The Role of MicroRNAs in the Pathophysiology of Osteoarthritis
Abstract
1. Introduction
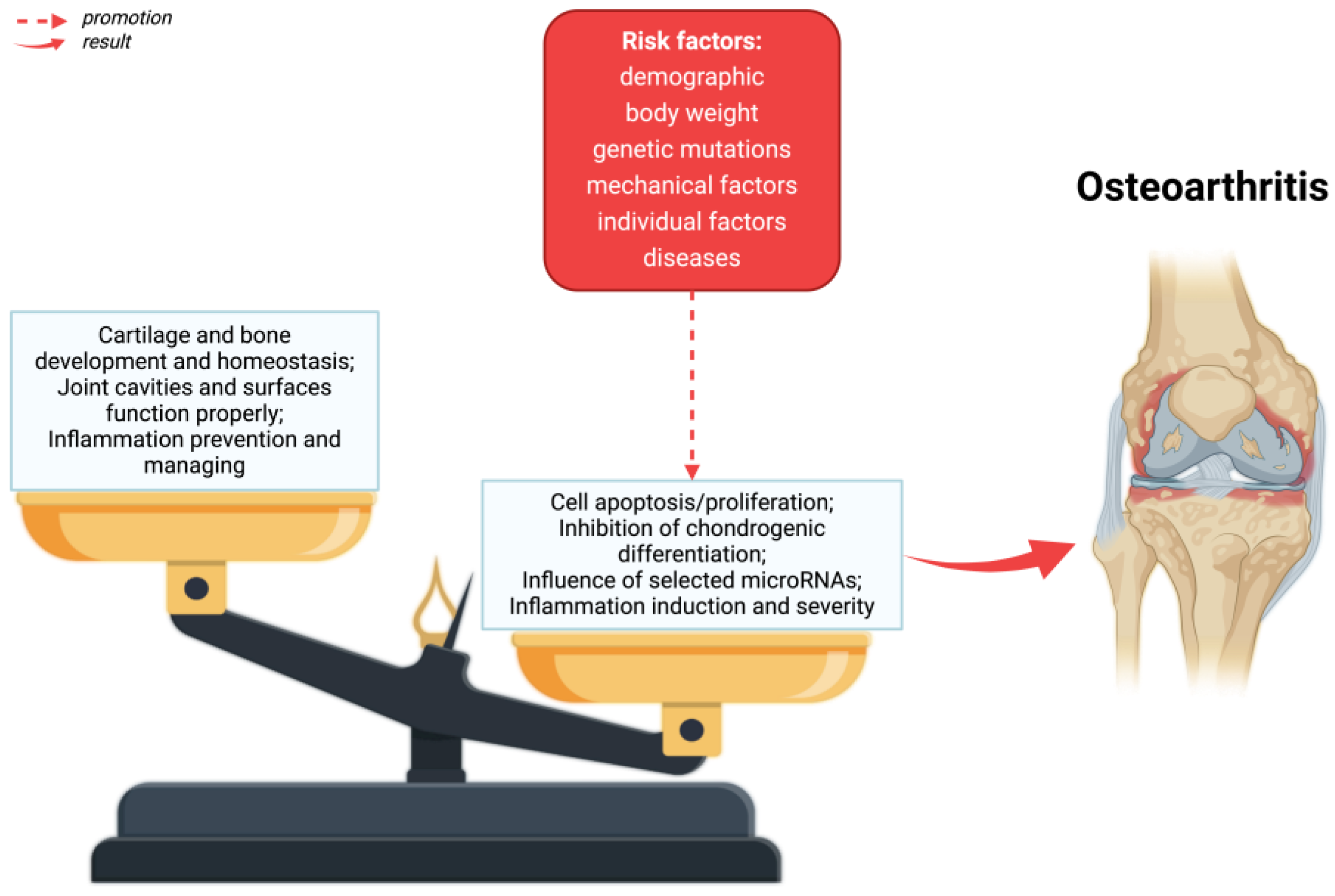
1.1. Osteoarthritis
1.2. Risk Factors
1.3. Clinical Relevance
2. Pathophysiology of Osteoarthritis
3. The Role of miRNAs in the Pathogenesis of Osteoarthritis
| MicroRNA | Level in OA | Potentially Action | Potential Target | Role in OA Pathogenesis | References |
|---|---|---|---|---|---|
| miR-9-5p | ↓ 1 | intensifying proliferation and suppressing chondrocyte apoptosis | MMP-13, PRTG | − 3 | [124] |
| miR-10a-5p | ↑ 2 | inhibiting chondrocyte proliferation, promoting chondrocyte apoptosis, and promoting cartilage matrix degradation | HOXA3 | + 4 | [127] |
| miR-22 | ↑ | the activation of metalloproteinases and aggrecanases and downregulation of cartilage structural proteins, cartilage degradation | PPARα, BMP-7 | + | [128] |
| miR-27b | ↑ | the fibrosis of the synovial membrane, influence on inflammatory processes, cartilage metabolism, and apoptosis of cartilage cells | MMP-13, COL1A1, α-SMA2, ADAMTS8, and CBFB | + | [144,145,146] |
| miR-34a-5p | ↑ | cell cycle arrest, promoting apoptosis, senescence, and proliferation | COL2A1, ACAN, ATG5, MMP13, ADAMTS5, IL-1β, and COL10A1 | + | [131] |
| miR-127-5p | ↓ | increasing the synthesis of cartilage extracellular matrix (ECM) | Osteoponin and MMP-13 | − | [147,148] |
| miR-128a | ↑ | impaired chondrocyte autophagy, the suppression of extracellular matrix deposition | Atg12, Bax, Bcl2, and cleaved caspase-3 | + | [137,138] |
| miR-138-5p | ↑ | the degradation of cartilage extracellular matrix (ECM) | FOXC1 and increase in IL-1β | + | [124] |
| miR-140 | ↓ | promoting chondrocyte differentiation | ADAMTS5 and AGGRECAN | − | [132] |
| miR-140-3p | ↓ | increase in the viability and migration capacity of chondrocytes | increase: SOX-9, COL2, ACAN, RUNX2, and SCX, decrease: COL1, COL6, COMP, TNC, and FMOD | − | [149] |
| miR-140-5p | ↓ | inhibits inflammation in the joint cavity, inhibits the progression of OA, promotes chondrogenesis, inhibits chondrocyte apoptosis, inhibits chondrocyte hypertrophy | IGFBP-5, IL-1β, IL-6, Syndecan-4, ADAMTS5, MMP-13, SMAD3, HMGB1, RALA, FUT1, HDAC4, and SMAD1 | − | [105,109,111,150,151,152,153,154,155,156] |
| mi-146 | ↑ | promoting the inflammatory response in the joint | TRAF6 and IRAQ1, | + | [157,158] |
| miR-146a-5p | ↑ | cartilage degradation, synovitis, neoangiogenesis, and osteoclastogenesis | TNF α, IL-1β, TRAF6 and IRAK1 genes, and MMP-13 | + | [114,124,159] |
| miR-149 | ↓ | promoting the synthesis of connective protein and proteoglycan, suppressing the inflammatory process | TNFα, IL1β, IL6, VCAM-1, and TAK1 | − | [134,135] |
| miR-210 | ↓ | promoting osteoblastic differentiation, anti-apoptotic effect, anti-inflammatory effect | AcvR1b and DR6 | − | [139,140,141,160] |
| miR-335-5p | ↑ | osteogenic and adipogenic differentiation, promoting ECM degradation | Wnt signaling pathway, IFNγ, HBP1, ACAN, MMP13, collagen X, and collagen II | + | [124,142] |
| miR-485-5p | ↑ | inhibiting the differentiation of BMSCs into chondroblasts and promoting the expression of inflammatory factors | SOX9 | + | [143] |
4. Potential Therapeutic Targets
5. Discussion
6. Conclusions
Funding
Conflicts of Interest
Abbreviations
| ACAN | Aggrecan |
| ACR | American College of Rheumatology |
| ADAMTS5 | A Disintegrin and Metalloproteinase with Thrombospondin Motifs 5 |
| ADAMTS8 | A Disintegrin and Metalloproteinase with Thrombospondin Motifs 8 |
| Atg12 | Autophagy Related 12 |
| ATG5 | Autophagy Related 5 |
| Bax | BCL2 Associated X, Apoptosis Regulator |
| Bcl2 | B-Cell Lymphoma 2 |
| BMP-7 | Bone Morphogenetic Protein 7 |
| CBFB | Core-Binding Factor Subunit Beta |
| COL10A1 | Collagen Type X Alpha 1 Chain |
| COL1A1 | Collagen Type I Alpha 1 Chain |
| COL2 | Collagen Type II |
| COL2A1 | Collagen Type II Alpha 1 Chain |
| COL6 | Collagen Type VI |
| COMP | Cartilage Oligomeric Matrix Protein |
| DR6 | Death Receptor 6 |
| ECM | Extracellular matrix |
| FMOD | Fibromodulin |
| FOXC1 | Forkhead Box C1 |
| FUT1 | Fucosyltransferase 1 |
| HDAC4 | Histone Deacetylase 4 |
| HMGB1 | High Mobility Group Box 1 |
| HOXA3 | Homeobox A3 |
| HBP1 | HMG-Box Transcription Factor 1 |
| IFNγ | Interferon Gamma |
| IGFBP-5 | Insulin-Like Growth Factor Binding Protein 5 |
| IL | interleukins |
| IRAK1 | Interleukin-1 Receptor-Associated Kinase 1 |
| MiRNA, MiR | microRNA |
| MMP-13 | Matrix Metallopeptidase 13 |
| NF-κB | Nuclear factor kappa B |
| OA | osteoarthritis |
| PARP | Poly (ADP-Ribose) Polymerase |
| PPARα/γ | Peroxisome Proliferator-Activated Receptor α/γ |
| PRTG | Protogenin |
| RALA | Ras-Like Proto-Oncogene A |
| RUNX2 | Runt-Related Transcription Factor 2 |
| SCX | Scleraxis |
| SMAD1 | SMAD Family Member 1 |
| SMAD3 | SMAD Family Member 3 |
| SOX9 | SRY-Box Transcription Factor 9 |
| TAK1 | TGF-Beta Activated Kinase 1 |
| TNC | Tenascin C |
| TNFα | Tumor Necrosis Factor Alpha |
| TRAF6 | TNF Receptor Associated Factor 6 |
| VCAM-1 | Vascular Cell Adhesion Molecule 1 |
| α-SMA2 | α Smooth Muscle Actin 2 |
References
- Yunus, M.H.M.; Nordin, A.; Kamal, H. Pathophysiological Perspective of Osteoarthritis. Medicina 2020, 56, 614. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Di, C.; Zhang, J.; Hu, S.; Jin, H.; Tong, P. Osteoarthritis Pathogenesis: A Review of Molecular Mechanisms. Calcif. Tissue Int. 2014, 95, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; March, L.; Chew, M. Osteoarthritis in 2020 and beyond: A Lancet Commission. Lancet 2020, 396, 1711–1712. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Liu, Q.; Yin, H.; Wang, K.; Diao, N.; Zhang, Y.; Lin, J.; Guo, A. Prevalence Trends of Site-Specific Osteoarthritis from 1990 to 2019: Findings from the Global Burden of Disease Study 2019. Arthritis Rheumatol. 2022, 74, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Thudium, C.S.; Bay-Jensen, A.C.; Maleitzke, T.; Geissler, S.; Duda, G.N.; Winkler, T. Biomarkers for Osteoarthritis: Current Status and Future Prospects. Best Pract. Res. Clin. Rheumatol. 2023, 37, 101852. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Li, H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, Regional Prevalence, Incidence and Risk Factors of Knee Osteoarthritis in Population-Based Studies. eClinicalMedicine 2020, 29–30, 100587. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Felson, D.T. Osteoarthritis. Br. Med. J. 2006, 332, 639. [Google Scholar] [CrossRef] [PubMed]
- Glyn-Jones, S.; Palmer, A.J.R.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Moseng, T.; Vliet Vlieland, T.P.M.; Battista, S.; Beckwée, D.; Boyadzhieva, V.; Conaghan, P.G.; Costa, D.; Doherty, M.; Finney, A.G.; Georgiev, T.; et al. EULAR Recommendations for the Non-Pharmacological Core Management of Hip and Knee Osteoarthritis: 2023 Update. Ann. Rheum. Dis. 2024, 83, 730–740. [Google Scholar] [CrossRef]
- Yao, Q.; Wu, X.; Tao, C.; Gong, W.; Chen, M.; Qu, M.; Zhong, Y.; He, T.; Chen, S.; Xiao, G. Osteoarthritis: Pathogenic Signaling Pathways and Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 56. [Google Scholar] [CrossRef]
- Little, C.B.; Hunter, D.J. Post-Traumatic Osteoarthritis: From Mouse Models to Clinical Trials. Nat. Rev. Rheumatol. 2013, 9, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.; van der Esch, M.; Hinman, R.S.; Peat, G.; de Zwart, A.; Quicke, J.G.; Runhaar, J.; Knoop, J.; van der Leeden, M.; de Rooij, M.; et al. How Does Hip Osteoarthritis Differ from Knee Osteoarthritis? Osteoarthr. Cartil. 2022, 30, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhang, Z.; Chen, Y.; Zhang, Y.; Xing, D.; Zhao, L.; Lin, J.; Mei, Y.; Lin, H.-Y.; Zheng, Y.; et al. Development and Formulation of the Classification Criteria for Osteoarthritis. Ann. Transl. Med. 2020, 8, 1068. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Lu, K.; Umar, M.; Zhu, Z.; Lu, W.W.; Speakman, J.R.; Chen, Y.; Tong, L.; Chen, D. Risk of Metabolic Abnormalities in Osteoarthritis: A New Perspective to Understand Its Pathological Mechanisms. Bone Res. 2023, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Chung, C.Y.; Sung, K.H.; Lee, S.Y.; Won, S.H.; Kim, T.G.; Choi, Y.; Kwon, S.S.; Kim, Y.H.; Park, M.S. Risk Factors for Osteoarthritis and Contributing Factors to Current Arthritic Pain in South Korean Older Adults. Yonsei Med. J. 2015, 56, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L. Osteoarthritis of the Knee. N. Engl. J. Med. 2021, 384, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Krakowski, P.; Karpiński, R.; Maciejewski, R.; Jonak, J.; Jurkiewicz, A. Short-Term Effects of Arthroscopic Microfracturation of Knee Chondral Defects in Osteoarthritis. Appl. Sci. 2020, 10, 8312. [Google Scholar] [CrossRef]
- Karpiński, R. Knee Joint Osteoarthritis Diagnosis Based on Selected Acoustic Signal Discriminants Using Machine Learning. Appl. Comput. Sci. 2022, 18, 71–85. [Google Scholar] [CrossRef]
- Lee, R.; Kean, W.F. Obesity and Knee Osteoarthritis. Inflammopharmacology 2012, 20, 53–58. [Google Scholar] [CrossRef]
- Michael, J.W.-P.; Schlüter-Brust, K.U.; Eysel, P. The Epidemiology, Etiology, Diagnosis, and Treatment of Osteoarthritis of the Knee. Dtsch. Arztebl. Int. 2010, 107, 152–162. [Google Scholar] [CrossRef]
- Karpiński, R.; Krakowski, P.; Jonak, J.; Machrowska, A.; Maciejewski, M. Comparison of Selected Classification Methods Based on Machine Learning as a Diagnostic Tool for Knee Joint Cartilage Damage Based on Generated Vibroacoustic Processes. Appl. Comput. Sci. 2023, 19, 136–150. [Google Scholar] [CrossRef]
- Felson, D.T. Clinical Practice. Osteoarthritis of the Knee. N. Engl. J. Med. 2006, 354, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Abramoff, B.; Caldera, F.E. Osteoarthritis: Pathology, Diagnosis, and Treatment Options. Med. Clin. N. Am. 2020, 104, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann-Górska, I.; Szczepański, L. Osteoarthritis. In Interna Szczeklika 2023; Szczeklik, A., Gajewski, P., Eds.; Medycyna Praktyczna: Kraków, Poland, 2023; pp. 2162–2170. [Google Scholar]
- Palazzo, C.; Nguyen, C.; Lefevre-Colau, M.M.; Rannou, F.; Poiraudeau, S. Risk Factors and Burden of Osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Aiello, F.C.; Szychlinska, M.A.; Di Rosa, M.; Castrogiovanni, P.; Mobasheri, A. Osteoarthritis in the XXIst Century: Risk Factors and Behaviours That Influence Disease Onset and Progression. Int. J. Mol. Sci. 2015, 16, 6093–6112. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, T.W.; McCabe, P.S.; McBeth, J. Update on the Epidemiology, Risk Factors and Disease Outcomes of Osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2018, 32, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, T.; Angelov, A.K. Modifiable Risk Factors in Knee Osteoarthritis: Treatment Implications. Rheumatol. Int. 2019, 39, 1145–1157. [Google Scholar] [CrossRef] [PubMed]
- Chojnacki, M.; Kwapisz, A.; Synder, M.; Szemraj, J. Osteoarthritis: Etiology, Risk Factors, Molecular Mechanisms. Postep. Hig. Med. Dosw. 2014, 68, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Moreno, M.; Rego, I.; Carreira-Garcia, V.; Blanco, F.J. Genetics in Osteoarthritis. Curr. Genom. 2008, 9, 542–547. [Google Scholar] [CrossRef]
- Hardcastle, S.A.; Dieppe, P.; Gregson, C.L.; Davey Smith, G.; Tobias, J.H. Osteoarthritis and Bone Mineral Density: Are Strong Bones Bad for Joints? Bonekey Rep. 2015, 4, 624. [Google Scholar] [CrossRef]
- Teichtahl, A.J.; Wang, Y.; Wluka, A.E.; Strauss, B.J.; Proietto, J.; Dixon, J.B.; Jones, G.; Cicuttini, F.M. Associations between Systemic Bone Mineral Density and Early Knee Cartilage Changes in Middle-Aged Adults without Clinical Knee Disease: A Prospective Cohort Study. Arthritis Res. Ther. 2017, 19, 98. [Google Scholar] [CrossRef] [PubMed]
- Nevitt, M.C.; Zhang, Y.; Javaid, M.K.; Neogi, T.; Curtis, J.R.; Niu, J.; McCulloch, C.E.; Segal, N.A.; Felson, D.T. High Systemic Bone Mineral Density Increases the Risk of Incident Knee OA and Joint Space Narrowing, but Not Radiographic Progression of Existing Knee OA: The MOST Study. Ann. Rheum. Dis. 2010, 69, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA—J. Am. Med. Assoc. 2021, 325, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.; Alarcon, G.; Appelrouth, D.; Bloch, D.; Borenstein, D.; Brandt, K.; Brown, C.; Cooke, T.D.; Daniel, W.; Gray, R.; et al. The American College of Rheumatology Criteria for the Classification and Reporting of Osteoarthritis of the Hand. Arthritis Rheum. 1990, 33, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.; Asch, E.; Bloch, D.; Bole, G.; Borenstein, D.; Brandt, K.; Christy, W.; Cooke, T.D.; Greenwald, R.; Hochberg, M.; et al. Development of Criteria for the Classification and Reporting of Osteoarthritis: Classification of Osteoarthritis of the Knee. Arthritis Rheum. 1986, 29, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, O.; Hayashi, M.; Horikawa, A.; Owada, H.; Miyamoto, R.; Mizukami, N.; Inui, T. The Role of MiR-217-5p in the Puromycin Aminonucleoside-Induced Morphological Change of Podocytes. Noncoding RNA 2022, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Strmsek, Z.; Kunej, T. MicroRNA Silencing by DNA Methylation in Human Cancer: A Literature Analysis. Noncoding RNA 2015, 1, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Grenda, A.; Budzyński, M.; Filip, A. Biogenesis of MicroRNAs and Their Role in the Development and Course of Selected Hematologic Disorders. Postep. Hig. Med. Dosw. 2013, 67, 174–185. [Google Scholar] [CrossRef]
- Pu, M.; Chen, J.; Tao, Z.; Miao, L.; Qi, X.; Wang, Y.; Ren, J. Regulatory Network of MiRNA on Its Target: Coordination between Transcriptional and Post-Transcriptional Regulation of Gene Expression. Cell. Mol. Life Sci. 2019, 76, 441–451. [Google Scholar] [CrossRef]
- Meister, G.; Tuschl, T. Mechanisms of Gene Silencing by Double-Stranded RNA. Nature 2004, 431, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Wakiyama, M.; Takimoto, K.; Ohara, O.; Yokoyama, S. Let-7 MicroRNA-Mediated MRNA Deadenylation and Translational Repression in a Mammalian Cell-Free System. Genes Dev. 2007, 21, 1857–1862. [Google Scholar] [CrossRef] [PubMed]
- Alwani, A.; Baj-Krzyworzeka, M. MiRNAs—Targets in Cancer Therapy. Postep. Biochem. 2021, 67. [Google Scholar] [CrossRef] [PubMed]
- Eastlack, S.C.; Alahari, S.K. MicroRNA and Breast Cancer: Understanding Pathogenesis, Improving Management. Noncoding RNA 2015, 1, 17–43. [Google Scholar] [CrossRef] [PubMed]
- Nedunchezhiyan, U.; Varughese, I.; Sun, A.R.J.; Wu, X.; Crawford, R.; Prasadam, I. Obesity, Inflammation, and Immune System in Osteoarthritis. Front. Immunol. 2022, 13, 907750. [Google Scholar] [CrossRef] [PubMed]
- Caldo, D.; Massarini, E.; Rucci, M.; Deaglio, S.; Ferracini, R. Epigenetics in Knee Osteoarthritis: A 2020–2023 Update Systematic Review. Life 2024, 14, 269. [Google Scholar] [CrossRef] [PubMed]
- Balaskas, P.; Goljanek-Whysall, K.; Clegg, P.D.; Fang, Y.; Cremers, A.; Smagul, A.; Welting, T.J.M.; Peffers, M.J. MicroRNA Signatures in Cartilage Ageing and Osteoarthritis. Biomedicines 2023, 11, 1189. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.C.; Chuang, S.M.; Hsu, C.J.; Tsai, C.H.; Wang, S.W.; Tang, C.H. CTGF Increases Vascular Endothelial Growth Factor-Dependent Angiogenesis in Human Synovial Fibroblasts by Increasing MiR-210 Expression. Cell Death Dis. 2014, 5, e1485. [Google Scholar] [CrossRef]
- Moran-Moguel, M.C.; Rio, S.P.D.; Mayorquin-Galvan, E.E.; Zavala-Cerna, M.G. Rheumatoid Arthritis and MiRNAs: A Critical Review through a Functional View. J. Immunol. Res. 2018, 2018, 2474529. [Google Scholar] [CrossRef]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-Grade Inflammation as a Key Mediator of the Pathogenesis of Osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef]
- Reyes, C.; Leyland, K.M.; Peat, G.; Cooper, C.; Arden, N.K.; Prieto-Alhambra, D. Association Between Overweight and Obesity and Risk of Clinically Diagnosed Knee, Hip, and Hand Osteoarthritis: A Population-Based Cohort Study. Arthritis Rheumatol. 2016, 68, 1869–1875. [Google Scholar] [CrossRef] [PubMed]
- Raud, B.; Gay, C.; Guiguet-Auclair, C.; Bonnin, A.; Gerbaud, L.; Pereira, B.; Duclos, M.; Boirie, Y.; Coudeyre, E. Level of Obesity Is Directly Associated with the Clinical and Functional Consequences of Knee Osteoarthritis. Sci. Rep. 2020, 10, 3601. [Google Scholar] [CrossRef] [PubMed]
- Galiniak, S.; Krawczyk-Marć, I.; Wawrzyniak, A.; Orkisz, S. Chondrocytes Apoptosis in Osteoarthritis. Postep. Hig. Med. Dosw. 2018, 72, 875–883. [Google Scholar] [CrossRef]
- Sandell, L.J.; Aigner, T. Articular Cartilage and Changes in Arthritis An Introduction: Cell Biology of Osteoarthritis. Arthritis Res. 2001, 3, 107. [Google Scholar] [CrossRef] [PubMed]
- Van Donkelaar, C.C.; Wilson, W. Mechanics of Chondrocyte Hypertrophy. Biomech. Model. Mechanobiol. 2012, 11, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Dreier, R. Hypertrophic Differentiation of Chondrocytes in Osteoarthritis: The Developmental Aspect of Degenerative Joint Disorders. Arthritis Res. Ther. 2010, 12, 216. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Kim, W.J. The Role of Matrix Metalloproteinase in Inflammation with a Focus on Infectious Diseases. Int. J. Mol. Sci. 2022, 23, 10546. [Google Scholar] [CrossRef] [PubMed]
- Takahata, Y.; Murakami, T.; Hata, K.; Nishimura, R. Molecular Mechanisms Involved in the Progression and Protection of Osteoarthritis. Curr. Mol. Pharmacol. 2020, 14, 165–169. [Google Scholar] [CrossRef]
- Yamamoto, K.; Wilkinson, D.; Bou-Gharios, G. Targeting Dysregulation of Metalloproteinase Activity in Osteoarthritis. Calcif. Tissue Int. 2021, 109, 277–290. [Google Scholar] [CrossRef]
- Mehana, E.S.E.; Khafaga, A.F.; El-Blehi, S.S. The Role of Matrix Metalloproteinases in Osteoarthritis Pathogenesis: An Updated Review. Life Sci. 2019, 234, 116786. [Google Scholar] [CrossRef]
- Stannus, O.; Jones, G.; Cicuttini, F.; Parameswaran, V.; Quinn, S.; Burgess, J.; Ding, C. Circulating Levels of IL-6 and TNF-α Are Associated with Knee Radiographic Osteoarthritis and Knee Cartilage Loss in Older Adults. Osteoarthr. Cartil. 2010, 18, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Attur, M.; Statnikov, A.; Samuels, J.; Li, Z.; Alekseyenko, A.V.; Greenberg, J.D.; Krasnokutsky, S.; Rybak, L.; Lu, Q.A.; Todd, J.; et al. Plasma Levels of Interleukin-1 Receptor Antagonist (IL1Ra) Predict Radiographic Progression of Symptomatic Knee Osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1915–1924. [Google Scholar] [CrossRef] [PubMed]
- Chow, Y.Y.; Chin, K.Y. The Role of Inflammation in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2020, 2020, 8293921. [Google Scholar] [CrossRef] [PubMed]
- Scanzello, C.R.; Umoh, E.; Pessler, F.; Diaz-Torne, C.; Miles, T.; DiCarlo, E.; Potter, H.G.; Mandl, L.; Marx, R.; Rodeo, S.; et al. Local Cytokine Profiles in Knee Osteoarthritis: Elevated Synovial Fluid Interleukin-15 Differentiates Early from End-Stage Disease. Osteoarthr. Cartil. 2009, 17, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Dell’Isola, A.; Steultjens, M. Classification of Patients with Knee Osteoarthritis in Clinical Phenotypes: Data from the Osteoarthritis Initiative. PLoS ONE 2018, 13, e019104. [Google Scholar] [CrossRef] [PubMed]
- Mimpen, J.Y.; Baldwin, M.J.; Cribbs, A.P.; Philpott, M.; Carr, A.J.; Dakin, S.G.; Snelling, S.J.B. Interleukin-17A Causes Osteoarthritis-Like Transcriptional Changes in Human Osteoarthritis-Derived Chondrocytes and Synovial Fibroblasts In Vitro. Front. Immunol. 2021, 12, 676173. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, D.; Long, L.; Deng, X.; Tao, R.; Huang, G. Correlation between Plasma, Synovial Fluid and Articular Cartilage Interleukin-18 with Radiographic Severity in 33 Patients with Osteoarthritis of the Knee. Clin. Exp. Med. 2014, 14, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Qi, C.; Liu, Y.; Gao, H.; Zhao, D.; Jiang, Y. Increased Frequency of Peripheral Blood Follicular Helper T Cells and Elevated Serum IL-21 Levels in Patients with Knee Osteoarthritis. Mol. Med. Rep. 2017, 15, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Deligne, C.; Casulli, S.; Pigenet, A.; Bougault, C.; Campillo-Gimenez, L.; Nourissat, G.; Berenbaum, F.; Elbim, C.; Houard, X. Differential Expression of Interleukin-17 and Interleukin-22 in Inflamed and Non-Inflamed Synovium from Osteoarthritis Patients. Osteoarthr. Cartil. 2015, 23, 1843–1852. [Google Scholar] [CrossRef]
- Yi, C.; Yi, Y.; Wei, J.; Jin, Q.; Li, J.; Sacitharan, P.K. Targeting IL-22 and IL-22R Protects against Experimental Osteoarthritis. Cell. Mol. Immunol. 2021, 18, 1329–1331. [Google Scholar] [CrossRef]
- Xiao, S.Q.; Cheng, M.; Wang, L.; Cao, J.; Fang, L.; Zhou, X.P.; He, X.J.; Hu, Y.F. The Role of Apoptosis in the Pathogenesis of Osteoarthritis. Int. Orthop. 2023, 47, 1895–1919. [Google Scholar] [CrossRef] [PubMed]
- Scanzello, C.R. Chemokines and Inflammation in Osteoarthritis: Insights from Patients and Animal Models. J. Orthop. Res. 2017, 35, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Doi, H.; Nishida, K.; Yorimitsu, M.; Komiyama, T.; Kadota, Y.; Tetsunaga, T.; Yoshida, A.; Kubota, S.; Takigawa, M.; Ozaki, T. Interleukin-4 Downregulates the Cyclic Tensile Stress-Induced Matrix Metalloproteinases-13 and Cathepsin b Expression by Rat Normal Chondrocytes. Acta Med. Okayama 2008, 62, 119–126. [Google Scholar] [PubMed]
- Van Meegeren, M.E.R.; Roosendaal, G.; Jansen, N.W.D.; Wenting, M.J.G.; Van Wesel, A.C.W.; Van Roon, J.A.G.; Lafeber, F.P.J.G. IL-4 Alone and in Combination with IL-10 Protects against Blood-Induced Cartilage Damage. Osteoarthr. Cartil. 2012, 20, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Tanzil, G.; Zreiqat, H.; Sabat, R.; Kohl, B.; Halder, A.; Muller, R.; John, T. Interleukin-10 and Articular Cartilage: Experimental Therapeutical Approaches in Cartilage Disorders. Curr. Gene Ther. 2009, 9, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Barker, T.; Rogers, V.E.; Henriksen, V.T.; Trawick, R.H.; Momberger, N.G.; Lynn Rasmussen, G. Circulating IL-10 Is Compromised in Patients Predisposed to Developing and in Patients with Severe Knee Osteoarthritis. Sci. Rep. 2021, 11, 1812. [Google Scholar] [CrossRef]
- Fernandes, T.L.; Gomoll, A.H.; Lattermann, C.; Hernandez, A.J.; Bueno, D.F.; Amano, M.T. Macrophage: A Potential Target on Cartilage Regeneration. Front. Immunol. 2020, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Molnar, V.; Matišić, V.; Kodvanj, I.; Bjelica, R.; Jeleč, Ž.; Hudetz, D.; Rod, E.; Čukelj, F.; Vrdoljak, T.; Vidović, D.; et al. Cytokines and Chemokines Involved in Osteoarthritis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9208. [Google Scholar] [CrossRef] [PubMed]
- Primorac, D.; Molnar, V.; Matišić, V.; Hudetz, D.; Jeleč, Ž.; Rod, E.; Čukelj, F.; Vidović, D.; Vrdoljak, T.; Dobričić, B.; et al. Comprehensive Review of Knee Osteoarthritis Pharmacological Treatment and the Latest Professional Societies’ Guidelines. Pharmaceuticals 2021, 14, 205. [Google Scholar] [CrossRef]
- Verbruggen, G.; Wittoek, R.; Vander Cruyssen, B.; Elewaut, D. Tumour Necrosis Factor Blockade for the Treatment of Erosive Osteoarthritis of the Interphalangeal Finger Joints: A Double Blind, Randomised Trial on Structure Modification. Ann. Rheum. Dis. 2012, 71, 891–898. [Google Scholar] [CrossRef]
- Cohen, S.B.; Proudman, S.; Kivitz, A.J.; Burch, F.X.; Donohue, J.P.; Burstein, D.; Sun, Y.N.; Banfield, C.; Vincent, M.S.; Ni, L.; et al. A Randomized, Double-Blind Study of AMG 108 (a Fully Human Monoclonal Antibody to IL-1R1) in Patients with Osteoarthritis of the Knee. Arthritis Res. Ther. 2011, 13, R125. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, X.; Eymard, F.; Richette, P. Biologic Agents in Osteoarthritis: Hopes and Disappointments. Nat. Rev. Rheumatol. 2013, 9, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, X.; Goupille, P.; Beaulieu, A.D.; Burch, F.X.; Bensen, W.G.; Conrozier, T.; Loeuille, D.; Kivitz, A.J.; Silver, D.; Appleton, B.E. Intraarticular Injection of Anakinra in Osteoarthritis of the Knee: A Multicenter, Randomized, Double-Blind, Placebo-Controlled Study. Arthritis Care Res. 2009, 61, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yin, J.; Gao, J.; Cheng, T.S.; Pavlos, N.J.; Zhang, C.; Zheng, M.H. Subchondral Bone in Osteoarthritis: Insight into Risk Factors and Microstructural Changes. Arthritis Res. Ther. 2013, 15, 223. [Google Scholar] [CrossRef] [PubMed]
- Bobinac, D.; Spanjol, J.; Zoricic, S.; Maric, I. Changes in Articular Cartilage and Subchondral Bone Histomorphometry in Osteoarthritic Knee Joints in Humans. Bone 2003, 32, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Cui, J.; Chu, L.; Zhang, W.; Xie, K.; Jiang, X.; He, Z.; Du, J.; Ai, S.; Sun, Q.; et al. Abnormal Subchondral Trabecular Bone Remodeling in Knee Osteoarthritis under the Influence of Knee Alignment. Osteoarthr. Cartil. 2022, 30, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Chen, Y.; Dou, C.; Dong, S. Microenvironment in Subchondral Bone: Predominant Regulator for the Treatment of Osteoarthritis. Ann. Rheum. Dis. 2021, 80, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kirkwood, C.L.; Sohn, J.; Lau, A.; Bayers-Thering, M.; Bali, S.K.; Rachala, S.; Marzo, J.M.; Anders, M.J.; Beier, F.; et al. Expansion of Myeloid-Derived Suppressor Cells Contributes to Metabolic Osteoarthritis through Subchondral Bone Remodeling. Arthritis Res. Ther. 2021, 23, 287. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, X.; Wang, S.; Jing, Y.; Su, J. Subchondral Bone Microenvironment in Osteoarthritis and Pain. Bone Res. 2021, 9, 20. [Google Scholar] [CrossRef]
- Burr, D.B.; Gallant, M.A. Bone Remodelling in Osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 665–673. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, Y.; Yu, Y.E.; Zhang, X.; Watts, T.; Zhou, B.; Wang, J.; Wang, T.; Zhao, W.; Chiu, K.Y.; et al. Subchondral Trabecular Rod Loss and Plate Thickening in the Development of Osteoarthritis. J. Bone Miner. Res. 2018, 33, 316–327. [Google Scholar] [CrossRef]
- Yokota, S.; Ishizu, H.; Miyazaki, T.; Takahashi, D.; Iwasaki, N.; Shimizu, T. Osteoporosis, Osteoarthritis, and Subchondral Insufficiency Fracture: Recent Insights. Biomedicines 2024, 12, 843. [Google Scholar] [CrossRef] [PubMed]
- Goldring, S.R.; Goldring, M.B. Changes in the Osteochondral Unit during Osteoarthritis: Structure, Function and Cartilage Bone Crosstalk. Nat. Rev. Rheumatol. 2016, 12, 632–644. [Google Scholar] [CrossRef]
- Wu, X.; Liyanage, C.; Plan, M.; Stark, T.; McCubbin, T.; Barrero, R.A.; Batra, J.; Crawford, R.; Xiao, Y.; Prasadam, I. Dysregulated Energy Metabolism Impairs Chondrocyte Function in Osteoarthritis. Osteoarthr. Cartil. 2023, 31, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Rayman, M.P.; Gualillo, O.; Sellam, J.; Van Der Kraan, P.; Fearon, U. The Role of Metabolism in the Pathogenesis of Osteoarthritis. Nat. Rev. Rheumatol. 2017, 13, 302–311. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, Z.; Sheng, P.; Mobasheri, A. The Role of Metabolism in Chondrocyte Dysfunction and the Progression of Osteoarthritis. Ageing Res. Rev. 2021, 66, 101249. [Google Scholar] [CrossRef] [PubMed]
- Maruotti, N.; Corrado, A.; Cantatore, F.P. Osteoblast Role in Osteoarthritis Pathogenesis. J. Cell. Physiol. 2017, 232, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Maglaviceanu, A.; Wu, B.; Kapoor, M. Fibroblast-like Synoviocytes: Role in Synovial Fibrosis Associated with Osteoarthritis. Wound Repair. Regen. 2021, 29, 642–649. [Google Scholar] [CrossRef]
- Li, T.; Peng, J.; Li, Q.; Shu, Y.; Zhu, P.; Hao, L. The Mechanism and Role of ADAMTS Protein Family in Osteoarthritis. Biomolecules 2022, 12, 959. [Google Scholar] [CrossRef]
- Grässel, S.; Zaucke, F.; Madry, H. Osteoarthritis: Novel Molecular Mechanisms Increase Our Understanding of the Disease Pathology. J. Clin. Med. 2021, 10, 1938. [Google Scholar] [CrossRef]
- Salman, L.A.; Ahmed, G.; Dakin, S.G.; Kendrick, B.; Price, A. Osteoarthritis: A Narrative Review of Molecular Approaches to Disease Management. Arthritis Res. Ther. 2023, 25, 27. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Tanzil, G. Experimental Therapeutics for the Treatment of Osteoarthritis. J. Exp. Pharmacol. 2021, 13, 101–125. [Google Scholar] [CrossRef]
- Swingler, T.E.; Niu, L.; Smith, P.; Paddy, P.; Le, L.; Barter, M.J.; Young, D.A.; Clark, I.M. The Function of MicroRNAs in Cartilage and Osteoarthritis. Clin. Exp. Rheumatol. 2019, 37, 40–47. [Google Scholar] [PubMed]
- Tardif, G.; Hum, D.; Pelletier, J.P.; Duval, N.; Martel-Pelletier, J. Regulation of the IGFBP-5 and MMP-13 Genes by the MicroRNAs MiR-140 and MiR-27a in Human Osteoarthritic Chondrocytes. BMC Musculoskelet. Disord. 2009, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, F.E.; Pais, H.; Schwach, F.; Lindow, M.; Kauppinen, S.; Moulton, V.; Dalmay, T. Experimental Identification of MicroRNA-140 Targets by Silencing and Overexpressing MiR-140. RNA 2008, 14, 2513–2520. [Google Scholar] [CrossRef]
- Pais, H.; Nicolas, F.E.; Soond, S.M.; Swingler, T.E.; Clark, I.M.; Chantry, A.; Moulton, V.; Dalmay, T. Analyzing MRNA Expression Identifies Smad3 as a MicroRNA-140 Target Regulated Only at Protein Level. RNA 2010, 16, 489–494. [Google Scholar] [CrossRef]
- Barter, M.J.; Tselepi, M.; Gõmez, R.; Woods, S.; Hui, W.; Smith, G.R.; Shanley, D.P.; Clark, I.M.; Young, D.A. Genome-Wide MicroRNA and Gene Analysis of Mesenchymal Stem Cell Chondrogenesis Identifies an Essential Role and Multiple Targets for MiR-140-5p. Stem Cells 2015, 33, 3266–3280. [Google Scholar] [CrossRef]
- Karlsen, T.A.; Jakobsen, R.B.; Mikkelsen, T.S.; Brinchmann, J.E. MicroRNA-140 Targets RALA and Regulates Chondrogenic Differentiation of Human Mesenchymal Stem Cells by Translational Enhancement of SOX9 and ACAN. Stem Cells Dev. 2014, 23, 290–304. [Google Scholar] [CrossRef]
- Nakamura, Y.; Inloes, J.B.; Katagiri, T.; Kobayashi, T. Chondrocyte-Specific MicroRNA-140 Regulates Endochondral Bone Development and Targets Dnpep to Modulate Bone Morphogenetic Protein Signaling. Mol. Cell. Biol. 2011, 31, 3019–3028. [Google Scholar] [CrossRef]
- Miyaki, S.; Sato, T.; Inoue, A.; Otsuki, S.; Ito, Y.; Yokoyama, S.; Kato, Y.; Takemoto, F.; Nakasa, T.; Yamashita, S.; et al. MicroRNA-140 Plays Dual Roles in Both Cartilage Development and Homeostasis. Genes Dev. 2010, 24, 1173–1185. [Google Scholar] [CrossRef]
- Papaioannou, G.; Inloes, J.B.; Nakamura, Y.; Paltrinieri, E.; Kobayashi, T. Let-7 and MiR-140 MicroRNAs Coordinately Regulate Skeletal Development. Proc. Natl. Acad. Sci. USA 2013, 110, E3291–E3300. [Google Scholar] [CrossRef] [PubMed]
- Paterson, M.R.; Kriegel, A.J. MiR-146a/b: A Family with Shared Seeds and Different Roots. Physiol. Genom. 2017, 49, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Nakasa, T.; Miyaki, S.; Ishikawa, M.; Deie, M.; Adachi, N.; Yasunaga, Y.; Asahara, H.; Ochi, M. Expression of MicroRNA-146a in Osteoarthritis Cartilage. Arthritis Rheum. 2009, 60, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gibson, G.; Kim, J.S.; Kroin, J.; Xu, S.; van Wijnen, A.J.; Im, H.J. MicroRNA-146a Is Linked to Pain-Related Pathophysiology of Osteoarthritis. Gene 2011, 480, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Gao, X.; Wang, J.; Yang, C.; Wang, Y.; Liu, Y.; Zou, W.; Liu, T. Hypoxia-Induced MicroRNA-146a Represses Bcl-2 through Traf6/IRAK1 but Not Smad4 to Promote Chondrocyte Autophagy. Biol. Chem. 2017, 398, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, J.; Dai, L.; Yu, D.; Chen, Q.; Zhang, X.; Dai, K. MiR-146a, an IL-1β Responsive MiRNA, Induces Vascular Endothelial Growth Factor and Chondrocyte Apoptosis by Targeting Smad4. Arthritis Res. Ther. 2012, 14, R75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, J.; Chu, J.; Yang, C.; Xiao, H.; Zhao, C.; Sun, Z.; Gao, X.; Chen, G.; Han, Z.; et al. MicroRNA-146a Induced by Hypoxia Promotes Chondrocyte Autophagy through Bcl-2. Cell. Physiol. Biochem. 2015, 37, 1442–1453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zheng, W.; Li, D.; Zheng, J. MiR-146a-5p Promotes Chondrocyte Apoptosis and Inhibits Autophagy of Osteoarthritis by Targeting NUMB. Cartilage 2021, 13, 1467S–1477S. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Wang, C.; He, Y.; Lu, A.; Li, T.; Zhang, B.; Shen, J. Silencing MiR-146a-5p Protects against Injury-Induced Osteoarthritis in Mice. Biomolecules 2023, 13, 123. [Google Scholar] [CrossRef]
- Guan, Y.J.; Li, J.; Yang, X.; Du, S.; Ding, J.; Gao, Y.; Zhang, Y.; Yang, K.; Chen, Q. Evidence That MiR-146a Attenuates Aging- and Trauma-Induced Osteoarthritis by Inhibiting Notch1, IL-6, and IL-1 Mediated Catabolism. Aging Cell 2018, 17, e12752. [Google Scholar] [CrossRef]
- Liu, J.N.; Lu, S.; Fu, C.M. MiR-146a Expression Profiles in Osteoarthritis in Different Tissue Sources: A Meta-Analysis of Observational Studies. J. Orthop. Surg. Res. 2022, 17, 148. [Google Scholar] [CrossRef]
- Papathanasiou, I.; Mourmoura, E.; Balis, C.; Tsezou, A. Impact of MiR-SNP Rs2910164 on MiR-146a Expression in Osteoarthritic Chondrocytes. Adv. Med. Sci. 2020, 65, 78–85. [Google Scholar] [CrossRef]
- Kopańska, M.; Szala, D.; Czech, J.; Gabło, N.; Gargasz, K.; Trzeciak, M.; Zawlik, I.; Snela, S. MiRNA Expression in the Cartilage of Patients with Osteoarthritis. J. Orthop. Surg. Res. 2017, 12, 51. [Google Scholar] [CrossRef]
- Hu, Q.; Ecker, M. Overview of MMP-13 as a Promising Target for the Treatment of Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 1742. [Google Scholar] [CrossRef]
- Song, J.; Kim, D.; Chun, C.H.; Jin, E.J. MicroRNA-9 Regulates Survival of Chondroblasts and Cartilage Integrity by Targeting Protogenin. Cell Commun. Signal. 2013, 11, 66. [Google Scholar] [CrossRef]
- Li, H.Z.; Xu, X.H.; Lin, N.; Wang, D.W.; Lin, Y.M.; Su, Z.Z.; Lu, H.D. Overexpression of MiR-10a-5p Facilitates the Progression of Osteoarthritis. Aging 2020, 12, 5948–5976. [Google Scholar] [CrossRef] [PubMed]
- Iliopoulos, D.; Malizos, K.N.; Oikonomou, P.; Tsezou, A. Integrative MicroRNA and Proteomic Approaches Identify Novel Osteoarthritis Genes and Their Collaborative Metabolic and Inflammatory Networks. PLoS ONE 2008, 3, e3740. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; Wang, Q.; Qin, H.; Cao, S.; Wei, Y.; Weng, J.; Yu, F.; Zeng, H. Osteoarthritis: Role of Peroxisome Proliferator-Activated Receptors. Int. J. Mol. Sci. 2023, 24, 13137. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Chanalaris, A.; Troeberg, L. ADAMTS and ADAM Metalloproteinases in Osteoarthritis—Looking beyond the ‘Usual Suspects’. Osteoarthr. Cartil. 2017, 25, 1000–1009. [Google Scholar] [CrossRef]
- Endisha, H.; Datta, P.; Sharma, A.; Nakamura, S.; Rossomacha, E.; Younan, C.; Ali, S.A.; Tavallaee, G.; Lively, S.; Potla, P.; et al. MicroRNA-34a-5p Promotes Joint Destruction during Osteoarthritis. Arthritis Rheumatol. 2021, 73, 426–439. [Google Scholar] [CrossRef]
- Miyaki, S.; Nakasa, T.; Otsuki, S.; Grogan, S.P.; Higashiyama, R.; Inoue, A.; Kato, Y.; Sato, T.; Lotz, M.K.; Asahara, H. MicroRNA-140 Is Expressed in Differentiated Human Articular Chondrocytes and Modulates Interleukin-1 Responses. Arthritis Rheum. 2009, 60, 2723–2730. [Google Scholar] [CrossRef] [PubMed]
- Law, Y.Y.; Lee, W.F.; Hsu, C.J.; Lin, Y.Y.; Tsai, C.H.; Huang, C.C.; Wu, M.H.; Tang, C.H.; Liu, J.F. MiR-Let-7c-5p and MiR-149-5p Inhibit Proinflammatory Cytokine Production in Osteoarthritis and Rheumatoid Arthritis Synovial Fibroblasts. Aging 2021, 13, 17227–17236. [Google Scholar] [CrossRef] [PubMed]
- Santini, P.; Politi, L.; Vedova, P.D.; Scandurra, R.; Scotto D’Abusco, A. The Inflammatory Circuitry of MiR-149 as a Pathological Mechanism in Osteoarthritis. Rheumatol. Int. 2014, 34, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, L.; Tian, H. MicroRNA-149 Improves Osteoarthritis via Repression of VCAM-1 and Inactivation of PI3K/AKT Pathway. Exp. Gerontol. 2023, 174, 112103. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wu, S.; Wu, Y.; Chen, L.; Pang, Q. MiR-149 Suppresses the Inflammatory Response of Chondrocytes in Osteoarthritis by down-Regulating the Activation of TAK1/NF-ΚB. Biomed. Pharmacother. 2018, 101, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Lian, W.S.; Ko, J.Y.; Wu, R.W.; Sun, Y.C.; Chen, Y.S.; Wu, S.L.; Weng, L.H.; Jahr, H.; Wang, F.S. MicroRNA-128a Represses Chondrocyte Autophagy and Exacerbates Knee Osteoarthritis by Disrupting Atg12. Cell Death Dis. 2018, 9, 919. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.-Y.; Wang, F.-S.; Lian, W.-S.; Fang, H.-C.; Kuo, S.-J. Cartilage-Specific Knockout of MiRNA-128a Expression Normalizes the Expression of Circadian Clock Genes (CCGs) and Mitigates the Severity of Osteoarthritis. Biomed. J. 2024, 47, 100629. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, Y.; Tokuzawa, Y.; Ninomiya, Y.; Yagi, K.; Yatsuka-Kanesaki, Y.; Suda, T.; Fukuda, T.; Katagiri, T.; Kondoh, Y.; Amemiya, T.; et al. MiR-210 Promotes Osteoblastic Differentiation through Inhibition of AcvR1b. FEBS Lett. 2009, 583, 2263–2268. [Google Scholar] [CrossRef]
- Qi, J.; Qiao, Y.; Wang, P.; Li, S.; Zhao, W.; Gao, C. MicroRNA-210 Negatively Regulates LPS-Induced Production of Proinflammatory Cytokines by Targeting NF-ΚB1 in Murine Macrophages. FEBS Lett. 2012, 586, 1201–1207. [Google Scholar] [CrossRef]
- Zhang, D.; Cao, X.; Li, J.; Zhao, G. MiR-210 Inhibits NF-ΚB Signaling Pathway by Targeting DR6 in Osteoarthritis. Sci. Rep. 2015, 5, 12775. [Google Scholar] [CrossRef]
- Lu, X.; Li, Y.; Chen, H.; Pan, Y.; Lin, R.; Chen, S. MiR-335-5P Contributes to Human Osteoarthritis by Targeting HBP1. Exp. Ther. Med. 2020, 21, 109. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.O.; Zhang, L.; Tang, Z.Y.; Gong, Z.M. MiR-485-5p Promotes the Development of Osteoarthritis by Inhibiting Cartilage Differentiation in BMSCs. Eur. Rev. Med. Pharmacol. Sci. 2021, 22, 3294–3302. [Google Scholar] [CrossRef]
- Akhtar, N.; Rasheed, Z.; Ramamurthy, S.; Anbazhagan, A.N.; Voss, F.R.; Haqqi, T.M. MicroRNA-27b Regulates the Expression of Matrix Metalloproteinase 13 in Human Osteoarthritis Chondrocytes. Arthritis Rheum. 2010, 62, 1361–1371. [Google Scholar] [CrossRef] [PubMed]
- Tavallaee, G.; Lively, S.; Rockel, J.S.; Ali, S.A.; Im, M.; Sarda, C.; Mitchell, G.M.; Rossomacha, E.; Nakamura, S.; Potla, P.; et al. Contribution of MicroRNA-27b-3p to Synovial Fibrotic Responses in Knee Osteoarthritis. Arthritis Rheumatol. 2022, 74, 1928–1942. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Xu, J.; Chen, L.; Wu, H.; Feng, W.; Zheng, Y.; Li, P.; Zhang, H.; Zhang, L.; Chi, G.; et al. MicroRNA-27b Targets CBFB to Inhibit Differentiation of Human Bone Marrow Mesenchymal Stem Cells into Hypertrophic Chondrocytes. Stem Cell Res. Ther. 2020, 11, 392. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.; Li, Y.; Zeng, C.; Deng, Z.; Gao, S.; Xiao, W.; Luo, W.; Jiang, W.; Li, L.; Lei, G. MicroRNA-127-5p Regulates Osteopontin Expression and Osteopontin-Mediated Proliferation of Human Chondrocytes. Sci. Rep. 2016, 6, 25032. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Cheon, E.J.; Lee, M.H.; Kim, H.A. MicroRNA-127-5p Regulates Matrix Metalloproteinase 13 Expression and Interleukin-1β-Induced Catabolic Effects in Human Chondrocytes. Arthritis Rheum. 2013, 65, 3141–3152. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, H.X.; Xu, D.; Xue, X.; Xu, X. The Anti-Inflammatory Effect of MiR-140-3p in BMSCs-Exosomes on Osteoarthritis. Acta Chir. Orthop. Traumatol. Cech. 2023, 90, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, T.A.; de Souza, G.A.; Ødegaard, B.; Engebretsen, L.; Brinchmann, J.E. MicroRNA-140 Inhibits Inflammation and Stimulates Chondrogenesis in a Model of Interleukin 1β-Induced Osteoarthritis. Mol. Ther. Nucleic Acids 2016, 5, e373. [Google Scholar] [CrossRef]
- Liang, Y.; Duan, L.; Xiong, J.; Zhu, W.; Liu, Q.; Wang, D.; Liu, W.; Li, Z.; Wang, D. E2 Regulates MMP-13 via Targeting MiR-140 in IL-1β-Induced Extracellular Matrix Degradation in Human Chondrocytes. Arthritis Res. Ther. 2016, 18, 105. [Google Scholar] [CrossRef]
- Li, W.; Zhao, S.; Yang, H.; Zhang, C.; Kang, Q.; Deng, J.; Xu, Y.; Ding, Y.; Li, S. Potential Novel Prediction of TMJ-OA: MiR-140-5p Regulates Inflammation through Smad/TGF-β Signaling. Front. Pharmacol. 2019, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shen, S.; Li, Z.; Li, W.; Weng, X. MIR-140-5p Affects Chondrocyte Proliferation, Apoptosis, and Inflammation by Targeting HMGB1 in Osteoarthritis. Inflamm. Res. 2020, 69, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hu, J.; Pan, Y.; Shan, Y.; Jiang, L.; Qi, X.; Jia, L. MiR-140-5p/MiR-149 Affects Chondrocyte Proliferation, Apoptosis, and Autophagy by Targeting FUT1 in Osteoarthritis. Inflammation 2018, 41, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Tuddenham, L.; Wheeler, G.; Ntounia-Fousara, S.; Waters, J.; Hajihosseini, M.K.; Clark, I.; Dalmay, T. The Cartilage Specific MicroRNA-140 Targets Histone Deacetylase 4 in Mouse Cells. FEBS Lett. 2006, 580, 4214–4217. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hu, Q.; Chen, Z.; Shen, B.; Yang, J.; Kang, P.; Zhou, Z.; Pei, F. MicroRNA-140 Suppresses Human Chondrocytes Hypertrophy by Targeting SMAD1 and Controlling the Bone Morphogenetic Protein Pathway in Osteoarthritis. Am. J. Med. Sci. 2018, 355, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Taganov, K.D.; Boldin, M.P.; Chang, K.J.; Baltimore, D. NF-ΚB-Dependent Induction of MicroRNA MiR-146, an Inhibitor Targeted to Signaling Proteins of Innate Immune Responses. Proc. Natl. Acad. Sci. USA 2006, 103, 12481–12486. [Google Scholar] [CrossRef]
- Jones, S.W.; Watkins, G.; Le Good, N.; Roberts, S.; Murphy, C.L.; Brockbank, S.M.V.; Needham, M.R.C.; Read, S.J.; Newham, P. The Identification of Differentially Expressed MicroRNA in Osteoarthritic Tissue That Modulate the Production of TNF-α and MMP13. Osteoarthr. Cartil. 2009, 17, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Skrzypa, M.; Szala, D.; Gablo, N.; Czech, J.; Pajak, J.; Kopanska, M.; Trzeciak, M.; Gargasz, K.; Snela, S.; Zawlik, I. MiRNA-146a-5p Is Upregulated in Serum and Cartilage Samples of Patients with Osteoarthritis. Pol. J. Surg. 2019, 91, 1–5. [Google Scholar] [CrossRef]
- Kapinas, K.; Delany, A.M. MicroRNA Biogenesis and Regulation of Bone Remodeling. Arthritis Res. Ther. 2011, 13, 220. [Google Scholar] [CrossRef]
- Liu, H.; Yan, L.; Li, X.; Li, D.; Wang, G.; Shen, N.N.; Li, J.J.; Wang, B. MicroRNA Expression in Osteoarthritis: A Meta-Analysis. Clin. Exp. Med. 2023, 23, 3737–3749. [Google Scholar] [CrossRef]
- Portal-Núñez, S.; Esbrit, P.; Alcaraz, M.J.; Largo, R. Oxidative Stress, Autophagy, Epigenetic Changes and Regulation by MiRNAs as Potential Therapeutic Targets in Osteoarthritis. Biochem. Pharmacol. 2016, 108, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, A.; Della Porta, G.; Peretti, G.M.; Maffulli, N. MicroRNA in Osteoarthritis: Physiopathology, Diagnosis and Therapeutic Challenge. Br. Med. Bull. 2019, 130, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.B.; Wen, K.; Wu, X.X.; Yao, Z.J. MiR-183 Regulates the Differentiation of Osteoblasts in the Development of Osteoporosis by Targeting Smad4. Acta Histochem. 2021, 123, 151786. [Google Scholar] [CrossRef] [PubMed]
- Briskin, D.; Wang, P.Y.; Bartel, D.P. The Biochemical Basis for the Cooperative Action of MicroRNAs. Proc. Natl. Acad. Sci. USA 2020, 117, 17764–17774. [Google Scholar] [CrossRef]
- Zhong, L.; Huang, X.; Karperien, M.; Post, J.N. Correlation between Gene Expression and Osteoarthritis Progression in Human. Int. J. Mol. Sci. 2016, 17, 1126. [Google Scholar] [CrossRef]

| Group of Risk Factors | Risk Factors | References |
|---|---|---|
| demographic | female; older age | [13,15,16,17,18,19,20,21,22,23] |
| body weight | overweight and obesity | [13,14,15,16,17,18,19] |
| genetic mutations | e.g., mutation of the COL2A1, COL11A, COL11A2, COL1A1, and COL9A1 gene | [17,21,29,30] |
| mechanical factors | professional work requiring frequent knee bending and significant use of manual dexterity; practicing competitive sports in the past; weakness of periarticular skeletal muscles; sedentary lifestyle; intense recreational running; past injuries; previous knee surgery | [16,17,18,21,22,24,25,26,27,28,29] |
| individual factors | high bone mineral density | [27,31,32,33] |
| diseases | disturbances of deep sensation | [24] |
| Categories | Factors |
|---|---|
| developmental and congenital defects | |
| local diseases | aseptic necrosis of the femoral head in children; congenital hip dysplasia; exfoliation of the bone epiphysis |
| mechanical factors | difference in the length of the lower limbs; valgus or varus; joint hypermobility syndrome |
| dieseases | |
| metabolic | ochronosis; hereditary hemochromatosis; Wilson’s disease; Gaucher’s disease |
| endocrine | acromegaly; hyperparathyroidism; diabetes; obesity; hypothyroidism |
| from the deposition of calcium salts | chondrocalcinosis; apatite arthropathy |
| endemic diseases | Kashin/Beck disease; Mseleni disease |
| other bone and joint diseases | local: fractures; aseptic necrosis; infections; gout |
| disseminated: rheumatoid arthritis; Paget’s disease; osteopeyrosis; osteochondritis; other inflammations | |
| neurodystrophy of bones and joint | |
| other diseases | hemoglobinopathies; caisson disease |
| other factors | |
| injuries | acute; chronic |
| external factors | frostbite |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szala, D.; Kopańska, M.; Trojniak, J.; Jabłoński, J.; Hanf-Osetek, D.; Snela, S.; Zawlik, I. The Role of MicroRNAs in the Pathophysiology of Osteoarthritis. Int. J. Mol. Sci. 2024, 25, 6352. https://doi.org/10.3390/ijms25126352
Szala D, Kopańska M, Trojniak J, Jabłoński J, Hanf-Osetek D, Snela S, Zawlik I. The Role of MicroRNAs in the Pathophysiology of Osteoarthritis. International Journal of Molecular Sciences. 2024; 25(12):6352. https://doi.org/10.3390/ijms25126352
Chicago/Turabian StyleSzala, Dariusz, Marta Kopańska, Julia Trojniak, Jarosław Jabłoński, Dorota Hanf-Osetek, Sławomir Snela, and Izabela Zawlik. 2024. "The Role of MicroRNAs in the Pathophysiology of Osteoarthritis" International Journal of Molecular Sciences 25, no. 12: 6352. https://doi.org/10.3390/ijms25126352
APA StyleSzala, D., Kopańska, M., Trojniak, J., Jabłoński, J., Hanf-Osetek, D., Snela, S., & Zawlik, I. (2024). The Role of MicroRNAs in the Pathophysiology of Osteoarthritis. International Journal of Molecular Sciences, 25(12), 6352. https://doi.org/10.3390/ijms25126352






