Noradrenaline and Adrenoreceptors Are Involved in the Regulation of Prostaglandin I2 Production in the Porcine Endometrium after Experimentally Induced Inflammation
Abstract
:1. Introduction
2. Results
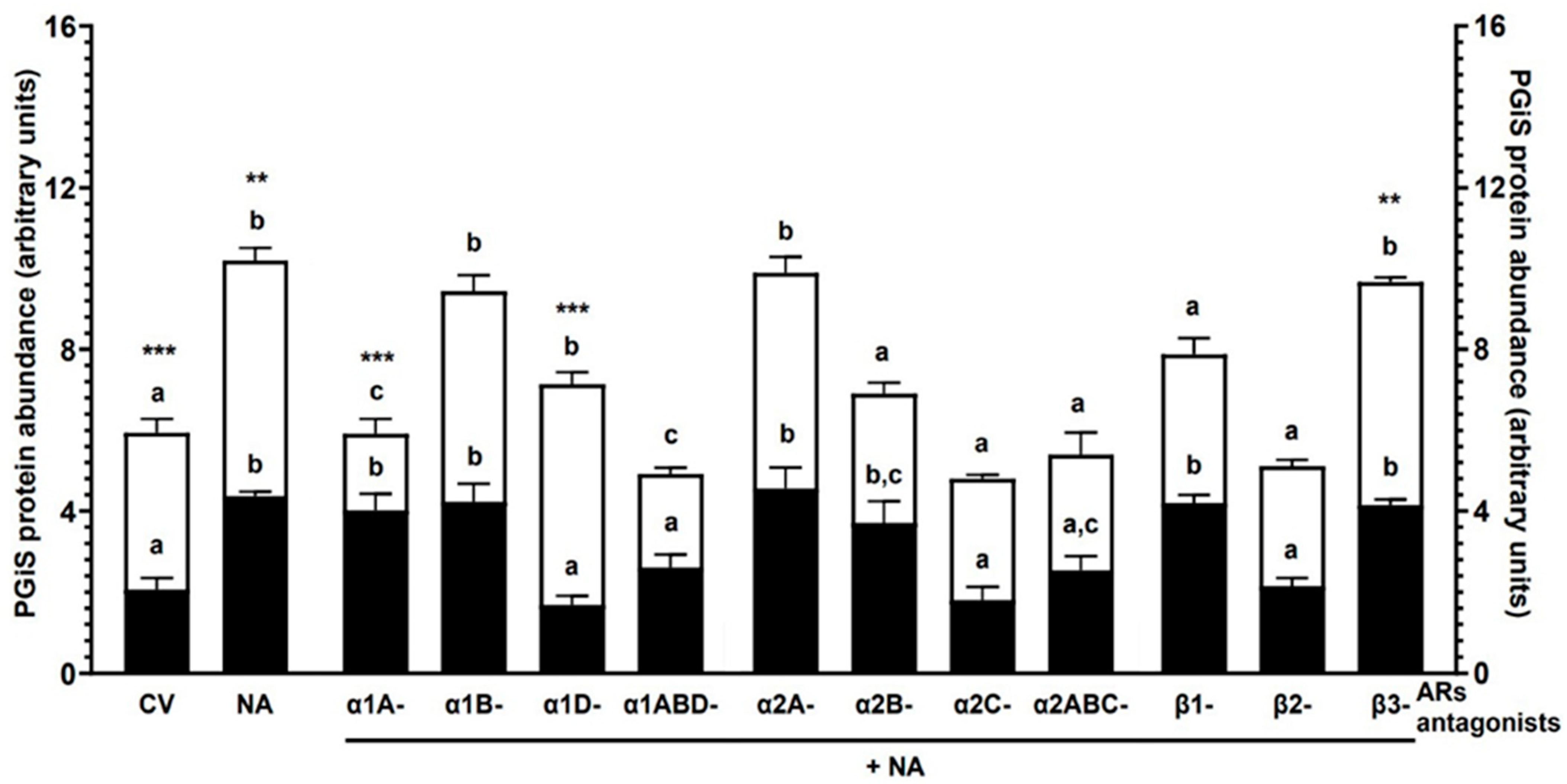
2.1. Experiment 1.1: The Effect of NA and/or AR Antagonists on PGIS Protein Abundance in Endometrial Explants with E. coli-Induced Inflammation In Vivo
2.1.1. AR Antagonists Alone
2.1.2. NA Alone
2.1.3. α1-AR Antagonists with NA
2.1.4. α2-AR Antagonists with NA
2.1.5. β-AR Antagonists with NA
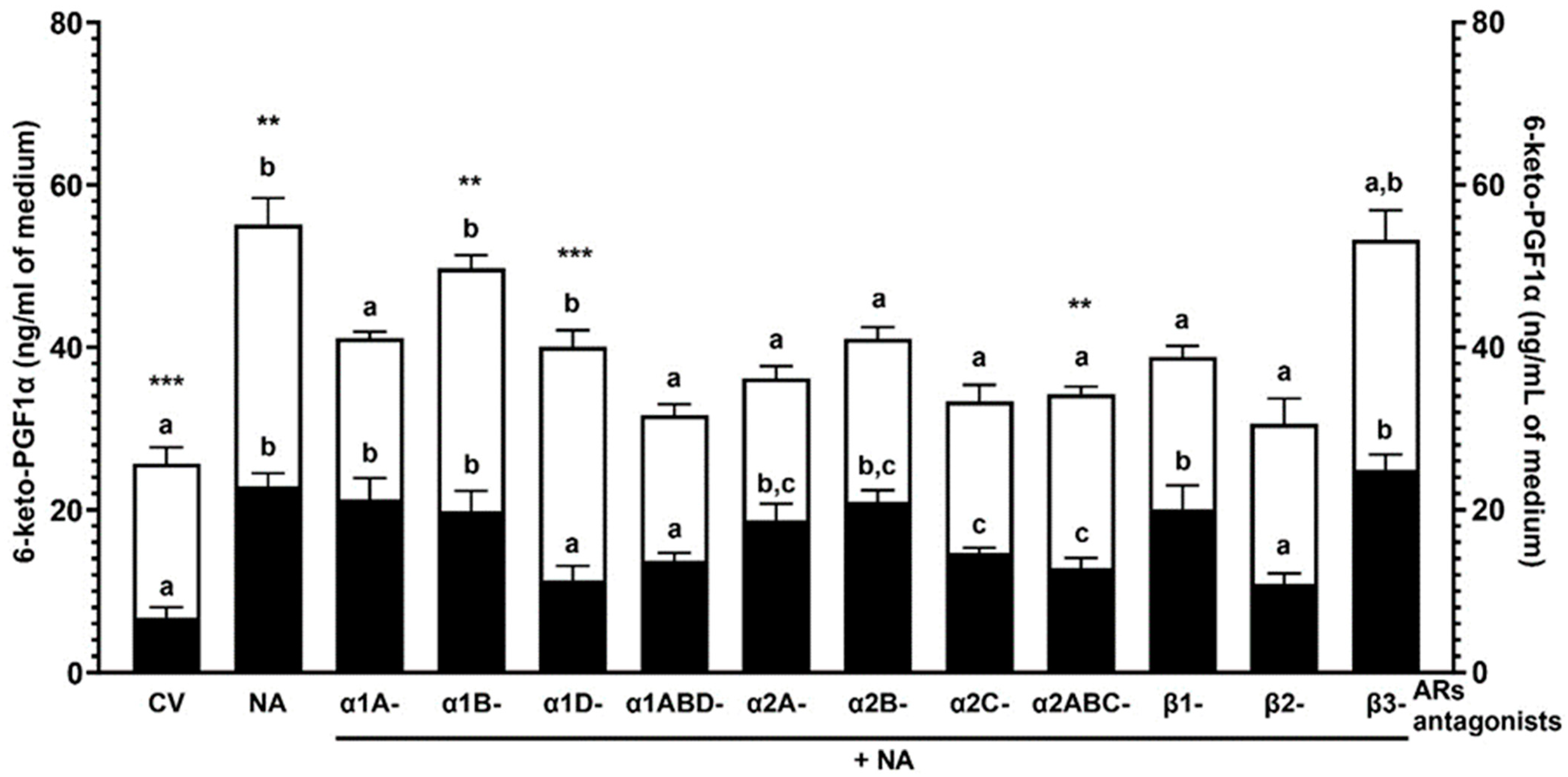
2.2. Experiment 1.2: The Effect of NA and/or AR Antagonists on the 6-keto-PGF1α Concentration in the Medium after Incubation of the Endometrial Explants with E. coli-Induced Inflammation In Vivo
2.2.1. AR Antagonists Alone
2.2.2. NA Alone
2.2.3. α1-AR Antagonists with NA
2.2.4. α2-AR Antagonists with NA
2.2.5. β-AR Antagonists with NA
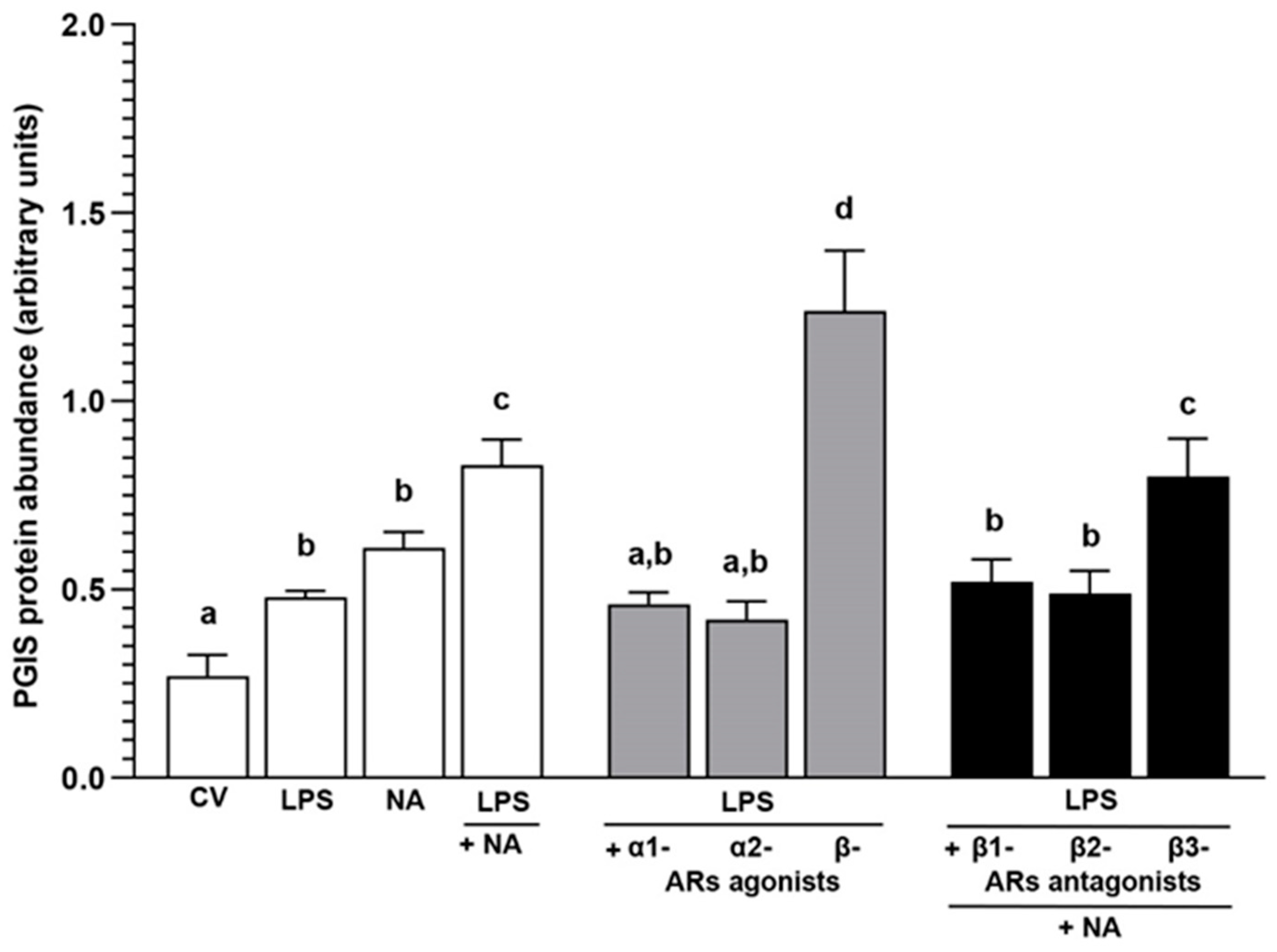
2.3. Experiment 2.1: The Effect of NA and/or AR Agonists and Antagonists on PGIS Protein Abundance in LPS-Treated Endometrial Epithelial Cells
2.3.1. AR Agonists and Antagonists Alone
2.3.2. LPS Alone or with NA, AR Agonists and Antagonists
2.4. Experiment 2.2: The Effect of NA and/or AR Agonists and Antagonists on the 6-keto-PGF1α Concentration in the Medium after Incubation of LPS-Treated Endometrial Epithelial Cells
2.4.1. AR Agonists and Antagonists Alone
2.4.2. LPS Alone or with NA, AR Agonists and Antagonists
3. Discussion
4. Materials and Methods
4.1. Experiment 1: Aimed to Determine the Effects of NA and/or AR Antagonists on PGIS Protein Abundance and 6-keto-PGF1α Concentration in Endometrial Explants with E. coli-Induced Inflammation In Vivo
4.1.1. Animals, Study Design and Collection of Uteri
4.1.2. Preparation and Treatment of Endometrial Explants with NA and/or AR Antagonists
4.2. Experiment 2: Aimed to Determine the Effects of NA and/or AR Agonists and Antagonists on PGIS Protein Abundance and 6-keto-PGF1α Medium Concentration in E. coli LPS-Treated Endometrial Epithelial Cells
4.2.1. Animals and Uteri Collection
4.2.2. Isolation of Endometrial Epithelial Cells
4.2.3. Treatment of Endometrial Epithelial Cells with LPS and/or NA, AR Agonists, Antagonists
4.3. Western Blot Analysis
4.4. ELISA Procedure
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tummaruk, P.; Kesdangsakonwut, S.; Prapasarakul, N.; Kaeoket, K. Endometritis in gilts: Reproductive data, bacterial culture, histopathology, and infiltration of immune cells in the endometrium. Comp. Clin. Pathol. 2010, 19, 575–584. [Google Scholar] [CrossRef]
- De Winter, P.J.J.; Verdonck, M.; De Kruif, A.; Devriese, L.A.; Haesebrouck, F. Bacterial endometritis and vaginal discharge in the sow: Prevalence of different bacterial species and experimental reproduction of the syndrome. Anim. Reprod. Sci. 1995, 37, 325–335. [Google Scholar] [CrossRef]
- Moreno, I.; Cicinelli, E.; Garcia-Grau, I.; Gonzalez-Monfort, M.; Bau, D.; Vilella, F.; De Ziegler, D.; Resta, L.; Valbuena, D.; Simon, C. The diagnosis of chronic endometritis in infertile asymptomatic women: A comparative study of histology, microbial cultures, hysteroscopy, and molecular microbiology. Am. J. Obstet. Gynecol. 2018, 218, 602.e1–602.e16. [Google Scholar] [CrossRef] [PubMed]
- Herath, S.; Fischer, D.P.; Werling, D.; Williams, E.J.; Lilly, S.T.; Dobson, H.; Bryant, C.E.; Sheldon, I.M. Expression and function of Toll-like receptor 4 in the endometrial cells of the uterus. Endocrinology 2006, 147, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Cronin, J.G.; Turner, M.L.; Goetze, L.; Bryant, C.E.; Sheldon, I.M. Toll-like receptor 4 and MYD88-dependent signaling mechanisms of the innate immune system are essential for the response to lipopolysaccharide by epithelial and stromal cells of the bovine endometrium. Biol. Reprod. 2012, 86, 51. [Google Scholar] [CrossRef] [PubMed]
- Krikun, G.; Trezza, J.; Shaw, J.; Rahman, M.; Guller, S.; Abrahams, V.M.; Lockwood, C.J. Lipopolysaccharide appears to activate human endometrial endothelial cells through TLR-4-dependent and TLR-4-independent mechanisms. Am. J. Reprod. Immunol. 2012, 68, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.L.; Healey, G.D.; Sheldon, I.M. Immunity and inflammation in the uterus. Reprod. Domest. Anim. 2012, 47 (Suppl. S4), 402–409. [Google Scholar] [CrossRef] [PubMed]
- Pascottini, O.B.; LeBlanc, S.J. Modulation of immune function in the bovine uterus peripartum. Theriogenology 2020, 150, 193–200. [Google Scholar] [CrossRef]
- Wiebe, M.; Pfarrer, C.; Górriz Martín, L.; Schmicke, M.; Hoedemaker, M.; Bollwein, H.; Heppelmann, M. In vitro effects of lipopolysaccharides on bovine uterine contractility. Reprod. Domest. Anim. 2021, 56, 172–182. [Google Scholar] [CrossRef]
- Jana, B.; Jaroszewski, J.; Czarzasta, J.; Włodarczyk, M.; Markiewicz, W. Synthesis of prostacyclin and its effect on the contractile activity of the inflamed porcine uterus. Theriogenology 2013, 79, 470–485. [Google Scholar] [CrossRef]
- Davies, P.; Bailey, P.J.; Goldenberg, M.M.; Ford-Hutchinson, A.W. The role of arachidonic acid oxygenation products in pain and inflammation. Annu. Rev. Immunol. 1984, 2, 335–357. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Ushikubi, F.; Matsuoka, T.; Hirata, M.; Yamasaki, A.; Sugimoto, Y.; Ichikawa, A.; Aze, Y.; Tanaka, T.; Yoshida, N.; et al. Altered pain perception and inflammatory response in mice lacking prostacyclin receptor. Nature 1997, 388, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Peter, A.T.; Bosu, W.T.; Luker, C.W. Plasma endotoxin and concentrations of stable metabolites of prostacyclin, tromboxane A2, and prostaglandin E2 in postpartum dairy cows. Prostaglandins 1987, 34, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Peter, A.T.; Bosu, W.T.; Gilbert, R.O. Absorption of Escherichia coli endotoxin (lipopolysaccharide) from the uteri of postpartum dairy cows. Theriogenology 1990, 33, 1011–1014. [Google Scholar] [CrossRef] [PubMed]
- Wasowicz, K.; Majewski, M.; Lakomy, M. Distribution of neurons innervating the uterus of the pig. J. Auton. Nerv. Syst. 1998, 25, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Taneike, T.; Narita, T.; Kitazawa, T.; Bando, S.; Teraoka, H.; Ohga, A. Binding and functional characterization of alpha-2 adrenoceptors in isolated swine myometrium. J. Auton. Pharmacol. 1995, 15, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Limon-Boulez, I.; Mhaouty-Kodja, S.; Coudouel, N.; Benoit de Coignac, A.; Legrand, C.; Maltier, J.P. The alpha1B-adrenergic receptor subtype activates the phospholipase C signaling pathway in rat myometrium at parturition. Biol. Reprod. 1997, 57, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Ducza, E.; Gáspár, R.; Falkay, G. Altered levels of mRNA expression and pharmacological reactivity of alpha1-adrenergic receptor subtypes in the late-pregnant rat myometrium. Mol. Reprod. Dev. 2002, 62, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Ontsouka, E.C.; Reist, M.; Graber, H.; Blum, J.W.; Steiner, A.; Hirsbrunner, G. Expression of messenger RNA coding for 5-HT receptor, alpha and beta adrenoreceptor (subtypes) during oestrus and dioestrus in the bovine uterus. J. Vet. Med. Ser. A Physiol. Pathol. Clin. Med. 2004, 51, 385–393. [Google Scholar] [CrossRef]
- Gáspár, R.; Gál, A.; Gálik, M.; Ducza, E.; Minorics, R.; Kolarovszki-Sipiczki, Z.; Klukovits, A.; Falkay, G. Different roles of alpha2-adrenoceptor subtypes in non-pregnant and late-pregnant uterine contractility in vitro in the rat. Neurochem. Int. 2007, 51, 311–318. [Google Scholar] [CrossRef]
- Parida, S.; Uttam Singh, T.; Ravi Prakash, V.; Mishra, S.K. Molecular and functional characteristics of β3-adrenoceptors in late pregnant mouse uterus: A comparison with β2-adrenoceptors. Eur. J. Pharmacol. 2013, 700, 74–79. [Google Scholar] [CrossRef]
- Bóta, J.; Hajagos-Tóth, J.; Ducza, E.; Samavati, R.; Borsodi, A.; Benyhe, S.; Gáspár, R. The effects of female sexual hormones on the expression and function of α1A- and α1D-adrenoceptor subtypes in the late-pregnant rat myometrium. Eur. J. Pharmacol. 2015, 769, 177–184. [Google Scholar] [CrossRef]
- Meller, K.A.; Całka, J.; Kaczmarek, M.; Jana, B. Expression of alpha and beta adrenergic receptors in the pig uterus during inflammation. Theriogenology 2018, 119, 96–104. [Google Scholar] [CrossRef]
- Quaas, L.; Zahradnik, P. The effects of alpha- and beta-adrenergic stimulation on contractility and prostaglandin (prostaglandins E2 and F2a and 6-keto-prostaglandin F1 alpha) production of pregnant human myometrial strips. Am. J. Obstet. Gynecol. 1985, 152, 852–856. [Google Scholar] [CrossRef]
- Chaud, M.A.; Franchi, A.M.; Gonzalez, E.T.; Gimeno, M.F.; Gimeno, A.L. Norepinephrine alters PGE2/PGE1 output ratio in isolated uterus from ovariectomized rats. Prostaglandins Leukot Med. 1986, 22, 201–210. [Google Scholar] [CrossRef]
- Jana, B.; Całka, J.; Palus, K.; Witek, K. Noradrenaline and adrenoreceptors regulate prostaglandin F2α formation in endometrium after experimentally-induced inflammation in the pig. Ann. Anim. Sci. 2023, 24, 453–463. [Google Scholar] [CrossRef]
- Jana, B.; Całka, J.; Bulc, M.; Witek, K. Role of noradrenaline and adrenoreceptors in regulating prostaglandin E2 synthesis cascade in inflamed endometrium of pigs. Int. J. Mol. Sci. 2023, 24, 5856. [Google Scholar] [CrossRef]
- Korzekwa, A.J.; Łupicka, M.; Socha, B.M.; Szczepańska, A.A.; Piotrowicz, E.; Barański, W. In vitro cow uterine response to Escherichia coli, leukotrienes and cytokines. Vet. Immunol. Immunopathol. 2016, 182, 59–62. [Google Scholar] [CrossRef]
- Czarzasta, J.; Meller, K.; Andronowska, A.; Jana, B. Lipopolysaccharide and cytokines modulate leukotriene (LT)B4 and LTC4 production by porcine endometrial endothelial cells. Reprod. Domest. Anim. 2018, 53, 101–109. [Google Scholar] [CrossRef]
- Nebigil, C.; Malik, K.U. Comparison of signal transduction mechanisms of alpha-2C and alpha-1A adrenergic receptor-stimulated prostaglandin synthesis. J. Pharmacol. Exp. Ther. 1992, 263, 987–996. [Google Scholar]
- Nishimiya, T.; Daniell, H.B.; Webb, J.G.; Oatis, J.; Walle, T.; Gaffney, T.E.; Halushka, P.V. Chronic treatment with propranolol enhances the synthesis of prostaglandins E2 and I2 by the aorta of spontaneously hypertensive rats. J. Pharmacol. Exp. Ther. 1990, 253, 207–213. [Google Scholar]
- Nadasy, G.L.; Szekacs, B.; Juhasz, I.; Monos, E. Pharmacological modulation of prostacyclin and thromboxane production of rat and cat venous tissue slices. Prostaglandins 1992, 44, 339–355. [Google Scholar] [CrossRef]
- Szekacs, B.; Nadasy, G.L.; Vajo, Z.; Juhasz, I.; Feher, J.; Monos, E. Prostacyclin and thromboxane production of rat and cat arterial tissue is altered independently by several vasoactive substances. Prostaglandins 1996, 52, 221–235. [Google Scholar] [CrossRef]
- Wang, H.L.; Voelkel, N.F. Norepinephrine induces lung vascular prostacyclin synthesis via alpha 1-adrenergic receptors. J. Appl. Physiol. 1989, 67, 330–338. [Google Scholar] [CrossRef]
- Skarzynski, D.J.; Uenoyama, Y.; Kotwica, J.; Okuda, K. Noradrenaline stimulates the production of prostaglandin f2alpha in cultured bovine endometrial cells. Biol. Reprod. 1999, 60, 277–282. [Google Scholar] [CrossRef]
- Ueda, F.; Ideguchi, K.; Taniguchi, N.; Kimura, K. Adrenergic regulation of prostaglandin biosynthesis in cultured rabbit gastric epithelial cells. Jpn. J. Pharmacol. 1994, 65, 113–120. [Google Scholar] [CrossRef]
- Gonzales, R.; Sherbourne, C.D.; Goldyne, M.E.; Levine, J.D. Noradrenaline-induced prostaglandin production by sympathetic postganglionic neurons is mediated by alpha 2-adrenergic receptors. J. Neurochem. 1991, 57, 1145–1150. [Google Scholar] [CrossRef]
- Schlachetzki, J.C.; Fiebich, B.L.; Haake, E.; de Oliveira, A.C.; Candelario-Jalil, E.; Heneka, M.T.; Hüll, M. Norepinephrine enhances the LPS-induced expression of COX-2 and secretion of PGE2 in primary rat microglia. J. Neuroinflamm. 2010, 7, 2. [Google Scholar] [CrossRef]
- Jana, B.; Całka, J.; Bulc, M.; Piotrowska-Tomala, K.K. Participation of acetylcholine and its receptors in the contractility of inflamed porcine uterus. Theriogenology 2020, 143, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Akins, E.L.; Morrissette, M.C. Gross ovarian changes during estrous cycle of swine. Am. J. Vet. Res. 1968, 29, 1953–1957. [Google Scholar]
- Blitek, A.; Morawska, E.; Kiewisz, J.; Ziecik, A.J. Effect of conceptus secretions on HOXA10 and PTGS2 gene expression, and PGE2 release in co-cultured luminal epithelial and stromal cells of the porcine endometrium at the time of early implantation. Theriogenology 2011, 76, 954–966. [Google Scholar] [CrossRef] [PubMed]
- Blitek, A.; Ziecik, A.J. Prostaglandins f and e secretion by porcine epithelial and stromal endometrial cells on different days of the oestrous cycle. Reprod. Domest. Anim. 2004, 39, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]


| Treatment | Group | PGIS Protein Abundance | 6-keto-PGF1α Concentration |
|---|---|---|---|
| (Arbitrary Units) | (ng/mL of Medium) | ||
| CV | CON | 2.07 ± 0.28 a | 6.77 ± 1.26 a |
| E. coli | 3.87 ± 0.34 a,*** | 18.92 ± 1.98 a,*** | |
| NA | CON | 4.37± 0.11 b | 22.92 ± 1.58 b |
| E. coli | 5.83 ± 0.31 b,** | 32.21 ± 3.24 b,** | |
| α1A anta. | CON | 1.74 ± 0.09 a | 5.99 ± 0.26 a |
| E. coli | 3.84 ± 0.12 a,*** | 15.99 ± 0.21 a,*** | |
| α1B anta. | CON | 1.93 ± 0.22 a | 7.59 ± 0.31 a |
| E. coli | 3.22 ± 0.33 a,** | 20.57 ± 0.26 a,*** | |
| α1D anta. | CON | 1.95 ± 0.52 a | 4.89 ± 0.12 a |
| E. coli | 3.13 ± 0.13 a,* | 19.67 ± 0.19 a,*** | |
| α1ABD anta. | CON | 2.25 ± 0.03 a | 8.11 ± 0.21 a |
| E. coli | 3.53 ± 0.09 a,* | 21.19 ± 0.31 a,*** | |
| α2A anta. | CON | 2.25 ± 0.23 a | 6.94 ± 0.19 a |
| E. coli | 3.39 ± 0.14 a,* | 15.93 ± 0.29 a,*** | |
| α2B anta. | CON | 1.95 ± 0.14 a | 7.55 ± 0.26 a |
| E. coli | 3.54 ± 0.41 a,*** | 18.47 ± 0.38 a,*** | |
| α2C anta. | CON | 2.22 ± 0.23 a | 8.14 ± 0.31 a |
| E. coli | 3.47 ± 0.34 a,** | 21.52 ± 0.34 a,*** | |
| α2ABC anta. | CON | 2.21 ± 0.12 a | 9.64 ± 0.38 a |
| E. coli | 3.31 ± 0.08 a,* | 17.85 ± 0.41 a,*** | |
| β1 anta. | CON | 2.29 ± 0.56 a | 4.69 ± 0.25 a |
| E. coli | 3.52 ± 0.13 a,** | 16.98 ± 0.39 a,*** | |
| β2 anta. | CON | 2.42 ± 0.04 a | 7.87 ± 0.2 a |
| E. coli | 3.31 ± 0.07 a | 20.35 ± 0.32 a,*** | |
| β3 anta. | CON | 1.84 ± 0.06 a | 6.69 ± 0.26 a |
| E. coli | 3.44 ± 0.09 a,*** | 18.48 ± 0.35 a,*** |
| Treatment | PGIS Protein Abundance | 6-keto-PGF1α Concentration |
|---|---|---|
| (Arbitrary Units) | (ng/mL of Medium) | |
| CV | 0.27 ± 0.03 a | 1.15 ± 007 a |
| NA | 0.48 ± 0.01 b,c | 2.34 ± 0.03 b |
| α1 agon. | 0.26 ± 0.05 a | 1.47 ± 0.09 a |
| α2 agon. | 0.22 ± 0.04 a | 1.23 ± 0.11 a |
| β agon. | 0.65 ± 0.03 c | 3.65 ± 0.07 c |
| β1 anta. | 0.25 ± 0.04 a | 1.41 ± 0.05 a |
| β2 anta. | 0.23 ± 0.05 a | 1.26 ± 0.13 a |
| β3 anta. | 0.21 ± 0.03 a | 1.32 ± 0.09 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jana, B.; Całka, J.; Andronowska, A.; Mówińska, A.; Witek, K.; Palus, K. Noradrenaline and Adrenoreceptors Are Involved in the Regulation of Prostaglandin I2 Production in the Porcine Endometrium after Experimentally Induced Inflammation. Int. J. Mol. Sci. 2024, 25, 6313. https://doi.org/10.3390/ijms25126313
Jana B, Całka J, Andronowska A, Mówińska A, Witek K, Palus K. Noradrenaline and Adrenoreceptors Are Involved in the Regulation of Prostaglandin I2 Production in the Porcine Endometrium after Experimentally Induced Inflammation. International Journal of Molecular Sciences. 2024; 25(12):6313. https://doi.org/10.3390/ijms25126313
Chicago/Turabian StyleJana, Barbara, Jarosław Całka, Aneta Andronowska, Aleksandra Mówińska, Krzysztof Witek, and Katarzyna Palus. 2024. "Noradrenaline and Adrenoreceptors Are Involved in the Regulation of Prostaglandin I2 Production in the Porcine Endometrium after Experimentally Induced Inflammation" International Journal of Molecular Sciences 25, no. 12: 6313. https://doi.org/10.3390/ijms25126313





