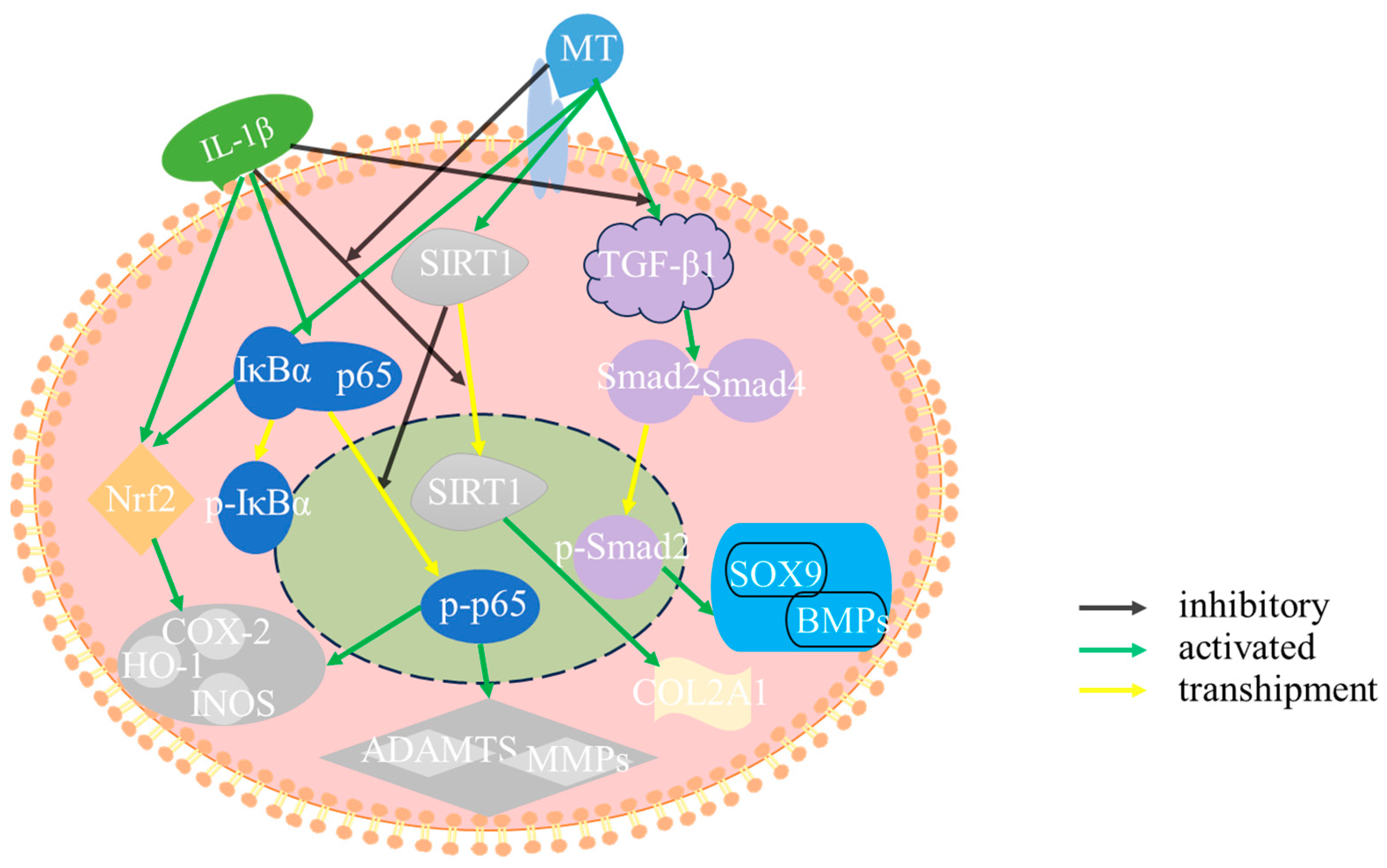
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Animal Ethics
4.3. Experimental Animal Grouping and Surgery
4.4. Safranin O-Fast Green Staining and Immunohistochemistry (IHC)
4.5. Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. Isolation and Culture of Primary Chondrocytes
4.7. Protein Extraction and Western Blot (WB)
4.8. RNA Extraction and Quantitative RT-PCR (q-PCR)
4.9. Immunofluorescence (IF)
4.10. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MT | Melatonin |
| OA | Osteoarthritis |
| ECM | Extracellular matrix |
| WB | Western blotting |
| IF | Immunofluorescence |
| ICH | Immunohistochemistry |
| qPCR | Real-time fluorescent quantitative PCR |
| ELISA | Enzyme Linked Immunosorbent assay |
| ACLT | Anterior Cruciate Ligament Transection |
| SD | Sprague Dawley |
| COL2A1 | Type II collagen |
| ADAMTS | A disintegrin and metalloprotease with thrombospondin motifs |
| MMPs | Matrix metalloproteinases |
| NF-κB | Nuclear factor kappa-B |
| Nrf2 | Nuclear factor erythroid-2-related factor 2 |
| HO-1 | Heme Oxygenase 1 |
| NQO1 | NADPH: Quinone Oxidoreductase 1 |
| SOD | Superoxide Dismutase |
| ROS | Reactive oxygen species |
| RNS | Reactive nitrogen species |
| iNOS | Induced nitric oxide synthase |
| IL-1β | Interleukin-1β |
| TNF-α | Tumor Necrosis Factor-α |
| STB | Subchondral trabecular bone |
| SBP | Subchondral bone plate |
| TGF-β | Transforming growth factor-beta |
| p-Smad2 | Phosphorylated mothers against decapentaplegic homolog 2 |
| BMPs | Bone morphogenetic proteins |
| SIRT1 | Silent information regulator transcript-1 |
| COX-2 | Cyclooxygenase-2 |
| PGE2 | Prostaglandin E2 |
| iNOS | Inducible nitric oxide synthase |
| EX527 | Selisistat |
| SOX9 | Sry-related HMG box-9 |
| Runx 2 | Core binding factor-α |
| RANKL | Receptor activator of nuclear factor-κB ligand |
| p65 | NF-κB p65 |
| p-p65 | Phosphorylated NF-κB p65 |
| IκBα | Inhibitor of nuclear factor kappa-B alpha |
| p-IκBα | Phosphorylated inhibitor of nuclear factor kappa-B alpha |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
Appendix A
Appendix B
Appendix C
Appendix D
Appendix E
- Cartilage surface: evaluating the smoothness and uniformity of the cartilage, graded from 0–3.
- Cartilage structure: assessing the thickness and integrity of the tissue structure, graded from 0–4.
- Cartilage staining: evaluating the uniformity of cartilage color, graded from 0–2.
- Subchondral bone: assessing the internal structure and morphology of the subchondral bone, graded from 0–3.
- Hard bone: evaluating the extent of damage and morphology of the hard bone, graded from 0–4.
References
- Jiang, Y.; Tuan, R.S. Origin and function of cartilage stem/progenitor cells in osteoarthritis. Nat. Rev. Rheumatol. 2015, 11, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Sandell, L.J.; Aigner, T. Articular cartilage and changes in arthritis. An introduction: Cell biology of osteoarthritis. Arthritis Res. 2001, 3, 107–113. [Google Scholar] [CrossRef]
- Takaishi, H.; Kimura, T.; Dalal, S.; Okada, Y.; D’Armiento, J. Joint diseases and matrix metalloproteinases: A role for MMP-13. Curr. Pharm. Biotechnol. 2008, 9, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Im, H.J.; Li, X.; Muddasani, P.; Kim, G.H.; Davis, F.; Rangan, J.; Forsyth, C.B.; Ellman, M.; Thonar, E.J. Basic fibroblast growth factor accelerates matrix degradation via a neuro-endocrine pathway in human adult articular chondrocytes. J. Cell Physiol. 2008, 215, 452–463. [Google Scholar] [CrossRef]
- Roughley, P.J. Articular cartilage and changes in arthritis: Noncollagenous proteins and proteoglycans in the extracellular matrix of cartilage. Arthritis Res. 2001, 3, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhang, L.; Dong, Y.; Tian, Z.; Chen, Y.; Dong, S. Tumour dormancy in inflammatory microenvironment: A promising therapeutic strategy for cancer-related bone metastasis. Cell Mol. Life Sci. 2020, 77, 5149–5169. [Google Scholar] [CrossRef] [PubMed]
- Aizah, N.; Chong, P.P.; Kamarul, T. Early Alterations of Subchondral Bone in the Rat Anterior Cruciate Ligament Transection Model of Osteoarthritis. Cartilage 2021, 13 (Suppl. S2), 1322S–1333S. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Vico, A.; Lardone, P.J.; Alvarez-Sanchez, N.; Rodriguez-Rodriguez, A.; Guerrero, J.M. Melatonin: Buffering the immune system. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [CrossRef] [PubMed]
- Maria, S.; Witt-Enderby, P.A. Melatonin effects on bone: Potential use for the prevention and treatment for osteopenia, osteoporosis, and periodontal disease and for use in bone-grafting procedures. J. Pineal Res. 2014, 56, 115–125. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Sanchez-Barcelo, E.; Mediavilla, M.D.; Reiter, R.J. Significance of high levels of endogenous melatonin in Mammalian cerebrospinal fluid and in the central nervous system. Curr. Neuropharmacol. 2010, 8, 162–167. [Google Scholar] [CrossRef]
- Lim, H.D.; Kim, Y.S.; Ko, S.H.; Yoon, I.J.; Cho, S.G.; Chun, Y.H.; Choi, B.J.; Kim, E.C. Cytoprotective and anti-inflammatory effects of melatonin in hydrogen peroxide-stimulated CHON-001 human chondrocyte cell line and rabbit model of osteoarthritis via the SIRT1 pathway. J. Pineal Res. 2012, 53, 225–237. [Google Scholar] [CrossRef]
- Gosset, M.; Pigenet, A.; Salvat, C.; Berenbaum, F.; Jacques, C. Inhibition of matrix metalloproteinase-3 and -13 synthesis induced by IL-1beta in chondrocytes from mice lacking microsomal prostaglandin E synthase-1. J. Immunol. 2010, 185, 6244–6252. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, D.A.; Guarente, L. Small-molecule allosteric activators of sirtuins. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 363–380. [Google Scholar] [CrossRef]
- Matsushita, T.; Sasaki, H.; Takayama, K.; Ishida, K.; Matsumoto, T.; Kubo, S.; Matsuzaki, T.; Nishida, K.; Kurosaka, M.; Kuroda, R. The overexpression of SIRT1 inhibited osteoarthritic gene expression changes induced by interleukin-1beta in human chondrocytes. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2013, 31, 531–537. [Google Scholar] [CrossRef]
- Shah, S.A.; Khan, M.; Jo, M.H.; Jo, M.G.; Amin, F.U.; Kim, M.O. Melatonin Stimulates the SIRT1/Nrf2 Signaling Pathway Counteracting Lipopolysaccharide (LPS)-Induced Oxidative Stress to Rescue Postnatal Rat Brain. CNS Neurosci. Ther. 2017, 23, 33–44. [Google Scholar] [CrossRef]
- Sacitharan, P.K.; Bou-Gharios, G.; Edwards, J.R. SIRT1 directly activates autophagy in human chondrocytes. Cell Death Discov. 2020, 6, 41. [Google Scholar] [CrossRef]
- Gabay, O.; Sanchez, C.; Dvir-Ginzberg, M.; Gagarina, V.; Zaal, K.J.; Song, Y.; He, X.H.; McBurney, M.W. Sirtuin 1 enzymatic activity is required for cartilage homeostasis in vivo in a mouse model. Arthritis Rheum. 2013, 65, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Moon, S.M.; Han, S.H.; Hwang, E.J.; Park, B.R.; Kim, J.S.; Kim, D.K.; Kim, C.S. Chondroprotective effects of aqueous extract of Anthriscus sylvestris leaves on osteoarthritis in vitro and in vivo through MAPKs and NF-κB signaling inhibition. Biomed. Pharmacother. 2018, 103, 1202–1211. [Google Scholar] [CrossRef]
- Li, B.; Guo, L.; Ku, T.; Chen, M.; Li, G.; Sang, N. PM2.5 exposure stimulates COX-2-mediated excitatory synaptic transmission via ROS-NF-κB pathway. Chemosphere 2018, 190, 124–134. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, J.; Li, T.; Zhang, Q.; Bu, S.; Wang, Q.; Lai, D. Melatonin ameliorates restraint stress-induced oxidative stress and apoptosis in testicular cells via NF-kappaB/iNOS and Nrf2/ HO-1 signaling pathway. Sci. Rep. 2017, 7, 9599. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, Y.; Chen, S.; Tan, Z.; Xiong, K.; Li, Y.; Ye, Y.; Luo, Z.P.; He, F.; Gong, Y. Rescue of proinflammatory cytokine-inhibited chondrogenesis by the antiarthritic effect of melatonin in synovium mesenchymal stem cells via suppression of reactive oxygen species and matrix metalloproteinases. Free Radic. Biol. Med. 2014, 68, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, J.; Fang, Q.; Gao, R.; Shi, Q.; Zhang, H.; Zhao, J. Upregulated heme oxygenase-1 expression of mouse mesenchymal stem cells resists to chemotherapy-induced bone marrow suppression. Chin. Med. J. 2014, 127, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Has, A.L.; Alotaibi, M.F.; Bin-Jumah, M.; Elgebaly, H.; Mahmoud, A.M. Olea europaea leaf extract up-regulates Nrf2/ARE/HO-1 signaling and attenuates cyclophosphamide-induced oxidative stress, inflammation and apoptosis in rat kidney. Biomed. Pharmacother. 2019, 111, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Lippross, S.; Beckmann, R.; Streubesand, N.; Ayub, F.; Tohidnezhad, M.; Campbell, G.; Kan, Y.W.; Horst, F.; Sonmez, T.T.; Varoga, D.; et al. Nrf2 deficiency impairs fracture healing in mice. Calcif. Tissue Int. 2014, 95, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, X.; Peng, Q.; Jin, Y.; Shi, G.; Fan, Z.; Zhou, Z. Four-Octyl Itaconate Protects Chondrocytes against H(2)O(2)-Induced Oxidative Injury and Attenuates Osteoarthritis Progression by Activating Nrf2 Signaling. Oxidative Med. Cell. Longev. 2022, 2022, 2206167. [Google Scholar] [CrossRef]
- Zhang, Q.; Bai, X.; Wang, R.; Zhao, H.; Wang, L.; Liu, J.; Li, M.; Chen, Z.; Wang, Z.; Li, L.; et al. 4-octyl Itaconate inhibits lipopolysaccharide (LPS)-induced osteoarthritis via activating Nrf2 signalling pathway. J. Cell Mol. Med. 2022, 26, 1515–1529. [Google Scholar] [CrossRef] [PubMed]
- Tossetta, G.; Fantone, S.; Goteri, G.; Giannubilo, S.R.; Ciavattini, A.; Marzioni, D. The Role of NQO1 in Ovarian Cancer. Int. J. Mol. Sci. 2023, 24, 7839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, T.; Zeng, S.; Zhang, X.; Zhou, F.; Gillies, M.C.; Zhu, L. The Role of Nrf2/sMAF Signalling in Retina Ageing and Retinal Dis-eases. Biomedicines 2023, 11, 1512. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zhang, K.J.; Li, G.C.; Chen, X.R.; Lin, J.J.; Li, J.W.; Lv, Z.Y.; Deng, Z.Z.; Dai, J.; Cao, W.; et al. CDDO-Im ameliorates osteoarthritis and inhibits chondrocyte apoptosis in mice via enhancing Nrf2-dependent autophagy. Acta Pharmacol. Sin. 2022, 43, 1793–1802. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Y.; Hou, M.; Liu, H.; Yang, H.; Chen, X.; Liu, T.; He, F.; Zhu, X. Melatonin Prevents Cartilage Degradation in Early-Stage Osteoarthritis Through Activation of miR-146a/NRF2/HO-1 Axis. J. Bone Miner. Res. 2022, 37, 1056–1072. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Huh, S.J.; Kang, B.J.; Shin, H. Directed Regeneration of Osteochondral Tissue by Hierarchical Assembly of Spatially Organized Composite Spheroids. Adv. Sci. 2022, 9, e2103525. [Google Scholar] [CrossRef]
- Cha, B.H.; Kim, J.H.; Kang, S.W.; Do, H.J.; Jang, J.W.; Choi, Y.R.; Park, H.; Kim, B.S.; Lee, S.H. Cartilage tissue formation from dedifferentiated chondrocytes by codelivery of BMP-2 and SOX-9 genes encoding bicistronic vector. Cell Transplant. 2013, 22, 1519–1528. [Google Scholar] [CrossRef]
- Zhen, G.; Wen, C.; Jia, X.; Li, Y.; Crane, J.L.; Mears, S.C.; Askin, F.B.; Frassica, F.J.; Chang, W.; Yao, J.; et al. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat. Med. 2013, 19, 704–712. [Google Scholar] [CrossRef]
- Wehrhan, F.; Amann, K.; Molenberg, A.; Lutz, R.; Neukam, F.W.; Schlegel, K.A. Critical size defect regeneration using PEG-mediated BMP-2 gene delivery and the use of cell occlusive barrier membranes—The osteopromotive principle revisited. Clin. Oral Implants Res. 2013, 24, 910–920. [Google Scholar] [CrossRef]
- Lefebvre, V. Roles and regulation of SOX transcription factors in skeletogenesis. Curr. Top. Dev. Biol. 2019, 133, 171–193. [Google Scholar] [CrossRef]
- Kim, E.J.; Cho, S.W.; Shin, J.O.; Lee, M.J.; Kim, K.S.; Jung, H.S. Ihh and Runx2/Runx3 signaling interact to coordinate early chondrogenesis: A mouse model. PLoS ONE 2013, 8, e55296. [Google Scholar] [CrossRef]
- Abed, E.; Bouvard, B.; Martineau, X.; Jouzeau, J.Y.; Reboul, P.; Lajeunesse, D. Elevated hepatocyte growth factor levels in osteoarthritis osteoblasts contribute to their altered response to bone morphogenetic protein-2 and reduced mineralization capacity. Bone 2015, 75, 111–119. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, G.; Cao, C.; Pang, J.; Chen, X. Bone morphogenetic protein-7 promotes chondrogenesis in human amniotic epithelial cells. Int. Orthop. 2011, 35, 941–948. [Google Scholar] [CrossRef]
- Pei, M.; He, F.; Wei, L.; Rawson, A. Melatonin enhances cartilage matrix synthesis by porcine articular chondrocytes. J. Pineal Res. 2009, 46, 181–187. [Google Scholar] [CrossRef]
- Gao, W.; Lin, M.; Liang, A.; Zhang, L.; Chen, C.; Liang, G.; Xu, C.; Peng, Y.; Chen, C.; Huang, D.; et al. Melatonin enhances chondrogenic differentiation of human mesenchymal stem cells. J. Pineal Res. 2014, 56, 62–70. [Google Scholar] [CrossRef]
- Abshirini, M.; Ilesanmi-Oyelere, B.L.; Kruger, M.C. Potential modulatory mechanisms of action by long-chain polyunsaturated fatty acids on bone cell and chondrocyte metabolism. Prog. Lipid Res. 2021, 83, 101113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, J.; Zhou, X.; Chen, X.; Chen, A.C.; Pi, B.; Pan, G.; Pei, M.; Yang, H.; Liu, T.; et al. Melatonin Prevents Osteoarthritis-Induced Cartilage Degradation via Targeting MicroRNA-140. Oxidative Med. Cell. Longev. 2019, 2019, 9705929. [Google Scholar] [CrossRef] [PubMed]
- Mayo, J.C.; Sainz, R.M.; Tan, D.X.; Hardeland, R.; Leon, J.; Rodriguez, C.; Reiter, R.J. Anti-inflammatory actions of melatonin and its metabolites, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK), in macrophages. J. Neuroimmunol. 2005, 165, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Mabey, T.; Honsawek, S.; Tanavalee, A.; Yuktanandana, P.; Wilairatana, V.; Poovorawan, Y. Plasma and synovial fluid inflammatory cytokine profiles in primary knee osteoarthritis. Biomarkers 2016, 21, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Mayo, J.C.; Sainz, R.M.; Gonzalez Menendez, P.; Cepas, V.; Tan, D.X.; Reiter, R.J. Melatonin and sirtuins: A “not-so unexpected” relationship. J. Pineal Res. 2017, 62, e12391. [Google Scholar] [CrossRef] [PubMed]
- Jung-Hynes, B.; Reiter, R.J.; Ahmad, N. Sirtuins, melatonin and circadian rhythms: Building a bridge between aging and cancer. J. Pineal Res. 2010, 48, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin and inflammation-Story of a double-edged blade. J. Pineal Res. 2018, 65, e12525. [Google Scholar] [CrossRef]
- Su, P.; Xu, C.; Zheng, S.; Zhou, T.; Zhou, H.; Chen, C. Study on the Effect and Mechanism of melatonin in Promoting Cartilage Repair. Master’s Thesis, The First Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China, 2020. [Google Scholar]
- Vriend, J.; Reiter, R.J. The Keap1-Nrf2-antioxidant response element pathway: A review of its regulation by melatonin and the proteasome. Mol. Cell Endocrinol. 2015, 401, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Arioz, B.I.; Tastan, B.; Tarakcioglu, E.; Tufekci, K.U.; Olcum, M.; Ersoy, N.; Bagriyanik, A.; Genc, K.; Genc, S. Melatonin Attenuates LPS-Induced Acute Depressive-Like Behaviors and Microglial NLRP3 Inflammasome Activation through the SIRT1/Nrf2 Pathway. Front. Immunol. 2019, 10, 1511. [Google Scholar] [CrossRef]
- Shintani, N.; Siebenrock, K.A.; Hunziker, E.B. TGF-ss1 enhances the BMP-2-induced chondrogenesis of bovine synovial explants and arrests downstream differentiation at an early stage of hypertrophy. PLoS ONE 2013, 8, e53086. [Google Scholar] [CrossRef]
- Turgut, M.; Oktem, G.; Uslu, S.; Yurtseven, M.E.; Aktug, H.; Uysal, A. The effect of exogenous melatonin administration on trabecular width, ligament thickness and TGF-beta(1) expression in degenerated intervertebral disk tissue in the rat. J. Clin. Neurosci. 2006, 13, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Qiu, X.; Gao, B.; Lian, C.; Peng, Y.; Liang, A.; Xu, C.; Gao, W.; Zhang, L.; Su, P.; et al. Melatonin-mediated miR-526b-3p and miR-590-5p upregulation promotes chondrogenic differentiation of human mesenchymal stem cells. J. Pineal Res. 2018, 65, e12483. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Zhang, M.; Liu, M.; Wang, Q.; Liu, M.; Zhao, Y. SOX9 Functions as a Master Regulator of Antler Growth by Controlling Multiple Cell Lineages. DNA Cell Biol. 2018, 37, 15–22. [Google Scholar] [CrossRef]
- Ko, J.Y.; Lee, J.; Lee, J.; Ryu, Y.H.; Im, G.I. SOX-6, 9-Transfected Adipose Stem Cells to Treat Surgically-Induced Osteoarthritis in Goats. Tissue Eng. Part A 2019, 25, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Hu, N.; Zhou, N.; Lin, L.; Zhao, C.; Yi, S.; Fan, T.; Bao, W.; Liang, X.; Chen, H.; et al. SOX9 potentiates BMP2-induced chondrogenic differentiation and inhibits BMP2-induced osteogenic differentiation. PLoS ONE 2014, 9, e89025. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.; Martson, A.; Vidya, R.; Chitnavis, S.; Harsulkar, A. Pathophysiological landscape of osteoarthritis. Adv. Clin. Chem. 2021, 100, 37–90. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.Y.; Jiang, T.; Huang, Z.F.; Chu, B.; Gu, J.; Zhao, X.; Liu, H.; Fan, J.; Yu, L.P.; Jiang, S.H.; et al. Fatty acids derived from apoptotic chondrocytes fuel macrophages FAO through MSR1 for facilitating BMSCs osteogenic differentiation. Redox Biol. 2022, 53, 102326. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, C.; Liu, P.; Huang, H.; Zhang, S.; Wang, X. Anti-Apoptosis and Autophagy Effects of melatonin Protect Rat Chondrocytes against Oxidative Stress via Regulation of AMPK/Foxo3 Pathways. Cartilage 2021, 13 (Suppl. S2), 1041S–1053S. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Qiu, J.; Huang, X. 8-Methoxypsoralen has Anti-inflammatory and Antioxidant Roles in Osteoarthritis Through SIRT1/NF-kappaB Pathway. Front. Pharmacol. 2021, 12, 692424. [Google Scholar] [CrossRef]
| Antibodies | Brands | Product Codes |
|---|---|---|
| SIRT1 | ABconal | A11267 |
| MMP3 | Affinity | AF0217 |
| MMP13 | Affinity | AF5355 |
| ADAMTS-4 | ABconal | A2525 |
| COX-2 | ABconal | A1253 |
| Nrf2 | ABconal | A0674 |
| COL2A1 | ABconal | A1560 |
| HO-1 | ABconal | A1346 |
| iNOS | ABconal | A3774 |
| TGF-β1 | ABconal | A2124 |
| p-Smad2 | ABconal | AP0269 |
| p65 | ABconal | A2547 |
| p-p65 | ABconal | AP0123 |
| IκBα | ABconal | A24742 |
| p-IκBα | ABconal | AP0614 |
| Aggrecan | ABconal | A8536 |
| RANKL | Absin | abs120177 |
| GAPDH | ABconal | AC001 |
| Forward Primer | Reverse Primer | |
|---|---|---|
| SIRT1 | 5′-TCAGTGTCATGGTTCCTTTGC-3′ | 5′-AATCTGCTCCTTTGCCACTCT-3′ |
| MMP3 | 5′-TTTGGCCGTCTCTTCCATCC-3′ | 5′-GCATCGATCTTCTGGACGGT-3′ |
| MMP13 | 5′-TTCTGGTCTTCTGGCACACG-3′ | 5′-TGGAGCTGCTTGTCCAGGT-3′ |
| ADAMTS-4 | 5′-CACCGAACCGACCTCTTCAA-3′ | 5′-GAGTTCCATCTGCCACCCGT-3′ |
| COX-2 | 5′-AGAAGCGAGGACCTGGGTTCAG-3′ | 5′-ACACCTCTCCACCGATGACCTG-3′ |
| Nrf2 | 5′-CGAGATATACGCAGGAGAGGTAAGA-3′ | 5′-GCTCGACAATGTTCTCCAGCTT-3′ |
| COL2A1 | 5′-GAACGGCGGCTTCCACTTCAG-3′ | 5′-CACACGCTAGACACTGGACT-3′ |
| BMP2 | 5′-GAGGAGAAGCCAGGTGTCT-3′ | 5′-GTCCACATACAAAGGGTGC-3′ |
| BMP7 | 5′-AAACAACGCAGCCAGAACCG-3′ | 5′-CCTCACAGTAGGCAGCATAGC-3′ |
| SOX9 | 5′-CTCCCAAAACAGACGTGCAA-3′ | 5′-CGAAGGTCTCGATGTTGGAGAT-3′ |
| Runx2 | 5′-TGCTGGAGTGATGTGGTTTTCT-3′ | 5′-CCCCTGTTGTGTTGTTTGGTAA-3′ |
| GAPDH | 5′-GATGCCCCCATGTTTGTGAT-3′ | 5′-GGCATGGACTGTGGTCATGAG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Qiu, D.; Miao, X.; Yang, W.; Li, S.; Cheng, X.; Tang, J.; Chen, H.; Ruan, H.; Liu, Y.; et al. Melatonin Delays Arthritis Inflammation and Reduces Cartilage Matrix Degradation through the SIRT1-Mediated NF-κB/Nrf2/TGF-β/BMPs Pathway. Int. J. Mol. Sci. 2024, 25, 6202. https://doi.org/10.3390/ijms25116202
Zhao M, Qiu D, Miao X, Yang W, Li S, Cheng X, Tang J, Chen H, Ruan H, Liu Y, et al. Melatonin Delays Arthritis Inflammation and Reduces Cartilage Matrix Degradation through the SIRT1-Mediated NF-κB/Nrf2/TGF-β/BMPs Pathway. International Journal of Molecular Sciences. 2024; 25(11):6202. https://doi.org/10.3390/ijms25116202
Chicago/Turabian StyleZhao, Mingchao, Di Qiu, Xue Miao, Wenyue Yang, Siyao Li, Xin Cheng, Jilang Tang, Hong Chen, Hongri Ruan, Ying Liu, and et al. 2024. "Melatonin Delays Arthritis Inflammation and Reduces Cartilage Matrix Degradation through the SIRT1-Mediated NF-κB/Nrf2/TGF-β/BMPs Pathway" International Journal of Molecular Sciences 25, no. 11: 6202. https://doi.org/10.3390/ijms25116202




