Cannabinoid Analgesia in Postoperative Pain Management: From Molecular Mechanisms to Clinical Reality
Abstract
1. Introduction
2. Cannabinoids and Therapeutic Potential in Pain Relief
2.1. Phytocannabinoids
Phytocannabinoids and Pain Relief
2.2. Endocannabinoids
Involvement of eCBs in the Regulation of Pain
2.3. Synthetic Cannabinoids
3. Molecular Mechanisms Underlying Analgesic Effects of Cannabinoids
4. Why Consider the Therapeutic Potential of Cannabinoids in Postoperative Pain Relief?
4.1. Activation of the ECS after Surgery
4.2. Cannabinoids Themselves Possess Analgesic Properties
4.3. Opioids—Cannabinoids
4.4. NSAIDs—Cannabinoids
4.5. Paracetamol—Cannabinoids
4.6. Local Anesthetics—Cannabinoids
4.7. α2-Adrenergic Receptors—Cannabinoids
5. Cautions and Limitations of Using Cannabinoids for Postoperative Pain Relief
5.1. Which Product Is Suitable for Postoperative Pain Control?
5.2. Pharmacokinetic Considerations and Routes of Administration of Cannabinoids in Postoperative Pain Relief
5.3. Potential Interactions of Cannabinoids in Postoperative Pain Relief
5.4. Acute Adverse Effects of Cannabinoids
5.5. Patients with a History of Cannabinoid Use
5.6. Contraindications
6. Clinical Trials Evaluating Cannabinoids for Postoperative Pain
7. Possible Explanations for the Lack of Analgesic Efficacy in Postoperative Pain Relief
8. Future Perspectives and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.A.; Herr, M.J. Physiology, Nociception. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2024. [Google Scholar]
- Attal, N.; Bouhassira, D.; Colvin, L. Advances and challenges in neuropathic pain: A narrative review and future directions. Br. J. Anaesth. 2023, 131, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Brennan, T.J.; Zahn, P.K.; Pogatzki-Zahn, E.M. Mechanisms of incisional pain. Anesthesiol. Clin. N. Am. 2005, 23, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Treede, R.D.; Meyer, R.A.; Raja, S.N.; Campbell, J.N. Peripheral and central mechanisms of cutaneous hyperalgesia. Prog. Neurobiol. 1992, 38, 397–421. [Google Scholar] [CrossRef] [PubMed]
- Hug, C.C., Jr. Opioids: Clinical use as anesthetic agents. J. Pain Symptom Manag. 1992, 7, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Peponis, T.; Kaafarani, H.M.A. What Is the Proper Use of Opioids in the Postoperative Patient? Adv. Surg. 2017, 51, 77–87. [Google Scholar] [CrossRef]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Manchikanti, L.; Kaye, A.M.; Knezevic, N.N.; McAnally, H.; Slavin, K.; Trescot, A.M.; Blank, S.; Pampati, V.; Abdi, S.; Grider, J.S.; et al. Responsible, Safe, and Effective Prescription of Opioids for Chronic Non-Cancer Pain: American Society of Interventional Pain Physicians (ASIPP) Guidelines. Pain Physician 2017, 20, S3–S92. [Google Scholar] [CrossRef]
- Alexander, J.C.; Patel, B.; Joshi, G.P. Perioperative use of opioids: Current controversies and concerns. Best Pract. Res. Clin. Anaesthesiol. 2019, 33, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Hah, J.M.; Bateman, B.T.; Ratliff, J.; Curtin, C.; Sun, E. Chronic Opioid Use After Surgery: Implications for Perioperative Management in the Face of the Opioid Epidemic. Anesth. Analg. 2017, 125, 1733–1740. [Google Scholar] [CrossRef]
- Quinlan, J.; Lobo, D.N.; Levy, N. Postoperative pain management: Time to get back on track. Anaesthesia 2020, 75 (Suppl. S1), e10–e13. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Hanus, L. A historical overview of chemical research on cannabinoids. Chem. Phys. Lipids 2000, 108, 1–13. [Google Scholar] [CrossRef]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Ben-Shabat, S.; Hanus, L.; Ligumsky, M.; Kaminski, N.E.; Schatz, A.R.; Gopher, A.; Almog, S.; Martin, B.R.; Compton, D.R.; et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995, 50, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Di, M.; Bisogno, T.; De Petrocellis, L. Endocannabinoids: New targets for drug development. Curr. Pharm. Des. 2000, 6, 1361–1380. [Google Scholar] [CrossRef] [PubMed]
- Romero-Sandoval, A.; Eisenach, J.C. Spinal cannabinoid receptor type 2 activation reduces hypersensitivity and spinal cord glial activation after paw incision. Anesthesiology 2007, 106, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Alkaitis, M.S.; Solorzano, C.; Landry, R.P.; Piomelli, D.; DeLeo, J.A.; Romero-Sandoval, E.A. Evidence for a role of endocannabinoids, astrocytes and p38 phosphorylation in the resolution of postoperative pain. PLoS ONE 2010, 5, e10891. [Google Scholar] [CrossRef] [PubMed]
- Howlett, A.C.; Barth, F.; Bonner, T.I.; Cabral, G.; Casellas, P.; Devane, W.A.; Felder, C.C.; Herkenham, M.; Mackie, K.; Martin, B.R.; et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002, 54, 161–202. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Shim, J.Y.; Assi, A.A.; Norford, D.; Howlett, A.C. CB1 cannabinoid receptor-G protein association: A possible mechanism for differential signaling. Chem. Phys. Lipids 2002, 121, 91–109. [Google Scholar] [CrossRef] [PubMed]
- Guindon, J.; Hohmann, A.G. Cannabinoid CB2 receptors: A therapeutic target for the treatment of inflammatory and neuropathic pain. Br. J. Pharmacol. 2008, 153, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.M.; Huang, S.M. Cannabinoid analgesia. Pharmacol. Ther. 2002, 95, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Kondo, S.; Sukagawa, A.; Nakane, S.; Shinoda, A.; Itoh, K.; Yamashita, A.; Waku, K. 2-Arachidonoylglycerol: A possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 1995, 215, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Malan, P.T., Jr.; Ibrahim, M.M.; Deng, H.; Liu, Q.; Mata, H.P.; Vanderah, T.; Porreca, F.; Makriyannis, A. CB2 cannabinoid receptor-mediated peripheral antinociception. Pain 2001, 93, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Clayton, N.; Marshall, F.H.; Bountra, C.; O’Shaughnessy, C.T. CB1 and CB2 cannabinoid receptors are implicated in inflammatory pain. Pain 2002, 96, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M.; Deng, H.; Zvonok, A.; Cockayne, D.A.; Kwan, J.; Mata, H.P.; Vanderah, T.W.; Lai, J.; Porreca, F.; Makriyannis, A.; et al. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: Pain inhibition by receptors not present in the CNS. Proc. Natl. Acad. Sci. USA 2003, 100, 10529–10533. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M.; Porreca, F.; Lai, J.; Albrecht, P.J.; Rice, F.L.; Khodorova, A.; Davar, G.; Makriyannis, A.; Vanderah, T.W.; Mata, H.P.; et al. CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc. Natl. Acad. Sci. USA 2005, 102, 3093–3098. [Google Scholar] [CrossRef] [PubMed]
- Nackley, A.G.; Makriyannis, A.; Hohmann, A.G. Selective activation of cannabinoid CB2 receptors suppresses spinal fos protein expression and pain behavior in a rat model of inflammation. Neuroscience 2003, 119, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, A.G.; Farthing, J.N.; Zvonok, A.M.; Makriyannis, A. Selective activation of cannabinoid CB2 receptors suppresses hyperalgesia evoked by intradermal capsaicin. J. Pharmacol. Exp. Ther. 2004, 308, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.A.; Wright, C.E.; Angus, J.A. Evidence that CB-1 and CB-2 cannabinoid receptors mediate antinociception in neuropathic pain in the rat. Pain 2004, 109, 124–131. [Google Scholar] [CrossRef]
- Valenzano, K.J.; Tafesse, L.; Lee, G.; Harrison, J.E.; Boulet, J.M.; Gottshall, S.L.; Mark, L.; Pearson, M.S.; Miller, W.; Shan, S.; et al. Pharmacological and pharmacokinetic characterization of the cannabinoid receptor 2 agonist, GW405833, utilizing rodent models of acute and chronic pain, anxiety, ataxia and catalepsy. Neuropharmacology 2005, 48, 658–672. [Google Scholar] [CrossRef] [PubMed]
- Sampson, P.B. Phytocannabinoid Pharmacology: Medicinal Properties of Cannabis sativa Constituents Aside from the “Big Two”. J. Nat. Prod. 2021, 84, 142–160. [Google Scholar] [CrossRef] [PubMed]
- Taura, F.; Sirikantaramas, S.; Shoyama, Y.; Yoshikai, K.; Shoyama, Y.; Morimoto, S. Cannabidiolic-acid synthase, the chemotype-determining enzyme in the fiber-type Cannabis sativa. FEBS Lett. 2007, 581, 2929–2934. [Google Scholar] [CrossRef] [PubMed]
- Radwan, M.M.; Chandra, S.; Gul, S.; ElSohly, M.A. Cannabinoids, Phenolics, Terpenes and Alkaloids of Cannabis. Molecules 2021, 26, 2774. [Google Scholar] [CrossRef] [PubMed]
- McPartland, J.M. Cannabis Systematics at the Levels of Family, Genus, and Species. Cannabis Cannabinoid Res. 2018, 3, 203–212. [Google Scholar] [CrossRef]
- Mechoulam, R. Cannabinoids as Therapeutic Agents, 1st ed.; Mechoulam, R., Ed.; Chapman and Hall/CRC: New York, NY, USA, 1986; p. 186. [Google Scholar] [CrossRef]
- Abel, E.L. Marihuana, the First Twelve Thousand Years; McGraw-Hill: New York, NY, USA, 1982; p. 304. [Google Scholar]
- Mehmedic, Z.; Chandra, S.; Slade, D.; Denham, H.; Foster, S.; Patel, A.S.; Ross, S.A.; Khan, I.A.; ElSohly, M.A. Potency trends of Delta9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. J. Forensic Sci. 2010, 55, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Al Ubeed, H.M.S.; Bhuyan, D.J.; Alsherbiny, M.A.; Basu, A.; Vuong, Q.V. A Comprehensive Review on the Techniques for Extraction of Bioactive Compounds from Medicinal Cannabis. Molecules 2022, 27, 604. [Google Scholar] [CrossRef]
- Abood, M.E.; Martin, B.R. Neurobiology of marijuana abuse. Trends Pharmacol. Sci. 1992, 13, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Hanus, L.O.; Hod, Y. Terpenes/Terpenoids in Cannabis: Are They Important? Med. Cannabis Cannabinoids 2020, 3, 25–60. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R. Marihuana chemistry. Science 1970, 168, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.R. Structural requirements for cannabinoid-induced antinociceptive activity in mice. Life Sci. 1985, 36, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hong, P.J.; May, C.; Rehman, Y.; Oparin, Y.; Hong, C.J.; Hong, B.Y.; AminiLari, M.; Gallo, L.; Kaushal, A.; et al. Medical cannabis or cannabinoids for chronic non-cancer and cancer related pain: A systematic review and meta-analysis of randomised clinical trials. BMJ 2021, 374, n1034. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.E.; Campbell, F. Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials. Br. J. Clin. Pharmacol. 2011, 72, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Strand, N.; D’Souza, R.S.; Karri, J.; Kalia, H.; Weisbein, J.; Kassa, B.J.; Hussain, N.; Chitneni, A.; Budwany, R.R.; Hagedorn, J.; et al. Medical Cannabis: A Review from the American Society of Pain and Neuroscience. J. Pain Res. 2023, 16, 4217–4228. [Google Scholar] [CrossRef] [PubMed]
- Abrams, D.I.; Jay, C.A.; Shade, S.B.; Vizoso, H.; Reda, H.; Press, S.; Kelly, M.E.; Rowbotham, M.C.; Petersen, K.L. Cannabis in painful HIV-associated sensory neuropathy: A randomized placebo-controlled trial. Neurology 2007, 68, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Andreae, M.H.; Carter, G.M.; Shaparin, N.; Suslov, K.; Ellis, R.J.; Ware, M.A.; Abrams, D.I.; Prasad, H.; Wilsey, B.; Indyk, D.; et al. Inhaled Cannabis for Chronic Neuropathic Pain: A Meta-analysis of Individual Patient Data. J. Pain 2015, 16, 1221–1232. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.J.; Toperoff, W.; Vaida, F.; van den Brande, G.; Gonzales, J.; Gouaux, B.; Bentley, H.; Atkinson, J.H. Smoked medicinal cannabis for neuropathic pain in HIV: A randomized, crossover clinical trial. Neuropsychopharmacology 2009, 34, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.S.; Marcotte, T.D.; Umlauf, A.; Gouaux, B.; Atkinson, J.H. Efficacy of Inhaled Cannabis on Painful Diabetic Neuropathy. J. Pain 2015, 16, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Ware, M.A.; Wang, T.; Shapiro, S.; Robinson, A.; Ducruet, T.; Huynh, T.; Gamsa, A.; Bennett, G.J.; Collet, J.P. Smoked cannabis for chronic neuropathic pain: A randomized controlled trial. Can. Med. Assoc. J. 2010, 182, E694–E701. [Google Scholar] [CrossRef] [PubMed]
- Wilsey, B.; Marcotte, T.; Tsodikov, A.; Millman, J.; Bentley, H.; Gouaux, B.; Fishman, S. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J. Pain 2008, 9, 506–521. [Google Scholar] [CrossRef] [PubMed]
- Bakheit, A.M. The pharmacological management of post-stroke muscle spasticity. Drugs Aging 2012, 29, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Koppel, B.S.; Brust, J.C.; Fife, T.; Bronstein, J.; Youssof, S.; Gronseth, G.; Gloss, D. Systematic review: Efficacy and safety of medical marijuana in selected neurologic disorders: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2014, 82, 1556–1563. [Google Scholar] [CrossRef] [PubMed]
- Passie, T.; Emrich, H.M.; Karst, M.; Brandt, S.D.; Halpern, J.H. Mitigation of post-traumatic stress symptoms by Cannabis resin: A review of the clinical and neurobiological evidence. Drug Test. Anal. 2012, 4, 649–659. [Google Scholar] [CrossRef] [PubMed]
- McGeeney, B.E. Cannabinoids and hallucinogens for headache. Headache 2013, 53, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.E.; Shiling, D.J.; Stillman, R.C.; Goldberg, N.H.; Seipp, C.A.; Barofsky, I.; Rosenberg, S.A. A prospective evaluation of delta-9-tetrahydrocannabinol as an antiemetic in patients receiving adriamycin and cytoxan chemotherapy. Cancer 1981, 47, 1746–1751. [Google Scholar] [CrossRef] [PubMed]
- Naftali, T.; Mechulam, R.; Lev, L.B.; Konikoff, F.M. Cannabis for inflammatory bowel disease. Dig. Dis. 2014, 32, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Schierenbeck, T.; Riemann, D.; Berger, M.; Hornyak, M. Effect of illicit recreational drugs upon sleep: Cocaine, ecstasy and marijuana. Sleep Med. Rev. 2008, 12, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Ware, M.A.; Fitzcharles, M.A.; Joseph, L.; Shir, Y. The effects of nabilone on sleep in fibromyalgia: Results of a randomized controlled trial. Anesth. Analg. 2010, 110, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.J. Medical marijuana: The conflict between scientific evidence and political ideology. Part two of two. J. Pain Palliat. Care Pharmacother. 2009, 23, 120–140. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.; Stjepanovic, D.; Caulkins, J.; Lynskey, M.; Leung, J.; Campbell, G.; Degenhardt, L. Public health implications of legalising the production and sale of cannabis for medicinal and recreational use. Lancet 2019, 394, 1580–1590. [Google Scholar] [CrossRef]
- Shover, C.L.; Humphreys, K. Six policy lessons relevant to cannabis legalization. Am. J. Drug Alcohol Abus. 2019, 45, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.; Wadsworth, E.; Leos-Toro, C.; Hammond, D.; International Cannabis Policy Study Team. Prevalence and forms of cannabis use in legal vs. illegal recreational cannabis markets. Int. J. Drug Policy 2020, 76, 102658. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.; Lynskey, M. Assessing the public health impacts of legalizing recreational cannabis use: The US experience. World Psychiatry 2020, 19, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Tashkin, D.P. Effects of marijuana smoking on the lung. Ann. Am. Thorac. Soc. 2013, 10, 239–247. [Google Scholar] [CrossRef]
- Wu, T.C.; Tashkin, D.P.; Djahed, B.; Rose, J.E. Pulmonary hazards of smoking marijuana as compared with tobacco. N. Engl. J. Med. 1988, 318, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Piomelli, D. The molecular logic of endocannabinoid signalling. Nat. Rev. Neurosci. 2003, 4, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Parker, L.A. The endocannabinoid system and the brain. Annu. Rev. Psychol. 2013, 64, 21–47. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef]
- Hillard, C.J. The Endocannabinoid Signaling System in the CNS: A Primer. Int. Rev. Neurobiol. 2015, 125, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Kogan, N.M.; Mechoulam, R. Beyond THC and Endocannabinoids. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 637–659. [Google Scholar] [CrossRef] [PubMed]
- Finn, D.P. Endocannabinoid-mediated modulation of stress responses: Physiological and pathophysiological significance. Immunobiology 2010, 215, 629–646. [Google Scholar] [CrossRef] [PubMed]
- De Laurentiis, A.; Correa, F.; Fernandez Solari, J. Endocannabinoid System in the Neuroendocrine Response to Lipopolysaccharide-induced Immune Challenge. J. Endocr. Soc. 2022, 6, bvac120. [Google Scholar] [CrossRef] [PubMed]
- Varvel, S.A.; Lichtman, A.H. Evaluation of CB1 receptor knockout mice in the Morris water maze. J. Pharmacol. Exp. Ther. 2002, 301, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Marsicano, G.; Wotjak, C.T.; Azad, S.C.; Bisogno, T.; Rammes, G.; Cascio, M.G.; Hermann, H.; Tang, J.; Hofmann, C.; Zieglgansberger, W.; et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature 2002, 418, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Ledent, C.; Parmentier, M.; Maldonado, R.; Valverde, O. Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology 2002, 159, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Ahmad, T.; Loureiro, M.; Zunder, J.; Laviolette, S.R. The role of cannabinoid transmission in emotional memory formation: Implications for addiction and schizophrenia. Front. Psychiatry 2014, 5, 73. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Goparaju, S.K.; Wang, L.; Liu, J.; Batkai, S.; Jarai, Z.; Fezza, F.; Miura, G.I.; Palmiter, R.D.; Sugiura, T.; et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature 2001, 410, 822–825. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M.; Bari, M.; Battista, N.; Finazzi-Agro, A. Endocannabinoid degradation, endotoxic shock and inflammation. Curr. Drug Targets Inflamm. Allergy 2002, 1, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Nagarkatti, P.; Pandey, R.; Rieder, S.A.; Hegde, V.L.; Nagarkatti, M. Cannabinoids as novel anti-inflammatory drugs. Future Med. Chem. 2009, 1, 1333–1349. [Google Scholar] [CrossRef] [PubMed]
- Hogestatt, E.D.; Zygmunt, P.M. Cardiovascular pharmacology of anandamide. Prostaglandins Leukot. Essent. Fat. Acids 2002, 66, 343–351. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.E. Endocannabinoids and the Cardiovascular System in Health and Disease. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2015; Volume 231, pp. 393–422. [Google Scholar] [CrossRef]
- Li, C.; Jones, P.M.; Persaud, S.J. Role of the endocannabinoid system in food intake, energy homeostasis and regulation of the endocrine pancreas. Pharmacol. Ther. 2011, 129, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Bellocchio, L.; Cervino, C.; Pasquali, R.; Pagotto, U. The endocannabinoid system and energy metabolism. J. Neuroendocrinol. 2008, 20, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.H.; Amoako, A.A.; Bambang, K.; Karasu, T.; Gebeh, A.; Lam, P.M.; Marzcylo, T.H.; Konje, J.C. Endocannabinoids and pregnancy. Clin. Chim. Acta 2010, 411, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Correa, F.; Wolfson, M.L.; Valchi, P.; Aisemberg, J.; Franchi, A.M. Endocannabinoid system and pregnancy. Reproduction 2016, 152, R191–R200. [Google Scholar] [CrossRef] [PubMed]
- Guindon, J.; Hohmann, A.G. The endocannabinoid system and pain. CNS Neurol. Disord. Drug Targets 2009, 8, 403–421. [Google Scholar] [CrossRef] [PubMed]
- Zogopoulos, P.; Vasileiou, I.; Patsouris, E.; Theocharis, S.E. The role of endocannabinoids in pain modulation. Fundam. Clin. Pharmacol. 2013, 27, 64–80. [Google Scholar] [CrossRef] [PubMed]
- Finn, D.P.; Haroutounian, S.; Hohmann, A.G.; Krane, E.; Soliman, N.; Rice, A.S.C. Cannabinoids, the endocannabinoid system, and pain: A review of preclinical studies. Pain 2021, 162, S5–S25. [Google Scholar] [CrossRef] [PubMed]
- Toth, B.I.; Dobrosi, N.; Dajnoki, A.; Czifra, G.; Olah, A.; Szollosi, A.G.; Juhasz, I.; Sugawara, K.; Paus, R.; Biro, T. Endocannabinoids modulate human epidermal keratinocyte proliferation and survival via the sequential engagement of cannabinoid receptor-1 and transient receptor potential vanilloid-1. J. Investig. Dermatol. 2011, 131, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.; Moreia-Pinto, B.; Felgueira, E.; Ribeiro, A.; Rebelo, I.; Fonseca, B.M. The major endocannabinoid anandamide (AEA) induces apoptosis of human granulosa cells. Prostaglandins Leukot. Essent. Fat. Acids 2021, 171, 102311. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Cascio, M.G.; Di Marzo, V. The endocannabinoid system: A general view and latest additions. Br. J. Pharmacol. 2004, 141, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 2020, 16, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Rezende, B.; Alencar, A.K.N.; de Bem, G.F.; Fontes-Dantas, F.L.; Montes, G.C. Endocannabinoid System: Chemical Characteristics and Biological Activity. Pharmaceuticals 2023, 16, 148. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.; Pryce, G.; Giovannoni, G.; Thompson, A.J. The therapeutic potential of cannabis. Lancet Neurol. 2003, 2, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Castillo, P.E.; Younts, T.J.; Chavez, A.E.; Hashimotodani, Y. Endocannabinoid signaling and synaptic function. Neuron 2012, 76, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Nomura, D.K.; Morrison, B.E.; Blankman, J.L.; Long, J.Z.; Kinsey, S.G.; Marcondes, M.C.; Ward, A.M.; Hahn, Y.K.; Lichtman, A.H.; Conti, B.; et al. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science 2011, 334, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Katona, I.; Freund, T.F. Multiple functions of endocannabinoid signaling in the brain. Annu. Rev. Neurosci. 2012, 35, 529–558. [Google Scholar] [CrossRef] [PubMed]
- Ligresti, A.; De Petrocellis, L.; Di Marzo, V. From Phytocannabinoids to Cannabinoid Receptors and Endocannabinoids: Pleiotropic Physiological and Pathological Roles Through Complex Pharmacology. Physiol. Rev. 2016, 96, 1593–1659. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G.; Howlett, A.C.; Abood, M.E.; Alexander, S.P.; Di Marzo, V.; Elphick, M.R.; Greasley, P.J.; Hansen, H.S.; Kunos, G.; Mackie, K.; et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: Beyond CB1 and CB2. Pharmacol. Rev. 2010, 62, 588–631. [Google Scholar] [CrossRef] [PubMed]
- Gomes, I.; Grushko, J.S.; Golebiewska, U.; Hoogendoorn, S.; Gupta, A.; Heimann, A.S.; Ferro, E.S.; Scarlata, S.; Fricker, L.D.; Devi, L.A. Novel endogenous peptide agonists of cannabinoid receptors. FASEB J. 2009, 23, 3020–3029. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Chicca, A.; Tamborrini, M.; Eisen, D.; Lerner, R.; Lutz, B.; Poetz, O.; Pluschke, G.; Gertsch, J. Identification and quantification of a new family of peptide endocannabinoids (Pepcans) showing negative allosteric modulation at CB1 receptors. J. Biol. Chem. 2012, 287, 36944–36967. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Zhao, L.; Jing, Y. Signaling molecules targeting cannabinoid receptors: Hemopressin and related peptides. Neuropeptides 2020, 79, 101998. [Google Scholar] [CrossRef] [PubMed]
- Fine, P.G.; Rosenfeld, M.J. The endocannabinoid system, cannabinoids, and pain. Rambam Maimonides Med. J. 2013, 4, e0022. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ovejero, D.; Arevalo-Martin, A.; Petrosino, S.; Docagne, F.; Hagen, C.; Bisogno, T.; Watanabe, M.; Guaza, C.; Di Marzo, V.; Molina-Holgado, E. The endocannabinoid system is modulated in response to spinal cord injury in rats. Neurobiol. Dis. 2009, 33, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.M.; Rajpal, S.; Sweeney, C.; Gerovac, T.A.; Allcock, B.; McChesney, S.; Patel, A.U.; Tilghman, J.I.; Miranpuri, G.S.; Resnick, D.K. Cannabinoid subtype-2 receptors modulate the antihyperalgesic effect of WIN 55,212-2 in rats with neuropathic spinal cord injury pain. Spine J. 2010, 10, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Sagar, D.R.; Gaw, A.G.; Okine, B.N.; Woodhams, S.G.; Wong, A.; Kendall, D.A.; Chapman, V. Dynamic regulation of the endocannabinoid system: Implications for analgesia. Mol. Pain 2009, 5, 59. [Google Scholar] [CrossRef]
- Svizenska, I.; Dubovy, P.; Sulcova, A. Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures—A short review. Pharmacol. Biochem. Behav. 2008, 90, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Racz, I.; Nadal, X.; Alferink, J.; Banos, J.E.; Rehnelt, J.; Martin, M.; Pintado, B.; Gutierrez-Adan, A.; Sanguino, E.; Manzanares, J.; et al. Crucial role of CB2 cannabinoid receptor in the regulation of central immune responses during neuropathic pain. J. Neurosci. 2008, 28, 12125–12135. [Google Scholar] [CrossRef] [PubMed]
- Benito, C.; Nunez, E.; Tolon, R.M.; Carrier, E.J.; Rabano, A.; Hillard, C.J.; Romero, J. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer’s disease brains. J. Neurosci. 2003, 23, 11136–11141. [Google Scholar] [CrossRef]
- Van Sickle, M.D.; Duncan, M.; Kingsley, P.J.; Mouihate, A.; Urbani, P.; Mackie, K.; Stella, N.; Makriyannis, A.; Piomelli, D.; Davison, J.S.; et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 2005, 310, 329–332. [Google Scholar] [CrossRef]
- Kibret, B.G.; Ishiguro, H.; Horiuchi, Y.; Onaivi, E.S. New Insights and Potential Therapeutic Targeting of CB2 Cannabinoid Receptors in CNS Disorders. Int. J. Mol. Sci. 2022, 23, 975. [Google Scholar] [CrossRef] [PubMed]
- Abuhasira, R.; Shbiro, L.; Landschaft, Y. Medical use of cannabis and cannabinoids containing products—Regulations in Europe and North America. Eur. J. Intern. Med. 2018, 49, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.C.; Nation, T.; McGregor, I.S. Prescribing medicinal cannabis. Aust. Prescr. 2020, 43, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.J.; Lichtman, A.H.; Piomelli, D.; Parker, L.A. Cannabinoids and Cancer Chemotherapy-Associated Adverse Effects. J. Natl. Cancer Inst. Monogr. 2021, 2021, 78–85. [Google Scholar] [CrossRef]
- Cunningham, D.; Bradley, C.J.; Forrest, G.J.; Hutcheon, A.W.; Adams, L.; Sneddon, M.; Harding, M.; Kerr, D.J.; Soukop, M.; Kaye, S.B. A randomized trial of oral nabilone and prochlorperazine compared to intravenous metoclopramide and dexamethasone in the treatment of nausea and vomiting induced by chemotherapy regimens containing cisplatin or cisplatin analogues. Eur. J. Cancer Clin. Oncol. 1988, 24, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.C.; Giudice, M.G. Nabilone for the Management of Pain. Pharmacotherapy 2016, 36, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Wissel, J.; Haydn, T.; Muller, J.; Brenneis, C.; Berger, T.; Poewe, W.; Schelosky, L.D. Low dose treatment with the synthetic cannabinoid Nabilone significantly reduces spasticity-related pain: A double-blind placebo-controlled cross-over trial. J. Neurol. 2006, 253, 1337–1341. [Google Scholar] [CrossRef]
- Walitt, B.; Klose, P.; Fitzcharles, M.A.; Phillips, T.; Hauser, W. Cannabinoids for fibromyalgia. Cochrane Database Syst. Rev. 2016, 7, CD011694. [Google Scholar] [CrossRef] [PubMed]
- Badowski, M.E.; Yanful, P.K. Dronabinol oral solution in the management of anorexia and weight loss in AIDS and cancer. Ther. Clin. Risk Manag. 2018, 14, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Bar-Sela, G.; Zalman, D.; Semenysty, V.; Ballan, E. The Effects of Dosage-Controlled Cannabis Capsules on Cancer-Related Cachexia and Anorexia Syndrome in Advanced Cancer Patients: Pilot Study. Integr. Cancer Ther. 2019, 18, 1534735419881498. [Google Scholar] [CrossRef] [PubMed]
- Abu-Sawwa, R.; Stehling, C. Epidiolex (Cannabidiol) Primer: Frequently Asked Questions for Patients and Caregivers. J. Pediatr. Pharmacol. Ther. 2020, 25, 75–77. [Google Scholar] [CrossRef]
- Sekar, K.; Pack, A. Epidiolex as adjunct therapy for treatment of refractory epilepsy: A comprehensive review with a focus on adverse effects. F1000Res 2019, 8, F1000 Faculty Rev-234. [Google Scholar] [CrossRef] [PubMed]
- Conte, A.; Vila Silvan, C. Review of Available Data for the Efficacy and Effectiveness of Nabiximols Oromucosal Spray (Sativex(R)) in Multiple Sclerosis Patients with Moderate to Severe Spasticity. Neurodegener. Dis. 2021, 21, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Meuth, S.G.; Henze, T.; Essner, U.; Trompke, C.; Vila Silvan, C. Tetrahydrocannabinol and cannabidiol oromucosal spray in resistant multiple sclerosis spasticity: Consistency of response across subgroups from the SAVANT randomized clinical trial. Int. J. Neurosci. 2020, 130, 1199–1205. [Google Scholar] [CrossRef]
- Perez, J.; Ribera, M.V. Managing neuropathic pain with Sativex: A review of its pros and cons. Expert Opin. Pharmacother. 2008, 9, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Tonstad, S. Rimonabant: A cannabinoid receptor blocker for the treatment of metabolic and cardiovascular risk factors. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicki, A.S. Rimonabant: Endocannabinoid inhibition for the metabolic syndrome. Int. J. Clin. Pract. 2006, 60, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Almeida, T.F.; Roizenblatt, S.; Tufik, S. Afferent pain pathways: A neuroanatomical review. Brain Res. 2004, 1000, 40–56. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, R.; Dickenson, A.H. Spinal cord mechanisms of pain. Br. J. Anaesth. 2008, 101, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef] [PubMed]
- Millan, M.J. The induction of pain: An integrative review. Prog. Neurobiol. 1999, 57, 1–164. [Google Scholar] [CrossRef]
- Chapman, C.R.; Tuckett, R.P.; Song, C.W. Pain and stress in a systems perspective: Reciprocal neural, endocrine, and immune interactions. J. Pain 2008, 9, 122–145. [Google Scholar] [CrossRef] [PubMed]
- Rittner, H.L.; Brack, A.; Stein, C. Pain and the immune system. Br. J. Anaesth. 2008, 101, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Dubner, R. Interactions between the immune and nervous systems in pain. Nat. Med. 2010, 16, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Pogatzki-Zahn, E.M.; Segelcke, D.; Schug, S.A. Postoperative pain-from mechanisms to treatment. Pain Rep. 2017, 2, e588. [Google Scholar] [CrossRef] [PubMed]
- Hillard, C.J.; Beatka, M.; Sarvaideo, J. Endocannabinoid Signaling and the Hypothalamic-Pituitary-Adrenal Axis. Compr. Physiol. 2016, 7, 1–15. [Google Scholar] [CrossRef]
- Imbe, H.; Iwai-Liao, Y.; Senba, E. Stress-induced hyperalgesia: Animal models and putative mechanisms. Front. Biosci. 2006, 11, 2179–2192. [Google Scholar] [CrossRef]
- Gangadharan, V.; Kuner, R. Pain hypersensitivity mechanisms at a glance. Dis. Models Mech. 2013, 6, 889–895. [Google Scholar] [CrossRef]
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef]
- Costigan, M.; Scholz, J.; Woolf, C.J. Neuropathic pain: A maladaptive response of the nervous system to damage. Annu. Rev. Neurosci. 2009, 32, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Besson, J.M. The neurobiology of pain. Lancet 1999, 353, 1610–1615. [Google Scholar] [CrossRef] [PubMed]
- Levy, D. Endogenous mechanisms underlying the activation and sensitization of meningeal nociceptors: The role of immuno-vascular interactions and cortical spreading depression. Curr. Pain Headache Rep. 2012, 16, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Torta, D.M.E.; Van Den Broeke, E.N.; Filbrich, L.; Jacob, B.; Lambert, J.; Mouraux, A. Intense pain influences the cortical processing of visual stimuli projected onto the sensitized skin. Pain 2017, 158, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.R.; Vierck, C.J. The Transition of Acute Postoperative Pain to Chronic Pain: An Integrative Overview of Research on Mechanisms. J. Pain 2017, 18, 359.e1–359.e38. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.; Bennett, D.L. Neuroinflammation and the generation of neuropathic pain. Br. J. Anaesth. 2013, 111, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Huh, Y.; Ji, R.R. Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J. Anesth. 2019, 33, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Ho, I.H.T.; Chan, M.T.V.; Wu, W.K.K.; Liu, X. Spinal microglia-neuron interactions in chronic pain. J. Leukoc. Biol. 2020, 108, 1575–1592. [Google Scholar] [CrossRef]
- Zahn, P.K.; Brennan, T.J. Lack of effect of intrathecally administered N-methyl-D-aspartate receptor antagonists in a rat model for postoperative pain. Anesthesiology 1998, 88, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Zahn, P.K.; Umali, E.; Brennan, T.J. Intrathecal non-NMDA excitatory amino acid receptor antagonists inhibit pain behaviors in a rat model of postoperative pain. Pain 1998, 74, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Sakashita, Y. The role of the spinal opioid receptor like1 receptor, the NK-1 receptor, and cyclooxygenase-2 in maintaining postoperative pain in the rat. Anesth. Analg. 1999, 89, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Conklin, D.; Eisenach, J.C. Cyclooxygenase-1 in the spinal cord plays an important role in postoperative pain. Pain 2003, 104, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Porreca, F.; Ossipov, M.H.; Gebhart, G.F. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002, 25, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Pogatzki, E.M.; Urban, M.O.; Brennan, T.J.; Gebhart, G.F. Role of the rostral medial medulla in the development of primary and secondary hyperalgesia after incision in the rat. Anesthesiology 2002, 96, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Obata, H.; Eisenach, J.C.; Hussain, H.; Bynum, T.; Vincler, M. Spinal glial activation contributes to postoperative mechanical hypersensitivity in the rat. J. Pain 2006, 7, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.R.; Tan, P.H.; Cheng, J.K.; Liu, Y.C.; Ji, R.R. Microglia: A promising target for treating neuropathic and postoperative pain, and morphine tolerance. J. Formos. Med. Assoc. 2011, 110, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Bair, M.; Descalzi, G. Reactive Astrocytes: Critical Players in the Development of Chronic Pain. Front. Psychiatry 2021, 12, 682056. [Google Scholar] [CrossRef]
- Maldonado, R.; Banos, J.E.; Cabanero, D. The endocannabinoid system and neuropathic pain. Pain 2016, 157 (Suppl. S1), S23–S32. [Google Scholar] [CrossRef] [PubMed]
- Heifets, B.D.; Castillo, P.E. Endocannabinoid signaling and long-term synaptic plasticity. Annu. Rev. Physiol. 2009, 71, 283–306. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Petrosino, S. Endocannabinoids and the regulation of their levels in health and disease. Curr. Opin. Lipidol. 2007, 18, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.N.; McEwen, B.S. Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Gorzalka, B.B.; Hill, M.N.; Hillard, C.J. Regulation of endocannabinoid signaling by stress: Implications for stress-related affective disorders. Neurosci. Biobehav. Rev. 2008, 32, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, H.B.; Walker, J.M. The expanding field of cannabimimetic and related lipid mediators. Br. J. Pharmacol. 2005, 144, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, A.G.; Suplita, R.L., 2nd. Endocannabinoid mechanisms of pain modulation. AAPS J. 2006, 8, E693–E708. [Google Scholar] [CrossRef] [PubMed]
- Murphy, L.L.; Munoz, R.M.; Adrian, B.A.; Villanua, M.A. Function of cannabinoid receptors in the neuroendocrine regulation of hormone secretion. Neurobiol. Dis. 1998, 5, 432–446. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. Cannabinoid receptor ligands: Clinical and neuropharmacological considerations, relevant to future drug discovery and development. Expert Opin. Investig. Drugs 2000, 9, 1553–1571. [Google Scholar] [CrossRef] [PubMed]
- Wootten, D.; Christopoulos, A.; Marti-Solano, M.; Babu, M.M.; Sexton, P.M. Mechanisms of signalling and biased agonism in G protein-coupled receptors. Nat. Rev. Mol. Cell Biol. 2018, 19, 638–653. [Google Scholar] [CrossRef] [PubMed]
- Nevalainen, T.; Irving, A.J. GPR55, a lysophosphatidylinositol receptor with cannabinoid sensitivity? Curr. Top. Med. Chem. 2010, 10, 799–813. [Google Scholar] [CrossRef]
- Ross, R.A. The enigmatic pharmacology of GPR55. Trends Pharmacol. Sci. 2009, 30, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Lauckner, J.E.; Jensen, J.B.; Chen, H.Y.; Lu, H.C.; Hille, B.; Mackie, K. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc. Natl. Acad. Sci. USA 2008, 105, 2699–2704. [Google Scholar] [CrossRef] [PubMed]
- Ryberg, E.; Larsson, N.; Sjogren, S.; Hjorth, S.; Hermansson, N.O.; Leonova, J.; Elebring, T.; Nilsson, K.; Drmota, T.; Greasley, P.J. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 2007, 152, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Irving, A.; Abdulrazzaq, G.; Chan, S.L.F.; Penman, J.; Harvey, J.; Alexander, S.P.H. Cannabinoid Receptor-Related Orphan G Protein-Coupled Receptors. Adv. Pharmacol. 2017, 80, 223–247. [Google Scholar] [CrossRef] [PubMed]
- LoVerme, J.; Russo, R.; La Rana, G.; Fu, J.; Farthing, J.; Mattace-Raso, G.; Meli, R.; Hohmann, A.; Calignano, A.; Piomelli, D. Rapid broad-spectrum analgesia through activation of peroxisome proliferator-activated receptor-alpha. J. Pharmacol. Exp. Ther. 2006, 319, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.; Lo Verme, J.; Fu, J.; Oveisi, F.; Blazquez, C.; Piomelli, D. Oleoylethanolamide stimulates lipolysis by activating the nuclear receptor peroxisome proliferator-activated receptor alpha (PPAR-alpha). J. Biol. Chem. 2004, 279, 27849–27854. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.E.; Kendall, D.A. Cannabinoid activation of peroxisome proliferator-activated receptors: Potential for modulation of inflammatory disease. Immunobiology 2010, 215, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, L.A. Molecular aspects of cannabinoid receptors. Crit. Rev. Neurobiol. 1997, 11, 143–166. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Bennett, A. Cannabinoids: A new group of agonists of PPARs. PPAR Res. 2007, 2007, 23513. [Google Scholar] [CrossRef] [PubMed]
- Singh Tahim, A.; Santha, P.; Nagy, I. Inflammatory mediators convert anandamide into a potent activator of the vanilloid type 1 transient receptor potential receptor in nociceptive primary sensory neurons. Neuroscience 2005, 136, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P. The pharmacological challenge to tame the transient receptor potential vanilloid-1 (TRPV1) nocisensor. Br. J. Pharmacol. 2008, 155, 1145–1162. [Google Scholar] [CrossRef] [PubMed]
- Lutz, B. Neurobiology of cannabinoid receptor signaling. Dialogues Clin. Neurosci. 2020, 22, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Deadwyler, S.A.; Hampson, R.E.; Bennett, B.A.; Edwards, T.A.; Mu, J.; Pacheco, M.A.; Ward, S.J.; Childers, S.R. Cannabinoids modulate potassium current in cultured hippocampal neurons. Recept. Channels 1993, 1, 121–134. [Google Scholar] [PubMed]
- Gomez del Pulgar, T.; Velasco, G.; Guzman, M. The CB1 cannabinoid receptor is coupled to the activation of protein kinase B/Akt. Biochem. J. 2000, 347, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Galve-Roperh, I.; Rueda, D.; Gomez del Pulgar, T.; Velasco, G.; Guzman, M. Mechanism of extracellular signal-regulated kinase activation by the CB1 cannabinoid receptor. Mol. Pharmacol. 2002, 62, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Molina-Holgado, F.; Pinteaux, E.; Heenan, L.; Moore, J.D.; Rothwell, N.J.; Gibson, R.M. Neuroprotective effects of the synthetic cannabinoid HU-210 in primary cortical neurons are mediated by phosphatidylinositol 3-kinase/AKT signaling. Mol. Cell. Neurosci. 2005, 28, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Karanian, D.A.; Brown, Q.B.; Makriyannis, A.; Kosten, T.A.; Bahr, B.A. Dual modulation of endocannabinoid transport and fatty acid amide hydrolase protects against excitotoxicity. J. Neurosci. 2005, 25, 7813–7820. [Google Scholar] [CrossRef] [PubMed]
- Karanian, D.A.; Karim, S.L.; Wood, J.T.; Williams, J.S.; Lin, S.; Makriyannis, A.; Bahr, B.A. Endocannabinoid enhancement protects against kainic acid-induced seizures and associated brain damage. J. Pharmacol. Exp. Ther. 2007, 322, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Hajos, N.; Katona, I.; Naiem, S.S.; MacKie, K.; Ledent, C.; Mody, I.; Freund, T.F. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur. J. Neurosci. 2000, 12, 3239–3249. [Google Scholar] [CrossRef] [PubMed]
- Kreitzer, A.C.; Regehr, W.G. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron 2001, 29, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Ohno-Shosaku, T.; Maejima, T.; Kano, M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron 2001, 29, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.I.; Kunos, G.; Nicoll, R.A. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron 2001, 31, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Ozaita, A.; Puighermanal, E.; Maldonado, R. Regulation of PI3K/Akt/GSK-3 pathway by cannabinoids in the brain. J. Neurochem. 2007, 102, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Price, T.J.; Patwardhan, A.; Akopian, A.N.; Hargreaves, K.M.; Flores, C.M. Modulation of trigeminal sensory neuron activity by the dual cannabinoid-vanilloid agonists anandamide, N-arachidonoyl-dopamine and arachidonyl-2-chloroethylamide. Br. J. Pharmacol. 2004, 141, 1118–1130. [Google Scholar] [CrossRef]
- Fischbach, T.; Greffrath, W.; Nawrath, H.; Treede, R.D. Effects of anandamide and noxious heat on intracellular calcium concentration in nociceptive drg neurons of rats. J. Neurophysiol. 2007, 98, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.G.; Weinreich, D.; Undem, B.J. Effect of olvanil and anandamide on vagal C-fiber subtypes in guinea pig lung. Br. J. Pharmacol. 2005, 146, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, A.M.; Jeske, N.A.; Price, T.J.; Gamper, N.; Akopian, A.N.; Hargreaves, K.M. The cannabinoid WIN 55,212-2 inhibits transient receptor potential vanilloid 1 (TRPV1) and evokes peripheral antihyperalgesia via calcineurin. Proc. Natl. Acad. Sci. USA 2006, 103, 11393–11398. [Google Scholar] [CrossRef] [PubMed]
- Price, T.J.; Patwardhan, A.; Akopian, A.N.; Hargreaves, K.M.; Flores, C.M. Cannabinoid receptor-independent actions of the aminoalkylindole WIN 55,212-2 on trigeminal sensory neurons. Br. J. Pharmacol. 2004, 142, 257–266. [Google Scholar] [CrossRef] [PubMed]
- McCarberg, B.H.; Barkin, R.L. The future of cannabinoids as analgesic agents: A pharmacologic, pharmacokinetic, and pharmacodynamic overview. Am. J. Ther. 2007, 14, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Mbvundula, E.C.; Rainsford, K.D.; Bunning, R.A. Cannabinoids in pain and inflammation. Inflammopharmacology 2004, 12, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Jeske, N.A.; Patwardhan, A.M.; Gamper, N.; Price, T.J.; Akopian, A.N.; Hargreaves, K.M. Cannabinoid WIN 55,212-2 regulates TRPV1 phosphorylation in sensory neurons. J. Biol. Chem. 2006, 281, 32879–32890. [Google Scholar] [CrossRef] [PubMed]
- Jordt, S.E.; Bautista, D.M.; Chuang, H.H.; McKemy, D.D.; Zygmunt, P.M.; Hogestatt, E.D.; Meng, I.D.; Julius, D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 2004, 427, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lo, Y.; Chen, I.; Simon, S.A. The responses of rat trigeminal ganglion neurons to capsaicin and two nonpungent vanilloid receptor agonists, olvanil and glyceryl nonamide. J. Neurosci. 1997, 17, 4101–4111. [Google Scholar] [CrossRef] [PubMed]
- Ishac, E.J.; Jiang, L.; Lake, K.D.; Varga, K.; Abood, M.E.; Kunos, G. Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br. J. Pharmacol. 1996, 118, 2023–2028. [Google Scholar] [CrossRef] [PubMed]
- Kathmann, M.; Bauer, U.; Schlicker, E.; Gothert, M. Cannabinoid CB1 receptor-mediated inhibition of NMDA- and kainate-stimulated noradrenaline and dopamine release in the brain. Naunyn Schmiedeberg’s Arch. Pharmacol. 1999, 359, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Nakazi, M.; Bauer, U.; Nickel, T.; Kathmann, M.; Schlicker, E. Inhibition of serotonin release in the mouse brain via presynaptic cannabinoid CB1 receptors. Naunyn Schmiedeberg’s Arch. Pharmacol. 2000, 361, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.I.; Nicoll, R.A. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 2001, 410, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Piser, T.M.; Seybold, V.S.; Thayer, S.A. Cannabinoid receptor agonists inhibit glutamatergic synaptic transmission in rat hippocampal cultures. J. Neurosci. 1996, 16, 4322–4334. [Google Scholar] [CrossRef] [PubMed]
- Barann, M.; Molderings, G.; Bruss, M.; Bonisch, H.; Urban, B.W.; Gothert, M. Direct inhibition by cannabinoids of human 5-HT3A receptors: Probable involvement of an allosteric modulatory site. Br. J. Pharmacol. 2002, 137, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Weidenfeld, J.; Feldman, S.; Mechoulam, R. Effect of the brain constituent anandamide, a cannabinoid receptor agonist, on the hypothalamo-pituitary-adrenal axis in the rat. Neuroendocrinology 1994, 59, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Steiner, M.A.; Wotjak, C.T. Role of the endocannabinoid system in regulation of the hypothalamic-pituitary-adrenocortical axis. Prog. Brain Res. 2008, 170, 397–432. [Google Scholar] [CrossRef]
- Iversen, L.; Chapman, V. Cannabinoids: A real prospect for pain relief? Curr. Opin. Pharmacol. 2002, 2, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Welch, S.P.; Thomas, C.; Patrick, G.S. Modulation of cannabinoid-induced antinociception after intracerebroventricular versus intrathecal administration to mice: Possible mechanisms for interaction with morphine. J. Pharmacol. Exp. Ther. 1995, 272, 310–321. [Google Scholar] [PubMed]
- Martin, W.J.; Lai, N.K.; Patrick, S.L.; Tsou, K.; Walker, J.M. Antinociceptive actions of cannabinoids following intraventricular administration in rats. Brain Res. 1993, 629, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, A.G.; Tsou, K.; Walker, J.M. Cannabinoid suppression of noxious heat-evoked activity in wide dynamic range neurons in the lumbar dorsal horn of the rat. J. Neurophysiol. 1999, 81, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Raffa, R.B.; Stone, D.J., Jr.; Hipp, S.J. Differential cholera-toxin sensitivity of supraspinal antinociception induced by the cannabinoid agonists delta9-THC, WIN 55,212-2 and anandamide in mice. Neurosci. Lett. 1999, 263, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Strangman, N.M.; Patrick, S.L.; Hohmann, A.G.; Tsou, K.; Walker, J.M. Evidence for a role of endogenous cannabinoids in the modulation of acute and tonic pain sensitivity. Brain Res. 1998, 813, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Welch, S.P.; Huffman, J.W.; Lowe, J. Differential blockade of the antinociceptive effects of centrally administered cannabinoids by SR141716A. J. Pharmacol. Exp. Ther. 1998, 286, 1301–1308. [Google Scholar] [PubMed]
- Walker, J.M.; Huang, S.M.; Strangman, N.M.; Tsou, K.; Sanudo-Pena, M.C. Pain modulation by release of the endogenous cannabinoid anandamide. Proc. Natl. Acad. Sci. USA 1999, 96, 12198–12203. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.D.; Aanonsen, L.; Hargreaves, K.M. Antihyperalgesic effects of spinal cannabinoids. Eur. J. Pharmacol. 1998, 345, 145–153. [Google Scholar] [CrossRef]
- Morisset, V.; Ahluwalia, J.; Nagy, I.; Urban, L. Possible mechanisms of cannabinoid-induced antinociception in the spinal cord. Eur. J. Pharmacol. 2001, 429, 93–100. [Google Scholar] [CrossRef]
- Hohmann, A.G.; Tsou, K.; Walker, J.M. Cannabinoid modulation of wide dynamic range neurons in the lumbar dorsal horn of the rat by spinally administered WIN55,212-2. Neurosci. Lett. 1998, 257, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.D.; Aanonsen, L.; Hargreaves, K.M. Hypoactivity of the spinal cannabinoid system results in NMDA-dependent hyperalgesia. J. Neurosci. 1998, 18, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Romero-Sandoval, A.; Nutile-McMenemy, N.; DeLeo, J.A. Spinal microglial and perivascular cell cannabinoid receptor type 2 activation reduces behavioral hypersensitivity without tolerance after peripheral nerve injury. Anesthesiology 2008, 108, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Wu, Y.; Tian, Z.; Xu, Y.; Wu, C.; Wang, Z. Microglial Cannabinoid CB2 Receptors in Pain Modulation. Int. J. Mol. Sci. 2023, 24, 2348. [Google Scholar] [CrossRef] [PubMed]
- Lichtman, A.H.; Martin, B.R. Cannabinoid-induced antinociception is mediated by a spinal alpha 2-noradrenergic mechanism. Brain Res. 1991, 559, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Pugh, G., Jr.; Smith, P.B.; Dombrowski, D.S.; Welch, S.P. The role of endogenous opioids in enhancing the antinociception produced by the combination of delta9-tetrahydrocannabinol and morphine in the spinal cord. J. Pharmacol. Exp. Ther. 1996, 279, 608–616. [Google Scholar] [PubMed]
- Fox, A.; Kesingland, A.; Gentry, C.; McNair, K.; Patel, S.; Urban, L.; James, I. The role of central and peripheral Cannabinoid1 receptors in the antihyperalgesic activity of cannabinoids in a model of neuropathic pain. Pain 2001, 92, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Malan, T.P., Jr.; Ibrahim, M.M.; Lai, J.; Vanderah, T.W.; Makriyannis, A.; Porreca, F. CB2 cannabinoid receptor agonists: Pain relief without psychoactive effects? Curr. Opin. Pharmacol. 2003, 3, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Hanus, L.; Breuer, A.; Tchilibon, S.; Shiloah, S.; Goldenberg, D.; Horowitz, M.; Pertwee, R.G.; Ross, R.A.; Mechoulam, R.; Fride, E. HU-308: A specific agonist for CB2, a peripheral cannabinoid receptor. Proc. Natl. Acad. Sci. USA 1999, 96, 14228–14233. [Google Scholar] [CrossRef] [PubMed]
- Malan, T.P., Jr.; Ibrahim, M.M.; Vanderah, T.W.; Makriyannis, A.; Porreca, F. Inhibition of pain responses by activation of CB2 cannabinoid receptors. Chem. Phys. Lipids 2002, 121, 191–200. [Google Scholar] [CrossRef]
- Ross, R.A. Anandamide and vanilloid TRPV1 receptors. Br. J. Pharmacol. 2003, 140, 790–801. [Google Scholar] [CrossRef]
- Starowicz, K.; Finn, D.P. Cannabinoids and Pain: Sites and Mechanisms of Action. Adv. Pharmacol. 2017, 80, 437–475. [Google Scholar] [CrossRef] [PubMed]
- Durnett-Richardson, J. Cannabinoids Modulate Pain by Multiple Mechanisms of Action. J. Pain 2000, 1, 2–14. [Google Scholar] [CrossRef]
- Bahr, B.A.; Karanian, D.A.; Makanji, S.S.; Makriyannis, A. Targeting the endocannabinoid system in treating brain disorders. Expert Opin. Investig. Drugs 2006, 15, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Galve-Roperh, I.; Aguado, T.; Palazuelos, J.; Guzman, M. Mechanisms of control of neuron survival by the endocannabinoid system. Curr. Pharm. Des. 2008, 14, 2279–2288. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.J.; Blair, R.E.; Falenski, K.W.; Martin, B.R.; DeLorenzo, R.J. The endogenous cannabinoid system regulates seizure frequency and duration in a model of temporal lobe epilepsy. J. Pharmacol. Exp. Ther. 2003, 307, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Khaspekov, L.G.; Brenz Verca, M.S.; Frumkina, L.E.; Hermann, H.; Marsicano, G.; Lutz, B. Involvement of brain-derived neurotrophic factor in cannabinoid receptor-dependent protection against excitotoxicity. Eur. J. Neurosci. 2004, 19, 1691–1698. [Google Scholar] [CrossRef] [PubMed]
- Karanian, D.A.; Brown, Q.B.; Makriyannis, A.; Bahr, B.A. Blocking cannabinoid activation of FAK and ERK1/2 compromises synaptic integrity in hippocampus. Eur. J. Pharmacol. 2005, 508, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Schomacher, M.; Muller, H.D.; Sommer, C.; Schwab, S.; Schabitz, W.R. Endocannabinoids mediate neuroprotection after transient focal cerebral ischemia. Brain Res. 2008, 1240, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, A.G.; Suplita, R.L.; Bolton, N.M.; Neely, M.H.; Fegley, D.; Mangieri, R.; Krey, J.F.; Walker, J.M.; Holmes, P.V.; Crystal, J.D.; et al. An endocannabinoid mechanism for stress-induced analgesia. Nature 2005, 435, 1108–1112. [Google Scholar] [CrossRef] [PubMed]
- Meng, I.D.; Johansen, J.P. Antinociception and modulation of rostral ventromedial medulla neuronal activity by local microinfusion of a cannabinoid receptor agonist. Neuroscience 2004, 124, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Finn, D.P.; Jhaveri, M.D.; Beckett, S.R.; Roe, C.H.; Kendall, D.A.; Marsden, C.A.; Chapman, V. Effects of direct periaqueductal grey administration of a cannabinoid receptor agonist on nociceptive and aversive responses in rats. Neuropharmacology 2003, 45, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br. J. Pharmacol. 2009, 156, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V. Targeting the endocannabinoid system: To enhance or reduce? Nat. Rev. Drug Discov. 2008, 7, 438–455. [Google Scholar] [CrossRef] [PubMed]
- Izzo, A.A.; Camilleri, M. Emerging role of cannabinoids in gastrointestinal and liver diseases: Basic and clinical aspects. Gut 2008, 57, 1140–1155. [Google Scholar] [CrossRef]
- Kearn, C.S.; Blake-Palmer, K.; Daniel, E.; Mackie, K.; Glass, M. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation: A mechanism for receptor cross-talk? Mol. Pharmacol. 2005, 67, 1697–1704. [Google Scholar] [CrossRef] [PubMed]
- Carriba, P.; Ortiz, O.; Patkar, K.; Justinova, Z.; Stroik, J.; Themann, A.; Muller, C.; Woods, A.S.; Hope, B.T.; Ciruela, F.; et al. Striatal adenosine A2A and cannabinoid CB1 receptors form functional heteromeric complexes that mediate the motor effects of cannabinoids. Neuropsychopharmacology 2007, 32, 2249–2259. [Google Scholar] [CrossRef]
- Pertwee, R.G. The therapeutic potential of drugs that target cannabinoid receptors or modulate the tissue levels or actions of endocannabinoids. AAPS J. 2005, 7, E625–E654. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V. Endocannabinoids: Synthesis and degradation. Rev. Physiol. Biochem. Pharmacol. 2008, 160, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Katona, I.; Freund, T.F. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat. Med. 2008, 14, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Margraf, A.; Ludwig, N.; Zarbock, A.; Rossaint, J. Systemic Inflammatory Response Syndrome After Surgery: Mechanisms and Protection. Anesth. Analg. 2020, 131, 1693–1707. [Google Scholar] [CrossRef] [PubMed]
- Richebe, P.; Capdevila, X.; Rivat, C. Persistent Postsurgical Pain: Pathophysiology and Preventative Pharmacologic Considerations. Anesthesiology 2018, 129, 590–607. [Google Scholar] [CrossRef] [PubMed]
- Lavand’homme, P. The progression from acute to chronic pain. Curr. Opin. Anesthesiol. 2011, 24, 545–550. [Google Scholar] [CrossRef]
- Bouhassira, D.; Lanteri-Minet, M.; Attal, N.; Laurent, B.; Touboul, C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 2008, 136, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, D.C.; Pogatzki-Zahn, E.M. Chronic post-surgical pain-update on incidence, risk factors and preventive treatment options. BJA Educ. 2022, 22, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Kehlet, H.; Jensen, T.S.; Woolf, C.J. Persistent postsurgical pain: Risk factors and prevention. Lancet 2006, 367, 1618–1625. [Google Scholar] [CrossRef]
- Fowler, C.J. Possible involvement of the endocannabinoid system in the actions of three clinically used drugs. Trends Pharmacol. Sci. 2004, 25, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.B.; Compton, D.R.; Welch, S.P.; Razdan, R.K.; Mechoulam, R.; Martin, B.R. The pharmacological activity of anandamide, a putative endogenous cannabinoid, in mice. J. Pharmacol. Exp. Ther. 1994, 270, 219–227. [Google Scholar] [PubMed]
- Calignano, A.; La Rana, G.; Giuffrida, A.; Piomelli, D. Control of pain initiation by endogenous cannabinoids. Nature 1998, 394, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.T.; Mackie, K.; Chiou, L.C. Alternative pain management via endocannabinoids in the time of the opioid epidemic: Peripheral neuromodulation and pharmacological interventions. Br. J. Pharmacol. 2023, 180, 894–909. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Urits, I.; Orhurhu, V.; Peck, J.; Orhurhu, M.S.; Giacomazzi, S.; Smoots, D.; Piermarini, C.; Manchikanti, L.; Kaye, A.D.; et al. The Role of the Cannabinoid System in Pain Control: Basic and Clinical Implications. Curr. Pain Headache Rep. 2020, 24, 35. [Google Scholar] [CrossRef] [PubMed]
- Haller, V.L.; Cichewicz, D.L.; Welch, S.P. Non-cannabinoid CB1, non-cannabinoid CB2 antinociceptive effects of several novel compounds in the PPQ stretch test in mice. Eur. J. Pharmacol. 2006, 546, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, N.; Pacher, P.; Tegeder, I.; Amaya, F.; Constantin, C.E.; Brenner, G.J.; Rubino, T.; Michalski, C.W.; Marsicano, G.; Monory, K.; et al. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat. Neurosci. 2007, 10, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Maione, S.; De Petrocellis, L.; de Novellis, V.; Moriello, A.S.; Petrosino, S.; Palazzo, E.; Rossi, F.S.; Woodward, D.F.; Di Marzo, V. Analgesic actions of N-arachidonoyl-serotonin, a fatty acid amide hydrolase inhibitor with antagonistic activity at vanilloid TRPV1 receptors. Br. J. Pharmacol. 2007, 150, 766–781. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.; Trovato, A.E.; Colleoni, M.; Giagnoni, G.; Zarini, E.; Croci, T. Effect of the cannabinoid CB1 receptor antagonist, SR141716, on nociceptive response and nerve demyelination in rodents with chronic constriction injury of the sciatic nerve. Pain 2005, 116, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Saez-Cassanelli, J.L.; Fontanella, G.H.; Delgado-Garcia, J.M.; Carrion, A.M. Functional blockage of the cannabinoid receptor type 1 evokes a kappa-opiate-dependent analgesia. J. Neurochem. 2007, 103, 2629–2639. [Google Scholar] [CrossRef] [PubMed]
- Lunn, C.A.; Fine, J.S.; Rojas-Triana, A.; Jackson, J.V.; Fan, X.; Kung, T.T.; Gonsiorek, W.; Schwarz, M.A.; Lavey, B.; Kozlowski, J.A.; et al. A novel cannabinoid peripheral cannabinoid receptor-selective inverse agonist blocks leukocyte recruitment in vivo. J. Pharmacol. Exp. Ther. 2006, 316, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Croci, T.; Zarini, E. Effect of the cannabinoid CB1 receptor antagonist rimonabant on nociceptive responses and adjuvant-induced arthritis in obese and lean rats. Br. J. Pharmacol. 2007, 150, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Oka, S.; Wakui, J.; Ikeda, S.; Yanagimoto, S.; Kishimoto, S.; Gokoh, M.; Nasui, M.; Sugiura, T. Involvement of the cannabinoid CB2 receptor and its endogenous ligand 2-arachidonoylglycerol in oxazolone-induced contact dermatitis in mice. J. Immunol. 2006, 177, 8796–8805. [Google Scholar] [CrossRef] [PubMed]
- Azim, S.; Nicholson, J.; Rebecchi, M.J.; Galbavy, W.; Feng, T.; Reinsel, R.; Volkow, N.D.; Benveniste, H.; Kaczocha, M. Endocannabinoids and acute pain after total knee arthroplasty. Pain 2015, 156, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. Cannabinoid receptors and pain. Prog. Neurobiol. 2001, 63, 569–611. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.D.; Kilo, S.; Hargreaves, K.M. Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CB1 receptors. Pain 1998, 75, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.J.; Loo, C.M.; Basbaum, A.I. Spinal cannabinoids are anti-allodynic in rats with persistent inflammation. Pain 1999, 82, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Rahn, E.J.; Hohmann, A.G. Cannabinoids as pharmacotherapies for neuropathic pain: From the bench to the bedside. Neurotherapeutics 2009, 6, 713–737. [Google Scholar] [CrossRef] [PubMed]
- Bloom, A.S.; Dewey, W.L.; Harris, L.S.; Brosius, K.K. 9-nor-9beta-hydroxyhexahydrocannabinol, a cannabinoid with potent antinociceptive activity: Comparisons with morphine. J. Pharmacol. Exp. Ther. 1977, 200, 263–270. [Google Scholar] [PubMed]
- Buxbaum, D.M. Analgesic activity of 9 -tetrahydrocannabinol in the rat and mouse. Psychopharmacologia 1972, 25, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.J.; Ramabadran, K.; Campos-Medeiros, M. A pharmacological analysis of levonantradol antinociception in mice. J. Clin. Pharmacol. 1981, 21, 327S–333S. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.M.; Hohmann, A.G.; Martin, W.J.; Strangman, N.M.; Huang, S.M.; Tsou, K. The neurobiology of cannabinoid analgesia. Life Sci. 1999, 65, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Alsalem, M.; Altarifi, A.; Haddad, M.; Aldossary, S.A.; Kalbouneh, H.; Aldaoud, N.; Saleh, T.; El-Salem, K. Antinociceptive and Abuse Potential Effects of Cannabinoid/Opioid Combinations in a Chronic Pain Model in Rats. Brain Sci. 2019, 9, 328. [Google Scholar] [CrossRef] [PubMed]
- Maguire, D.R.; France, C.P. Antinociceptive effects of mixtures of mu opioid receptor agonists and cannabinoid receptor agonists in rats: Impact of drug and fixed-dose ratio. Eur. J. Pharmacol. 2018, 819, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Welch, S.P.; Stevens, D.L. Antinociceptive activity of intrathecally administered cannabinoids alone, and in combination with morphine, in mice. J. Pharmacol. Exp. Ther. 1992, 262, 10–18. [Google Scholar] [PubMed]
- Bloom, A.S.; Dewey, W.L. A comparison of some pharmacological actions of morphine and delta9-tetrahydrocannabinol in the mouse. Psychopharmacology 1978, 57, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Lichtman, A.H.; Smith, F.L.; Martin, B.R. Evidence that the antinociceptive tail-flick response is produced independently from changes in either tail-skin temperature or core temperature. Pain 1993, 55, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.B.; Martin, B.R. Spinal mechanisms of delta9-tetrahydrocannabinol-induced analgesia. Brain Res. 1992, 578, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Thorat, S.N.; Bhargava, H.N. Evidence for a bidirectional cross-tolerance between morphine and delta9-tetrahydrocannabinol in mice. Eur. J. Pharmacol. 1994, 260, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Cravatt, B.F.; Lichtman, A.H. The endogenous cannabinoid system and its role in nociceptive behavior. J. Neurobiol. 2004, 61, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Goya, P.; Jagerovic, N.; Hernandez-Folgado, L.; Martin, M.I. Cannabinoids and neuropathic pain. Mini Rev. Med. Chem. 2003, 3, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Bisogno, T.; Maurelli, S.; Melck, D.; De Petrocellis, L.; Di Marzo, V. Biosynthesis, uptake, and degradation of anandamide and palmitoylethanolamide in leukocytes. J. Biol. Chem. 1997, 272, 3315–3323. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Blumberg, P.M.; Szallasi, A. Endovanilloid signaling in pain. Curr. Opin. Neurobiol. 2002, 12, 372–379. [Google Scholar] [CrossRef]
- Anand, U.; Otto, W.R.; Sanchez-Herrera, D.; Facer, P.; Yiangou, Y.; Korchev, Y.; Birch, R.; Benham, C.; Bountra, C.; Chessell, I.P.; et al. Cannabinoid receptor CB2 localisation and agonist-mediated inhibition of capsaicin responses in human sensory neurons. Pain 2008, 138, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Aviram, J.; Samuelly-Leichtag, G. Efficacy of Cannabis-Based Medicines for Pain Management: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pain Physician 2017, 20, E755–E796. [Google Scholar] [CrossRef] [PubMed]
- Petzke, F.; Tolle, T.; Fitzcharles, M.A.; Hauser, W. Cannabis-Based Medicines and Medical Cannabis for Chronic Neuropathic Pain. CNS Drugs 2022, 36, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B. History of cannabis and its preparations in saga, science, and sobriquet. Chem. Biodivers. 2007, 4, 1614–1648. [Google Scholar] [CrossRef] [PubMed]
- Buggy, D.J.; Toogood, L.; Maric, S.; Sharpe, P.; Lambert, D.G.; Rowbotham, D.J. Lack of analgesic efficacy of oral delta-9-tetrahydrocannabinol in postoperative pain. Pain 2003, 106, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Kraft, B.; Frickey, N.A.; Kaufmann, R.M.; Reif, M.; Frey, R.; Gustorff, B.; Kress, H.G. Lack of analgesia by oral standardized cannabis extract on acute inflammatory pain and hyperalgesia in volunteers. Anesthesiology 2008, 109, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Naef, M.; Curatolo, M.; Petersen-Felix, S.; Arendt-Nielsen, L.; Zbinden, A.; Brenneisen, R. The analgesic effect of oral delta-9-tetrahydrocannabinol (THC), morphine, and a THC-morphine combination in healthy subjects under experimental pain conditions. Pain 2003, 105, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Naef, M.; Russmann, S.; Petersen-Felix, S.; Brenneisen, R. Development and pharmacokinetic characterization of pulmonal and intravenous delta-9-tetrahydrocannabinol (THC) in humans. J. Pharm. Sci. 2004, 93, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.; Schulteis, G.; Atkinson, J.H.; Wolfson, T.; Lazzaretto, D.; Bentley, H.; Gouaux, B.; Abramson, I. Dose-dependent effects of smoked cannabis on capsaicin-induced pain and hyperalgesia in healthy volunteers. Anesthesiology 2007, 107, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Campbell, F.A.; Tramer, M.R.; Carroll, D.; Reynolds, D.J.; Moore, R.A.; McQuay, H.J. Are cannabinoids an effective and safe treatment option in the management of pain? A qualitative systematic review. BMJ 2001, 323, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Fitzcharles, M.A.; Baerwald, C.; Ablin, J.; Hauser, W. Efficacy, tolerability and safety of cannabinoids in chronic pain associated with rheumatic diseases (fibromyalgia syndrome, back pain, osteoarthritis, rheumatoid arthritis): A systematic review of randomized controlled trials. Schmerz 2016, 30, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Mucke, M.; Phillips, T.; Radbruch, L.; Petzke, F.; Hauser, W. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2018, 3, CD012182. [Google Scholar] [CrossRef] [PubMed]
- Phillips, T.J.; Cherry, C.L.; Cox, S.; Marshall, S.J.; Rice, A.S. Pharmacological treatment of painful HIV-associated sensory neuropathy: A systematic review and meta-analysis of randomised controlled trials. PLoS ONE 2010, 5, e14433. [Google Scholar] [CrossRef] [PubMed]
- Stockings, E.; Campbell, G.; Hall, W.D.; Nielsen, S.; Zagic, D.; Rahman, R.; Murnion, B.; Farrell, M.; Weier, M.; Degenhardt, L. Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: A systematic review and meta-analysis of controlled and observational studies. Pain 2018, 159, 1932–1954. [Google Scholar] [CrossRef] [PubMed]
- Johal, H.; Devji, T.; Chang, Y.; Simone, J.; Vannabouathong, C.; Bhandari, M. Cannabinoids in Chronic Non-Cancer Pain: A Systematic Review and Meta-Analysis. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2020, 13, 1179544120906461. [Google Scholar] [CrossRef] [PubMed]
- Fisher, E.; Moore, R.A.; Fogarty, A.E.; Finn, D.P.; Finnerup, N.B.; Gilron, I.; Haroutounian, S.; Krane, E.; Rice, A.S.C.; Rowbotham, M.; et al. Cannabinoids, cannabis, and cannabis-based medicine for pain management: A systematic review of randomised controlled trials. Pain 2021, 162, S45–S66. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Gordon, D.B.; de Leon-Casasola, O.A.; Rosenberg, J.M.; Bickler, S.; Brennan, T.; Carter, T.; Cassidy, C.L.; Chittenden, E.H.; Degenhardt, E.; et al. Management of Postoperative Pain: A Clinical Practice Guideline From the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J. Pain 2016, 17, 131–157. [Google Scholar] [CrossRef] [PubMed]
- Inturrisi, C.E. Clinical pharmacology of opioids for pain. Clin. J. Pain 2002, 18, S3–S13. [Google Scholar] [CrossRef] [PubMed]
- Oderda, G.M.; Gan, T.J.; Johnson, B.H.; Robinson, S.B. Effect of opioid-related adverse events on outcomes in selected surgical patients. J. Pain Palliat. Care Pharmacother. 2013, 27, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Kirksey, M.A.; Duong, S.; Wu, C.L. A Review of Opioid-Sparing Modalities in Perioperative Pain Management: Methods to Decrease Opioid Use Postoperatively. Anesth. Analg. 2017, 125, 1749–1760. [Google Scholar] [CrossRef] [PubMed]
- Weber, L.; Yeomans, D.C.; Tzabazis, A. Opioid-induced hyperalgesia in clinical anesthesia practice: What has remained from theoretical concepts and experimental studies? Curr. Opin. Anesthesiol. 2017, 30, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.A.; Hedrick, T.L.; Jayaram, J.; Argoff, C.; Gulur, P.; Holubar, S.D.; Gan, T.J.; Mythen, M.G.; Miller, T.E.; Shaw, A.D.; et al. American Society for Enhanced Recovery and Perioperative Quality Initiative Joint Consensus Statement on Perioperative Management of Patients on Preoperative Opioid Therapy. Anesth. Analg. 2019, 129, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.A.; Caplan, R.A.; Stephens, L.S.; Posner, K.L.; Terman, G.W.; Voepel-Lewis, T.; Domino, K.B. Postoperative opioid-induced respiratory depression: A closed claims analysis. Anesthesiology 2015, 122, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Nagappa, M.; Weingarten, T.N.; Montandon, G.; Sprung, J.; Chung, F. Opioids, respiratory depression, and sleep-disordered breathing. Best Pract. Res. Clin. Anaesthesiol. 2017, 31, 469–485. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.I.; Nagarekha, D.; Hegade, G.; Marutheesh, M. Postoperative nausea and vomiting: A simple yet complex problem. Anesth. Essays Res. 2016, 10, 388–396. [Google Scholar] [CrossRef] [PubMed]
- de Boer, H.D.; Detriche, O.; Forget, P. Opioid-related side effects: Postoperative ileus, urinary retention, nausea and vomiting, and shivering. A review of the literature. Best Pract. Res. Clin. Anaesthesiol. 2017, 31, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Dorn, S.; Lembo, A.; Cremonini, F. Opioid-induced bowel dysfunction: Epidemiology, pathophysiology, diagnosis, and initial therapeutic approach. Am. J. Gastroenterol. Suppl. 2014, 2, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Farmer, A.D.; Holt, C.B.; Downes, T.J.; Ruggeri, E.; Del Vecchio, S.; De Giorgio, R. Pathophysiology, diagnosis, and management of opioid-induced constipation. Lancet Gastroenterol. Hepatol. 2018, 3, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Kurz, A.; Sessler, D.I. Opioid-induced bowel dysfunction: Pathophysiology and potential new therapies. Drugs 2003, 63, 649–671. [Google Scholar] [CrossRef] [PubMed]
- Verhamme, K.M.; Sturkenboom, M.C.; Stricker, B.H.; Bosch, R. Drug-induced urinary retention: Incidence, management and prevention. Drug Saf. 2008, 31, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Devlin, J.W.; Roberts, R.J. Pharmacology of commonly used analgesics and sedatives in the ICU: Benzodiazepines, propofol, and opioids. Crit. Care Clin. 2009, 25, 431–449, vii. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.; Oderda, G.M.; Ashburn, M.A.; Lipman, A.G. Adverse events associated with postoperative opioid analgesia: A systematic review. J. Pain 2002, 3, 159–180. [Google Scholar] [CrossRef] [PubMed]
- Athanasos, P.; Smith, C.S.; White, J.M.; Somogyi, A.A.; Bochner, F.; Ling, W. Methadone maintenance patients are cross-tolerant to the antinociceptive effects of very high plasma morphine concentrations. Pain 2006, 120, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Chia, Y.Y.; Liu, K.; Wang, J.J.; Kuo, M.C.; Ho, S.T. Intraoperative high dose fentanyl induces postoperative fentanyl tolerance. Can. J. Anesth. 1999, 46, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Guignard, B.; Bossard, A.E.; Coste, C.; Sessler, D.I.; Lebrault, C.; Alfonsi, P.; Fletcher, D.; Chauvin, M. Acute opioid tolerance: Intraoperative remifentanil increases postoperative pain and morphine requirement. Anesthesiology 2000, 93, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Joseph, E.K.; Reichling, D.B.; Levine, J.D. Shared mechanisms for opioid tolerance and a transition to chronic pain. J. Neurosci. 2010, 30, 4660–4666. [Google Scholar] [CrossRef] [PubMed]
- Vinik, H.R.; Kissin, I. Rapid development of tolerance to analgesia during remifentanil infusion in humans. Anesth. Analg. 1998, 86, 1307–1311. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.; Bigelow, G.E.; Stitzer, M.L.; Liebson, I.A. Acute physical dependence in humans: Repeated naloxone-precipitated withdrawal after a single dose of methadone. Drug Alcohol Depend. 1991, 27, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Angst, M.S.; Clark, J.D. Opioid-induced hyperalgesia: A qualitative systematic review. Anesthesiology 2006, 104, 570–587. [Google Scholar] [CrossRef] [PubMed]
- Angst, M.S.; Koppert, W.; Pahl, I.; Clark, D.J.; Schmelz, M. Short-term infusion of the mu-opioid agonist remifentanil in humans causes hyperalgesia during withdrawal. Pain 2003, 106, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.F.; Clark, D.J.; Angst, M.S. Opioid tolerance and hyperalgesia in chronic pain patients after one month of oral morphine therapy: A preliminary prospective study. J. Pain 2006, 7, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, D.; Martinez, V. Opioid-induced hyperalgesia in patients after surgery: A systematic review and a meta-analysis. Br. J. Anaesth. 2014, 112, 991–1004. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Gomes, T.; Zheng, H.; Mamdani, M.M.; Juurlink, D.N.; Bell, C.M. Long-term analgesic use after low-risk surgery: A retrospective cohort study. Arch. Intern. Med. 2012, 172, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Bateman, B.T.; Franklin, J.M.; Bykov, K.; Avorn, J.; Shrank, W.H.; Brennan, T.A.; Landon, J.E.; Rathmell, J.P.; Huybrechts, K.F.; Fischer, M.A.; et al. Persistent opioid use following cesarean delivery: Patterns and predictors among opioid-naive women. Am. J. Obstet. Gynecol. 2016, 215, 353.e1–353.e18. [Google Scholar] [CrossRef] [PubMed]
- Bates, C.; Laciak, R.; Southwick, A.; Bishoff, J. Overprescription of postoperative narcotics: A look at postoperative pain medication delivery, consumption and disposal in urological practice. J. Urol. 2011, 185, 551–555. [Google Scholar] [CrossRef]
- Dowell, D.; Haegerich, T.M.; Chou, R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. JAMA 2016, 315, 1624–1645. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.; Cunningham, K.; Fitzgerald, K.; Finnerty, E. Opioid consumption following outpatient upper extremity surgery. J. Hand Surg. 2012, 37, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Curatolo, M.; Sveticic, G. Drug combinations in pain treatment: A review of the published evidence and a method for finding the optimal combination. Best Pract. Res. Clin. Anaesthesiol. 2002, 16, 507–519. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, A.; Lirk, P. Multimodal Analgesia. Anesthesiol. Clin. 2022, 40, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Alger, B.E. Retrograde signaling in the regulation of synaptic transmission: Focus on endocannabinoids. Prog. Neurobiol. 2002, 68, 247–286. [Google Scholar] [CrossRef] [PubMed]
- Kano, M.; Ohno-Shosaku, T.; Hashimotodani, Y.; Uchigashima, M.; Watanabe, M. Endocannabinoid-mediated control of synaptic transmission. Physiol. Rev. 2009, 89, 309–380. [Google Scholar] [CrossRef] [PubMed]
- Schlicker, E.; Kathmann, M. Modulation of transmitter release via presynaptic cannabinoid receptors. Trends Pharmacol. Sci. 2001, 22, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Price, D.D.; Lu, J.; Keniston, L.; Mayer, D.J. Two distinctive antinociceptive systems in rats with pathological pain. Neurosci. Lett. 2000, 280, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, J.; Corchero, J.; Romero, J.; Fernandez-Ruiz, J.J.; Ramos, J.A.; Fuentes, J.A. Pharmacological and biochemical interactions between opioids and cannabinoids. Trends Pharmacol. Sci. 1999, 20, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Fontana, A.; Cadas, H.; Schinelli, S.; Cimino, G.; Schwartz, J.C.; Piomelli, D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature 1994, 372, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Cadas, H.; Gaillet, S.; Beltramo, M.; Venance, L.; Piomelli, D. Biosynthesis of an endogenous cannabinoid precursor in neurons and its control by calcium and cAMP. J. Neurosci. 1996, 16, 3934–3942. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.; Siniscalco, D.; Trovato, A.E.; Comelli, F.; Sotgiu, M.L.; Colleoni, M.; Maione, S.; Rossi, F.; Giagnoni, G. AM404, an inhibitor of anandamide uptake, prevents pain behaviour and modulates cytokine and apoptotic pathways in a rat model of neuropathic pain. Br. J. Pharmacol. 2006, 148, 1022–1032. [Google Scholar] [CrossRef]
- Hojo, M.; Sudo, Y.; Ando, Y.; Minami, K.; Takada, M.; Matsubara, T.; Kanaide, M.; Taniyama, K.; Sumikawa, K.; Uezono, Y. mu-Opioid receptor forms a functional heterodimer with cannabinoid CB1 receptor: Electrophysiological and FRET assay analysis. J. Pharmacol. Sci. 2008, 108, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Babalonis, S.; Walsh, S.L. Therapeutic potential of opioid/cannabinoid combinations in humans: Review of the evidence. Eur. Neuropsychopharmacol. 2020, 36, 206–216. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Peigneur, S.; Hendrickx, L.A.; Tytgat, J. Targeting Cannabinoid Receptors: Current Status and Prospects of Natural Products. Int. J. Mol. Sci. 2020, 21, 5064. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.B.; Mukherjee, S.; Fan, Y.; Garrison, T.R.; Daza, A.V.; Grayson, G.K.; Hooker, B.A.; Dart, M.J.; Sullivan, J.P.; Meyer, M.D. In vitro pharmacological characterization of AM1241: A protean agonist at the cannabinoid CB2 receptor? Br. J. Pharmacol. 2006, 149, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.C.; Mackie, K. An Introduction to the Endogenous Cannabinoid System. Biol. Psychiatry 2016, 79, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Cichewicz, D.L.; Martin, Z.L.; Smith, F.L.; Welch, S.P. Enhancement mu opioid antinociception by oral delta9-tetrahydrocannabinol: Dose-response analysis and receptor identification. J. Pharmacol. Exp. Ther. 1999, 289, 859–867. [Google Scholar] [PubMed]
- Fuentes, J.A.; Ruiz-Gayo, M.; Manzanares, J.; Vela, G.; Reche, I.; Corchero, J. Cannabinoids as potential new analgesics. Life Sci. 1999, 65, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Bhattacharya, S.K. Cannabis-induced potentiation of morphine analgesia in rat--role of brain monoamines. Indian J. Med. Res. 1979, 70, 275–280. [Google Scholar] [PubMed]
- Reche, I.; Fuentes, J.A.; Ruiz-Gayo, M. Potentiation of delta 9-tetrahydrocannabinol-induced analgesia by morphine in mice: Involvement of mu- and kappa-opioid receptors. Eur. J. Pharmacol. 1996, 318, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.L.; Cichewicz, D.; Martin, Z.L.; Welch, S.P. The enhancement of morphine antinociception in mice by delta9-tetrahydrocannabinol. Pharmacol. Biochem. Behav. 1998, 60, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Cichewicz, D.L.; Haller, V.L.; Welch, S.P. Changes in opioid and cannabinoid receptor protein following short-term combination treatment with delta9-tetrahydrocannabinol and morphine. J. Pharmacol. Exp. Ther. 2001, 297, 121–127. [Google Scholar] [PubMed]
- Reche, I.; Ruiz-Gayo, M.; Fuentes, J.A. Inhibition of opioid-degrading enzymes potentiates delta9-tetrahydrocannabinol-induced antinociception in mice. Neuropharmacology 1998, 37, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Yesilyurt, O.; Dogrul, A.; Gul, H.; Seyrek, M.; Kusmez, O.; Ozkan, Y.; Yildiz, O. Topical cannabinoid enhances topical morphine antinociception. Pain 2003, 105, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Levin, D.N.; Dulberg, Z.; Chan, A.W.; Hare, G.M.; Mazer, C.D.; Hong, A. A randomized-controlled trial of nabilone for the prevention of acute postoperative nausea and vomiting in elective surgery. Can. J. Anesth. 2017, 64, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Seeling, W.; Kneer, L.; Buchele, B.; Gschwend, J.E.; Maier, L.; Nett, C.; Simmet, T.; Steffen, P.; Schneider, M.; Rockemann, M. [Delta9-tetrahydrocannabinol and the opioid receptor agonist piritramide do not act synergistically in postoperative pain]. Anaesthesist 2006, 55, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Miranda, H.F.; Sierralta, F.; Pinardi, G. Neostigmine interactions with non steroidal anti-inflammatory drugs. Br. J. Pharmacol. 2002, 135, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.A.; Warner, T.D. Cyclo-oxygenase-2: Pharmacology, physiology, biochemistry and relevance to NSAID therapy. Br. J. Pharmacol. 1999, 128, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.L.; DeWitt, D.L.; Garavito, R.M. Cyclooxygenases: Structural, cellular, and molecular biology. Annu. Rev. Biochem. 2000, 69, 145–182. [Google Scholar] [CrossRef] [PubMed]
- Warner, T.D.; Mitchell, J.A. Cyclooxygenases: New forms, new inhibitors, and lessons from the clinic. FASEB J. 2004, 18, 790–804. [Google Scholar] [CrossRef] [PubMed]
- Miranda, H.F.; Puig, M.M.; Prieto, J.C.; Pinardi, G. Synergism between paracetamol and nonsteroidal anti-inflammatory drugs in experimental acute pain. Pain 2006, 121, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Fimiani, C.; Liberty, T.; Aquirre, A.J.; Amin, I.; Ali, N.; Stefano, G.B. Opiate, cannabinoid, and eicosanoid signaling converges on common intracellular pathways nitric oxide coupling. Prostaglandins Other Lipid Mediat. 1999, 57, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Reichman, M.; Nen, W.; Hokin, L.E. Delta9-tetrahydrocannabinol increases arachidonic acid levels in guinea pig cerebral cortex slices. Mol. Pharmacol. 1988, 34, 823–828. [Google Scholar] [PubMed]
- Guindon, J.; Beaulieu, P. Antihyperalgesic effects of local injections of anandamide, ibuprofen, rofecoxib and their combinations in a model of neuropathic pain. Neuropharmacology 2006, 50, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Guindon, J.; De Lean, A.; Beaulieu, P. Local interactions between anandamide, an endocannabinoid, and ibuprofen, a nonsteroidal anti-inflammatory drug, in acute and inflammatory pain. Pain 2006, 121, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Ulugol, A.; Ozyigit, F.; Yesilyurt, O.; Dogrul, A. The additive antinociceptive interaction between WIN 55,212-2, a cannabinoid agonist, and ketorolac. Anesth. Analg. 2006, 102, 443–447. [Google Scholar] [CrossRef]
- Diaz-Reval, M.I.; Cardenas, Y.; Huerta, M.; Trujillo, X.; Sanchez-Pastor, E.A.; Gonzalez-Trujano, M.E.; Virgen-Ortiz, A.; Perez-Hernandez, M.G. Activation of Peripheral Cannabinoid Receptors Synergizes the Effect of Systemic Ibuprofen in a Pain Model in Rat. Pharmaceuticals 2022, 15, 910. [Google Scholar] [CrossRef] [PubMed]
- Paunescu, H.; Coman, O.A.; Coman, L.; Ghita, I.; Georgescu, S.R.; Draghia, F.; Fulga, I. Cannabinoid system and cyclooxygenases inhibitors. J. Med. Life 2011, 4, 11–20. [Google Scholar] [PubMed]
- Klinger-Gratz, P.P.; Ralvenius, W.T.; Neumann, E.; Kato, A.; Nyilas, R.; Lele, Z.; Katona, I.; Zeilhofer, H.U. Acetaminophen Relieves Inflammatory Pain through CB1 Cannabinoid Receptors in the Rostral Ventromedial Medulla. J. Neurosci. 2018, 38, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Mallet, C.; Daulhac, L.; Bonnefont, J.; Ledent, C.; Etienne, M.; Chapuy, E.; Libert, F.; Eschalier, A. Endocannabinoid and serotonergic systems are needed for acetaminophen-induced analgesia. Pain 2008, 139, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Hama, A.T.; Sagen, J. Cannabinoid receptor-mediated antinociception with acetaminophen drug combinations in rats with neuropathic spinal cord injury pain. Neuropharmacology 2010, 58, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Ottani, A.; Leone, S.; Sandrini, M.; Ferrari, A.; Bertolini, A. The analgesic activity of paracetamol is prevented by the blockade of cannabinoid CB1 receptors. Eur. J. Pharmacol. 2006, 531, 280–281. [Google Scholar] [CrossRef] [PubMed]
- Dani, M.; Guindon, J.; Lambert, C.; Beaulieu, P. The local antinociceptive effects of paracetamol in neuropathic pain are mediated by cannabinoid receptors. Eur. J. Pharmacol. 2007, 573, 214–215. [Google Scholar] [CrossRef] [PubMed]
- Mallet, C.; Barriere, D.A.; Ermund, A.; Jonsson, B.A.; Eschalier, A.; Zygmunt, P.M.; Hogestatt, E.D. TRPV1 in brain is involved in acetaminophen-induced antinociception. PLoS ONE 2010, 5, e12748. [Google Scholar] [CrossRef]
- Hogestatt, E.D.; Jonsson, B.A.; Ermund, A.; Andersson, D.A.; Bjork, H.; Alexander, J.P.; Cravatt, B.F.; Basbaum, A.I.; Zygmunt, P.M. Conversion of acetaminophen to the bioactive N-acylphenolamine AM404 via fatty acid amide hydrolase-dependent arachidonic acid conjugation in the nervous system. J. Biol. Chem. 2005, 280, 31405–31412. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, J.; Urban, L.; Capogna, M.; Bevan, S.; Nagy, I. Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience 2000, 100, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Farquhar-Smith, W.P.; Egertova, M.; Bradbury, E.J.; McMahon, S.B.; Rice, A.S.; Elphick, M.R. Cannabinoid CB1 receptor expression in rat spinal cord. Mol. Cell. Neurosci. 2000, 15, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Salio, C.; Doly, S.; Fischer, J.; Franzoni, M.F.; Conrath, M. Neuronal and astrocytic localization of the cannabinoid receptor-1 in the dorsal horn of the rat spinal cord. Neurosci. Lett. 2002, 329, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Szallasi, A.; Blumberg, P.M. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999, 51, 159–212. [Google Scholar] [PubMed]
- Kang, S.; Kim, C.H.; Lee, H.; Kim, D.Y.; Han, J.I.; Chung, R.K.; Lee, G.Y. Antinociceptive synergy between the cannabinoid receptor agonist WIN 55,212-2 and bupivacaine in the rat formalin test. Anesth. Analg. 2007, 104, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Tonner, P.H. Additives used to reduce perioperative opioid consumption 1: Alpha2-agonists. Best Pract. Res. Clin. Anaesthesiol. 2017, 31, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Boyd, R.E. Alpha2-adrenergic receptor agonists as analgesics. Curr. Top. Med. Chem. 2001, 1, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Giovannitti, J.A., Jr.; Thoms, S.M.; Crawford, J.J. Alpha-2 adrenergic receptor agonists: A review of current clinical applications. Anesth. Prog. 2015, 62, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.L.; Wu, Z.Z.; Zhou, H.Y.; Chen, S.R.; Zhang, H.M.; Li, D.P. Modulation of pain transmission by G-protein-coupled receptors. Pharmacol. Ther. 2008, 117, 141–161. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, J.; Julian, M.; Carrascosa, A. Role of the cannabinoid system in pain control and therapeutic implications for the management of acute and chronic pain episodes. Curr. Neuropharmacol. 2006, 4, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Holdcroft, A.; Maze, M.; Dore, C.; Tebbs, S.; Thompson, S. A multicenter dose-escalation study of the analgesic and adverse effects of an oral cannabis extract (Cannador) for postoperative pain management. Anesthesiology 2006, 104, 1040–1046. [Google Scholar] [CrossRef]
- Guillaud, J.; Legagneux, F.; Paulet, C.; Leoni, J.; Lassner, J. Essai du lvonantradol pour l’analgsie postopratoire. Cah. D’anesthsiologie 1983, 31, 243–248. [Google Scholar]
- Jain, A.K.; Ryan, J.R.; McMahon, F.G.; Smith, G. Evaluation of intramuscular levonantradol and placebo in acute postoperative pain. J. Clin. Pharmacol. 1981, 21, 320S–326S. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, P. Effects of nabilone, a synthetic cannabinoid, on postoperative pain. Can. J. Anesth. 2006, 53, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Hickernell, T.R.; Lakra, A.; Berg, A.; Cooper, H.J.; Geller, J.A.; Shah, R.P. Should Cannabinoids Be Added to Multimodal Pain Regimens After Total Hip and Knee Arthroplasty? J. Arthroplast. 2018, 33, 3637–3641. [Google Scholar] [CrossRef] [PubMed]
- Alaia, M.J.; Li, Z.I.; Chalem, I.; Hurley, E.T.; Vasavada, K.; Gonzalez-Lomas, G.; Rokito, A.S.; Jazrawi, L.M.; Kaplan, K. Cannabidiol for Postoperative Pain Control After Arthroscopic Rotator Cuff Repair Demonstrates No Deficits in Patient-Reported Outcomes Versus Placebo: 1-Year Follow-up of a Randomized Controlled Trial. Orthop. J. Sports Med. 2024, 12, 23259671231222265. [Google Scholar] [CrossRef] [PubMed]
- Haffar, A.; Khan, I.A.; Abdelaal, M.S.; Banerjee, S.; Sharkey, P.F.; Lonner, J.H. Topical Cannabidiol (CBD) After Total Knee Arthroplasty Does Not Decrease Pain or Opioid Use: A Prospective Randomized Double-Blinded Placebo-Controlled Trial. J. Arthroplast. 2022, 37, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Narang, G.; Moore, J.; Wymer, K.; Chang, Y.H.; Lim, E.; Adeleye, O.; Humphreys, M.R.; Stern, K.L. Effect of Cannabidiol Oil on Post-ureteroscopy Pain for Urinary Calculi: A Randomized, Double-blind, Placebo-controlled Trial. J. Urol. 2023, 209, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Kalliomaki, J.; Segerdahl, M.; Webster, L.; Reimfelt, A.; Huizar, K.; Annas, P.; Karlsten, R.; Quiding, H. Evaluation of the analgesic efficacy of AZD1940, a novel cannabinoid agonist, on post-operative pain after lower third molar surgical removal. Scand. J. Pain 2013, 4, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Ostenfeld, T.; Price, J.; Albanese, M.; Bullman, J.; Guillard, F.; Meyer, I.; Leeson, R.; Costantin, C.; Ziviani, L.; Nocini, P.F.; et al. A randomized, controlled study to investigate the analgesic efficacy of single doses of the cannabinoid receptor-2 agonist GW842166, ibuprofen or placebo in patients with acute pain following third molar tooth extraction. Clin. J. Pain 2011, 27, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.; Straube, S.; Fisher, E.; Eccleston, C. Cannabidiol (CBD) Products for Pain: Ineffective, Expensive, and With Potential Harms. J. Pain 2024, 25, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Khelemsky, Y.; Goldberg, A.T.; Hurd, Y.L.; Winkel, G.; Ninh, A.; Qian, L.; Oprescu, A.; Ciccone, J.; Katz, D.J. Perioperative Patient Beliefs Regarding Potential Effectiveness of Marijuana (Cannabinoids) for Treatment of Pain: A Prospective Population Survey. Reg. Anesth. Pain Med. 2017, 42, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gutierrez, M.S.; Navarrete, F.; Gasparyan, A.; Austrich-Olivares, A.; Sala, F.; Manzanares, J. Cannabidiol: A Potential New Alternative for the Treatment of Anxiety, Depression, and Psychotic Disorders. Biomolecules 2020, 10, 1575. [Google Scholar] [CrossRef] [PubMed]
- Vigil, J.M.; Stith, S.S.; Brockelman, F.; Keeling, K.; Hall, B. Systematic combinations of major cannabinoid and terpene contents in Cannabis flower and patient outcomes: A proof-of-concept assessment of the Vigil Index of Cannabis Chemovars. J. Cannabis Res. 2023, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Gaston, T.E.; Friedman, D. Pharmacology of cannabinoids in the treatment of epilepsy. Epilepsy Behav. 2017, 70, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Grotenhermen, F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin. Pharmacokinet. 2003, 42, 327–360. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.J.; Galettis, P.; Schneider, J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br. J. Clin. Pharmacol. 2018, 84, 2477–2482. [Google Scholar] [CrossRef] [PubMed]
- Huestis, M.A. Human cannabinoid pharmacokinetics. Chem. Biodivers. 2007, 4, 1770–1804. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.W.; Bhatia, A.; Buzon-Tan, A.; Walker, S.; Ilangomaran, D.; Kara, J.; Venkatraghavan, L.; Prabhu, A.J. Weeding Out the Problem: The Impact of Preoperative Cannabinoid Use on Pain in the Perioperative Period. Anesth. Analg. 2019, 129, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Mlost, J.; Bryk, M.; Starowicz, K. Cannabidiol for Pain Treatment: Focus on Pharmacology and Mechanism of Action. Int. J. Mol. Sci. 2020, 21, 8870. [Google Scholar] [CrossRef] [PubMed]
- British Medical Association. Therapeutic Uses of Cannabis; Harwood Academic Publishers: Amsterdam, The Netherlands, 1997; 142p. [Google Scholar]
- Zieglgansberger, W.; Brenneisen, R.; Berthele, A.; Wotjak, C.T.; Bandelow, B.; Tolle, T.R.; Lutz, B. Chronic Pain and the Endocannabinoid System: Smart Lipids—A Novel Therapeutic Option? Med. Cannabis Cannabinoids 2022, 5, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G.; Gibson, T.M.; Stevenson, L.A.; Ross, R.A.; Banner, W.K.; Saha, B.; Razdan, R.K.; Martin, B.R. O-1057, a potent water-soluble cannabinoid receptor agonist with antinociceptive properties. Br. J. Pharmacol. 2000, 129, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, B. Gandhi, J.R.R. Oral cavity as a site for bioadhesive drug delivery. Adv. Drug Deliv. Rev. 1994, 13, 43–74. [Google Scholar] [CrossRef]
- Martin, J.H.; Schneider, J.; Lucas, C.J.; Galettis, P. Exogenous Cannabinoid Efficacy: Merely a Pharmacokinetic Interaction? Clin. Pharmacokinet. 2018, 57, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Hua, S. Advances in Nanoparticulate Drug Delivery Approaches for Sublingual and Buccal Administration. Front. Pharmacol. 2019, 10, 1328. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudinoodezh, H.; Telukutla, S.R.; Bhangu, S.K.; Bachari, A.; Cavalieri, F.; Mantri, N. The Transdermal Delivery of Therapeutic Cannabinoids. Pharmaceutics 2022, 14, 438. [Google Scholar] [CrossRef] [PubMed]
- Challapalli, P.V.; Stinchcomb, A.L. In vitro experiment optimization for measuring tetrahydrocannabinol skin permeation. Int. J. Pharm. 2002, 241, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Stinchcomb, A.L.; Valiveti, S.; Hammell, D.C.; Ramsey, D.R. Human skin permeation of Delta8-tetrahydrocannabinol, cannabidiol and cannabinol. J. Pharm. Pharmacol. 2004, 56, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Fantegrossi, W.E.; Moran, J.H.; Radominska-Pandya, A.; Prather, P.L. Distinct pharmacology and metabolism of K2 synthetic cannabinoids compared to Delta9-THC: Mechanism underlying greater toxicity? Life Sci. 2014, 97, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.; Gupta, V.; Keshock, M.C. Tetrahydrocannabinol (THC). In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2023. [Google Scholar]
- Englund, A.; Stone, J.M.; Morrison, P.D. Cannabis in the arm: What can we learn from intravenous cannabinoid studies? Curr. Pharm. Des. 2012, 18, 4906–4914. [Google Scholar] [CrossRef] [PubMed]
- Raft, D.; Gregg, J.; Ghia, J.; Harris, L. Effects of intravenous tetrahydrocannabinol on experimental and surgical pain. Psychological correlates of the analgesic response. Clin. Pharmacol. Ther. 1977, 21, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, J.E.; Ohlsson, A.; Agurell, S.; Hollister, L.; Gillespie, H. Clinical effects and plasma levels of delta9-tetrahydrocannabinol (delta9-THC) in heavy and light users of cannabis. Psychopharmacology 1981, 74, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Maykut, M.O. Health consequences of acute and chronic marihuana use. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 1985, 9, 209–238. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, A.; Lindgren, J.E.; Andersson, S.; Agurell, S.; Gillespie, H.; Hollister, L.E. Single-dose kinetics of deuterium-labelled cannabidiol in man after smoking and intravenous administration. Biomed. Environ. Mass Spectrom. 1986, 13, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Casati, S.; Angeli, I.; Ravelli, A.; Del Fabbro, M.; Minoli, M.; Orioli, M. 11-OH-THC in hair as marker of active cannabis consumption: Estimating a reliable cut-off by evaluation of 672 THC-positive hair samples. Forensic. Sci. Int. 2019, 304, 109951. [Google Scholar] [CrossRef] [PubMed]
- Martin-Santos, R.; Crippa, J.A.; Batalla, A.; Bhattacharyya, S.; Atakan, Z.; Borgwardt, S.; Allen, P.; Seal, M.; Langohr, K.; Farre, M.; et al. Acute effects of a single, oral dose of d9-tetrahydrocannabinol (THC) and cannabidiol (CBD) administration in healthy volunteers. Curr. Pharm. Des. 2012, 18, 4966–4979. [Google Scholar] [CrossRef] [PubMed]
- Zendulka, O.; Dovrtelova, G.; Noskova, K.; Turjap, M.; Sulcova, A.; Hanus, L.; Jurica, J. Cannabinoids and Cytochrome P450 Interactions. Curr. Drug Metab. 2016, 17, 206–226. [Google Scholar] [CrossRef] [PubMed]
- Ujvary, I.; Hanus, L. Human Metabolites of Cannabidiol: A Review on Their Formation, Biological Activity, and Relevance in Therapy. Cannabis Cannabinoid Res. 2016, 1, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Heuberger, J.A.; Guan, Z.; Oyetayo, O.O.; Klumpers, L.; Morrison, P.D.; Beumer, T.L.; van Gerven, J.M.; Cohen, A.F.; Freijer, J. Population pharmacokinetic model of THC integrates oral, intravenous, and pulmonary dosing and characterizes short- and long-term pharmacokinetics. Clin. Pharmacokinet. 2015, 54, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.J.; Galettis, P.; Song, S.; Solowij, N.; Reuter, S.E.; Schneider, J.; Martin, J.H. Cannabinoid Disposition After Human Intraperitoneal Use: AnInsight Into Intraperitoneal Pharmacokinetic Properties in Metastatic Cancer. Clin. Ther. 2018, 40, 1442–1447. [Google Scholar] [CrossRef]
- Toennes, S.W.; Ramaekers, J.G.; Theunissen, E.L.; Moeller, M.R.; Kauert, G.F. Comparison of cannabinoid pharmacokinetic properties in occasional and heavy users smoking a marijuana or placebo joint. J. Anal. Toxicol. 2008, 32, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Skopp, G.; Potsch, L. Cannabinoid concentrations in spot serum samples 24–48 hours after discontinuation of cannabis smoking. J. Anal. Toxicol. 2008, 32, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Doohan, P.T.; Oldfield, L.D.; Arnold, J.C.; Anderson, L.L. Cannabinoid Interactions with Cytochrome P450 Drug Metabolism: A Full-Spectrum Characterization. AAPS J. 2021, 23, 91. [Google Scholar] [CrossRef] [PubMed]
- Lopera, V.; Rodriguez, A.; Amariles, P. Clinical Relevance of Drug Interactions with Cannabis: A Systematic Review. J. Clin. Med. 2022, 11, 1154. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, C.; Peyraube, R.; Fagiolino, P.; Oricchio, F.; Cunetti, L.; Vazquez, M. Human Data on Pharmacokinetic Interactions of Cannabinoids: A Narrative Review. Curr. Pharm. Des. 2024, 30, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Kocis, P.T.; Wadrose, S.; Wakefield, R.L.; Ahmed, A.; Calle, R.; Gajjar, R.; Vrana, K.E. CANNabinoid Drug Interaction Review (CANN-DIR). Med. Cannabis Cannabinoids 2023, 6, 1–7. [Google Scholar] [CrossRef]
- Russo, E.B. Current Therapeutic Cannabis Controversies and Clinical Trial Design Issues. Front. Pharmacol. 2016, 7, 309. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, M.; Guevara, N.; Maldonado, C.; Guido, P.C.; Schaiquevich, P. Potential Pharmacokinetic Drug-Drug Interactions between Cannabinoids and Drugs Used for Chronic Pain. Biomed. Res. Int. 2020, 2020, 3902740. [Google Scholar] [CrossRef] [PubMed]
- Arellano, A.L.; Papaseit, E.; Romaguera, A.; Torrens, M.; Farre, M. Neuropsychiatric and General Interactions of Natural and Synthetic Cannabinoids with Drugs of Abuse and Medicines. CNS Neurol. Disord. Drug Targets 2017, 16, 554–566. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, S.E.; Strawn, J.R.; Poweleit, E.A.; Sarangdhar, M.; Ramsey, L.B. The Impact of Marijuana on Antidepressant Treatment in Adolescents: Clinical and Pharmacologic Considerations. J. Pers. Med. 2021, 11, 615. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Yamaori, S.; Okamoto, Y.; Yamamoto, I.; Watanabe, K. Cannabidiol is a potent inhibitor of the catalytic activity of cytochrome P450 2C19. Drug Metab. Pharmacokinet. 2013, 28, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Yamaori, S.; Okamoto, Y.; Yamamoto, I.; Watanabe, K. Cannabidiol, a major phytocannabinoid, as a potent atypical inhibitor for CYP2D6. Drug Metab. Pharmacokinet. 2011, 39, 2049–2056. [Google Scholar] [CrossRef] [PubMed]
- Yamaori, S.; Ebisawa, J.; Okushima, Y.; Yamamoto, I.; Watanabe, K. Potent inhibition of human cytochrome P450 3A isoforms by cannabidiol: Role of phenolic hydroxyl groups in the resorcinol moiety. Life Sci. 2011, 88, 730–736. [Google Scholar] [CrossRef]
- Balachandran, P.; Elsohly, M.; Hill, K.P. Cannabidiol Interactions with Medications, Illicit Substances, and Alcohol: A Comprehensive Review. J. Gen. Intern. Med. 2021, 36, 2074–2084. [Google Scholar] [CrossRef] [PubMed]
- MacCallum, C.A.; Russo, E.B. Practical considerations in medical cannabis administration and dosing. Eur. J. Intern. Med. 2018, 49, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Dewey, W.L. Cannabinoid pharmacology. Pharmacol. Rev. 1986, 38, 151–178. [Google Scholar] [CrossRef] [PubMed]
- Hampson, R.E.; Deadwyler, S.A. Cannabinoids, hippocampal function and memory. Life Sci. 1999, 65, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Heyser, C.J.; Hampson, R.E.; Deadwyler, S.A. Effects of delta-9-tetrahydrocannabinol on delayed match to sample performance in rats: Alterations in short-term memory associated with changes in task specific firing of hippocampal cells. J. Pharmacol. Exp. Ther. 1993, 264, 294–307. [Google Scholar] [PubMed]
- Adams, I.B.; Martin, B.R. Cannabis: Pharmacology and toxicology in animals and humans. Addiction 1996, 91, 1585–1614. [Google Scholar] [CrossRef] [PubMed]
- Hollister, L.E. Cannabis—1988. Acta Psychiatr. Scand. Suppl. 1988, 345, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Hollister, L.E. Health aspects of cannabis. Pharmacol. Rev. 1986, 38, 1–20. [Google Scholar] [CrossRef]
- Barnett, G.; Licko, V.; Thompson, T. Behavioral pharmacokinetics of marijuana. Psychopharmacology 1985, 85, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Huestis, M.A.; Sampson, A.H.; Holicky, B.J.; Henningfield, J.E.; Cone, E.J. Characterization of the absorption phase of marijuana smoking. Clin. Pharmacol. Ther. 1992, 52, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Johns, A. Psychiatric effects of cannabis. Br. J. Psychiatry 2001, 178, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Stanley, C.P.; Hind, W.H.; Tufarelli, C.; O’Sullivan, S.E. Cannabidiol causes endothelium-dependent vasorelaxation of human mesenteric arteries via CB1 activation. Cardiovasc. Res. 2015, 107, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Stanley, C.P.; Hind, W.H.; Tufarelli, C.; O’Sullivan, S.E. The endocannabinoid anandamide causes endothelium-dependent vasorelaxation in human mesenteric arteries. Pharmacol. Res. 2016, 113, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, B.; Baranowska-Kuczko, M.; Schlicker, E. Triphasic blood pressure responses to cannabinoids: Do we understand the mechanism? Br. J. Pharmacol. 2012, 165, 2073–2088. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, D.A.; Heishman, S.J.; Preston, K.L.; Nelson, R.A.; Moolchan, E.T.; Huestis, M.A. The cannabinoid CB1 receptor antagonist rimonabant attenuates the hypotensive effect of smoked marijuana in male smokers. Am. Heart J. 2006, 151, 754.e751–754.e755. [Google Scholar] [CrossRef] [PubMed]
- Huestis, M.A.; Gorelick, D.A.; Heishman, S.J.; Preston, K.L.; Nelson, R.A.; Moolchan, E.T.; Frank, R.A. Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch. Gen. Psychiatry 2001, 58, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.T. Cardiovascular system effects of marijuana. J. Clin. Pharmacol. 2002, 42, 58S–63S. [Google Scholar] [CrossRef] [PubMed]
- Tashkin, D.P.; Levisman, J.A.; Abbasi, A.S.; Shapiro, B.J.; Ellis, N.M. Short-term effects of smoked marihuana on left ventricular function in man. Chest 1977, 72, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Perez-Reyes, M. he Psychologic and Physiologic Effects of Active Cannabinoids. In Marihuana and Medicine; Humana Press: Totowa, NJ, USA, 1999. [Google Scholar]
- Shook, J.E.; Burks, T.F. Psychoactive cannabinoids reduce gastrointestinal propulsion and motility in rodents. J. Pharmacol. Exp. Ther. 1989, 249, 444–449. [Google Scholar] [PubMed]
- Hollmann, M.W.; Rathmell, J.P.; Lirk, P. Optimal postoperative pain management: Redefining the role for opioids. Lancet 2019, 393, 1483–1485. [Google Scholar] [CrossRef]
- Hall, W.; Solowij, N. Adverse effects of cannabis. Lancet 1998, 352, 1611–1616. [Google Scholar] [CrossRef]
- Beaulieu, P.; Boulanger, A.; Desroches, J.; Clark, A.J. Medical cannabis: Considerations for the anesthesiologist and pain physician. Can. J. Anesth. 2016, 63, 608–624. [Google Scholar] [CrossRef] [PubMed]
- Laudanski, K.; Wain, J. Considerations for Cannabinoids in Perioperative Care by Anesthesiologists. J. Clin. Med. 2022, 11, 558. [Google Scholar] [CrossRef] [PubMed]
- Mallat, A.; Roberson, J.; Brock-Utne, J.G. Preoperative marijuana inhalation--an airway concern. Can. J. Anesth. 1996, 43, 691–693. [Google Scholar] [CrossRef] [PubMed]
- Boyce, S.H.; Quigley, M.A. Uvulitis and partial upper airway obstruction following cannabis inhalation. Emerg. Med. 2002, 14, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Guarisco, J.L.; Cheney, M.L.; LeJeune, F.E., Jr.; Reed, H.T. Isolated uvulitis secondary to marijuana use. Laryngoscope 1988, 98, 1309–1312. [Google Scholar] [CrossRef] [PubMed]
- White, S.M. Cannabis abuse and laryngospasm. Anaesthesia 2002, 57, 622–623. [Google Scholar] [CrossRef] [PubMed]
- Ghuran, A.; Nolan, J. Recreational drug misuse: Issues for the cardiologist. Heart 2000, 83, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Kuczkowski, K.M. Anesthetic implications of drug abuse in pregnancy. J. Clin. Anesth. 2003, 15, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Badowski, S.; Smith, G. Cannabis use during pregnancy and postpartum. Can. Fam. Physician 2020, 66, 98–103. [Google Scholar]
- Conner, S.N.; Bedell, V.; Lipsey, K.; Macones, G.A.; Cahill, A.G.; Tuuli, M.G. Maternal Marijuana Use and Adverse Neonatal Outcomes: A Systematic Review and Meta-analysis. Obstet. Gynecol. 2016, 128, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Grant, K.S.; Petroff, R.; Isoherranen, N.; Stella, N.; Burbacher, T.M. Cannabis use during pregnancy: Pharmacokinetics and effects on child development. Pharmacol. Ther. 2018, 182, 133–151. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Medina, M.B.; Perea, M.; Torales, J.; Ventriglio, A.; Vitrani, G.; Aguilar, L.; Roncero, C. Cannabis consumption and psychosis or schizophrenia development. Int. J. Soc. Psychiatry 2018, 64, 690–704. [Google Scholar] [CrossRef] [PubMed]
- Sellers, E.M.; Schoedel, K.; Bartlett, C.; Romach, M.; Russo, E.B.; Stott, C.G.; Wright, S.; White, L.; Duncombe, P.; Chen, C.F. A Multiple-Dose, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group QT/QTc Study to Evaluate the Electrophysiologic Effects of THC/CBD Spray. Clin. Pharmacol. Drug Dev. 2013, 2, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Hurd, Y.L.; Manzoni, O.J.; Pletnikov, M.V.; Lee, F.S.; Bhattacharyya, S.; Melis, M. Cannabis and the Developing Brain: Insights into Its Long-Lasting Effects. J. Neurosci. 2019, 39, 8250–8258. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; Di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S.; et al. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA 2015, 313, 2456–2473. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, F.W.; Hussain, N.; Weaver, T.; Brull, R. Analgesic efficacy of cannabinoids for acute pain management after surgery: A systematic review and meta-analysis. Reg. Anesth. Pain Med. 2020, 45, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Stevens, A.J.; Higgins, M.D. A systematic review of the analgesic efficacy of cannabinoid medications in the management of acute pain. Acta Anaesthesiol. Scand. 2017, 61, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.A.; Ghuran, A.; Vadamalai, V.; Antonios, T.F. Cardiovascular complications induced by cannabis smoking: A case report and review of the literature. J. Emerg. Med. 2005, 22, 679–680. [Google Scholar] [CrossRef] [PubMed]
- Kleine-Brueggeney, M.; Greif, R.; Brenneisen, R.; Urwyler, N.; Stueber, F.; Theiler, L.G. Intravenous Delta-9-Tetrahydrocannabinol to Prevent Postoperative Nausea and Vomiting: A Randomized Controlled Trial. Anesth. Analg. 2015, 121, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Alaia, M.J.; Hurley, E.T.; Vasavada, K.; Markus, D.H.; Britton, B.; Gonzalez-Lomas, G.; Rokito, A.S.; Jazrawi, L.M.; Kaplan, K. Buccally Absorbed Cannabidiol Shows Significantly Superior Pain Control and Improved Satisfaction Immediately After Arthroscopic Rotator Cuff Repair: A Placebo-Controlled, Double-Blinded, Randomized Trial. Am. J. Sports Med. 2022, 50, 3056–3063. [Google Scholar] [CrossRef] [PubMed]
- Allegri, M.; Clark, M.R.; De Andres, J.; Jensen, T.S. Acute and chronic pain: Where we are and where we have to go. Minerva Anestesiol. 2012, 78, 222–235. [Google Scholar] [PubMed]
- Gupta, A.; Kaur, K.; Sharma, S.; Goyal, S.; Arora, S.; Murthy, R.S. Clinical aspects of acute post-operative pain management & its assessment. J. Adv. Pharm. Technol. Res. 2010, 1, 97–108. [Google Scholar] [PubMed]
- Miller, L.K.; Devi, L.A. The highs and lows of cannabinoid receptor expression in disease: Mechanisms and their therapeutic implications. Pharmacol. Rev. 2011, 63, 461–470. [Google Scholar] [CrossRef]
- D’Souza, D.C.; Perry, E.; MacDougall, L.; Ammerman, Y.; Cooper, T.; Wu, Y.T.; Braley, G.; Gueorguieva, R.; Krystal, J.H. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: Implications for psychosis. Neuropsychopharmacology 2004, 29, 1558–1572. [Google Scholar] [CrossRef] [PubMed]
- Jennings, J.M.; Angerame, M.R.; Eschen, C.L.; Phocas, A.J.; Dennis, D.A. Cannabis Use Does Not Affect Outcomes After Total Knee Arthroplasty. J. Arthroplast. 2019, 34, 1667–1669. [Google Scholar] [CrossRef] [PubMed]
- Mackie, K. Distribution of cannabinoid receptors in the central and peripheral nervous system. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar] [CrossRef]
- Pazos, M.R.; Nunez, E.; Benito, C.; Tolon, R.M.; Romero, J. Functional neuroanatomy of the endocannabinoid system. Pharmacol. Biochem. Behav. 2005, 81, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.S.; Mackie, K. Distribution of the Endocannabinoid System in the Central Nervous System. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2015; Volume 231, pp. 59–93. [Google Scholar] [CrossRef]
- Tao, R.; Li, C.; Jaffe, A.E.; Shin, J.H.; Deep-Soboslay, A.; Yamin, R.; Weinberger, D.R.; Hyde, T.M.; Kleinman, J.E. Cannabinoid receptor CNR1 expression and DNA methylation in human prefrontal cortex, hippocampus and caudate in brain development and schizophrenia. Transl. Psychiatry 2020, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Kunos, G.; Osei-Hyiaman, D.; Batkai, S.; Sharkey, K.A.; Makriyannis, A. Should peripheral CB1 cannabinoid receptors be selectively targeted for therapeutic gain? Trends Pharmacol. Sci. 2009, 30, 1–7. [Google Scholar] [CrossRef]
- Atwood, B.K.; Mackie, K. CB2: A cannabinoid receptor with an identity crisis. Br. J. Pharmacol. 2010, 160, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M.; Bab, I.; Biro, T.; Cabral, G.A.; Dey, S.K.; Di Marzo, V.; Konje, J.C.; Kunos, G.; Mechoulam, R.; Pacher, P.; et al. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol. Sci. 2015, 36, 277–296. [Google Scholar] [CrossRef] [PubMed]
- Dhopeshwarkar, A.; Mackie, K. CB2 Cannabinoid receptors as a therapeutic target-what does the future hold? Mol. Pharmacol. 2014, 86, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Pernia-Andrade, A.J.; Kato, A.; Witschi, R.; Nyilas, R.; Katona, I.; Freund, T.F.; Watanabe, M.; Filitz, J.; Koppert, W.; Schuttler, J.; et al. Spinal endocannabinoids and CB1 receptors mediate C-fiber-induced heterosynaptic pain sensitization. Science 2009, 325, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.A.; Desjardins, P.J.; Turk, D.C.; Dworkin, R.H.; Katz, N.P.; Kehlet, H.; Ballantyne, J.C.; Burke, L.B.; Carragee, E.; Cowan, P.; et al. Research design considerations for single-dose analgesic clinical trials in acute pain: IMMPACT recommendations. Pain 2016, 157, 288–301. [Google Scholar] [CrossRef] [PubMed]
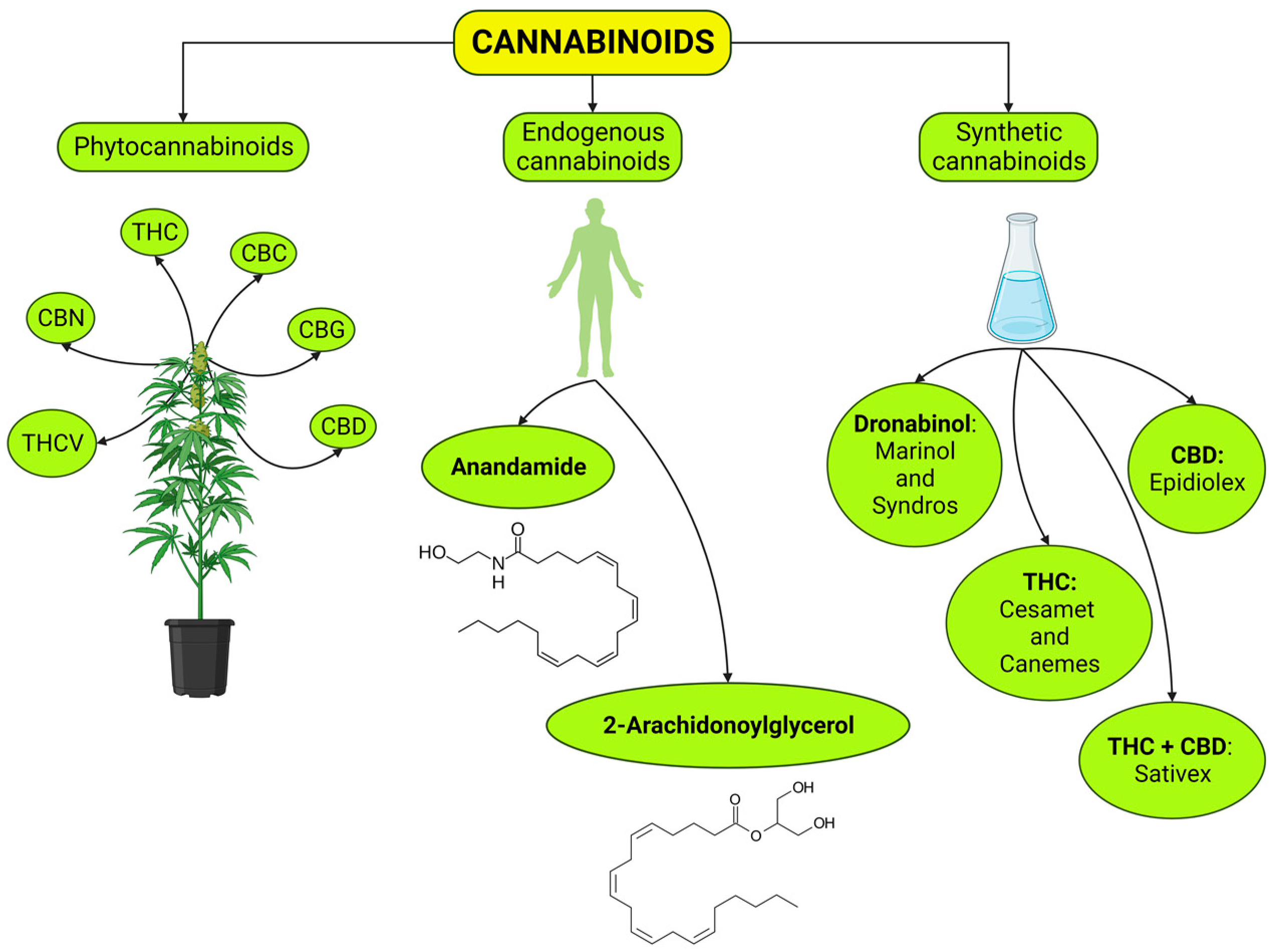
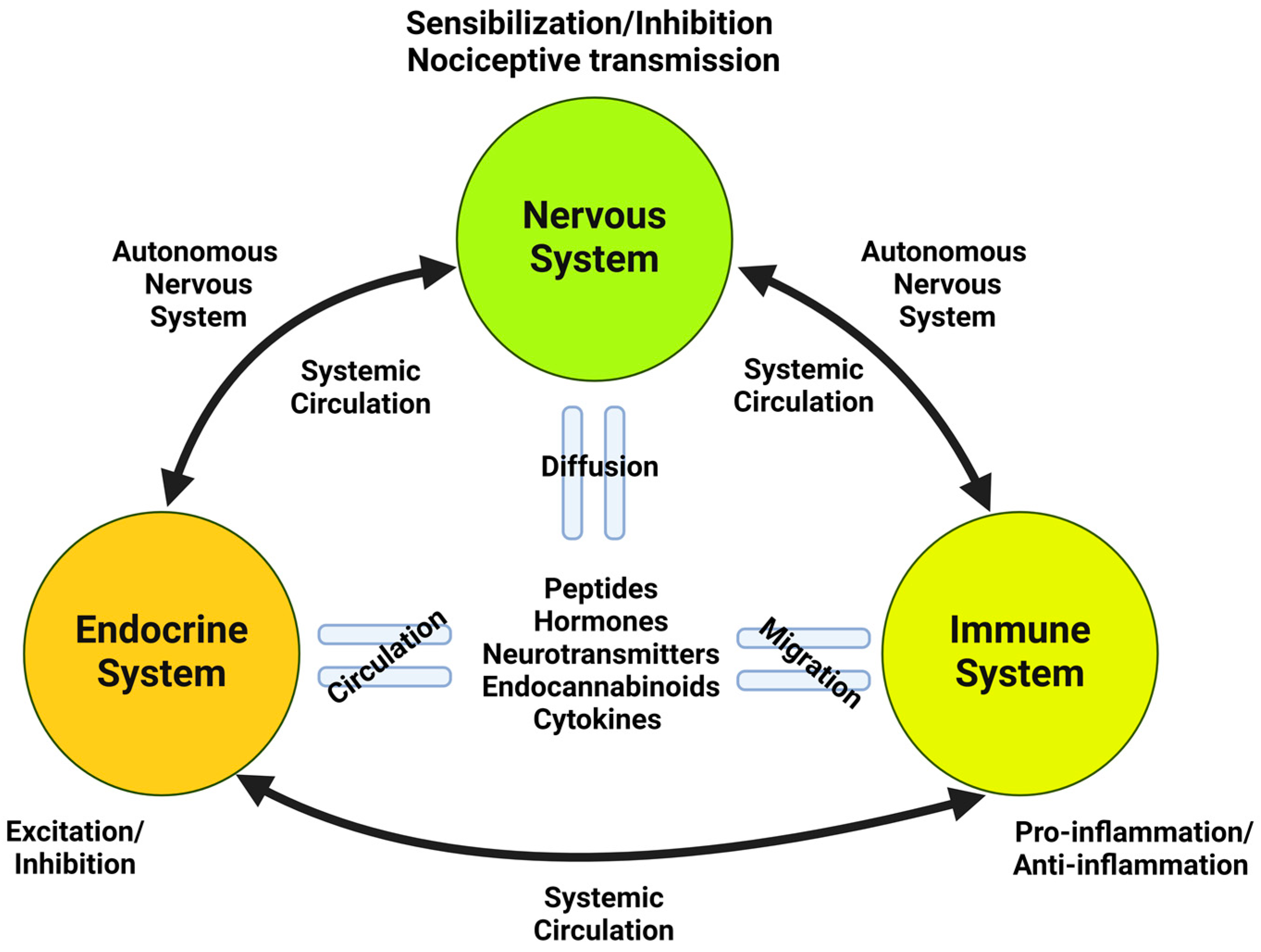

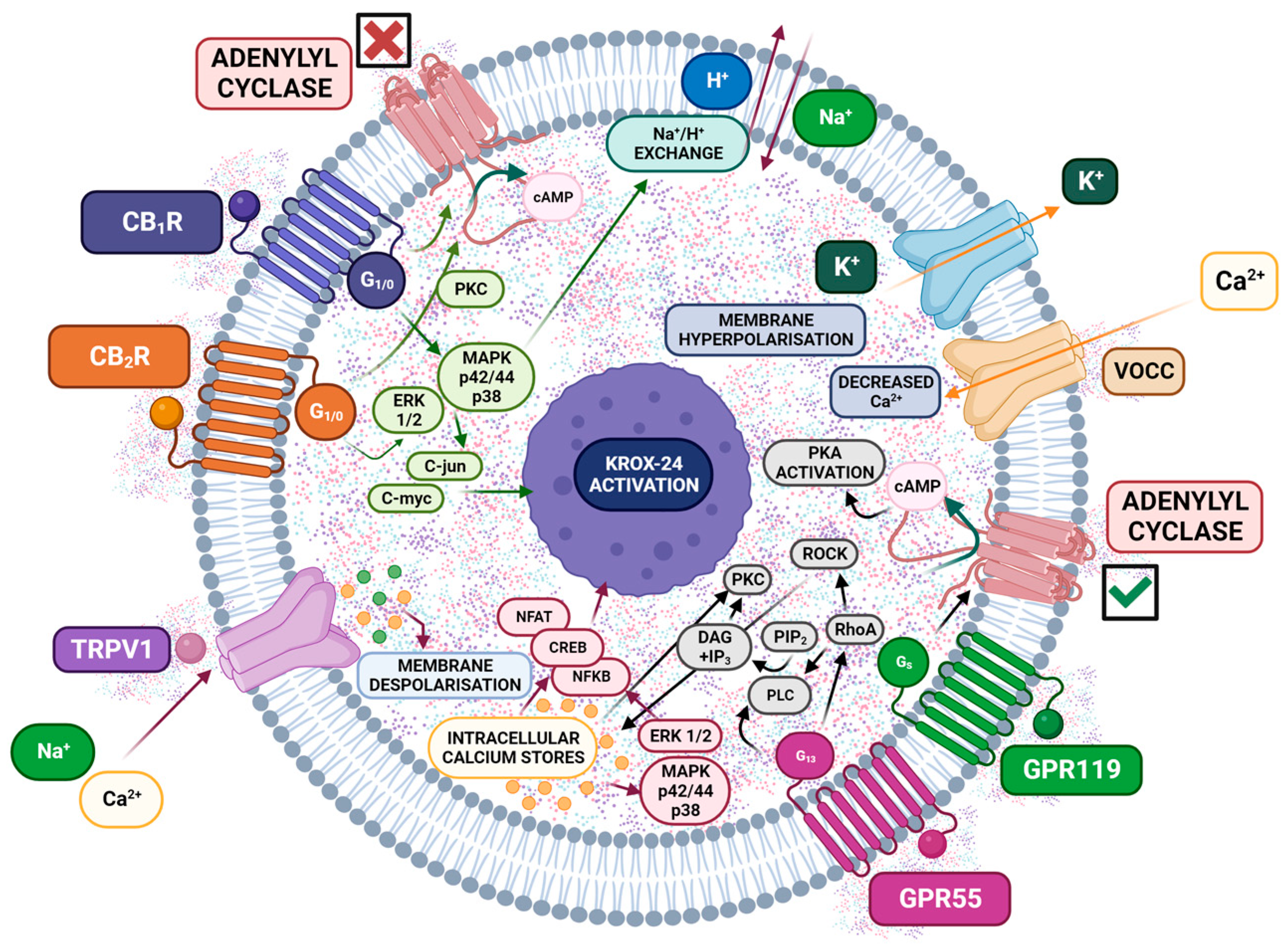
| Pulmonary Route | Oral | Other Routes | ||||
|---|---|---|---|---|---|---|
| Smoking | Vaporization | Oral Transmucosal | Topical | |||
| Characteristics |
|
|
| Nabiximols oral spray is currently the only cannabis-based prescription that delivers a standardized dosage of CBD/THC in a 1:1 ratio with extensive research. | Topical is ideal for localized symptoms (dermatological conditions, arthritis), with limited research evidence. | |
| Clinical effects | Onset (min) | 5–10 min | 60–180 min | 15–45 min | Variable | |
| Duration (h) | 2–4 h | 6–8 h | 6–8 h | Variable | ||
| Advantages | Rapid action benefits acute or episodic symptoms (nausea/pain). | Less odor, convenient and discreet, prolonged effect. Advantage for chronic disease/symptoms. | Pharmaceutical form (nabiximols) available, with documented efficacy and safety. | Less systemic effect, suitable for localized symptoms. | ||
| Disadvantages | Dexterity is required, vaporizers may be expensive, and not all are portable. | Titration challenges due to delayed onset. | Expensive, spotty availability. | Only local effects. | ||
| Side Effect | Most Common | Common | Rare |
|---|---|---|---|
| Drowsiness/fatigue | × | ||
| Dizziness | × | ||
| Cough, phlegm, bronchitis (smoking only) | × | ||
| Anxiety | × | ||
| Nausea | × | ||
| Cognitive effects | × | ||
| Euphoria | × | ||
| Blurred vision | × | ||
| Headache | × | ||
| Orthostatic hypotension | × | ||
| Toxic psychosis/paranoia | × | ||
| Depression | × | ||
| Ataxia/dyscoordination | × | ||
| Tachycardia (after titration) | × | ||
| Cannabis hyperemesis | × | ||
| Diarrhea | × |
| Type and Design of the Study | Subjects | Surgical Procedure | Primary Outcome | Postoperative Analgesia | Cannabinoid Intervention | Main Results | Reference |
|---|---|---|---|---|---|---|---|
| RCT (double blind, placebo-controlled, crossover design) | 56 patients | Acute Fracture or trauma | N/S | N/S | Levonantradol I.M. Preoperative regimen. Single injection: 1, 1.5, 2, 2.5, and 3 mg. | Pain relief with the four doses; analgesia persisted for more than 6 h with the 2.5 and 3 mg doses. | Jain et al., 1981 [349] |
| Placebo-controlled, single-dose | 100 patients | Renal surgery with lumbar incision | N/S | Noramidopyrine (metamizol), Camylofine (anti-cholinergic drug) | Levonantradol I.M. Postoperative regimen. Single injection: 1 and 2 mg. | No significant difference compared with placebo. | Guillaud et al., 1983 [348] |
| RCT (double blind, placebo-controlled, single dose, parallel) | 40 patients | Elective abdominal hysterectomy | Pain scores | Morphine PCA | THC P.O. Postoperative regimen. Single dose: 5 mg. | No significant difference compared with placebo. Increased awareness of surroundings is more frequently reported with THC | Buggy et al., 2003 [259] |
| RCT (double-blind, placebo-controlled) | 41 patients | Orthopedic, gynecology, urology, and plastic or general surgery | Opioid consumption at 24 h | Morphine PCA | Nabilone P.O. Preoperative and postoperative regimen. 1 and 2 mg. | No significant difference compared with placebo. | Beaulieu et al., 2006 [350] |
| RCT (double-blind, placebo-controlled) | 100 patients | Radical retropubic prostatectomy | Opioid consumption at 48 h | Piritramide PCA | Dronabinol P.O. Preoperative (evening before operation) and postoperative until the morning of the 2nd postoperative day) regimen. 8 doses of 5 mg. | No significant difference between dronabinol and placebo groups in the self-administration of post-operative piritramide. | Seeling et al., 2006 [322] |
| Dose escalating study | 65 patients | Various major Surgeries (included orthopedic, gynecologic, urology, plastics, and general) | N/S | Morphine PCA | Cannador P.O. Postoperative regimen. Single dose: 5, 10, 15 and 24 mg. | Significant dose-related improvements in rescue analgesia requirements in the 10 and 15 mg groups. Study ended because of a serious vasovagal adverse event in a patient receiving 15 mg. | Holdcroft et al., 2006 [347] |
| CT (double-blind, placebo-controlled) | 112 patients | Third molar tooth extraction | Pain scores up to 10 h postsurgery | 500 mg acetaminophen, 15 mg codeine phosphate | GW842166 P.O. Preoperative regimen. Single dose: 100 and 800 mg. | In comparison to ibuprofen, single doses of GW842166 (100 and 800 mg) failed to demonstrate clinically meaningful analgesia in the setting of acute dental pain. | Ostenfeld et al., 2011 [353] |
| RCT (double-blind, placebo-controlled) | 150 patients | Removal of impacted lower third molar tooth | Area under the curve pain scores | 1000 mg acetaminophen | AZD1940 P.O. Preoperative regimen. Single dose: 800 µg. | No significant differences compared with placebo. | Kalliomaki et al., 2013 [352] |
| RCT (double-blind, placebo-controlled) | 99 patients | Arthroscopic rotator cuff repair | Pain scores Patient satisfaction with pain control Opioid consumption at days 1, 2, 7, and 14 | 5 mg oxycodone 325 mg acetaminophen | CBD P.O. Postoperative regimen. Repeated dose (three times a day, 14 days): 25 and 50 mg | Days 1 and 2: Lower pain score in the CBD group. Higher patient satisfaction with pain control. No statistical difference between groups in opioid consumption Days 7 and 14: No significant differences compared with placebo. | Alaia et al., 2022 [422] |
| RCT (double-blind, placebo-controlled) | 80 patients | Total knee arthroplasty | Pain and sleep scores Cumulative postoperative opioid use | 1000 mg acetaminophen 300 mg gabapentin 15 mg meloxicam 5 mg oxycodone | CBD topical. Postoperative regimen. Repeated dose (three times a day, 14 days): 120 mg/ounce | No significant differences compared with placebo. | Haffar et al., 2022 [421] |
| RCT (double-blind, placebo-controlled) | 94 patients | Ureteroscopy with stent placement for urinary stone disease | Pain scores Postoperative opioid use | 5 mg oxycodone | CBD P.O. Postoperative regimen. Repeated dose (3 days): 20 mg | No significant differences compared with placebo. | Narang et al., 2023 [424] |
| RCT (double-blind, placebo-controlled) | 83 patients (follow-up) | Arthroscopic rotator cuff repair | Pain scores Patient satisfaction with pain control Opioid consumption at 7 and 14 days | 5 mg oxycodone 325 mg acetaminophen | CBD P.O. Postoperative regimen. Repeated dose (three times a day, 14 days): 25 and 50 mg | No significant differences compared with placebo. | Alaia et al., 2024 [423] |
| Related to the symptoms | Postoperative pain is usually localized. In this context, the activation of the endocannabinoid system is minor compared to that in chronic pain, and cannabinoids are mainly associated with the relief of neuropathic pain. |
| Pain assessment is highly subjective, and the quantification and comparison between study groups are generally inconclusive. | |
| Related to the product | On the one hand, the composition of phytocannabinoids is very heterogeneous. On the other hand, studies of its components separately do not provide the same results as when the whole plant is analyzed. |
| Human clinical trials on postoperative pain used almost exclusively THC or an analog. | |
| Few studies are testing a mixture of THC/CBD or CBD without THC in humans for the treatment of postoperative pain. | |
| Lack of knowledge about cannabinoid interaction (synergic, antagonistic, entourage effect). | |
| Related to the administration route | In the systemic routes, the clinical doses seem limited by the appearance of side effects, primarily psychotropic. |
| There is no record of perimedullary administration at the clinical level of cannabinoid compounds, with robust and proven analgesic preclinical evidence for this route of administration. | |
| Related to the studies | Short-term duration (mostly single-dose administration and a short follow-up period). |
| There is considerable variation in the doses and therapeutic guidelines employed. | |
| There are no 100% effective standard treatments against which to compare the effect of cannabinoids. | |
| A small number of patients in multiple operative settings. | |
| No homogenous groups (healthy patients, patients with diseases of different etiologies). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrascosa, A.J.; Navarrete, F.; Saldaña, R.; García-Gutiérrez, M.S.; Montalbán, B.; Navarro, D.; Gómez-Guijarro, F.M.; Gasparyan, A.; Murcia-Sánchez, E.; Torregrosa, A.B.; et al. Cannabinoid Analgesia in Postoperative Pain Management: From Molecular Mechanisms to Clinical Reality. Int. J. Mol. Sci. 2024, 25, 6268. https://doi.org/10.3390/ijms25116268
Carrascosa AJ, Navarrete F, Saldaña R, García-Gutiérrez MS, Montalbán B, Navarro D, Gómez-Guijarro FM, Gasparyan A, Murcia-Sánchez E, Torregrosa AB, et al. Cannabinoid Analgesia in Postoperative Pain Management: From Molecular Mechanisms to Clinical Reality. International Journal of Molecular Sciences. 2024; 25(11):6268. https://doi.org/10.3390/ijms25116268
Chicago/Turabian StyleCarrascosa, Antonio J., Francisco Navarrete, Raquel Saldaña, María S. García-Gutiérrez, Belinda Montalbán, Daniela Navarro, Fernando M. Gómez-Guijarro, Ani Gasparyan, Elena Murcia-Sánchez, Abraham B. Torregrosa, and et al. 2024. "Cannabinoid Analgesia in Postoperative Pain Management: From Molecular Mechanisms to Clinical Reality" International Journal of Molecular Sciences 25, no. 11: 6268. https://doi.org/10.3390/ijms25116268
APA StyleCarrascosa, A. J., Navarrete, F., Saldaña, R., García-Gutiérrez, M. S., Montalbán, B., Navarro, D., Gómez-Guijarro, F. M., Gasparyan, A., Murcia-Sánchez, E., Torregrosa, A. B., Pérez-Doblado, P., Gutiérrez, L., & Manzanares, J. (2024). Cannabinoid Analgesia in Postoperative Pain Management: From Molecular Mechanisms to Clinical Reality. International Journal of Molecular Sciences, 25(11), 6268. https://doi.org/10.3390/ijms25116268











