A Cross-Sectional Exploratory Study of Cardiovascular Risk Biomarkers in Non-Obese Women with and without Polycystic Ovary Syndrome: Association with Vitamin D
Abstract
:1. Introduction
2. Results
2.1. Cardiovascular Risk Protein Levels
2.2. Functional and Pathway Enrichment for Dysregulated Proteins
2.3. Correlation Analyses between 25(OH)D3, 1,25(OH)2D3 and Cardiovascular Risk Proteins
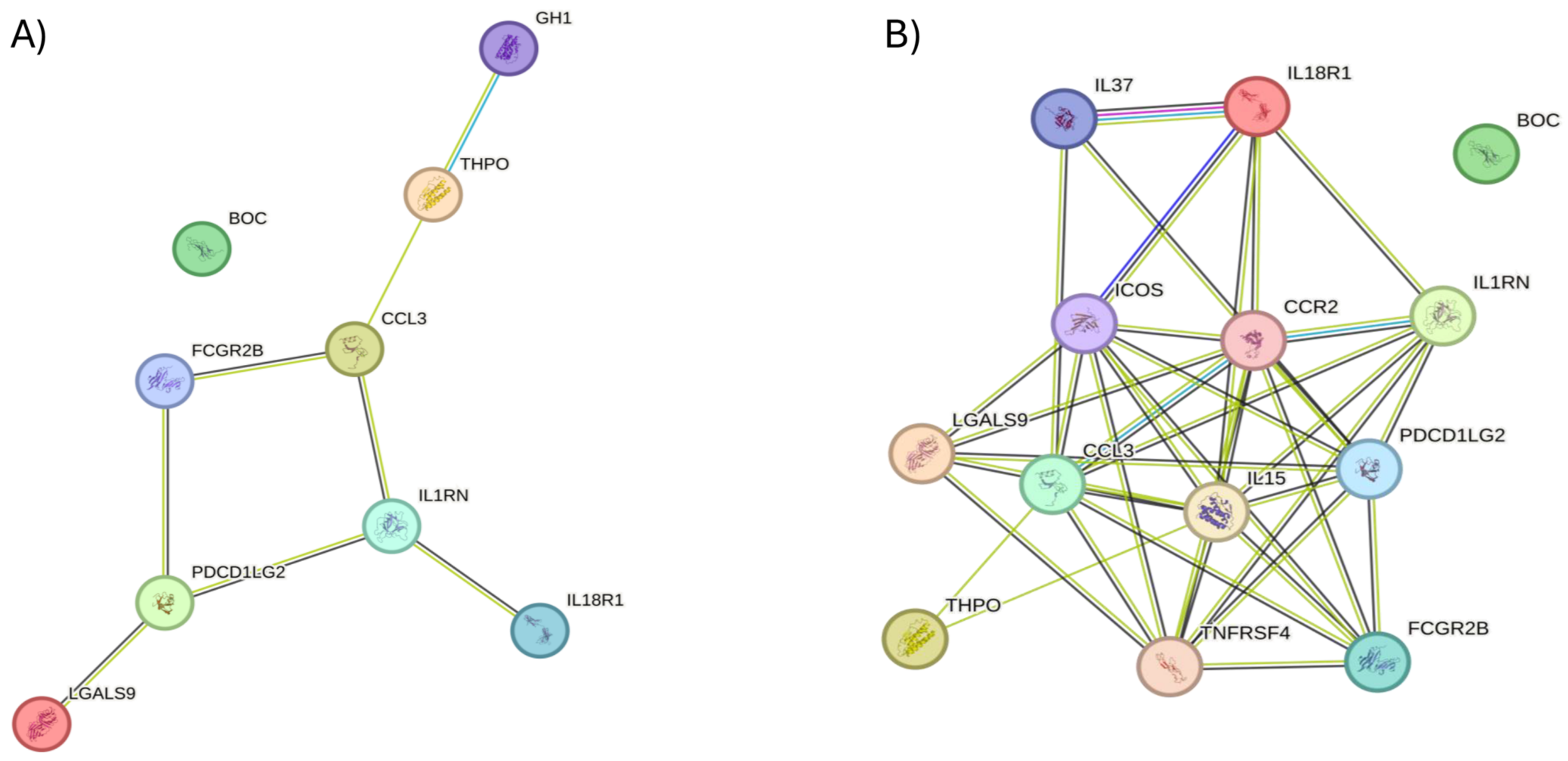
2.4. Protein–Protein Interactions
2.5. Multivariable Regression and Predictive Value of the Variables
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Statistics
4.3. Systems Biology Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shroff, R.; Kerchner, A.; Maifeld, M.; Van Beek, E.J.R.; Jagasia, D.; Dokras, A. Young obese women with polycystic ovary syndrome have evidence of early coronary atherosclerosis. J. Clin. Endocrinol. Metab. 2007, 92, 4609–4614. [Google Scholar] [CrossRef]
- Legro, R.S.; Arslanian, S.A.; Ehrmann, D.A.; Hoeger, K.M.; Murad, M.H.; Pasquali, R.; Welt, C.K. Diagnosis and treatment of polycystic ovary syndrome: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2013, 98, 4565–4592. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Kouli, C.R.; Bergiele, A.T.; Filandra, F.A.; Tsianateli, T.C.; Spina, G.G.; Zapanti, E.D.; Bartzis, M.I. A Survey of the Polycystic Ovary Syndrome in the Greek Island of Lesbos: Hormonal and Metabolic Profile. J. Clin. Endocrinol. Metab. 1999, 84, 4006–4011. [Google Scholar] [CrossRef]
- Sathyapalan, T.; Atkin, S.L. Mechanisms in endocrinology: Recent advances in cardiovascular aspects of polycystic ovary syndrome. Eur. J. Endocrinol. 2012, 166, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Sathyapalan, T.; Shepherd, J.; Coady, A.-M.; Kilpatrick, E.S.; Atkin, S.L. Atorvastatin reduces malondialdehyde concentrations in patients with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2012, 97, 3951–3955. [Google Scholar] [CrossRef]
- Bannigida, D.M.; Nayak, B.S.; Vijayaraghavan, R. Insulin resistance and oxidative marker in women with PCOS. Arch. Physiol. Biochem. 2020, 126, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Niinuma, S.A.; Lubbad, L.; Lubbad, W.; Moin, A.S.M.; Butler, A.E. The Role of Heat Shock Proteins in the Pathogenesis of Polycystic Ovarian Syndrome: A Review of the Literature. Int. J. Mol. Sci. 2023, 24, 1838. [Google Scholar] [CrossRef]
- Moin, A.S.M.; Sathyapalan, T.; Butler, A.E.; Atkin, S.L. Coagulation factor dysregulation in polycystic ovary syndrome is an epiphenomenon of obesity. Clin. Endocrinol. 2023, 98, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.D.; Narayanaswamy, A.K.; Farewell, D.; Rees, D.A. Complement activation in polycystic ovary syndrome occurs in the postprandial and fasted state and is influenced by obesity and insulin sensitivity. Clin. Endocrinol. 2020, 94, 74–84. [Google Scholar] [CrossRef]
- Yang, S.; Li, Q.; Song, Y.; Tian, B.; Cheng, Q.; Qing, H.; Zhong, L.; Xia, W. Serum complement C3 has a stronger association with insulin resistance than high-sensitivity C-reactive protein in women with polycystic ovary syndrome. Fertil. Steril. 2011, 95, 1749–1753. [Google Scholar] [CrossRef]
- Moin, A.S.M.; Sathyapalan, T.; Butler, A.E.; Atkin, S.L. Classical and alternate complement factor overexpression in non-obese weight matched women with polycystic ovary syndrome does not correlate with Vitamin D. Front. Endocrinol. 2022, 13, 935750. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.E.; Moin, A.S.M.; Sathyapalan, T.; Atkin, S.L. Components of the Complement Cascade Differ in Polycystic Ovary Syndrome. Int. J. Mol. Sci. 2022, 23, 12232. [Google Scholar] [CrossRef] [PubMed]
- Legro, R.S. Obesity and PCOS: Implications for diagnosis and treatment. Semin. Reprod. Med. 2012, 30, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Ma, L.; Xia, X.; Ying, T.; Zhou, M.; Zou, S.; Yu, H.; Yin, J. Efficacy of Bariatric Surgery in the Treatment of Women with Obesity and Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2022, 107, e3217–e3229. [Google Scholar] [CrossRef] [PubMed]
- Alalami, H.; Sathyapalan, T.; Atkin, S.L. Cardiovascular profile of pharmacological agents used for the management of polycystic ovary syndrome. Ther. Adv. Endocrinol. Metab. 2018, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Fauser, B.C.; Tarlatzis, B.C.; Rebar, R.W.; Legro, R.S.; Balen, A.H.; Lobo, R.; Carmina, E.; Chang, J.; Yildiz, B.O.; Laven, J.S.; et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): The Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil. Steril. 2011, 97, 28–38.e25. [Google Scholar] [CrossRef]
- Setji, T.L.; Holland, N.D.; Sanders, L.L.; Pereira, K.C.; Diehl, A.M.; Brown, A.J. Nonalcoholic steatohepatitis and nonalcoholic fatty liver disease in young women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2006, 91, 1741–1747. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.C.; Lyall, H.; Petrie, J.R.; Gould, G.W.; Connell, J.M.; Sattar, N. Low grade chronic inflammation in women with polycystic ovarian syndrome. J. Clin. Endocrinol. Metab. 2001, 86, 2453–2455. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Hennekens, C.H.; Buring, J.E.; Rifai, N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N. Engl. J. Med. 2000, 342, 836–843. [Google Scholar] [CrossRef]
- Baptiste, C.G.; Battista, M.-C.; Trottier, A.; Baillargeon, J.-P. Insulin and hyperandrogenism in women with polycystic ovary syndrome. J. Steroid Biochem. Mol. Biol. 2009, 122, 42–52. [Google Scholar] [CrossRef]
- Cunningham, T.K.; Allgar, V.; Dargham, S.R.; Kilpatrick, E.; Sathyapalan, T.; Maguiness, S.; Mokhtar Rudin, H.R.; Abdul Ghani, N.M.; Latiff, A.; Atkin, S.L. Association of Vitamin D Metabolites with Embryo Development and Fertilization in Women with and without PCOS Undergoing Subfertility Treatment. Front. Endocrinol. 2019, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S.; Rafison, B.; Witzel, S.; Reyes, R.E.; Shieh, A.; Chun, R.; Zavala, K.; Hewison, M.; Liu, P.T. Regulation of the extrarenal CYP27B1-hydroxylase. J. Steroid Biochem. Mol. Biol. 2014, 144 Pt A, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Lösel, R.; Wehling, M. Nongenomic actions of steroid hormones. Nat. Rev. Mol. Cell Biol. 2003, 4, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Lisse, T.S.; Vadivel, K.; Bajaj, S.P.; Chun, R.F.; Hewison, M.; Adams, J.S. The heterodimeric structure of heterogeneous nuclear ribonucleoprotein C1/C2 dictates 1,25-dihydroxyvitamin D-directed transcriptional events in osteoblasts. Bone Res. 2014, 2, 14011. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.; Haselhorst, U.; Tan, S.; Quadbeck, B.; Schmidt, M.; Roesler, S.; Kimmig, R.; Mann, K.; Janssen, O. Low Serum 25-hydroxyvitamin D concentrations are associated with insulin resistance and obesity in women with polycystic ovary syndrome. Exp. Clin. Endocrinol. Diabetes 2006, 114, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Li, H.W.R.; Brereton, R.E.; Anderson, R.A.; Wallace, A.M.; Ho, C.K. Vitamin D deficiency is common and associated with metabolic risk factors in patients with polycystic ovary syndrome. Metabolism 2011, 60, 1475–1481. [Google Scholar] [CrossRef]
- Lerchbaum, E.; Obermayer-Pietsch, B. Vitamin D and fertility: A systematic review. Eur. J. Endocrinol. 2012, 166, 765–778. [Google Scholar] [CrossRef]
- Selimoglu, H.; Duran, C.; Kiyici, S.; Ersoy, C.; Guclu, M.; Ozkaya, G.; Tuncel, E.; Erturk, E.; Imamoglu, S. The effect of vitamin D replacement therapy on insulin resistance and androgen levels in women with polycystic ovary syndrome. J. Endocrinol. Investig. 2009, 33, 234–238. [Google Scholar] [CrossRef]
- Krul-Poel, Y.; Snackey, C.; Louwers, Y.; Lips, P.; Lambalk, C.; Laven, J.; Simsek, S. The role of vitamin D in metabolic disturbances in polycystic ovary syndrome (PCOS): A systematic review. Eur. J. Endocrinol. 2013, 169, 853–865. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Vitamin D Deficiency: Effects on Oxidative Stress, Epigenetics, Gene Regulation, and Aging. Biology 2019, 8, 30. [Google Scholar] [CrossRef]
- Cannell, J.J.; Grant, W.B.; Holick, M.F. Vitamin D and inflammation. Dermato-Endocrinology 2014, 6, e983401. [Google Scholar] [CrossRef] [PubMed]
- Krajewska, M.; Witkowska-Sędek, E.; Rumińska, M.; Stelmaszczyk-Emmel, A.; Sobol, M.; Majcher, A.; Pyrżak, B. Vitamin D Effects on Selected Anti-Inflammatory and Pro-Inflammatory Markers of Obesity-Related Chronic Inflammation. Front. Endocrinol. 2022, 13, 920340. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Ma, Z.; Ma, Q.; Wu, Z.; Fan, P.; Zhou, X.; Chen, L.; Zhou, S.; Goltzman, D.; Miao, D.; et al. 1,25(OH)2D3 inhibits hepatocellular carcinoma development through reducing secretion of inflammatory cytokines from immunocytes. Curr. Med. Chem. 2013, 20, 4131–4141. [Google Scholar] [CrossRef] [PubMed]
- Korf, H.; Wenes, M.; Stijlemans, B.; Takiishi, T.; Robert, S.; Miani, M.; Eizirik, D.L.; Gysemans, C.; Mathieu, C. 1,25-Dihydroxyvitamin D3 curtails the inflammatory and T cell stimulatory capacity of macrophages through an IL-10-dependent mechanism. Immunobiology 2012, 217, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Guillot, X.; Semerano, L.; Saidenberg-Kermanac’h, N.; Falgarone, G.; Boissier, M.C. Vitamin D and inflammation. Jt. Bone Spine 2010, 77, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, E.; Suchta, K.; Grymowicz, M.; Calik-Ksepka, A.; Smolarczyk, K.; Duszewska, A.M.; Smolarczyk, R.; Meczekalski, B. Chronic Low Grade Inflammation in Pathogenesis of PCOS. Int. J. Mol. Sci. 2021, 22, 3789. [Google Scholar] [CrossRef] [PubMed]
- Vasyukova, E.; Zaikova, E.; Kalinina, O.; Gorelova, I.; Pyanova, I.; Bogatyreva, E.; Vasilieva, E.; Grineva, E.; Popova, P. Inflammatory and Anti-Inflammatory Parameters in PCOS Patients Depending on Body Mass Index: A Case-Control Study. Biomedicines 2023, 11, 2791. [Google Scholar] [CrossRef] [PubMed]
- Rezayat, F.; Hajiaghayi, M.; Ghasemi, N.; Mesdaghi, M.; Tehrani, F.R.; Mosaffa, N. Inflammatory Responses of Women with Polycystic Ovary Syndrome in Vitro Differ from Healthy Women. Int. J. Mol. Cell Med. 2023, 12, 70–80. [Google Scholar] [CrossRef]
- Wang, J.; Yin, T.; Liu, S. Dysregulation of immune response in PCOS organ system. Front. Immunol. 2023, 14, 1169232. [Google Scholar] [CrossRef]
- O’Brien, M.J.; Shu, Q.; Stinson, W.A.; Tsou, P.-S.; Ruth, J.H.; Isozaki, T.; Campbell, P.L.; Ohara, R.A.; Koch, A.E.; Fox, D.A.; et al. A unique role for galectin-9 in angiogenesis and inflammatory arthritis. Arthritis Res. Ther. 2018, 20, 31. [Google Scholar] [CrossRef]
- Krautter, F.; Hussain, M.T.; Zhi, Z.; Lezama, D.R.; Manning, J.E.; Brown, E.; Marigliano, N.; Raucci, F.; Recio, C.; Chimen, M.; et al. Galectin-9: A novel promoter of atherosclerosis progression. Atherosclerosis 2022, 363, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, H.-B.; Zhou, L.; Cui, X.-Q.; Fan, X.-H. CCL3 participates in the development of rheumatoid arthritis by activating AKT. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 6625–6632. [Google Scholar] [CrossRef] [PubMed]
- Lentzsch, S.; Gries, M.; Janz, M.; Bargou, R.; Dorken, B.; Mapara, M.Y. Macrophage inflammatory protein 1-alpha (MIP-1α) triggers migration and signaling cascades mediating survival and proliferation in multiple myeloma (MM) cells. Blood 2003, 101, 3568–3573. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Fang, L.; Li, Y.; Yan, Y.; Thakur, A.; Cheng, J.; Sun, Y. Association of circulating monocyte chemoattractant protein-1 levels with polycystic ovary syndrome: A meta-analysis. Am. J. Reprod. Immunol. 2021, 86, e13407. [Google Scholar] [CrossRef] [PubMed]
- Sims, J.E. IL-1 and IL-18 receptors, and their extended family. Curr. Opin. Immunol. 2002, 14, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Cheung, H.; Chen, N.-J.; Cao, Z.; Ono, N.; Ohashi, P.S.; Yeh, W.-C. Accessory protein-like is essential for IL-18-mediated signaling. J. Immunol. 2005, 174, 5351–5357. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin 1 and interleukin 18 as mediators of inflammation and the aging process. Am. J. Clin. Nutr. 2006, 83, 447S–455S. [Google Scholar] [CrossRef] [PubMed]
- Imaoka, H.; Takenaka, S.-I.; Kawayama, T.; Oda, H.; Kaku, Y.; Matsuoka, M.; Sakazaki, Y.; O’byrne, P.M.; Hoshino, T. Increased serum levels of soluble il-18 receptor complex in patients with allergic asthma. Allergol. Int. 2013, 62, 513–515. [Google Scholar] [CrossRef]
- Ahmad, R.; Thomas, R.; Kochumon, S.; Sindhu, S. Increased adipose tissue expression of IL-18R and its ligand IL-18 associates with inflammation and insulin resistance in obesity. Immun. Inflamm. Dis. 2017, 5, 318–335. [Google Scholar] [CrossRef]
- Yang, Y.; Qiao, J.; Li, R.; Li, M.Z. Is interleukin-18 associated with polycystic ovary syndrome? Reprod. Biol. Endocrinol. 2011, 9, 7. [Google Scholar] [CrossRef]
- Ezumi, Y.; Takayama, H.; Okuma, M. Thrombopoietin, c-Mpl ligand, induces tyrosine phosphorylation of Tyk2, JAK2, and STAT3, and enhances agonists-induced aggregation in platelets in vitro. FEBS Lett. 1995, 374, 48–52. [Google Scholar] [CrossRef]
- Dereli, D.; Ozgen, G.; Buyukkececi, F.; Guney, E.; Yilmaz, C. Platelet dysfunction in lean women with polycystic ovary syndrome and association with insulin sensitivity. J. Clin. Endocrinol. Metab. 2003, 88, 2263–2268. [Google Scholar] [CrossRef]
- Maury, E.; Brichard, S.M.; Pataky, Z.; Carpentier, A.; Golay, A.; Bobbioni-Harsch, E. Effect of obesity on growth-related oncogene factor-alpha, thrombopoietin, and tissue inhibitor metalloproteinase-1 serum levels. Obesity 2010, 18, 1503–1509. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, S.P.; Brewer, M.T.; Verderber, E.; Heimdal, P.; Brandhuber, B.J.; Thompson, R.C. Interleukin 1 receptor antagonist is a member of the interleukin 1 gene family: Evolution of a cytokine control mechanism. Proc. Natl. Acad. Sci. USA 1991, 88, 5232–5236. [Google Scholar] [CrossRef] [PubMed]
- Rashid, N.; Nigam, A.; Saxena, P.; Jain, S.K.; Wajid, S. Association of IL-1β, IL-1Ra and FABP1 gene polymorphisms with the metabolic features of polycystic ovary syndrome. Inflamm. Res. 2017, 66, 621–636. [Google Scholar] [CrossRef] [PubMed]
- Luotola, K.; Piltonen, T.T.; Puurunen, J.; Tapanainen, J.S. IL-1 receptor antagonist levels are associated with glucose tolerance in polycystic ovary syndrome. Clin. Endocrinol. 2016, 85, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Rajabian, Z.; Kalani, F.; Taghiloo, S.; Tehrani, M.; Rafiei, A.; Hosseini-Khah, Z.; Hosseini, V.; Ajami, A. Over-Expression of Immunosuppressive Molecules, PD-L1 and PD-L2, in Ulcerative Colitis Patients. Iran J. Immunol. 2019, 16, 62–70. [Google Scholar] [CrossRef]
- Kawamoto, E.; Masui-Ito, A.; Eguchi, A.; Soe, Z.Y.; Prajuabjinda, O.; Darkwah, S.; Park, E.J.; Imai, H.; Shimaoka, M. Integrin and PD-1 Ligand Expression on Circulating Extracellular Vesicles in Systemic Inflammatory Response Syndrome and Sepsis. Shock 2019, 52, 13–22. [Google Scholar] [CrossRef]
- Smith, K.G.; Clatworthy, M.R. Fcγ RIIB in autoimmunity and infection: Evolutionary and therapeutic implications. Nat. Rev. Immunol. 2010, 10, 328–343. [Google Scholar] [CrossRef]
- Clatworthy, M.R.; Smith, K.G. Fcγ RIIb balances efficient pathogen clearance and the cytokine-mediated consequences of sepsis. J. Exp. Med. 2004, 199, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, S.; Liu, J.; Zhang, T.; Shen, Q.; Yu, Y.; Cao, X. Immune complex/Ig negatively regulate TLR4-triggered inflammatory response in macrophages through Fc gamma RIIb-dependent PGE2 production. J. Immunol. 2009, 182, 554–562. [Google Scholar] [CrossRef]
- El Shikh, M.E.; El Sayed, R.; Szakal, A.K.; Tew, J.G. Follicular dendritic cell (FDC)-FcgammaRIIB engagement via immune complexes induces the activated FDC phenotype associated with secondary follicle development. Eur. J. Immunol. 2006, 36, 2715–2724. [Google Scholar] [CrossRef] [PubMed]
- Premoli, A.C.; Santana, L.F.; Ferriani, R.A.; Moura, M.D.; De Sá, M.F.; Reis, R.M. Growth hormone secretion and insulin-like growth factor-1 are related to hyperandrogenism in nonobese patients with polycystic ovary syndrome. Fertil. Steril. 2005, 83, 1852–1855. [Google Scholar] [CrossRef]
- Amisi, C.A. Markers of insulin resistance in Polycystic ovary syndrome women: An update. World J. Diabetes 2022, 13, 129–149. [Google Scholar] [CrossRef]
- Aboeldalyl, S.; James, C.; Seyam, E.; Ibrahim, E.M.; Shawki, H.E.-D.; Amer, S. The Role of Chronic Inflammation in Polycystic Ovarian Syndrome—A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2021, 22, 2734. [Google Scholar] [CrossRef]
- Gao, L.; Zhao, Y.; Wu, H.; Lin, X.; Guo, F.; Li, J.; Long, Y.; Zhou, B.; She, J.; Zhang, C.; et al. Polycystic Ovary Syndrome Fuels Cardiovascular Inflammation and Aggravates Ischemic Cardiac Injury. Circulation 2023, 148, 1958–1973. [Google Scholar] [CrossRef] [PubMed]
- Gholami, F.; Moradi, G.; Zareei, B.; Rasouli, M.A.; Nikkhoo, B.; Roshani, D.; Ghaderi, E. The association between circulating 25-hydroxyvitamin D and cardiovascular diseases: A meta-analysis of prospective cohort studies. BMC Cardiovasc. Disord. 2019, 19, 248. [Google Scholar] [CrossRef]
- Amjadi, F.; Zandieh, Z.; Mehdizadeh, M.; Ajdary, M.; Aghamajidi, A.; Raoufi, E.; Aflatoonian, R. Molecular signature of immunological mechanism behind impaired endometrial receptivity in polycystic ovarian syndrome. Arq. Bras. Endocrinol. Metabol. 2022, 66, 303–311. [Google Scholar] [CrossRef]
- Tal, R.; Seifer, D.B.; Grazi, R.V.; Malter, H.E. Angiopoietin-1 and angiopoietin-2 are altered in polycystic ovarian syndrome (PCOS) during controlled ovarian stimulation. Vasc. Cell 2013, 5, 18. [Google Scholar] [CrossRef]
- Di Pietro, M.; Pascuali, N.; Parborell, F.; Abramovich, D. Ovarian angiogenesis in polycystic ovary syndrome. Reproduction 2018, 155, R199–R209. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, H.; Li, K.; Liu, R.; Qiu, S.; Lei, X.; Yang, M.; Lai, Y.; He, J.; Liang, Z. Dickkopf1 (DKK1) as a Potential Biomarker in Polycystic Ovary Syndrome and Insulin Resistance: A Cross-Sectional Study. Res. Sq. 2023, 1. [Google Scholar] [CrossRef]
- Asadi, K.K.; Amiri, K.; Ghasemi, H.; Amiri, I. Comparison of Follicular Fluid Glycosaminoglycan and Hydroxyproline Concentration in Women with Polycystic Ovary Syndrome with Healthy Women. Avicenna J. Med. Biochem. 2023, 11, 146–150. [Google Scholar] [CrossRef]
- Ullah, A.; Wang, M.-J.; Wang, Y.-X.; Shen, B. CXC chemokines influence immune surveillance in immunological disorders: Polycystic ovary syndrome and endometriosis. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2023, 1869, 166704. [Google Scholar] [CrossRef] [PubMed]
- Hatziagelaki, E.; Pergialiotis, V.; Kannenberg, J.M.; Trakakis, E.; Tsiavou, A.; Markgraf, D.F.; Carstensen-Kirberg, M.; Pacini, G.; Roden, M.; Dimitriadis, G.; et al. Association between Biomarkers of Low-grade Inflammation and Sex Hormones in Women with Polycystic Ovary Syndrome. Exp. Clin. Endocrinol. Diabetes 2020, 128, 723–730. [Google Scholar] [CrossRef]
- Adams, J.; Liu, Z.; Ren, Y.A.; Wun, W.-S.; Zhou, W.; Kenigsberg, S.; Librach, C.; Valdes, C.; Gibbons, W.; Richards, J. Enhanced Inflammatory Transcriptome in the Granulosa Cells of Women with Polycystic Ovarian Syndrome. J. Clin. Endocrinol. Metab. 2016, 101, 3459–3468. [Google Scholar] [CrossRef]
- Nunemaker, C.S.; Chung, H.G.; Verrilli, G.M.; Corbin, K.L.; Upadhye, A.; Sharma, P.R. Increased serum CXCL1 and CXCL5 are linked to obesity, hyperglycemia, and impaired islet function. J. Endocrinol. 2014, 222, 267–276. [Google Scholar] [CrossRef]
- Nissou, M.-F.; Guttin, A.; Zenga, C.; Berger, F.; Issartel, J.-P.; Wion, D. Additional clues for a protective role of vitamin d in neurodegenerative diseases: 1,25-dihydroxyvitamin d3 triggers an anti-inflammatory response in brain pericytes. J. Alzheimer’s Dis. 2014, 42, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, M.; Öktem, M.; Altinkaya, S.; Öktem, E.; Elbeg, S.; Erdem, A.; Erdem, M. Elevated PAPP-A levels in lean patients with polycystic ovary syndrome. Taiwan. J. Obstet. Gynecol. 2018, 57, 394–398. [Google Scholar] [CrossRef]
- Eshre, R.; ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar]
- World Health Organization. Waist Circumference and Waist–Hip Ratio; Report of a WHO Expert Consultation; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Javed, Z.; Papageorgiou, M.; Deshmukh, H.; Kilpatrick, E.S.; Mann, V.; Corless, L.; Abouda, G.; Rigby, A.S.; Atkin, S.L.; Sathyapalan, T. A Randomized, Controlled Trial of Vitamin D Supplementation on Cardiovascular Risk Factors, Hormones, and Liver Markers in Women with Polycystic Ovary Syndrome. Nutrients 2019, 11, 188. [Google Scholar] [CrossRef] [PubMed]
- Kahal, H.; Halama, A.; Aburima, A.; Bhagwat, A.M.; Butler, A.E.; Grauman, J.; Suhre, K.; Sathyapalan, T.; Atkin, S.L. Effect of induced hypoglycemia on inflammation and oxidative stress in type 2 diabetes and control subjects. Sci. Rep. 2020, 10, 4750. [Google Scholar] [CrossRef]
- Gold, L.; Ayers, D.; Bertino, J.; Bock, C.; Bock, A.; Brody, E.N.; Carter, J.; Cunningham, V.; Dalby, A.; Eaton, B.; et al. Aptamer-based multiplexed proteomic technology for biomarker discovery. PLoS ONE 2010, 5, e15004. [Google Scholar] [CrossRef] [PubMed]
- Suhre, K.; Arnold, M.; Bhagwat, A.M.; Cotton, R.J.; Engelke, R.; Raffler, J.; Sarwath, H.; Thareja, G.; Wahl, A.; DeLisle, R.K.; et al. Connecting genetic risk to disease end points through the human blood plasma proteome. Nat. Commun. 2017, 8, 14357. [Google Scholar] [CrossRef]
- Kraemer, S.; Vaught, J.D.; Bock, C.; Gold, L.; Katilius, E.; Keeney, T.R.; Kim, N.; Saccomano, N.A.; Wilcox, S.K.; Zichi, D.; et al. From SOMAmer-based biomarker discovery to diagnostic and clinical applications: A SOMAmer-based, streamlined multiplex proteomic assay. PLoS ONE 2011, 6, e26332. [Google Scholar] [CrossRef] [PubMed]

| Control (n = 29) | PCOS (n = 29) | p-Value | |
|---|---|---|---|
| Age (years) | 32.5 ± 4.1 | 31 ± 6.4 | 0.14 |
| BMI (kg/m2) | 24.8 ± 1.1 | 25.9 ± 1.8 | 0.56 |
| Fasting glucose (nmol/L) | 4.9 ± 0.4 | 4.7 ± 0.8 | 0.06 |
| HbA1C (mmol/mol) | 30.9 ± 6.5 | 31.8 ± 3.0 | 0.9 |
| HOMA-IR | 1.8 ± 1.0 | 1.9 ± 1.6 | 0.97 |
| SHBG (nmol/L) | 104.2 ± 80.3 | 71.7 ± 62.2 | 0.01 |
| Free androgen index (FAI) | 1.3 ± 0.5 | 4.1 ± 2.9 ± 4.08 | 0.0001 |
| CRP (mg L−1) | 2.34 ± 2.34 | 2.77 ± 2.57 | 0.43 |
| AMH (ng/mL) | 24.3 ± 13.1 | 57.2 ± 14.2 | 0.0001 |
| 25(OH)D3 (laboratory reference range 20–40 ng/mL) | 23.77 (13.72–33.24) | 21.80 (15.63–30.46) | 0.86 |
| 1,25(OH)2D3 (laboratory reference range 0.02–0.08 ng/mL) | 0.04 (0.03–0.05) | 0.05 (0.035–0.06) | 0.53 |
| Genes | Target Protein |
|---|---|
| BMP-6 | Bone morphogenetic protein 6 |
| SLAF7 | SLAM family member 7 |
| ATS13 | A disintegrin and metalloproteinase with thrombospondin motifs 13 |
| Angiopoietin-1 | Angiopoietin-1 |
| Adrenomedullin | Adrenomedullin |
| ATS13 | A disintegrin and metalloproteinase with thrombospondin motifs 13 |
| SRCN1 | Proto-oncogene tyrosine-protein kinase Src |
| IL-6 | Interleukin-6 |
| TRAIL R1 | Tumor necrosis factor receptor superfamily member 10A |
| IDUA | Alpha-L-iduronidase |
| RANK | Tumor necrosis factor receptor superfamily member 11A |
| TRAIL R2 | Tumor necrosis factor receptor superfamily member 10B |
| Marapsin | Serine protease 27 |
| sTie-2 | Angiopoietin-1 receptor, soluble |
| TF | Tissue Factor |
| PDGF Rb | Platelet-derived growth factor receptor beta |
| IL-27 | Interleukin-27 |
| Gro-a (CXCL1) | Growth-regulated alpha protein |
| PIGR | Polymeric immunoglobulin receptor |
| sRAGE | Advanced glycosylation end product-specific receptor, soluble |
| Mn SOD | Superoxide dismutase [Mn], mitochondrial |
| HGH | Somatotropin |
| FST | Follistatin |
| IL-1Ra | Interleukin-1 receptor antagonist protein |
| PIGF | Placenta growth factor |
| BOC | Brother of CDO |
| LEG9 | Galectin-9 |
| IL-18 Ra | Interleukin-18 receptor 1 |
| FCG2B | Low-affinity immunoglobulin gamma Fc region receptor II-b |
| Tpo | Thrombopoietin |
| MIP-1a | C-C motif chemokine 3 |
| SLAF5 | SLAM family member 5 |
| PAPPA | Pappalysin-1 |
| Renin | Renin |
| TSP2 | Thrombospondin-2 |
| Lymphotactin | Lymphotactin |
| IL-16 | Interleukin-16 |
| TARC | C-C motif chemokine 17 |
| MMP-7 | Matrilysin |
| Bone proteoglycan II | Decorin |
| DKK1 | Dickkopf-related protein 1 |
| ART | Agouti-related protein |
| HB-EGF | Heparin-binding EGF-like growth factor |
| GDF2 | Growth/differentiation factor 2 |
| MMP-12 | Macrophage metalloelastase |
| ACE2 | Angiotensin-converting enzyme 2 |
| PD-L2 | Programmed cell death 1 ligand 2 |
| TACI | Tumor necrosis factor receptor superfamily member 13B |
| Leptin | Leptin |
| sCD4 | T-cell surface glycoprotein CD4 |
| IgE | Immunoglobulin E |
| FGF23 | Fibroblast growth factor 23 |
| BNP-32 | Brain natriuretic peptide 32 |
| IL-2 sRa | Interleukin-2 receptor subunit alpha |
| CVRPs | logFC | Av Expression | T | p Value |
|---|---|---|---|---|
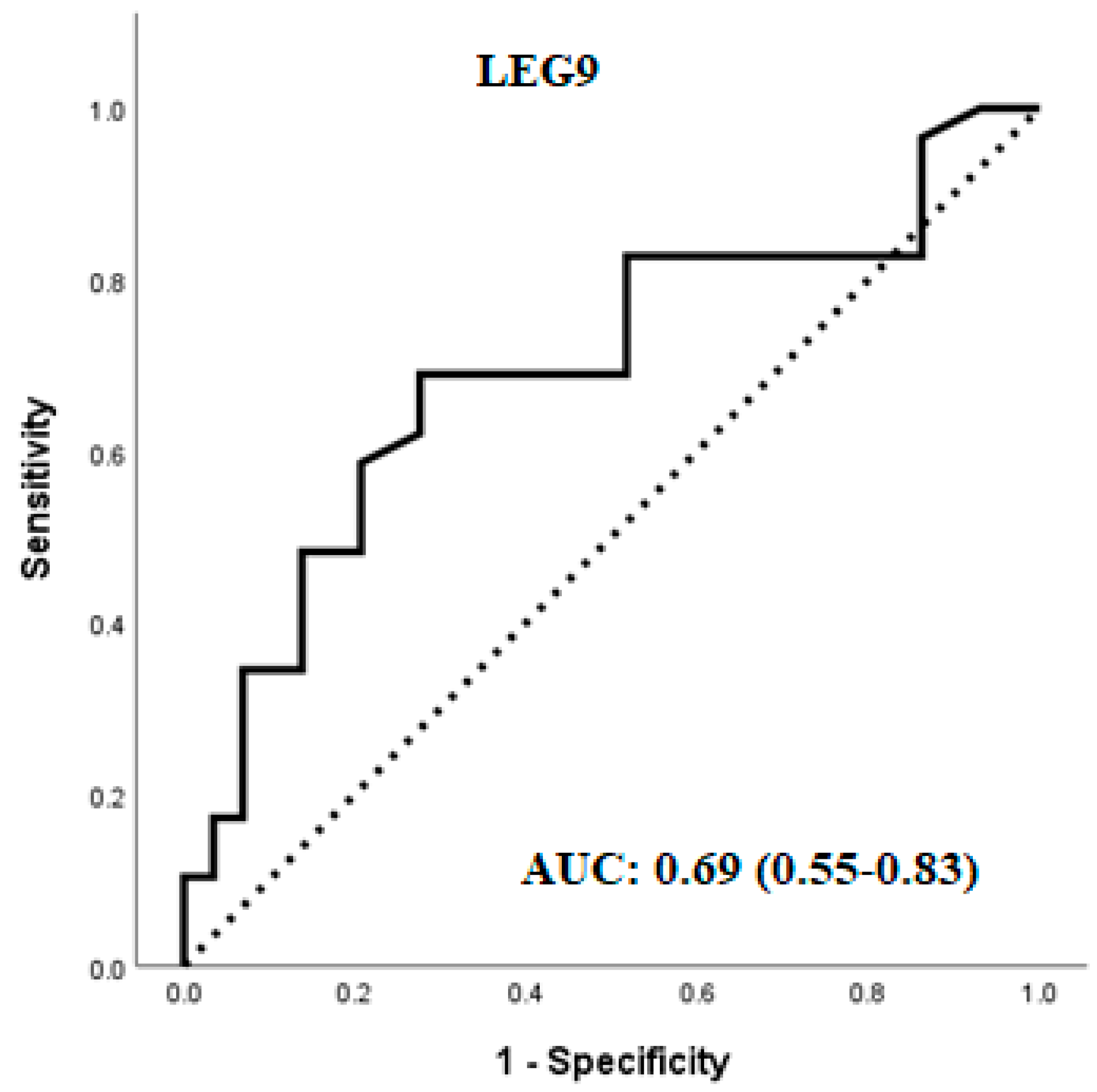
| LEG9 | 0.24 | 9.95 | 2.4 | 0.02 |
| BOC | 0.50 | 10.33 | 2.4 | 0.02 |
| MIP-1a | 0.27 | 8.87 | 2.4 | 0.02 |
| IL-18 Ra | 0.36 | 12.98 | 2.3 | 0.03 |
| Tpo | 0.29 | 6.92 | 2.2 | 0.03 |
| IL-1Ra | 0.39 | 11.93 | 2.2 | 0.03 |
| PD-L2 | 0.26 | 11.55 | 2.2 | 0.03 |
| FCG2B | 0.45 | 10.64 | 2.1 | 0.04 |
| HGH | 0.24 | 7.94 | 2.0 | 0.04 |
| GO Terms | Count | p-Value | Benjamini |
|---|---|---|---|
| Biological Processes—Upregulated genes | |||
| GO:0006954—inflammatory response | 4 | 5.2 × 10−4 | 5.5 × 10−2 |
| GO:0006955—immune response | 4 | 8.6 × 10−4 | 5.5 × 10−2 |
| GO:0032760—positive regulation of TNF production | 3 | 9.0 × 10−4 | 5.5 × 10−2 |
| Pathway enrichment—Upregulated genes | |||
| KEGG_Pathway term: cytokine–cytokine receptor interaction | 5 | 4.4 × 10−5 | 1.1 × 10−3 |
| PCOS | ||
|---|---|---|
| 25(OH)D3 | ||
| r | p | |
| IDUA | −0.570 | 0.01 |
| LEG9 | 0.518 | 0.02 |
| Angiopoietin-1 | −0.500 | 0.02 |
| DKK1 | −0.458 | 0.04 |
| Gro-a (CXCL1) | −0.437 | 0.04 |
| CONTROL | ||
| 25(OH)D3 | ||
| r | p | |
| MMP7 | 0.41 | 0.03 |
| 1,25(OH)2D3 | ||
| TARC | 0.68 | 0.001 |
| PAPPA | −0.48 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nandakumar, M.; Das, P.; Sathyapalan, T.; Butler, A.E.; Atkin, S.L. A Cross-Sectional Exploratory Study of Cardiovascular Risk Biomarkers in Non-Obese Women with and without Polycystic Ovary Syndrome: Association with Vitamin D. Int. J. Mol. Sci. 2024, 25, 6330. https://doi.org/10.3390/ijms25126330
Nandakumar M, Das P, Sathyapalan T, Butler AE, Atkin SL. A Cross-Sectional Exploratory Study of Cardiovascular Risk Biomarkers in Non-Obese Women with and without Polycystic Ovary Syndrome: Association with Vitamin D. International Journal of Molecular Sciences. 2024; 25(12):6330. https://doi.org/10.3390/ijms25126330
Chicago/Turabian StyleNandakumar, Manjula, Priya Das, Thozhukat Sathyapalan, Alexandra E. Butler, and Stephen L. Atkin. 2024. "A Cross-Sectional Exploratory Study of Cardiovascular Risk Biomarkers in Non-Obese Women with and without Polycystic Ovary Syndrome: Association with Vitamin D" International Journal of Molecular Sciences 25, no. 12: 6330. https://doi.org/10.3390/ijms25126330





