CircRNA Regulation of T Cells in Cancer: Unraveling Potential Targets
Abstract
:1. Introduction
2. T Cells in Cancer
2.1. Changes in the Function and Status of T Cells in TME
2.2. Immunotherapy Targeting T Cells
3. Structure and Function of circRNAs
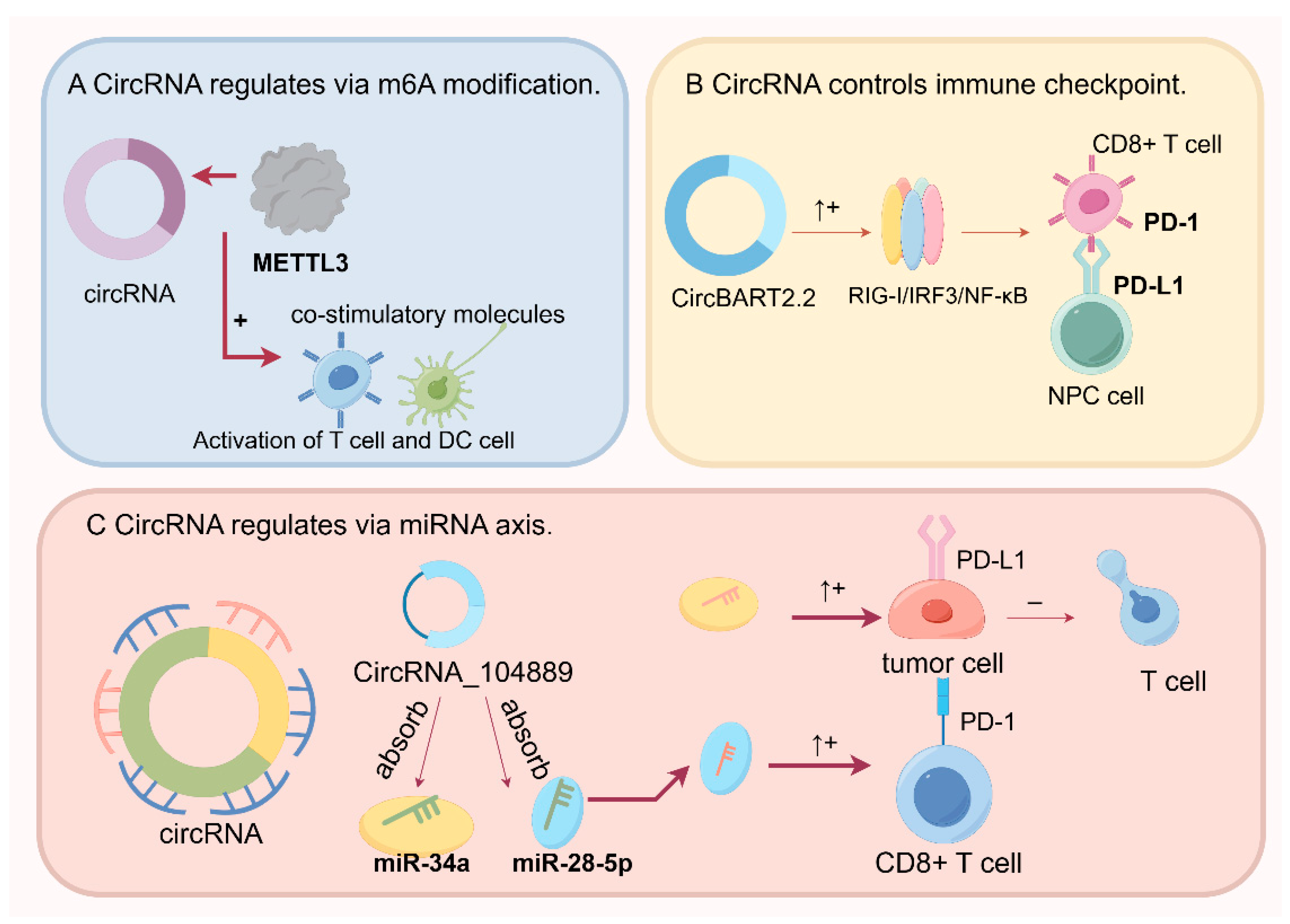
4. CircRNA Regulation of T Cells in Cancer
4.1. CircRNA Regulation by N6-Methyladenosine (m6A) Modification in Cancer
4.2. Regulation of the miRNA Axis by CircRNAs in Cancer Progression
4.3. CircRNAs Regulate Immune Checkpoints
4.4. CircRNAs Trigger the Tumor Immune Response
5. Potential Clinical Applications
5.1. Tumor Immunotherapy Targets
5.2. Tumor Diagnostic and Prognostic Markers
5.3. Modulation of Tumor Metastasis and Invasion
6. Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iglesias-Escudero, M.; Arias-Gonzalez, N.; Martinez-Caceres, E. Regulatory cells and the effect of cancer immunotherapy. Mol. Cancer 2023, 22, 26. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Zheng, X.; Niu, M.; Zhu, S.; Ge, H.; Wu, K. Combination strategies with PD-1/PD-L1 blockade: Current advances and future directions. Mol. Cancer 2022, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Latchman, Y.; Wood, C.R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I.; Iwai, Y.; Long, A.J.; Brown, J.A.; Nunes, R.; et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001, 2, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Chen, Y.; Li, X.; Long, S.; Shi, Y.; Yu, Y.; Wu, W.; Han, L.; Wang, S. The role of PD-1/PD-L1 and application of immune-checkpoint inhibitors in human cancers. Front. Immunol. 2022, 13, 964442. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kao, K.C.; Vilbois, S.; Tsai, C.H.; Ho, P.C. Metabolic communication in the tumour-immune microenvironment. Nat. Cell Biol. 2022, 24, 1574–1583. [Google Scholar] [CrossRef] [PubMed]
- Thommen, D.S.; Schumacher, T.N. T Cell Dysfunction in Cancer. Cancer Cell 2018, 33, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Chow, A.; Perica, K.; Klebanoff, C.A.; Wolchok, J.D. Clinical implications of T cell exhaustion for cancer immunotherapy. Nat. Rev. Clin. Oncol. 2022, 19, 775–790. [Google Scholar] [CrossRef] [PubMed]
- Kersten, K.; Hu, K.H.; Combes, A.J.; Samad, B.; Harwin, T.; Ray, A.; Rao, A.A.; Cai, E.; Marchuk, K.; Artichoker, J.; et al. Spatiotemporal co-dependency between macrophages and exhausted CD8+ T cells in cancer. Cancer Cell 2022, 40, 624–638.e9. [Google Scholar] [CrossRef]
- Franco, F.; Jaccard, A.; Romero, P.; Yu, Y.-R.; Ho, P.-C. Metabolic and epigenetic regulation of T-cell exhaustion. Nat. Metab. 2020, 2, 1001–1012. [Google Scholar] [CrossRef]
- Taniuchi, I. CD4 Helper and CD8 Cytotoxic T Cell Differentiation. Annu. Rev. Immunol. 2018, 36, 579–601. [Google Scholar] [CrossRef] [PubMed]
- Ando, M.; Ito, M.; Srirat, T.; Kondo, T.; Yoshimura, A. Memory T cell, exhaustion, and tumor immunity. Immunol. Med. 2019, 43, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, L.S.; Jakobsen, T.; Hager, H.; Kjems, J. The emerging roles of circRNAs in cancer and oncology. Nat. Rev. Clin. Oncol. 2021, 19, 188–206. [Google Scholar] [CrossRef] [PubMed]
- Amaya, L.; Grigoryan, L.; Li, Z.; Lee, A.; Wender, P.A.; Pulendran, B.; Chang, H.Y. Circular RNA vaccine induces potent T cell responses. Proc. Natl. Acad. Sci. USA 2023, 120, e2302191120. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Pan, S.; Chen, X.; Wang, Z.W.; Zhu, X. The role of lncRNAs and circRNAs in the PD-1/PD-L1 pathway in cancer immunotherapy. Mol. Cancer 2021, 20, 116. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, P.; Fan, L.; Wu, M. The Potential Role of circRNA in Tumor Immunity Regulation and Immunotherapy. Front. Immunol. 2018, 9, 9. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer–immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zheng, N.; Li, J.; Li, C.; Zheng, D.; Jiang, X.; Ge, X.; Liu, M.; Liu, L.; Song, Z.; et al. N6-methyladenosine-modified circular RNA QSOX1 promotes colorectal cancer resistance to anti-CTLA-4 therapy through induction of intratumoral regulatory T cells. Drug Resist. Updates 2022, 65, 100886. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Z.; Liang, J.; Liu, J.; Hao, J.; Wan, Q.; Liu, J.; Luo, C.; Lu, Z. CircRNA has_circ_0069313 induced OSCC immunity escape by miR-325-3p-Foxp3 axes in both OSCC cells and Treg cells. Aging 2022, 14, 4376–4389. [Google Scholar] [CrossRef]
- Crespo, J.; Sun, H.; Welling, T.H.; Tian, Z.; Zou, W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr. Opin. Immunol. 2013, 25, 214–221. [Google Scholar] [CrossRef]
- Zhao, B.; Li, H.; Xia, Y.; Wang, Y.; Wang, Y.; Shi, Y.; Xing, H.; Qu, T.; Wang, Y.; Ma, W. Immune checkpoint of B7-H3 in cancer: From immunology to clinical immunotherapy. J. Hematol. Oncol. 2022, 15, 153. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.-T.; Jin, W.-L. B7-H3/CD276: An Emerging Cancer Immunotherapy. Front. Immunol. 2021, 12, 701006. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gong, R.; Zhao, C.; Lei, K.; Sun, X.; Ren, H. Human FOXP3 and tumour microenvironment. Immunology 2022, 168, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wu, S.; Mou, Z.; Zhou, Q.; Dai, X.; Ou, Y.; Chen, X.; Chen, Y.; Xu, C.; Hu, Y.; et al. Exosome-derived circTRPS1 promotes malignant phenotype and CD8+ T cell exhaustion in bladder cancer microenvironments. Mol. Ther. 2022, 30, 1054–1070. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Chen, G.; Zhao, Y.; Gao, H.; Li, L.; Yin, Y.; Jiang, J.; Wang, L.; Mang, Y.; Gao, Y.; et al. Exosome-derived circCCAR1 promotes CD8 + T-cell dysfunction and anti-PD1 resistance in hepatocellular carcinoma. Mol. Cancer 2023, 22, 55. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.W.; Zhu, S.Q.; Pei, X.; Qiu, B.Q.; Xiong, D.; Long, X.; Lin, K.; Lu, F.; Xu, J.J.; Wu, Y.B. Cancer cell-derived exosomal circUSP7 induces CD8(+) T cell dysfunction and anti-PD1 resistance by regulating the miR-934/SHP2 axis in NSCLC. Mol. Cancer 2021, 20, 144. [Google Scholar] [CrossRef] [PubMed]
- Madden, M.Z.; Rathmell, J.C. The Complex Integration of T-cell Metabolism and Immunotherapy. Cancer Discov. 2021, 11, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Couzin-Frankel, J. Cancer Immunotherapy. Science 2013, 342, 1432–1433. [Google Scholar] [CrossRef] [PubMed]
- June, C.H.; Blazar, B.R.; Riley, J.L. Engineering lymphocyte subsets: Tools, trials and tribulations. Nat. Rev. Immunol. 2009, 9, 704–716. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef]
- Zhu, Q.; Man, S.M.; Gurung, P.; Liu, Z.; Vogel, P.; Lamkanfi, M.; Kanneganti, T.-D. Cutting Edge: STING Mediates Protection against Colorectal Tumorigenesis by Governing the Magnitude of Intestinal Inflammation. J. Immunol. 2014, 193, 4779–4782. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T cell immunotherapy for human cancer. Science 2018, 359, 1361–1365. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, A.P.; Stadtmauer, E.A.; Binder-Scholl, G.K.; Goloubeva, O.; Vogl, D.T.; Lacey, S.F.; Badros, A.Z.; Garfall, A.; Weiss, B.; Finklestein, J.; et al. NY-ESO-1–specific TCR–engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat. Med. 2015, 21, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Eyquem, J.; Mansilla-Soto, J.; Giavridis, T.; van der Stegen, S.J.; Hamieh, M.; Cunanan, K.M.; Odak, A.; Gonen, M.; Sadelain, M. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 2017, 543, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.R.; Rodriguez, A.; Shepphird, J.; Brown, C.E.; Badie, B. Chimeric Antigen Receptors T Cell Therapy in Solid Tumor: Challenges and Clinical Applications. Front. Immunol. 2017, 8, 1850. [Google Scholar] [CrossRef] [PubMed]
- Baulu, E.; Gardet, C.; Chuvin, N.; Depil, S. TCR-engineered T cell therapy in solid tumors: State of the art and perspectives. Sci. Adv. 2023, 9, eadf3700. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Chen, X.; Liang, C.; Ling, Y.; Yang, X.; Ye, X.; Zhang, H.; Yang, P.; Cui, X.; Ren, Y.; et al. A Noncoding Regulatory RNAs Network Driven by Circ-CDYL Acts Specifically in the Early Stages Hepatocellular Carcinoma. Hepatology 2020, 71, 130–147. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, S.; Liang, Q.; Liu, Y.; Liu, L.A.-O. Unraveling the multifaceted role of EpCAM in colorectal cancer: An integrated review of its function and interplay with non-coding RNAs. Med. Oncol. 2023, 41, 35. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, T.; She, Y.; Wu, K.; Gu, S.; Li, L.; Dong, C.; Chen, C.; Zhou, Y.A.-O. N6-methyladenosine-modified circIGF2BP3 inhibits CD8+ T-cell responses to facilitate tumor immune evasion by promoting the deubiquitination of PD-L1 in non-small cell lung cancer. Mol. Cancer 2021, 20, 105. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. Immune Checkpoint Targeting in Cancer Therapy: Toward Combination Strategies with Curative Potential. Cell 2015, 161, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, C.; Bao, C.; Chen, L.; Lin, M.; Wang, X.; Zhong, G.; Yu, B.; Hu, W.; Dai, L.; et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat. Struct. Mol. Biol. 2015, 22, 256–264. [Google Scholar] [CrossRef]
- Anastasiadou, E.; Jacob, L.S.; Slack, F.J. Non-coding RNA networks in cancer. Nat. Rev. Cancer 2018, 18, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Han, Y.; Meng, J.; Zhong, B.; Chen, J.; Zhang, H.; Qin, J.; Pang, J.; Liu, L. Small extrachromosomal circular DNA (eccDNA): Major functions in evolution and cancer. Mol. Cancer 2021, 20, 113. [Google Scholar] [CrossRef]
- Yang, L.; Jia, R.; Ge, T.; Ge, S.; Zhuang, A.; Chai, P.; Fan, X. Extrachromosomal circular DNA: Biogenesis, structure, functions and diseases. Signal Transduct. Target. Ther. 2022, 7, 342. [Google Scholar] [CrossRef]
- Patop, I.L.; Wust, S.; Kadener, S. Past, present, and future of circRNAs. EMBO J. 2019, 38, e100836. [Google Scholar] [CrossRef] [PubMed]
- Tay, Y.; Rinn, J.; Pandolfi, P.P. The multilayered complexity of ceRNA crosstalk and competition. Nature 2014, 505, 344–352. [Google Scholar] [CrossRef]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA circles function as efficient microRNA sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef]
- Hansen, T.B.; Kjems, J.; Damgaard, C.K. Circular RNA and miR-7 in cancer. Cancer Res. 2013, 73, 5609–5612. [Google Scholar] [CrossRef]
- Zheng, X.B.; Zhang, M.; Xu, M.Q. Detection and characterization of ciRS-7: A potential promoter of the development of cancer. Neoplasma 2017, 64, 321–328. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Wang, Y.; Ren, F.; Sun, D.; Yan, Y.; Kong, X.; Bu, J.; Liu, M.; Xu, S. circRNA-002178 act as a ceRNA to promote PDL1/PD1 expression in lung adenocarcinoma. Cell Death Dis. 2020, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-L. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat. Rev. Mol. Cell Biol. 2020, 21, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Li, B.; Xia, Y.; Xuan, Z.; Li, Z.; Xie, L.; Gu, C.; Lv, J.; Lu, C.; Jiang, T.; et al. CircTHBS1 drives gastric cancer progression by increasing INHBA mRNA expression and stability in a ceRNA- and RBP-dependent manner. Cell Death Dis. 2022, 13, 266. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Xu, D.; Xiong, Y.; Yue, J.; Ihira, K.; Konno, Y.; Watari, H. The Expression, Functions and Mechanisms of Circular RNAs in Gynecological Cancers. Cancers 2020, 12, 1472. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xia, C.; Tang, H.; Jiang, Y.; Wang, S.; Zhang, X.; Huang, T.; Yuan, X.; Wang, J.; Peng, L. Circular RNAs Involve in Immunity of Digestive Cancers from Bench to Bedside: A Review. Front. Immunol. 2022, 13, 833058. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Shao, X.; Liu, H.; Liu, Q.; Lu, J.; Li, W. The circRNA_102911/miR-129-5p/SOX6 axis is involved with T lymphocyte immune function in elderly patients with laparoscopic left hepatectomy for hepatolithiasis. Exp. Ther. Med. 2021, 21, 150. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fan, X.; Mao, M.; Song, X.; Wu, P.; Zhang, Y.; Jin, Y.; Yang, Y.; Chen, L.L.; Wang, Y.; et al. Extensive translation of circular RNAs driven by N(6)-methyladenosine. Cell Res. 2017, 27, 626–641. [Google Scholar] [CrossRef]
- Müller, S.; Appel, B. In vitro circularization of RNA. RNA Biol. 2017, 14, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Long, Z.; Yang, T.; Wang, S.; Zhong, W.; Hu, F.; Teoh, J.Y.; Lu, J.; Mao, X. M6A-modified circRBM33 promotes prostate cancer progression via PDHA1-mediated mitochondrial respiration regulation and presents a potential target for ARSI therapy. Int. J. Biol. Sci. 2023, 19, 1543–1563. [Google Scholar] [CrossRef]
- Zhang, L.; Hou, C.; Chen, C.; Guo, Y.; Yuan, W.; Yin, D.; Liu, J.; Sun, Z. The role of N(6)-methyladenosine (m(6)A) modification in the regulation of circRNAs. Mol. Cancer 2020, 19, 105. [Google Scholar] [CrossRef]
- Lou, X.; Wang, J.J.; Wei, Y.Q.; Sun, J.J. Emerging role of RNA modification N6-methyladenosine in immune evasion. Cell Death Dis. 2021, 12, 300. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Zhu, Y.; Li, J.; Zeng, J.; Wu, L. ALKBH5-mediated m6A modification of circCCDC134 facilitates cervical cancer metastasis by enhancing HIF1A transcription. J. Exp. Clin. Cancer Res. 2022, 41, 261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Hou, J.; Mei, C.; Wang, X.; Wang, Y.; Wang, K. Effect of circular RNAs and N6-methyladenosine (m6A) modification on cancer biology. Biomed. Pharmacother. 2023, 159, 114260. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, X.; Huang, M.; Liu, J.; Gu, Y.; Ma, L.; Zhou, Q.; Cao, X. Mettl3-mediated mRNA m(6)A methylation promotes dendritic cell activation. Nat. Commun. 2019, 10, 1898. [Google Scholar] [CrossRef] [PubMed]
- Vesely, M.D.; Kershaw, M.H.; Schreiber, R.D.; Smyth, M.J. Natural Innate and Adaptive Immunity to Cancer. Annu. Rev. Immunol. 2011, 29, 235–271. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Chen, Y.G. Circular RNAs in Immune Response and Viral Infection. Trends Biochem. Sci. 2020, 45, 1022–1034. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, H.Y.; Dai, X.Y.; Zhang, X.; Huang, Y.Z.; Shi, L.; Wei, J.F.; Ding, Q. CircMETTL3, upregulated in a m6A-dependent manner, promotes breast cancer progression. Int. J. Biol. Sci. 2021, 17, 1178–1190. [Google Scholar] [CrossRef] [PubMed]
- Seimiya, T.; Otsuka, M.; Iwata, T.; Shibata, C.; Tanaka, E.; Suzuki, T.; Koike, K. Emerging Roles of Exosomal Circular RNAs in Cancer. Front. Cell Dev. Biol. 2020, 8, 568366. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Y.; Guo, S.; Wei, N.; Xue, R.; Li, W.; Dong, G.; Li, J.; Tian, X.; Chen, C.; Qiu, S.; et al. ciRS-7 Promotes the Proliferation and Migration of Papillary Thyroid Cancer by Negatively Regulating the miR-7/Epidermal Growth Factor Receptor Axis. Biomed. Res. Int. 2020, 2020, 9875636. [Google Scholar] [CrossRef]
- Liu, L.; Liu, F.-B.; Huang, M.; Xie, K.; Xie, Q.-S.; Liu, C.-H.; Shen, M.-J.; Huang, Q. Circular RNA ciRS-7 promotes the proliferation and metastasis of pancreatic cancer by regulating miR-7-mediated EGFR/STAT3 signaling pathway. Hepatobiliary Pancreat. Dis. Int. 2019, 18, 580–586. [Google Scholar] [CrossRef]
- Xiang, P.; Ge, T.; Zhou, J.; Zhang, Y. Protective role of circRNA CCND1 in ulcerative colitis via miR-142-5p/NCOA3 axis. BMC Gastroenterol. 2023, 23, 18. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Jia, L.; Luo, L.; Xu, X.; Xiang, Y.; Ren, Y.; Ren, D.; Shen, L.; Liang, T. Critical Roles of Circular RNA in Tumor Metastasis via Acting as a Sponge of miRNA/isomiR. Int. J. Mol. Sci. 2022, 23, 7024. [Google Scholar] [CrossRef]
- Sadeghi Rad, H.; Monkman, J.; Warkiani, M.E.; Ladwa, R.; O’Byrne, K.; Rezaei, N.; Kulasinghe, A. Understanding the tumor microenvironment for effective immunotherapy. Med. Res. Rev. 2021, 41, 1474–1498. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.J.; Croessmann, S.; Lin, J.; Phyo, Z.H.; Charmsaz, S.; Danilova, L.; Mohan, A.A.; Gross, N.E.; Chen, F.; Dong, J.; et al. Systemic inhibition of PTPN22 augments anticancer immunity. J. Clin. Investig. 2021, 131, e146950. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yao, Y.; Jin, M. Circ-0001068 is a novel biomarker for ovarian cancer and inducer of PD1 expression in T cells. Aging 2020, 12, 19095–19106. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Wang, J.; Xiong, F.; Jiang, X.; Zhu, K.; Wang, Y.; Mo, Y.; Gong, Z.; Zhang, S.; He, Y.; et al. Epstein-Barr Virus-Encoded Circular RNA CircBART2.2 Promotes Immune Escape of Nasopharyngeal Carcinoma by Regulating PD-L1. Cancer Res. 2021, 81, 5074–5088. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, H.; Fu, L.; Xu, T. Investigating the Underlying Mechanisms of Circular RNAs and Their Application in Clinical Research of Cervical Cancer. Front. Genet. 2021, 12, 653051. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Zhu, X.; Ye, S.; Zhang, J.; Liao, J.; Zhang, N.; Zeng, X.; Wang, J.; Yang, B.; Zhang, Y.; et al. Tumour circular RNAs elicit anti-tumour immunity by encoding cryptic peptides. Nature 2024, 625, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Li, W.; Liang, Z.; Wu, X.; Cheng, S.; Peng, J.; Zeng, K.; Li, W.; Lan, P.; Yang, X.; et al. Mutant KRAS-activated circATXN7 fosters tumor immunoescape by sensitizing tumor-specific T cells to activation-induced cell death. Nat. Commun. 2024, 15, 499. [Google Scholar] [CrossRef]
- Xu, Q.; Deng, B.; Li, M.; Chen, Y.; Zhuan, L. circRNA-UBAP2 promotes the proliferation and inhibits apoptosis of ovarian cancer though miR-382-5p/PRPF8 axis. J. Ovarian Res. 2020, 13, 81. [Google Scholar] [CrossRef]
- Lin, C.; Xi, Y.; Yu, H.; Wang, Z.; Chen, X.; Shen, W. circRNA TCFL5 Promote Esophageal Cancer Progression by Modulating M2 Macrophage Polarization via the miR-543-FMNL2 Axis. J. Oncol. 2022, 2022, 5075615. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Li, J.; Luo, M.; Wei, Q. Inhibitory role of circRNA_100395 in the proliferation and metastasis of prostate cancer cells. J. Int. Med. Res. 2021, 49, 0300060521992215. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.P.; Liu, C.G.; Qiu, F.; Xu, Y.Q.; Xing, F.; Yin, J.Q.; Han, S.J.; Yu, H.; Han, Y.; Jing, X.; et al. CircRNA_100395 protects breast carcinoma deterioration by targeting MAPK6. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 12216–12223. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Yang, J.; Pathak, J.L.; Wang, H.; Zha, J.; Wei, Y.; Tang, H.; Ge, L. CircRNA_104889 promotes lung adenocarcinoma cell invasion via sponging miR4458. Cancer Cell Int. 2020, 20, 432. [Google Scholar] [CrossRef]
- Lv, Y.S.; Wang, C.; Li, L.X.; Han, S.; Li, Y. Effects of circRNA_103993 on the proliferation and apoptosis of NSCLC cells through miR-1271/ERG signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 8384–8393. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, G.; Wu, Z.; Dong, Y.; Shi, Y.; Yang, F.; Chen, X.; Wang, J.; Du, S.; Xu, H.; et al. Exosomal circ-PTPN22 and circ-ADAMTS6 mark T cell exhaustion and neutrophil extracellular traps in Asian intrahepatic cholangiocarcinoma. Mol. Ther. Nucleic Acids 2023, 31, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Shao, N.; Song, L.; Sun, X. Exosomal circ_PIP5K1A regulates the progression of non-small cell lung cancer and cisplatin sensitivity by miR-101/ABCC1 axis. Mol. Cell Biochem. 2021, 476, 2253–2267. [Google Scholar] [CrossRef]
- Katopodi, T.; Petanidis, S.; Domvri, K.; Zarogoulidis, P.; Anestakis, D.; Charalampidis, C.; Tsavlis, D.; Bai, C.; Huang, H.; Freitag, L.; et al. Kras-driven intratumoral heterogeneity triggers infiltration of M2 polarized macrophages via the circHIPK3/PTK2 immunosuppressive circuit. Sci. Rep. 2021, 11, 15455. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, Y.; Shen, Q.; Chen, Q.; Zhu, X.J.; Jiang, S.S.; Zhang, Q. CircRNA_MYLK promotes malignant progression of ovarian cancer through regulating microRNA-652. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5281–5291. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, C. Upregulated hsa_circRNA_100269 inhibits the growth and metastasis of gastric cancer through inactivating PI3K/Akt axis. PLoS ONE 2021, 16, e0250603. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, H.; Li, P. Upregulation of hsa_circRNA_102958 Indicates Poor Prognosis and Promotes Ovarian Cancer Progression Through miR-1205/SH2D3A Axis. Cancer Manag. Res. 2020, 12, 4045–4053. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Liu, G.; Chen, X.; Li, Q.; Fang, F.; Shen, X. Hsa_circ_0044301 Regulates Gastric Cancer Cell’s Proliferation, Migration, and Invasion by Modulating the Hsa-miR-188-5p/DAXX Axis and MAPK Pathway. Cancers 2022, 14, 4183. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Yang, D.; Zhang, B.; Zhao, Y.; Zheng, Z.; Zhang, T. Regulatory mechanisms and clinical applications of tumor-driven exosomal circRNAs in cancers. Int. J. Med. Sci. 2023, 20, 818–835. [Google Scholar] [CrossRef] [PubMed]
- Dammes, N.; Peer, D. Paving the Road for RNA Therapeutics. Trends Pharmacol. Sci. 2020, 41, 755–775. [Google Scholar] [CrossRef] [PubMed]
- Loan Young, T.; Chang Wang, K.; James Varley, A.; Li, B. Clinical delivery of circular RNA: Lessons learned from RNA drug development. Adv. Drug Deliv. Rev. 2023, 197, 114826. [Google Scholar] [CrossRef] [PubMed]
- Wortzel, I.; Dror, S.; Kenific, C.M.; Lyden, D. Exosome-Mediated Metastasis: Communication from a Distance. Dev. Cell 2019, 49, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.; Dong, P.Y.; Yang, G.M.; Gurunathan, S. A comprehensive review on the composition, biogenesis, purification, and multifunctional role of exosome as delivery vehicles for cancer therapy. Biomed. Pharmacother. 2023, 165, 115087. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Peng, K.; Yang, K.; Ma, W.; Qi, S.; Yu, X.; He, J.; Lin, X.; Yu, G. Circular RNA cancer vaccines drive immunity in hard-to-treat malignancies. Theranostics 2022, 12, 6422–6436. [Google Scholar] [CrossRef]
- Lv, J.; Li, K.; Yu, H.; Han, J.; Zhuang, J.; Yu, R.; Cheng, Y.; Song, Q.; Bai, K.; Cao, Q.; et al. HNRNPL induced circFAM13B increased bladder cancer immunotherapy sensitivity via inhibiting glycolysis through IGF2BP1/PKM2 pathway. J. Exp. Clin. Cancer Res. 2023, 42, 41. [Google Scholar] [CrossRef]
- Chen, Z.Q.; Zuo, X.L.; Cai, J.; Zhang, Y.; Han, G.Y.; Zhang, L.; Ding, W.Z.; Wu, J.D.; Wang, X.H. Hypoxia-associated circPRDM4 promotes immune escape via HIF-1α regulation of PD-L1 in hepatocellular carcinoma. Exp. Hematol. Oncol. 2023, 12, 17. [Google Scholar] [CrossRef]
- Huang, X.Y.; Zhang, P.F.; Wei, C.Y.; Peng, R.; Lu, J.C.; Gao, C.; Cai, J.B.; Yang, X.; Fan, J.; Ke, A.W.; et al. Circular RNA circMET drives immunosuppression and anti-PD1 therapy resistance in hepatocellular carcinoma via the miR-30-5p/snail/DPP4 axis. Mol. Cancer 2020, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Su, W.; Zhong, C.; Yang, T.; Chen, W.; Chen, G.; Liu, Z.; Wu, K.; Zhong, W.; Li, B.; et al. An Eight-CircRNA Assessment Model for Predicting Biochemical Recurrence in Prostate Cancer. Front. Cell Dev. Biol. 2020, 8, 599494. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Z.; Liu, P.; Ai, S.; Sun, F.; Hu, Q.; Dong, Y.; Xia, X.; Guan, W.; Liu, S. Novel CircRNAs in Hub ceRNA Axis Regulate Gastric Cancer Prognosis and Microenvironment. Front. Med. 2021, 8, 771206. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Gao, Q.; Chen, Y.; Zhang, Z.; Du, Y.; Liu, Y.; Zhang, G.; Li, S.; Wang, G.; Chen, X.; et al. Identification of CircRNA signature associated with tumor immune infiltration to predict therapeutic efficacy of immunotherapy. Nat. Commun. 2023, 14, 2540. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Lu, Q.; Yu, H.; Zhang, X.M. The Circular RNA circFGFR4 Facilitates Resistance to Anti-PD-1 of Triple-Negative Breast Cancer by Targeting the miR-185-5p/CXCR4 Axis. Cancer Manag. Res. 2023, 15, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Muftuoglu, C.; Mert, U.; Akagunduz, O.O.; Tavlayan, E.; Al-Omar, A.; Asadi, M.; Caner, A. Profiling of circRNA expressions in radiation-treated head and neck cancer cells and the potential role of circPVT1. Arch. Oral Biol. 2023, 150, 105690. [Google Scholar] [CrossRef] [PubMed]
- Buratin, A.; Borin, C.; Tretti Parenzan, C.; Dal Molin, A.; Orsi, S.; Binatti, A.; Simon, K.; Paganin, M.; Serafin, V.; Gaffo, E.; et al. CircFBXW7 in patients with T-cell ALL: Depletion sustains MYC and NOTCH activation and leukemia cell viability. Exp. Hematol. Oncol. 2023, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Wang, Z.; Jiang, J.; Tong, C.; Wu, L. A novel molecular mechanism mediated by circCCDC134 regulates non-small cell lung cancer progression. Thorac. Cancer 2023, 14, 1958–1968. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, P.; Zhang, G. Identification of circRNA-miRNA-Immune-Related mRNA Regulatory Network in Gastric Cancer. Front. Oncol. 2022, 12, 816884. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, Z.; Wang, Y.; Yang, Y.; Fan, R.; Gao, K.; Zhang, H.; Xie, Z.; Jiang, W. Immune Microenvironment Change and Involvement of Circular RNAs in TIL Cells of Recurrent Nasopharyngeal Carcinoma. Front. Cell Dev. Biol. 2021, 9, 722224. [Google Scholar] [CrossRef]
- Ma, C.; Qin, J.; Zhang, J.; Wang, X.; Wu, D.; Li, X. Construction and analysis of circular RNA molecular regulatory networks in clear cell renal cell carcinoma. Mol. Med. Rep. 2020, 21, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Cai, W.H.; Wang, M.F.; Deng, Y.J.; Huang, L.L.; Cao, Y.J. Microarray Profile of Circular RNAs Identifies hsa_circ_0001583 as A New Circular RNA Biomarker for Breast Cancer: A Retrospective Study. Cell J. 2022, 24, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, X.; Xu, J.; Liao, Y.; Deng, M.; Wang, X.; Li, J. Differential expression and bioinformatics analysis of exosome circRNAs in pancreatic ductal adenocarcinoma. Transl. Oncol. 2023, 33, 101686. [Google Scholar] [CrossRef] [PubMed]
- Mei, M.; Wang, Y.; Song, W.; Li, Z.; Wang, Q.; Li, J.; Zhang, M. CircADARB1 serves as a new biomarker in natural killer T-cell lymphoma and a potential regulator of p-Stat3. Cancer Cell Int. 2021, 21, 594. [Google Scholar] [CrossRef] [PubMed]
- Lux, S.; Blätte, T.J.; Gillissen, B.; Richter, A.; Cocciardi, S.; Skambraks, S.; Schwarz, K.; Schrezenmeier, H.; Döhner, H.; Döhner, K.; et al. Deregulated expression of circular RNAs in acute myeloid leukemia. Blood Adv. 2021, 5, 1490–1503. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Yu, X.H.; Luo, S.S.; Han, H. Comprehensive circular RNA profiling reveals that circular RNA100783 is involved in chronic CD28-associated CD8(+)T cell ageing. Immun. Ageing 2015, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lu, Y. Circular RNA: Biosynthesis in vitro. Front. Bioeng. Biotechnol. 2021, 9, 787881. [Google Scholar] [CrossRef] [PubMed]
- Karami Fath, M.; Akhavan Masouleh, R.; Afifi, N.; Loghmani, S.; Tamimi, P.; Fazeli, A.; Mousavian, S.A.; Falsafi, M.M.; Barati, G. PI3K/AKT/mTOR signaling pathway modulation by circular RNAs in breast cancer progression. Pathol. Res. Pract. 2023, 241, 154279. [Google Scholar] [CrossRef]
- Xue, C.; Li, G.; Zheng, Q.; Gu, X.; Bao, Z.; Lu, J.; Li, L. The functional roles of the circRNA/Wnt axis in cancer. Mol. Cancer 2022, 21, 108. [Google Scholar] [CrossRef]



| Therapy | Description | Current Limitations | References |
|---|---|---|---|
| Immune checkpoint inhibitors | Blockade of immune checkpoint receptors on T cells, such as PD-1, B7-H3, and CTLA-4, releases the brakes on antitumor T cell activity | Response only in a subset of patients; acquired resistance after the initial response | [30] |
| STING agonists | Activation of the STING pathway enhances dendritic cell maturation and function, leading to increased T cell-mediated antitumor immunity | No phase III trial data are available yet; mechanisms linking STING signaling and cancer immunity have not been fully elucidated | [31] |
| Tumor RNA vaccines | Tumor antigen-encoding mRNA or self-amplifying RNA directly translates antigens to stimulate antitumor T cell responses | High tumor heterogeneity challenges universal antigen selection; technically immature, currently in early clinical testing | [32] |
| CAR-T cell therapy | T cells engineered to express chimeric antigen receptors recognize and kill tumor cells expressing target antigens | Severe cytokine release syndrome; defining optimal antigens for safety and efficacy challenging | [33] |
| TCR-T cell therapy | T cells engineered to express tumor antigen-specific TCRs have enhanced recognition and cytotoxicity against cancers | Risk of antigen escape; severe toxicities including cytokine release syndrome and on-target off-tumor effects | [34] |
| CRISPR/Cas9-engineered T cells | CRISPR/Cas9 gene editing to enhance T cell proliferation, trafficking, and antitumor cytotoxicity | Low editing efficiency leads to uncertain efficacy; safety concerns due to potential genomic damage | [35] |
| circRNA | Expression | Location | Cancer | Model | Pathway | Result | Ref. |
|---|---|---|---|---|---|---|---|
| circUSP7 | ↑ | Exosome | NSCLC | HuNSG mice | circUSP7/miR-934/SHP2 | Accelerates CD8+ T cell exhaustion; Promotes resistance to anti-PD1 therapy. | [26] |
| circRNA-UBAP2 | ↑ | Cytoplasm | OC | Tumor cells | circRNA-UBAP2/miR-382-5p/PRPF8 | Regulates cell proliferation and apoptosis of ovarian cancer. | [80] |
| circRNA TCFL5 | ↑ | Cytoplasm | EC | Tumor cells | circRNA TCFL5-FMNL2/miR-543 axis | circRNA TCFL5 encourages cell proliferation, migration, and invasion of KYSE150 and Eca109. | [81] |
| circCCAR1 | ↑ | Exosome | HCC | HuNSG mice | circCCAR1/miR-127-5p/WTAP | Promotes growth and metastasis of tumor cells; promotes resistance to anti-PD1 therapy. | [25] |
| circRNA-002178 | ↑ | Exosome | LUAD | Anti-Human CD279 PE Mice | circRNA-002178/miR-34a/PD-L1/ circRNA-002178/miR-28-5p/PD-1 | Enhances PD-1 and PD-L1 expression. | [51] |
| circRNA_100395 | ↓ | Cytoplasm | PC | Tumor cells | circRNA_100395/miR-1228 | Inhibits cell proliferation; alters cell cycle distribution; reduces cell migration and invasion. | [82] |
| circRNA_100395 | ↓ | Cytoplasm | BC | Tumor cells | circRNA_100395/MAPK6 | Weakens proliferative and migratory abilities. | [83] |
| circRNA_104889 | ↑ | Cytoplasm | LUAD | Tumor cells | circRNA_104889/ERK1/2 circRNA_104889/miR4458/CASP3 | Promotes migration and invasion of lung adenocarcinoma cells. | [84] |
| circRNA_103993 | ↑ | Cytoplasm | NSCLC | Tumor cells | circRNA_103993/miR-1271/ERG | Regulates proliferation and apoptosis of NSCLC cells. | [85] |
| circ_PTPN22 | ↑ | Exosome | ICC | Tumor cells | _ | Promotes expression level of regulatory T cells, M1 macrophages, and immune checkpoint gene. | [86] |
| circ_PIP5K1A | ↑ | exosome | NSCLC | Tumor cells | circ_PIP5K1A/miR-101/ABCC1 | Regulates the progression of non-small cell lung cancer and cisplatin sensitivity. | [87] |
| circBART2.2 | ↑ | exogenous circRNA | NPC | Nude mouse | circBART2.2/RIG-I/IRF3/NF-κB/PD-L1 | Upregulates PD-L1 to inhibit T cell killing of NPC cells and promotes T cell apoptosis. | [76] |
| circRNA-HIPK3, PTK2 | ↑ | Exosome | LC | C57BL/6 Mice | Circhipk3/PTK2/CD163/CD206/Kras | Promotes growth and metastatic potential and survival in lung tumor. | [88] |
| circRNA_MYLK | ↑ | Cytoplasm | OC | Tumor cells | circRNA_MYLK/miRNA-652 | Promotes the malignant progression of OC | [89] |
| hsa_circRNA_100269 | ↓ | Cytoplasm | GC | BALB/Cnude mice | hsa_circRNA_100269/PT3K/Atk | Induces the development of GC cells;suppresses cell cycle arrest and apoptosis in GC cells. | [90] |
| hsa_circRNA_102958 | ↑ | Cytoplasm | OC | Tumorcells | hsa_circRNA_102958/miR-1205/SH2D3A | Promotes ovarian cancer progression. | [91] |
| hsa_circ_0044301 | ↑ | cytoplasm | GC | BALB/c nude mice | hsa_circ_0044301/miRNA-188-5p/DAXX (ERK1/2) | Influences GC progression; regulates the role of the downstream target DAXX. | [92] |
| circRNA | Targeting T-Cell | Cancer | Signaling Pathway | Result | Ref. |
|---|---|---|---|---|---|
| circFAM13B | CD8 T cell | BC | _ | Repress immune evasion and enhance immunotherapy sensitivity. | [99] |
| circMET | CD8 T cell | HCC | miR-30-5p/Snail/dipeptidyl peptidase 4(DPP4)/CXCL10 | Induces HCC development and immune tolerance. | [101] |
| circPRDM4 | CD8 T cell | HCC | Promotes the expression of PD-L1 and the escape of immune cells by CD8+ T cells. | [100] | |
| circ_14736 and circ_17720 | CD8 T cell | PCa | _ | Serves as a disease prognosis marker. | [102] |
| circRNA DYRK1A_017, circRNA FLNA_118 | Regulatory T cell | GC | EMT/NFκβ-TNFα | Serves as the target of immunotherapy. | [103] |
| circTMTC3 and circFAM117B | CD8 T cell | Melanoma | miR-142-5p/PD-L1 | Increases PD-L1 expression and reduces T cell activity leading to immune escape. | [104] |
| circFGFR4 | CD8 T cell | TNBC | miR-1-185p/CXCR5 | Serves as a biomarker for predicting sensitivity to anti-PD-1 immunotherapy and as an immunotherapeutic target. | [105] |
| circPVT1 | _ | HNSC | _ | Improves and understands radiotherapy efficacy in HNCs. | [106] |
| circFBXW7 | _ | T-ALL | _ | Suppresses tumors. | [107] |
| circCCDC134 | _ | NSCLC | miR-625-5p/NFAT5 | Serves as the diagnostic and therapeutic target. | [108] |
| hsa_circ_0003763, hsa_circ_0004928, hsa_circ_0040573 | _ | GC | Rap1 and Ras/MAP2K1 | Regulates the development of GC. | [109] |
| hsa_circ_0000831 … | Treg and CD4+/CD8+ T cell | NPC | _ | Serves as potential biomarkers. | [110] |
| hsa_circ_0031594 … | _ | CCRCC | _ | Serves as potential biomarkers. | [111] |
| hsa_circ_0001583 | _ | BC | _ | Serves as potential biomarkers. | [112] |
| hsa_circ_0000247 | _ | PDAC | _ | Serves as potential biomarkers. | [113] |
| circADARB1 | _ | NKTCL | _ | Serves as a biomarker to assist diagnosis. | [114] |
| circBCL11B | _ | AML | _ | Serves as biomarker and potential novel pathway dependencies | [115] |
| circRNA_100783 | CD28-related CD8(+) T cell | _ | _ | Serves as potential biomarkers. | [116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Yin, S.; Yang, K.; Zhang, B.; Wu, X.; Zhang, M.; Gao, D. CircRNA Regulation of T Cells in Cancer: Unraveling Potential Targets. Int. J. Mol. Sci. 2024, 25, 6383. https://doi.org/10.3390/ijms25126383
Li Z, Yin S, Yang K, Zhang B, Wu X, Zhang M, Gao D. CircRNA Regulation of T Cells in Cancer: Unraveling Potential Targets. International Journal of Molecular Sciences. 2024; 25(12):6383. https://doi.org/10.3390/ijms25126383
Chicago/Turabian StyleLi, Zelin, Shuanshuan Yin, Kangping Yang, Baojie Zhang, Xuanhuang Wu, Meng Zhang, and Dian Gao. 2024. "CircRNA Regulation of T Cells in Cancer: Unraveling Potential Targets" International Journal of Molecular Sciences 25, no. 12: 6383. https://doi.org/10.3390/ijms25126383





