Prenatal Factors in the Development of Allergic Diseases
Abstract
1. Introduction
1.1. Mechanisms Inducing Allergy
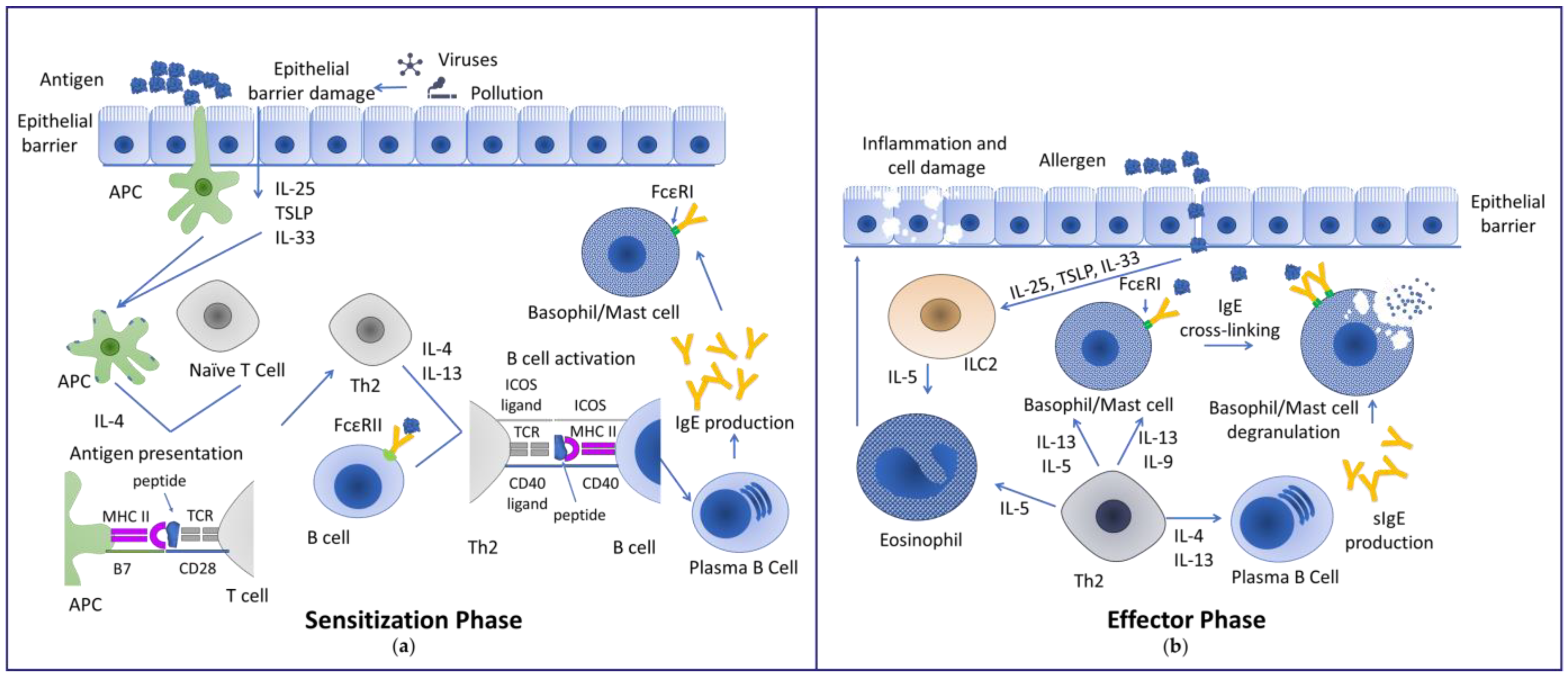
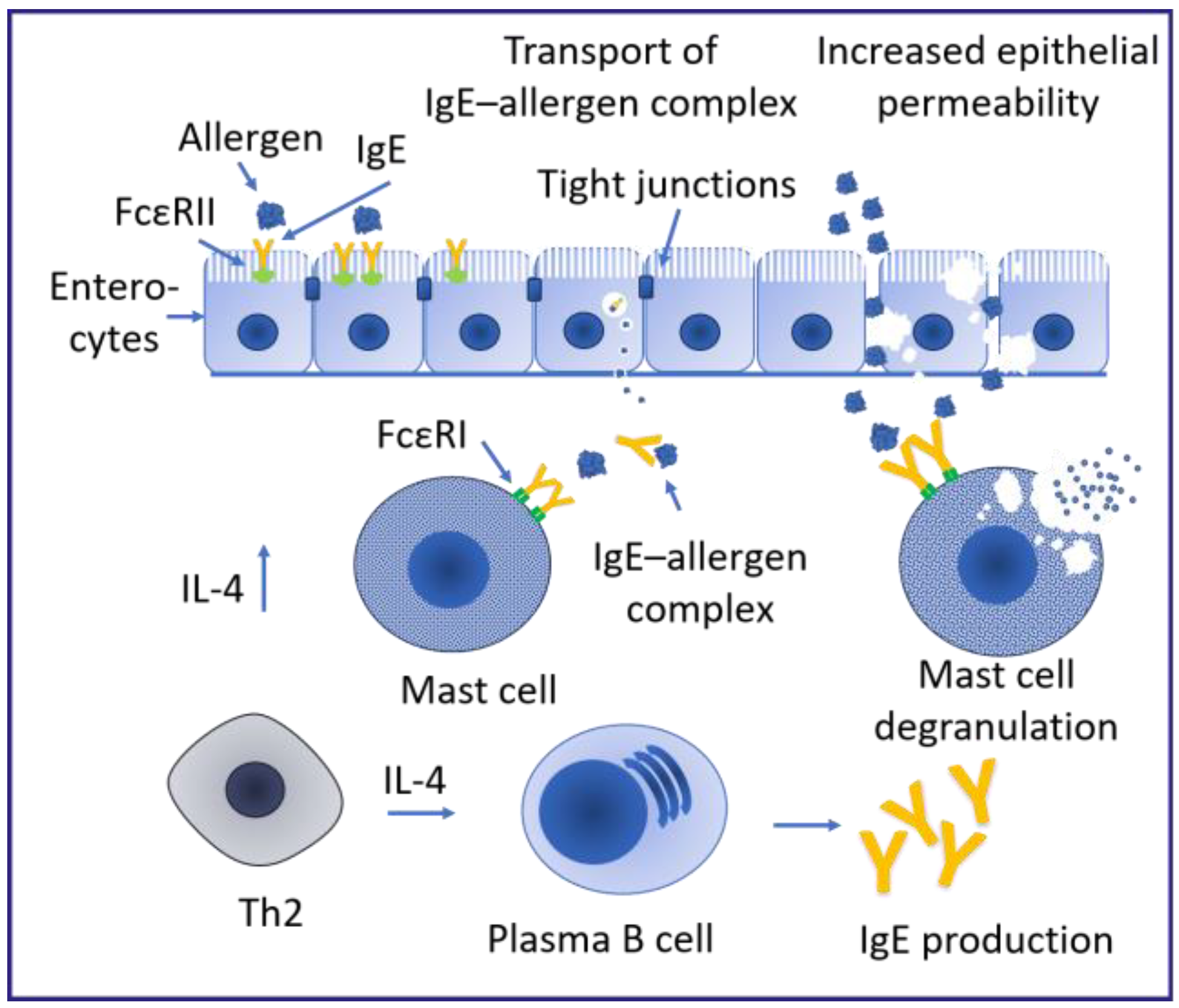
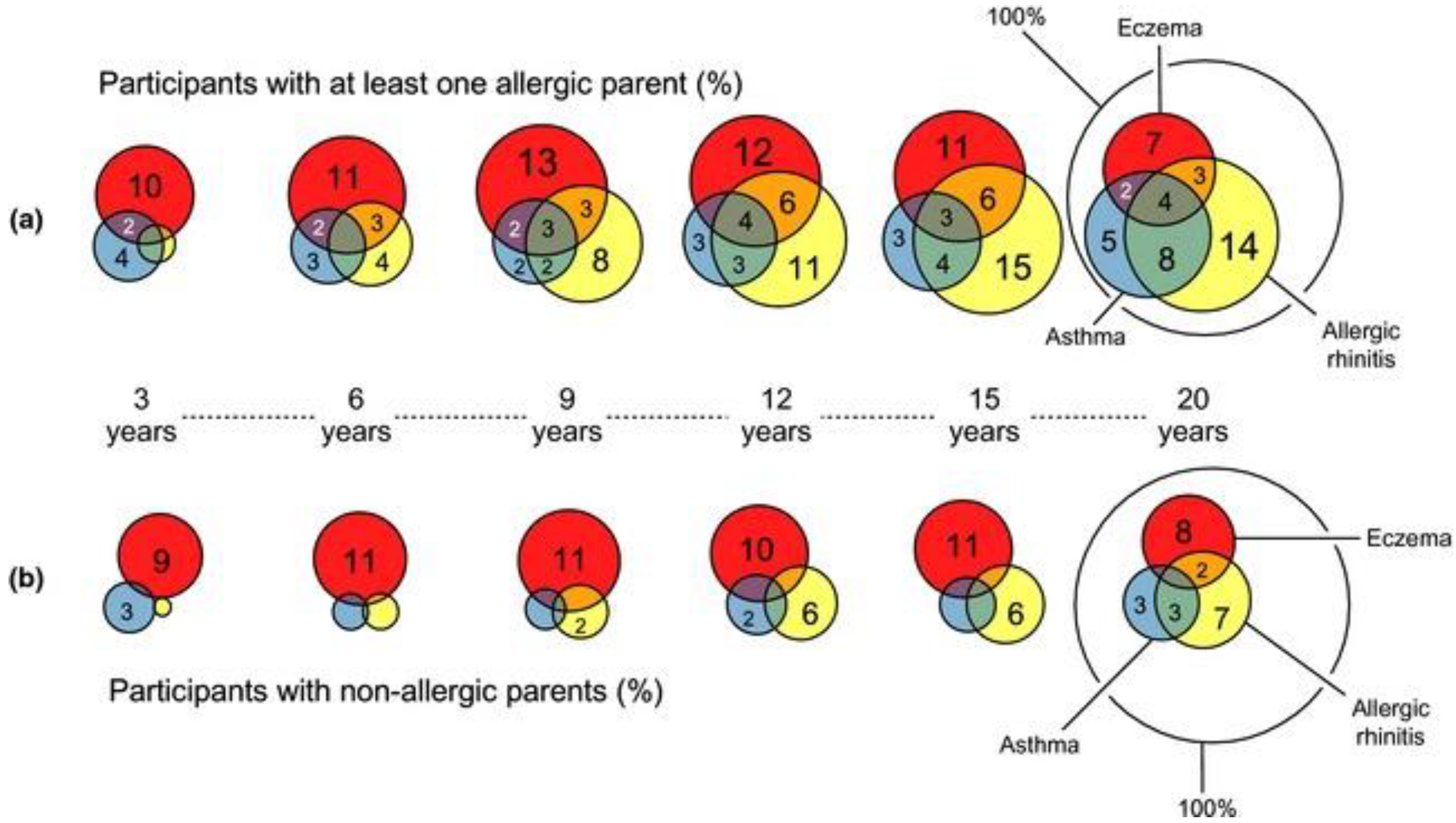
1.2. Disease Progression in Association with Sensitizations
2. Prenatal Risk Factors in the Development of Allergic Diseases

3. Prenatal Protective Factors in the Development of Allergic Diseases
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lake, I.R.; Jones, N.R.; Agnew, M.; Goodess, C.M.; Giorgi, F.; Hamaoui-Laguel, L.; Semenov, M.A.; Solmon, F.; Storkey, J.; Vautard, R.; et al. Climate Change and Future Pollen Allergy in Europe. Environ. Health Perspect. 2017, 125, 385–391. [Google Scholar] [CrossRef]
- Edwards-Salmon, S.E.; Padmanabhan, S.L.; Kuruvilla, M.; Levy, J.M. Increasing Prevalence of Allergic Disease and Its Impact on Current Practice. Curr. Otorhinolaryngol. Rep. 2022, 10, 278–284. [Google Scholar] [CrossRef]
- Golden, D.B.K. WAO White Book on Allergy Update 2013; Pawankar, R., Canonica, G.W., Holgate, S.T., Lockey, R.F., Blaiss, M.S., Eds.; World Allergy Organization (WAO): Milwaukee, WI, USA, 2013; ISBN 9780323809122. [Google Scholar]
- Dierick, B.J.H.; van der Molen, T.; Flokstra-de Blok, B.M.J.; Muraro, A.; Postma, M.J.; Kocks, J.W.H.; van Boven, J.F.M. Burden and Socioeconomics of Asthma, Allergic Rhinitis, Atopic Dermatitis and Food Allergy. Expert Rev. Pharmacoecon. Outcomes Res. 2020, 20, 437–453. [Google Scholar] [CrossRef]
- Canonica, G.W.; Bachert, C.; Hellings, P.; Ryan, D.; Valovirta, E.; Wickman, M.; De Beaumont, O.; Bousquet, J. Allergen Immunotherapy (AIT): A Prototype of Precision Medicine. World Allergy Organ. J. 2015, 8, 31. [Google Scholar] [CrossRef]
- Johansson, S.G.O.; Bousquet, J.; Dreborg, S.; Haahtela, T.; Kowalski, M.L.; Mygind, N.; Ring, J.; Van Cauwenberge, P.; Wu, B. A Revised Nomenclature for Allergy—An EAACI Position Statement from the EAACI Nomenclature Task Force. Allergy 2001, 56, 813–824. [Google Scholar] [CrossRef]
- Seité, S.; Kuo, A.M.S.; Taieb, C.; Strugar, T.L.; Lio, P. Self-Reported Prevalence of Allergies in the USA and Impact on Skin–an Epidemiological Study on a Representative Sample of American Adults. Int. J. Environ. Res. Public Health 2020, 17, 3360. [Google Scholar] [CrossRef]
- Jutel, M.; Agache, I.; Zemelka-Wiacek, M.; Akdis, M.; Chivato, T.; Del Giacco, S.; Gajdanowicz, P.; Gracia, I.E.; Klimek, L.; Lauerma, A. Nomenclature of Allergic Diseases and Hypersensitivity Reactions: Adapted to Modern Needs: An EAACI Position Paper. Allergy 2023, 78, 2851–2874. [Google Scholar] [CrossRef]
- Bever, H.P. Early Events in Atopy. Eur. J. Pediatr. 2002, 161, 542–546. [Google Scholar] [CrossRef]
- Annunziato, F.; Romagnani, C.; Romagnani, S. The 3 Major Types of Innate and Adaptive Cell-Mediated Effector Immunity. J. Allergy Clin. Immunol. 2015, 135, 626–635. [Google Scholar] [CrossRef]
- Gauvreau, G.M.; Bergeron, C.; Boulet, L.P.; Cockcroft, D.W.; Côté, A.; Davis, B.E.; Leigh, R.; Myers, I.; O’Byrne, P.M.; Sehmi, R. Sounding the Alarmins—The Role of Alarmin Cytokines in Asthma. Allergy Eur. J. Allergy Clin. Immunol. 2023, 78, 402–417. [Google Scholar] [CrossRef]
- Gevaert, P.; Wong, K.; Millette, L.A.; Carr, T.F. The Role of IgE in Upper and Lower Airway Disease: More Than Just Allergy! Clin. Rev. Allergy Immunol. 2022, 62, 200–215. [Google Scholar] [CrossRef]
- Varricchi, G.; Bencivenga, L.; Poto, R.; Pecoraro, A.; Shamji, M.H.; Rengo, G. The Emerging Role of T Follicular Helper (TFH) Cells in Aging: Influence on the Immune Frailty. Ageing Res. Rev. 2020, 61, 101071. [Google Scholar] [CrossRef]
- Miyake, K.; Shibata, S.; Yoshikawa, S.; Karasuyama, H. Basophils and Their Effector Molecules in Allergic Disorders. Allergy Eur. J. Allergy Clin. Immunol. 2021, 76, 1693–1706. [Google Scholar] [CrossRef]
- Nakano, N.; Kitaura, J. Mucosal Mast Cells as Key Effector Cells in Food Allergies. Cells 2022, 11, 329. [Google Scholar] [CrossRef]
- Hammad, H.; Lambrecht, B.N. The Basic Immunology of Asthma. Cell 2021, 184, 1469–1485. [Google Scholar] [CrossRef]
- Sztandera-Tymoczek, M.; Szuster-Ciesielska, A. Fungal Aeroallergens—The Impact of Climate Change. J. Fungi 2023, 9, 544. [Google Scholar] [CrossRef]
- Seidler, C.A.; Zeindl, R.; Fernández-Quintero, M.L.; Tollinger, M.; Liedl, K.R. Allergenicity and Conformational Diversity of Allergens. Allergies 2024, 4, 1–16. [Google Scholar] [CrossRef]
- Morianos, I.; Semitekolou, M. Dendritic Cells: Critical Regulators of Allergic Asthma. Int. J. Mol. Sci. 2020, 21, 7930. [Google Scholar] [CrossRef]
- Varricchi, G.; Raap, U.; Rivellese, F.; Marone, G.; Gibbs, B.F. Human Mast Cells and Basophils—How Are They Similar How Are They Different? Immunol. Rev. 2018, 282, 8–34. [Google Scholar] [CrossRef]
- Engeroff, P.; Vogel, M. The Role of CD23 in the Regulation of Allergic Responses. Allergy Eur. J. Allergy Clin. Immunol. 2021, 76, 1981–1989. [Google Scholar] [CrossRef]
- Reginald, K.; Eckl-Dorna, J.; Zafred, D.; Focke-Tejkl, M.; Lupinek, C.; Niederberger, V.; Keller, W.; Valenta, R. Different Modes of IgE Binding to CD23 Revealed with Major Birch Allergen, Bet v 1-Specific Monoclonal IgE. Immunol. Cell Biol. 2013, 91, 167–172. [Google Scholar] [CrossRef]
- Engeroff, P.; Caviezel, F.; Mueller, D.; Thoms, F.; Bachmann, M.F.; Vogel, M. CD23 Provides a Noninflammatory Pathway for IgE-Allergen Complexes. J. Allergy Clin. Immunol. 2020, 145, 301–311.e4. [Google Scholar] [CrossRef]
- Yokota, A.; Kikutani, H.; Yamamoto, A.; Sugiyama, K.; Suemura, M.; Tashiro, Y.; Kishimoyo, T.; Kikutani, H. Two Forms of the Low-Affinity Fc Receptor for IgE Differentially Mediate Endocytosis and Phagocytosis: Identification of the Critical Cytoplasmic Domains. Proc. Natl. Acad. Sci. USA 1992, 89, 5030–5034. [Google Scholar] [CrossRef]
- Peng, W.; Grobe, W.; Walgenbach-Brünagel, G.; Flicker, S.; Yu, C.; Sylvester, M.; Allam, J.-P.; Oldenburg, J.; Garbi, N.; Valenta, R.; et al. Distinct Expression and Function of FcεRII in Human B Cells and Monocytes. J. Immunol. 2017, 198, 3033–3044. [Google Scholar] [CrossRef]
- Chan, M.A.; Gigliotti, N.M.; Matangkasombut, P.; Gauld, S.B.; Cambier, J.C.; Rosenwasser, L.J. CD23-Mediated Cell Signaling in Human B Cells Differs from Signaling in Cells of the Monocytic Lineage. Clin. Immunol. 2010, 137, 330–336. [Google Scholar] [CrossRef]
- Cheng, L.E.; Wang, Z.-E.; Locksley, R.M. Murine B Cells Regulate Serum IgE Levels in a CD23-Dependent Manner. J. Immunol. 2010, 185, 5040–5047. [Google Scholar] [CrossRef]
- Getahun, A.; Hjelm, F.; Heyman, B. IgE Enhances Antibody and T Cell Responses In Vivo via CD23+ B Cells. J. Immunol. 2015, 175, 1473–1482. [Google Scholar] [CrossRef]
- Tu, Y.; Salim, S.; Bourgeois, J.; Di Leo, V.; Irvine, E.J.; Marshall, J.K.; Perdue, M.H. CD23-Mediated IgE Transport across Human Intestinal Epithelium: Inhibition by Blocking Sites of Translation or Binding. Gastroenterology 2005, 129, 928–940. [Google Scholar] [CrossRef]
- Ristivojević, M.K.; Apostolović, D.; Smiljanić, K. Enterocytes in Food Hypersensitivity Reactions. Animals 2021, 11, 2713. [Google Scholar] [CrossRef]
- Yu, L.C.H.; Yang, P.C.; Berin, M.C.; Di Leo, V.; Conrad, D.H.; McKay, D.M.; Satoskar, A.R.; Perdue, M.H. Enhanced Transepithelial Antigen Transport in Intestine of Allergic Mice Is Mediated by IgE/CD23 and Regulated by Interleukin-4. Gastroenterology 2001, 121, 370–381. [Google Scholar] [CrossRef]
- Li, H.; Chehade, M.; Liu, W.; Xiong, H.; Mayer, L.; Berin, M.C. Allergen-IgE Complexes Trigger CD23-Dependent CCL20 Release From Human Intestinal Epithelial Cells. Gastroenterology 2007, 133, 1905–1915. [Google Scholar] [CrossRef]
- Nešić, A.; Stam, A.; Čavić, M.; Ten Klooster, J.P.; Pieters, R.; Smit, J.; Gavrović-Jankulović, M. Activation of Epithelial Cells by the Major Kiwifruit Allergen Act d 1 in Human and Mouse-Derived Intestinal Model. J. Funct. Foods 2019, 62, 103556. [Google Scholar] [CrossRef]
- Gough, H.; Grabenhenrich, L.; Reich, A.; Eckers, N.; Nitsche, O.; Schramm, D.; Beschorner, J.; Hoffmann, U.; Schuster, A.; Bauer, C.P.; et al. Allergic Multimorbidity of Asthma, Rhinitis and Eczema over 20 Years in the German Birth Cohort MAS. Pediatr. Allergy Immunol. 2015, 26, 431–437. [Google Scholar] [CrossRef]
- Hill, D.A.; Spergel, J.M. The Atopic March: Critical Evidence and Clinical Relevance. Ann. Allergy Asthma Immunol. 2018, 120, 131–137. [Google Scholar] [CrossRef]
- Wong, C.Y.; Yeh, K.W.; Huang, J.L.; Su, K.W.; Tsai, M.H.; Hua, M.C.; Liao, S.L.; Lai, S.H.; Chen, L.C.; Chiu, C.Y. Longitudinal Analysis of Total Serum IgE Levels with Allergen Sensitization and Atopic Diseases in Early Childhood. Sci. Rep. 2020, 10, 21278. [Google Scholar] [CrossRef]
- Maiello, N.; Comberiati, P.; Giannetti, A.; Ricci, G.; Carello, R.; Galli, E. New Directions in Understanding Atopic March Starting from Atopic Dermatitis. Children 2022, 9, 450. [Google Scholar] [CrossRef]
- Belgrave, D.C.M.; Granell, R.; Simpson, A.; Guiver, J.; Bishop, C.; Buchan, I.; Henderson, A.J.; Custovic, A. Developmental Profiles of Eczema, Wheeze, and Rhinitis: Two Population-Based Birth Cohort Studies. PLoS Med. 2014, 11, e1001748. [Google Scholar] [CrossRef]
- Siroux, V.; Ballardini, N.; Soler, M.; Lupinek, C.; Boudier, A.; Pin, I.; Just, J.; Nadif, R.; Anto, J.M.; Melen, E. The Asthma-rhinitis Multimorbidity Is Associated with IgE Polysensitization in Adolescents and Adults. Allergy 2018, 73, 1447–1458. [Google Scholar] [CrossRef]
- Forster, F.; Ege, M.J.; Gerlich, J.; Weinmann, T.; Kreißl, S.; Weinmayr, G.; Genuneit, J.; Nowak, D.; von Mutius, E.; Vogelberg, C.; et al. Trajectories of Asthma and Allergy Symptoms from Childhood to Adulthood. Allergy Eur. J. Allergy Clin. Immunol. 2022, 77, 1192–1203. [Google Scholar] [CrossRef]
- Kilanowski, A.; Thiering, E.; Wang, G.; Kumar, A.; Kress, S.; Flexeder, C.; Bauer, C.P.; Berdel, D.; von Berg, A.; Bergström, A.; et al. Allergic Disease Trajectories up to Adolescence: Characteristics, Early-Life, and Genetic Determinants. Allergy Eur. J. Allergy Clin. Immunol. 2023, 78, 836–850. [Google Scholar] [CrossRef]
- Tsuge, M.; Ikeda, M.; Matsumoto, N.; Yorifuji, T.; Tsukahara, H. Current Insights into Atopic March. Children 2021, 8, 1067. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Huang, Y.L.; Tsai, M.H.; Tu, Y.L.; Hua, M.C.; Yao, T.C.; Yeh, K.W.; Huang, J.L. Sensitization to Food and Inhalant Allergens in Relation to Atopic Diseases in Early Childhood: A Birth Cohort Study. PLoS ONE 2014, 9, e102809. [Google Scholar] [CrossRef]
- Melén, E.; Bergström, A.; Kull, I.; Almqvist, C.; Andersson, N.; Asarnoj, A.; Borres, M.P.; Georgellis, A.; Pershagen, G.; Westman, M.; et al. Male Sex Is Strongly Associated with IgE-Sensitization to Airborne but Not Food Allergens: Results up to Age 24 Years from the BAMSE Birth Cohort. Clin. Transl. Allergy 2020, 10, 15. [Google Scholar] [CrossRef]
- Eller, E.; Kjaer, H.F.; Høst, A.; Andersen, K.E.; Bindslev-Jensen, C. Food Allergy and Food Sensitization in Early Childhood: Results from the DARC Cohort. Allergy Eur. J. Allergy Clin. Immunol. 2009, 64, 1023–1029. [Google Scholar] [CrossRef]
- Bergmann, R.L.; Edenharter, G.; Bergmann, K.E.; Guggenmoos-Holzmann, I.; Forster, J.; Bauer, C.P.; Wahn, V.; Zepp, F.; Wahn, U. Predictability of Early Atopy by Cord-Blood IgE and Parental History. Clin. Exp. Allergy 1997, 27, 752–760. [Google Scholar] [CrossRef]
- Hon, K.L.E.; Leung, T.F.; Ching, G.; Chow, C.M.; Luk, V.; Ko, W.S.F.; Ng, P.C. Patterns of Food and Aeroallergen Sensitization in Childhood Eczema. Acta Paediatr. Int. J. Paediatr. 2008, 97, 1734–1737. [Google Scholar] [CrossRef]
- de Jong, N.W.; Elbert, N.J.; Mensink-Bout, S.M.; van der Valk, J.P.M.; Pasmans, S.G.M.A.; Jaddoe, V.W.V.; de Jongste, J.C.; van Wijk, R.G.; Duijts, L. Parental and Child Factors Associated with Inhalant and Food Allergy in a Population-Based Prospective Cohort Study: The Generation R Study. Eur. J. Pediatr. 2019, 178, 1507–1517. [Google Scholar] [CrossRef]
- Alduraywish, S.A.; Standl, M.; Lodge, C.J.; Abramson, M.J.; Allen, K.J.; Erbas, B.; von Berg, A.; Heinrich, J.; Lowe, A.J.; Dharmage, S.C. Is There a March from Early Food Sensitization to Later Childhood Allergic Airway Disease? Results from Two Prospective Birth Cohort Studies. Pediatr. Allergy Immunol. 2017, 28, 30–37. [Google Scholar] [CrossRef]
- Lindqvist, M.; Leth-Møller, K.B.; Linneberg, A.; Kull, I.; Bergström, A.; Georgellis, A.; Borres, M.P.; Ekebom, A.; Van Hage, M.; Melén, E.; et al. Natural Course of Pollen-Induced Allergic Rhinitis from Childhood to Adulthood: A 20-Year Follow Up. Allergy 2024, 79, 884–893. [Google Scholar] [CrossRef]
- Wickman, M.; Lupinek, C.; Andersson, N.; Belgrave, D.; Asarnoj, A.; Benet, M.; Pinart, M.; Wieser, S.; Garcia-Aymerich, J.; Baar, A.; et al. Detection of IgE Reactivity to a Handful of Allergen Molecules in Early Childhood Predicts Respiratory Allergy in Adolescence. EBioMedicine 2017, 26, 91–99. [Google Scholar] [CrossRef]
- Westman, M.; Åberg, K.; Apostolovic, D.; Lupinek, C.; Gattinger, P.; Mittermann, I.; Andersson, N.; Melén, E.; Bergström, A.; Anto, J.M.; et al. Sensitization to Grass Pollen Allergen Molecules in a Birth Cohort—Natural Phl p 4 as an Early Indicator of Grass Pollen Allergy. J. Allergy Clin. Immunol. 2020, 145, 1174–1181.e6. [Google Scholar] [CrossRef]
- Siroux, V.; Boudier, A.; Bousquet, J.; Dumas, O.; Just, J.; Le Moual, N.; Nadif, R.; Varraso, R.; Valenta, R.; Pin, I. Trajectories of IgE Sensitization to Allergen Molecules from Childhood to Adulthood and Respiratory Health in the EGEA Cohort. Allergy Eur. J. Allergy Clin. Immunol. 2022, 77, 609–618. [Google Scholar] [CrossRef]
- Anto, J.M.; Bousquet, J.; Akdis, M.; Auffray, C.; Keil, T.; Momas, I.; Postma, D.S.; Valenta, R.; Wickman, M.; Cambon-Thomsen, A. Mechanisms of the Development of Allergy (MeDALL): Introducing Novel Concepts in Allergy Phenotypes. J. Allergy Clin. Immunol. 2017, 139, 388–399. [Google Scholar] [CrossRef]
- Ghiani, A.; Aina, R.; Asero, R.; Bellotto, E.; Citterio, S. Ragweed Pollen Collected along High-Traffic Roads Shows a Higher Allergenicity than Pollen Sampled in Vegetated Areas. Allergy Eur. J. Allergy Clin. Immunol. 2012, 67, 887–894. [Google Scholar] [CrossRef]
- Luoma, R.; Koivikko, A. Occurrence of Atopic Diseases in Three Generations. Scand. J. Soc. Med. 1982, 10, 49–56. [Google Scholar] [CrossRef]
- Tariq, S.M.; Matthews, S.M.; Hakim, E.A.; Stevens, M.; Arshad, S.H.; Hide, D.W. The Prevalence of and Risk Factors for Atopy in Early Childhood: A Whole Population Birth Cohort Study. J. Allergy Clin. Immunol. 1998, 101, 587–593. [Google Scholar] [CrossRef]
- Tariq, S.M.; Arshad, S.H.; Matthews, S.M.; Hakim, E.A. Elevated Cord Serum IgE Increases the Risk of Aeroallergen Sensitization without Increasing Respiratory Allergic Symptoms in Early Childhood. Clin. Exp. Allergy 1999, 29, 1042–1048. [Google Scholar] [CrossRef]
- Liu, X.; Agerbo, E.; Schlünssen, V.; Wright, R.J.; Li, J.; Munk-Olsen, T. Maternal Asthma Severity and Control during Pregnancy and Risk of Offspring Asthma. J. Allergy Clin. Immunol. 2018, 141, 886–892.e3. [Google Scholar] [CrossRef]
- Venter, C.; Palumbo, M.P.; Sauder, K.A.; Glueck, D.H.; Liu, A.H.; Yang, I.V.; Ben-Abdallah, M.; Fleischer, D.M.; Dabelea, D. Incidence and Timing of Offspring Asthma, Wheeze, Allergic Rhinitis, Atopic Dermatitis, and Food Allergy and Association with Maternal History of Asthma and Allergic Rhinitis. World Allergy Organ. J. 2021, 14, 100526. [Google Scholar] [CrossRef]
- McCready, C.; Haider, S.; Little, F.; Nicol, M.P.; Workman, L.; Gray, D.M.; Granell, R.; Stein, D.J.; Custovic, A.; Zar, H.J. Early Childhood Wheezing Phenotypes and Determinants in a South African Birth Cohort: Longitudinal Analysis of the Drakenstein Child Health Study. Lancet Child Adolesc. Health 2023, 7, 127–135. [Google Scholar] [CrossRef]
- Saito-Abe, M.; Yamamoto-Hanada, K.; Pak, K.; Iwamoto, S.; Sato, M.; Miyaji, Y.; Mezawa, H.; Nishizato, M.; Yang, L.; Kumasaka, N.; et al. How a Family History of Allergic Diseases Influences Food Allergy in Children: The Japan Environment and Children’s Study. Nutrients 2022, 14, 4323. [Google Scholar] [CrossRef]
- Kim, N.Y.; Kim, G.R.; Kim, J.H.; Baek, J.H.; Yoon, J.W.; Jee, H.M.; Baek, H.S.; Jung, Y.H.; Choi, S.H.; Kim, K.E.; et al. Food Allergen Sensitization in Young Children with Typical Signs and Symptoms of Immediatetype Food Allergies: A Comparison between Monosensitized and Polysensitized Children. Korean J. Pediatr. 2015, 58, 330–335. [Google Scholar] [CrossRef]
- Bazaral, M.; Orgel, H.A.; Hamburger, R.N. Genetics of IgE and Allergy: Serum IgE Levels in Twins. J. Allergy Clin. Immunol. 1974, 54, 288–304. [Google Scholar] [CrossRef]
- Kivistö, J.E.; Clarke, A.; Dery, A.; De Schryver, S.; Shand, G.; Huhtala, H.; Mäkelä, M.J.; Asai, Y.; Nadeau, K.; Harada, L.; et al. Genetic and Environmental Susceptibility to Food Allergy in a Registry of Twins. J. Allergy Clin. Immunol. Pract. 2019, 7, 2916–2918. [Google Scholar] [CrossRef]
- Hedman, A.M.; Anne, R.K.; van Hage, M.; Almqvist, C.; Nordlund, B. Genetic Effects of Allergen-Specific IgE Levels on Exhaled Nitric Oxide in Schoolchildren with Asthma: The STOPPA Twin Study. Pediatr. Allergy Immunol. 2021, 32, 709–719. [Google Scholar] [CrossRef]
- Cookson, W.O.C.M.; Young, R.P.; Sandford, A.J.; Moffatt, M.F.; Shirakawa, T.; Sharp, P.A.; Faux, J.A.; Le Souef, P.N.; Julier, C.; Lathrop, G.M.; et al. Maternal Inheritance of Atopic IgE Responsiveness on Chromosome 11 Q. Lancet 1992, 340, 381–384. [Google Scholar] [CrossRef]
- Gheerbrant, H.; Guillien, A.; Vernet, R.; Lupinek, C.; Pison, C.; Pin, I.; Demenais, F.; Nadif, R.; Bousquet, J.; Pickl, W.F. Associations between Specific IgE Sensitization to 26 Respiratory Allergen Molecules and HLA Class II Alleles in the EGEA Cohort. Allergy 2021, 76, 2575–2586. [Google Scholar] [CrossRef]
- Yao, T.; Chung, R.; Lin, C.; Tsai, P.; Chang, W.; Yeh, K.; Tsai, M.; Liao, S.; Hua, M.; Lai, S. Genetic Loci Determining Total Immunoglobulin E Levels from Birth through Adulthood. Allergy 2019, 74, 621–625. [Google Scholar] [CrossRef]
- Park, J.; Jang, H.; Kim, M.; Yeon, J.; Hee, Y. Predicting Allergic Diseases in Children Using Genome-Wide Association Study (GWAS) Data and Family History. World Allergy Organ. J. 2021, 14, 100539. [Google Scholar] [CrossRef]
- Portelli, M.A.; Hodge, E.; Sayers, I. Genetic Risk Factors for the Development of Allergic Disease Identified by Genome-wide Association. Clin. Exp. Allergy 2015, 45, 21–31. [Google Scholar] [CrossRef]
- Bønnelykke, K.; Sparks, R.; Waage, J.; Millner, J. Genetics of Allergy and Allergic Sensitization:- Common Variants and Rare Mutations. Curr. Opin. Immunol. 2015, 36, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, S.; O’Sullivan, M.; Illig, T.; Baurecht, H.; Depner, M.; Rodriguez, E.; Ruether, A.; Klopp, N.; Vogelberg, C.; Weiland, S.K.; et al. Filaggrin Mutations, Atopic Eczema, Hay Fever, and Asthma in Children. J. Allergy Clin. Immunol. 2008, 121, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Johansson, E.; Bagini Myers, J.M.; Martin, L.J.; He, H.; Ryan, P.; LeMasters, G.K.; Bernstein, D.I.; Lockey, J.; Hershey, G.K.K. Identification of Two Early Life Eczema and Non-Eczema Phenotypes with High Risk of Asthma Development. Clin. Exp. Allergy 2019, 49, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Nedoszytko, B.; Reszka, E.; Gutowska-Owsiak, D.; Trzeciak, M.; Lange, M.; Jarczak, J.; Niedoszytko, M.; Jablonska, E.; Romantowski, J.; Strapagiel, D.; et al. Genetic and Epigenetic Aspects of Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 6484. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, N.; Alhamwe, B.A.; Caraballo, L.; Ding, M.; Ferrante, A.; Garn, H.; Garssen, J.; Hii, C.S.; Irvine, J.; Llinás-Caballero, K.; et al. Perinatal and Early-Life Nutrition, Epigenetics, and Allergy. Nutrients 2021, 13, 724. [Google Scholar] [CrossRef]
- Danielewicz, H.; Gurgul, A.; Dębińska, A.; Myszczyszyn, G.; Szmatoła, T.; Myszkal, A.; Jasielczuk, I.; Drabik-Chamerska, A.; Hirnle, L.; Boznański, A. Maternal Atopy and Offspring Epigenome-Wide Methylation Signature. Epigenetics 2021, 16, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Reese, S.E.; Xu, C.J.; den Dekker, H.T.; Lee, M.K.; Sikdar, S.; Ruiz-Arenas, C.; Merid, S.K.; Rezwan, F.I.; Page, C.M.; Ullemar, V.; et al. Epigenome-Wide Meta-Analysis of DNA Methylation and Childhood Asthma. J. Allergy Clin. Immunol. 2019, 143, 2062–2074. [Google Scholar] [CrossRef] [PubMed]
- Potaczek, D.P.; Harb, H.; Michel, S.; Alhamwe, B.A.; Renz, H.; Tost, J. Epigenetics and Allergy: From Basic Mechanisms to Clinical Applications. Epigenomics 2017, 9, 539–571. [Google Scholar] [CrossRef] [PubMed]
- Weidner, J.; Bartel, S.; Kılıç, A.; Zissler, U.M.; Renz, H.; Schwarze, J.; Schmidt-Weber, C.B.; Maes, T.; Rebane, A.; Krauss-Etschmann, S.; et al. Spotlight on MicroRNAs in Allergy and Asthma. Allergy 2020, 76, 1661–1678. [Google Scholar] [CrossRef]
- Herberth, G.; Bauer, M.; Gasch, M.; Hinz, D.; Röder, S.; Olek, S.; Kohajda, T.; Rolle-Kampcyzk, U.; von Bergen, M.; Sack, U.; et al. Mechanisms of Allergy and Clinical Immunology Maternal and Cord Blood MiR-223 Expression Associates with Prenatal Tobacco Smoke Exposure and Low Regulatory T-Cell Numbers. J. Allergy Clin. Immunol. 2014, 133, 543–550. [Google Scholar] [CrossRef]
- Richmond, R.C.; Simpkin, A.J.; Woodward, G.; Gaunt, T.R.; Lyttleton, O.; McArdle, W.L.; Ring, S.M.; Smith, A.D.A.C.; Timpson, N.J.; Tilling, K.; et al. Prenatal Exposure to Maternal Smoking and Offspring DNA Methylation across the Lifecourse: Findings from the Avon Longitudinal Study of Parents and Children (ALSPAC). Hum. Mol. Genet. 2015, 24, 2201–2217. [Google Scholar] [CrossRef] [PubMed]
- Gould, H.J.; Sutton, B.J. IgE in Allergy and Asthma Today. Nat. Rev. Immunol. 2008, 8, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.A.; Vonk, J.M.; Baurecht, H.; Marenholz, I.; Tian, C.; Hoffman, J.D.; Helmer, Q.; Tillander, A.; Ullemar, V.; Van Dongen, J.; et al. Shared Genetic Origin of Asthma, Hay Fever and Eczema Elucidates Allergic Disease Biology. Nat. Genet. 2017, 49, 1752–1757. [Google Scholar] [CrossRef] [PubMed]
- Potaczek, D.P.; Kabesch, M. Current Concepts of IgE Regulation and Impact of Genetic Determinants. Clin. Exp. Allergy 2012, 42, 852–871. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.A.; Gigliotti, N.M.; Aubin, B.G.; Rosenwasser, L.J. FCER2 (CD23) Asthma-Related Single Nucleotide Polymorphisms. Am. J. Respir. Cell Mol. Biol. 2014, 50, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Bégin, P.; Kang, H.; Henderson, M.; Lewin, A.; Lee, G.E.; Healy-Profitós, J.; Auger, N. Maternal Autoimmune Disease and Risk of Hospitalization for Autoimmune Disease, Allergy, and Cancer in Offspring. Pediatr. Allergy Immunol. 2022, 33, e13728. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, A.; Dimich-Ward, H.; Becker, A.; Watson, W.; Dybuncio, A.; Carlsten, C.; Chan-Yeung, M. Elevated Cord Blood IgE Is Associated with Recurrent Wheeze and Atopy at 7 Yrs in a High Risk Cohort. Pediatr. Allergy Immunol. 2009, 20, 710–713. [Google Scholar] [CrossRef]
- Karmaus, W.; Arshad, S.H.; Sadeghnejad, A.; Twiselton, R. Does Maternal Immunoglobulin E Decrease with Increasing Order of Live Offspring? Investigation into Maternal Immune Tolerance. Clin. Exp. Allergy 2004, 34, 853–859. [Google Scholar] [CrossRef]
- Hernández, E.; Barraza-Villarreal, A.; Escamilla-Núñez, M.C.; Hernández-Cadena, L.; Sly, P.D.; Neufeld, L.M.; Ramakishnan, U.; Romieu, I. Prenatal Determinants of Cord Blood Total Immunoglobulin e Levels in Mexican Newborns. Allergy Asthma Proc. 2013, 35, 27–34. [Google Scholar] [CrossRef]
- Avrech, O.M.; Samra, Z.; Lazarovich, Z.; Jacobovich, A.; Sompolinsky, D. Efficacy of Placental Barrier for Immunoglobulins—Correlations between Maternal, Paternal and Fetal Immunoglobulin Levels. Int. Arch. Allergy Immunol. 1994, 103, 160–165. [Google Scholar] [CrossRef]
- Lima, J.O.; Zhang, L.; Atkinson, T.P.; Philips, J.; Dasanayake, A.P.; Schroeder, H.W. Early Expression of Iε, CD23 (FcεRII), IL-4Rα, and IgE in the Human Fetus. J. Allergy Clin. Immunol. 2000, 106, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Bønnelykke, K.; Pipper, C.B.; Bisgaard, H. Transfer of Maternal IgE Can Be a Common Cause of Increased IgE Levels in Cord Blood. J. Allergy Clin. Immunol. 2010, 126, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Bertino, E.; Bisson, C.; Coscia, A.; Fabris, C.; Monti, G.; Testa, T.; Conti, A. Relationship between Maternal- and Fetal- Specific IgE. Pediatr. Allerg 2006, 17, 484–488. [Google Scholar] [CrossRef] [PubMed]
- De Amici, M.; Perotti, F.; Marseglia, G.L.; Ierullo, A.M.; Bollani, L.; Decembrino, L.; Licari, A.; Quaglini, S.; Stronati, M.; Spinillo, A. Cord and Blood Levels of Newborn IgE: Correlation, Role and Influence of Maternal IgE. Immunobiology 2017, 222, 450–453. [Google Scholar] [CrossRef]
- Sadeghnejad, A.; Karmaus, W.; Davis, S.; Kurukulaaratchy, R.J.; Matthews, S.; Arshad, S.H. Raised Cord Serum Immunoglobulin E Increases the Risk of Allergic Sensitisation at Ages 4 and 10 and Asthma at Age 10. Thorax 2004, 59, 936–942. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pastor-Vargas, C.; Maroto, A.S.; Díaz-Perales, A.; Villalba, M.; Esteban, V.; Ruiz-Ramos, M.; de Alba, M.R.; Vivanco, F.; Cuesta-Herranz, J. Detection of Major Food Allergens in Amniotic Fluid: Initial Allergenic Encounter during Pregnancy. Pediatr. Allergy Immunol. 2016, 27, 716–720. [Google Scholar] [CrossRef]
- Jones, C.A.; Warner, J.A.; Warner, J.O. Fetal Swallowing of IgE. Lancet 1998, 351, 1859. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Wood, R.A.; Stablein, D.; Lindblad, R.; Burks, A.W.; Liu, A.H.; Jones, S.M.; Fleischer, D.M.; Leung, D.Y.M.; Sampson, H.A. Maternal Consumption of Peanut during Pregnancy Is Associated with Peanut Sensitization in Atopic Infants. J. Allergy Clin. Immunol. 2010, 126, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.S.; Kakuma, R. Maternal Dietary Antigen Avoidance during Pregnancy or Lactation, or Both, for Preventing or Treating Atopic Disease in the Child. Evid.-Based Child Health 2014, 9, 447–483. [Google Scholar] [CrossRef]
- Thornton, C.A.; Holloway, J.A.; Popplewell, E.J.; Shute, J.K.; Boughton, J.; Warner, J.O. Fetal Exposure to Intact Immunoglobulin E Occurs via the Gastrointestinal Tract. Clin. Exp. Allergy 2003, 33, 306–311. [Google Scholar] [CrossRef]
- Thornton, C.A.; MacFarlane, T.V.; Holt, P.G. The Hygiene Hypothesis Revisited: Role of Materno-Fetal Interactions. Curr. Allergy Asthma Rep. 2010, 10, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Msallam, R.; Balla, J.; Rathore, A.P.S.; Kared, H.; Malleret, B.; Saron, W.A.A.; Liu, Z.; Hang, J.W.; Dutertre, C.A.; Larbi, A.; et al. Fetal Mast Cells Mediate Postnatal Allergic Responses Dependent on Maternal IgE. Science 2020, 370, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Seymour, B.W.P.; Friebertshauser, K.E.; Peake, J.L.; Pinkerton, K.E.; Coffman, R.L.; Gershwin, L.J. Gender Differences in the Allergic Response of Mice Neonatally Exposed to Environmental Tobacco Smoke. Dev. Immunol. 2002, 9, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Lødrup Carlsen, K.C.; Carlsen, K.H. Effects of Maternal and Early Tobacco Exposure on the Development of Asthma and Airway Hyperreactivity. Curr. Opin. Allergy Clin. Immunol. 2001, 1, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.; Illi, S.; Sommerfeld, C.; Niggemann, B.; Völkel, K.; Madloch, C.; Grüber, C.; Nickel, R.; Forster, J.; Wahn, U. Transient Early Wheeze Is Not Associated with Impaired Lung Function in 7-yr-Old Children. Eur. Respir. J. 2003, 21, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Berger, K.; Eskenazi, B.; Balmes, J.; Holland, N.; Calafat, A.M.; Harley, K.G. Associations between Prenatal Maternal Urinary Concentrations of Personal Care Product Chemical Biomarkers and Childhood Respiratory and Allergic Outcomes in the CHAMACOS Study. Environ. Int. 2018, 121, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Huang, R.; Hua, L.; Guo, Y.; Huang, L.; Zhao, Y.; Wang, X.; Zhang, J. Prenatal Exposure to Perfluoroalkyl and Polyfluoroalkyl Substances and Childhood Atopic Dermatitis: A Prospective Birth Cohort Study. Environ. Health A Glob. Access Sci. Source 2018, 17, 8. [Google Scholar] [CrossRef]
- Karmaus, W.; Kheirkhah Rahimabad, P.; Pham, N.; Mukherjee, N.; Chen, S.; Anthony, T.M.; Arshad, H.S.; Rathod, A.; Sultana, N.; Jones, A.D. Association of Metabolites, Nutrients, and Toxins in Maternal and Cord Serum with Asthma, IgE, SPT, FeNO, and Lung Function in Offspring. Metabolites 2023, 13, 737. [Google Scholar] [CrossRef]
- Galli, E.; Picardo, M.; Chini, L.; Passi, S.; Moschese, V.; Terminali, O.; Paone, F.; Fraioli, G.; Rossi, P. Analysis of Polyunsaturated Fatty Acids in Newborn Sera: A Screening Tool for Atopic Disease? Br. J. Dermatol. 1994, 130, 752–756. [Google Scholar] [CrossRef]
- Murk, W.; Risnes, K.R.; Bracken, M.B. Prenatal or Early-Life Exposure to Antibiotics and Risk of Childhood Asthma: A Systematic Review. Pediatrics 2011, 127, 1125–1138. [Google Scholar] [CrossRef]
- Björksten, B.; Finnström’, O.; Wichmanb, K. Intrauterine Exposure to the Beta-Adrenergic Receptor-Blocking Agent Metoprolol and Allergy. Int Arch Allergy Appl Immunol. 1988, 87, 59–62. [Google Scholar]
- Michel, F.B.; Bousquet, J.; Greillier, P.; Robinet-Levy, M.; Coulomb, Y. Comparison of Cord Blood Immunoglobulin E Concentrations and Maternal Allergy for the Prediction of Atopic Diseases in Infancy. J. Allergy Clin. Immunol. 1980, 65, 422–430. [Google Scholar] [CrossRef]
- Dib, G.; Doya, L.J.; Ismaeel, M.; Ismaeel, Y. The predictive value of umbilical cord blood total IgE levels in allergic diseases. Russ. Open Med. J. 2022, 11, e0105. [Google Scholar] [CrossRef]
- Scirica, C.V.; Gold, D.R.; Ryan, L.; Abulkerim, H.; Celedón, J.C.; Platts-Mills, T.A.E.; Naccara, L.M.; Weiss, S.T.; Litonjua, A.A. Predictors of Cord Blood IgE Levels in Children at Risk for Asthma and Atopy. J. Allergy Clin. Immunol. 2007, 119, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Z.; Chu, S.S.; Xia, Y.K.; Wang, D.D.; Wang, X. Environmental Exposure during Pregnancy and the Risk of Childhood Allergic Diseases. World J. Pediatr. 2021, 17, 467–475. [Google Scholar] [CrossRef]
- Brew, B.K.; Lundholm, C.; Viktorin, A.; Lichtenstein, P.; Larsson, H.; Almqvist, C. Longitudinal Depression or Anxiety in Mothers and Offspring Asthma: A Swedish Populationbased Study. Int. J. Epidemiol. 2018, 47, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Simister, N.E. Placental Transport of Immunoglobulin G. Vaccine 2003, 21, 3365–3369. [Google Scholar] [CrossRef] [PubMed]
- Malek, A.; Sager, R.; Schneider, H. Maternal—Fetal Transport of Immunoglobulin G and Its Subclasses During the Third Trimester of Human Pregnancy. Am. J. Reprod. Immunol. 1994, 32, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Jenmalm, M.C.; Björkstén, B. Cord Blood Levels of Immunoglobulin G Subclass Antibodies to Food and Inhalant Allergens in Relation to Maternal Atopy and the Development of Atopic Disease during the First 8 Years of Life. Clin. Exp. Allergy 2000, 30, 34–40. [Google Scholar] [CrossRef]
- Victor, J.R.; Fusaro, A.E.; Da Silva Duarte, A.J.; Sato, M.N. Preconception Maternal Immunization to Dust Mite Inhibits the Type I Hypersensitivity Response of Offspring. J. Allergy Clin. Immunol. 2003, 111, 269–277. [Google Scholar] [CrossRef]
- Jarrett, E.E.; Hall, E. IgE Suppression by Maternal IgG. Immunology 1983, 48, 49–58. [Google Scholar] [PubMed]
- Victor, J.R. Allergen-Specific IgG as a Mediator of Allergy Inhibition: Lessons from Mother to Child. Hum. Vaccines Immunother. 2017, 13, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Victor, J.R.; Muniz, B.P.; Fusaro, A.E.; de Brito, C.A.; Taniguchi, E.F.; Duarte, A.J.S.; Sato, M.N. Maternal Immunization with Ovalbumin Prevents Neonatal Allergy Development and Up-Regulates Inhibitory Receptor FcγRIIB Expression on B Cells. BMC Immunol. 2010, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Dorofeeva, Y.; Shilovskiy, I.; Tulaeva, I.; Focke-Tejkl, M.; Flicker, S.; Kudlay, D.; Khaitov, M.; Karsonova, A.; Riabova, K.; Karaulov, A.; et al. Past, Presence, and Future of Allergen Immunotherapy Vaccines. Allergy Eur. J. Allergy Clin. Immunol. 2020, 76, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Prescott, S.L.; Macaubas, C.; Smallacombe, T.; Holt, B.J.; Sly, P.D.; Holt, P.G. Development of Allergen-Specific T-Cell Memory in Atopic and Normal Children. Lancet 1999, 353, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Perveen, K.; Quach, A.; McPhee, A.; Prescott, S.L.; Barry, S.C.; Hii, C.S.; Ferrante, A. Validation of Monoclonal Anti-PKC Isozyme Antibodies for Flow Cytometry Analyses in Human T Cell Subsets and Expression in Cord Blood T Cells. Sci. Rep. 2019, 9, 9263. [Google Scholar] [CrossRef] [PubMed]
- Holt, P.G.; Strickland, D.H.; Custovic, A. Targeting Maternal Immune Function during Pregnancy for Asthma Prevention in Offspring: Harnessing the “Farm Effect”? J. Allergy Clin. Immunol. 2020, 146, 270–272. [Google Scholar] [CrossRef] [PubMed]
- Holt, P.G.; Jones, C.A. The Development of the Immune System during Pregnancy and Early Life. Allergy Eur. J. Allergy Clin. Immunol. 2000, 55, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Bunyavanich, S.; Rifas-Shiman, S.L.; Platts-Mills, T.A.; Workman, L.; Sordillo, J.E.; Camargo Jr, C.A.; Gillman, M.W.; Gold, D.R.; Litonjua, A.A. Peanut, Milk, and Wheat Intake during Pregnancy Is Associated with Reduced Allergy and Asthma in Children. J. Allergy Clin. Immunol. 2014, 133, 1373–1382. [Google Scholar] [CrossRef]
- Venter, C.; Palumbo, M.P.; Glueck, D.H.; Sauder, K.A.; O’Mahony, L.; Fleischer, D.M.; Ben-Abdallah, M.; Ringham, B.M.; Dabelea, D. The Maternal Diet Index in Pregnancy Is Associated with Offspring Allergic Diseases: The Healthy Start Study. Allergy Eur. J. Allergy Clin. Immunol. 2022, 77, 162–172. [Google Scholar] [CrossRef]
- Lupinek, C.; Hochwallner, H.; Johansson, C.; Mie, A.; Rigler, E.; Scheynius, A.; Alm, J.; Valenta, R. Maternal Allergen-Specific IgG Might Protect the Child against Allergic Sensitization. J. Allergy Clin. Immunol. 2019, 144, 536–548. [Google Scholar] [CrossRef]
- Marchant, A.; Sadarangani, M.; Garand, M.; Dauby, N.; Verhasselt, V.; Pereira, L.; Bjornson, G.; Jones, C.E.; Halperin, S.A.; Edwards, K.M.; et al. Maternal Immunisation: Collaborating with Mother Nature. Lancet Infect. Dis. 2017, 17, e197–e208. [Google Scholar] [CrossRef]
- Venter, C.; Agostoni, C.; Arshad, S.H.; Ben-Abdallah, M.; Du Toit, G.; Fleischer, D.M.; Greenhawt, M.; Glueck, D.H.; Groetch, M.; Lunjani, N.; et al. Dietary Factors during Pregnancy and Atopic Outcomes in Childhood: A Systematic Review from the European Academy of Allergy and Clinical Immunology. Pediatr. Allergy Immunol. 2020, 31, 889–912. [Google Scholar] [CrossRef]
- Brzozowska, A.; Podlecka, D.; Jankowska, A.; Król, A.; Kaleta, D.; Trafalska, E.; Nowakowska-Świrta, E.; Kałużny, P.; Hanke, W.; Bal-Gierańczyk, K.; et al. Maternal Diet during Pregnancy and Risk of Allergic Diseases in Children up to 7–9 Years Old from Polish Mother and Child Cohort Study. Environ. Res. 2022, 208, 112682. [Google Scholar] [CrossRef]
- Harb, H.; Irvine, J.; Amarasekera, M.; Hii, C.S.; Kesper, D.A.; Ma, Y.F.; D’Vaz, N.; Renz, H.; Potaczek, D.P.; Prescott, S.L.; et al. The Role of PKCζ in Cord Blood T-Cell Maturation towards Th1 Cytokine Profile and Its Epigenetic Regulation by Fish Oil. Biosci. Rep. 2017, 37, BSR20160485. [Google Scholar] [CrossRef]
- Tsuji, S.; Adachi, Y.; Tsuchida, A.; Hamazaki, K.; Matsumura, K.; Inadera, H.; Study, T.J.E.; Japan Environment and Children’s Study Group; Allergology International. Association of Allergies in Children Younger than 3 Years with Levels of Maternal Intake of N-3 Polyunsaturated Fatty Acids or Fish during Pregnancy: A Nationwide Birth Cohort Study, the Japan Environment and Children’s Study. Allergol. Int. 2024, 73, 282–289. [Google Scholar] [CrossRef]

| Type I Response (IVa) | Type II Response (IVb) | Type III Response (IVc) | |
|---|---|---|---|
| Role | Intracellular pathogen (viruses, Mycobacterium tuberculosis) | Helminths, poisons, venoms | Extracellular pathogens (bacteria, fungi) |
| Key components | Type 1 CD4+ T cells (Th1) Type 1 innate lymphoid cells (ILC1) Natural Killer cells (NK) Natural Killer T cells (NK-T) Type 1 CD8+ cytotoxic lymphocytes (Tc1) | Type 2 CD4+ T cells (Th2) Type 2 innate lymphoid cells (ILC2) Type 2 CD8+ cytotoxic lymphocytes (Tc2) | Type 3 CD4+ T cells (Th17) Type 3 innate lymphoid cells (ILC3) Type 3 CD8+ cytotoxic lymphocytes (Tc17) |
| Mediators | Interferon-gamma (IFN-γ), Tumour Necrosis Factor (TNF), Interleukin-12 (IL-12) | IL-4, IL-5, IL-9, IL-13, (IL21) | IL-8, IL-17, IL-22, TNF, Granulocyte-macrophage colony-stimulating factor (GM-CSF) |
| Antibodies | IgG1, IgG2, IgG3 | IgE | |
| Effector cells | Macrophages, neutrophils | Eosinophils, mast cells, basophils | Mononuclear phagocytes, neutrophils |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grijincu, M.; Buzan, M.-R.; Zbîrcea, L.-E.; Păunescu, V.; Panaitescu, C. Prenatal Factors in the Development of Allergic Diseases. Int. J. Mol. Sci. 2024, 25, 6359. https://doi.org/10.3390/ijms25126359
Grijincu M, Buzan M-R, Zbîrcea L-E, Păunescu V, Panaitescu C. Prenatal Factors in the Development of Allergic Diseases. International Journal of Molecular Sciences. 2024; 25(12):6359. https://doi.org/10.3390/ijms25126359
Chicago/Turabian StyleGrijincu, Manuela, Maria-Roxana Buzan, Lauriana-Eunice Zbîrcea, Virgil Păunescu, and Carmen Panaitescu. 2024. "Prenatal Factors in the Development of Allergic Diseases" International Journal of Molecular Sciences 25, no. 12: 6359. https://doi.org/10.3390/ijms25126359
APA StyleGrijincu, M., Buzan, M.-R., Zbîrcea, L.-E., Păunescu, V., & Panaitescu, C. (2024). Prenatal Factors in the Development of Allergic Diseases. International Journal of Molecular Sciences, 25(12), 6359. https://doi.org/10.3390/ijms25126359








