Monitoring and Evaluation of the Corrosion Behavior in Seawater of the Low-Alloy Steels BVDH36 and LRAH36
Abstract
:1. Introduction
2. Results and Discussion
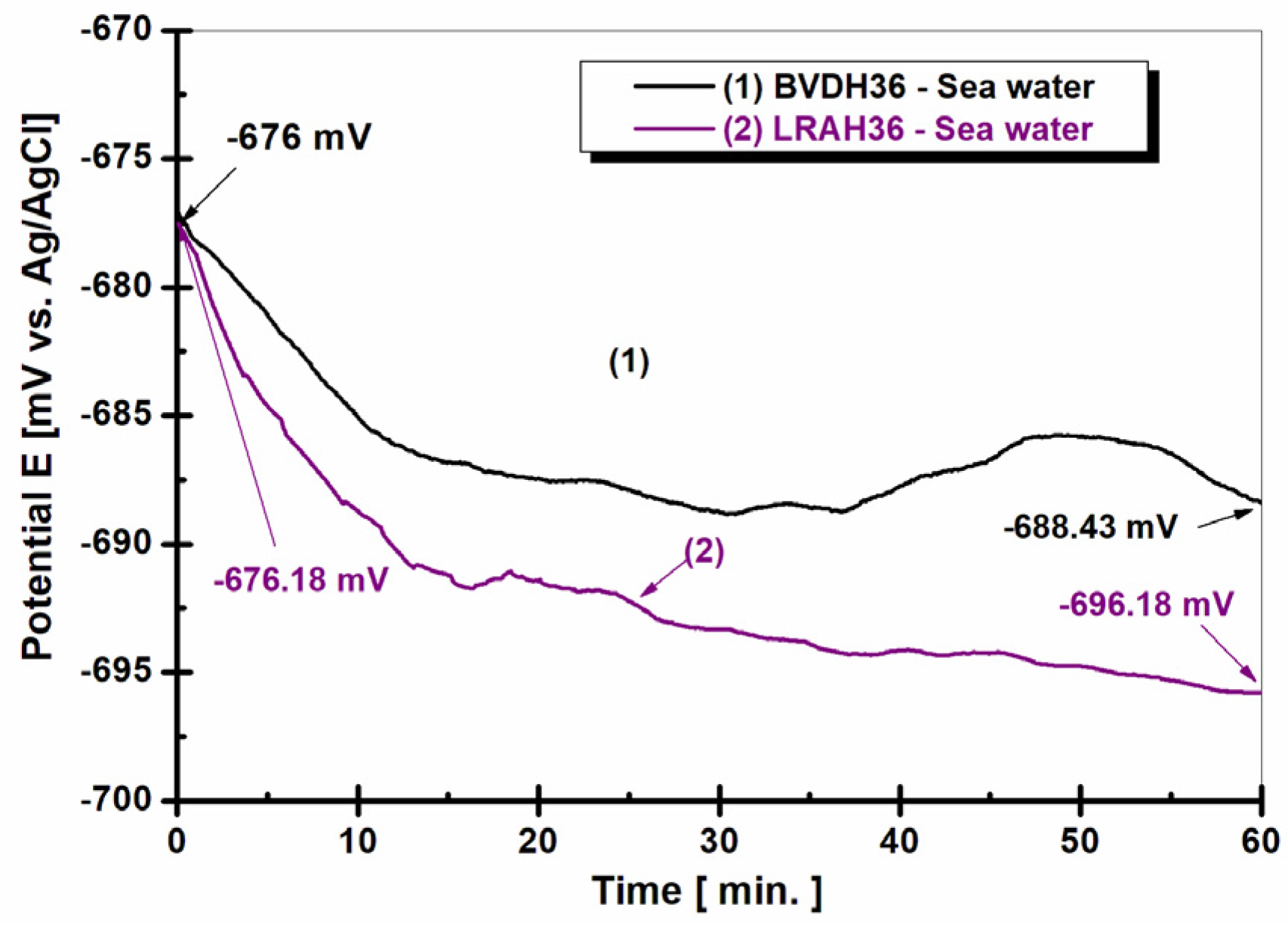
2.1. The Evolution of the Free Potential
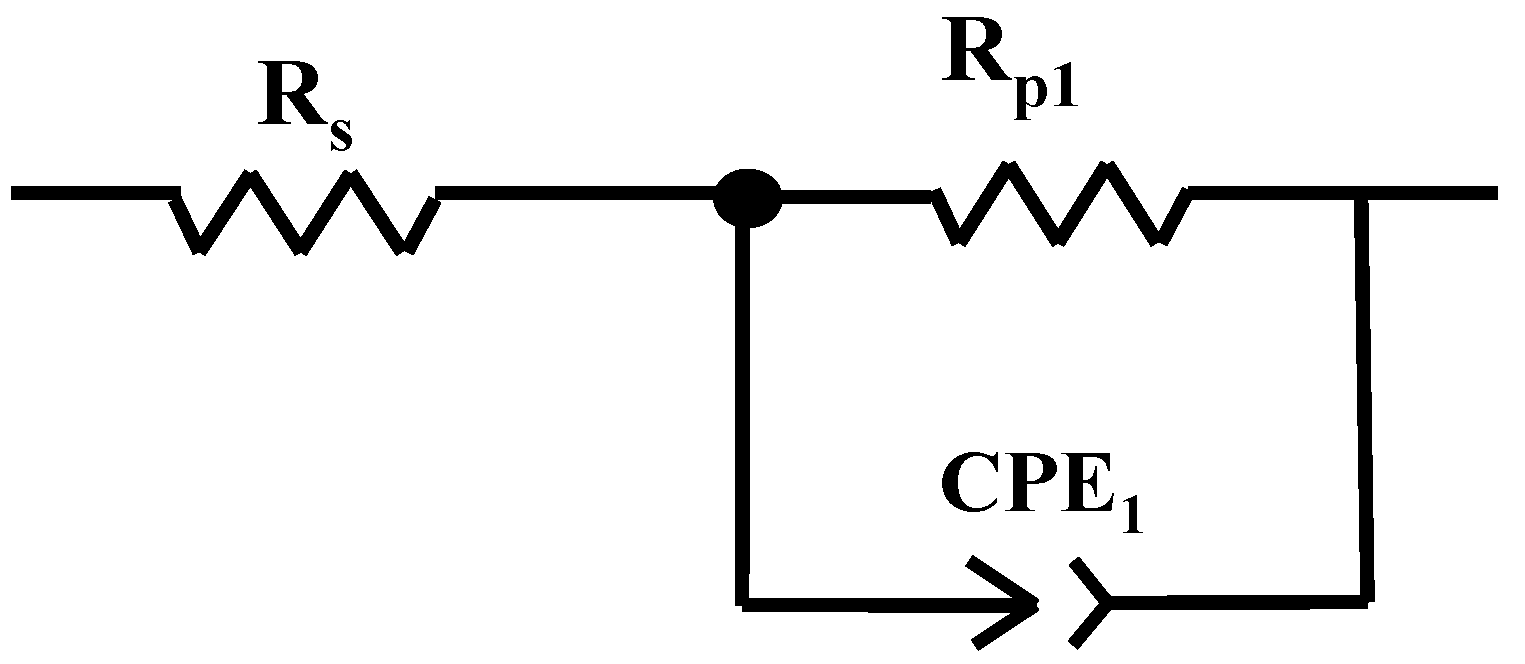
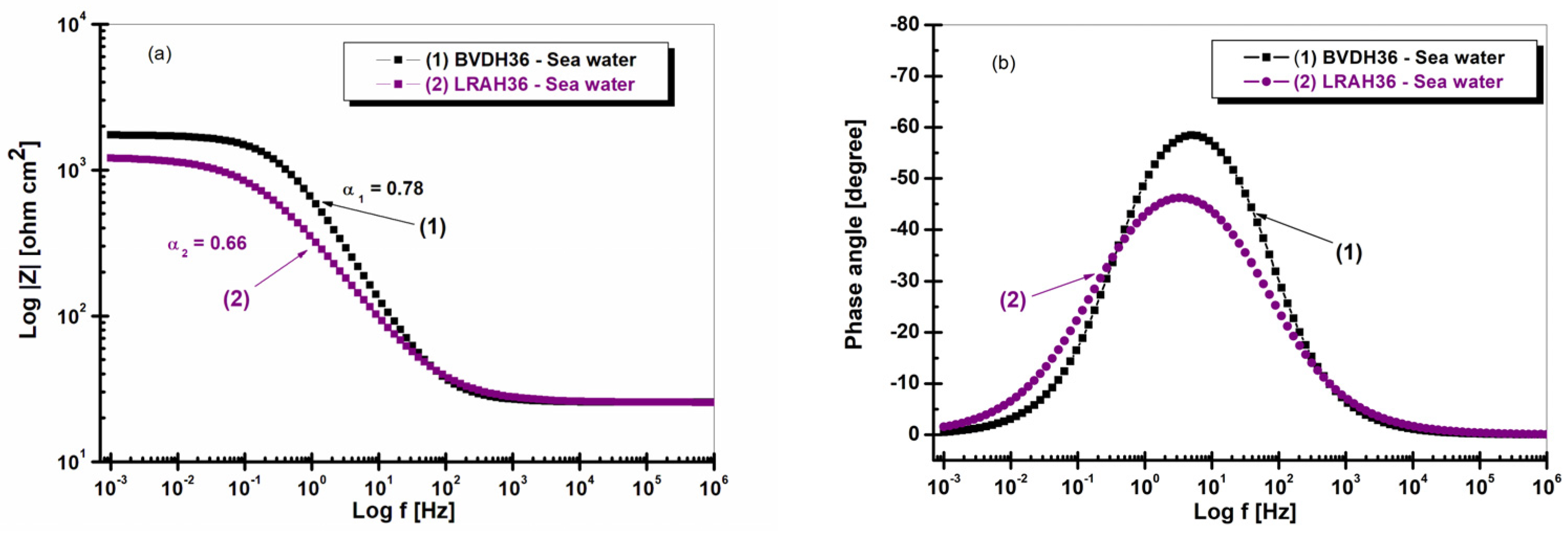
2.2. Electrochemical Impedance Spectroscopy (EIS) after 1 h of Immersion
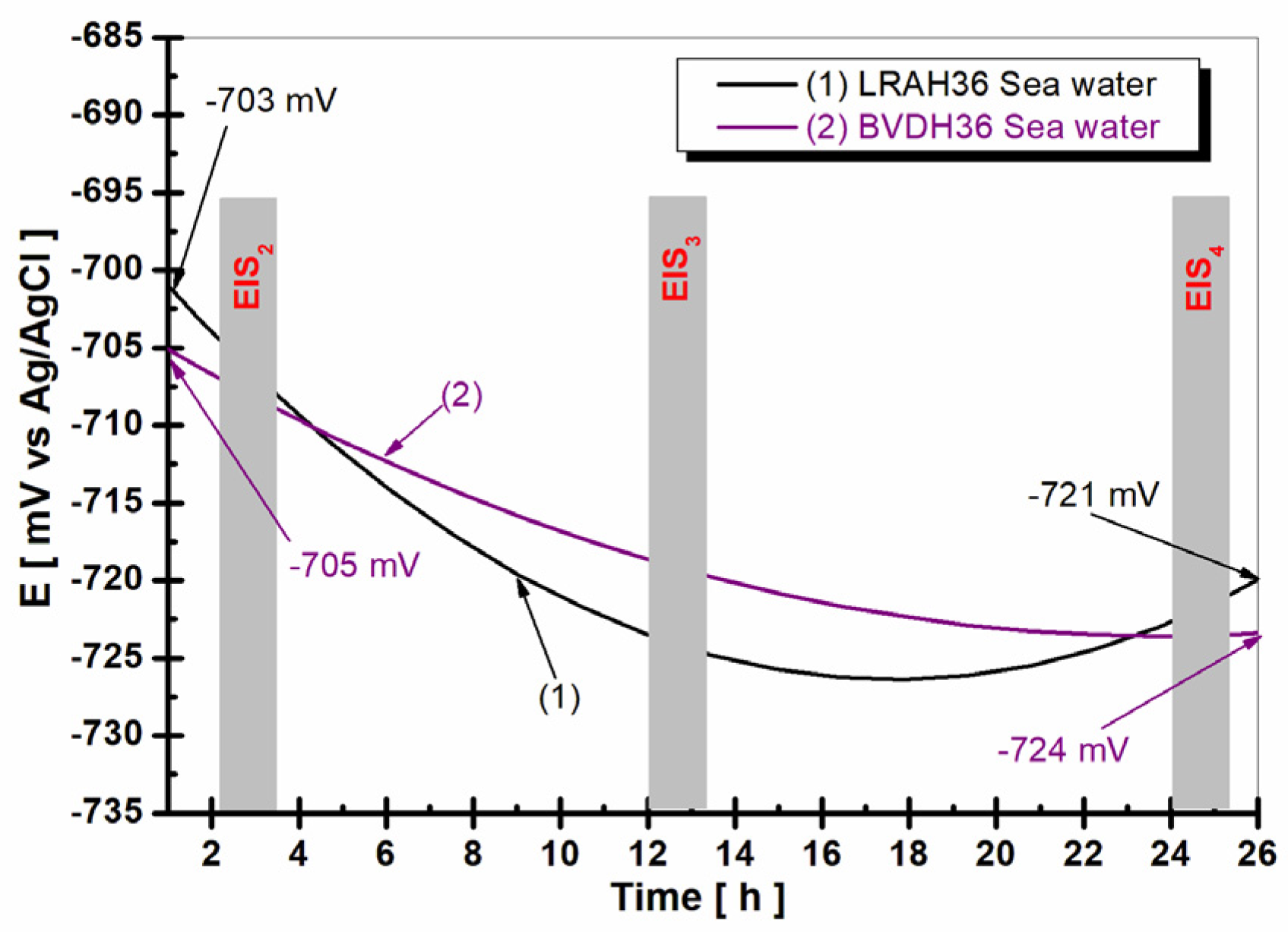
2.3. Evolution of Open Circuit Potential in Seawater for 26 h of LRAH36 Steel and BVDH36 Steel
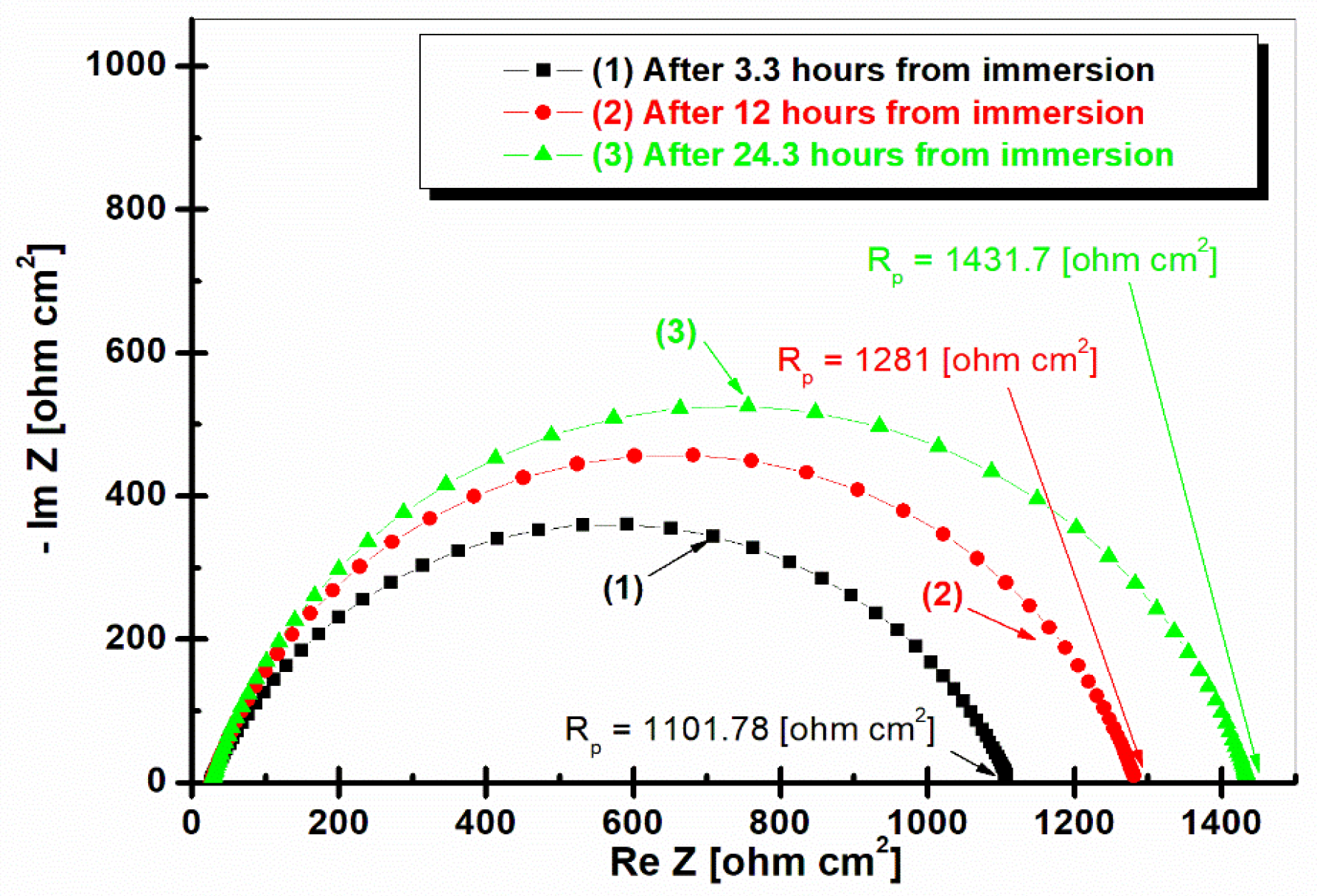
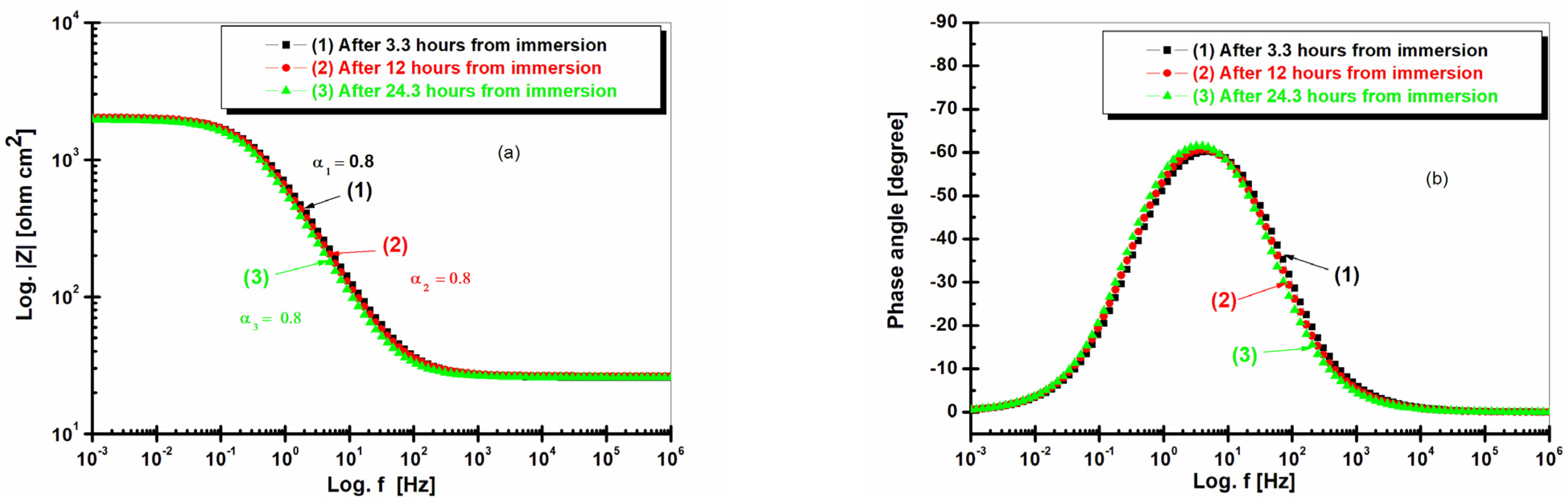
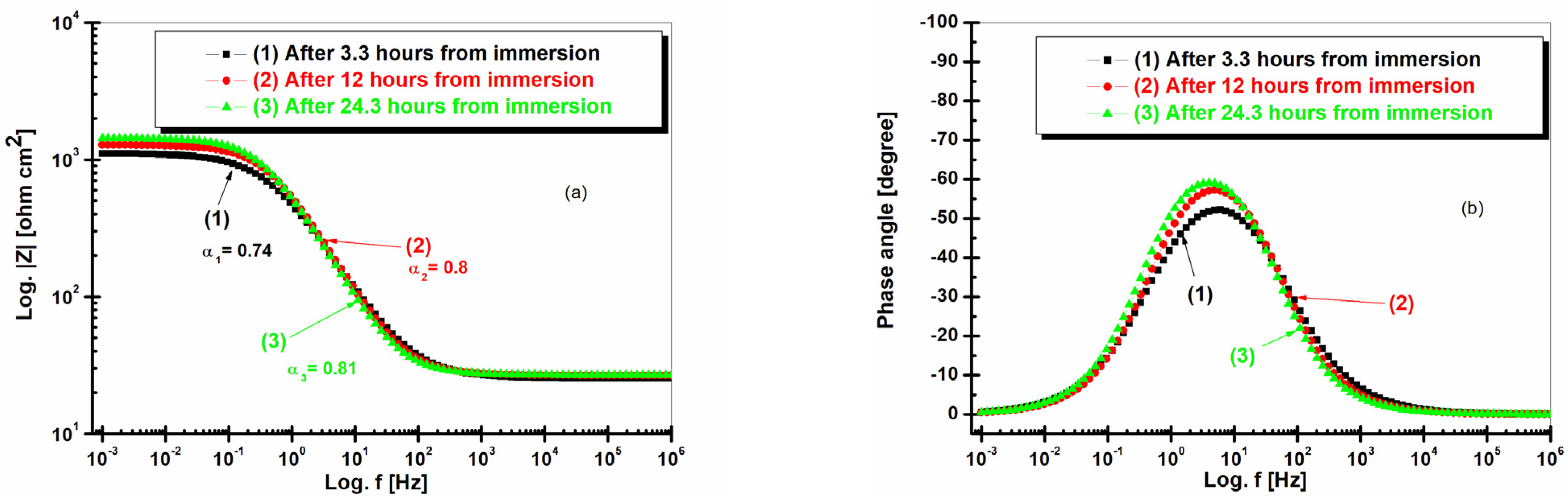
2.4. Electrochemical Impedance Spectroscopy (EIS) after 3.3 h, 12 h, and 24.3 h of Immersion in Seawater of LRAH36 and BVDH36 Steel Samples
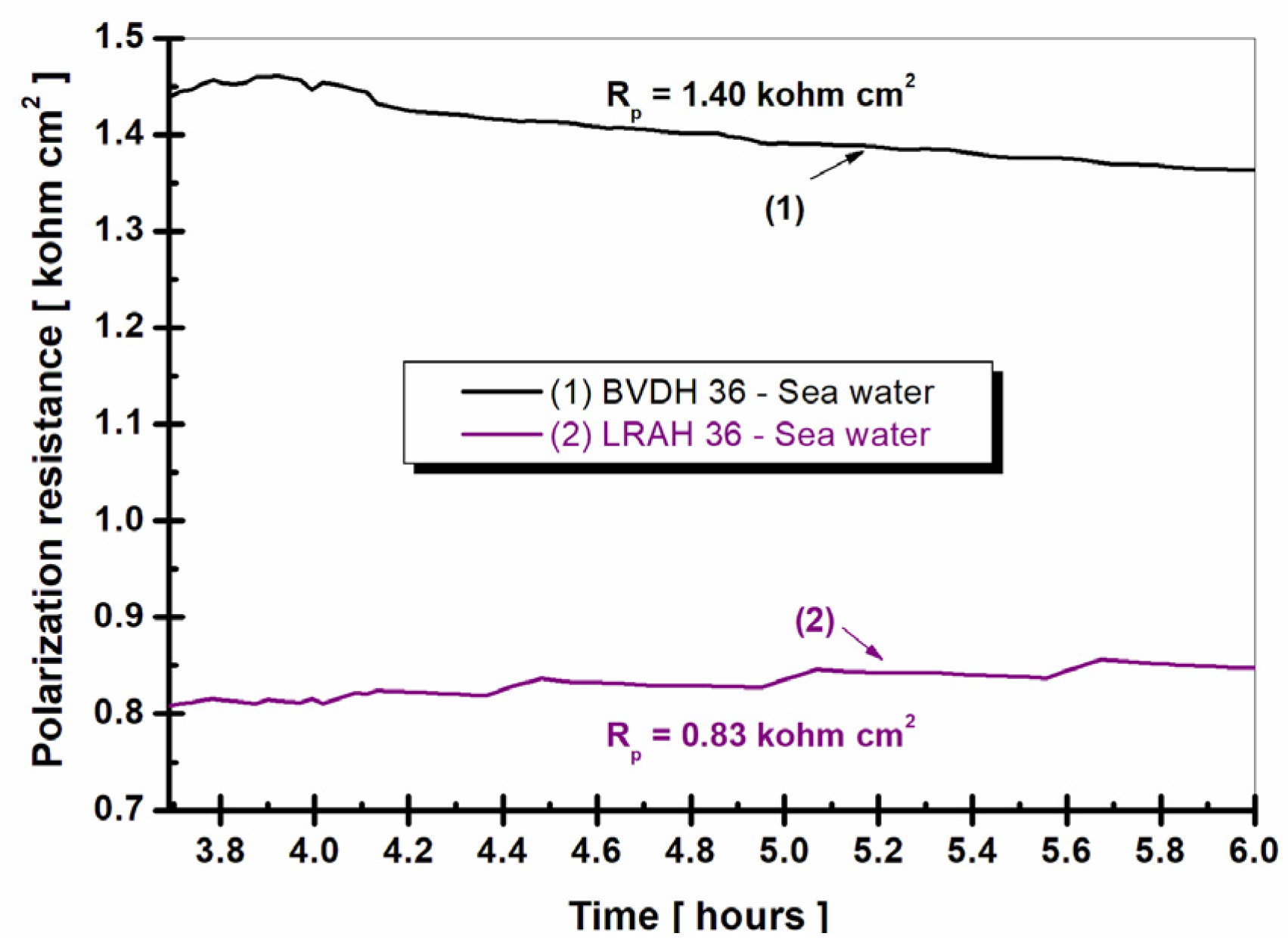
2.5. Polarization Resistance and Corrosion Rate
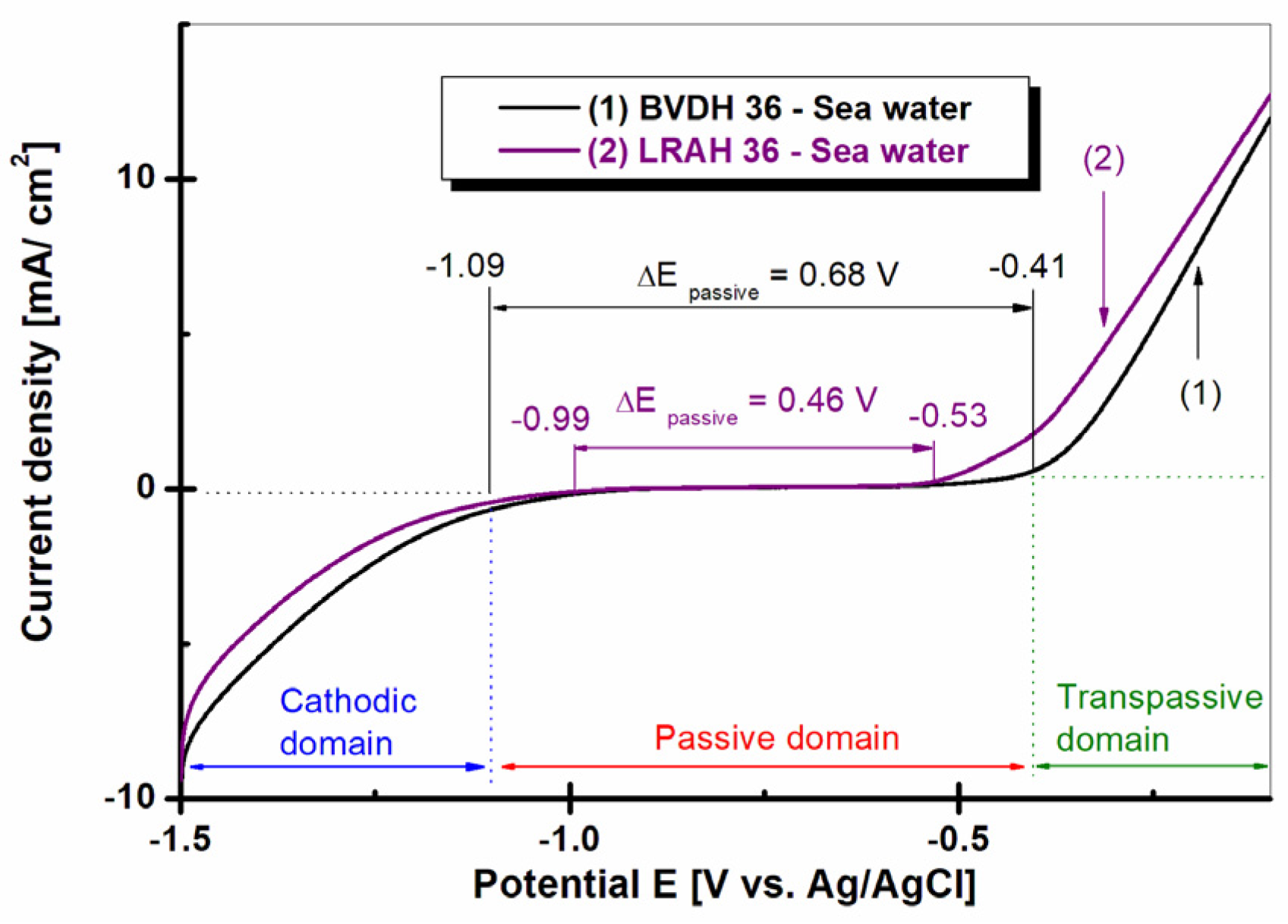
2.6. Potentiodynamic Polarization Plots, Logarithmically Scaled for Current Density, Obtained in Seawater for Steels BVDH36 and LRAH36 (Tafel Representation)
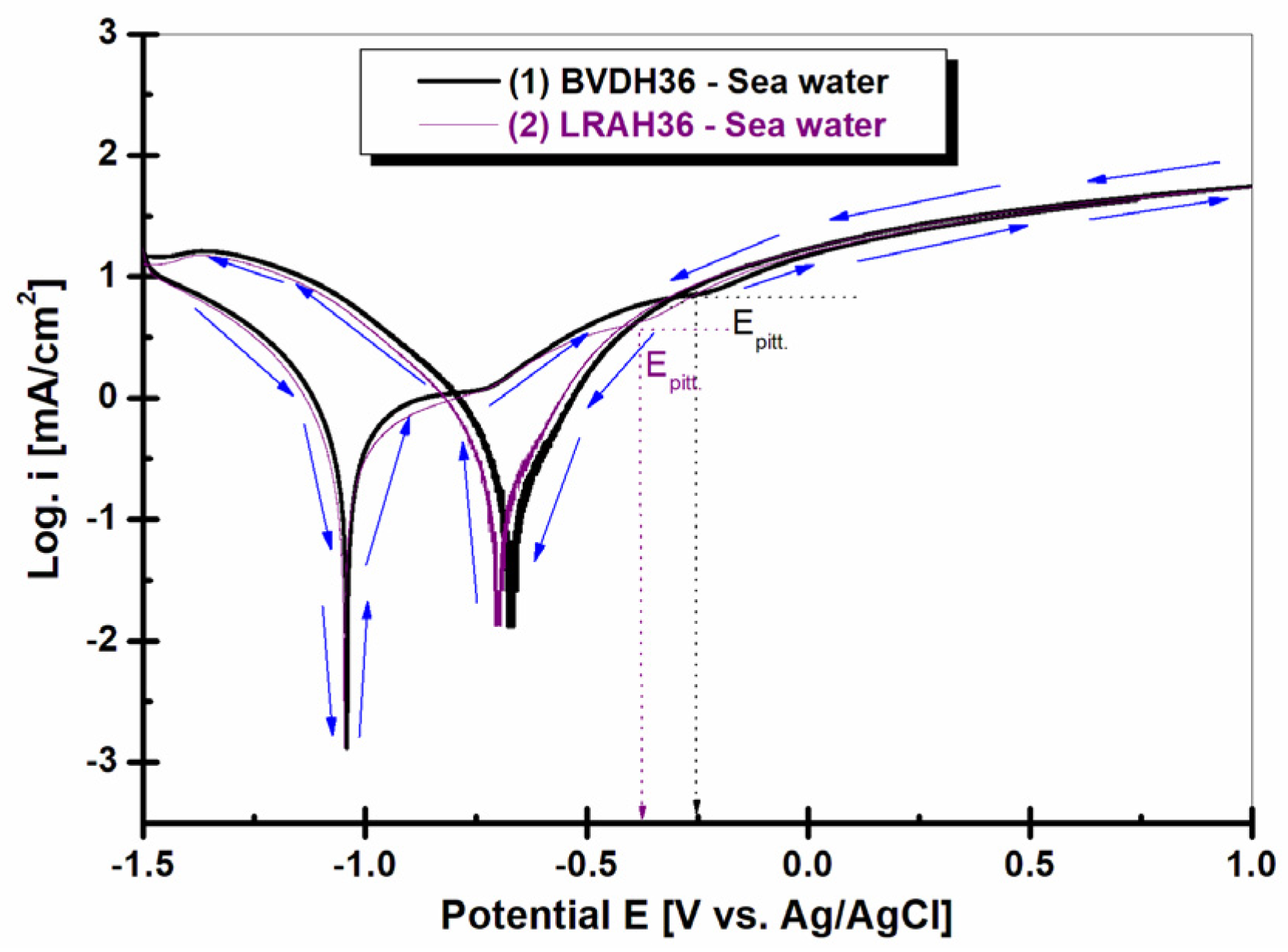
2.7. Cyclic Voltammetry
2.8. Optical Microscopy of BVDH36 and LRAH36 Steels before and after Corrosion
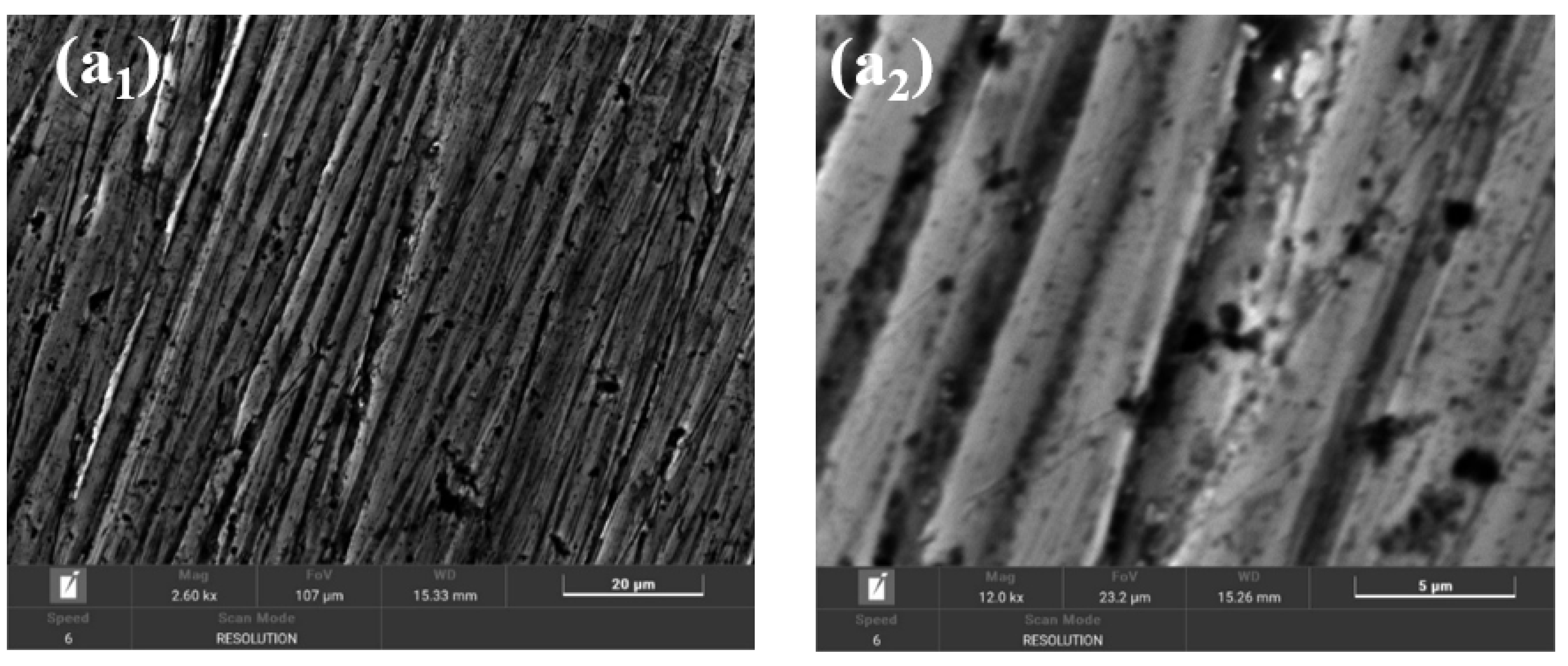
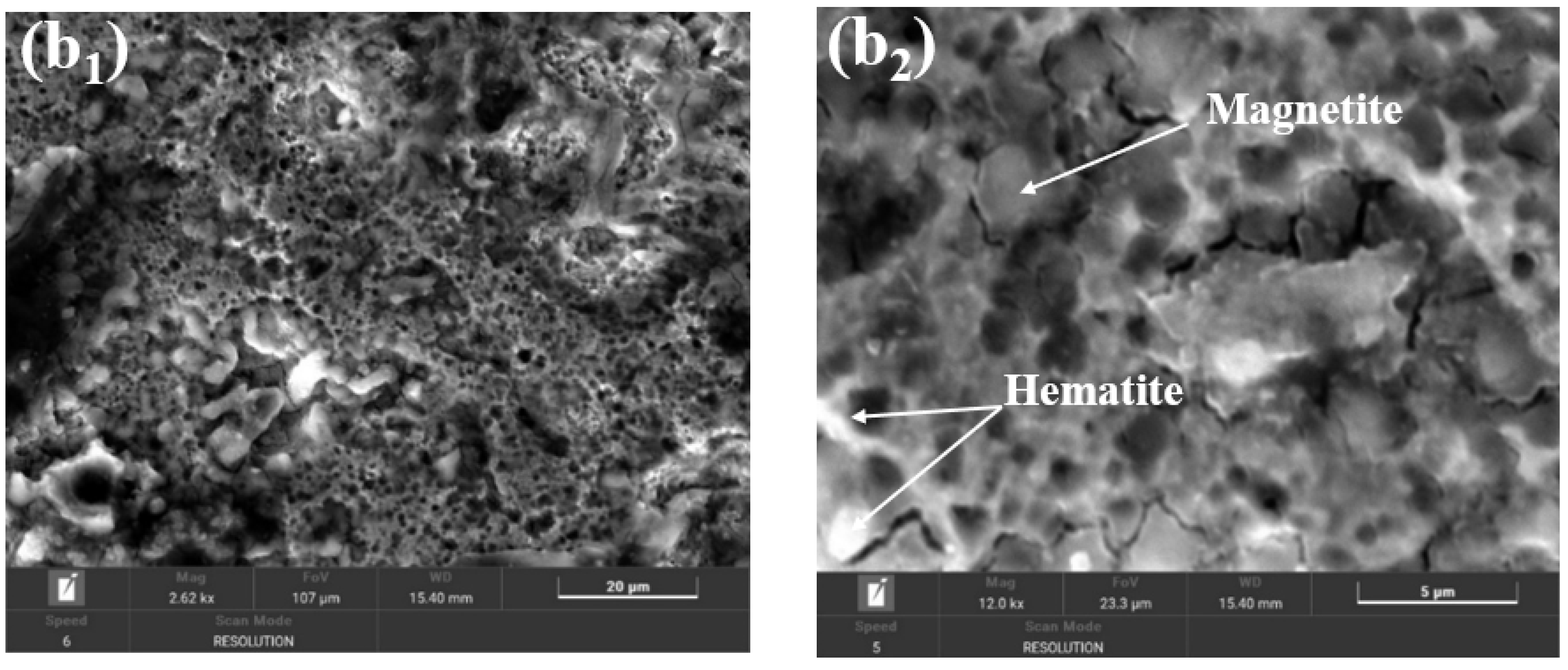
2.9. Morphological and Compositional Characterization of the Surfaces of BVDH36 and LRAH36 Steels before and after Corrosion by Scanning Electron Microscopy (SEM-EDX)
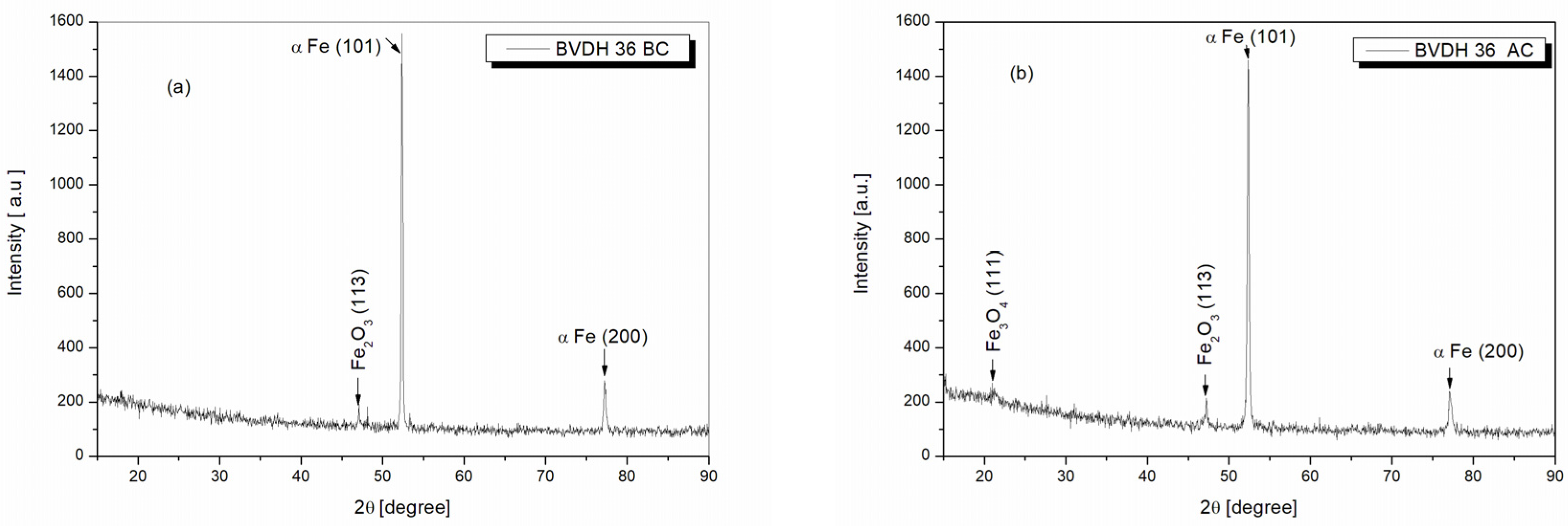
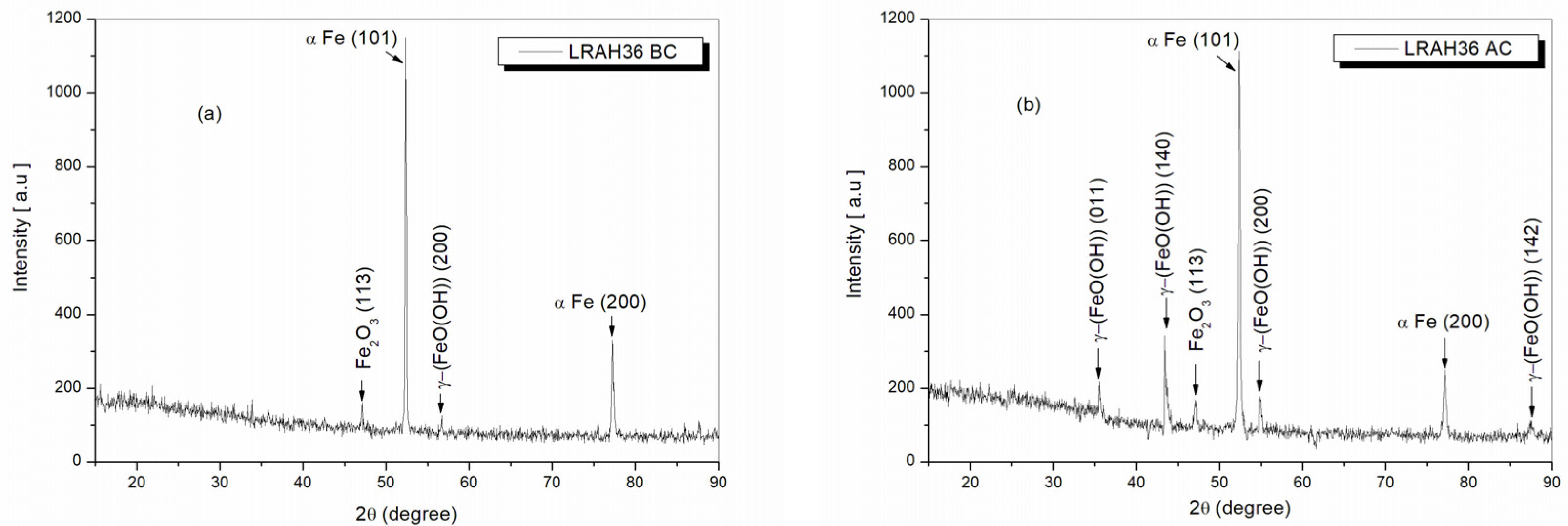
2.10. Structural Analysis of BVDH36 and LRAH36 Steels before and after Corrosion Using the XRD Technique
3. Materials and Methods
3.1. Materials
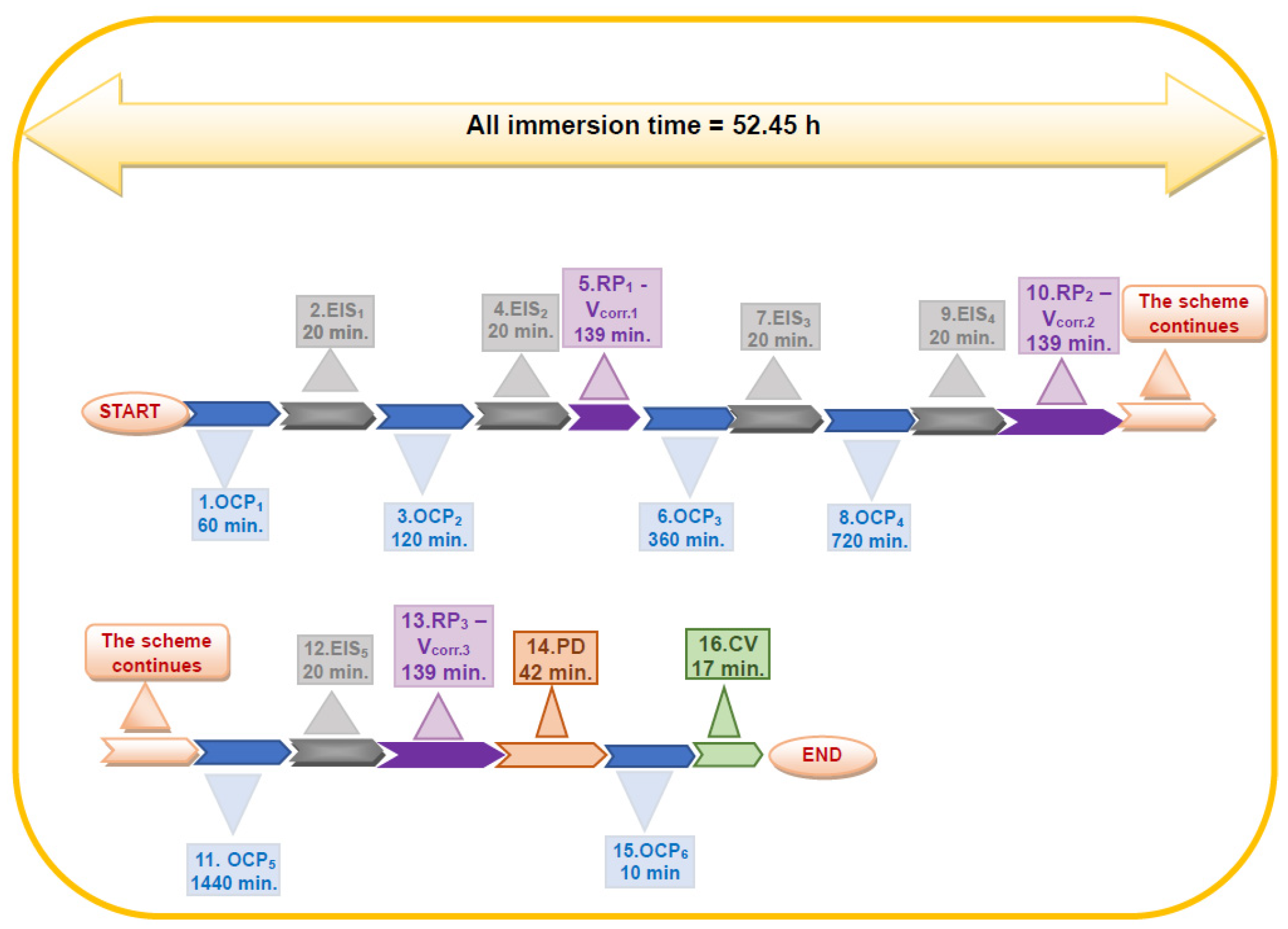
3.2. Electrochemical Test
3.3. Characterization of Low-Alloy Steels before and after Electrochemical Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Moubaraki, A.H.; Obot, I.B. Corrosion challenges in petroleum refinery operations: Sources, mechanisms, mitigation, and future outlook. J. Saudi Chem. Soc. 2021, 25, 101370–101397. [Google Scholar] [CrossRef]
- Yan, L.; Deng, W.; Wang, N.; Xue, X.; Hua, J.; Chen, Z. Anti corrosion reinforcements using coating technologies—A Review. Polymers 2022, 14, 4782. [Google Scholar] [CrossRef] [PubMed]
- Benea, L.; Mardare, L.; Simionescu, N. Anticorrosion performances of modified polymeric coatings on E32 naval steel in sea water. Prog. Org. Coat. 2018, 123, 120–127. [Google Scholar] [CrossRef]
- Benea, L.; Simionescu, N.; Mardare, L. The effect of polymeric protective layers and the immersion time on the corrosion behavior of naval steel in natural seawater. J. Mater. Res. Technol. 2020, 9, 13174–13184. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, Y.; Cai, F.; Lu, D.; Wang, X. Study on corrosion behavior and mechanism of AISI 4135 steel in marine environments based on field exposure experiment. Sci. Total Environ. 2022, 830, 154864. [Google Scholar] [CrossRef] [PubMed]
- Motlatle, A.M.; Ray, S.S.; Ojijo, V.; Scriba, M.R. Polyester-based coatings for corrosion protection. Polymers 2022, 14, 3413. [Google Scholar] [CrossRef] [PubMed]
- Gudić, S.; Vrsalović, L.; Matošin, A.; Krolo, J.; Oguzie, E.E.; Nagod, A. Corrosion behavior of stainless steel in seawater in the presence of sulfide. Appl. Sci. 2023, 13, 4366. [Google Scholar] [CrossRef]
- Melchers, R.E. Modelling long term corrosion of steel infrastructure in natural marine environments. In Understanding Biocorrosion Fundamentals and Applications, 1st ed.; Liengen, T., Féron, D., Basséguy, R., Beech, I.B., Eds.; Woodhead Publishing: Sawston, UK, 2014; pp. 213–241. [Google Scholar] [CrossRef]
- Plieth, W. Corrosion and Corrosion Protection. In Electrochemistry for Materials Science, 1st ed.; Plieth, W., Ed.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 291–321. [Google Scholar] [CrossRef]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical impedance spectroscopy (EIS): Principles, construction, and biosensing applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef]
- Humooudi, A.; Hamoudi, M.R.; Ali, B.M. Corrosion mitigation in crude oil process by implementation of desalting unit in erbil refinery. ASRJETS 2017, 36, 224–241. [Google Scholar]
- Fan, W.; Zhang, Y.; Tian, H.; Wang, W.; Li, W. Corrosion behavior of two low alloy steel in simulative deep-sea environment coupling to titanium alloy. Colloids Interface Sci. Commun. 2019, 29, 40–46. [Google Scholar] [CrossRef]
- Brown, A.; Wright, R.; Mevenkamp, L.; Hauton, C. A comparative experimental approach to ecotoxicology in shallow-water and deep-sea holothurians suggests similar behavioural responses. Aquat. Toxicol. 2017, 191, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Kark, S.; Brokovich, E.; Mazor, T.; Levin, N. Emerging conservation challenges and prospects in an era of offshore hydrocarbon exploration and exploitation. Conserv. Biol. J. Soc. Conserv. Biol. 2015, 29, 1573–1585. [Google Scholar] [CrossRef] [PubMed]
- Lubinevsky, H.; Hyams-Kaphzan, O.; Almogi-Labin, A.; Silverman, J.; Harlavan, Y.; Crouvi, O.; Herut, B.; Kanari, M.; Tom, M. Deep-sea soft bottom infaunal communities of the Levantine Basin (SE Mediterranean) and their shaping factors. Mar. Biol. 2017, 164, 36. [Google Scholar] [CrossRef]
- Azumi, K.; Yamamoto, G.; Seo, M. Pursuit of electronic property change of the passive film on titanium. Zairyo-To-Kankyo 2009, 46, 176–179. [Google Scholar] [CrossRef]
- Finšgar, M.; Jackson, J. Application of corrosion inhibitors for steels in acidic media for the oil and gas industry: A review. Corros. Sci. 2014, 86, 17–41. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Tzeng, H.J.; Wei, L.I.; Wang, L.H.; Oung, J.C.; Shih, H.C. Corrosion resistance and mechanical properties of low-alloy steels under atmospheric conditions. Corros. Sci. 2005, 47, 1001–1021. [Google Scholar] [CrossRef]
- Xing, S.H.; Li, Y.; Wharton, J.A.; Fan, W.J.; Liu, G.Y.; Zhang, F.; Zhao, X.D. Effect of dissolved oxygen and coupled resistance on the galvanic corrosion of Cr-Ni lowalloy steel/90-10 cupronickel under simulated deep sea condition. Mater. Corros. 2017, 68, 1123–1128. [Google Scholar] [CrossRef]
- Wei, X.; Fu, D.; Chen, M.; Wu, W.; Wu, D.; Liu, C. Data mining to effect of key alloying elements on corrosion resistance of low alloy steels in Sanya seawater environment alloying elements. J. Mater. Sci. Technol. 2020, 64, 222–232. [Google Scholar] [CrossRef]
- Jia, C.; Shao, Y.; Guo, L.; Liu, Y. Mechanical properties of corroded high strength low alloy steel plate. J. Constr. Steel. Res. 2020, 172, 106160. [Google Scholar] [CrossRef]
- Shi, G.; Ban, H.Y.; Shi, Y.J.; Wang, Y.Q. Overview of research progress for high strength steel structures. J. Eng. Mech. 2013, 30, 1–13. [Google Scholar]
- Kainuma, S.; Jeong, Y.-S.; Ahn, J.-H. Investigation on the stress concentration effect at the corroded surface achieved by atmospheric exposure test. Mater. Sci. Eng. A 2014, 602, 89–97. [Google Scholar] [CrossRef]
- Islam, M.R.; Sumi, Y. Geometrical effects of pitting corrosion on strength and deformability of steel rectangular plates subjected to uniaxial tension and pure bending. J. Jpn. Soc. Nav. Archit. Ocean Eng. 2011, 14, 9–17. [Google Scholar] [CrossRef]
- Nakai, T.; Matsushita, H.; Yamamoto, N.; Arai, H. Effect of pitting corrosion on local strength of hold frames of bulk carriers (1st report). Mar. Struct. 2004, 17, 403–432. [Google Scholar] [CrossRef]
- Nakai, T.; Matsushita, H.; Yamamoto, N. Effect of pitting corrosion on the ultimatestrength of steel plates subjected to in plane compression and bending. J. Mar. Sci. Technol. 2006, 11, 52–64. [Google Scholar] [CrossRef]
- Ahmmad, M.M.; Sumi, Y. Strength and deformability of corroded steel plates under quasi-static tensile load. J. Mar. Sci. Technol. 2010, 15, 1–15. [Google Scholar] [CrossRef]
- Garbatov, Y.; Guedes Soares, C.; Parunov, J.; Kodvanj, J. Tensile strength assessment of corroded small scale specimens. Corros. Sci. 2014, 85, 296–303. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, X.H.; Guedes Soares, C. Experimental analysis of residual ultimate strength of stiffened panels with pitting corrosion under compression. Eng. Struct. 2017, 152, 70–86. [Google Scholar] [CrossRef]
- Sultana, S.; Wang, Y.; Sobey, A.J.; Wharton, J.A.; Shenoi, R.A. Influence of corrosion on the ultimate compressive strength of steel plates and stiffened panels. Thin-Walled Struct. 2015, 96, 95–104. [Google Scholar] [CrossRef]
- Nakai, T.; Matsushita, H.; Yamamoto, N. Effect of pitting corrosion on strength of web plates subjected to patch loading. Thin-Walled Struct. 2006, 44, 10–19. [Google Scholar] [CrossRef]
- Nakai, T.; Matsushita, H.; Yamamoto, N. Effect of pitting corrosion on local strength of hold frames of bulk carriers (2nd report)—Lateral-distortional buckling and local face buckling. Mar. Struct. 2004, 17, 612–641. [Google Scholar] [CrossRef]
- Saad-Eldeen, S.; Garbatov, Y.; Guedes Soares, C. Compressive strength assessment of amoderately corroded box girder. Mar. Syst. Ocean Technol. 2011, 6, 27–37. [Google Scholar] [CrossRef]
- Paik, J.K.; Lee, J.M.; Ko, M.J. Ultimate shear strength of plate elements with pit corrosion wastage. Thin-Walled Struct. 2004, 42, 1161–1176. [Google Scholar] [CrossRef]
- Wang, Y.; Wharton, J.A.; Shenoi, R.A. Ultimate strength analysis of aged steel-plated structures exposed to marine corrosion damage: A review. Corros. Sci. 2014, 86, 42–60. [Google Scholar] [CrossRef]
- Kainuma, S.; Jeong, Y.-S.; Ahn, J.-H.; Yamagami, T.; Tsukamoto, S. Behavior and stress of orthotropic deck with bulb rib by surface corrosion. J. Constr. Steel Res. 2015, 113, 135–145. [Google Scholar] [CrossRef]
- Wang, R.; Shenoi, R.A.; Sobey, A. Ultimate strength assessment of plated steel structures with random pitting corrosion damage. J. Constr. Steel Res. 2018, 143, 331–342. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, R.; Yang, J.; Xiao, D.; Luo, D.; Zhou, W.; Tuo, C.; Wu, H.; Niu, G. Effect of tempering on corrosion behavior and mechanism of low alloy steel in wet atmosphere. J. Mater. Res. Technol. 2022, 2, 4077–4096. [Google Scholar] [CrossRef]
- Fu, J.; Wan, S.; Yang, Y.; Su, Q.; Han, W.; Zhu, Y. Accelerated corrosion behavior of weathering steel Q345qDNH for bridge in industrial atmosphere. Constr. Build. Mater. 2021, 306, 124864. [Google Scholar] [CrossRef]
- Wu, W.; Dai, Z.; Liu, Z.; Liu, C.; Li, X. Synergy of Cu and Sb to enhance the resistance of 3%Ni weathering steel to marine atmospheric corrosion. Corros. Sci. 2021, 183, 109353. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, W.; Sun, Z. Effect of chloride ion on corrosion resistance of Ni-advanced weathering steel in simulated tropical marine atmosphere. Constr. Build. Mater. 2021, 266, 120937–120949. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, W.; Chen, L.; Dong, B.; Yang, W.; Fan, Y.; Zhao, Y. On how the corrosion behavior and the functions of Cu, Ni and Mo of the weathering steel in environments with different NaCl Concentrations. Corros. Sci. 2021, 192, 109851. [Google Scholar] [CrossRef]
- Gong, K.; Wu, M.; Liu, G. Comparative study on corrosion behaviour of rusted X100 steel in dry/wet cycle and immersion environments. Constr. Build. Mater. 2020, 235, 117440–117451. [Google Scholar] [CrossRef]
- Zhao, T.; Liu, K.; Li, Q. Comparison of the rusting behaviors of S450EW weathering steel under continuous spray and wet/dry cycling. Constr. Build. Mater. 2021, 309, 125211. [Google Scholar] [CrossRef]
- Wu, H.; Lei, H.; Chen, Y.F.; Qiao, J. Comparison on corrosion behaviour and mechanical properties of structural steel exposed between urban industrial atmosphere and laboratory simulated environment. Constr. Build. Mater. 2019, 211, 228–243. [Google Scholar] [CrossRef]
- Wu, W.; Cheng, X.; Zhao, J.; Li, X. Benefit of the corrosion product film formed on a new weathering steel containing 3% nickel under marine atmosphere in Maldives. Corros. Sci. 2020, 165, 108416. [Google Scholar] [CrossRef]
- Oso’rio, W.R.; Peixoto, L.C.; Garcia, L.R.; Garcia, A. Electrochemical corrosion response of a low carbon heat treated steel in a NaCl solution. Mater. Corros. 2009, 60, 804–812. [Google Scholar] [CrossRef]
- Yuan, F.; Wei, G.; Gao, S.; Lu, S.; Liu, H.; Li, S.; Fang, X.; Chen, Y. Tuning the pitting performance of a Cr-13 type martensitic stainless steel by tempering time. Corros. Sci. 2022, 203, 110346. [Google Scholar] [CrossRef]
- Rai, P.K.; Shekhar, S.; Mondal, K. Development of gradient microstructure in mild steel and grain size dependence of its electrochemical response. Corros. Sci. 2018, 138, 85–95. [Google Scholar] [CrossRef]
- Wang, P.; Ma, L.; Cheng, X.; Li, X. Effect of grain size and crystallographic orientation on the corrosion behaviors of low alloy steel. J. Alloys Compd. 2021, 857, 158258. [Google Scholar] [CrossRef]
- Chen, H.; Cui, H.; He, Z.; Lu, L.; Huang, Y. Influence of chloride deposition rate on rust layer protectiveness and corrosion severity of mild steel in tropical coastal atmosphere. Mater. Chem. Phys. 2021, 259, 123971. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Wei, Y.; Wang, Z. Effect of different UV intensity on corrosion behavior of carbon steel exposed to simulated Nansha atmospheric environment. Mater. Chem. Phys. 2019, 237, 121855. [Google Scholar] [CrossRef]
- Feliu, S.; Morcillo, M.; Chico, B. Effect of distance from sea on atmospheric corrosion rate. Corrosion 1999, 55, 883–891. [Google Scholar] [CrossRef]
- Almeida, E.; Morcillo, M.; Rosales, B. Atmospheric corrosion of mild steel Part II—Marine atmospheres. Mater. Corros. 2000, 51, 865–874. [Google Scholar] [CrossRef]
- Corvo, F.; Perez, T.; Dzib, L.R.; Martin, Y.; Castañeda, A.; Gonzalez, E.; Perez, J. Outdoor-indoor corrosion of metals in tropical coastal atmospheres. Corros. Sci. 2008, 50, 220–230. [Google Scholar] [CrossRef]
- Corvo, F.; Mendoza, A.R.; Autie, M.; Betancourt, N. Role of water adsorption and salt content in atmospheric corrosion products of steel. Corros. Sci. 1997, 39, 815–820. [Google Scholar] [CrossRef]
- Angelo, L.D.; Verdingovas, V.; Ferrero, L.; Bolzacchini, E.; Ambat, R. Effect of hygroscopic atmospheric particles deposition on the corrosion reliability of electronics. Eur. Corros. Congr. 2016, 2, 956–965. [Google Scholar]
- Ma, Y.; Li, Y.; Wang, F. The atmospheric corrosion kinetics of low carbon steel in a tropical marine environment. Corros. Sci. 2010, 5, 1796–1800. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Y.; Wang, F. Corrosion of low carbon steel in atmospheric environments of different chloride content. Corros. Sci. 2009, 51, 997–1006. [Google Scholar] [CrossRef]
- Hao, L.; Zhang, S.; Dong, J.; Ke, W. Evolution of corrosion of MnCuP weathering steel submitted to wet/dry cyclic tests in a simulated coastal atmosphere. Corros. Sci. 2012, 58, 175–180. [Google Scholar] [CrossRef]
- Rocca, E.; Faiz, H.; Dillmann, P.; Neff, D.; Mirambet, F. Electrochemical behavior of thick rust layers on steel artefact: Mechanism of corrosion inhibition. Electrochim. Acta 2019, 316, 219–227. [Google Scholar] [CrossRef]
- Nishimura, T. Electrochemical behavior of rust formed on carbon steel in a wet/dry environment containing chloride ions. Corrosion 2000, 56, 935–941. [Google Scholar] [CrossRef]
- Li, S.; Hihara, L.H. In situ Raman spectroscopic study of NaCl particle-induced marine atmospheric corrosion of carbon steel. J. Electrochem. Soc. 2012, 159, C147–C154. [Google Scholar] [CrossRef]
- Dubois, F.; Mendibide, C.; Pagnier, T.; Perrard, F.; Duret, C. Raman mapping of corrosion products formed onto spring steels during salt spray experiments. A correlation between the scale composition and the corrosion resistance. Corros. Sci. 2008, 50, 3401–3409. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Y.; Wang, F. The effect of β-FeOOH on the corrosion behavior of low carbon steel exposed in tropic marine environment. Mater. Chem. Phys. 2008, 112, 844–852. [Google Scholar] [CrossRef]
- Wan, Y.; Tan, J.; Zhu, S.; Cui, J.; Zhang, K.; Wang, X.; Shen, X.; Li, Y.; Zhu, X. Insight into atmospheric pitting corrosion of carbon steel via a dual-beam FIB/SEM system associated with high-resolution TEM. Corros. Sci. 2019, 152, 226–233. [Google Scholar] [CrossRef]
- Almeida, E.; Diamantino, T.C.; de Sousa, O. Marine paints: The particular case of antifouling paints. Prog. Org. Coat. 2007, 59, 2–20. [Google Scholar] [CrossRef]
- Tracton, A.A. Coatings materials and surface coatings. In Coatings Technology Handbook, 3rd ed.; CRC Press: Florida, US, USA, 2006; pp. 1–528. [Google Scholar]
- Ren, Z.; Ernst, F. Stress–corrosion cracking of AISI 316L stainless steel in seawater environments: Effect of surface machining. Metals 2020, 10, 1324. [Google Scholar] [CrossRef]
- Melchers, R.E. Long-term durability of marine reinforced concrete structures. J. Mar. Sci. Eng. 2020, 8, 290. [Google Scholar] [CrossRef]
- Duan, T.; Peng, W.; Ding, K.; Guo, W.; Hou, J.; Cheng, W.; Liu, S.; Xu, L. Long-term field exposure corrosion behavior investigation of 316L stainless steel in the deep sea environment. Ocean Eng. 2019, 189, 106405. [Google Scholar] [CrossRef]
- Rajput, A.; Paik, J.K. Effects of naturally-progressed corrosion on the chemical and mechanical properties of structural steels. Structures 2021, 29, 2120–2138. [Google Scholar] [CrossRef]
- Liu, J.G.; Li, Y.T.; Hou, B.R. Corrosion behaviour of DH36 steel used for oil platform in splash zones. Corros. Sci. Technol. 2015, 14, 190–194. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, J.; Zheng, Y.Y. Electrochemical techniques for determining corrosion rate of rusted steel in seawater. Corros. Sci. 2011, 53, 208–216. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, P.; Huang, S.; Chi, Y. Corrosion mechanism of low alloy steel in NaCl solution with CO2 and H2S. Int. J. Electrochem. Sci. 2016, 11, 1293–1309. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, L.; Zhu, Y.; Ai, F.; Li, H.; Li, Y.; Jiang, Z. The effect of immersion corrosion time on electrochemical corrosion behavior and the corrosion mechanism of EH47 ship steel in seawater. Metals 2021, 11, 1317. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, S.; Smith, K.; Yu, K.; Hu, H.; Jiang, W.; Li, Y. Characterization of corrosion scale formed on stainless steel delivery pipe for reclaimed water treatment. Water Res. 2016, 88, 816–825. [Google Scholar] [CrossRef]
- Zafar, F.; Bano, H.; Mahmood, A.; Corvo, F.; Rodriguez, J. Physicochemical studies of mild steel corrosion and atmospheric corrosivity mapping of Karachi: An important harbor city of modern Maritime Silk Route. Mater. Corros. 2020, 71, 1557–1575. [Google Scholar] [CrossRef]
- Sharma, V.; Sharma, V.; Goyat, M.S.; Hooda, A.; Pandey, J.K.; Kumar, A.; Gupta, R.; Upadhyay, A.K.; Prakash, R.; Kirabira, J.B.; et al. Recent progress in nano-oxides and CNTs based corrosion resistant superhydrophobic coatings: A critical review. Prog. Org. Coat. 2020, 140, 105512. [Google Scholar] [CrossRef]
- McMahon, M.E.; Santucci, R.J., Jr.; Glover, C.F.; Kannan, B.; Walsh, Z.R.; Scully, J.R. A review of modern assessment methods for metal and metal-oxide based primers for substrate corrosion protection. Front. Mater. 2019, 6, 190. [Google Scholar] [CrossRef]






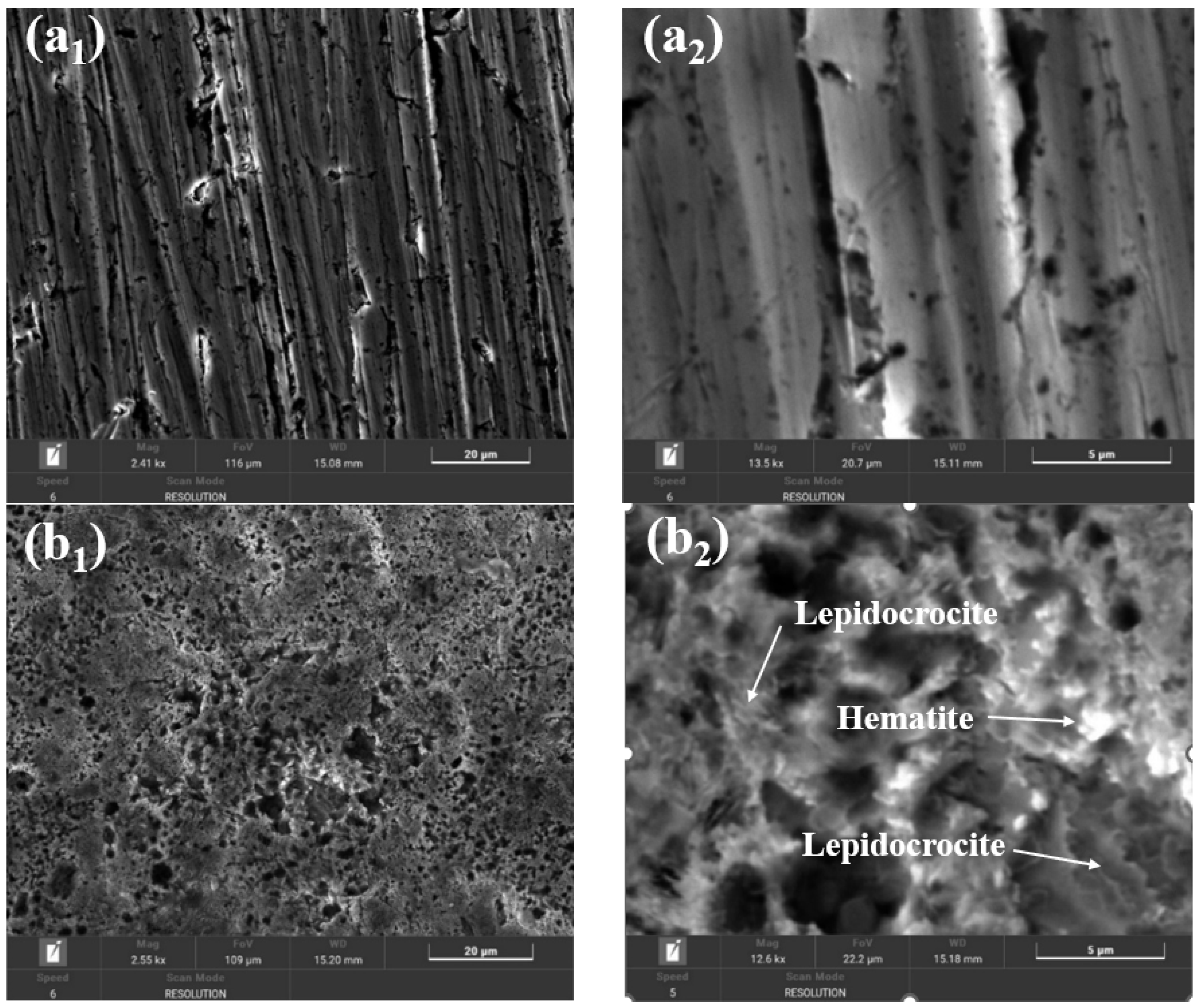
| Chemical Elements | Wt % | σ % |
|---|---|---|
| Fe | 90.2% | 1.3% |
| C | 6.6% | 1.3% |
| Mn | 1.7% | 0.1% |
| Si | 1.2% | 0.1% |
| Ni | 0.1% | 0.1% |
| P | 0.1% | 0.1% |
| Al | 0.1% | 0.1% |
| Chemical Elements | Wt % | σ % |
|---|---|---|
| Fe | 68.5% | 0.6% |
| C | 15.6% | 0.6% |
| O | 14.8% | 0.3% |
| Mn | 0.9% | 0.1% |
| Ni | 0.1% | 0.1% |
| Cu | 0.1% | 0.1% |
| Chemical Elements | Wt % | σ % |
|---|---|---|
| Fe | 91.5% | 1.3% |
| C | 6% | 1.2% |
| Mn | 1.6% | 0.1% |
| Si | 0.5% | 0.1% |
| Mo | 0.2% | 0.3% |
| Ni | 0.1% | 0.1% |
| Al | 0.1% | 0.1% |
| Chemical Elements | Wt % | σ % |
|---|---|---|
| Fe | 80.7% | 0.7% |
| C | 10.8% | 0.8% |
| O | 6.4% | 0.3% |
| Mn | 1.2% | 0.1% |
| Cu | 0.3% | 0.1% |
| Si | 0.3% | 0.1% |
| Ni | 0.1% | 0.1% |
| Al | 0.1% | 0.1% |
| P | 0.1% | 0.1% |
| Type of Sample | 2θ | Intensity Peak | Miller Indices (hkl) | Crystallographic Phases | COD (Crystallography Open Database) |
|---|---|---|---|---|---|
| BVDH36 before corrosion | 47.20° | 130 | (113) | Fe2O3 (hematite) | COD 96-210-8029 |
| 52.35° | 1557 | (101) | α Fe | COD 96-110-0109 | |
| 77.20° | 278 | (200) | α Fe | COD 96-110-0109 | |
| BVDH36 after corrosion | 21.44° | 232 | (111) | Fe3O4 (magnetite) | COD 96-900-2321 |
| 47.20° | 209 | (113) | Fe2O3 (hematite) | COD 96-210-8029 | |
| 52.35° | 1458 | (101) | α Fe | COD 96-110-0109 | |
| 77.20° | 200 | (200) | α Fe | COD 96-110-0109 | |
| LRAH36 before corrosion | 47.20° | 109 | (113) | Fe2O3 | COD 96-210-8029 |
| 52.35° | 1150 | (101) | α Fe | COD 96-110-0109 | |
| 54.97° | 93 | (200) | γ-(FeO(OH)) lepidocrocite | COD 96-901-5157 | |
| 77.20° | 244 | (200) | α Fe | COD 96-110-0109 | |
| LRAH36 after corrosion | 35.53° | 195 | (011) | γ-(FeO(OH)) lepidocrocite | COD 96-901-5157 |
| 43.70° | 202 | (140) | γ-(FeO(OH)) lepidocrocite | COD 96-901-5157 | |
| 47.20° | 127 | (113) | Fe2O3 | COD 96-210-8029 | |
| 52.35° | 1113 | (101) | α Fe | COD 96-110-0109 | |
| 54.97° | 168 | (200) | γ-(FeO(OH)) lepidocrocite | COD 96-901-5157 | |
| 77.20° | 183 | (200) | α Fe | COD 96-110-0109 | |
| 87.58° | 111 | (142) | γ-(FeO(OH)) lepidocrocite | COD 96-901-5157 |
| C | Mn | Si | P | S | Al | Cu | Cr | Ni | V | Mo | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [%] | [%] | [%] | [%] | [%] | [%] | [%] | [%] | [%] | [%] | [%] | [%] |
| 0.17 | 1.19 | 0.18 | 0.011 | 0.006 | 0.039 | 0.05 | 0.02 | 0.05 | 0.003 | 0.008 | 98.3 |
| C | Mn | Si | P | S | Al | Cu | Cr | Ni | V | Mo | Ti | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [%] | [%] | [%] | [%] | [%] | [%] | [%] | [%] | [%] | [%] | [%] | [%] | [%] |
| 0.16 | 1.20 | 0.23 | 0.01 | 0.011 | 0.03 | 0.05 | 0.03 | 0.04 | 0.003 | 0.006 | 0.001 | 98.2 |
| Black Sea Water | pH | Conductivity [mS/cm] | Salinity [ppt] |
|---|---|---|---|
| 8.31 | 22.2 | 13.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazilu, A.; Benea, L.; Axente, E.R. Monitoring and Evaluation of the Corrosion Behavior in Seawater of the Low-Alloy Steels BVDH36 and LRAH36. Int. J. Mol. Sci. 2024, 25, 6405. https://doi.org/10.3390/ijms25126405
Mazilu A, Benea L, Axente ER. Monitoring and Evaluation of the Corrosion Behavior in Seawater of the Low-Alloy Steels BVDH36 and LRAH36. International Journal of Molecular Sciences. 2024; 25(12):6405. https://doi.org/10.3390/ijms25126405
Chicago/Turabian StyleMazilu, Adrian, Lidia Benea, and Elena Roxana Axente. 2024. "Monitoring and Evaluation of the Corrosion Behavior in Seawater of the Low-Alloy Steels BVDH36 and LRAH36" International Journal of Molecular Sciences 25, no. 12: 6405. https://doi.org/10.3390/ijms25126405
APA StyleMazilu, A., Benea, L., & Axente, E. R. (2024). Monitoring and Evaluation of the Corrosion Behavior in Seawater of the Low-Alloy Steels BVDH36 and LRAH36. International Journal of Molecular Sciences, 25(12), 6405. https://doi.org/10.3390/ijms25126405







