Efficacy of Human Recombinant Growth Hormone in Females of a Non-Obese Hyperglycemic Mouse Model after Birth with Low Birth Weight
Abstract
1. Introduction
2. Results
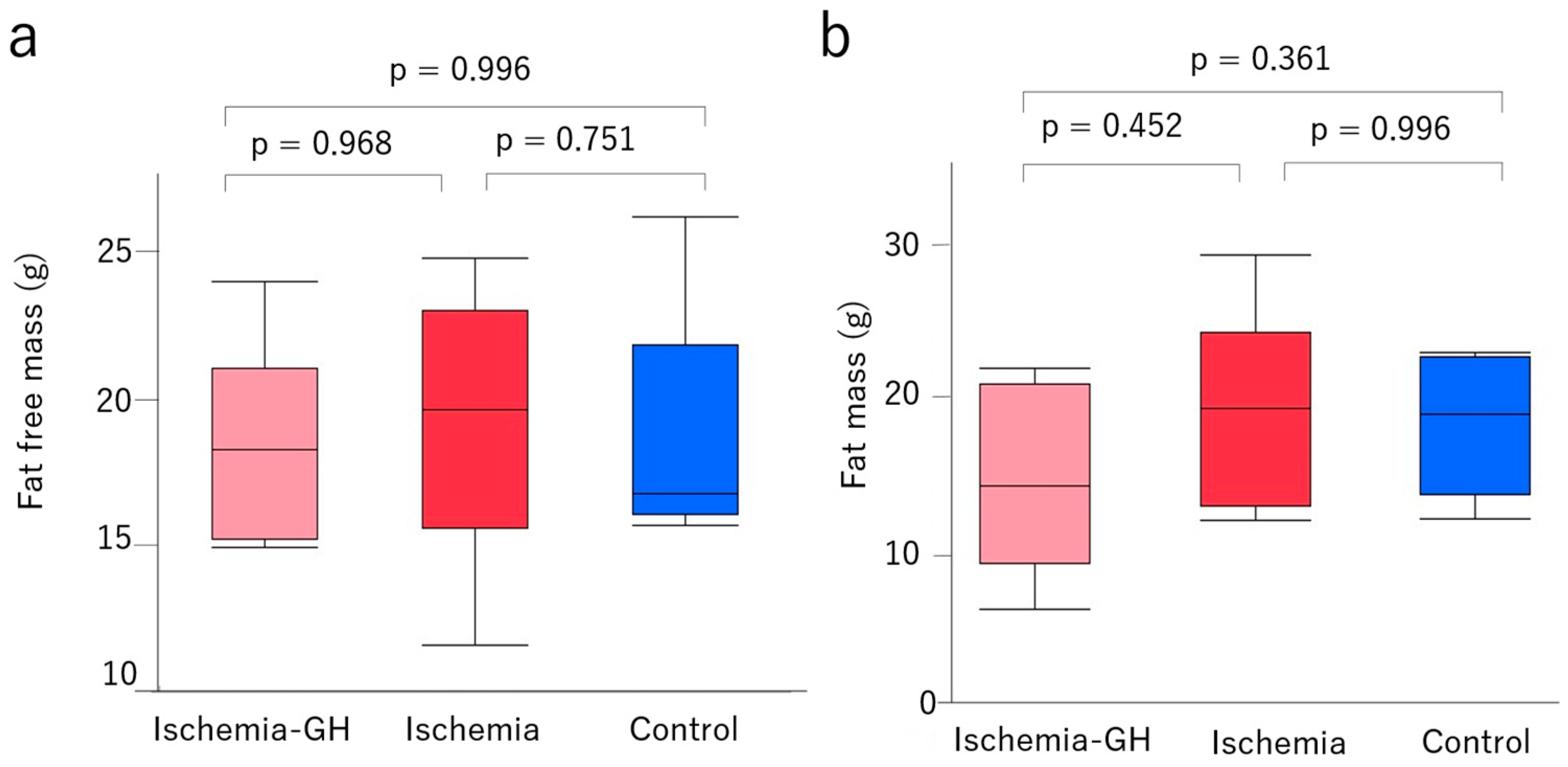
2.1. Body Weight and Body Composition
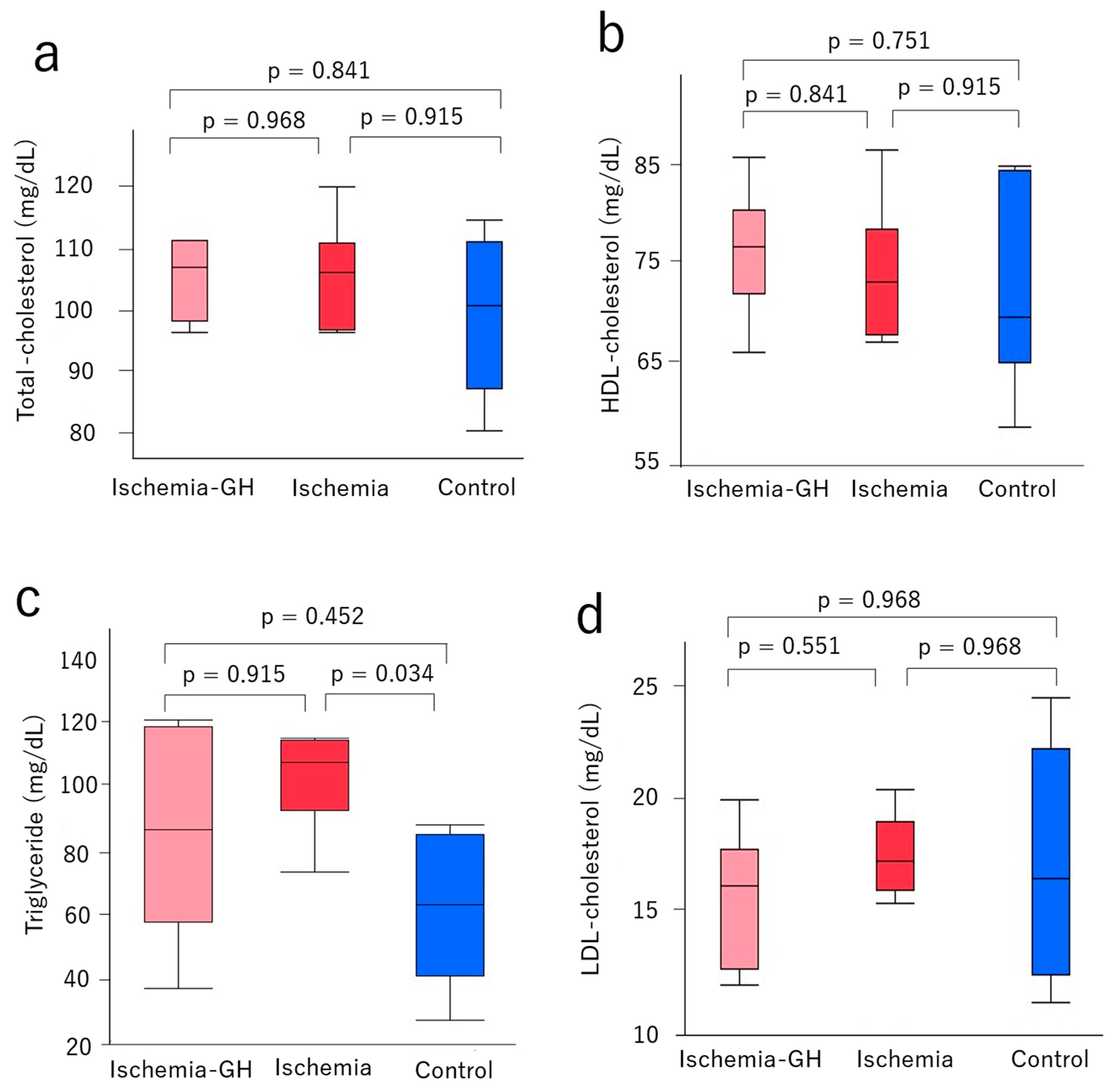
2.2. Glucose Metabolism and Serum Lipoproteins
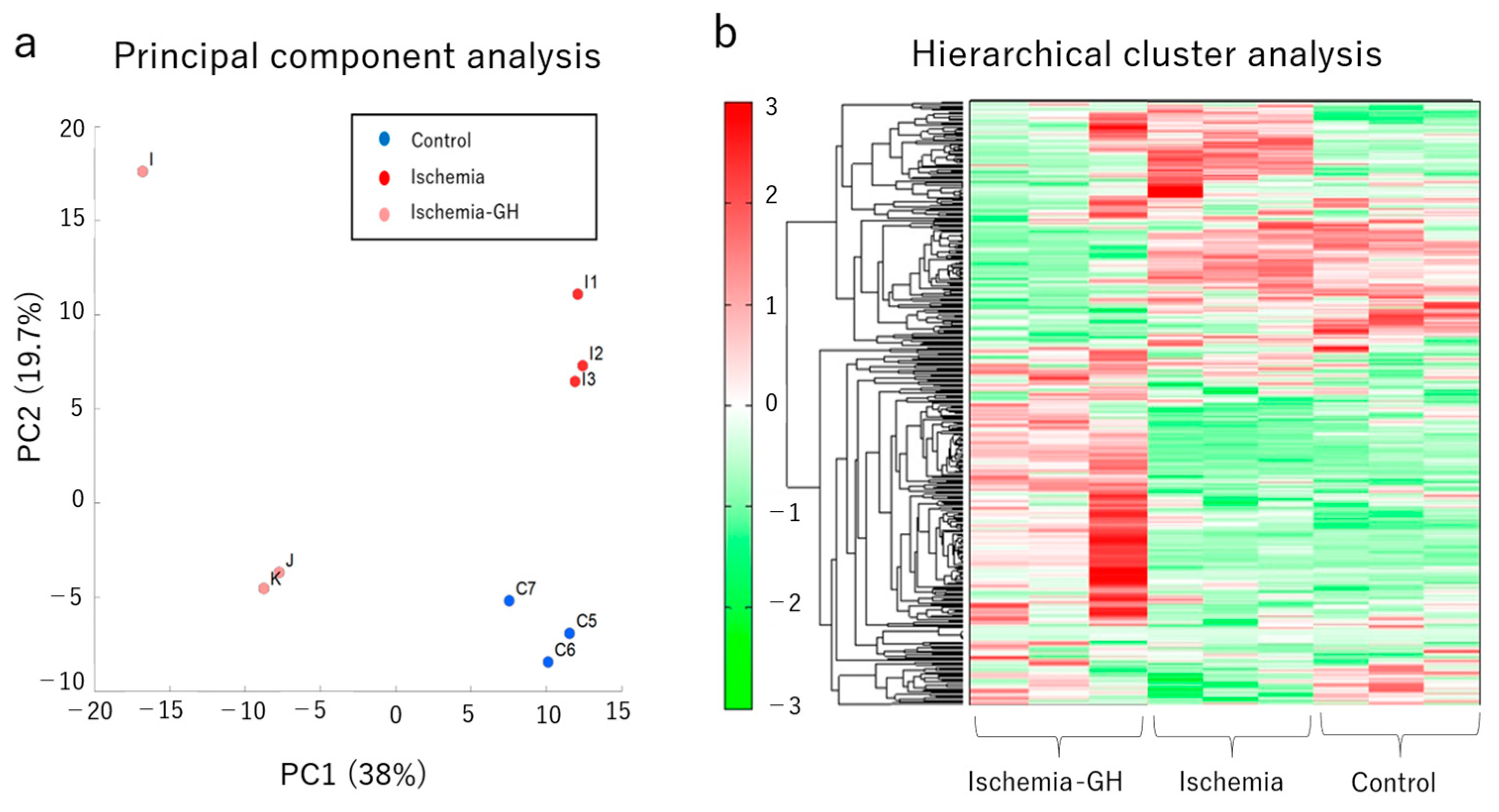
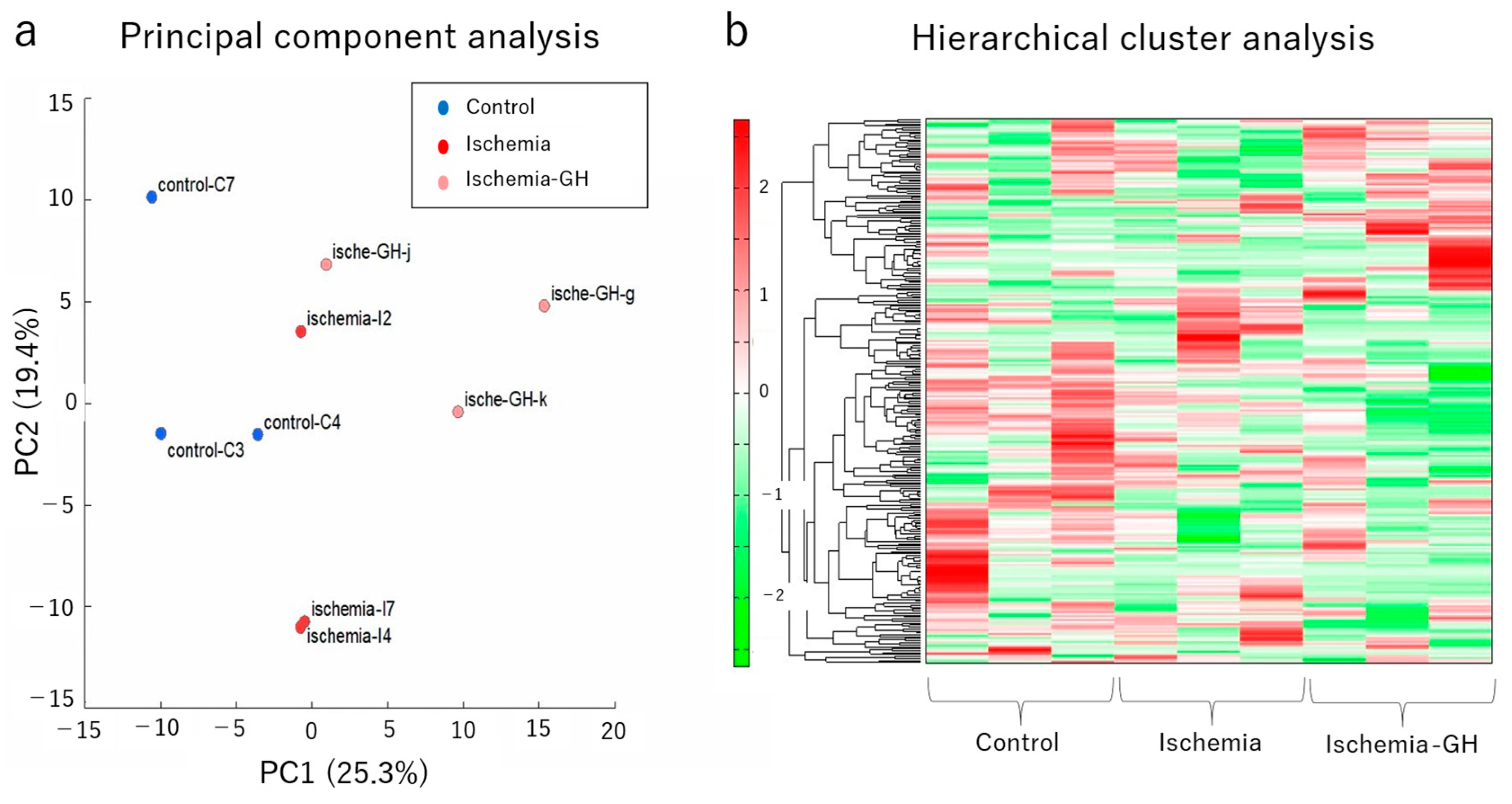
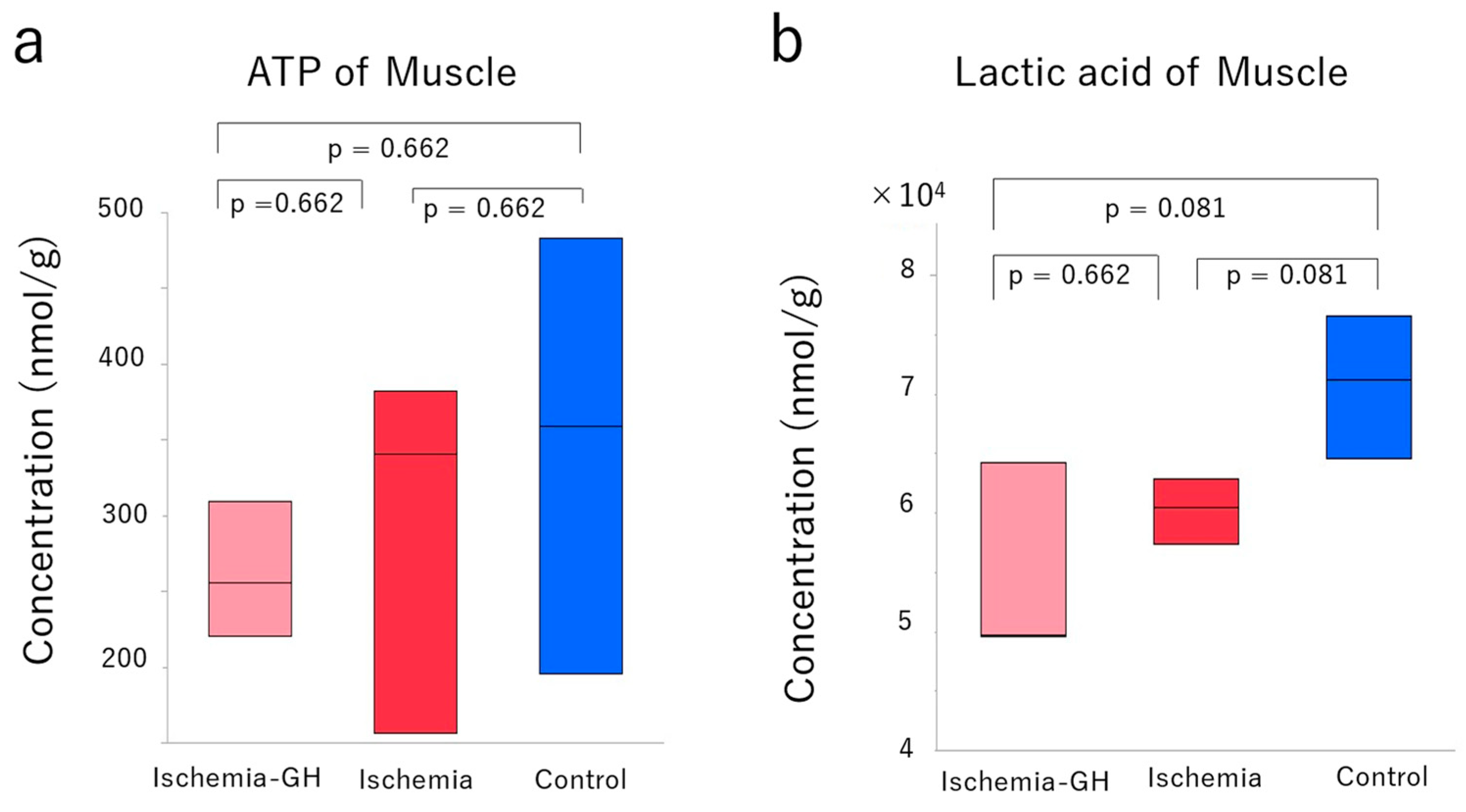
2.3. Metabolome Analyses
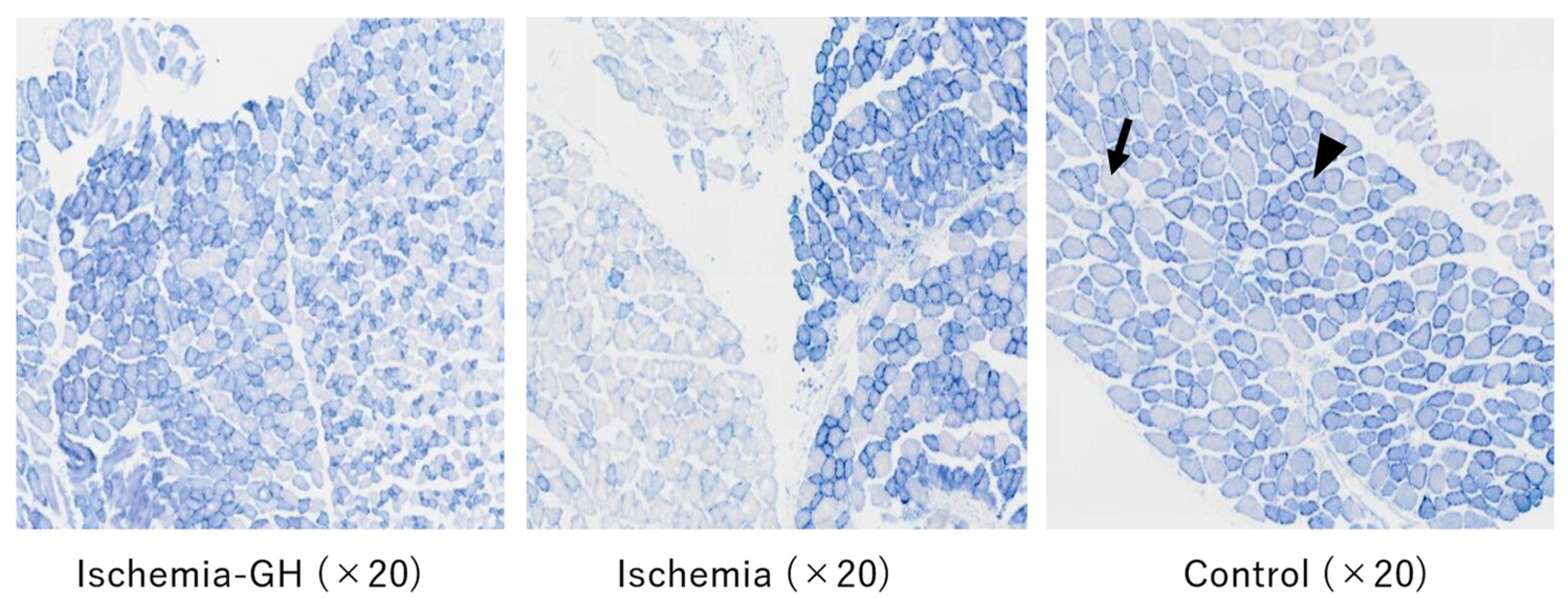
2.4. Muscle Tissue Analysis
3. Discussion
3.1. Insulin Resistance Related to Skeletal Muscle
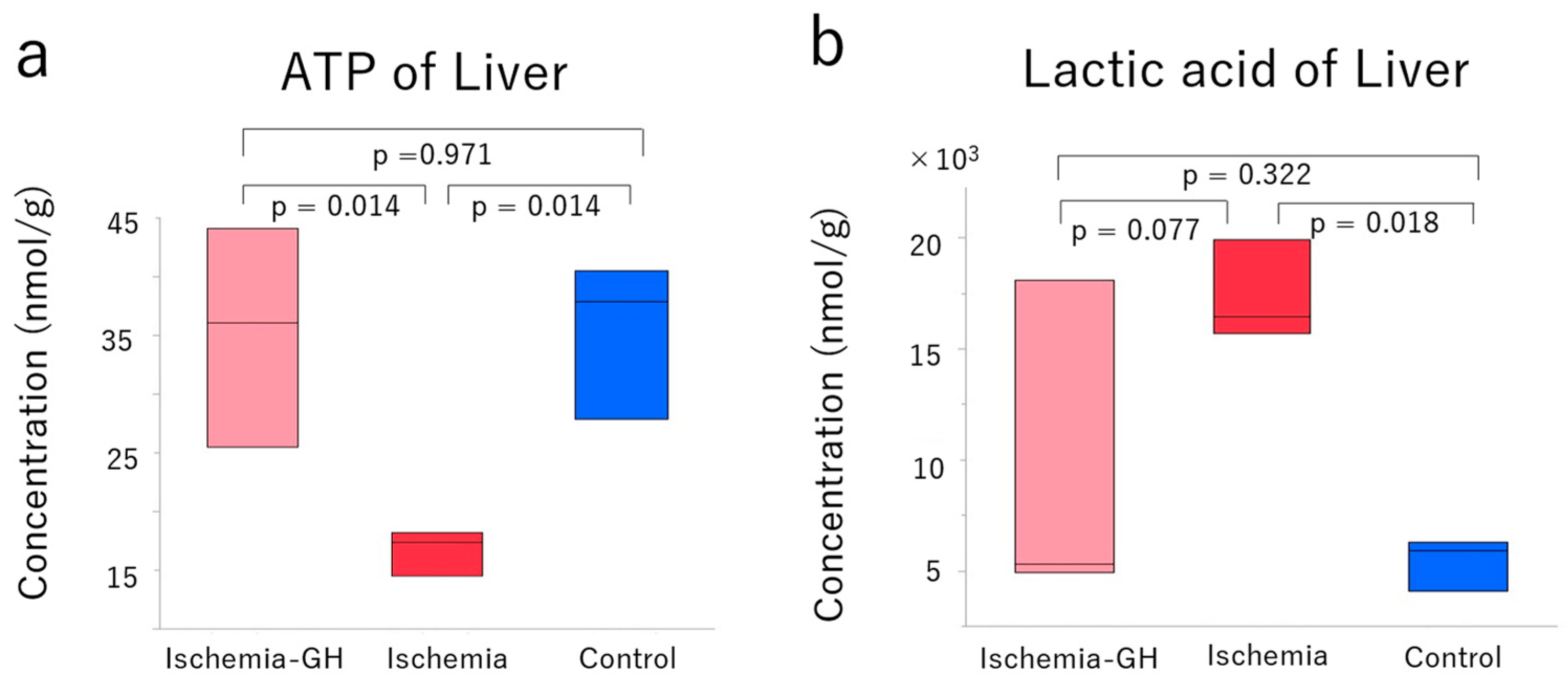
3.2. Mitochondrial Dysfunction of Liver
3.3. Improvement of Insulin Resistance
3.4. Limitations
3.5. Future Directions
4. Materials and Methods
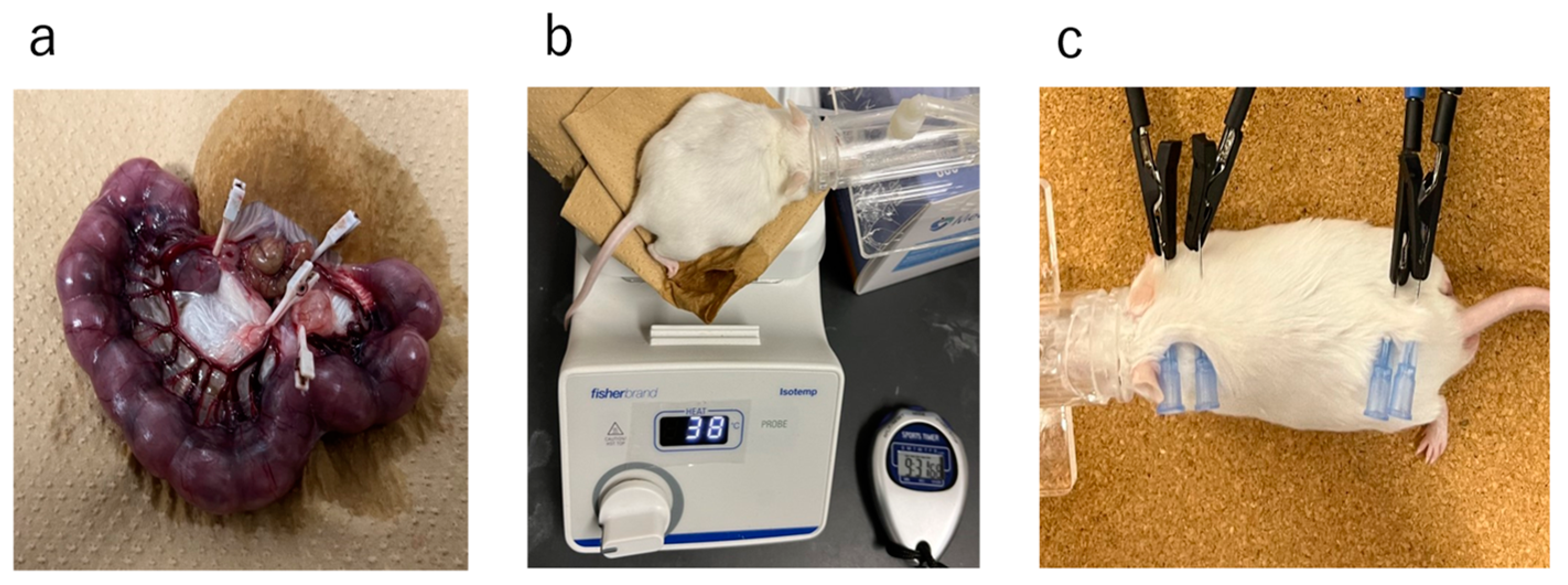
4.1. Study Design and Protocol
4.2. Body Weight and Body Composition Analyses
4.3. Glucose Metabolism and Serum Lipoprotein
4.4. Metabolome Analysis in Liver and Muscle
4.5. Muscle Tissue Analyses
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barker, D.J.; Hales, C.N.; Fall, C.H.; Osmond, C.; Phipps, K.; Clark, P.M. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): Relation to reduced fetal. Diabetologia 1993, 36, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.; Osmond, C.; Forsén, T.J.; Kajantie, E.; Eriksson, J.G. Trajectories of growth among children who have coronary events as adults. N. Engl. J. Med. 2005, 353, 1802–1809. [Google Scholar] [CrossRef] [PubMed]
- Fleming, T.P.; Watkins, A.J.; Velazquez, M.A.; Mathers, J.C.; Prentice, A.M.; Stephenson, J.; Barker, M.; Saffery, R.; Yajnik, C.S.; Eckert, J.J.; et al. Origins of lifetime health around the time of conception: Causes and consequences. Lancet 2018, 391, 1842–1852. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.A. Living with the past: Evolution, development, and patterns of disease. Science 2004, 305, 1733–1736. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.A.; Pinal, C. The developmental origins of adult disease. Matern. Child Nutr. 2005, 1, 130–141. [Google Scholar] [CrossRef] [PubMed]
- de Boo, H.A.; Harding, J.E. The developmental origins of the adult disease (Barker) hypothesis. Aust. N. Z. J. Obstet. Gynaecol. 2006, 46, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health, Labor and Welfare in Japan. Vital Statistics in Japan. Available online: https://www.mhlw.go.jp/toukei/list/81-1a.html (accessed on 1 April 2024).
- Kuwabara, R.; Urakami, T.; Yoshida, K.; Morioka, I. Case of type 2 diabetes possibly caused by excessive accumulation of visceral fat in a child born small-for-gestational age. J. Diabetes Investig. 2020, 11, 1366–1369. [Google Scholar] [CrossRef] [PubMed]
- Nagano, N.; Kaneko, C.; Ohashi, S.; Seya, M.; Takigawa, I.; Masunaga, K.; Morioka, I. Non-obese type 2 diabetes with a history of being an extremely preterm small-for-gestational-age infant without early adiposity. Int. J. Environ. Res. Public Health 2022, 19, 8560. [Google Scholar] [CrossRef] [PubMed]
- Urakami, T.; Kuwabara, R.; Habu, M.; Okuno, M.; Suzuki, J.; Takahashi, S.; Mugishima, H. Clinical characteristics of non-obese children with type 2 diabetes mellitus without involvement of beta-cell autoimmunity. Diabetes Res. Clin. Pract. 2013, 99, 105–111. [Google Scholar] [CrossRef]
- Sone, H.; Ito, H.; Ohashi, Y.; Akanuma, Y.; Yamada, N.; Japan Diabetes Complication Study Group. Obesity and type 2 diabetes in Japanese patients. Lancet 2003, 361, 85. [Google Scholar] [CrossRef]
- Katayama, D.; Nagano, N.; Shimizu, S.; Nakazaki, K.; Matsuda, K.; Takunaga, W.; Fuwa, K.; Aoki, R.; Morioka, I. A non-obese hyperglycemic mouse model that develops after birth with low birthweight. Biomedicines 2022, 10, 1642. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, R.H.; Arends, N.J.Y.; Bakker-van Waarde, W.M.; Jansen, M.; van Mil, E.G.A.H.; Mulder, J.; Odink, R.J.; Reeser, M.; Rongen-Westerlaken, C.; Stokvis-Brantsma, W.H.; et al. Long-term effects of growth hormone (GH) treatment on body composition and bone mineral density in short children born small and for-gestational-age: Six-year follow-up of a randomized controlled GH trial. Clin. Endocrinol. 2007, 67, 485–492. [Google Scholar] [CrossRef] [PubMed]
- van der Kaay, D.; Bakker, B.; van der Hulst, F.; Mul, D.; Mulder, J.; Schroor, E.; van Elswijk, D.; Rowaan, I.; Willeboer, M.; de Ridder, M.; et al. Randomized GH trial with two different dosages in combination with a GnRH analogue in short small for gestational age children: Effects on metabolic profile and serum GH, IGF1, and IGFBP3 levels. Eur. J. Endocrinol. 2010, 162, 887–895. [Google Scholar] [CrossRef] [PubMed]
- de Gouveia Buff Passone, C.; Franco, R.R.; Ito, S.S.; Trindade, E.; Polak, M.; Damiani, D.; Bernardo, W.M. Growth hormone treatment in Prader-Willi syndrome patients: Systematic review and meta-analysis. BMJ Paediatr. Open 2020, 4, e000630. [Google Scholar] [CrossRef] [PubMed]
- de Lind van Wijngaarden, R.F.; Siemensma, E.P.C.; Festen, D.A.M.; Otten, B.J.; van Mil, E.G.A.H.; Rotteveel, J.; Odink, R.J.H.; Bindels-de Heus, G.C.B.K.; van Leeuwen, M.; Haring, D.A.J.P.; et al. Efficacy and safety of long-term continuous growth hormone treatment in children with Prader-Willi syndrome. J. Clin. Endocrinol. Metab. 2009, 94, 4205–4215. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, J.O.; Thuesen, L.; Müller, J.; Ovesen, O.; Skakkebaek, N.E.; Christiansen, J.S. Three years of growth hormone treatment in growth hormone-deficient adults: Near normalization of body composition and physical performance. Eur. J. Endocrinol. 1994, 130, 224–228. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Czech, M.P. The GLUT4 glucose transporter. Cell Metab. 2007, 5, 237–252. [Google Scholar] [CrossRef]
- Leto, D.; Saltiel, A.R. Regulation of glucose transport by insulin: Traffic control of GLUT4. Nat. Rev. Mol. Cell Biol. 2012, 13, 383–396. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Peraldi, P.; Budavari, A.; Ellis, R.; White, M.F.; Spiegelman, B.M. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science 1996, 271, 665–668. [Google Scholar] [CrossRef]
- Shibata, T.; Takaguri, A.; Ichihara, K.; Satoh, K. Inhibition of the TNF-alpha-induced serine phosphorylation of IRS-1 at 636/639 by AICAR. J. Pharmacol. Sci. 2013, 122, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, S.; Fukuda, D.; Higashikuni, Y.; Tanaka, K.; Hirata, Y.; Murata, C.; Kim-Kaneyama, J.R.; Sato, F.; Bando, M.; Yagi, S.; et al. Obesity-induced DNA released from adipocytes stimulates chronic adipose tissue inflammation and insulin resistance. Sci. Adv. 2016, 2, e1501332. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Shinozawa, Y.; Iijima, Y.; Yu, B.C.; Sone, M.; Ooi, Y.; Watanaka, Y.; Chida, K.; Hakuno, F.; Takahashi, S. Tumor necrosis factor (TNF)-alpha-induced repression of GKAP42 protein levels through cGMP-dependent kinase (cGK)-Ialpha causes insulin resistance in 3T3-L1 adipocytes. J. Biol. Chem. 2015, 290, 5881–5892. [Google Scholar] [CrossRef] [PubMed]
- Hickey, M.S.; Carey, J.O.; Azevedo, J.L.; Houmard, J.A.; Pories, W.J.; Israel, R.G.; Dohm, G.L. Skeletal muscle fiber composition is related to adiposity and in vitro glucose transport rate in humans. Am. J. Physiol. 1995, 268, E453–E457. [Google Scholar] [CrossRef] [PubMed]
- Mårin, P.; Andersson, B.; Krotkiewski, M.; Björntorp, P. Muscle fiber composition and capillary density in women and men with NIDDM. Diabetes Care 1994, 17, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, B.; Ghosh, S.; Dysart, M.W.; Kanaan, G.N.; Chu, A.; Blais, A.; Rajamanickam, K.; Tsai, E.C.; Patti, M.-E.; Harper, M.-E. Low birth weight is associated with adiposity, impaired skeletal muscle energetics and weight loss resistance in mice. Int. J. Obes. 2015, 39, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Ayling, C.M.; Moreland, B.H.; Zanelli, J.M.; Schulster, D. Human growth hormone treatment of hypophysectomized rats increases the proportion of type-1 fibres in skeletal muscle. J. Endocrinol. 1989, 123, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Loughna, P.T.; Bates, P.C. Interactions between growth hormone and nutrition in hypophysectomised rats: Skeletal muscle myosin heavy chain mRNA. Biochem. Biophys. Res. Commun. 1994, 198, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Aroniadou-Anderjaska, V.; Lemon, P.W.; Gilloteaux, J. Effects of exogenous growth hormone on skeletal muscle of young female rats. Tissue Cell 1996, 28, 719–724. [Google Scholar] [CrossRef]
- Holtmann, B.; Wiese, S.; Samsam, M.; Grohmann, K.; Penninca, D.; Marini, R.; Sendtner, M. Triple knock-out of CNTF, LIF, and CT-1 defines cooperative and distinct roles of these neurotrophic factors for motoneuron maintenance and function. J. Neurosci. 2005, 25, 1778–1787. [Google Scholar] [CrossRef]
- Messa, G.A.M.; Piasecki, M.; Rittweger, J.; McPhee, J.S.; Koltai, E.; Radak, Z.; Simunic, B.; Heinonen, A.; Suominen, H.; Korhonen, M.T.; et al. Absence of an aging-related increase in fiber type grouping in athletes and non-athletes. Scand. J. Med. Sci. Sports 2020, 30, 2057–2069. [Google Scholar] [CrossRef]
- Coletti, C.; Acosta, G.F.; Keslacy, S.; Coletti, D. Exercise-mediated reinnervation of skeletal muscle in elderly people: An update. Eur. J. Transl. Myol. 2022, 32, 10416. [Google Scholar] [CrossRef] [PubMed]
- Caicedo, D.; Díaz, O.; Devesa, P.; Devesa, J. Growth hormone (GH) and the cardiovascular system. Int. J. Mol. Sci. 2018, 19, 290. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.; Wang, X.; Yang, C.; Dong, M.; Wang, F. Recombinant human growth hormone inhibits lipotoxicity, oxidative stress, and apoptosis in a mouse model of diabetic cardiomyopathy. Oxid. Med. Cell Longev. 2021, 2021, 3899356. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, S.; Marzetti, E.; Borst, S.E.; Leeuwenburgh, C. Modulation of GH/IGF-1 axis: Potential strategies to counteract sarcopenia in older adults. Mech. Ageing Dev. 2008, 129, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Perrini, S.; Laviola, L.; Carreira, M.C.; Cignarelli, A.; Natalicchio, A.; Giorgino, F. The GH/IGF1 axis and signaling pathways in the muscle and bone: Mechanisms underlying age-related skeletal muscle wasting and osteoporosis. J. Endocrinol. 2010, 205, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; Urakami, T.; Morioka, I. Greater insulin resistance in short children born small-for-gestational age than in children with growth hormone therapy. Pediatr. Int. 2021, 63, 1180–1184. [Google Scholar] [CrossRef] [PubMed]
- Breukhoven, P.E.; Kerkhof, G.F.; van Dijk, M.; Hokken-Joelega, A.C.S. Long-term impact of GH treatment during childhood on body composition and fat distribution in young adults born SGA. J. Clin. Endocrinol. Metab. 2011, 96, 3710–3716. [Google Scholar] [CrossRef] [PubMed]
- Bakker, N.E.; Kuppens, R.J.; Siemensma, E.P.C.; Tummers-de Lind van Wijngaarden, R.F.A.; Bindels-de Heus, G.C.B.; Bocca, G.; Haring, D.A.J.P.; Hoorweg-Nijman, J.J.G.; Houdijk, E.C.A.M.; Jira, P.E.; et al. Eight years of growth hormone treatment in children with Prader-Willi syndrome: Maintaining the positive effects. J. Clin. Endocrinol. Metab. 2013, 98, 4013–4022. [Google Scholar] [CrossRef]
- Feng, Z.; Hanson, R.W.; Berger, N.A.; Trubitsyn, A. Reprogramming of energy metabolism as a driver of aging. Oncotarget 2016, 7, 15410–15420. [Google Scholar] [CrossRef]
- Ardail, D.; Debon, A.; Perret-Vivancos, C.; Biol-N’Garagba, M.-C.; Krantic, S.; Lobie, P.E.; Morel, G. Growth hormone internalization in mitochondria decreases respiratory chain activity. Neuroendocrinology 2010, 91, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Granger, D.N.; Kvietys, P.R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015, 6, 524–551. [Google Scholar] [CrossRef] [PubMed]
- Rasola, A.; Bernardi, P. The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis 2007, 12, 815–833. [Google Scholar] [CrossRef] [PubMed]
- Clayton, P.E.; Cianfarani, S.; Czernichow, P.; Johannsson, G.; Rapaport, R.; Rogol, A. Management of the child born small for gestational age through to adulthood: A consensus statement of the International Societies of Pediatric Endocrinology and the Growth Hormone Research Society. J. Clin. Endocrinol. Metab. 2007, 92, 804–810. [Google Scholar] [CrossRef]
- Kubo, K.-I.; Deguchi, K.; Nagai, T.; Ito, Y.; Yoshida, K.; Endo, T.; Benner, S.; Shan, W.; Kitazawa, A.; Aramaki, M.; et al. Association of impaired neuronal migration with cognitive deficits in extremely preterm infants. JCI Insight 2017, 2, e88609. [Google Scholar] [CrossRef] [PubMed]
- Yokoya, S.; Tanaka, T.; Itabashi, K.; Osada, H.; Hirai, H.; Seino, Y. Efficacy and safety of growth hormone treatment in Japanese children with small-for-gestational-age short stature in accordance with Japanese guidelines. Clin. Pediatr. Endocrinol. 2018, 27, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Lukaski, H.C.; Johnson, P.E.; Bolonchuk, W.W.; Lykken, G.I. Assessment of fat-free mass using bioelectrical impedance measurements of the human body. Am. J. Clin. Nutr. 1985, 41, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Usui, S.; Hara, Y.; Hosaki, S.; Okazaki, M. A new on-line dual enzymatic method for simultaneous quantification of cholesterol and triglycerides in lipoproteins by HPLC. J. Lipid Res. 2002, 43, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, M.; Yamashita, S. Recent advances in analytical methods on lipoprotein subclasses: Calculation of particle numbers from lipid levels by gel permeation HPLC using “Spherical Particle Model”. J. Oleo Sci. 2016, 65, 265–282. [Google Scholar] [CrossRef]
- Okazaki, M.; Usui, S.; Ishigami, M.; Sakai, N.; Nakamura, T.; Matsuzawa, Y.; Yamashita, S. Identification of unique lipoprotein subclasses for visceral obesity by component analysis of cholesterol profile in high-performance liquid chromatography. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 578–584. [Google Scholar] [CrossRef]
- Ohashi, Y.; Hirayama, A.; Ishikawa, T.; Nakamura, S.; Shimizu, K.; Ueno, Y.; Tomita, M.; Soga, T. Depiction of metabolome changes in histidine-starved Escherichia coli by CE-TOFMS. Mol. Biosyst. 2008, 4, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Ooga, T.; Sato, H.; Nagashima, A.; Sasaki, K.; Tomita, M.; Soga, T.; Ohashi, Y. Metabolomic anatomy of an animal model revealing homeostatic imbalances in dyslipidaemia. Mol. Biosyst. 2011, 7, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Wong, D.T.; Hirayama, A.; Soga, T.; Tomita, M. Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics 2010, 6, 78–95. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Fujimori, T.; Sato, H.; Ishikawa, G.; Kami, K.; Ohashi, Y. Statistical hypothesis testing of factor loading in principal component analysis and its application to metabolite set enrichment analysis. BMC Bioinform. 2014, 15, 51. [Google Scholar] [CrossRef]
- Junker, B.H.; Klukas, C.; Schreiber, F. VANTED: A system for advanced data analysis and visualization in the context of biological networks. BMC Bioinform. 2006, 7, 109. [Google Scholar] [CrossRef]




| Major Category | Compound Name | Comparative Analysis | |||||
|---|---|---|---|---|---|---|---|
| Ischemia-GH vs. Ischemia | Ischemia-GH vs. Control | Ischemia vs. Control | |||||
| Ratio 1 | p-Value 2 | Ratio 1 | p-Value 2 | Ratio 1 | p-Value 2 | ||
| Anti-oxidant | Ascorbic acid | 1.0 | 0.802 | 1.1 | 0.566 | 1.1 | 0.184 |
| Anti-oxidant | Carnosine | 1.3 | 0.451 | 1.1 | 0.740 | 0.9 | 0.684 |
| Anti-oxidant | Hypotaurine | 1.4 | 0.076 | 1.8 | 0.041 * | 1.3 | 0.359 |
| Oxidative stress | 3-Indoxylsulfuric acid | 0.8 | 0.175 | 1.2 | 0.296 | 1.6 | 0.024 * (0.024 *) |
| Oxidative stress | Cysteine | 3.1 | 0.265 | 6.8 | 0.188 | 2.3 | 0.032 * (0.032) |
| Oxidative stress | Methionine sulfoxide | 1.2 | 0.723 | 1.4 | 0.564 | 1.2 | 0.444 |
| Oxidative stress | N,N-Dimethylglycine | 0.5 | 0.109 | 0.5 | 0.001 ** | 0.9 | 0.677 |
| Oxidative stress | S-Adenosylmethionine | 0.3 | 0.004 ** | 0.6 | 0.055 | 1.6 | 0.005 ** |
| Major Category | Compound Name | Comparative Analysis | |||||
|---|---|---|---|---|---|---|---|
| Ischemia-GH vs. Ischemia | Ischemia-GH vs. Control | Ischemia vs. Control | |||||
| Ratio 1 | p-Value 2 | Ratio 1 | p-Value 2 | Ratio 1 | p-Value 2 | ||
| Anti-oxidant | Ascorbic acid | 1.3 | 0.333 | 0.9 | 0.712 | 0.7 | 0.177 |
| Anti-oxidant | Carnosine | 0.9 | 0.762 | 0.8 | 0.517 | 0.9 | 0.822 |
| Anti-oxidant | Hypotaurine | 1.1 | 0.745 | 1.1 | 0.792 | 1.0 | 0.829 |
| Oxidative stress | 3-Indoxylsulfuric acid | 0.8 | N.A. | 0.9 | N.A. | 1.1 | 0.593 |
| Oxidative stress | Cysteine | 1.6 | 0.638 | 1.1 | 0.948 | 0.7 | 0.753 |
| Oxidative stress | Methionine sulfoxide | 0.7 | 0.104 | 0.6 | 0.153 | 0.9 | 0.749 |
| Oxidative stress | N,N-Dimethylglycine | 0.9 | 0.306 | 1.1 | 0.426 | 1.2 | 0.004 * |
| Oxidative stress | S-Adenosylmethionine | 0.9 | 0.593 | 0.9 | 0.396 | 1.0 | 0.718 |
| Type 1 Fibers | Type 2 Fibers | Total | |
|---|---|---|---|
| Ischemia-GH | 578 | 422 | 1000 |
| Ischemia | 288 | 712 | 1000 |
| Control | 573 | 427 | 1000 |
| Total | 1439 | 1561 | 3000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokunaga, W.; Nagano, N.; Matsuda, K.; Nakazaki, K.; Shimizu, S.; Okuda, K.; Aoki, R.; Fuwa, K.; Murakami, H.; Morioka, I. Efficacy of Human Recombinant Growth Hormone in Females of a Non-Obese Hyperglycemic Mouse Model after Birth with Low Birth Weight. Int. J. Mol. Sci. 2024, 25, 6294. https://doi.org/10.3390/ijms25126294
Tokunaga W, Nagano N, Matsuda K, Nakazaki K, Shimizu S, Okuda K, Aoki R, Fuwa K, Murakami H, Morioka I. Efficacy of Human Recombinant Growth Hormone in Females of a Non-Obese Hyperglycemic Mouse Model after Birth with Low Birth Weight. International Journal of Molecular Sciences. 2024; 25(12):6294. https://doi.org/10.3390/ijms25126294
Chicago/Turabian StyleTokunaga, Wataru, Nobuhiko Nagano, Kengo Matsuda, Kimitaka Nakazaki, Shoichi Shimizu, Koh Okuda, Ryoji Aoki, Kazumasa Fuwa, Hitohiko Murakami, and Ichiro Morioka. 2024. "Efficacy of Human Recombinant Growth Hormone in Females of a Non-Obese Hyperglycemic Mouse Model after Birth with Low Birth Weight" International Journal of Molecular Sciences 25, no. 12: 6294. https://doi.org/10.3390/ijms25126294
APA StyleTokunaga, W., Nagano, N., Matsuda, K., Nakazaki, K., Shimizu, S., Okuda, K., Aoki, R., Fuwa, K., Murakami, H., & Morioka, I. (2024). Efficacy of Human Recombinant Growth Hormone in Females of a Non-Obese Hyperglycemic Mouse Model after Birth with Low Birth Weight. International Journal of Molecular Sciences, 25(12), 6294. https://doi.org/10.3390/ijms25126294







