Unraveling the Roles of Neuropeptides in the Chemosensation of the Root-Knot Nematode Meloidogyne javanica
Abstract
1. Introduction
2. Results
2.1. TRE Treatment Causes Transcriptional Changes in M. javanica
2.2. M. javanica Primarily Responds to TRE at 1 h
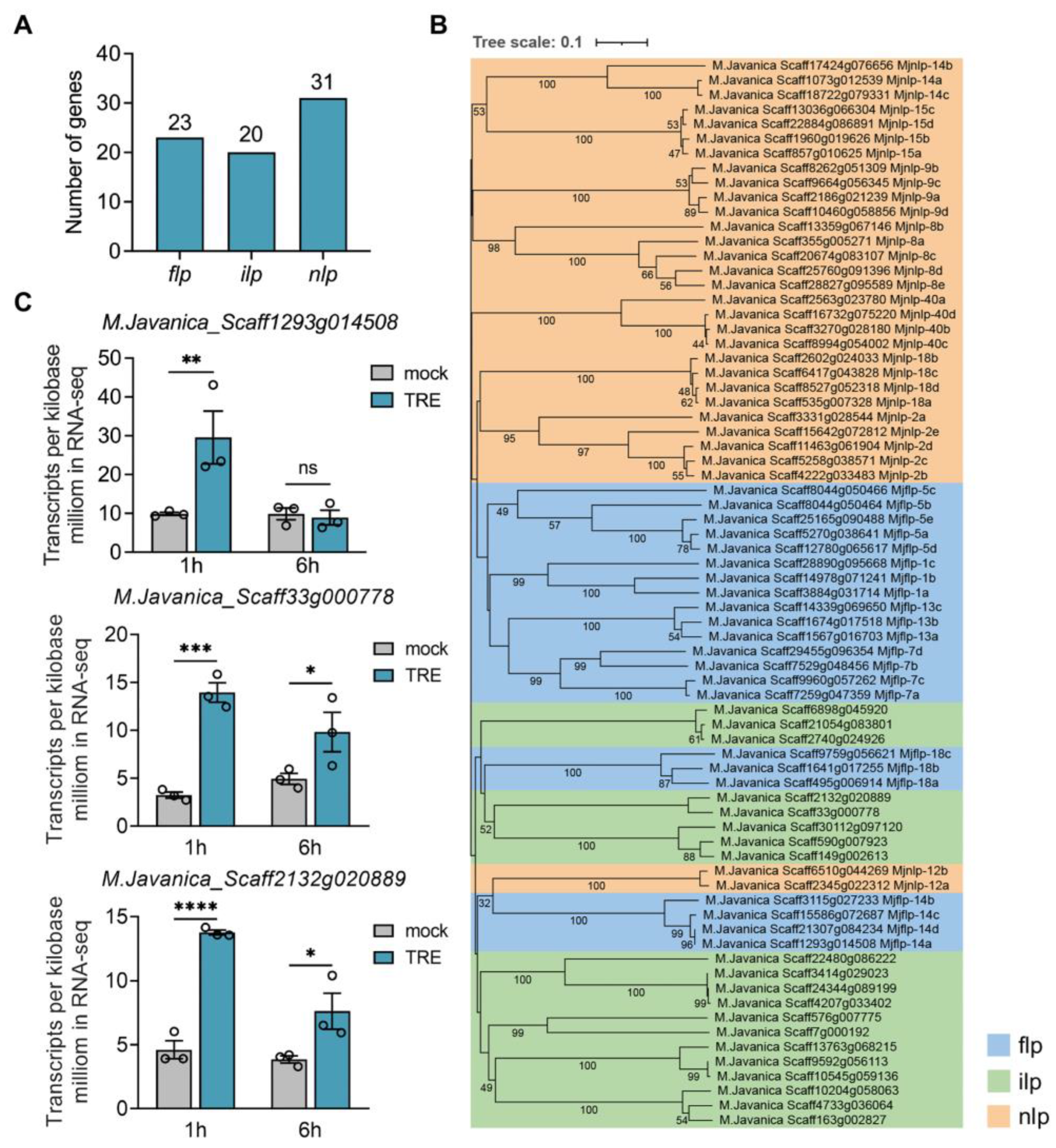
2.3. Neuropeptide genes in M. javanica
2.4. The Precursors of MjFLP-14 Neuropeptides (Pro-Neuropeptide) Contain Two Neuropeptides
2.5. MjFLP-14-2 Affects the Root Chemotaxis of M. javanica
3. Discussion
4. Materials and Methods
4.1. Nematode Propagation
4.2. Tomato Root Exudate (TRE) Collection
4.3. Sample Collection for RNA-Seq
4.4. RNA Extraction, Library Preparation, and RNA-Seq
4.5. Analysis of RNA-Seq Data
4.6. BLAST Searches for Neuropeptide Genes in M. javanica
4.7. Post-BLAST Analysis
4.8. Phylogenetic Tree Construction
4.9. Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
4.10. Peptide Synthesis and Root Attraction Assay
4.11. Statistics Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, J.; Gheysen, G.; Fenoll, C. Genomics and Molecular Genetics of Plant-Nematode Interactions; Springer: Dordrecht, The Netherlands, 2011; pp. 3–20. [Google Scholar]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.L.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.H.; Finckh, M.R.; Hallmann, J. Oilseed radish/black oat subsidiary crops can help regulate plant-parasitic nematodes under non-inversion tillage in an organic wheat-potato rotation. Nematology 2017, 19, 1135–1146. [Google Scholar] [CrossRef]
- Poveda, J.; Abril-Urias, P.; Escobar, C. Biological Control of Plant-Parasitic Nematodes by Filamentous Fungi Inducers of Resistance: Trichoderma, Mycorrhizal and Endophytic Fungi. Front. Microbiol. 2020, 11, 992. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, S.; Li, H.; Wang, R.; Zhang, K.Q.; Xu, J. Fungi-Nematode Interactions: Diversity, Ecology, and Biocontrol Prospects in Agriculture. J. Fungi 2020, 6, 206. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.; Vicente, C.S.L.; Menéndez, E.; Faria, J.M.S.; Rusinque, L.; Camacho, M.J.; Inácio, M.L. The Fight against Plant-Parasitic Nematodes: Current Status of Bacterial and Fungal Biocontrol Agents. Pathogens 2022, 11, 1178. [Google Scholar] [CrossRef] [PubMed]
- Tsegay, M.W.; Wallau, M.O.; Liu, C.; Dubeux, J.C.; Grabau, Z.J. Crop rotation for management of plant-parasitic nematodes in forage corn production. Agron. J. 2023, 116, 313–325. [Google Scholar] [CrossRef]
- Milligan, S.B.; Bodeau, J.; Yaghoobi, J.; Kaloshian, I.; Zabel, P.; Williamson, V.M. The Root Knot Nematode Resistance Gene Mi from Tomato Is a Member of the Leucine Zipper, Nucleotide Binding, Leucine-Rich Repeat Family of Plant Genes. Plant Cell 1998, 10, 1307–1319. [Google Scholar] [CrossRef] [PubMed]
- Bozbuga, R.; Dasgan, H.Y.; Akhoundnejad, Y.; Imren, M.; Günay, O.C.; Toktay, H. Effect of Mi Gene and Nematode Resistance on Tomato Genotypes Using Molecular and Screening Assay. Cytol. Genet. 2020, 54, 154–164. [Google Scholar] [CrossRef]
- Jacquet, M.; Bongiovanni, M.; Martinez, M.; Verschave, P.; Wajnberg, E.; Castagnone-Sereno, P. Variation in resistance to the root-knot nematode Meloidogyne incognita in tomato genotypes bearing the Mi gene. Plant Pathol. 2005, 54, 93–99. [Google Scholar] [CrossRef]
- El-Sappah, A.H.; Islam, M.M.; El-awady, H.; Yan, S.; Qi, S.; Liu, J.; Cheng, G.T.; Liang, Y. Tomato Natural Resistance Genes in Controlling the Root-Knot Nematode. Genes 2019, 10, 925. [Google Scholar] [CrossRef]
- Kimber, M.J.; Fleming, C.C. Neuromuscular function in plant parasitic nematodes: A target for novel control strategies? Parasitology 2005, 131, 129–142. [Google Scholar] [CrossRef]
- Mousley, A.; Maule, A.G.; Halton, D.W.; Marks, N.J. Inter-phyla studies on neuropeptides: The potential for broad-spectrum anthelmintic and/or endectocide discovery. Parasitology 2005, 131, 143–167. [Google Scholar] [CrossRef]
- McVeigh, P.; Alexander-Bowman, S.; Veal, E.; Mousley, A.; Marks, N.J.; Maule, A.G. Neuropeptide-like protein diversity in phylum Nematoda. Int. J. Parasitol. 2008, 38, 1493–1503. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Kim, K. Neuropeptides. WormBook. 2008. Available online: http://www.wormbook.org/chapters/www_neuropeptides/neuropeptides.html (accessed on 5 June 2024).
- Kimura, K.D.; Tissenbaum, H.A.; Liu, Y.; Ruvkun, G. daf-2, an Insulin Receptor–Like Gene That Regulates Longevity and Diapause in Caenorhabditis elegans. Science 1997, 277, 942–946. [Google Scholar] [CrossRef]
- Bargmann, C.I. Neurobiology of the Caenorhabditis elegans Genome. Science 1998, 282, 2028–2033. [Google Scholar] [CrossRef] [PubMed]
- McVeigh, P.; Atkinson, L.; Marks, N.J.; Mousley, A.; Dalzell, J.J.; Sluder, A.; Hammerland, L.; Maule, A.G. Parasite neuropeptide biology: Seeding rational drug target selection? Int. J. Parasitol. Drugs Drug Resist. 2012, 2, 76–91. [Google Scholar] [CrossRef] [PubMed]
- Bhat, U.S.; Shahi, N.; Surendran, S.; Babu, K. Neuropeptides and Behaviors: How Small Peptides Regulate Nervous System Function and Behavioral Outputs. Front. Mol. Neurosci. 2021, 14, 786471. [Google Scholar] [CrossRef]
- Abad, P.; Gouzy, J.; Aury, J.-M.; Castagnone-Sereno, P.; Danchin, E.G.J.; Deleury, E.; Perfus-Barbeoch, L.; Anthouard, V.; Artiguenave, F.; Blok, V.C.; et al. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat. Biotechnol. 2008, 26, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Blanc-Mathieu, R.; Perfus-Barbeoch, L.; Aury, J.-M.; Da Rocha, M.; Gouzy, J.; Sallet, E.; Martin-Jimenez, C.; Bailly-Bechet, M.; Castagnone-Sereno, P.; Flot, J.-F.; et al. Hybridization and polyploidy enable genomic plasticity without sex in the most devastating plant-parasitic nematodes. PLoS Genet. 2017, 13, e1006777. [Google Scholar] [CrossRef]
- Koutsovoulos, G.D.; Poullet, M.; Elashry, A.; Kozlowski, D.K.L.; Sallet, E.; Da Rocha, M.; Perfus-Barbeoch, L.; Martin-Jimenez, C.; Frey, J.E.; Ahrens, C.H.; et al. Genome assembly and annotation of Meloidogyne enterolobii, an emerging parthenogenetic root-knot nematode. Sci. Data 2020, 7, 324. [Google Scholar] [CrossRef]
- Kimber, M.J.; McKinney, S.; McMaster, S.; Day, T.A.; Fleming, C.C.; Maule, A.G. flp gene disruption in a parasitic nematode reveals motor dysfunction and unusual neuronal sensitivity to RNA interference. FASEB J. 2007, 21, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Banakar, P.; Sharma, A.; Lilley, C.J.; Gantasala, N.P.; Kumar, M.; Rao, U. Combinatorial in vitro RNAi of two neuropeptide genes and a pharyngeal gland gene on Meloidogyne incognita. Nematology 2015, 17, 155–167. [Google Scholar] [CrossRef]
- Dash, M.; Dutta, T.K.; Phani, V.; Papolu, P.K.; Shivakumara, T.N.; Rao, U. RNAi-mediated disruption of neuropeptide genes, nlp-3 and nlp-12, cause multiple behavioral defects in Meloidogyne incognita. Biochem. Biophys. Res. Commun. 2017, 490, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Warnock, N.D.; Wilson, L.; Patten, C.; Fleming, C.C.; Maule, A.G.; Dalzell, J.J. Nematode neuropeptides as transgenic nematicides. PLoS Pathog. 2017, 13, e1006237. [Google Scholar] [CrossRef] [PubMed]
- Čepulytė, R.; Danquah, W.B.; Bruening, G.; Williamson, V.M. Potent Attractant for Root-Knot Nematodes in Exudates from Seedling Root Tips of Two Host Species. Sci. Rep. 2018, 8, 10847. [Google Scholar] [CrossRef] [PubMed]
- Beets, I.; Zels, S.; Vandewyer, E.; Demeulemeester, J.; Caers, J.; Baytemur, E.; Courtney, A.; Golinelli, L.; Hasakioğulları, İ.; Schafer, W.R.; et al. System-wide mapping of peptide-GPCR interactions in C. elegans. Cell Rep. 2023, 42, 113058. [Google Scholar] [CrossRef] [PubMed]
- Pu, L.; Wang, J.; Lu, Q.; Nilsson, L.; Philbrook, A.; Pandey, A.; Zhao, L.; Schendel, R.V.; Koh, A.; Peres, T.V.; et al. Dissecting the genetic landscape of GPCR signaling through phenotypic profiling in C. elegans. Nat. Commun. 2023, 14, 8410. [Google Scholar] [CrossRef]
- Ferkey, D.M.; Sengupta, P.; L’Etoile, N.D. Chemosensory signal transduction in Caenorhabditis elegans. Genetics 2021, 217, iyab004. [Google Scholar] [CrossRef] [PubMed]
- Dyer, S.; Weir, R.; Cox, D.; Cheseto, X.; Torto, B.; Dalzell, J.J. Ethylene Response Factor (ERF) genes modulate plant root exudate composition and the attraction of plant parasitic nematodes. Int. J. Parasitol. 2019, 49, 999–1003. [Google Scholar] [CrossRef]
- Oota, M.; Toyoda, S.; Kotake, T.; Wada, N.; Hashiguchi, M.; Akashi, R.; Ishikawa, H.; Favery, B.; Tsai, A.Y.-L.; Sawa, S. Rhamnogalacturonan-I as a nematode chemoattractant from Lotus corniculatus L. super-growing root culture. Front. Plant Sci. 2023, 13, 1008725. [Google Scholar] [CrossRef]
- Oota, M.; Tsai, A.Y.-L.; Aoki, D.; Matsushita, Y.; Toyoda, S.; Fukushima, K.; Saeki, K.; Toda, K.; Perfus-Barbeoch, L.; Favery, B.; et al. Identification of Naturally Occurring Polyamines as Root-Knot Nematode Attractants. Mol. Plant 2020, 13, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Huang, M.H.; Li, C.J.; Hua, C.; Qin, R.F.; Chang, D.D.; Jiang, D.; Zhao, L.; Wang, X.; Yu, J.Y.; et al. Responses of infective juveniles of the soybean cyst nematode (Heterodera glycines) and the root-knot nematodes (Meloidogyne hapla, M. incognita) to amino acids. Nematology 2022, 24, 1049–1062. [Google Scholar] [CrossRef]
- Gahoi, S.; Gautam, B. Identification and analysis of insulin like peptides in nematode secretomes provide targets for parasite control. Bioinformation 2016, 12, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.A.; Lilley, C.J.; McCarthy, J.; Atkinson, H.J.; Urwin, P.E. Plant-parasitic nematodes respond to root exudate signals with host-specific gene expression patterns. PLoS Pathog. 2019, 15, e1007503. [Google Scholar] [CrossRef] [PubMed]
- McVeigh, P.; Leech, S.; Mair, G.R.; Marks, N.J.; Geary, T.G.; Maule, A.G. Analysis of FMRFamide-like peptide (FLP) diversity in phylum Nematoda. Int. J. Parasitol. 2005, 35, 1043–1060. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.J.G.; McVeigh, P.; McMaster, S.; Fleming, C.C.; Maule, A.G. FMRFamide-like peptides in root knot nematodes and their potential role in nematode physiology. J. Helminthol. 2010, 84, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.; Falquet, L.; Vandewyer, E.; Beets, I.; Glauser, D.A. Signaling via the FLP-14/FRPR-19 neuropeptide pathway sustains nociceptive response to repeated noxious stimuli in C. elegans. PLoS Genet. 2021, 17, e1009880. [Google Scholar] [CrossRef]
- Papolu, P.K.; Gantasala, N.P.; Kamaraju, D.; Banakar, P.; Sreevathsa, R.; Rao, U. Utility of Host Delivered RNAi of Two FMRF Amide Like Peptides, flp-14 and flp-18, for the Management of Root Knot Nematode, Meloidogyne incognita. PLoS ONE 2013, 8, e80603. [Google Scholar] [CrossRef] [PubMed]
- Kumari, C.; Dutta, T.K.; Chaudhary, S.; Banakar, P.; Papolu, P.K.; Rao, U. Molecular characterization of FMRFamide-like peptides in Meloidogyne graminicola and analysis of their knockdown effect on nematode infectivity. Gene 2017, 619, 50–60. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Peymen, K.; Watteyne, J.; Frooninckx, L.; Schoofs, L.; Beets, I. The FMRFamide-Like Peptide Family in Nematodes. Front. Endocrinol. 2014, 5, 90. [Google Scholar] [CrossRef] [PubMed]
- Teufel, F.; Almagro Armenteros, J.J.; Johansen, A.R.; Gíslason, M.H.; Pihl, S.I.; Tsirigos, K.D.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 6.0 predicts all five types of signal peptides using protein language models. Nat. Biotechnol. 2022, 40, 1023–1025. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]




| Sample | Raw Data | Clean Data | Survival Percentage (%) | Mapping Rate (%) |
|---|---|---|---|---|
| mock1h_1 | 38,356,574 | 37,765,286 | 98.46 | 95.21 |
| mock1h_2 | 35,490,768 | 34,994,387 | 98.60 | 95.85 |
| mock1h_3 | 32,613,765 | 32,155,106 | 98.59 | 96.32 |
| TRE1h_1 | 29,664,312 | 29,282,765 | 98.71 | 95.21 |
| TRE1h_2 | 34,020,431 | 33,620,422 | 98.82 | 95.87 |
| TRE1h_3 | 31,189,106 | 30,799,201 | 98.75 | 95.81 |
| mock6h_1 | 32,607,581 | 32,126,436 | 98.52 | 94.50 |
| mock6h_2 | 34,724,144 | 34,277,033 | 98.71 | 95.69 |
| mock6h_3 | 42,798,243 | 42,222,837 | 98.66 | 95.69 |
| TRE6h_1 | 33,362,074 | 32,944,845 | 98.75 | 95.26 |
| TRE6h_2 | 36,609,225 | 36,141,861 | 98.72 | 95.11 |
| TRE6h_3 | 33,611,572 | 33,078,735 | 98.41 | 95.22 |
| Total | 415,047,795 | 409,408,914 |
| Gene ID | log2FC | Description | |
|---|---|---|---|
| 1 | M.Javanica_Scaff1717g017816 | 10.01 | Zinc finger nuclear hormone receptor-type |
| 2 | M.Javanica_Scaff8002g050303 | 8.70 | - |
| 3 | M.Javanica_Scaff13615g067812 | 8.55 | Zinc finger nuclear hormone receptor-type |
| 4 | M.Javanica_Scaff2813g025362 | 8.01 | 7TM GPCR serpentine receptor class g (Srg) |
| 5 | M.Javanica_Scaff275g004293 | 7.51 | - |
| 6 | M.Javanica_Scaff28572g095259 | 7.48 | 7TM GPCR serpentine receptor class d (Srd) |
| 7 | M.Javanica_Scaff7668g048992 | 7.41 | - |
| 8 | M.Javanica_Scaff11760g062770 | 7.09 | - |
| 9 | M.Javanica_Scaff14283g069509 | 6.97 | - |
| 10 | M.Javanica_Scaff4004g032378 | 6.91 | - |
| 11 | M.Javanica_Scaff13439g067345 | 6.51 | Zinc finger nuclear hormone receptor-type |
| 12 | M.Javanica_Scaff1298g014556 | 6.39 | Pectin lyase fold/virulence factor |
| 13 | M.Javanica_Scaff21028g083757 | 6.29 | - |
| 14 | M.Javanica_Scaff275g004289 | 6.16 | - |
| 15 | M.Javanica_Scaff4732g036058 | 5.81 | - |
| 16 | M.Javanica_Scaff4717g035993 | 5.72 | 7TM GPCR chemoreceptor (Srsx) |
| 17 | M.Javanica_Scaff22685g086570 | 5.70 | - |
| 18 | M.Javanica_Scaff22685g086569 | 5.56 | - |
| 19 | M.Javanica_Scaff5658g040440 | 5.43 | CAP superfamily |
| 20 | M.Javanica_Scaff28396g095016 | 5.36 | - |
| Gene ID | log2FC | Description | |
|---|---|---|---|
| 1 | M.Javanica_Scaff4047g032589 | 13.20 | - |
| 2 | M.Javanica_Scaff1588g016853 | 12.14 | - |
| 3 | M.Javanica_Scaff1298g014556 | 10.27 | Pectin lyase fold/virulence factor |
| 4 | M.Javanica_Scaff1588g016854 | 10.22 | - |
| 5 | M.Javanica_Scaff13615g067812 | 9.05 | Zinc finger nuclear hormone receptor-type |
| 6 | M.Javanica_Scaff2019g020060 | 8.71 | - |
| 7 | M.Javanica_Scaff14881g071003 | 8.18 | - |
| 8 | M.Javanica_Scaff8002g050303 | 8.05 | - |
| 9 | M.Javanica_Scaff4004g032378 | 7.55 | - |
| 10 | M.Javanica_Scaff110g002058 | 7.53 | - |
| 11 | M.Javanica_Scaff7797g049511 | 7.50 | - |
| 12 | M.Javanica_Scaff12043g063606 | 7.13 | - |
| 13 | M.Javanica_Scaff1896g019155 | 6.95 | - |
| 14 | M.Javanica_Scaff5267g038619 | 6.92 | - |
| 15 | M.Javanica_Scaff7479g048256 | 6.92 | - |
| 16 | M.Javanica_Scaff17664g077171 | 6.87 | Ribonuclease H-like superfamily |
| 17 | M.Javanica_Scaff410g005950 | 6.84 | NAD(P)-binding domain superfamily |
| 18 | M.Javanica_Scaff4857g036668 | 6.58 | Zona pellucida domain |
| 19 | M.Javanica_Scaff13439g067345 | 6.52 | Zinc finger nuclear hormone receptor-type |
| 20 | M.Javanica_Scaff879g010834 | 6.21 | Pectin lyase fold/virulence factor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, C.; Zhang, L. Unraveling the Roles of Neuropeptides in the Chemosensation of the Root-Knot Nematode Meloidogyne javanica. Int. J. Mol. Sci. 2024, 25, 6300. https://doi.org/10.3390/ijms25126300
Mo C, Zhang L. Unraveling the Roles of Neuropeptides in the Chemosensation of the Root-Knot Nematode Meloidogyne javanica. International Journal of Molecular Sciences. 2024; 25(12):6300. https://doi.org/10.3390/ijms25126300
Chicago/Turabian StyleMo, Chenmi, and Lei Zhang. 2024. "Unraveling the Roles of Neuropeptides in the Chemosensation of the Root-Knot Nematode Meloidogyne javanica" International Journal of Molecular Sciences 25, no. 12: 6300. https://doi.org/10.3390/ijms25126300
APA StyleMo, C., & Zhang, L. (2024). Unraveling the Roles of Neuropeptides in the Chemosensation of the Root-Knot Nematode Meloidogyne javanica. International Journal of Molecular Sciences, 25(12), 6300. https://doi.org/10.3390/ijms25126300





