Effects of Alkaline Extraction pH on Amino Acid Compositions, Protein Secondary Structures, Thermal Stability, and Functionalities of Brewer’s Spent Grain Proteins
Abstract
:1. Introduction
2. Results and Discussion
2.1. Ideal Conditions of Temperature and BSG-to-Solvent Ratio
2.2. Effects of Extraction pH on BSG Proteins’ Physicochemical Properties
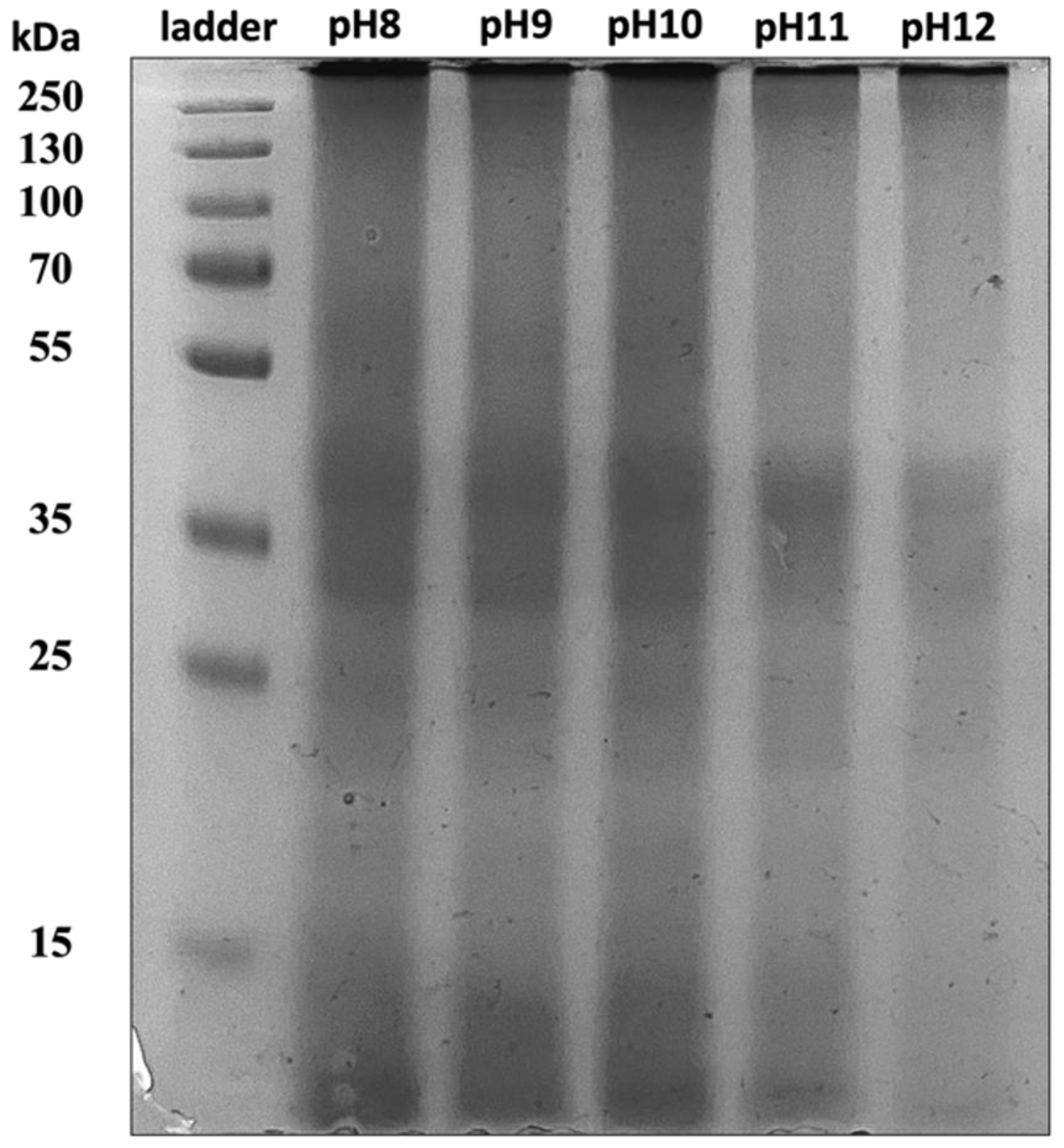
2.2.1. Molecular Weight by Gel Electrophoresis
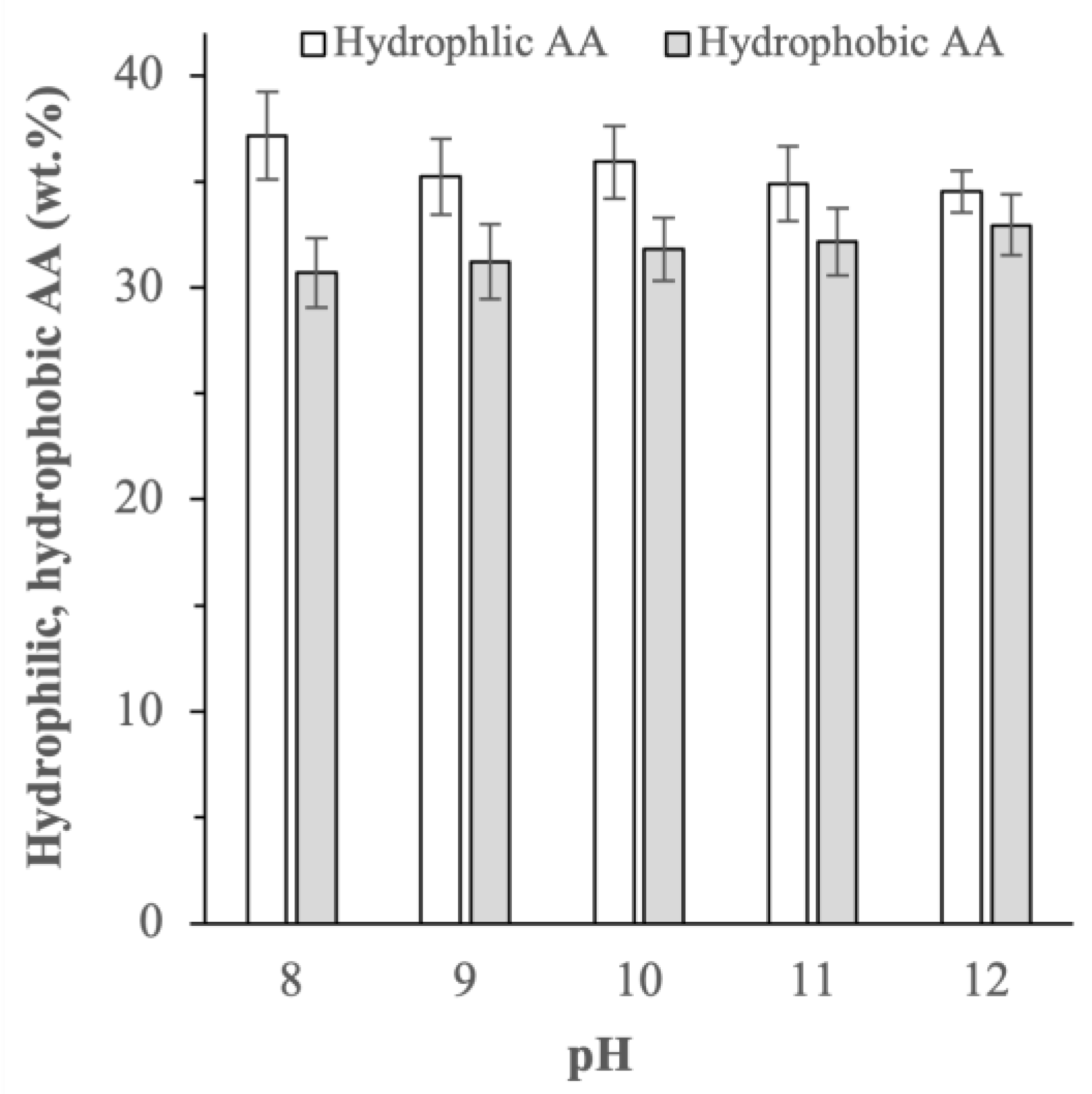
2.2.2. Amino Acid Compositions
2.2.3. Protein Secondary Structures
2.2.4. Thermal Stability
2.3. Effects of Extraction pH on BSG Proteins’ Functional Properties
2.3.1. Water and Oil Holding Capacity
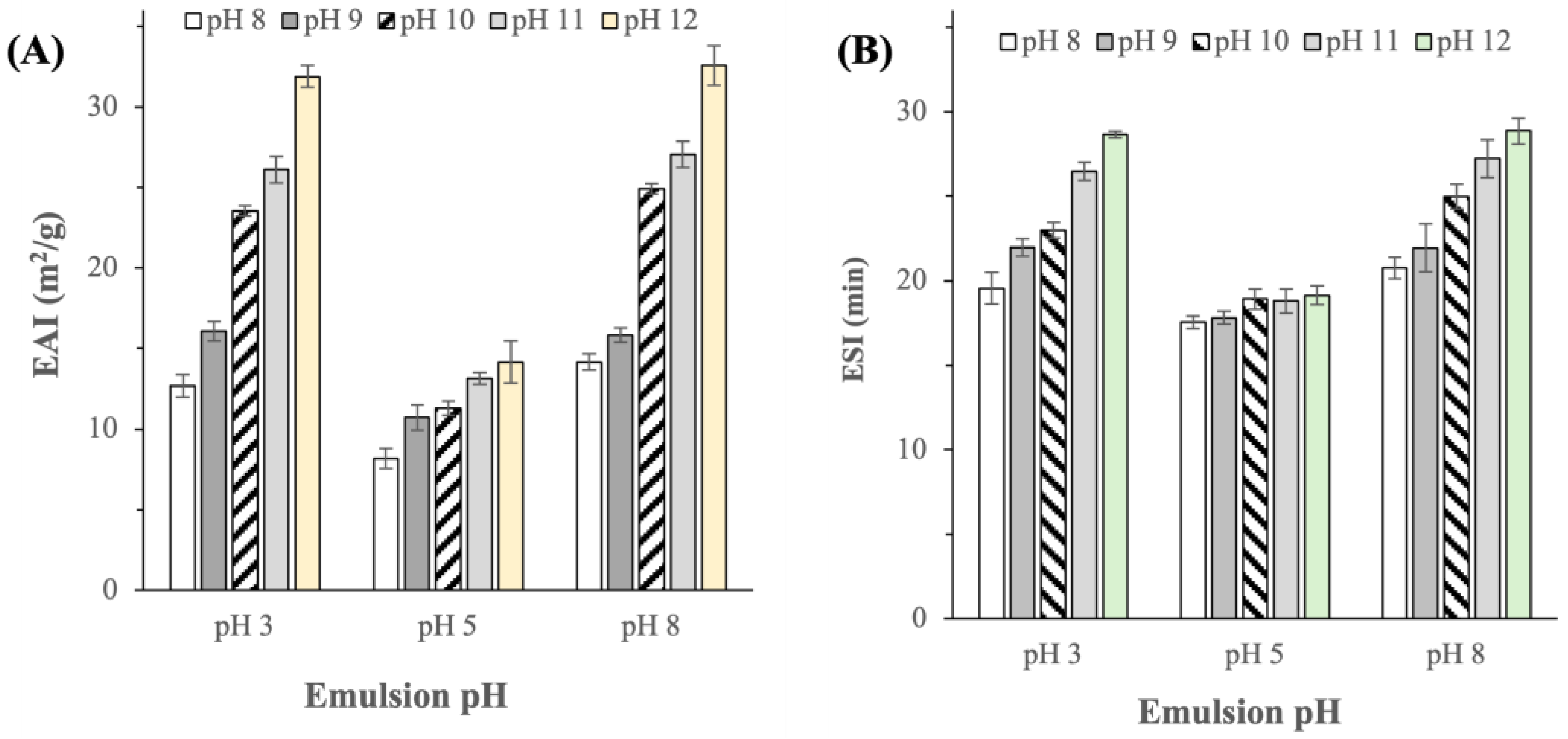
2.3.2. Emulsifying Activity and Stability Indexes
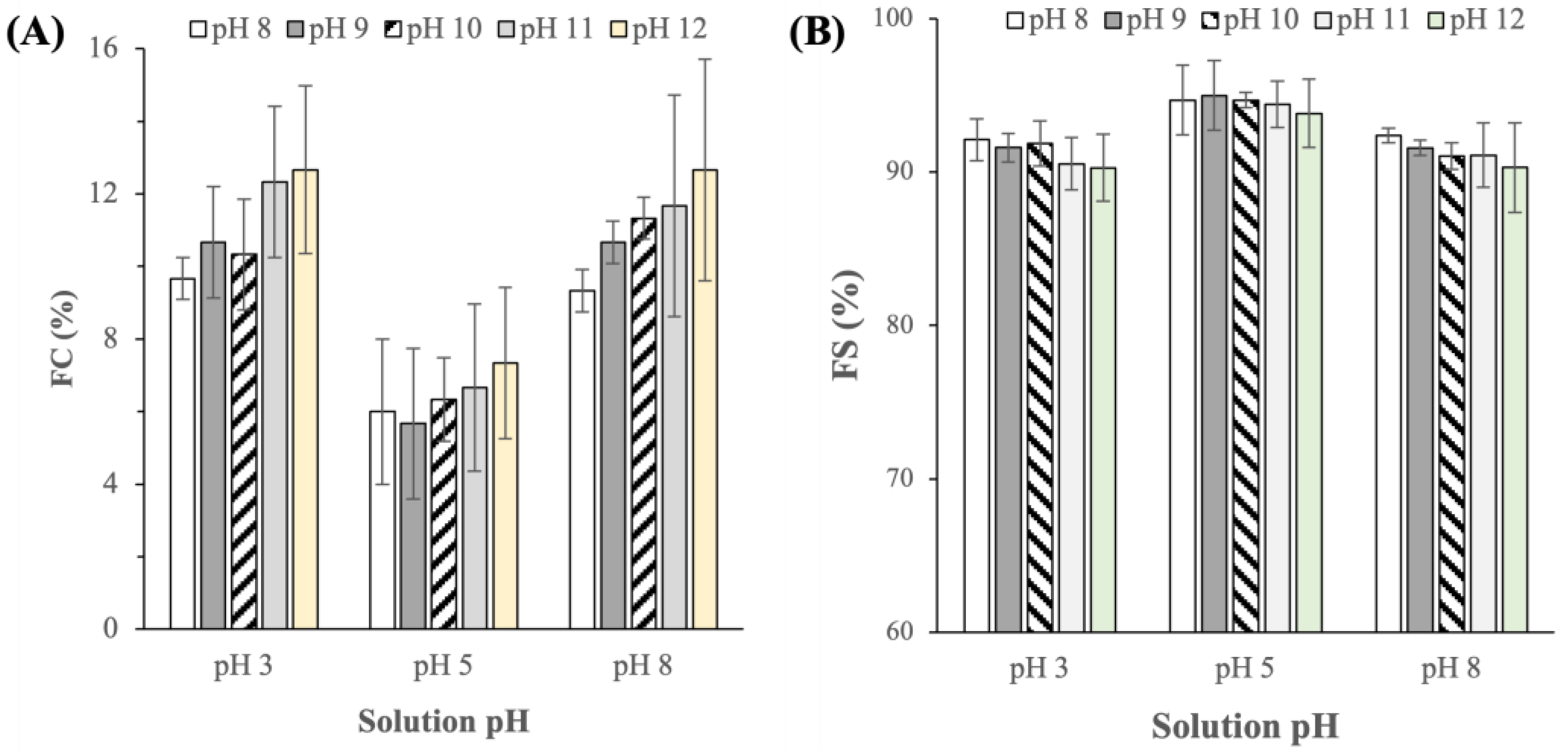
2.3.3. Foaming Capacity and Stability
3. Materials and Methods
3.1. Materials
3.2. Methods
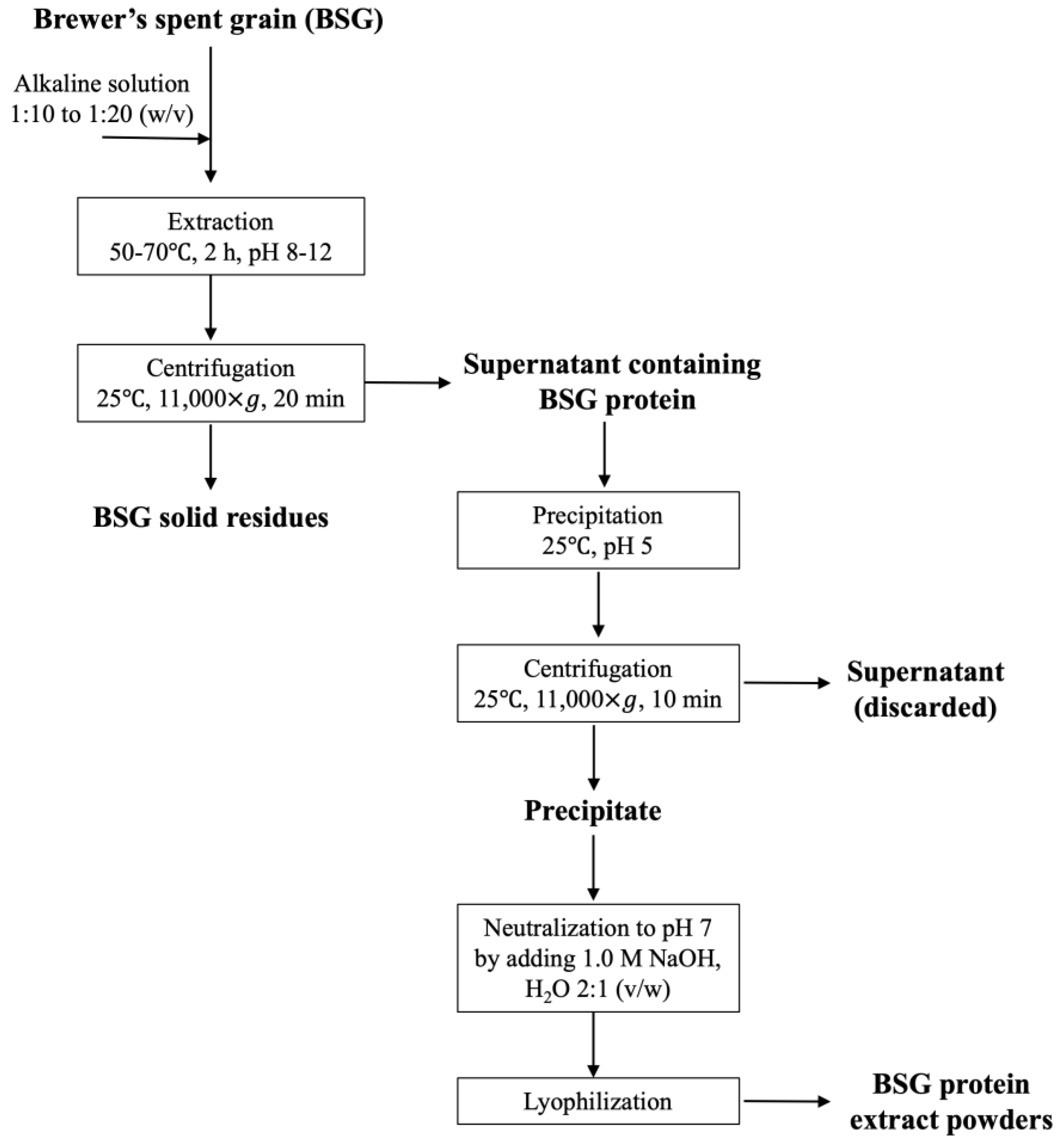
3.2.1. Extraction and Isolation of BSG Proteins
3.2.2. Extraction Yield and Extracted Protein Content
3.2.3. Gel Electrophoresis
3.2.4. Amino Acid Compositions
3.2.5. Protein Secondary Structures
3.2.6. Thermal Stability
3.2.7. Water and Oil Holding Capacities
3.2.8. Emulsifying Properties
3.2.9. Foaming Properties
3.2.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ Spent Grain: Generation, Characteristics and Potential Applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Lynch, K.M.; Steffen, E.J.; Arendt, E.K. Brewers’ Spent Grain: A Review with an Emphasis on Food and Health. J. Inst. Brew. 2016, 122, 553–568. [Google Scholar] [CrossRef]
- Mitri, S.; Salameh, S.-J.; Khelfa, A.; Leonard, E.; Maroun, R.G.; Louka, N.; Koubaa, M. Valorization of Brewers’ Spent Grains: Pretreatments and Fermentation, a Review. Fermentation 2022, 8, 50. [Google Scholar] [CrossRef]
- Devnani, B.; Moran, G.C.; Grossmann, L. Extraction, Composition, Functionality, and Utilization of Brewer’s Spent Grain Protein in Food Formulations. Foods 2023, 12, 1543. [Google Scholar] [CrossRef]
- Vieira, E.; Rocha, M.A.M.; Coelho, E.; Pinho, O.; Saraiva, J.A.; Ferreira, I.M.P.L.V.O.; Coimbra, M.A. Valuation of Brewer’s Spent Grain Using a Fully Recyclable Integrated Process for Extraction of Proteins and Arabinoxylans. Ind. Crops Prod. 2014, 52, 136–143. [Google Scholar] [CrossRef]
- Karlsen, F.; Lund, I.; Skov, P.V. Optimisation of Alkaline Extraction of Protein from Brewer’s Spent Grain. J. Inst. Brew. 2022, 128, 150–161. [Google Scholar] [CrossRef]
- da Silva, A.M.M.; Almeida, F.S.; da Silva, M.F.; Goldbeck, R.; Sato, A.C.K. How Do pH and Temperature Influence Extraction Yield, Physicochemical, Functional, and Rheological Characteristics of Brewer Spent Grain Protein Concentrates? Food Bioprod. Process. 2023, 139, 34–45. [Google Scholar] [CrossRef]
- Li, W.; Yang, H.; Coldea, T.E.; Zhao, H. Modification of Structural and Functional Characteristics of Brewer’s Spent Grain Protein by Ultrasound Assisted Extraction. LWT 2021, 139, 110582. [Google Scholar] [CrossRef]
- González-García, E.; Marina, M.L.; García, M.C. Impact of the Use of Pressurized Liquids on the Extraction and Functionality of Proteins and Bioactives from Brewer’s Spent Grain. Food Chem. 2021, 359, 129874. [Google Scholar] [CrossRef]
- Junttila, M.H. Extraction of Brewers’ Spent Grain in near Subcritical Conditions: A Method to Obtain High Protein Contents Extracts. J. Agric. Food Res. 2022, 10, 100378. [Google Scholar] [CrossRef]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein Content and Amino Acid Composition of Commercially Available Plant-Based Protein Isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef]
- Jarpa-Parra, M.; Bamdad, F.; Wang, Y.; Tian, Z.; Temelli, F.; Han, J.; Chen, L. Optimization of Lentil Protein Extraction and the Influence of Process pH on Protein Structure and Functionality. LWT—Food Sci. Technol. 2014, 57, 461–469. [Google Scholar] [CrossRef]
- Shen, L.; Wang, X.; Wang, Z.; Wu, Y.; Chen, J. Studies on Tea Protein Extraction Using Alkaline and Enzyme Methods. Food Chem. 2008, 107, 929–938. [Google Scholar] [CrossRef]
- Ikram, S.; Huang, L.; Zhang, H.; Wang, J.; Yin, M. Composition and Nutrient Value Proposition of Brewers Spent Grain. J. Food Sci. 2017, 82, 2232–2242. [Google Scholar] [CrossRef] [PubMed]
- Connolly, A.; Piggott, C.O.; FitzGerald, R.J. Technofunctional Properties of a Brewers’ Spent Grain Protein-Enriched Isolate and Its Associated Enzymatic Hydrolysates. LWT—Food Sci. Technol. 2014, 59, 1061–1067. [Google Scholar] [CrossRef]
- Niemi, P.; Martins, D.; Buchert, J.; Faulds, C.B. Pre-Hydrolysis with Carbohydrases Facilitates the Release of Protein from Brewer’s Spent Grain. Bioresour. Technol. 2013, 136, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Celus, I.; Brijs, K.; Delcour, J.A. The Effects of Malting and Mashing on Barley Protein Extractability. J. Cereal Sci. 2006, 44, 203–211. [Google Scholar] [CrossRef]
- Chin, Y.L.; Chai, K.F.; Chen, W.N. Upcycling of Brewers’ Spent Grains via Solid-State Fermentation for the Production of Protein Hydrolysates with Antioxidant and Techno-Functional Properties. Food Chem. X 2021, 13, 100184. [Google Scholar] [CrossRef]
- Meng, G.-T.; Ma, C.-Y. Fourier-Transform Infrared Spectroscopic Study of Globulin from Phaseolus Angularis (Red Bean). Int. J. Biol. Macromol. 2001, 29, 287–294. [Google Scholar] [CrossRef]
- Tang, C.-H.; Choi, S.-M.; Ma, C.-Y. Study of Thermal Properties and Heat-Induced Denaturation and Aggregation of Soy Proteins by Modulated Differential Scanning Calorimetry. Int. J. Biol. Macromol. 2007, 40, 96–104. [Google Scholar] [CrossRef]
- Hejna, A.; Barczewski, M.; Kosmela, P.; Aniśko, J.; Szulc, J.; Skórczewska, K.; Piasecki, A.; Kuang, T. More than Just a Beer—Brewers’ Spent Grain, Spent Hops, and Spent Yeast as Potential Functional Fillers for Polymer Composites. Waste Manag. 2024, 180, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Xu, T.-R.; Fu, Y.-H.; Cai, M.; Xia, Q.-L.; Guan, R.-F.; Zou, X.-G.; Sun, P.-L. Effects of Ultrasonic Pre-Treatment on Physicochemical Properties of Proteins Extracted from Cold-Pressed Sesame Cake. Food Res. Int. 2021, 139, 109907. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.K.; Grossmann, L.; Nolden, A.A.; McClements, D.J.; Kinchla, A.J. Functional and Physical Properties of Commercial Pulse Proteins Compared to Soy Derived Protein. Future Foods 2022, 6, 100155. [Google Scholar] [CrossRef]
- Benelhadj, S.; Gharsallaoui, A.; Degraeve, P.; Attia, H.; Ghorbel, D. Effect of pH on the Functional Properties of Arthrospira (Spirulina) Platensis Protein Isolate. Food Chem. 2016, 194, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Withana-Gamage, T.S.; Wanasundara, J.P.; Pietrasik, Z.; Shand, P.J. Physicochemical, Thermal and Functional Characterisation of Protein Isolates from Kabuli and Desi Chickpea (Cicer arietinum L.): A Comparative Study with Soy (Glycine Max) and Pea (Pisum sativum L.). J. Sci. Food Agric. 2011, 91, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Wang, Y.; Selomulya, C. Dairy and Plant Proteins as Natural Food Emulsifiers. Trends Food Sci. Technol. 2020, 105, 261–272. [Google Scholar] [CrossRef]
- Lupatini Menegotto, A.L.; de Souza, L.E.S.; Colla, L.M.; Costa, J.A.V.; Sehn, E.; Bittencourt, P.R.S.; de Moraes Flores, É.L.; Canan, C.; Colla, E. Investigation of Techno-Functional and Physicochemical Properties of Spirulina Platensis Protein Concentrate for Food Enrichment. LWT 2019, 114, 108267. [Google Scholar] [CrossRef]
- Gundogan, R.; Can Karaca, A. Physicochemical and Functional Properties of Proteins Isolated from Local Beans of Turkey. LWT 2020, 130, 109609. [Google Scholar] [CrossRef]
- Murray, B.S. Recent Developments in Food Foams. Curr. Opin. Colloid Interface Sci. 2020, 50, 101394. [Google Scholar] [CrossRef]
- Amagliani, L.; O’Regan, J.; Schmitt, C.; Kelly, A.L.; O’Mahony, J.A. Characterisation of the Physicochemical Properties of Intact and Hydrolysed Rice Protein Ingredients. J. Cereal Sci. 2019, 88, 16–23. [Google Scholar] [CrossRef]
- Vogelsang-O’Dwyer, M.; Petersen, I.L.; Joehnke, M.S.; Sørensen, J.C.; Bez, J.; Detzel, A.; Busch, M.; Krueger, M.; O’Mahony, J.A.; Arendt, E.K.; et al. Comparison of Faba Bean Protein Ingredients Produced Using Dry Fractionation and Isoelectric Precipitation: Techno-Functional, Nutritional and Environmental Performance. Foods 2020, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Adhikari, B.; He, Z.; Qin, F.; Huang, X.; Chen, J. Improving the Foaming Properties of Soy Protein Isolate Through Partial Enzymatic Hydrolysis. Dry. Technol. 2013, 31, 1545–1552. [Google Scholar] [CrossRef]
- Chin, Y.L. Valorization of Brewer’s Spent Grains for Food Applications. Ph.D. Dissertation, Nanyang Technological University, Singapore, 2024. [Google Scholar]
- Qin, F.; Johansen, A.Z.; Mussatto, S.I. Evaluation of Different Pretreatment Strategies for Protein Extraction from Brewer’s Spent Grains. Ind. Crops Prod. 2018, 125, 443–453. [Google Scholar] [CrossRef]
- Pérez-Palacios, T.; Barroso, M.A.; Ruiz, J.; Antequera, T. A Rapid and Accurate Extraction Procedure for Analysing Free Amino Acids in Meat Samples by GC-MS. Int. J. Anal. Chem. 2015, 2015, 209214. [Google Scholar] [CrossRef]
- Sadat, A.; Joye, I.J. Peak Fitting Applied to Fourier Transform Infrared and Raman Spectroscopic Analysis of Proteins. Appl. Sci. 2020, 10, 5918. [Google Scholar] [CrossRef]



| pH | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|
| Extracted protein content (g/100 g of lyophilized BSG) | 5.2 | 6.7 0.3 | 8.9 | 9.4 0.1 | 11.7 0.2 |
| Amino Acid Composition (wt.%) | pH 8 | pH 9 | pH 10 | pH 11 | pH 12 | -Value |
|---|---|---|---|---|---|---|
| L-Alanine | 6.21 0.01 | 6.32 0.31 | 5.88 0.01 | 5.83 0.14 | 5.68 0.33 | 0.018 |
| Glycine | 1.95 0.40 | 2.82 0.58 | 3.01 0.47 | 2.84 0.37 | 2.79 0.17 | 0.074 |
| L-Valine | 4.48 0.59 | 5.51 0.36 | 5.49 0.14 | 4.96 0.10 | 4.80 0.43 | 0.027 |
| L-Leucine | 5.61 0.26 | 6.77 0.29 | 7.55 0.19 | 7.61 0.22 | 7.51 0.06 | 0.001 |
| Isoleucine | 4.99 0.36 | 4.75 0.57 | 4.61 0.53 | 4.42 0.52 | 4.70 0.42 | 0.696 |
| L-Threonine | 5.04 0.13 | 5.70 0.13 | 5.44 0.01 | 5.30 0.08 | 5.09 0.39 | 0.012 |
| L-Proline | 7.70 0.19 | 7.10 0.28 | 6.72 0.13 | 6.83 0.21 | 6.59 0.17 | 0.002 |
| L-Methionine | 2.10 0.12 | 1.71 0.30 | 1.82 0.57 | 1.94 0.52 | 2.14 0.29 | 0.648 |
| L-Serine | 4.42 0.20 | 4.50 0.15 | 4.19 0.39 | 4.06 0.26 | 4.04 0.08 | 0.144 |
| L-Phenylalanine | 7.31 0.35 | 6.15 0.57 | 6.46 0.02 | 7.40 0.27 | 8.11 0.29 | <0.0005 |
| L-Aspartic acid | 8.01 0.14 | 7.14 0.73 | 7.55 0.37 | 7.14 0.63 | 7.57 0.21 | 0.211 |
| L-Glutamic acid | 17.40 0.13 | 15.41 0.20 | 14.63 0.15 | 14.62 0.17 | 14.33 0.28 | <0.0005 |
| L-Ornithine | 5.69 0.64 | 4.91 0.22 | 5.70 0.65 | 5.49 0.55 | 5.10 0.14 | 0.252 |
| L-Lysine | 6.05 0.53 | 7.78 0.29 | 8.04 0.21 | 7.66 0.08 | 7.52 0.16 | 0.0005 |
| L-Histidine | 3.55 0.02 | 3.30 0.48 | 3.18 0.52 | 3.97 0.14 | 3.82 0.32 | 0.092 |
| L-Tyrosine | 8.32 0.03 | 8.22 0.17 | 7.66 0.32 | 7.89 0.37 | 8.13 0.32 | 0.078 |
| L-Cystine | 1.16 0.18 | 1.90 0.36 | 2.08 0.62 | 2.05 0.43 | 2.08 0.19 | 0.068 |
| Assignment (%) | pH 8 | pH 9 | pH 10 | pH 11 | pH 12 | -Value |
|---|---|---|---|---|---|---|
| -helix | 8.4 0.3 | 8.4 0.4 | 10.4 0.4 | 11.2 0.4 | 11.8 0.3 | <0.0005 |
| -sheet | 28.9 1.2 | 29.8 1.3 | 29.9 1.1 | 30.7 1.2 | 28.8 0.7 | 0.281 |
| -turn | 52.5 2.2 | 49.1 2.1 | 46.0 1.6 | 48.0 1.8 | 43.2 1.1 | 0.001 |
| Random coil | 10.2 0.4 | 12.7 0.5 | 13.7 0.5 | 10.1 0.4 | 16.2 0.4 | <0.0005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadinoto, K.; Ling, J.K.-U.; Pu, S.; Tran, T.-T. Effects of Alkaline Extraction pH on Amino Acid Compositions, Protein Secondary Structures, Thermal Stability, and Functionalities of Brewer’s Spent Grain Proteins. Int. J. Mol. Sci. 2024, 25, 6369. https://doi.org/10.3390/ijms25126369
Hadinoto K, Ling JK-U, Pu S, Tran T-T. Effects of Alkaline Extraction pH on Amino Acid Compositions, Protein Secondary Structures, Thermal Stability, and Functionalities of Brewer’s Spent Grain Proteins. International Journal of Molecular Sciences. 2024; 25(12):6369. https://doi.org/10.3390/ijms25126369
Chicago/Turabian StyleHadinoto, Kunn, Jordy Kim-Ung Ling, Siyu Pu, and The-Thien Tran. 2024. "Effects of Alkaline Extraction pH on Amino Acid Compositions, Protein Secondary Structures, Thermal Stability, and Functionalities of Brewer’s Spent Grain Proteins" International Journal of Molecular Sciences 25, no. 12: 6369. https://doi.org/10.3390/ijms25126369






