Tumor-Homing Peptides as Crucial Component of Magnetic-Based Delivery Systems: Recent Developments and Pharmacoeconomical Perspective
Abstract
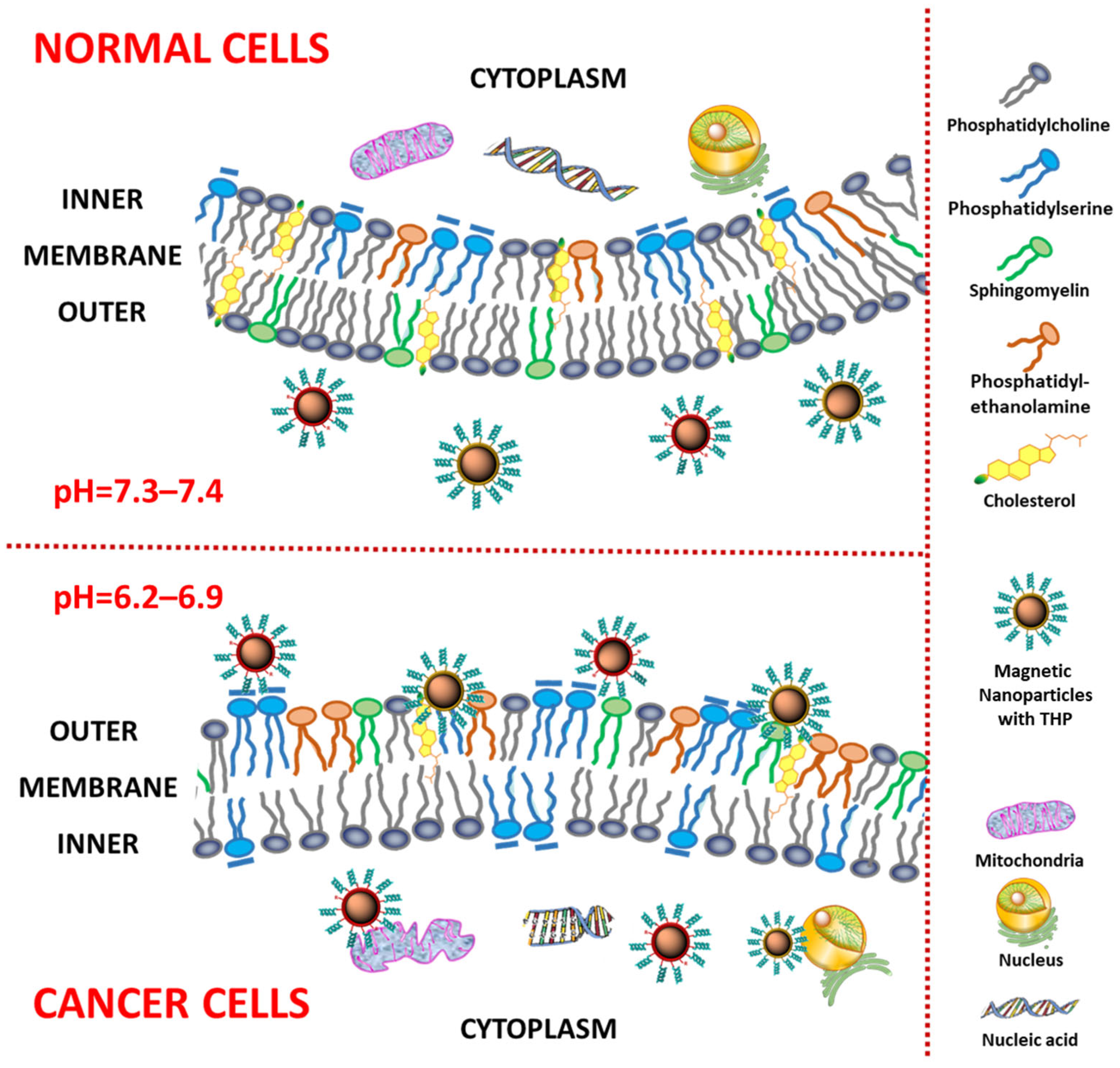
:1. Introduction
2. Cancer Membrane—Targeting Receptors
3. Tumor-Homing Peptides (THPs)—Characteristics
3.1. RGD Peptides—Characteristics
3.2. Cyclic RGD—Characteristics
3.3. iRGD—Characteristics
3.4. NGR Peptides—Characteristics
3.5. Cell-Penetrating Peptides (CPPs)—Characteristics
3.6. Machine Learning Approaches for Designing THP
4. Homing Peptides for Drug Delivery
4.1. THPs for Drug Delivery
4.2. THPs for Blood–Brain Barrier (BBB) Delivery
5. Homing Peptides for Imaging
6. Magnetic Nanoparticles as a Platform for Tumor-Homing Peptides
6.1. Methods of Preparation and Functionalization of Magnetic Nanoparticles
- -
- -
- -
- -
- Copper Catalyzed Azide Alkyne Cycloaddition (reaction of alkyne-modified MNP with azido derivative of polypeptide) [159];
- -
- Schiff base formation between a carbonyl group presented on the surface of MNP and an amino group of the THP [160].
6.2. Biomedical Application of Magnetic Nanoparticles
6.2.1. MNPs in Cancer Imaging
| Imaging Method | Targeting Peptide | Nanocarrier | Advantages | Ref. |
|---|---|---|---|---|
| MRI | LTVSPWY | LTVSPWY-PEG-CS MNPs |
| [161] |
| A54 | A54-GFP-coated MNPs |
| [151] | |
| A54-Dex-PLGA/DOX/SPIO |
| [162] | ||
| iRGD | iRGD-SPIO |
| [67] | |
| CKAAKN | CKAAKN–HA–VES@USPIO NPs |
| [167] |
| Diagnostic Method | Targeting Peptide | Nanocarrier | Advantages | Ref. |
|---|---|---|---|---|
| Magnetic field | YSA | MNPs-YSA peptide conjugates |
| [169] |
| Whole-body imaging | CREKA | CREKA-SPIO |
| [170] |
| Sensitive monitoring of the magnetic relaxation of IONPs with the use of MPS | IONPs-N/IONPs-N-P/IONPs-N-P with protease |
| [171] | |
| MRI | A54 | Dex-PLGA/DOX/SPIO |
| [162] |
| MRI | - | MNPs |
| [172] |
6.2.2. MNPs in Hyperthermia Treatment
| Carrier | Ligand | Agent/ Tag | Tumor | Result | Ref. |
|---|---|---|---|---|---|
| Magneto-liposome | cRGD | DOX/ICG | In vitro: lung, breast, skin, brain, and liver cancer In vivo: BALB/c mice murine immuno-competent fibrosarcoma tumor model | Combinatorial tumor therapy (chemo-radio-hyperthermia) Insignificant cardiac toxicity | [179] |
| Fe3O4@PMAO-PEG | RGD | ND | In vitro compatibility assay: Vero cells | Prototype system for further in vivo evaluation | [180] |
| Fe3O4@PMAO | RGD | ND | In vivo: rats bearing hepatic implants of colon adenocarcinoma | Therapeutic approach for poorly vascularized liver tumors | [181] |
| Fe3O4 | EGFR— targeted peptide (YHWYGYTPQNVI) | ND | In vitro: lung cancer (NSCLC) In vivo: mouse orthotopic lung tumor model | Effective anticancer treatment modality for the treatment of NSCLC based on targeted magnetic hyperthermia | [182] |
| TMNPs, i.e., Fe3O4@Mn0.5Zn0.5Fe2O4@CoFe2O4 | LN1 CPP | ND | In vitro: prostate cancer | Reduction in cancer cell aggressiveness | [183] |
| SPIONs-PEG | membranotropic peptide gH625 | Cyanine 5.5 | In vitro: breast cancer cells | Prototype of nanoplatform for cancer theranostics involving magnetic resonance imaging, optical imaging (infrared), drug delivery, and hyperthermia | [184] |
6.2.3. Different Biomedical Applications of MNPs
| Condition | Targeting Peptide | Nanocarrier | Advantages | Ref. |
|---|---|---|---|---|
| Conditions associated with Gram(+)/Gram(−) bacteria | Gly-Ala-Phe-Pro-His-Arg | Silica-coated iron oxide NPs |
| [185] |
| GBM | NFL peptide | pSiNRs |
| [186] |
| ALI | iNOS PNAs and CPPs | SCKs |
| [187] |
| LC | TAT-functionalized IONPs | Fe3O4 + TAT |
| [188] |
| UTIs | rGO/MPND with pyrene–PEG | rGO |
| [189] |
| BC | Fe-Arg-MTX | IOMNPs |
| [190] |
7. Clinical and Cost-Effectiveness Analysis of Application THPs with MNPs
8. Future Perspectives
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Khot, V.M.; Salunkhe, A.B.; Pricl, S.; Bauer, J.; Thorat, N.D.; Townley, H. Nanomedicine-driven molecular targeting, drug delivery, and therapeutic approaches to cancer chemoresistance. Drug Discov. Today 2021, 26, 724–739. [Google Scholar] [CrossRef]
- Yavari, B.; Mahjub, R.; Saidijam, M.; Raigani, M.; Soleimani, M. The Potential Use of Peptides in Cancer Treatment. Curr. Protein Pept. Sci. 2018, 19, 759–770. [Google Scholar] [CrossRef]
- Xiao, Y.F.; Jie, M.M.; Li, B.S.; Hu, C.J.; Xie, R.; Tang, B.; Yang, S.M. Peptide-Based Treatment: A Promising Cancer Therapy. J. Immunol. Res. 2015, 2015, 761820. [Google Scholar] [CrossRef]
- Chatzisideri, T.; Leonidis, G.; Sarli, V. Cancer-targeted delivery systems based on peptides. Future Med. Chem. 2018, 10, 2201–2226. [Google Scholar] [CrossRef]
- Tran, S.; DeGiovanni, P.J.; Piel, B.; Rai, P. Cancer nanomedicine: A review of recent success in drug delivery. Clin. Transl. Med. 2017, 6, 44. [Google Scholar] [CrossRef]
- Jain, K.K. Advances in the field of nanooncology. BMC Med. 2010, 8, 83. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Cheng, R.; Yang, Z.; Tian, Z.M. Nanotechnology for Cancer Therapy Based on Chemotherapy. Molecules 2018, 23, 826. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef]
- Hossen, S.; Hossain, M.K.; Basher, M.K.; Mia, M.N.H.; Rahman, M.T.; Uddin, M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res. 2019, 15, 1–18. [Google Scholar] [CrossRef]
- Vangijzegem, T.; Lecomte, V.; Ternad, I.; Van Leuven, L.; Muller, R.N.; Stanicki, D.; Laurent, S. Superparamagnetic Iron Oxide Nanoparticles (SPION): From Fundamentals to State-of-the-Art Innovative Applications for Cancer Therapy. Pharmaceutics 2023, 15, 236. [Google Scholar] [CrossRef]
- Ma, Z.; Mohapatra, J.; Wei, K.; Liu, J.P.; Sun, S. Magnetic Nanoparticles: Synthesis, Anisotropy, and Applications. Chem. Rev. 2023, 123, 3904–3943. [Google Scholar] [CrossRef]
- Stueber, D.D.; Villanova, J.; Aponte, I.; Xiao, Z.; Colvin, V.L. Magnetic Nanoparticles in Biology and Medicine: Past, Present, and Future Trends. Pharmaceutics 2021, 13, 943. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Mignani, S.; Majoral, J.-P.; Shen, M.; Shi, X. Construction of iron oxide nanoparticle-based hybrid platforms for tumor imaging and therapy. Chem. Soc. Rev. 2018, 47, 1874–1900. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Lindsley, C.W. Pharmacoeconomics and the medicinal chemist. ACS Med. Chem. Lett. 2014, 5, 1066–1068. [Google Scholar] [CrossRef]
- Milewska, S.; Niemirowicz-Laskowska, K.; Siemiaszko, G.; Nowicki, P.; Wilczewska, A.Z.; Car, H. Current Trends and Challenges in Pharmacoeconomic Aspects of Nanocarriers as Drug Delivery Systems for Cancer Treatment. Int. J. Nanomed. 2021, 16, 6593–6644. [Google Scholar] [CrossRef] [PubMed]
- Ruoslahti, E. Tumor penetrating peptides for improved drug delivery. Adv. Drug Deliv. Rev. 2017, 110–111, 3–12. [Google Scholar] [CrossRef]
- Laakkonen, P.; Vuorinen, K. Homing peptides as targeted delivery vehicles. Integr. Biol. 2010, 2, 326–337. [Google Scholar] [CrossRef]
- Seyyednia, E.; Oroojalian, F.; Baradaran, B.; Mojarrad, J.S.; Mokhtarzadeh, A.; Valizadeh, H. Nanoparticles modified with vasculature-homing peptides for targeted cancer therapy and angiogenesis imaging. J. Control. Release 2021, 338, 367–393. [Google Scholar] [CrossRef]
- Vadevoo, S.M.P.; Gurung, S.; Lee, H.-S.; Gunassekaran, G.R.; Lee, S.-M.; Yoon, J.-W.; Lee, Y.-K.; Lee, B. Peptides as multifunctional players in cancer therapy. Exp. Mol. Med. 2023, 55, 1099–1109. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, Y.; Wei, X.; Li, N.; Huang, H.; Xie, Z.; Zhang, H.; Yang, G.; Li, M.; Li, T.; et al. Tumor homing-penetrating and nanoenzyme-augmented 2D phototheranostics against hypoxic solid tumors. Acta Biomater. 2022, 150, 391–401. [Google Scholar] [CrossRef]
- Muro, E.; Atilla-Gokcumen, G.E.; Eggert, U.S. Lipids in cell biology: How can we understand them better? Mol. Biol. Cell 2014, 25, 1819–1823. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.L.; Preta, G. Lipids in the cell: Organisation regulates function. Cell Mol. Life Sci. 2018, 75, 1909–1927. [Google Scholar] [CrossRef] [PubMed]
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, C. The mystery of membrane organization: Composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 2017, 18, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Li, C.M.; Haratipour, P.; Lingeman, R.G.; Perry, J.J.P.; Gu, L.; Hickey, R.J.; Malkas, L.H. Novel Peptide Therapeutic Approaches for Cancer Treatment. Cells 2021, 10, 2908. [Google Scholar] [CrossRef] [PubMed]
- Yamaji-Hasegawa, A.; Tsujimoto, M. Asymmetric distribution of phospholipids in biomembranes. Biol. Pharm. Bull. 2006, 29, 1547–1553. [Google Scholar] [CrossRef] [PubMed]
- Ran, S.; Downes, A.; Thorpe, P.E. Increased exposure of anionic phospholipids on the surface of tumor blood vessels. Cancer Res. 2002, 62, 6132–6140. [Google Scholar] [PubMed]
- Stafford, J.H.; Thorpe, P.E. Increased exposure of phosphatidylethanolamine on the surface of tumor vascular endothelium. Neoplasia 2011, 13, 299–308. [Google Scholar] [CrossRef]
- Barceló-Coblijn, G.; Martin, M.L.; de Almeida, R.F.; Noguera-Salvà, M.A.; Marcilla-Etxenike, A.; Guardiola-Serrano, F.; Lüth, A.; Kleuser, B.; Halver, J.E.; Escribá, P.V. Sphingomyelin and sphingomyelin synthase (SMS) in the malignant transformation of glioma cells and in 2-hydroxyoleic acid therapy. Proc. Natl. Acad. Sci. USA 2011, 108, 19569–19574. [Google Scholar] [CrossRef]
- Preetha, A.; Huilgol, N.; Banerjee, R. Comparison of paclitaxel penetration in normal and cancerous cervical model monolayer membranes. Colloids Surf. B Biointerfaces 2006, 53, 179–186. [Google Scholar] [CrossRef]
- Zhao, L.; Feng, S.S.; Go, M.L. Investigation of molecular interactions between paclitaxel and DPPC by Langmuir film balance and differential scanning calorimetry. J. Pharm. Sci. 2004, 93, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Chiangjong, W.; Chutipongtanate, S.; Hongeng, S. Anticancer peptide: Physicochemical property, functional aspect and trend in clinical application (Review). Int. J. Oncol. 2020, 57, 678–696. [Google Scholar] [CrossRef] [PubMed]
- Logozzi, M.; Spugnini, E.; Mizzoni, D.; Di Raimo, R.; Fais, S. Extracellular acidity and increased exosome release as key phenotypes of malignant tumors. Cancer Metastasis Rev. 2019, 38, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Cardone, R.A.; Casavola, V.; Reshkin, S.J. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat. Rev. Cancer 2005, 5, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, N.; Fialho, A.M. Perturbing the Dynamics and Organization of Cell Membrane Components: A New Paradigm for Cancer-Targeted Therapies. Int. J. Mol. Sci. 2018, 19, 3871. [Google Scholar] [CrossRef] [PubMed]
- Escribá, P.V. Membrane-lipid therapy: A historical perspective of membrane-targeted therapies—From lipid bilayer structure to the pathophysiological regulation of cells. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1493–1506. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, D.W.; Ramamoorthy, A. Studies on anticancer activities of antimicrobial peptides. Biochim. Biophys. Acta 2008, 1778, 357–375. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, E.G.; Dobroff, A.S.; Taborda, C.P.; Travassos, L.R. Antifungal and antitumor models of bioactive protective peptides. An. Acad. Bras. Cienc. 2009, 81, 503–520. [Google Scholar] [CrossRef] [PubMed]
- Droin, N.; Hendra, J.B.; Ducoroy, P.; Solary, E. Human defensins as cancer biomarkers and antitumour molecules. J. Proteom. 2009, 72, 918–927. [Google Scholar] [CrossRef]
- Simons, K.; Ikonen, E. How cells handle cholesterol. Science 2000, 290, 1721–1726. [Google Scholar] [CrossRef]
- Harris, F.; Dennison, S.R.; Singh, J.; Phoenix, D.A. On the selectivity and efficacy of defense peptides with respect to cancer cells. Med. Res. Rev. 2013, 33, 190–234. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ding, J.; Wang, X.; Wei, K.; Weng, J.; Wang, J. One-pot synthesis and functionalisation of Fe2O3@C-NH2 nanoparticles for imaging and therapy. IET Nanobiotechnol. 2014, 8, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Hoppenz, P.; Els-Heindl, S.; Beck-Sickinger, A.G. Peptide-Drug Conjugates and Their Targets in Advanced Cancer Therapies. Front. Chem. 2020, 8, 571. [Google Scholar] [CrossRef] [PubMed]
- EHJ, D. Integrins: An Overview of Structural and Functional Aspects. In Madame Curie Bioscience Database [Internet]; Landes Bioscience: Austin, TX, USA, 2000–2013. [Google Scholar]
- Liu, Z.; Wang, F.; Chen, X. Integrin alpha(v)beta(3)-Targeted Cancer Therapy. Drug Dev. Res. 2008, 69, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Seshacharyulu, P.; Ponnusamy, M.P.; Haridas, D.; Jain, M.; Ganti, A.K.; Batra, S.K. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin. Ther. Targets 2012, 16, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Sriram, K.; Insel, P.A. G Protein-Coupled Receptors as Targets for Approved Drugs: How Many Targets and How Many Drugs? Mol. Pharmacol. 2018, 93, 251–258. [Google Scholar] [CrossRef]
- Basith, S.; Cui, M.; Macalino, S.J.Y.; Park, J.; Clavio, N.A.B.; Kang, S.; Choi, S. Exploring G Protein-Coupled Receptors (GPCRs) Ligand Space via Cheminformatics Approaches: Impact on Rational Drug Design. Front. Pharmacol. 2018, 9, 128. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.X.; Liu, L.T.; Qi, X.R. Development of small interfering RNA delivery system using PEI-PEG-APRPG polymer for antiangiogenic vascular endothelial growth factor tumor-targeted therapy. Int. J. Nanomed. 2011, 6, 1661–1673. [Google Scholar] [CrossRef]
- Hao, Z.; Fan, W.; Hao, J.; Wu, X.; Zeng, G.Q.; Zhang, L.J.; Nie, S.F.; Wang, X.D. Efficient delivery of micro RNA to bone-metastatic prostate tumors by using aptamer-conjugated atelocollagen in vitro and in vivo. Drug Deliv. 2016, 23, 874–881. [Google Scholar] [CrossRef]
- Gostaviceanu, A.; Gavrilaş, S.; Copolovici, L.; Copolovici, D.M. Membrane-Active Peptides and Their Potential Biomedical Application. Pharmaceutics 2023, 15, 2091. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- David, A. Peptide ligand-modified nanomedicines for targeting cells at the tumor microenvironment. Adv. Drug Deliv. Rev. 2017, 119, 120–142. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, N.; Caraglia, M.; Grimaldi, A.; Marfella, R.; Servillo, L.; Paolisso, G.; Balestrieri, M.L. Vascular-homing peptides for targeted drug delivery and molecular imaging: Meeting the clinical challenges. Biochim. Biophys. Acta 2014, 1846, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ghabraie, E.; Kemker, I.; Tonali, N.; Ismail, M.; Dodero, V.I.; Sewald, N. Phenothiazine-Biaryl-Containing Fluorescent RGD Peptides. Chemistry 2020, 26, 12036–12042. [Google Scholar] [CrossRef] [PubMed]
- Liolios, C.; Sachpekidis, C.; Kolocouris, A.; Dimitrakopoulou-Strauss, A.; Bouziotis, P. PET Diagnostic Molecules Utilizing Multimeric Cyclic RGD Peptide Analogs for Imaging Integrin α. Molecules 2021, 26, 1792. [Google Scholar] [CrossRef]
- Shi, J.; Wang, F.; Liu, S. Radiolabeled cyclic RGD peptides as radiotracers for tumor imaging. Biophys. Rep. 2016, 2, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Zuo, H. iRGD: A Promising Peptide for Cancer Imaging and a Potential Therapeutic Agent for Various Cancers. J. Oncol. 2019, 2019, 9367845. [Google Scholar] [CrossRef]
- Cho, H.J.; Lee, S.J.; Park, S.J.; Paik, C.H.; Lee, S.M.; Kim, S.; Lee, Y.S. Activatable iRGD-based peptide monolith: Targeting, internalization, and fluorescence activation for precise tumor imaging. J. Control. Release 2016, 237, 177–184. [Google Scholar] [CrossRef]
- Yin, H.; Yang, J.; Zhang, Q.; Wang, H.; Xu, J.; Zheng, J. iRGD as a tumor-penetrating peptide for cancer therapy (Review). Mol. Med. Rep. 2017, 15, 2925–2930. [Google Scholar] [CrossRef]
- Sugahara, K.N.; Teesalu, T.; Karmali, P.P.; Kotamraju, V.R.; Agemy, L.; Girard, O.M.; Hanahan, D.; Mattrey, R.F.; Ruoslahti, E. Tissue-penetrating delivery of compounds and nanoparticles into tumors. Cancer Cell 2009, 16, 510–520. [Google Scholar] [CrossRef]
- Sugahara, K.N.; Braun, G.B.; de Mendoza, T.H.; Kotamraju, V.R.; French, R.P.; Lowy, A.M.; Teesalu, T.; Ruoslahti, E. Tumor-penetrating iRGD peptide inhibits metastasis. Mol. Cancer Ther. 2015, 14, 120–128. [Google Scholar] [CrossRef]
- Teesalu, T.; Sugahara, K.N.; Kotamraju, V.R.; Ruoslahti, E. C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration. Proc. Natl. Acad. Sci. USA 2009, 106, 16157–16162. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, Y.; Li, J.; Peng, Z.H.; Sheinin, Y.; Zhou, J.; Oupický, D. Tumor-Penetrating Nanoparticles for Enhanced Anticancer Activity of Combined Photodynamic and Hypoxia-Activated Therapy. ACS Nano 2017, 11, 2227–2238. [Google Scholar] [CrossRef]
- Sugahara, K.N.; Teesalu, T.; Karmali, P.P.; Kotamraju, V.R.; Agemy, L.; Greenwald, D.R.; Ruoslahti, E. Coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs. Science 2010, 328, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Pellinen, T.; Ivaska, J. Integrin traffic. J. Cell Sci. 2006, 119, 3723–3731. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Yoshioka, Y.; Morishige, T.; Eto, Y.; Narimatsu, S.; Kawai, Y.; Mizuguchi, H.; Gao, J.Q.; Mukai, Y.; Okada, N.; et al. Tumor vascular targeted delivery of polymer-conjugated adenovirus vector for cancer gene therapy. Mol. Ther. 2011, 19, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Zuo, H.D.; Yao, W.W.; Chen, T.W.; Zhu, J.; Zhang, J.J.; Pu, Y.; Liu, G.; Zhang, X.M. The effect of superparamagnetic iron oxide with iRGD peptide on the labeling of pancreatic cancer cells in vitro: A preliminary study. BioMed. Res. Int. 2014, 2014, 852352. [Google Scholar] [CrossRef] [PubMed]
- Seidi, K.; Jahanban-Esfahlan, R.; Monhemi, H.; Zare, P.; Minofar, B.; Daei Farshchi Adli, A.; Farajzadeh, D.; Behzadi, R.; Mesgari Abbasi, M.; Neubauer, H.A.; et al. NGR (Asn-Gly-Arg)-targeted delivery of coagulase to tumor vasculature arrests cancer cell growth. Oncogene 2018, 37, 3967–3980. [Google Scholar] [CrossRef] [PubMed]
- Seidi, K.; Neubauer, H.A.; Moriggl, R.; Jahanban-Esfahlan, R.; Javaheri, T. Tumor target amplification: Implications for nano drug delivery systems. J. Control. Release 2018, 275, 142–161. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Ding, Z.; Li, X.; Wei, H.; Chen, Y. Research Progress of Radiolabeled Asn-Gly-Arg (NGR) Peptides for Imaging and Therapy. Mol. Imaging 2020, 19, 1536012120934957. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.; Santo, D.; Domingues, C.; Veiga, F.; Faneca, H.; Figueiras, A. Recent advances in peptide-targeted micelleplexes: Current developments and future perspectives. Int. J. Pharm. 2021, 597, 120362. [Google Scholar] [CrossRef]
- Felício, M.R.; Silva, O.N.; Gonçalves, S.; Santos, N.C.; Franco, O.L. Peptides with Dual Antimicrobial and Anticancer Activities. Front. Chem. 2017, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Kebebe, D.; Liu, Y.; Wu, Y.; Vilakhamxay, M.; Liu, Z.; Li, J. Tumor-targeting delivery of herb-based drugs with cell-penetrating/tumor-targeting peptide-modified nanocarriers. Int. J. Nanomed. 2018, 13, 1425–1442. [Google Scholar] [CrossRef] [PubMed]
- Ciobanasu, C.; Dragomir, I.; Apetrei, A. The penetrating properties of the tumor homing peptide LyP-1 in model lipid membranes. J. Pept. Sci. 2019, 25, e3145. [Google Scholar] [CrossRef]
- Taylor, R.E.; Zahid, M. Cell Penetrating Peptides, Novel Vectors for Gene Therapy. Pharmaceutics 2020, 12, 225. [Google Scholar] [CrossRef] [PubMed]
- Paulus, J.; Sewald, N. Small molecule– and peptide–drug conjugates addressing integrins: A story of targeted cancer treatment. J. Pept. Sci. 2024, 30, e3561. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kapoor, P.; Gautam, A.; Chaudhary, K.; Kumar, R.; Chauhan, J.S.; Tyagi, A.; Raghava, G.P.S. Computational approach for designing tumor homing peptides. Sci. Rep. 2013, 3, 1607. [Google Scholar] [CrossRef] [PubMed]
- Shoombuatong, W.; Schaduangrat, N.; Pratiwi, R.; Nantasenamat, C. THPep: A machine learning-based approach for predicting tumor homing peptides. Comput. Biol. Chem. 2019, 80, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Charoenkwan, P.; Chiangjong, W.; Nantasenamat, C.; Moni, M.A.; Lio’, P.; Manavalan, B.; Shoombuatong, W. SCMTHP: A New Approach for Identifying and Characterizing of Tumor-Homing Peptides Using Estimated Propensity Scores of Amino Acids. Pharmaceutics 2022, 14, 122. [Google Scholar] [CrossRef]
- Guan, J.; Yao, L.; Chung, C.-R.; Chiang, Y.-C.; Lee, T.-Y. StackTHPred: Identifying Tumor-Homing Peptides through GBDT-Based Feature Selection with Stacking Ensemble Architecture. Int. J. Mol. Sci. 2023, 24, 10348. [Google Scholar] [CrossRef]
- Zou, H.; Yang, F.; Yin, Z. Identification of tumor homing peptides by utilizing hybrid feature representation. J. Biomol. Struct. Dyn. 2023, 41, 3405–3412. [Google Scholar] [CrossRef]
- Charoenkwan, P.; Schaduangrat, N.; Lio, P.; Moni, M.A.; Manavalan, B.; Shoombuatong, W. NEPTUNE: A novel computational approach for accurate and large-scale identification of tumor homing peptides. Comput. Biol. Med. 2022, 148, 105700. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.; Marrero-Ponce, Y.; Rodríguez, H.; Agüero-Chapin, G.; Antunes, A.; Aguilera-Mendoza, L.; Martinez-Rios, F. A Novel Network Science and Similarity-Searching-Based Approach for Discovering Potential Tumor-Homing Peptides from Antimicrobials. Antibiotics 2022, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Fang, X.; Yang, Y.; Wang, C. Peptide-Enabled Targeted Delivery Systems for Therapeutic Applications. Front. Bioeng. Biotechnol. 2021, 9, 701504. [Google Scholar] [CrossRef] [PubMed]
- Svensen, N.; Walton, J.G.; Bradley, M. Peptides for cell-selective drug delivery. Trends Pharmacol. Sci. 2012, 33, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Maeda, M.; Kida, S.; Hojo, K.; Eto, Y.; Gaob, J.Q.; Kurachi, S.; Sekiguchi, F.; Mizuguchi, H.; Hayakawa, T.; Mayumi, T.; et al. Design and synthesis of a peptide-PEG transporter tool for carrying adenovirus vector into cells. Bioorg. Med. Chem. Lett. 2005, 15, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Ogawara, K.; Kułdo, J.M.; Oosterhuis, K.; Kroesen, B.J.; Rots, M.G.; Trautwein, C.; Kimura, T.; Haisma, H.J.; Molema, G. Functional inhibition of NF-kappaB signal transduction in alphavbeta3 integrin expressing endothelial cells by using RGD-PEG-modified adenovirus with a mutant IkappaB gene. Arthritis Res. Ther. 2006, 8, R32. [Google Scholar] [CrossRef] [PubMed]
- Ogawara, K.; Rots, M.G.; Kok, R.J.; Moorlag, H.E.; Van Loenen, A.M.; Meijer, D.K.; Haisma, H.J.; Molema, G. A novel strategy to modify adenovirus tropism and enhance transgene delivery to activated vascular endothelial cells in vitro and in vivo. Hum. Gene Ther. 2004, 15, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Xiong, Z.; Cheng, Z.; Cai, W.; Gambhir, S.S.; Xing, L.; Chen, X. In vivo bioluminescence tumor imaging of RGD peptide-modified adenoviral vector encoding firefly luciferase reporter gene. Mol. Imaging Biol. 2007, 9, 126–134. [Google Scholar] [CrossRef]
- Kim, P.H.; Kim, T.I.; Yockman, J.W.; Kim, S.W.; Yun, C.O. The effect of surface modification of adenovirus with an arginine-grafted bioreducible polymer on transduction efficiency and immunogenicity in cancer gene therapy. Biomaterials 2010, 31, 1865–1874. [Google Scholar] [CrossRef]
- Eto, Y.; Gao, J.Q.; Sekiguchi, F.; Kurachi, S.; Katayama, K.; Maeda, M.; Kawasaki, K.; Mizuguchi, H.; Hayakawa, T.; Tsutsumi, Y.; et al. PEGylated adenovirus vectors containing RGD peptides on the tip of PEG show high transduction efficiency and antibody evasion ability. J. Gene Med. 2005, 7, 604–612. [Google Scholar] [CrossRef]
- Shen, J.; Meng, Q.; Sui, H.; Yin, Q.; Zhang, Z.; Yu, H.; Li, Y. iRGD conjugated TPGS mediates codelivery of paclitaxel and survivin shRNA for the reversal of lung cancer resistance. Mol. Pharm. 2014, 11, 2579–2591. [Google Scholar] [CrossRef]
- Schiffelers, R.M.; Ansari, A.; Xu, J.; Zhou, Q.; Tang, Q.; Storm, G.; Molema, G.; Lu, P.Y.; Scaria, P.V.; Woodle, M.C. Cancer siRNA therapy by tumor selective delivery with ligand-targeted sterically stabilized nanoparticle. Nucleic Acids Res. 2004, 32, e149. [Google Scholar] [CrossRef] [PubMed]
- Christie, R.J.; Matsumoto, Y.; Miyata, K.; Nomoto, T.; Fukushima, S.; Osada, K.; Halnaut, J.; Pittella, F.; Kim, H.J.; Nishiyama, N.; et al. Targeted polymeric micelles for siRNA treatment of experimental cancer by intravenous injection. ACS Nano 2012, 6, 5174–5189. [Google Scholar] [CrossRef]
- Malhotra, M.; Tomaro-Duchesneau, C.; Saha, S.; Prakash, S. Systemic siRNA Delivery via Peptide-Tagged Polymeric Nanoparticles, Targeting PLK1 Gene in a Mouse Xenograft Model of Colorectal Cancer. Int. J. Biomater. 2013, 2013, 252531. [Google Scholar] [CrossRef]
- Dou, S.; Yang, X.Z.; Xiong, M.H.; Sun, C.Y.; Yao, Y.D.; Zhu, Y.H.; Wang, J. ScFv-decorated PEG-PLA-based nanoparticles for enhanced siRNA delivery to Her2⁺ breast cancer. Adv. Healthc. Mater. 2014, 3, 1792–1803. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Tang, C.; Yin, C. Dual-targeting and pH/redox-responsive multi-layered nanocomplexes for smart co-delivery of doxorubicin and siRNA. Biomaterials 2015, 60, 42–52. [Google Scholar] [CrossRef]
- Gao, L.Y.; Liu, X.Y.; Chen, C.J.; Wang, J.C.; Feng, Q.; Yu, M.Z.; Ma, X.F.; Pei, X.W.; Niu, Y.J.; Qiu, C.; et al. Core-shell type lipid/rPAA-Chol polymer hybrid nanoparticles for in vivo siRNA delivery. Biomaterials 2014, 35, 2066–2078. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, D.W.; Davis, M.E. Impact of tumor-specific targeting and dosing schedule on tumor growth inhibition after intravenous administration of siRNA-containing nanoparticles. Biotechnol. Bioeng. 2008, 99, 975–985. [Google Scholar] [CrossRef]
- Parker, A.L.; Fisher, K.D.; Oupicky, D.; Read, M.L.; Nicklin, S.A.; Baker, A.H.; Seymour, L.W. Enhanced gene transfer activity of peptide-targeted gene-delivery vectors. J. Drug Target. 2005, 13, 39–51. [Google Scholar] [CrossRef]
- Vurro, F.; Jabalera, Y.; Mannucci, S.; Glorani, G.; Sola-Leyva, A.; Gerosa, M.; Romeo, A.; Romanelli, M.G.; Malatesta, M.; Calderan, L.; et al. Improving the Cellular Uptake of Biomimetic Magnetic Nanoparticles. Nanomaterials 2021, 11, 766. [Google Scholar] [CrossRef]
- Stevenson, M.; Hale AB, H.; Hale, S.J.; Green, N.K.; Black, G.; Fisher, K.; Ulbrich, K.; Fabra, A.; Seymour, L.W. Incorporation of a laminin-derived peptide (SIKVAV) on polymer-modified adenovirus permits tumor-specific targeting via alpha6-integrins. Cancer Gene Ther. 2007, 14, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Soudy, R.; Byeon, N.; Raghuwanshi, Y.; Ahmed, S.; Lavasanifar, A.; Kaur, K. Engineered Peptides for Applications in Cancer-Targeted Drug Delivery and Tumor Detection. Mini Rev. Med. Chem. 2017, 17, 1696–1712. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Xie, J.; Chen, X. Peptide-based probes for targeted molecular imaging. Biochemistry 2010, 49, 1364–1376. [Google Scholar] [CrossRef] [PubMed]
- Colone, M.; Calcabrini, A.; Stringaro, A. Drug Delivery Systems of Natural Products in Oncology. Molecules 2020, 25, 4560. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.Y.; Xu, Y.M.; Lau, A.T.Y. Recent Progress of Nanocarrier-Based Therapy for Solid Malignancies. Cancers 2020, 12, 2783. [Google Scholar] [CrossRef] [PubMed]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef] [PubMed]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Navya, P.N.; Kaphle, A.; Srinivas, S.P.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019, 6, 23. [Google Scholar] [CrossRef]
- Morales-Cruz, M.; Delgado, Y.; Castillo, B.; Figueroa, C.M.; Molina, A.M.; Torres, A.; Milián, M.; Griebenow, K. Smart Targeting To Improve Cancer Therapeutics. Drug Des. Devel Ther. 2019, 13, 3753–3772. [Google Scholar] [CrossRef]
- Dissanayake, S.; Denny, W.A.; Gamage, S.; Sarojini, V. Recent developments in anticancer drug delivery using cell penetrating and tumor targeting peptides. J. Control. Release 2017, 250, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Gessner, I.; Neundorf, I. Nanoparticles Modified with Cell-Penetrating Peptides: Conjugation Mechanisms, Physicochemical Properties, and Application in Cancer Diagnosis and Therapy. Int. J. Mol. Sci. 2020, 21, 2536. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.; Kapoor, P.; Chaudhary, K.; Kumar, R.; Raghava, G.P.; Consortium, O.S.D.D. Tumor homing peptides as molecular probes for cancer therapeutics, diagnostics and theranostics. Curr. Med. Chem. 2014, 21, 2367–2391. [Google Scholar] [CrossRef] [PubMed]
- Arcangeli, C.; Lico, C.; Baschieri, S.; Mancuso, M. Characterization Of Blood-Brain Barrier Crossing And Tumor Homing Peptides By Molecular Dynamics Simulations. Int. J. Nanomed. 2019, 14, 10123–10136. [Google Scholar] [CrossRef] [PubMed]
- Bor, G.; Hosta-Rigau, L. Next Generation of Brain Cancer Nanomedicines to Overcome the Blood–Brain Barrier (BBB): Insights on Transcytosis, Perivascular Tumor Growth, and BBB Models. Adv. Ther. 2023, 6, 2300161. [Google Scholar] [CrossRef]
- Wanjale, M.V.; Kumar, G.S.V. Peptides as a therapeutic avenue for nanocarrier-aided targeting of glioma. Expert Opin. Drug Deliv. 2017, 14, 811–824. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, W. Recent advances in brain tumor-targeted nano-drug delivery systems. Expert Opin. Drug Deliv. 2012, 9, 671–686. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Yang, Z.; Zhang, S.; Cao, S.; Pang, Z.; Yang, X.; Jiang, X. Glioma-homing peptide with a cell-penetrating effect for targeting delivery with enhanced glioma localization, penetration and suppression of glioma growth. J. Control. Release 2013, 172, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Pethő, L.; Mező, G.; Schlosser, G. Overcharging Effect in Electrospray Ionization Mass Spectra of Daunomycin-Tuftsin Bioconjugates. Molecules 2019, 24, 2981. [Google Scholar] [CrossRef]
- Baranyai, Z.; Biri-Kovács, B.; Krátký, M.; Szeder, B.; Debreczeni, M.L.; Budai, J.; Kovács, B.; Horváth, L.; Pári, E.; Németh, Z.; et al. Cellular Internalization and Inhibition Capacity of New Anti-Glioma Peptide Conjugates: Physicochemical Characterization and Evaluation on Various Monolayer- and 3D-Spheroid-Based in Vitro Platforms. J. Med. Chem. 2021, 64, 2982–3005. [Google Scholar] [CrossRef]
- von Wronski, M.A.; Raju, N.; Pillai, R.; Bogdan, N.J.; Marinelli, E.R.; Nanjappan, P.; Ramalingam, K.; Arunachalam, T.; Eaton, S.; Linder, K.E.; et al. Tuftsin Binds Neuropilin-1 through a Sequence Similar to That Encoded by Exon 8 of Vascular Endothelial Growth Factor. J. Biol. Chem. 2006, 281, 5702–5710. [Google Scholar] [CrossRef] [PubMed]
- Roth, L.; Agemy, L.; Kotamraju, V.R.; Braun, G.; Teesalu, T.; Sugahara, K.N.; Hamzah, J.; Ruoslahti, E. Transtumoral targeting enabled by a novel neuropilin-binding peptide. Oncogene 2012, 31, 3754–3763. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.B.; Wang, Z.; Wang, Q.S.; Han, Y.J.; Wang, M.; Zhou, W.L.; Li, H.S. Use of Labelled tLyP-1 as a Novel Ligand Targeting the NRP Receptor to Image Glioma. PLoS ONE 2015, 10, e0137676. [Google Scholar] [CrossRef] [PubMed]
- Larue, L.; Kenzhebayeva, B.; Al-Thiabat, M.G.; Jouan–Hureaux, V.; Mohd–Gazzali, A.; Wahab, H.A.; Boura, C.; Yeligbayeva, G.; Nakan, U.; Frochot, C.; et al. tLyp–1: A peptide suitable to target NRP–1 receptor. Bioorg. Chem. 2023, 130, 106200. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Lyon, J.G.; Patil, K.; Mokarram, N.; Kim, C.; Bellamkonda, R.V. Bacterial Carriers for Glioblastoma Therapy. Mol. Ther.-Oncolytics 2017, 4, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Feng, L.; Fan, L.; Zha, Y.; Guo, L.; Zhang, Q.; Chen, J.; Pang, Z.; Wang, Y.; Jiang, X.; et al. Targeting the brain with PEG-PLGA nanoparticles modified with phage-displayed peptides. Biomaterials 2011, 32, 4943–4950. [Google Scholar] [CrossRef] [PubMed]
- Ruan, S.; Yuan, M.; Zhang, L.; Hu, G.; Chen, J.; Cun, X.; Zhang, Q.; Yang, Y.; He, Q.; Gao, H. Tumor microenvironment sensitive doxorubicin delivery and release to glioma using angiopep-2 decorated gold nanoparticles. Biomaterials 2015, 37, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; He, Q.; Liu, J.; Chen, Y.; Ma, M.; Zhang, L.; Shi, J. Nuclear-Targeted Drug Delivery of TAT Peptide-Conjugated Monodisperse Mesoporous Silica Nanoparticles. J. Am. Chem. Soc. 2012, 134, 5722–5725. [Google Scholar] [CrossRef] [PubMed]
- Jafari, B.; Pourseif, M.M.; Barar, J.; Rafi, M.A.; Omidi, Y. Peptide-mediated drug delivery across the blood-brain barrier for targeting brain tumors. Expert Opin. Drug Deliv. 2019, 16, 583–605. [Google Scholar] [CrossRef]
- Mamelak, A.N.; Jacoby, D.B. Targeted delivery of antitumoral therapy to glioma and other malignancies with synthetic chlorotoxin (TM-601). Expert Opin. Drug Deliv. 2007, 4, 175–186. [Google Scholar] [CrossRef]
- Dardevet, L.; Rani, D.; Aziz, T.A.; Bazin, I.; Sabatier, J.-M.; Fadl, M.; Brambilla, E.; De Waard, M. Chlorotoxin: A Helpful Natural Scorpion Peptide to Diagnose Glioma and Fight Tumor Invasion. Toxins 2015, 7, 1079–1101. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.-F.; Farquhar, C.E.; Fadzen, C.M.; Scott, B.; Zhuang, P.; von Spreckelsen, N.; Loas, A.; Hartrampf, N.; Pentelute, B.L.; Lawler, S.E. A Tumor-Homing Peptide Platform Enhances Drug Solubility, Improves Blood–Brain Barrier Permeability and Targets Glioblastoma. Cancers 2022, 14, 2207. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Cho, C.H. Peptides as targeting probes against tumor vasculature for diagnosis and drug delivery. J. Transl. Med. 2012, 10 (Suppl. 1), S1. [Google Scholar] [CrossRef] [PubMed]
- Anani, T.; Rahmati, S.; Sultana, N.; David, A.E. MRI-traceable theranostic nanoparticles for targeted cancer treatment. Theranostics 2021, 11, 579–601. [Google Scholar] [CrossRef] [PubMed]
- Koudrina, A.; DeRosa, M.C. Advances in Medical Imaging: Aptamer- and Peptide-Targeted MRI and CT Contrast Agents. ACS Omega 2020, 5, 22691–22701. [Google Scholar] [CrossRef] [PubMed]
- Kondo, E.; Iioka, H.; Saito, K. Tumor-homing peptide and its utility for advanced cancer medicine. Cancer Sci. 2021, 112, 2118–2125. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, J.; Xu, Y.; Zhan, Y.; Li, Y.; Song, T.; Zheng, J.; Yang, H. Application of Phage-Displayed Peptides in Tumor Imaging Diagnosis and Targeting Therapy. Int. J. Pept. Res. Ther. 2020, 27, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Shubayev, V.I.; Pisanic, T.R.; Jin, S. Magnetic nanoparticles for theragnostics. Adv. Drug Deliv. Rev. 2009, 61, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Jain, T.K.; Richey, J.; Strand, M.; Leslie-Pelecky, D.L.; Flask, C.A.; Labhasetwar, V. Magnetic nanoparticles with dual functional properties: Drug delivery and magnetic resonance imaging. Biomaterials 2008, 29, 4012–4021. [Google Scholar] [CrossRef]
- Larsen, E.K.; Nielsen, T.; Wittenborn, T.; Rydtoft, L.M.; Lokanathan, A.R.; Hansen, L.; Østergaard, L.; Kingshott, P.; Howard, K.A.; Besenbacher, F.; et al. Accumulation of magnetic iron oxide nanoparticles coated with variably sized polyethylene glycol in murine tumors. Nanoscale 2012, 4, 2352–2361. [Google Scholar] [CrossRef]
- Yu, M.; Zheng, J. Clearance Pathways and Tumor Targeting of Imaging Nanoparticles. ACS Nano 2015, 9, 6655–6674. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Lee, J.S.H.; Zhang, M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef] [PubMed]
- Turrina, C.; Schoenen, M.; Milani, D.; Klassen, A.; Rojas Gonzaléz, D.M.; Cvirn, G.; Mela, P.; Berensmeier, S.; Slabu, I.; Schwaminger, S.P. Application of magnetic iron oxide nanoparticles: Thrombotic activity, imaging and cytocompatibility of silica-coated and carboxymethyl dextrane-coated particles. Colloids Surf. B Biointerfaces 2023, 228, 113428. [Google Scholar] [CrossRef] [PubMed]
- Rarokar, N.; Yadav, S.; Saoji, S.; Bramhe, P.; Agade, R.; Gurav, S.; Khedekar, P.; Subramaniyan, V.; Wong, L.S.; Kumarasamy, V. Magnetic nanosystem a tool for targeted delivery and diagnostic application: Current challenges and recent advancement. Int. J. Pharm. X 2024, 7, 100231. [Google Scholar] [CrossRef] [PubMed]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef]
- Chen, Y.; Hou, S. Recent progress in the effect of magnetic iron oxide nanoparticles on cells and extracellular vesicles. Cell Death Discov. 2023, 9, 195. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Nandi, A.; Jha, E.; Sinha, A.; Mohanty, S.; Panda, P.K.; Mishra, S.; Verma, S.K.; Suar, M. 2—Magnetic nanoparticles: Fabrication, characterization, properties, and application for environment sustainability. In Magnetic Nanoparticle-Based Hybrid Materials; Ehrmann, A., Nguyen, T.A., Ahmadi, M., Farmani, A., Nguyen-Tri, P., Eds.; Woodhead Publishing: Sawston, UK, 2021; pp. 33–64. [Google Scholar]
- Ling, D.; Lee, N.; Hyeon, T. Chemical synthesis and assembly of uniformly sized iron oxide nanoparticles for medical applications. Acc. Chem. Res. 2015, 48, 1276–1285. [Google Scholar] [CrossRef]
- Próspero, A.G.; Quini, C.C.; Bakuzis, A.F.; Fidelis-de-Oliveira, P.; Moretto, G.M.; Mello, F.P.; Calabresi, M.F.; Matos, R.V.; Zandoná, E.A.; Zufelato, N.; et al. Real-time in vivo monitoring of magnetic nanoparticles in the bloodstream by AC biosusceptometry. J. Nanobiotechnology 2017, 15, 22. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.F.; Jiang, J.S.; Yang, Y.; Du, B.; Qian, M.; Zhang, P. Immobilization of homing peptide on magnetite nanoparticles and its specificity in vitro. J. Biomed. Mater. Res. A 2008, 84, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Sakurai, R.; Wakimoto, T.; Kobayashi, K.; Xu, B.; Toku, Y.; Song, G.; Luo, Q.; Ju, Y. TLyP-1-Conjugated Core-Shell Nanoparticles, Fe3O4NPs@mSiO2, for Tumor-Targeted Drug Delivery. Appl. Surf. Sci. 2019, 474, 17–24. [Google Scholar] [CrossRef]
- Niemirowicz, K.; Prokop, I.; Wilczewska, A.Z.; Wnorowska, U.; Piktel, E.; Wątek, M.; Savage, P.B.; Bucki, R. Magnetic nanoparticles enhance the anticancer activity of cathelicidin LL-37 peptide against colon cancer cells. Int. J. Nanomed. 2015, 10, 3843–3853. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Bai, H.; Ma, M.; Wang, C.; Xu, H.; Gu, N.; Zhang, Y. Fe3O4@Pt Nanozymes Combining with CXCR4 Antagonists to Synergistically Treat Acute Myeloid Leukemia. Nano Today 2021, 37, 101106. [Google Scholar] [CrossRef]
- Hamilton, A.M.; Aidoudi-Ahmed, S.; Sharma, S.; Kotamraju, V.R.; Foster, P.J.; Sugahara, K.N.; Ruoslahti, E.; Rutt, B.K. Nanoparticles coated with the tumor-penetrating peptide iRGD reduce experimental breast cancer metastasis in the brain. J. Mol. Med. 2015, 93, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Xie, J.; Xu, H.; Behera, D.; Michalski, M.H.; Biswal, S.; Wang, A.; Chen, X. Triblock copolymer coated iron oxide nanoparticle conjugate for tumor integrin targeting. Biomaterials 2009, 30, 6912–6919. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kotamraju, V.R.; Mölder, T.; Tobi, A.; Teesalu, T.; Ruoslahti, E. Tumor-Penetrating Nanosystem Strongly Suppresses Breast Tumor Growth. Nano Lett. 2017, 17, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Mann, A.P.; Mölder, T.; Kotamraju, V.R.; Mattrey, R.; Teesalu, T.; Ruoslahti, E. Vascular changes in tumors resistant to a vascular disrupting nanoparticle treatment. J. Control. Release 2017, 268, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Q.; Liu, L.; Sun, T.; Zhou, W.; Chen, Q.; Lu, Y.; He, X.; Zhang, Y.; Ruan, C.; et al. Double-sided effect of tumor microenvironment on platelets targeting nanoparticles. Biomaterials 2018, 183, 258–267. [Google Scholar] [CrossRef]
- Jiang, J.; Gan, Z.; Yang, Y.; Bing, D.; Min, Q.; Ping, Z. A Novel Magnetic Fluid Based on Starch-Coated Mgnetite Nanoparticles Functionalized with Homing Peptide. J. Nanoparticle Res. 2009, 11, 1321–1330. [Google Scholar] [CrossRef]
- Jie, L.Y.; Cai, L.L.; Wang, L.J.; Ying, X.Y.; Yu, R.S.; Zhang, M.M.; Du, Y.Z. Actively-targeted LTVSPWY peptide-modified magnetic nanoparticles for tumor imaging. Int. J. Nanomed. 2012, 7, 3981–3989. [Google Scholar] [CrossRef]
- Situ, J.Q.; Wang, X.J.; Zhu, X.L.; Xu, X.L.; Kang, X.Q.; Hu, J.B.; Lu, C.Y.; Ying, X.Y.; Yu, R.S.; You, J.; et al. Multifunctional SPIO/DOX-loaded A54 Homing Peptide Functionalized Dextran-g-PLGA Micelles for Tumor Therapy and MR Imaging. Sci. Rep. 2016, 6, 35910. [Google Scholar] [CrossRef]
- Huang, Y.W.; Lee, H.J.; Tolliver, L.M.; Aronstam, R.S. Delivery of nucleic acids and nanomaterials by cell-penetrating peptides: Opportunities and challenges. BioMed. Res. Int. 2015, 2015, 834079. [Google Scholar] [CrossRef]
- Jones, S.; Uusna, J.; Langel, Ü.; Howl, J. Intracellular Target-Specific Accretion of Cell Penetrating Peptides and Bioportides: Ultrastructural and Biological Correlates. Bioconjugate Chem. 2016, 27, 121–129. [Google Scholar] [CrossRef]
- Raucher, D.; Ryu, J.S. Cell-penetrating peptides: Strategies for anticancer treatment. Trends Mol. Med. 2015, 21, 560–570. [Google Scholar] [CrossRef]
- Bruno, B.J.; Miller, G.D.; Lim, C.S. Basics and recent advances in peptide and protein drug delivery. Ther. Deliv. 2013, 4, 1443–1467. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Lu, N.; Zhou, Y.; Xuan, S.; Zhang, J.; Giampieri, F.; Zhang, Y.; Yang, F.; Yu, R.; Battino, M.; et al. Targeting Pancreatic Cancer Cells with Peptide-Functionalized Polymeric Magnetic Nanoparticles. Int. J. Mol. Sci. 2019, 20, 2988. [Google Scholar] [CrossRef]
- Luo, Z.; Du, H. Prospect of Different Types of Magnetic Nanoparticles in Stem Cell Therapy. Stem Cell Rev. Rep. 2020, 16, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Scarberry, K.E.; Dickerson, E.B.; McDonald, J.F.; Zhang, Z.J. Magnetic nanoparticle-peptide conjugates for in vitro and in vivo targeting and extraction of cancer cells. J. Am. Chem. Soc. 2008, 130, 10258–10262. [Google Scholar] [CrossRef]
- Simberg, D.; Duza, T.; Park, J.H.; Essler, M.; Pilch, J.; Zhang, L.; Derfus, A.M.; Yang, M.; Hoffman, R.M.; Bhatia, S.; et al. Biomimetic amplification of nanoparticle homing to tumors. Proc. Natl. Acad. Sci. USA 2007, 104, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Arami, H.; Krishnan, K.M. Detection of Cancer-Specific Proteases Using Magnetic Relaxation of Peptide-Conjugated Nanoparticles in Biological Environment. Nano Lett. 2016, 16, 3668–3674. [Google Scholar] [CrossRef]
- Rastogi, A.; Yadav, K.; Mishra, A.; Singh, M.S.; Chaudhary, S.; Manohar, R.; Parmar, A.S. Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems. Nanotechnol. Rev. 2022, 11, 544–574. [Google Scholar] [CrossRef]
- Crezee, J.; Franken, N.A.P.; Oei, A.L. Hyperthermia-Based Anti-Cancer Treatments. Cancers 2021, 13, 1240. [Google Scholar] [CrossRef] [PubMed]
- Datta, N.R.; Ordóñez, S.G.; Gaipl, U.S.; Paulides, M.M.; Crezee, H.; Gellermann, J.; Marder, D.; Puric, E.; Bodis, S. Local hyperthermia combined with radiotherapy and-/or chemotherapy: Recent advances and promises for the future. Cancer Treat. Rev. 2015, 41, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Behrouzkia, Z.; Joveini, Z.; Keshavarzi, B.; Eyvazzadeh, N.; Aghdam, R.Z. Hyperthermia: How Can It Be Used? Oman Med. J. 2016, 31, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Zhang, Y.; Liu, C.; Zhang, M.; Han, S. Application of Radiosensitizers in Cancer Radiotherapy. Int. J. Nanomed. 2021, 16, 1083–1102. [Google Scholar] [CrossRef] [PubMed]
- Roizin-Towle, L.; Pirro, J.P. The response of human and rodent cells to hyperthermia. Int. J. Radiat. Oncol. Biol. Phys. 1991, 20, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Gavilán, H.; Avugadda, S.K.; Fernández-Cabada, T.; Soni, N.; Cassani, M.; Mai, B.T.; Chantrell, R.; Pellegrino, T. Magnetic nanoparticles and clusters for magnetic hyperthermia: Optimizing their heat performance and developing combinatorial therapies to tackle cancer. Chem. Soc. Rev. 2021, 50, 11614–11667. [Google Scholar] [CrossRef] [PubMed]
- Shetake, N.G.; Ali, M.; Kumar, A.; Bellare, J.; Pandey, B.N. Theranostic magnetic nanoparticles enhance DNA damage and mitigate doxorubicin-induced cardio-toxicity for effective multi-modal tumor therapy. Biomater. Adv. 2022, 142, 213147. [Google Scholar] [CrossRef] [PubMed]
- Arriortua, O.K.; Insausti, M.; Lezama, L.; Gil de Muro, I.; Garaio, E.; de la Fuente, J.M.; Fratila, R.M.; Morales, M.P.; Costa, R.; Eceiza, M.; et al. RGD-Functionalized Fe(3)O(4) nanoparticles for magnetic hyperthermia. Colloids Surf. B Biointerfaces 2018, 165, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Arriortua, O.K.; Garaio, E.; Herrero de la Parte, B.; Insausti, M.; Lezama, L.; Plazaola, F.; García, J.A.; Aizpurua, J.M.; Sagartzazu, M.; Irazola, M.; et al. Antitumor magnetic hyperthermia induced by RGD-functionalized Fe(3)O(4) nanoparticles, in an experimental model of colorectal liver metastases. Beilstein J. Nanotechnol. 2016, 7, 1532–1542. [Google Scholar] [CrossRef]
- Sadhukha, T.; Wiedmann, T.S.; Panyam, J. Inhalable magnetic nanoparticles for targeted hyperthermia in lung cancer therapy. Biomaterials 2013, 34, 5163–5171. [Google Scholar] [CrossRef]
- Nica, V.; Marino, A.; Pucci, C.; Şen, Ö.; Emanet, M.; De Pasquale, D.; Carmignani, A.; Petretto, A.; Bartolucci, M.; Lauciello, S.; et al. Cell-Membrane-Coated and Cell-Penetrating Peptide-Conjugated Trimagnetic Nanoparticles for Targeted Magnetic Hyperthermia of Prostate Cancer Cells. ACS Appl. Mater. Interfaces 2023, 15, 30008–30028. [Google Scholar] [CrossRef]
- Perillo, E.; Hervé-Aubert, K.; Allard-Vannier, E.; Falanga, A.; Galdiero, S.; Chourpa, I. Synthesis and in vitro evaluation of fluorescent and magnetic nanoparticles functionalized with a cell penetrating peptide for cancer theranosis. J. Colloid. Interface Sci. 2017, 499, 209–217. [Google Scholar] [CrossRef]
- Zhang, W.; Taheri-Ledari, R.; Hajizadeh, Z.; Zolfaghari, E.; Ahghari, M.R.; Maleki, A.; Hamblin, M.R.; Tian, Y. Enhanced activity of vancomycin by encapsulation in hybrid magnetic nanoparticles conjugated to a cell-penetrating peptide. Nanoscale 2020, 12, 3855–3870. [Google Scholar] [CrossRef]
- Chaix, A.; Griveau, A.; Defforge, T.; Grimal, V.; Le Borgne, B.; Gautier, G.; Eyer, J. Cell penetrating peptide decorated magnetic porous silicon nanorods for glioblastoma therapy and imaging. RSC Adv. 2022, 12, 11708–11714. [Google Scholar] [CrossRef]
- Shrestha, R.; Shen, Y.; Pollack, K.A.; Taylor, J.S.; Wooley, K.L. Dual peptide nucleic acid- and peptide-functionalized shell cross-linked nanoparticles designed to target mRNA toward the diagnosis and treatment of acute lung injury. Bioconjugate Chem. 2012, 23, 574–585. [Google Scholar] [CrossRef]
- Hauser, A.K.; Anderson, K.W.; Hilt, J.Z. Peptide conjugated magnetic nanoparticles for magnetically mediated energy delivery to lung cancer cells. Nanomedicine 2016, 11, 1769–1785. [Google Scholar] [CrossRef]
- Halouane, F.; Jijie, R.; Meziane, D.; Li, C.; Singh, S.K.; Bouckaert, J.; Jurazek, J.; Kurungot, S.; Barras, A.; Li, M.; et al. Selective isolation and eradication of E. coli associated with urinary tract infections using anti-fimbrial modified magnetic reduced graphene oxide nanoheaters. J. Mater. Chem. B 2017, 5, 8133–8142. [Google Scholar] [CrossRef]
- Attari, E.; Nosrati, H.; Danafar, H.; Kheiri Manjili, H. Methotrexate anticancer drug delivery to breast cancer cell lines by iron oxide magnetic based nanocarrier. J. Biomed. Mater. Res. A 2019, 107, 2492–2500. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ (accessed on 24 March 2024).
- International Clinical Trials; ICTRP Search Portal. 2024. Available online: https://www.who.int/clinical-trials-registry-platform/the-ictrp-search-portal (accessed on 24 March 2024).
- 68Ga- THP- PSMA PET/CT Imaging in High Risk Primary Prostate Cancer or Biochemical Recurrence of Prostate Cancer (PRONOUNCED). 12 June 2019. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03617588 (accessed on 24 March 2024).
- Pre-Operative Nodal Staging of Thyroid Cancer Using USPIO MRI: Preliminary Study. April 2016. Available online: https://classic.clinicaltrials.gov/ProvidedDocs/33/NCT01815333/Prot_SAP_000.pdf (accessed on 24 March 2024).
- Clinical and Technical Feasibility of a Ultrasuperparamagnetic Nanoparticle Iron Oxide (USPIO)-Enhanced Magnetic Resonance Lymph Node Imaging. June 2019. Available online: https://www.clinicaltrials.gov/study/NCT01815333 (accessed on 24 March 2024).
- Pre-Operative Staging of Pancreatic Cancer Using Superparamagnetic Iron Oxide Magnetic Resonance Imaging (SPIO MRI). February 2013. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT00920023 (accessed on 24 March 2024).
- Kim, J.; Kim, P.H.; Kim, S.W.; Yun, C.O. Enhancing the therapeutic efficacy of adenovirus in combination with biomaterials. Biomaterials 2012, 33, 1838–1850. [Google Scholar] [CrossRef]
- Thundimadathil, J. Cancer treatment using peptides: Current therapies and future prospects. J. Amino Acids 2012, 2012, 967347. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Wang, Y.; Gao, H.; Wei, G.; Huang, Y.; Yu, H.; Gan, Y.; Mei, L.; Chen, H.; et al. Recent progress in drug delivery. Acta Pharm. Sin. B 2019, 9, 1145–1162. [Google Scholar] [CrossRef] [PubMed]
- Cutrera, J.; Dibra, D.; Xia, X.; Hasan, A.; Reed, S.; Li, S. Discovery of a linear peptide for improving tumor targeting of gene products and treatment of distal tumors by IL-12 gene therapy. Mol. Ther. 2011, 19, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Sorolla, A.; Wang, E.; Golden, E.; Duffy, C.; Henriques, S.T.; Redfern, A.D.; Blancafort, P. Precision medicine by designer interference peptides: Applications in oncology and molecular therapeutics. Oncogene 2020, 39, 1167–1184. [Google Scholar] [CrossRef] [PubMed]
- Liscano, Y.; Oñate-Garzón, J.; Delgado, J.P. Peptides with Dual Antimicrobial-Anticancer Activity: Strategies to Overcome Peptide Limitations and Rational Design of Anticancer Peptides. Molecules 2020, 25, 4245. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnology 2018, 16, 1–33. [Google Scholar] [CrossRef]


| Core Type | Av. Diameter | Interaction with THP | Application | Interaction with the Drug | Ref. |
|---|---|---|---|---|---|
| PEG polymer nanoparticles | 20–50 nm | covalent; conjugation by NHS ester | siRNA delivery | non-covalent interactions | [49] |
| chemical complex | - | covalent; conjugation by maleimide | siRNA delivery | non-covalent interactions | [50] |
| PEG polymer nanoparticles | nd | covalent; conjugation by maleimide | adenovirus vector carrier | covalent (by maleimide or NHS ester) | [86,87,88,89,90,91] |
| PEG polymer nanoparticles | 141–160 nm | covalent; Michael reaction | paclitaxel delivery | non-covalent interactions | [92] |
| PEG-PEI polymer nanoparticles | 100 nm | covalent; conjugation by NHS ester | siRNA delivery | non-covalent interactions | [93] |
| PEG micelles | 32 nm | covalent; reaction with acetal | siRNA delivery | covalent (by maleimide or NHS ester) | [94] |
| chitosan–PEG micelles | 260 nm | covalent; esterification reaction | siRNA delivery | non-covalent interactions | [95] |
| PEG-PLA nanoparticles | 120 nm | covalent; conjugation by maleimide | siRNA delivery | non-covalent interactions | [96] |
| mesoporous silica nanoparticles | 51.2 nm | non-covalent interactions | doxorubicin and siRNA | non-covalent interactions | [97] |
| cationic liposome and PAA hybrid nanoparticles | 93 nm | non-covalent interactions | siRNA delivery | non-covalent interactions | [98] |
| cyclodextrin | nd | non-covalent interactions | siRNA delivery | non-covalent interactions | [99] |
| pHPMA polyplexes | 90–100 nm | covalent; amide bond formation | DNA delivery | non-covalent interactions | [100] |
| biomimetic magnetic nanoparticles@PLGA copolymer | 220 nm | covalent; esterification reaction | drug delivery | nd | [101] |
| Core | Ligand/Vector | Agent | Effect | Ref. |
|---|---|---|---|---|
| Atelocollagen | Aptamer APT A10-3.2 | miR15a, miR-16-1 | reduction in damage to the bone tissue improvement in the therapeutic effect | [50] |
| PEG | Ac-YGGRGDTP(beta)A)(2)K-PEG-(beta)AC | Ads | modified adenovirus exhibited high gene expression even in a CAR-negative cell | [86] |
| PEG | RGD-Ads | dnIkappaB | gene therapy/tool in rheumatoid arthritis and in IBD therapy | [87] |
| Pluronic P85/PEI/TPGS | iRGD | PTX and survivin shRNA | powerful approach for the reversal and therapy of lung cancer resistance | [92] |
| PEG-b-PLL(2IT) | cRGD | SiRNAs | antiangiogenic therapy—suppression of angiogenesis and tumor growth rate | [94] |
| Chitosan–PEG | CP15 peptide | PLK1-siRNA | lack of systemic toxicity/potential approach for cancer therapy | [95] |
| Multi-layered nanocomplexes MNS/PAH-Cit/GTC | TAT peptide | DOX/VEGF-siRNA | strong anticancer effect | [97] |
| PLGA/lipid | iRGD | ICG/TPZ | delivery platform for PDT and hypoxia-activated chemotherapy | [63] |
| Peptide | Target | Agent | Ref. |
|---|---|---|---|
| Interleukin 13 peptide (IL-13p) | IL13Rα2 | docetaxel | [119] |
| Tuftsin (TKPR) | Neuropilin-1 (NRP-1) | anthracyclines salicylanilides | [120,121,122] |
| tLyP-1 | Neuropilin-1 (NRP-1) | 5–carboxyfluorescein (FAM), 18F–fluoride | [123,124,125] |
| Azurin (Paz) | TKR | - | [126] |
| TGN | BBB AS1411 aptamer | docetaxel | [127,128] |
| Angiopep-2 | LRP1 | doxorubicin | [128] |
| Trans-activating transcriptional activator (TAT peptide) | Nucleus | siRNA expression plasmid, docetaxel, paclitaxel | [129,130] |
| Chlorotoxin (CTX) | Tumor cell surface receptor; MMP-2 | platinum | [131,132] |
| BTP-7 | dg-Bcan protein | camptothecin | [133] |
| I. 68Ga-THP-PSMA PET/CT Imaging in High-Risk Primary Prostate Cancer or Biochemical Recurrence of Prostate Cancer (PRONOUNCED) [193] (49 Participants) Condition or Disease: Prostate Cancer | ||||
| Selected Study Details | Arms and Interventions | Brief Description | Clinical Analysis | Cost-Effectiveness Analysis |
| Official Study Title: A Phase II, Open-label Study to Assess Safety and Clinical Utility of 68Ga-THP-PSMA PET/CT in Patients With High Risk Primary Prostate Cancer or Biochemical Recurrence After Radical Treatment (PRONOUNCED Study) Study Phase: 2 Study Objectives: Diagnostic Study Design: Interventional (Single Group Assignment), Open Label Study Status: Completed (June, 2019) | Arm: 1. Experimental: Single i.v. administration of Gallium-68 THP-PSMA Intervention/treatment: 1. Drug: Gallium-68 THP-PSMA (other name: THG-001) | Brief Summary:
| Primary Outcome Measures: 1. Change in Patient Management—Measured as % of patients who had a change in management plan as a result of 68Ga-THP-PSMA PET/CT documented after scan, compared with their pre-scan management plan Results:
1. Safety—Treatment of Emergent AEs Safety was assessed by
| Increase in
|
| II. Pre-Operative Nodal Staging of Thyroid Cancer Using USPIO MRI: Preliminary Study (12 Participants) [194] Condition or Disease: Thyroid Cancer | ||||
| Selected Study Details | Arms and Interventions | Brief Description | Clinical Analysis | Cost-Effectiveness Analysis |
| Official Study Title: Pre-Operative Nodal Staging of Thyroid Cancer Using Ultra-Small Superparamagnetic Iron Oxide Magnetic Resonance Imaging (USPIO MRI): Preliminary Study Study Phase: N/A Study Objectives: Diagnostic Study Design: Interventional (Single Group Assignment, Open Label) Study Status: Completed (April, 2016) | Arm: 1. Experimental: Nanoparticle MRI Within 48–72 h after ferumoxytol infusion, a scan will be performed Intervention/treatment: 1. Drug: Ferumoxytol i.v. administration at dose of 6 mg/kg of body weight, up to a maximum dose of 510 mg, delivered at a rate of up to 1 mL/s (other name: iron oxide-ferumoxytol) 2. Device: Nanoparticle MRI within 48–72 h after i.v. ferumoxytol infusion, and a scan will be performed | Brief Summary:
| Primary Outcome Measures:
| Increase in
|
| III. Clinical and Technical Feasibility of an Ultrasuperparamagnetic Nanoparticle Iron Oxide (USPIO)-Enhanced Magnetic Resonance Lymph Node Imaging [195] (10 Participants) Condition or Disease: Cancer of Lymph Nodes | ||||
| Selected Study Details | Arms and Interventions | Brief Description | Clinical Analysis | Cost-Effectiveness Analysis |
| Official Study Title: Clinical and Technical Feasibility of a Ultrasuperparamagnetic Nanoparticle Iron Oxide (USPIO)-Enhanced Magnetic Resonance Lymph Node Imaging Study Phase: N/A Study Objectives: Diagnostic Study Design: Interventional (Single Group Assignment), Open Label Study Status: Completed (July 2019) | Arm: 1. Experimental: Feraheme MRI within 48–72 h after i.v. administration of Feraheme® Intervention/treatment: 1. Drug: Feraheme i.v. administration at dose of 6 mg of iron/kg (maximum: 510 mg/dose) at a rate of 1 mL/s (30 mg/s) or slower after initial MRI (other name: ferumoxytole) 2. Procedure: MRI MRI scan performed:
| Brief Summary:
| Primary Outcome Measures: 1. No. of patients with SI change in a lymph node—comparison between the pre- and post-contrast Results:
| Increase in
|
| IV. Pre-Operative Staging of Pancreatic Cancer Using Superparamagnetic Iron Oxide Magnetic Resonance Imaging (SPIO MRI) [196] (35 Participants) Condition or Disease: Pancreatic Cancer | ||||
| Selected Study Details | Arms and Interventions | Brief Description | Clinical Analysis | Cost-Effectiveness Analysis |
| Official Study Title: Improved Pre-Operative Staging of Pancreatic Cancer Using Superparamagnetic Iron Oxide Magnetic Resonance Imaging (SPIO MRI) Study Phase: 4 Study Objectives: Diagnostic Study Design: Interventional (Single Group Assignment), Open Label Study Status: Completed (February 2013) | Arm: 1. Experimental: SPIO MRI Intervention/treatment: 1. Drug: SPIO MRI Two MRIs will be performed over a 2-day period. The second scan will be performed 48 h after i.v. administration of ferumoxytol (other names: SPIO MRI, USPIO, feruoxytol) | Brief Summary:
| Primary Outcome Measures:
| Increase in
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milewska, S.; Sadowska, A.; Stefaniuk, N.; Misztalewska-Turkowicz, I.; Wilczewska, A.Z.; Car, H.; Niemirowicz-Laskowska, K. Tumor-Homing Peptides as Crucial Component of Magnetic-Based Delivery Systems: Recent Developments and Pharmacoeconomical Perspective. Int. J. Mol. Sci. 2024, 25, 6219. https://doi.org/10.3390/ijms25116219
Milewska S, Sadowska A, Stefaniuk N, Misztalewska-Turkowicz I, Wilczewska AZ, Car H, Niemirowicz-Laskowska K. Tumor-Homing Peptides as Crucial Component of Magnetic-Based Delivery Systems: Recent Developments and Pharmacoeconomical Perspective. International Journal of Molecular Sciences. 2024; 25(11):6219. https://doi.org/10.3390/ijms25116219
Chicago/Turabian StyleMilewska, Sylwia, Anna Sadowska, Natalia Stefaniuk, Iwona Misztalewska-Turkowicz, Agnieszka Z. Wilczewska, Halina Car, and Katarzyna Niemirowicz-Laskowska. 2024. "Tumor-Homing Peptides as Crucial Component of Magnetic-Based Delivery Systems: Recent Developments and Pharmacoeconomical Perspective" International Journal of Molecular Sciences 25, no. 11: 6219. https://doi.org/10.3390/ijms25116219





