The Expression of Forkhead Box P3 T Regulatory Lymphocytes as a Prognostic Factor in Malignant Melanomas
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Patient Selection and Data Collection
4.2. Immunohistochemistry Analysis
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Cancer Institute (NIH), Surveillance, Epidemiology, and End Results (SEER) Program. Cancer Stat Facts: Melanoma of the Skin 2020. Available online: https://seer.cancer.gov/statfacts/html/melan.html (accessed on 26 May 2024).
- Ballantine, K.R.; Watson, H.; Macfarlane, S.; Winstanley, M.; Corbett, R.P.; Spearing, R.; Stevanovic, V.; Yi, M.; Sullivan, M.J. Small Numbers, Big Challenges: Adolescent and Young Adult Cancer Incidence and Survival in New Zealand. J. Adolesc. Young Adult Oncol. 2017, 6, 277–285. [Google Scholar] [CrossRef]
- Watson, M.; Geller, A.C.; Tucker, M.A.; Guy, G.P., Jr.; Weinstock, M.A. Melanoma burden and recent trends among non-Hispanic whites aged 15–49, United States. Prev. Med. 2016, 91, 294–298. [Google Scholar] [CrossRef][Green Version]
- Guy, G.P., Jr.; Zhang, Y.; Ekwueme, D.U.; Rim, S.H.; Watson, M. The potential impact of reducing indoor tanning on melanoma prevention and treatment costs in the United States: An economic analysis. J. Am. Acad. Dermatol. 2017, 76, 226–233. [Google Scholar] [CrossRef]
- Specenier, P. Ipilimumab in melanoma. Expert Rev. Anticancer Ther. 2016, 16, 811–826. [Google Scholar] [CrossRef] [PubMed]
- McDermott, D.; Haanen, J.; Chen, T.T.; Lorigan, P.; O’Day, S. Efficacy and safety of ipilimumab in metastatic melanoma patients surviving more than two years following treatment in a phase III trial (MDX010-20). Ann. Oncol. 2013, 24, 2694–2698. [Google Scholar] [CrossRef]
- Luther, C.; Swami, U.; Zhang, J.; Milhem, M.; Zakharia, Y. Advanced stage melanoma therapies: Detailing the present and exploring the future. Crit. Rev. Oncol. Hematol. 2019, 133, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Karaszewska, B.; Schachter, J.; Rutkowski, P.; Mackiewicz, A.; Stroiakovski, D.; Lichinitser, M.; Dummer, R.; Grange, F.; Mortier, L.; et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N. Engl. J. Med. 2015, 372, 30–39. [Google Scholar] [CrossRef]
- Schachter, J.; Ribas, A.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival results of a multicentre, randomized, open-label phase 3 study (KEYNOTE-006). Lancet 2017, 390, 1853–1862. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomized, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef]
- Long, G.V.; Atkinson, V.; Cebon, J.S.; Jameson, M.B.; Fitzharris, B.M.; McNeil, C.M.; Hill, A.G.; Ribas, A.; Atkins, M.B.; A Thompson, J.; et al. Standard-dose pembrolizumab in combination with reduced-dose ipilimumab for patients with advanced melanoma (KEYNOTE-029): An open-label, phase 1b trial. Lancet Oncol. 2017, 18, 1202–1210. [Google Scholar] [CrossRef]
- Corneliu Jinga, D.; Ciuleanu, T.; Negru, S.; Aldea, C.; Gales, L.; Bacanu, F.; Oprean, C.; Manolache, M.; Zob, D.; Curescu, S.; et al. Effectiveness and safety profile of ipilimumab therapy in previously treated patients with unresectable or metastatic melanoma—The Romanian Patient Access Program. J. BUON 2017, 22, 1287–1295. [Google Scholar]
- Restifo, N.P.; Smyth, M.J.; Snyder, A. Acquired resistance to immunotherapy and future challenges. Nat. Rev. Cancer 2016, 16, 121–126. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.S.; Long, G.V.; Scolyer, R.A.; Teng, M.W.; Smyth, M.J. Resistance to PD1/PDL1 checkpoint inhibition. Cancer Treat. Rev. 2017, 52, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, T.F. Identifying and overcoming immune resistance mechanisms in the melanoma tumor microenvironment. Clin. Cancer Res. 2006, 12 Pt 2, 2326s–2330s. [Google Scholar] [CrossRef]
- Nayak, L.; Lee, E.Q.; Wen, P.Y. Epidemiology of brain metastases. Curr. Oncol. Rep. 2012, 14, 48–54. [Google Scholar] [CrossRef]
- Balch, C.M.; Gershenwald, J.E.; Soong, S.J.; Thompson, J.F.; Atkins, M.B.; Byrd, D.R.; Buzaid, A.C.; Cochran, A.J.; Coit, D.G.; Ding, S.; et al. Final version of 2009 AJCC melanoma staging and classification. J. Clin. Oncol. 2009, 27, 6199–6206. [Google Scholar] [CrossRef] [PubMed]
- Camisaschi, C.; Vallacchi, V.; Castelli, C.; Rivoltini, L.; Rodolfo, M. Immune cells in the melanoma microenvironment hold information for prediction of the risk of recurrence and response to treatment. Expert Rev. Mol. Diagn. 2014, 14, 643–646. [Google Scholar] [CrossRef]
- Maio, M. Melanoma as a model tumour for immuno-oncology. Ann. Oncol. 2012, 23 (Suppl. 8), viii10–viii14. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, T.F.; Schreiber, H.; Fu, Y.X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013, 14, 1014–1022. [Google Scholar] [CrossRef]
- Lawrence, M.S.; Stojanov, P.; Polak, P.; Kryukov, G.V.; Cibulskis, K.; Sivachenko, A.; Carter, S.L.; Stewart, C.; Mermel, C.H.; Roberts, S.A.; et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013, 499, 214–218. [Google Scholar] [CrossRef]
- Hayward, N.K.; Wilmott, J.S.; Waddell, N.; Johansson, P.A.; Field, M.A.; Nones, K.; Patch, A.-M.; Kakavand, H.; Alexandrov, L.B.; Burke, H.; et al. Whole-genome landscapes of major melanoma subtypes. Nature 2017, 545, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Incorvaia, L.; Badalamenti, G.; Rinaldi, G.; Iovanna, J.L.; Olive, D.; Swayden, M.; Terruso, L.; Vincenzi, B.; Fulfaro, F.; Bazan, V.; et al. Can the plasma PD-1 levels predict the presence and efficiency of tumor-infiltrating lymphocytes in patients with metastatic melanoma? Ther. Adv. Med. Oncol. 2019, 11, 1758835919848872. [Google Scholar] [CrossRef] [PubMed]
- Sini, M.C.; Doneddu, V.; Paliogiannis, P.; Casula, M.; Colombino, M.; Manca, A.; Botti, G.; Ascierto, P.A.; Lissia, A.; Cossu, A.; et al. Genetic alterations in main candidate genes during melanoma progression. Oncotarget 2018, 9, 8531–8541. [Google Scholar] [CrossRef] [PubMed]
- Letca, A.F.; Ungureanu, L.; Şenilă, S.C.; Grigore, L.E.; Pop, Ş.; Fechete, O.; Vesa, S.C.; Cosgarea, R. Regression and Sentinel Lymph Node Status in Melanoma Progression. Med. Sci. Monit. 2018, 24, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Azimi, F.; Scolyer, R.A.; Rumcheva, P.; Moncrieff, M.; Murali, R.; McCarthy, S.W.; Saw, R.P.; Thompson, J.F. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J. Clin. Oncol. 2012, 30, 2678–2683. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.E.; Busam, K.J.; From, L.; Kricker, A.; Armstrong, B.K.; Anton-Culver, H.; Gruber, S.B.; Gallagher, R.P.; Zanetti, R.; Rosso, S.; et al. Tumor-infiltrating lymphocyte grade in primary melanomas is independently associated with melanoma-specific survival in the population-based genes, environment and melanoma study. J. Clin. Oncol. 2013, 31, 4252–4259. [Google Scholar] [CrossRef]
- Gata, V.A.; Lisencu, C.I.; Vlad, C.I.; Piciu, D.; Irimie, A.; Achimas-Cadariu, P. Tumor infiltrating lymphocytes as a prognostic factor in malignant melanoma. Review of the literature. J. BUON 2017, 22, 592–598. [Google Scholar]
- Alexandru Gata, V.; Milan Kubelac, P.; Buiga, R.; Vlad, I.C.; Valean, D.; Muntean, M.V.; Morariu, D.S.; Bonci, E.-A.; Irimie, A.; Dina, C.; et al. The value of tumor infiltrating lymphocytes as prognostic factor for lymph node status and survival amongst patients with cutaneous malignant melanoma. J. BUON 2020, 25, 2700–2707. [Google Scholar] [PubMed]
- Lee, N.; Zakka, L.R.; Mihm, M.C., Jr.; Schatton, T. Tumour-infiltrating lymphocytes in melanoma prognosis and cancer immunotherapy. Pathology 2016, 48, 177–187. [Google Scholar] [CrossRef]
- deLeeuw, R.J.; Kost, S.E.; Kakal, J.A.; Nelson, B.H. The prognostic value of FoxP3+ tumor-infiltrating lymphocytes in cancer: A critical review of the literature. Clin. Cancer Res. 2012, 18, 3022–3029. [Google Scholar] [CrossRef]
- Reissfelder, C.; Stamova, S.; Gossmann, C.; Braun, M.; Bonertz, A.; Walliczek, U.; Grimm, M.; Rahbari, N.N.; Koch, M.; Saadati, M.; et al. Tumor-specific cytotoxic T lymphocyte activity determines colorectal cancer patient prognosis. J. Clin. Investig. 2015, 125, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Schalper, K.A.; Brown, J.; Carvajal-Hausdorf, D.; McLaughlin, J.; Velcheti, V.; Syrigos, K.N.; Herbst, R.S.; Rimm, D.L. Objective measurement and clinical significance of TILs in non-small cell lung cancer. J. Natl. Cancer Inst. 2015, 107, dju435. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Mikami, N.; Wing, J.B.; Tanaka, A.; Ichiyama, K.; Ohkura, N. Regulatory T Cells and Human Disease. Annu. Rev. Immunol. 2020, 38, 541–566. [Google Scholar] [CrossRef] [PubMed]
- Newton, R.; Priyadharshini, B.; Turka, L.A. Immunometabolism of regulatory T cells. Nat. Immunol. 2016, 17, 618–625. [Google Scholar] [CrossRef] [PubMed]
- van der Veeken, J.; Gonzalez, A.J.; Cho, H.; Arvey, A.; Hemmers, S.; Leslie, C.S.; Rudensky, A.Y. Memory of Inflammation in Regulatory T Cells. Cell 2016, 166, 977–990. [Google Scholar] [CrossRef]
- Gershon, R.K.; Kondo, K. Cell interactions in the induction of tolerance: The role of thymic lymphocytes. Immunology 1970, 18, 723–737. [Google Scholar] [PubMed]
- Gershon, R.K.; Cohen, P.; Hencin, R.; Liebhaber, S.A. Suppressor T cells. J. Immunol. 1972, 108, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Möller, G. Do suppressor T cells exist? Scand. J. Immunol. 1988, 27, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Wing, K.; Miyara, M. Regulatory T cells—A brief history and perspective. Eur. J. Immunol. 2007, 37 (Suppl. 1), S116–S123. [Google Scholar] [CrossRef]
- Attias, M.; Al-Aubodah, T.; Piccirillo, C.A. Mechanisms of human FoxP3(+) T(reg) cell development and function in health and disease. Clin. Exp. Immunol. 2019, 197, 36–51. [Google Scholar] [CrossRef]
- Leslie, C.; Bowyer, S.E.; White, A.; Grieu-Iacopetta, F.; Trevenen, M.; Iacopetta, B.; Amanuel, B.; Millward, M. FOXP3+ T regulatory lymphocytes in primary melanoma are associated with BRAF mutation but not with response to BRAF inhibitor. Pathology 2015, 47, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Chen, N.; Ge, C.; Li, R.; Li, Z.; Zeng, B.; Li, C.; Wang, Y.; Xue, Y.; Song, X.; et al. Prognostic value of tumor-infiltrating lymphocytes in melanoma: A systematic review and meta-analysis. Oncoimmunology 2019, 8, 1593806. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Anaka, M.; Deb, S.; Freyer, C.; Ebert, L.M.; Chueh, A.C.; Al-Obaidi, S.; Behren, A.; Jayachandran, A.; Cebon, J.; et al. FOXP3 over-expression inhibits melanoma tumorigenesis via effects on proliferation and apoptosis. Oncotarget 2014, 5, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Quaglino, P.; Osella-Abate, S.; Marenco, F.; Nardò, T.; Gado, C.; Novelli, M.; Savoia, P.; Bernengo, M.G. FoxP3 expression on melanoma cells is related to early visceral spreading in melanoma patients treated by electrochemotherapy. Pigment Cell Melanoma Res. 2011, 24, 734–736. [Google Scholar] [CrossRef] [PubMed]
- Knol, A.C.; Nguyen, J.M.; Quéreux, G.; Brocard, A.; Khammari, A.; Dréno, B. Prognostic value of tumor-infiltrating Foxp3+ T-cell subpopulations in metastatic melanoma. Exp. Dermatol. 2011, 20, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Balatoni, T.; Mohos, A.; Papp, E.; Sebestyén, T.; Liszkay, G.; Oláh, J.; Varga, A.; Lengyel, Z.; Emri, G.; Gaudi, I.; et al. Tumor-infiltrating immune cells as potential biomarkers predicting response to treatment and survival in patients with metastatic melanoma receiving ipilimumab therapy. Cancer Immunol. Immunother. 2018, 67, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Shang, B.; Liu, Y.; Jiang, S.J. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: A systematic review and meta-analysis. Sci. Rep. 2015, 5, 15179. [Google Scholar] [CrossRef] [PubMed]
- Gerber, A.L.; Münst, A.; Schlapbach, C.; Shafighi, M.; Kiermeir, D.; Hüsler, R.; Hunger, R. High expression of FOXP3 in primary melanoma is associated with tumour progression. Br. J. Dermatol. 2014, 170, 103–109. [Google Scholar] [CrossRef]
- Deng, G.; Song, X.; Fujimoto, S.; Piccirillo, C.A.; Nagai, Y.; Greene, M.I. Foxp3 Post-translational Modifications and Treg Suppressive Activity. Front. Immunol. 2019, 10, 2486. [Google Scholar] [CrossRef]
- Hall, B.M.; Verma, N.D.; Tran, G.T.; Hodgkinson, S.J. Distinct regulatory CD4+ T cell subsets; differences between naïve and antigen specific T regulatory cells. Curr. Opin. Immunol. 2011, 23, 641–647. [Google Scholar] [CrossRef]
- Feuerer, M.; Hill, J.A.; Mathis, D.; Benoist, C. Foxp3+ regulatory T cells: Differentiation, specification, subphenotypes. Nat. Immunol. 2009, 10, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Josse, J.; Tanioka, M.; Miyachi, Y.; Husson, F.; Ono, M. Regulatory T Cells in Melanoma Revisited by a Computational Clustering of FOXP3+ T Cell Subpopulations. J. Immunol. 2016, 196, 2885–2892. [Google Scholar] [CrossRef]
- Leonard, J.D.; Gilmore, D.C.; Dileepan, T.; Nawrocka, W.I.; Chao, J.L.; Schoenbach, M.H.; Jenkins, M.K.; Adams, E.J.; Savage, P.A. Identification of Natural Regulatory T Cell Epitopes Reveals Convergence on a Dominant Autoantigen. Immunity 2017, 47, 107–117.e8. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Simons, D.L.; Lu, X.; Tu, T.Y.; Solomon, S.; Wang, R.; Rosario, A.; Avalos, C.; Schmolze, D.; Yim, J.; et al. Connecting blood and intratumoral T(reg) cell activity in predicting future relapse in breast cancer. Nat. Immunol. 2019, 20, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- d’Hennezel, E.; Bin Dhuban, K.; Torgerson, T.; Piccirillo, C.A. The immunogenetics of immune dysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J. Med. Genet. 2012, 49, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Barbi, J.; Pan, F. The regulation of immune tolerance by FOXP3. Nat. Rev. Immunol. 2017, 17, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A. IL-2: The first effective immunotherapy for human cancer. J. Immunol. 2014, 192, 5451–5458. [Google Scholar] [CrossRef] [PubMed]
- de Wolf, C.; van de Bovenkamp, M.; Hoefnagel, M. Regulatory perspective on in vitro potency assays for human T cells used in antitumor immunotherapy. Cytotherapy 2018, 20, 601–622. [Google Scholar] [CrossRef]
- Yu, J.; Ren, X.; Cao, S.; Zhang, W.; Hao, X. Th1 polarization and apoptosis-inducing activity of CD4+ T -cells in cytokine-induced killers might favor the antitumor cytotoxicity of cytokine-induced killers in vivo. Cancer Biother. Radiopharm. 2006, 21, 276–284. [Google Scholar] [CrossRef]
- Yu, P.; Fu, Y.X. Tumor-infiltrating T lymphocytes: Friends or foes? Lab. Investig. 2006, 86, 231–245. [Google Scholar] [CrossRef]
- Oh, S.; Perera, L.P.; Terabe, M.; Ni, L.; Waldmann, T.A.; Berzofsky, J.A. IL-15 as a mediator of CD4+ help for CD8+ T cell longevity and avoidance of TRAIL-mediated apoptosis. Proc. Natl. Acad. Sci. USA 2008, 105, 5201–5206. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Jiang, C.; Li, C.; Liu, L.; Li, K.; Jian, Z.; Gao, T. Foxp3 expression in melanoma cells as a possible mechanism of resistance to immune destruction. Cancer Immunol. Immunother. 2011, 60, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Jiang, C.L.; Yan, W.; Zhang, Y.H.; Yang, J.T.; Zhang, C.; Yan, B.; Zhang, W.; Han, W.; Wang, J.Z.; et al. FOXP3 expression and clinical characteristics of hepatocellular carcinoma. World J. Gastroenterol. 2010, 16, 5502–5509. [Google Scholar] [CrossRef] [PubMed]
- Winerdal, M.E.; Marits, P.; Winerdal, M.; Hasan, M.; Rosenblatt, R.; Tolf, A.; Selling, K.; Sherif, A.; Winqvist, O. FOXP3 and survival in urinary bladder cancer. BJU Int. 2011, 108, 1672–1678. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.L.; Morari, E.C.; Nonogaki, S.; Soares, F.A.; Vassallo, J.; Ward, L.S. Foxp3 expression is associated with aggressiveness in differentiated thyroid carcinomas. Clinics 2012, 67, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Chung, N.G.; Yoo, N.J.; Lee, S.H. No mutation in the FOXP3 gene in acute leukemias. Leuk. Res. 2011, 35, e10. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Yao, Y.; Jie, W.; Zhang, M.; Hu, X.; Zhao, Y.; Wang, S.; Yin, J.; Song, Y. Up-regulation of Foxp3 participates in progression of cervical cancer. Cancer Immunol. Immunother. 2013, 62, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Arakawa, A.; Kitoh, A.; Miyara, M.; Kato, M.; Kore-Eda, S.; Sakaguchi, S.; Miyachi, Y.; Tanioka, M.; Ono, M. Perturbations of both non-regulatory and regulatory FOXP3+ T cells in patients with malignant melanoma. Br. J. Dermatol. 2011, 164, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.; Crow, J.; Kahmke, R.; Fisher, S.R.; Su, Z.; Lee, W.T. FoxP3 and indoleamine 2,3-dioxygenase immunoreactivity in sentinel nodes from melanoma patients. Am. J. Otolaryngol. 2014, 35, 689–694. [Google Scholar] [CrossRef]
- Chłopik, A.; Selim, M.A.; Peng, Y.; Wu, C.L.; Tell-Marti, G.; Paral, K.M.; Shalin, S.C.; Kraft, S.; Hsu, C.-K.; Shea, C.R.; et al. Prognostic role of tumoral PDL1 expression and peritumoral FoxP3+ lymphocytes in vulvar melanomas. Hum. Pathol. 2018, 73, 176–183. [Google Scholar] [CrossRef]
- Gershenwald, J.E.; Scolyer, R.A. Melanoma Staging: American Joint Committee on Cancer (AJCC) 8th Edition and Beyond. Ann. Surg. Oncol. 2018, 25, 2105–2110. [Google Scholar] [CrossRef] [PubMed]
- Michielin, O.; van Akkooi, A.C.J.; Ascierto, P.A.; Dummer, R.; Keilholz, U. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1884–1901. [Google Scholar] [CrossRef] [PubMed]
- Varghese, F.; Bukhari, A.B.; Malhotra, R.; De, A. IHC Profiler: An open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS ONE 2014, 9, e96801. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | Number (%) (n = 79) |
|---|---|
| Clark scale | 2: 3/79 (3.8) 3: 25/79 (31.65) 4: 44/79 (55.7) 5: 7/79 (8.86) |
| Ulceration (Yes vs. No) | 54/78 (69.23) |
| Perineural invasion (Yes vs. No) | 4/78 (5.13) |
| Angiolymphatic invasion (Yes vs. No) | 8/78 (10.26) |
| Regression (Yes vs. No) | 24/78 (30.77) |
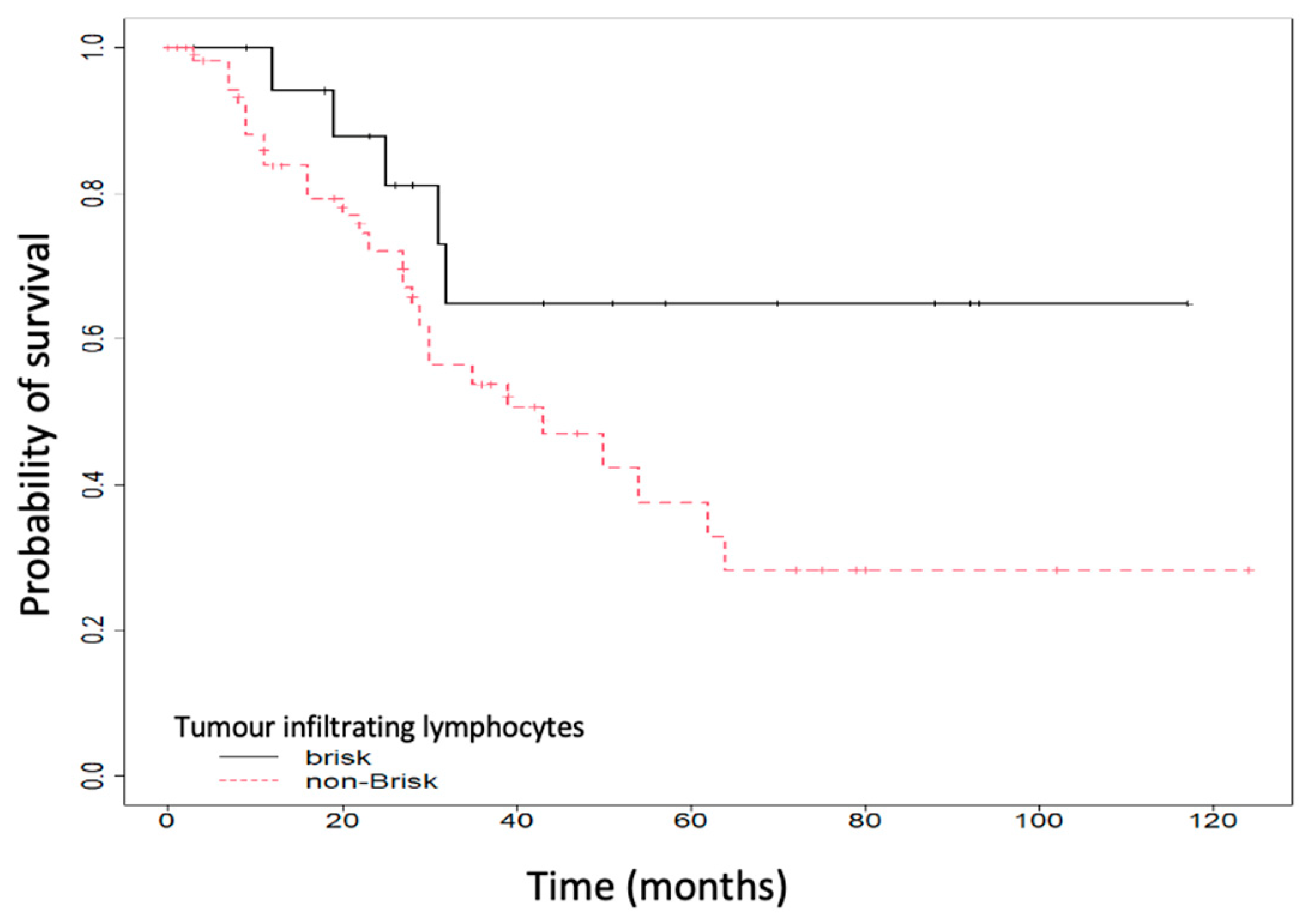
| TILs a (brisk vs. non-Brisk) | 20/79 (25.32) |
| Microsatellitosis (Yes vs. No) | 13/78 (16.67) |
| pT b (3a vs. 3b) | 27/79 (34.18) |
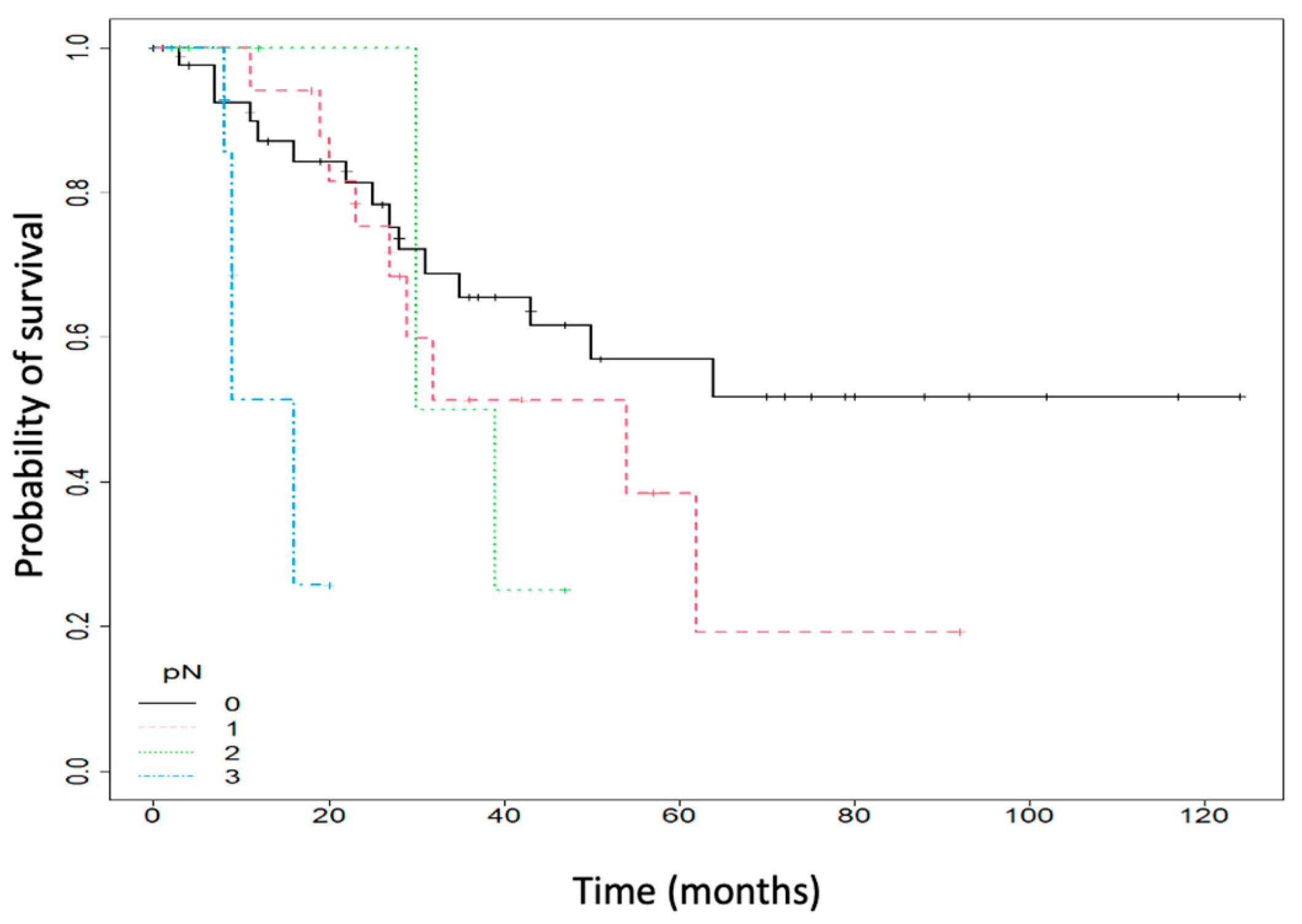
| pN c | 0: 46/79 (58.23) 1: 18/79 (22.78) 2: 8/79 (10.13) 3: 7/79 (8.86) |
| FOXP3 d expression (Negativ vs. Pozitiv) | 15/79 (18.99) |
| Tumor relapse (Yes vs. No) | 29/79 (36.71) |
| Death (Yes vs. No) | 31/79 (39.24) |
| FOXP3 a Expression | Negative (n = 15) | Positive (n = 64) | p-Value |
|---|---|---|---|
| Histological type, number (%) | Acral: 0 (0) Nodular melanoma: 1 (6.67) Superficial spreading melanoma: 14 (93.33) | Acral: 1 (1.56) Nodular melanoma: 14 (21.87) Superficial spreading melanoma: 49 (76.56) | 0.707 |
| Ulceration (Yes), number (%) | 12 (80) | 42 (66.67) | 0.37 |
| Perineural invasion (Yes), number (%) | 1 (6.67) | 3 (4.76) | 1 |
| Angiolymphatic invasion (Yes), number (%) | 0 (0) | 8 (12.7) | 0.342 |
| Regression (Yes), number (%) | 5 (33.33) | 19 (30.16) | 1 |
| TILs b (brisk), number (%) | 6 (40) | 14 (21.88) | 0.189 |
| Microsatellitosis (Yes), number | 2 (13.33) | 11 (17.46) | 1 |
| Melanoma site, number (%) | Head and neck: 3 (20) Arms and legs: 8 (53.33) Thorax and abdomen: 4 (26.67) | Head and neck: 14 (21.88) Arms and legs: 19 (29.69) Thorax and abdomen: 31 (48.44) | 0.191 |
| Tumor relapse (Yes), number (%) | 1 (6.67) | 28 (43.75) | 0.007 |
| Death (Yes), number (%) | 1 (6.67) | 30 (46.88) | 0.004 |
| pN c, nr (%) | 0: 14 (93.33) 1: 1 (6.67) 2: 0 (0) 3: 0 (0) | 0: 32 (50) 1: 17 (26.56) 2: 8 (12.5) 3: 7 (10.94) | 0.03 |
| HR a Unadjusted | (95% CI) | p-Value | HR Adjusted | (95% CI) | p-Value | |
|---|---|---|---|---|---|---|
| Breslow index (mm) | 0.96 | (0.58–1.59) | 0.87 | 0.96 | (0.57–1.62) | 0.887 |
| Ulcerative (Yes vs. No) | 0.52 | (0.26–1.06) | 0.073 | 0.72 | (0.34–1.55) | 0.407 |
| TILS b (brisk vs. non-Brisk) | 0.42 | (0.16–1.11) | 0.08 | 0.72 | (0.26–2.04) | 0.542 |
| pN c | 1.81 | (1.22–2.68) | 0.003 | 1.38 | (0.92–2.07) | 0.119 |
| FOXP3 d score (Positive vs. Negative) | 17.34 | (2.34–128.6) | 0.005 | 12.77 | (1.67–97.77) | 0.014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gâta, V.A.; Pașca, A.; Roman, A.; Muntean, M.V.; Morariu, D.Ș.; Bonci, E.A.; Dina, C.; Ungureanu, L. The Expression of Forkhead Box P3 T Regulatory Lymphocytes as a Prognostic Factor in Malignant Melanomas. Int. J. Mol. Sci. 2024, 25, 6377. https://doi.org/10.3390/ijms25126377
Gâta VA, Pașca A, Roman A, Muntean MV, Morariu DȘ, Bonci EA, Dina C, Ungureanu L. The Expression of Forkhead Box P3 T Regulatory Lymphocytes as a Prognostic Factor in Malignant Melanomas. International Journal of Molecular Sciences. 2024; 25(12):6377. https://doi.org/10.3390/ijms25126377
Chicago/Turabian StyleGâta, Vlad Alexandru, Andrei Pașca, Andrei Roman, Maximilian Vlad Muntean, Dragoș Ștefan Morariu, Eduard Alexandru Bonci, Constantin Dina, and Loredana Ungureanu. 2024. "The Expression of Forkhead Box P3 T Regulatory Lymphocytes as a Prognostic Factor in Malignant Melanomas" International Journal of Molecular Sciences 25, no. 12: 6377. https://doi.org/10.3390/ijms25126377
APA StyleGâta, V. A., Pașca, A., Roman, A., Muntean, M. V., Morariu, D. Ș., Bonci, E. A., Dina, C., & Ungureanu, L. (2024). The Expression of Forkhead Box P3 T Regulatory Lymphocytes as a Prognostic Factor in Malignant Melanomas. International Journal of Molecular Sciences, 25(12), 6377. https://doi.org/10.3390/ijms25126377






