Associations between Serum Kallistatin Levels and Markers of Glucose Homeostasis, Inflammation, and Lipoprotein Metabolism in Patients with Type 2 Diabetes and Nondiabetic Obesity
Abstract
1. Introduction
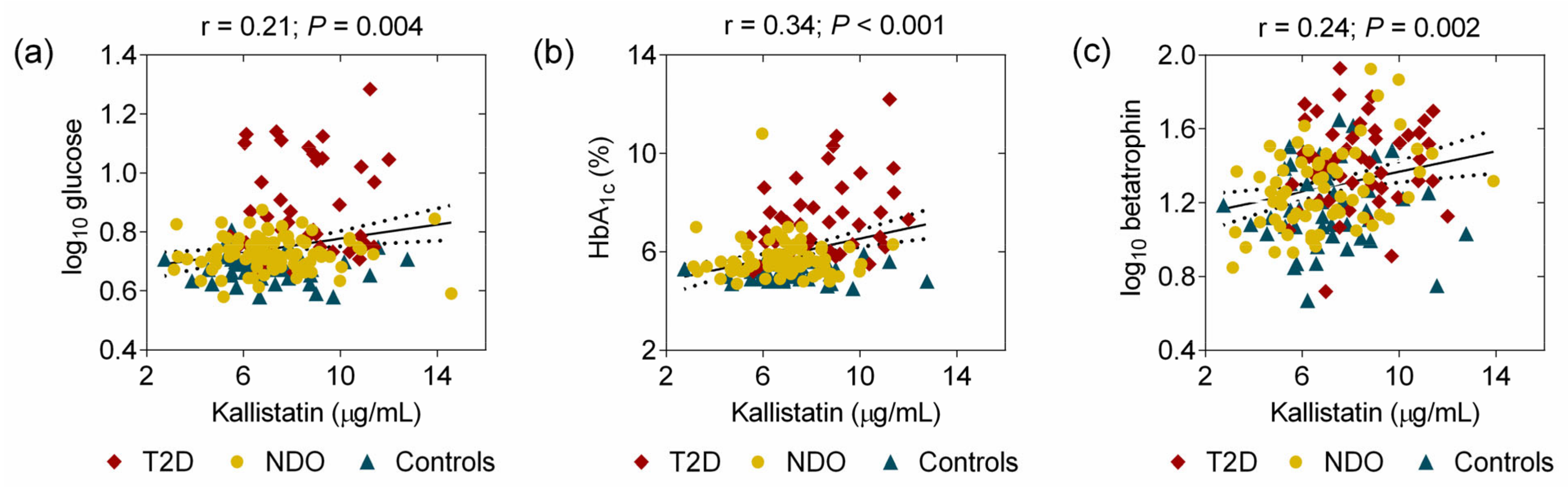
2. Results
3. Discussion
4. Materials and Methods
4.1. Enrolment of Study Subjects
4.2. Determination of Routine Laboratory Parameters
4.3. Determination of Lipoprotein Subfractions
4.4. Determination of Kallistatin
4.5. Determination of Betatrophin
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chai, K.X.; Ward, D.C.; Chao, J.; Chao, L. Molecular cloning, sequence analysis, and chromosomal localization of the human protease inhibitor 4 (kallistatin) gene (PI4). Genomics 1994, 23, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Chai, K.X.; Chen, L.M.; Chao, J.; Chao, L. Kallistatin: A novel human serine proteinase inhibitor. Molecular cloning, tissue distribution, and expression in Escherichia coli. J. Biol. Chem. 1993, 268, 24498–24505. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zou, J.; Yu, X.; Yin, S.; Tang, C. The antiatherogenic function of kallistatin and its potential mechanism. Acta Biochim. Biophys. Sin. 2020, 52, 583–589. [Google Scholar] [CrossRef] [PubMed]
- El-Asrar, M.A.; Andrawes, N.G.; Ismail, E.A.; Salem, S.M. Kallistatin as a marker of microvascular complications in children and adolescents with type 1 diabetes mellitus: Relation to carotid intima media thickness. Vasc. Med. 2015, 20, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Gateva, A.; Assyov, Y.; Velikova, T.; Kamenov, Z. Increased kallistatin levels in patients with obesity and prediabetes compared to normal glucose tolerance. Endocr. Res. 2017, 42, 163–168. [Google Scholar] [CrossRef] [PubMed]
- McBride, J.D.; Jenkins, A.J.; Liu, X.; Zhang, B.; Lee, K.; Berry, W.L.; Janknecht, R.; Griffin, C.T.; Aston, C.E.; Lyons, T.J.; et al. Elevated circulation levels of an antiangiogenic SERPIN in patients with diabetic microvascular complications impair wound healing through suppression of Wnt signaling. J. Investig. Dermatol. 2014, 134, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; He, X.; Cheng, R.; Chen, Q.; Shan, C.; Chen, L.; Ma, J.X. Diabetes-induced upregulation of kallistatin levels exacerbates diabetic nephropathy via RAS activation. FASEB J. 2020, 34, 8428–8441. [Google Scholar] [CrossRef] [PubMed]
- Vekic, J.; Zeljkovic, A.; Stefanovic, A.; Jelic-Ivanovic, Z.; Spasojevic-Kalimanovska, V. Obesity and dyslipidemia. Metabolism 2019, 92, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Thambiah, S.C.; Lai, L.C. Diabetic dyslipidaemia. Pract. Lab. Med. 2021, 26, e00248. [Google Scholar] [CrossRef]
- Zhu, H.; Chao, J.; Kotak, I.; Guo, D.; Parikh, S.J.; Bhagatwala, J.; Dong, Y.; Patel, S.Y.; Houk, C.; Chao, L. Plasma kallistatin is associated with adiposity and cardiometabolic risk in apparently healthy African American adolescents. Metabolism 2013, 62, 642–646. [Google Scholar] [CrossRef]
- Przybyłowski, P.; Wasilewski, G.; Koc-Żórawska, E.; Małyszko, J. Kallistatin Concentration and Hypertension in Heart Transplant Recipients. Transplant. Proc. 2018, 50, 2105–2109. [Google Scholar] [CrossRef] [PubMed]
- Lőrincz, H.; Ratku, B.; Csiha, S.; Seres, I.; Szabó, Z.; Paragh, G.; Harangi, M.; Somodi, S. Impaired Organokine Regulation in Non-Diabetic Obese Subjects: Halfway to the Cardiometabolic Danger Zone. Int. J. Mol. Sci. 2023, 24, 4115. [Google Scholar] [CrossRef] [PubMed]
- Lőrincz, H.; Csiha, S.; Ratku, B.; Somodi, S.; Sztanek, F.; Seres, I.; Paragh, G.; Harangi, M. Gender-Dependent Associations between Serum Betatrophin Levels and Lipoprotein Subfractions in Diabetic and Nondiabetic Obese Patients. Int. J. Mol. Sci. 2023, 24, 16504. [Google Scholar] [CrossRef] [PubMed]
- Chary, A.; Tohidi, M.; Hedayati, M. Association of LDL-cholesterol subfractions with cardiovascular disorders: A systematic review. BMC Cardiovasc. Disord. 2023, 23, 533. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, H.; Vincent, V.; Sen, A.; Singh, A.; Roy, A. Changing Perspectives on HDL: From Simple Quantity Measurements to Functional Quality Assessment. J. Lipids 2021, 2021, 5585521. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.H.; Yeh, Y.H.; Chen, W.J.; Lin, K.H. Emerging regulation and function of betatrophin. Int. J. Mol. Sci. 2014, 15, 23640–23657. [Google Scholar] [CrossRef] [PubMed]
- Abu-Farha, M.; Abubaker, J.; Al-Khairi, I.; Cherian, P.; Noronha, F.; Hu, F.B.; Behbehani, K.; Elkum, N. Higher plasma betatrophin/ANGPTL8 level in Type 2 Diabetes subjects does not correlate with blood glucose or insulin resistance. Sci. Rep. 2015, 5, 10949. [Google Scholar] [CrossRef] [PubMed]
- Espes, D.; Martinell, M.; Carlsson, P.O. Increased circulating betatrophin concentrations in patients with type 2 diabetes. Int. J. Endocrinol. 2014, 2014, 323407. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ambrosi, J.; Pascual, E.; Catalán, V.; Rodríguez, A.; Ramírez, B.; Silva, C.; Gil, M.J.; Salvador, J.; Frühbeck, G. Circulating betatrophin concentrations are decreased in human obesity and type 2 diabetes. J. Clin. Endocrinol. Metab. 2014, 99, E2004–E2009. [Google Scholar] [CrossRef]
- Mahser, A.; Serel, A.; Bozkaya, G.; Tatar, B.; Tatar, E. Relationship of Serum Kallistatin Level With Arterial Stiffness and Metabolic Parameters in Obesity-Related Chronic Kidney Disease. Exp. Clin. Transplant. 2024, 22, 243–246. [Google Scholar] [CrossRef]
- Nowicki, G.J.; Ślusarska, B.; Polak, M.; Naylor, K.; Kocki, T. Relationship between Serum Kallistatin and Afamin and Anthropometric Factors Associated with Obesity and of Being Overweight in Patients after Myocardial Infarction and without Myocardial Infarction. J. Clin. Med. 2021, 10, 5792. [Google Scholar] [CrossRef] [PubMed]
- Calan, M.; Guler, A.; Unal Kocabas, G.; Alarslan, P.; Bicer, M.; Imamoglu, C.; Yuksel, A.; Bozkaya, G.; Bilgir, O. Association of kallistatin with carotid intima-media thickness in women with polycystic ovary syndrome. Minerva Endocrinol. 2018, 43, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.; Yin, H.; Yao, Y.Y.; Shen, B.; Smith, R.S.; Chao, L. Novel role of kallistatin in protection against myocardial ischemia-reperfusion injury by preventing apoptosis and inflammation. Hum. Gene Ther. 2006, 17, 1201–1213. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Li, P.; Zhang, J.; Hagiwara, M.; Shen, B.; Bledsoe, G.; Chang, E.; Chao, L.; Chao, J. Novel role of kallistatin in vascular repair by promoting mobility, viability, and function of endothelial progenitor cells. J. Am. Heart Assoc. 2014, 3, e001194. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Bledsoe, G.; Yang, Z.R.; Fan, H.; Chao, L.; Chao, J. Human kallistatin administration reduces organ injury and improves survival in a mouse model of polymicrobial sepsis. Immunology 2014, 142, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Gao, L.; Hsu, Y.T.; Bledsoe, G.; Hagiwara, M.; Chao, L.; Chao, J. Kallistatin attenuates endothelial apoptosis through inhibition of oxidative stress and activation of Akt-eNOS signaling. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1419–H1427. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Gao, L.; Shen, B.; Chao, L.; Chao, J. Kallistatin inhibits vascular inflammation by antagonizing tumor necrosis factor-alpha-induced nuclear factor kappaB activation. Hypertension 2010, 56, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.F.; Yang, H.Y.; Xing, Y.M.; Lin, J.S.; Diao, Y. Recombinant human kallistatin inhibits angiogenesis by blocking VEGF signaling pathway. J. Cell. Biochem. 2014, 115, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Gómez-Ambrosi, J.; Rodríguez, A.; Ramírez, B.; Valentí, V.; Moncada, R.; Becerril, S.; Unamuno, X.; Silva, C.; Salvador, J.; et al. Novel protective role of kallistatin in obesity by limiting adipose tissue low grade inflammation and oxidative stress. Metabolism 2018, 87, 123–135. [Google Scholar] [CrossRef]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto, A.M., Jr.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G.; et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 2008, 359, 2195–2207. [Google Scholar] [CrossRef]
- German, C.A.; Liao, J.K. Understanding the molecular mechanisms of statin pleiotropic effects. Arch. Toxicol. 2023, 97, 1529–1545. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45, S17–S38. [Google Scholar] [CrossRef] [PubMed]
- Lőrincz, H.; Katkó, M.; Harangi, M.; Somodi, S.; Gaál, K.; Fülöp, P.; Paragh, G.; Seres, I. Strong correlations between circulating chemerin levels and lipoprotein subfractions in nondiabetic obese and nonobese subjects. Clin. Endocrinol. 2014, 81, 370–377. [Google Scholar] [CrossRef]

| Controls (n = 49) | NDO (n = 106) | T2D (n = 62) | |
|---|---|---|---|
| Male/Female (n) | 13/36 | 23/83 | 22/40 |
| BMI (kg/m2) | 24.7 ± 2.8 | 42.6 ± 8.1 * | 43.1 ± 9.1 § |
| Waist circumference (cm) | 85.2 ± 12.3 | 123.7 ± 17.4 * | 128.3 ± 18.5 § |
| Age (years) | 43.2 ± 9.1 | 44.3 ± 12.5 | 47.6 ± 7.7 |
| Lipid parameters | |||
| Total cholesterol (mmol/L) | 5 ± 0.8 | 5 ± 0.8 | 5 ± 1.2 |
| Triglyceride (mmol/L) | 1.1 (0.9–1.5) | 1.45 (1.1–1.9) | 1.7 (1.2–2.7) §,# |
| LDL-C (mmol/L) | 2.9 ± 0.5 | 3.2 ± 0.7 | 3 ± 0.9 |
| HDL-C (mmol/L) | 1.5 ± 0.4 | 1.3 ± 0.3 | 1.2 ± 0.3 § |
| Lipoprotein subfractions | |||
| VLDL (%) | 17.69 ± 3.2 | 19.9 ± 4.1 | 20.8 ± 5.2 |
| IDL (C-A;%) | 26.6 ± 6.3 | 25.3 ± 4 | 24.6 ± 3.9 |
| Large LDL (1–2;%) | 23.2 ± 6.1 | 28 ± 4.7 * | 26.9 ± 5.4 § |
| Small-dense LDL (3–7;%) | 0.6 (0–1.9) | 1.15 (0–2.4) | 1.65 (0–3.3) § |
| Mean LDL size (nm) | 27.3 (27–27.4) | 27.1 (26.9–27.3) | 26.9 (26.9–27.3) §,# |
| Large HDL (1–3;%) | 29 ± 8.7 | 22.9 ± 7 * | 18.9 ± 6.5 §,# |
| Intermediate HDL (4–7;%) | 50.3 ± 4.6 | 51.1 ± 3.7 | 48.8 ± 4.1 |
| Small HDL (8–10;%) | 20.7 ± 6.3 | 26 ± 6.8 * | 32.2 ± 7.7 §,# |
| Routine laboratory parameters | |||
| Glucose (mmol/L) | 4.8 (4.5–5.1) | 5.2 (4.9–5.8) * | 6.4 (5.5–10.5) §,# |
| HbA1C (%) | 5.1 ± 0.3 | 5.7 ± 0.8 | 7.2 ± 1.7 §,# |
| Insulin (mU/L) | 10.9 (6.6–12.9) (n = 16) | 15 (11.2–21.6) | 25.4 (14.1–31.5) (n = 16) § |
| hsCRP (mg/L) | 1.3 (0.6–2.5) | 8 (3.4–15.7) * | 6.8 (3.1–13.7) § |
| eGFR (mL/1.73 m2) | 90 (90–90) | 90 (90–90) | 90 (90–90) |
| sTSH (mU/L) | 1.65 (1.18–2.11) | 1.95 (1.46–2.67) | 2.29 (1.34–15.2) (n = 33) |
| γ-GTP (U/L) | 19 (16–28) | 28.5 (19–44) * | 35 (25–53) § |
| AST (U/L) | 19 (17–24) | 20 (17–27) | 25 (17–30) |
| ALT (U/L) | 17.5 (13–25) | 26 (18–35) * | 28 (21–44) § |
| Betatrophin (ng/mL) | 15.35 (11.72–23.88) | 18.22 (13.14–26.12) | 26.85 (19.00–37.10) §,# |
| Controls (n = 49) | NDO (n = 106) | T2D (n = 62) | ||||
|---|---|---|---|---|---|---|
| Variable | r | p | r | p | r | p |
| Antropometric parameters | ||||||
| Age (years) | −0.21 | 0.139 | 0.06 | 0.552 | 0.059 | 0.659 |
| BMI (kg/m2) | −0.15 | 0.345 | −0.07 | 0.455 | −0.18 | 0.184 |
| Waist circumference (cm) | 0.14 | 0.417 | −0.04 | 0.738 | 0.02 | 0.898 |
| Markers of glucose metabolism | ||||||
| Glucose (mmol/L) | −0.07 | 0.653 | 0.02 | 0.869 | 0.22 | 0.150 |
| HbA1C (%) | −0.01 | 0.978 | −0.02 | 0.872 | 0.40 | 0.009 |
| Insulin (mU/L) | 0.30 | 0.260 | 0.13 | 0.209 | −0.11 | 0.697 |
| Betatrophin (ng/mL) | −0.03 | 0.841 | 0.35 | 0.003 | 0.07 | 0.611 |
| Markers of inflammation and liver enzymes | ||||||
| hsCRP (mg/L) | −0.54 | 0.002 | −0.23 | 0.030 | 0.02 | 0.932 |
| γ-GTP (U/L) | −0.08 | 0.642 | −0.04 | 0.737 | 0.20 | 0.203 |
| AST (U/L) | 0.18 | 0.218 | −0.02 | 0.883 | 0.16 | 0.305 |
| ALT (U/L) | −0.01 | 0.971 | 0.01 | 0.904 | 0.23 | 0.126 |
| Lipid parameters | ||||||
| Total cholesterol (mmol/L) | 0.15 | 0.304 | 0.13 | 0.201 | 0.19 | 0.196 |
| Triglyceride (mmol/L) | −0.30 | 0.090 | 0.10 | 0.332 | 0.46 | 0.015 |
| LDL-C (mmol/L) | 0.11 | 0.460 | 0.10 | 0.333 | 0.06 | 0.706 |
| HDL-C (mmol/L) | 0.18 | 0.212 | 0.02 | 0.831 | −0.31 | 0.036 |
| Controls (n = 49) | NDO (n = 106) | T2D (n = 62) | ||||
|---|---|---|---|---|---|---|
| Variable | r | p | r | p | r | p |
| LDL subfraction test | ||||||
| VLDL (%) | 0.02 | 0.896 | 0.40 | <0.001 | 0.37 | 0.004 |
| IDL (C-A;%) | −0.13 | 0.378 | −0.25 | 0.010 | −0.27 | 0.040 |
| Large LDL (1–2;%) | −0.04 | 0.802 | −0.13 | 0.190 | −0.18 | 0.170 |
| Small LDL (3–7;%) | 0.17 | 0.230 | 0.26 | 0.007 | 0.30 | 0.022 |
| Mean LDL size (nm) | −0.10 | 0.482 | −0.28 | 0.004 | −0.37 | 0.004 |
| HDL subfraction test | ||||||
| HDL-1 (%) | 0.01 | 0.990 | −0.28 | 0.004 | 0.09 | 0.494 |
| HDL-2 (%) | 0.02 | 0.978 | −0.26 | 0.008 | −0.17 | 0.219 |
| HDL-3 (%) | 0.04 | 0.783 | −0.08 | 0.439 | −0.37 | 0.005 |
| HDL-4 (%) | 0.02 | 0.876 | −0.05 | 0.644 | −0.34 | 0.009 |
| HDL-5 (%) | −0.29 | 0.043 | 0.08 | 0.444 | −0.28 | 0.034 |
| HDL-6 (%) | −0.30 | 0.034 | 0.02 | 0.857 | −0.18 | 0.183 |
| HDL-7 (%) | −0.06 | 0.671 | −0.01 | 0.983 | 0.03 | 0.854 |
| HDL-8 (%) | 0.04 | 0.789 | 0.34 | <0.001 | 0.34 | 0.010 |
| HDL-9 (%) | 0.09 | 0.539 | 0.31 | 0.001 | 0.42 | 0.001 |
| HDL-10 (%) | 0.30 | 0.036 | 0.12 | 0.226 | 0.32 | 0.018 |
| Large HDL (1–3;%) | 0.03 | 0.861 | −0.24 | 0.013 | −0.23 | 0.074 |
| Intermediate HDL (4–7;%) | −0.32 | 0.026 | 0.02 | 0.835 | −0.32 | 0.014 |
| Small HDL (8–10;%) | 0.20 | 0.170 | 0.24 | 0.013 | 0.36 | 0.005 |
| Controls | NDO | T2D | ||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||
| Predictor | hsCRP | Betatrophin | hsCRP | HbA1c |
| β* | −0.54 | 0.362 | −0.31 | 0.489 |
| p-value | 0.001 | 0.007 | 0.001 | 0.018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lőrincz, H.; Csiha, S.; Ratku, B.; Somodi, S.; Sztanek, F.; Paragh, G.; Harangi, M. Associations between Serum Kallistatin Levels and Markers of Glucose Homeostasis, Inflammation, and Lipoprotein Metabolism in Patients with Type 2 Diabetes and Nondiabetic Obesity. Int. J. Mol. Sci. 2024, 25, 6264. https://doi.org/10.3390/ijms25116264
Lőrincz H, Csiha S, Ratku B, Somodi S, Sztanek F, Paragh G, Harangi M. Associations between Serum Kallistatin Levels and Markers of Glucose Homeostasis, Inflammation, and Lipoprotein Metabolism in Patients with Type 2 Diabetes and Nondiabetic Obesity. International Journal of Molecular Sciences. 2024; 25(11):6264. https://doi.org/10.3390/ijms25116264
Chicago/Turabian StyleLőrincz, Hajnalka, Sára Csiha, Balázs Ratku, Sándor Somodi, Ferenc Sztanek, György Paragh, and Mariann Harangi. 2024. "Associations between Serum Kallistatin Levels and Markers of Glucose Homeostasis, Inflammation, and Lipoprotein Metabolism in Patients with Type 2 Diabetes and Nondiabetic Obesity" International Journal of Molecular Sciences 25, no. 11: 6264. https://doi.org/10.3390/ijms25116264
APA StyleLőrincz, H., Csiha, S., Ratku, B., Somodi, S., Sztanek, F., Paragh, G., & Harangi, M. (2024). Associations between Serum Kallistatin Levels and Markers of Glucose Homeostasis, Inflammation, and Lipoprotein Metabolism in Patients with Type 2 Diabetes and Nondiabetic Obesity. International Journal of Molecular Sciences, 25(11), 6264. https://doi.org/10.3390/ijms25116264









