The Role of Z Chromosome Localization Gene psmd9 in Spermatogenesis of Cynoglossus semilaevis
Abstract
:1. Introduction
2. Results
2.1. Cloning and Sequence Analysis of psmd9
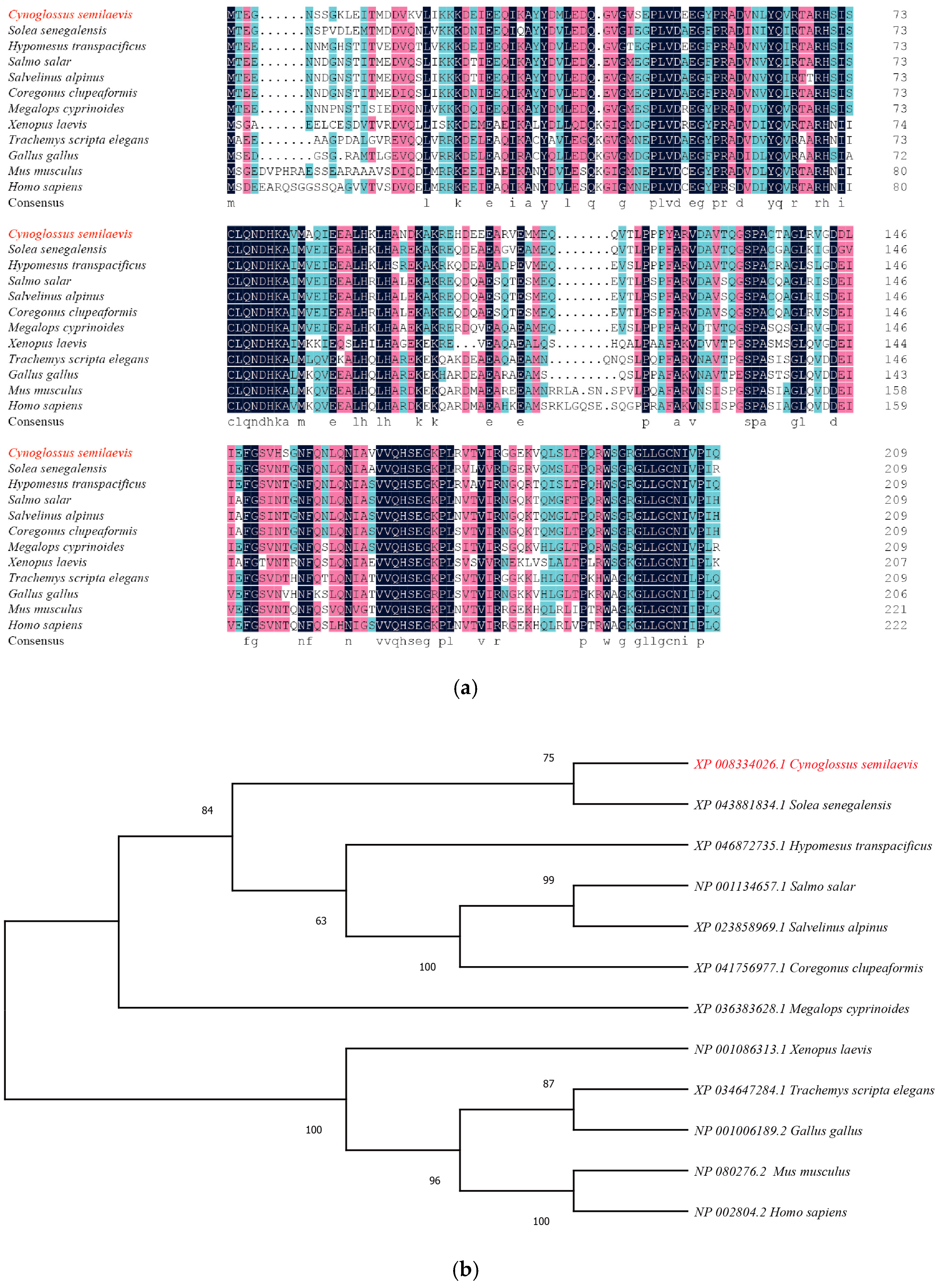
2.2. Multisequence Alignment and Molecular Phylogenetic Analysis
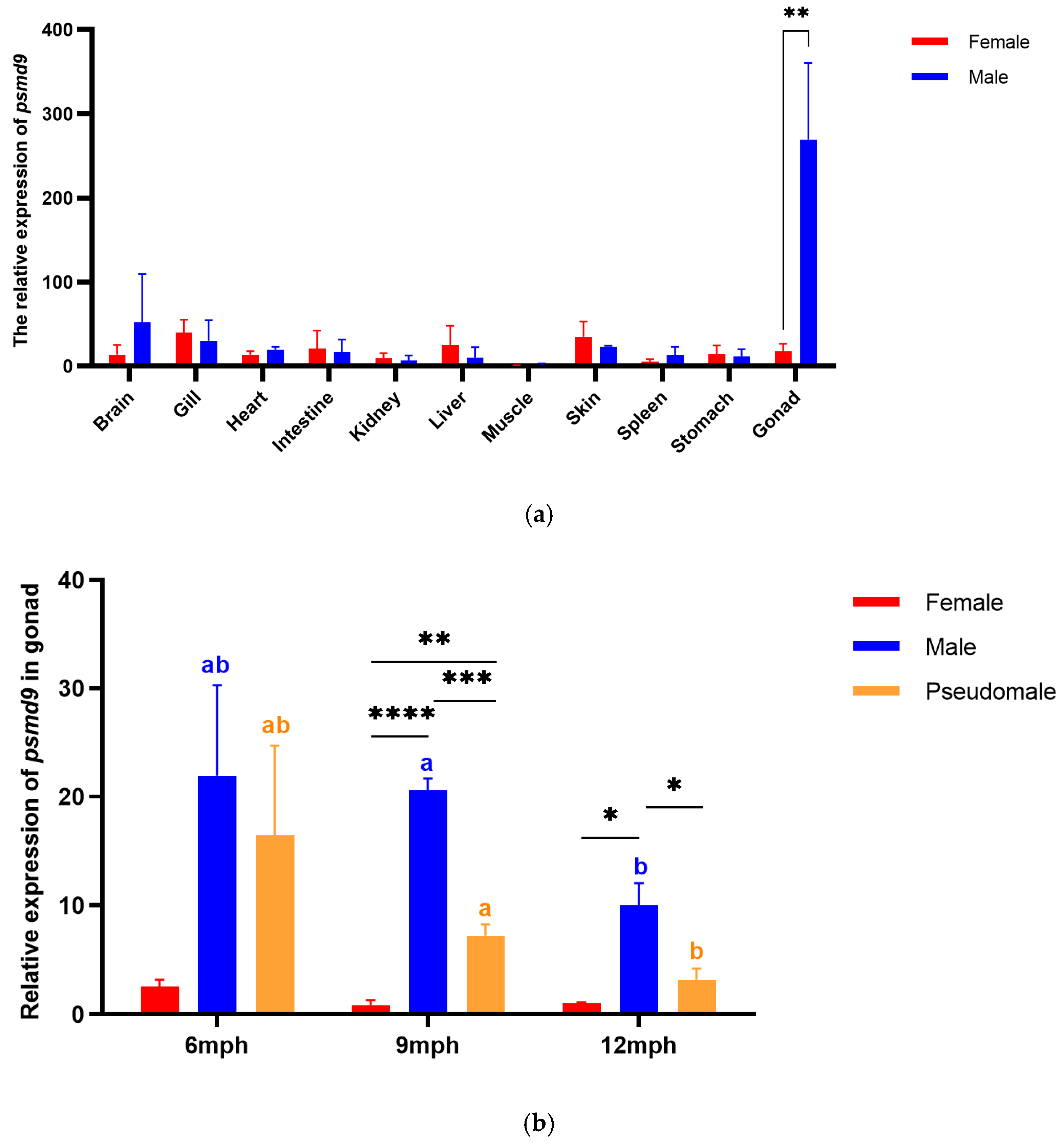
2.3. Expression Pattern of psmd9
2.4. FISH of psmd9 in Gonads
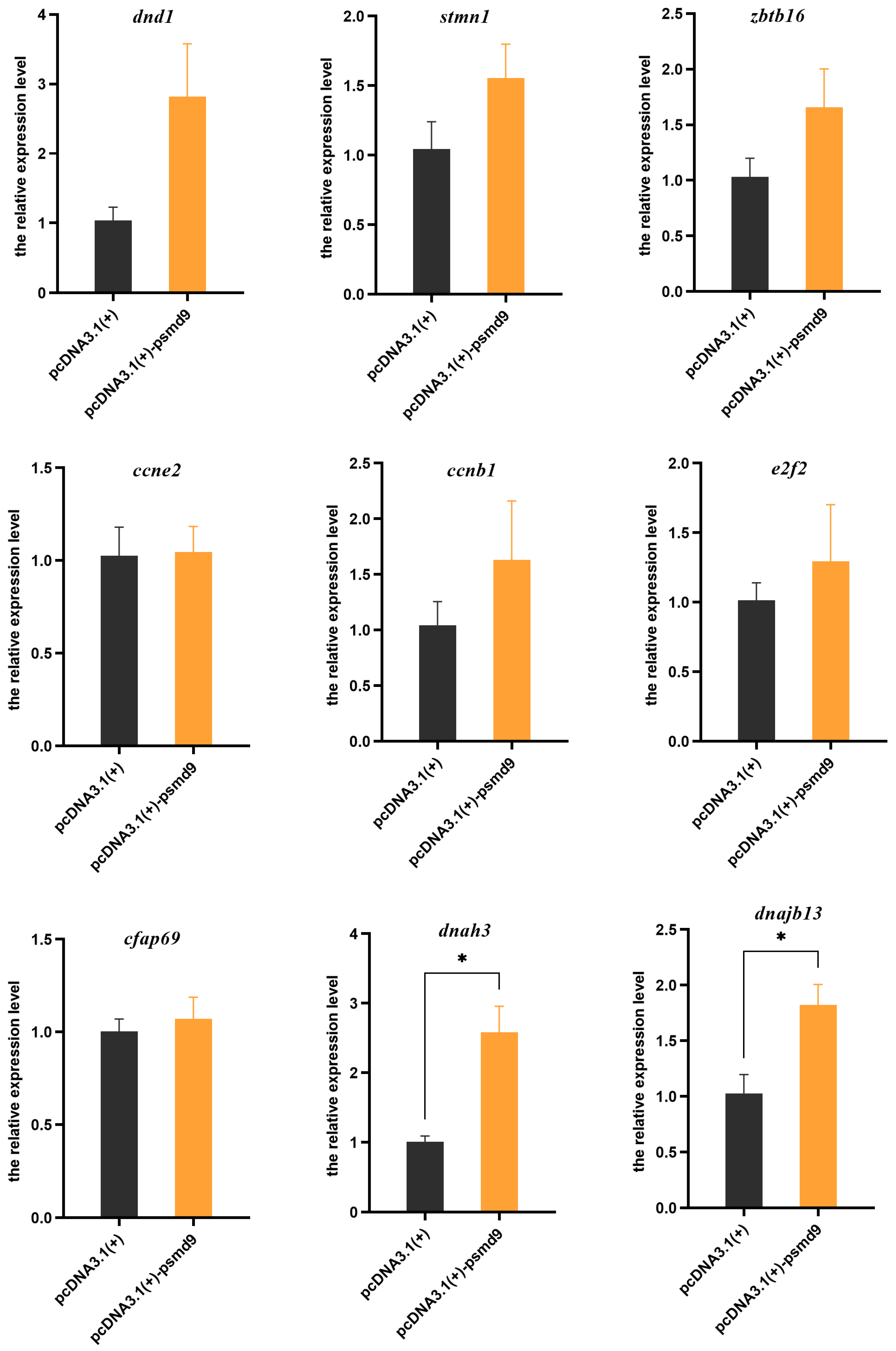
2.5. In Vitro Overexpression of psmd9 and its Effect on Other Genes
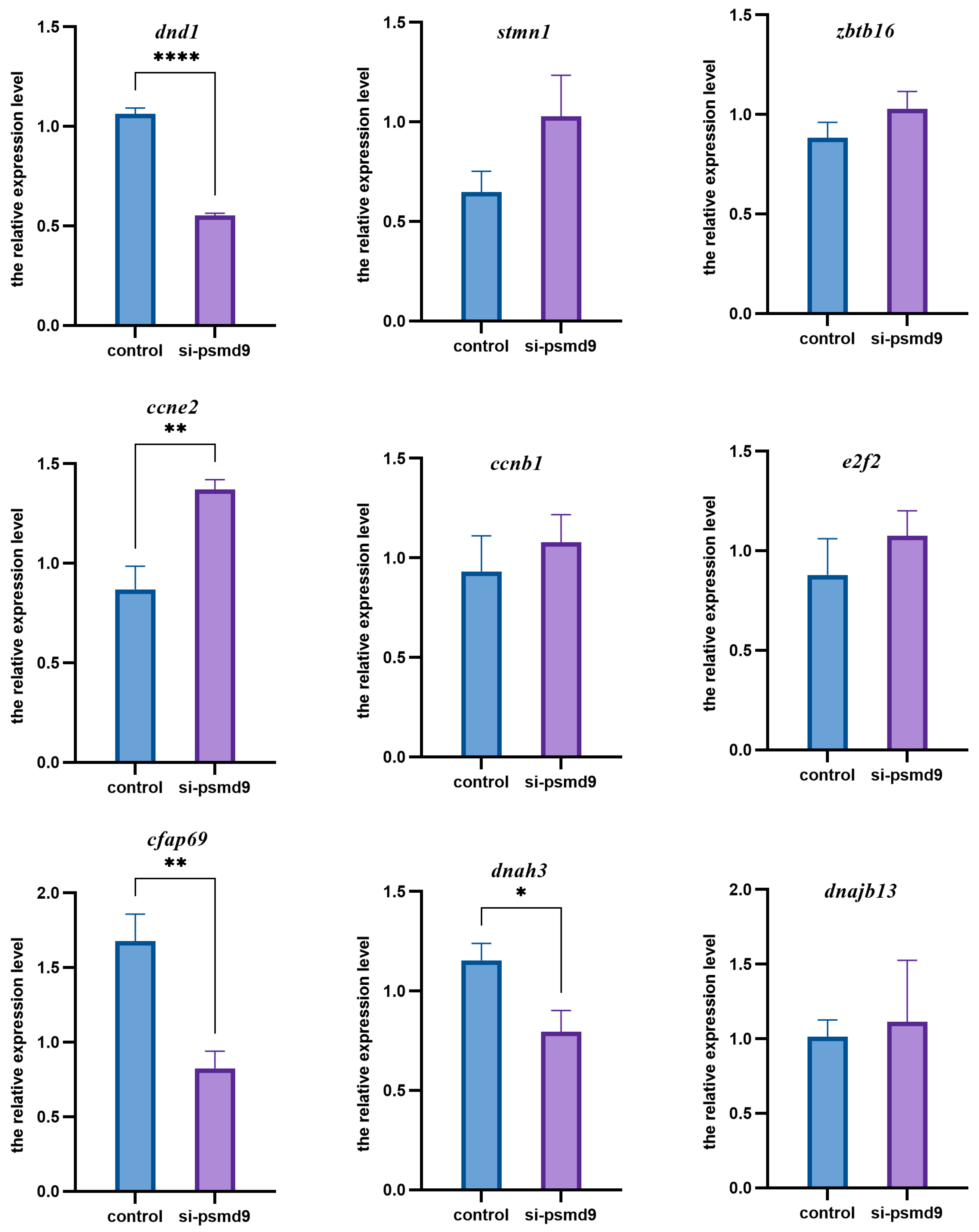
2.6. psmd9 Knockdown and Its Effect on Other Genes
3. Discussion
4. Materials and Methods
4.1. Fish and Tissue Collection
4.2. CDS Cloning of psmd9
4.3. Real-Time Quantitative PCR
4.4. Fluorescence In Situ Hybridization of psmd9
4.5. Culture and Transfection of C. semilaevis Testicular Cell Line
4.6. siRNA-Mediated Knockdown of psmd9 in Testicular Cell Line
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, R.; Agarwal, A. Spermatogenesis: An Overview. In Sperm Chromatin: Biological and Clinical Applications in Male Infertility and Assisted Reproduction; Zini, A., Agarwal, A., Eds.; Springer: New York, NY, USA, 2011; pp. 19–44. [Google Scholar]
- Renkawitz-Pohl, R.; Hempel, L.; Hollmann, M.; Schäfer, M.A. 1.4—Spermatogenesis. In Comprehensive Molecular Insect Science; Gilbert, L.I., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 157–177. [Google Scholar]
- Schulz, R.W.; de França, L.R.; Lareyre, J.-J.; LeGac, F.; Chiarini-Garcia, H.; Nobrega, R.H.; Miura, T. Spermatogenesis in fish. Gen. Comp. Endocrinol. 2010, 165, 390–411. [Google Scholar] [CrossRef]
- Hess, R.A.; de Franca, L.R. Spermatogenesis and Cycle of the Seminiferous Epithelium. In Molecular Mechanisms in Spermatogenesis; Cheng, C.Y., Ed.; Springer: New York, NY, USA, 2008; pp. 1–15. [Google Scholar]
- Li, X.-Y.; Mei, J.; Ge, C.-T.; Liu, X.-L.; Gui, J.-F. Sex determination mechanisms and sex control approaches in aquaculture animals. Sci. China Life Sci. 2022, 65, 1091–1122. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-P.; Shen, Z.-G. Sex Control in Aquaculture. In Sex Control in Aquaculture; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 1–34. [Google Scholar]
- Chen, S.-L.; Xu, W.-T. Sex Identification and Control in Half-Smooth Tongue Sole. In Sex Control in Aquaculture; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 547–563. [Google Scholar]
- Hu, L.; Siqing, C.; Haiqing, L.; Hongbo, J.; Meiyu, W. Study on sex ratio and comparison of morphological variation between genders of cultured half-smooth tongue sole(Cynoglossus semilaevis). J. Fish. China 2012, 36, 1331–1336. [Google Scholar]
- Shao, C.; Li, Q.; Chen, S.; Zhang, P.; Lian, J.; Hu, Q.; Sun, B.; Jin, L.; Liu, S.; Wang, Z.; et al. Epigenetic modification and inheritance in sexual reversal of fish. Genome Res. 2014, 24, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, G.; Shao, C.; Huang, Q.; Liu, G.; Zhang, P.; Song, W.; An, N.; Chalopin, D.; Volff, J.N.; et al. Whole-genome sequence of a flatfish provides insights into ZW sex chromosome evolution and adaptation to a benthic lifestyle. Nat. Genet. 2014, 46, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Li, H.; Dong, Z.; Cui, Z.; Zhang, N.; Meng, L.; Zhu, Y.; Liu, Y.; Li, Y.; Guo, H.; et al. Ubiquitin ligase gene neurl3 plays a role in spermatogenesis of half-smooth tongue sole (Cynoglossus semilaevis) by regulating testis protein ubiquitination. Gene 2016, 592, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Zhu, Y.; Zhang, N.; Liu, W.; Liu, Y.; Shao, C.; Wang, N.; Chen, S. Cloning and characterization of tesk1, a novel spermatogenesis-related gene, in the tongue sole (Cynoglossus semilaevis). PLoS ONE 2014, 9, e107922. [Google Scholar] [CrossRef]
- Meng, L.; Xu, W.; Zhu, Y.; Zhang, N.; Shao, C.; Liu, Y.; Chen, S. Molecular characterization and expression analysis of strbp in Chinese tongue sole (Cynoglossus semilaevis). Theriogenology 2018, 118, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-P. Discriminant Analysis of Sexual Morphology Juvenile and Integrative Analysis of Omics Reveals the Clues Why Pseudomales Are Not Able to Produce W Sperms in Cynoglossus semilaevis. Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2022. [Google Scholar]
- Wang, H.Y.; Liu, X.; Chen, J.Y.; Huang, Y.; Lu, Y.; Tan, F.; Liu, Q.; Yang, M.; Li, S.; Zhang, X.; et al. Single-cell-resolution transcriptome map revealed novel genes involved in testicular germ cell progression and somatic cells specification in Chinese tongue sole with sex reversal. Sci. China Life Sci. 2023, 66, 1151–1169. [Google Scholar] [CrossRef]
- Christie, J.; Anthony, C.M.; Harish, M.; Mudartha, D.; Ud Din Farooqee, S.B.; Venkatraman, P. The interaction network of the proteasome assembly chaperone PSMD9 regulates proteostasis. FEBS J. 2023, 290, 5581–5604. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Zhao, F.; Zhao, Z.; Li, X.; Wang, J.; Huang, B.; Chen, A. Comprehensive analysis of PSMD family members and validation of PSMD9 as a potential therapeutic target in human glioblastoma. CNS Neurosci. Ther. 2023, 30, e14366. [Google Scholar] [CrossRef]
- Jafarian, A.; Sadeghi, M.R.; Pejhan, N.; Salehkhou, S.; Lakpour, N.; Akhondi, M.M. Regeneration of spermatogenesis in a mouse model of azoospermia by follicle-stimulating hormone and oestradiol. Andrologia 2014, 46, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Sangith, N.; Srinivasaraghavan, K.; Sahu, I.; Desai, A.; Medipally, S.; Somavarappu, A.K.; Verma, C.; Venkatraman, P. Discovery of novel interacting partners of PSMD9, a proteasomal chaperone: Role of an Atypical and versatile PDZ-domain motif interaction and identification of putative functional modules. FEBS Open Bio 2014, 4, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.K.; Liu, X.Z.; Wen, H.S.; Xu, Y.; Zhang, L.J. Histological observation on gonadal sex differentiation in Cynoglossus semilaevis Günther. Mar. Fish. Res. 2006, 27, 55–61. [Google Scholar]
- Liang, Z.; Chen, S.; Zhang, J.; Song, W.; Liu, S. Gonadal development process observation of half-smooth tongue sole in rearing population. J. South. Agric. 2012, 43, 2074–2078. [Google Scholar]
- Wang, Y. Spermatogenesis and Fertilization Process of Cynoglossus semilaevis Günther. Ph.D. Thesis, Ocean University of China, Qingdao, China, 2008. [Google Scholar]
- Kang, X.; Liang, C.; Guo, M.; Ge, S.; Mu, S.; Su, W.; Liu, X. Ultrastructure of Spermatogenesis and Spermiogenesis in Half-smooth tongue sole Cynoglossus semilaevis. Curr. Zool. 2008, 54, 356–365. [Google Scholar]
- Song, H.; Weng, Y.; Liu, Z.; Fang, Y. A Historical Study on Spermatogenesis in Cynoglossus semilaevis. J. Appl. Oceanogr. 2009, 28, 19–24. [Google Scholar]
- Akmal, M.; Widodo, M.A.; Sumitro, S.B.; Purnomo, B.B. The important role of protamine in spermatogenesis and quality of sperm: A mini review. Asian Pac. J. Reprod. 2016, 5, 357–360. [Google Scholar] [CrossRef]
- Schmid, C.; Heng, H.H.; Rubin, C.; Ye, C.J.; Krawetz, S.A. Sperm nuclear matrix association of the PRM1→PRM2→TNP2 domain is independent of Alu methylation. Mol. Hum. Reprod. 2001, 7, 903–911. [Google Scholar] [CrossRef]
- Park, J.I.; Bell, G.W.; Yamashita, Y.M. Derepression of Y-linked multicopy protamine-like genes interferes with sperm nuclear compaction in D. melanogaster. Proc. Natl. Acad. Sci. USA 2023, 120, e2220576120. [Google Scholar] [CrossRef]
- Ud Din Farooqee, S.B.; Christie, J.; Venkatraman, P. PSMD9 ribosomal protein network maintains nucleolar architecture and WT p53 levels. Biochem. Biophys. Res. Commun. 2021, 563, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.-Q.; Wang, Y.; Luo, C.; Tan, Y.-M.; Li, Y.; Tan, C.; Tu, C.; Zhang, Q.-J.; Hu, L.; Zhang, H.; et al. Bi-allelic variants in DNAH3 cause male infertility with asthenoteratozoospermia in humans and mice. Hum. Reprod. Open 2024, 2024, hoae003. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Kinoshita, M.; Yuan, L. Spatiotemporal association of DNAJB13 with the annulus during mouse sperm flagellum development. BMC Dev. Biol. 2009, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Sironen, A.; Shoemark, A.; Patel, M.; Loebinger, M.R.; Mitchison, H.M. Sperm defects in primary ciliary dyskinesia and related causes of male infertility. Cell. Mol. Life Sci. 2020, 77, 2029–2048. [Google Scholar] [CrossRef] [PubMed]
- Kedde, M.; Strasser, M.J.; Boldajipour, B.; Vrielink, J.A.F.O.; Slanchev, K.; le Sage, C.; Nagel, R.; Voorhoeve, P.M.; van Duijse, J.; Ørom, U.A.; et al. RNA-Binding Protein Dnd1 Inhibits MicroRNA Access to Target mRNA. Cell 2007, 131, 1273–1286. [Google Scholar] [CrossRef]
- Imai, A.; Matsuda, K.; Niimi, Y.; Suzuki, A. Loss of Dead end1 induces testicular teratomas from primordial germ cells that failed to undergo sexual differentiation in embryonic testes. Sci. Rep. 2023, 13, 6398. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, R.; Giebeler, N.; Nevzorova, Y.A.; Bangen, J.-M.; Fahrenkamp, D.; Lambertz, D.; Haas, U.; Hu, W.; Gassler, N.; Cubero, F.J.; et al. Cyclin E1 and cyclin-dependent kinase 2 are critical for initiation, but not for progression of hepatocellular carcinoma. Proc. Natl. Acad. Sci. USA 2018, 115, 9282–9287. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.N.; Amiri-Yekta, A.; Martinez, G.; Saut, A.; Tek, J.; Stouvenel, L.; Lorès, P.; Karaouzène, T.; Thierry-Mieg, N.; Satre, V.; et al. Absence of CFAP69 Causes Male Infertility due to Multiple Morphological Abnormalities of the Flagella in Human and Mouse. Am. J. Hum. Genet. 2018, 102, 636–648. [Google Scholar] [CrossRef] [PubMed]
- McClintock, T.S.; Glasser, C.E.; Bose, S.C.; Bergman, D.A. Tissue expression patterns identify mouse cilia genes. Physiol. Genomics 2008, 32, 198–206. [Google Scholar] [CrossRef]
- Shiraishi, K.; Naito, K.; Yoshida, K.-I. Vasectomy impairs spermatogenesis through germ cell apoptosis mediated by the p53-Bax pathway in rats. J. Urol. 2001, 166, 1565–1571. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.; Gao, F.; Liang, M.M.; Hu, Q.; Song, W.; Shao, C.; Lv, W. SCAR-transformation of sex-specific SSR marker and its application in half-smooth tongue sole (Cynoglossus semiliaevis). J. Agric. Biotechnol. 2014, 22, 787–792. [Google Scholar]
- Li, Z.; Yang, L.; Wang, J.; Shi, W.; Pawar, R.A.; Liu, Y.; Xu, C.; Cong, W.; Hu, Q.; Lu, T.; et al. β-Actin is a useful internal control for tissue-specific gene expression studies using quantitative real-time PCR in the half-smooth tongue sole Cynoglossus semilaevis challenged with LPS or Vibrio anguillarum. Fish Shellfish Immunol. 2010, 29, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y.; Tan, L.; Ma, W.; Zhao, X.; Shao, C.; Wang, Q. The Role of the Heat Shock Cognate Protein 70 Genes in Sex Determination and Differentiation of Chinese Tongue Sole (Cynoglossus semilaevis). Int. J. Mol. Sci. 2023, 24, 3761. [Google Scholar] [CrossRef] [PubMed]


| Primer Name | Sequence (5′–3′) | Purpose |
|---|---|---|
| psmd9-F | AACAGACAACAGGAAGCG | Partial fragment amplification |
| psmd9-R | CAGTTTTATCCTGTTTACCTC | |
| psmd9-EcoR1-F | tagtccagtgtggtggaattcATGAAGATGACAGAGGGAAACAGTT | Construction of overexpression vector |
| psmd9-Xho1-R | aacgggccctctagactcgagTCACCGCTGAATTGGAACAAT | |
| psmd9-qF | ACCAGGGTGTTGGTGTTTCTG | RT-qPCR |
| psmd9-qR | CGTTTGGCTTTATCATTCGCA | |
| zbtb16-qF | GCCGAAGTCAGTCAGATGGG | |
| zbtb16-qR | GCGTCCGATTTGTGATTGATA | |
| dnd1-qF | GCTGTGTAAGTTGCTTGATGTTGT | |
| dnd1-qR | GCCTGTGAAGGGTGCGG | |
| stmn1-qF | TTCCCTTGTCTGCCCCC | |
| stmn1-qR | CTTCTTTTTCATGTTCACGCTTC | |
| ccne2-qF | GAGCAGGAAAACAGTGGTGAAG | |
| ccne2-qR | GAATGTGTGGAAGTGACAGAAGG | |
| ccnb1-qF | ACCGCTTTCTTCAGGACCAC | |
| ccnb1-qR | TCTCCTCAAACTTAGACGCCAG | |
| e2f2-qF | CAGTGGCTGGTGGGAGATG | |
| e2f2-qR | CAGGGATTTCTCCGCTCG | |
| cfap69-qF | TGTTGAACCTGTTGGTGTTGATG | |
| cfap69-qR | GTGGTGGATTTGGGCGG | |
| dnah3-qF | TCTTTTGGGAGGTTAGGCAAG | |
| dnah3-qR | GTGGGATTTAGATGGGGTCG | |
| dnajb13-qF | TCTGGAGATGGCTCTGACTGG | |
| dnajb13-qR | GCACAATGTCGTTGATGGGTAT | |
| β-actin-qF | GAGTAGCCACGCTCTGTC | |
| β-actin-qR | GCTGTGCTGTCCCTGTA | |
| sex-F | CCTAAATGATGGATGTAGATTCTGTC | Sex identification |
| sex-R | GATCCAGAGAAAA-TAAACCCAGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, Y.; Liu, Q.; Wang, H.; Wang, Q.; Shao, C. The Role of Z Chromosome Localization Gene psmd9 in Spermatogenesis of Cynoglossus semilaevis. Int. J. Mol. Sci. 2024, 25, 6372. https://doi.org/10.3390/ijms25126372
Zhang Y, Wang Y, Liu Q, Wang H, Wang Q, Shao C. The Role of Z Chromosome Localization Gene psmd9 in Spermatogenesis of Cynoglossus semilaevis. International Journal of Molecular Sciences. 2024; 25(12):6372. https://doi.org/10.3390/ijms25126372
Chicago/Turabian StyleZhang, Yuman, Yue Wang, Qian Liu, Hongyan Wang, Qian Wang, and Changwei Shao. 2024. "The Role of Z Chromosome Localization Gene psmd9 in Spermatogenesis of Cynoglossus semilaevis" International Journal of Molecular Sciences 25, no. 12: 6372. https://doi.org/10.3390/ijms25126372






