Naphthoquinone-Quinolone Hybrids with Antitumor Effects on Breast Cancer Cell Lines—From the Synthesis to 3D-Cell Culture Effects
Abstract
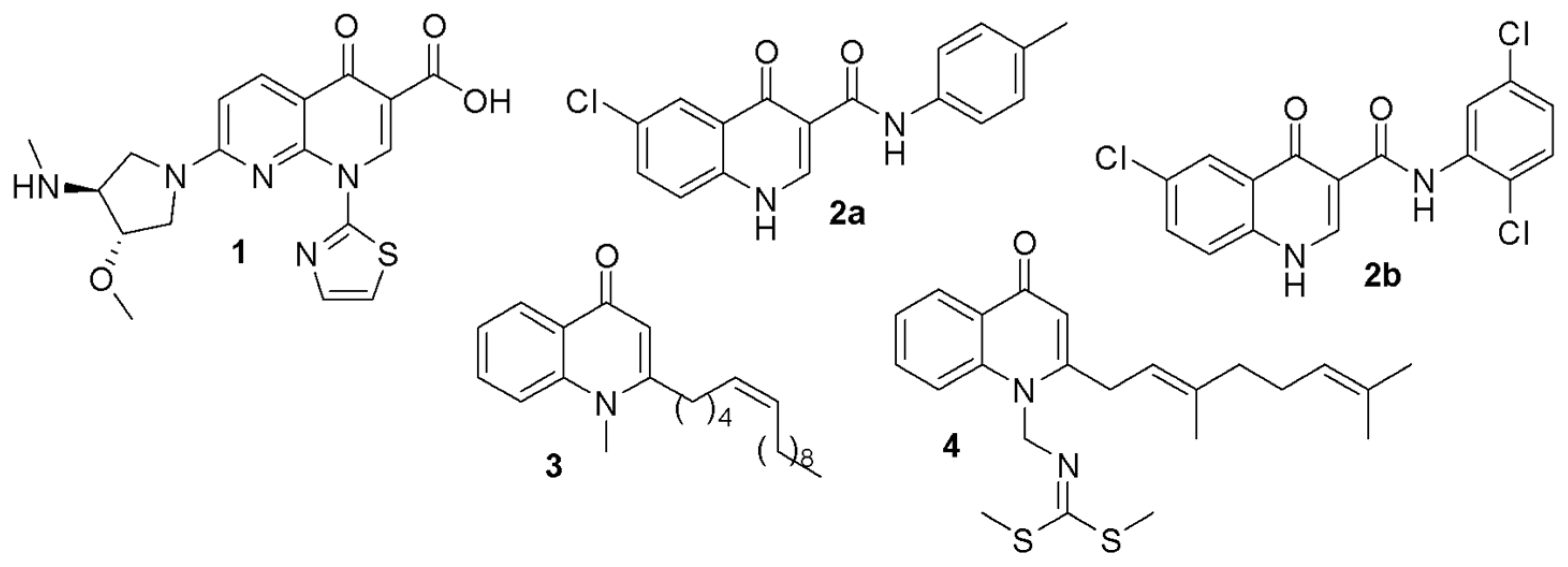
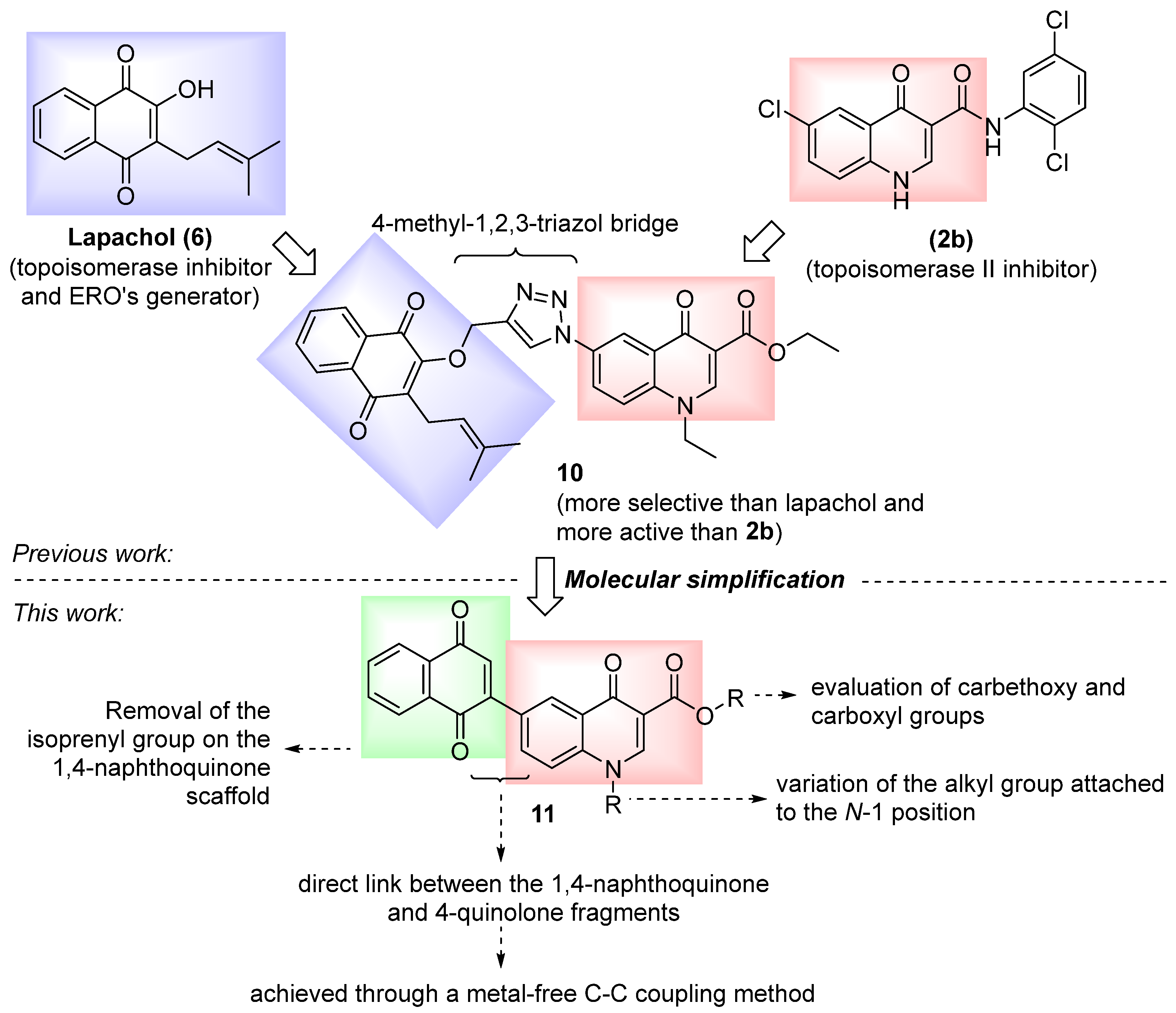
1. Introduction
2. Results and Discussion
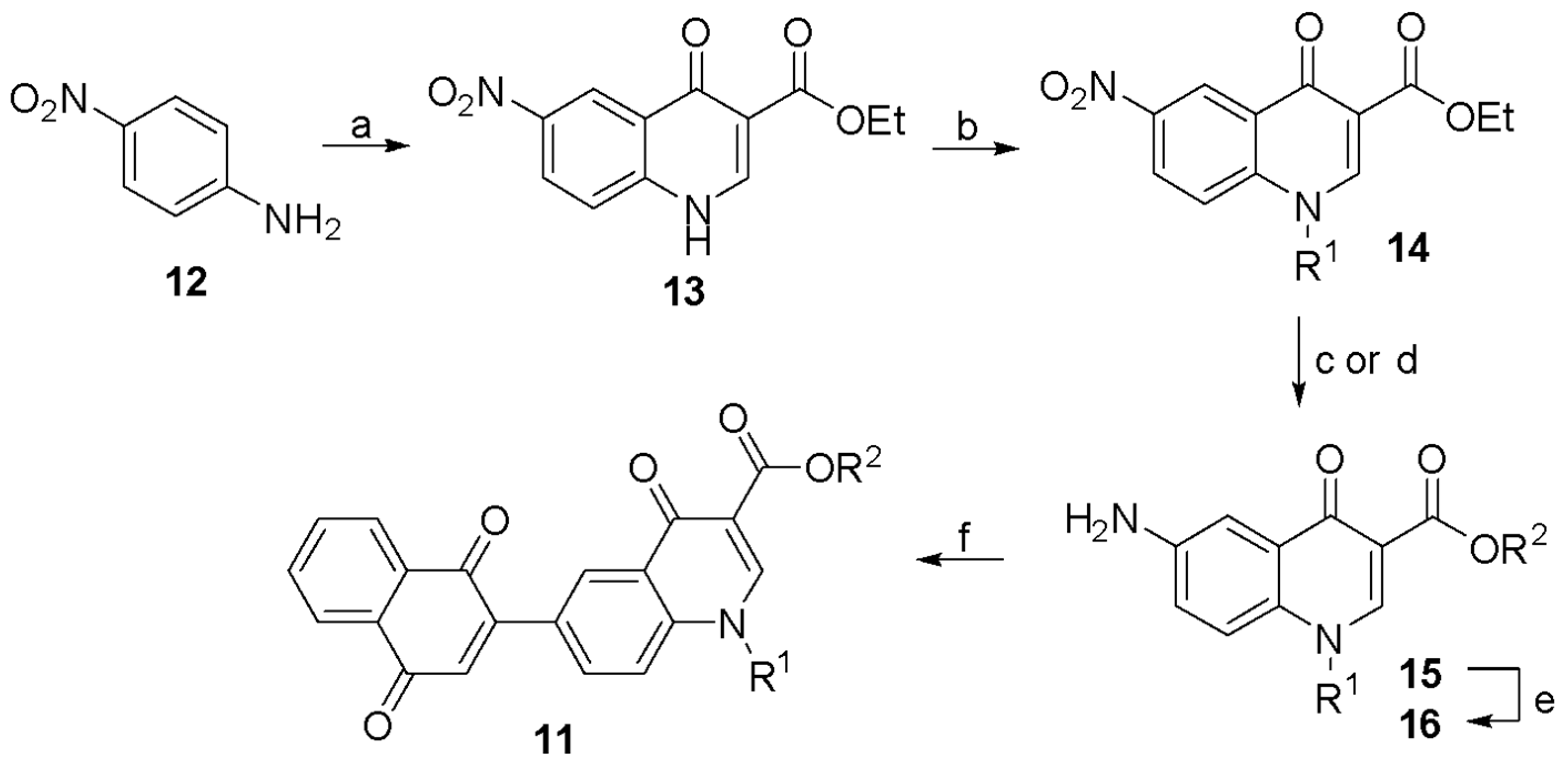
2.1. Chemical Synthesis
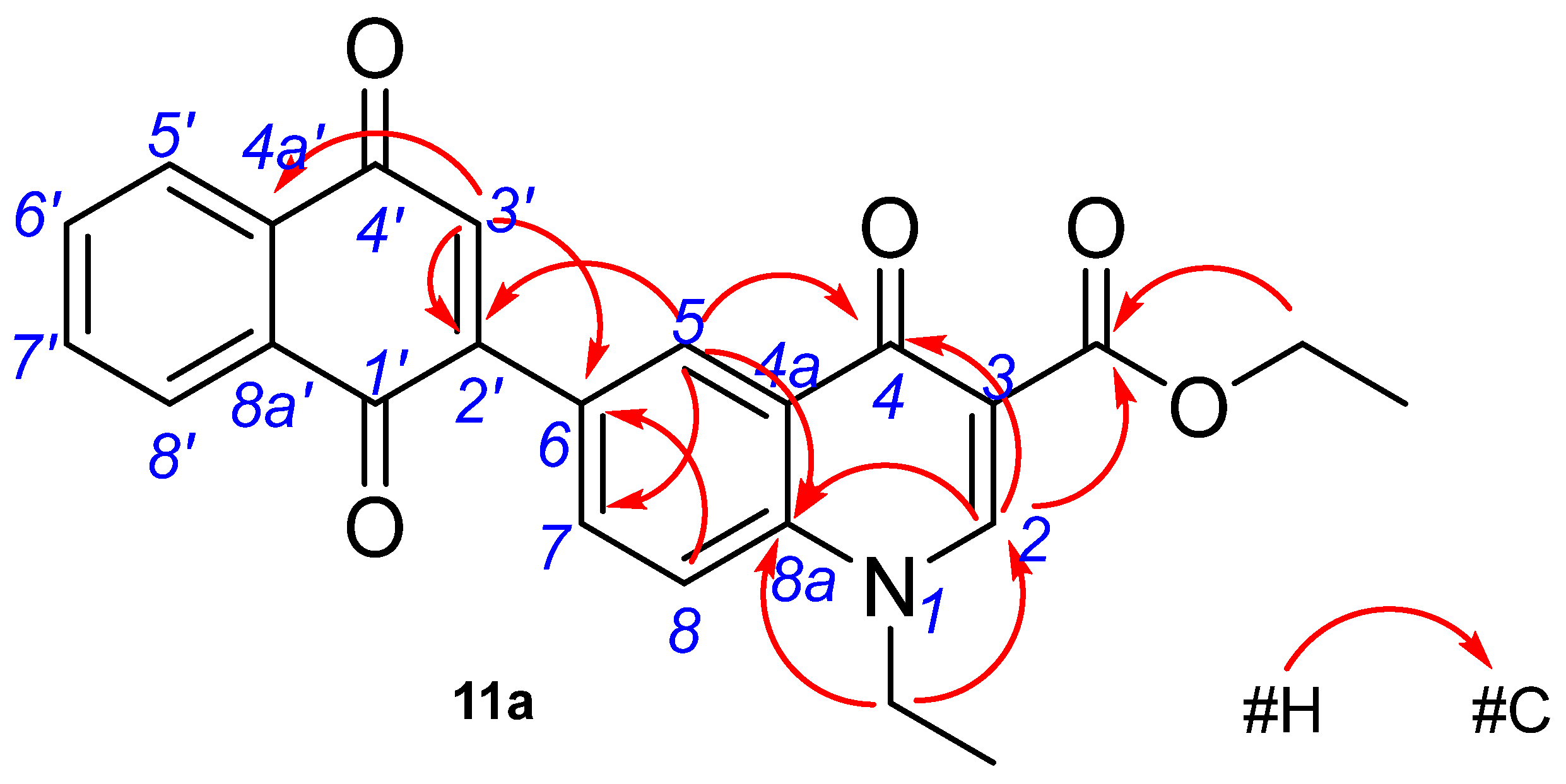
2.2. Structure Characterization
2.3. Antitumor Effects
2.3.1. Cytotoxicity
2.3.2. Selectivity Indices (SI)
2.3.3. Apoptosis Induction
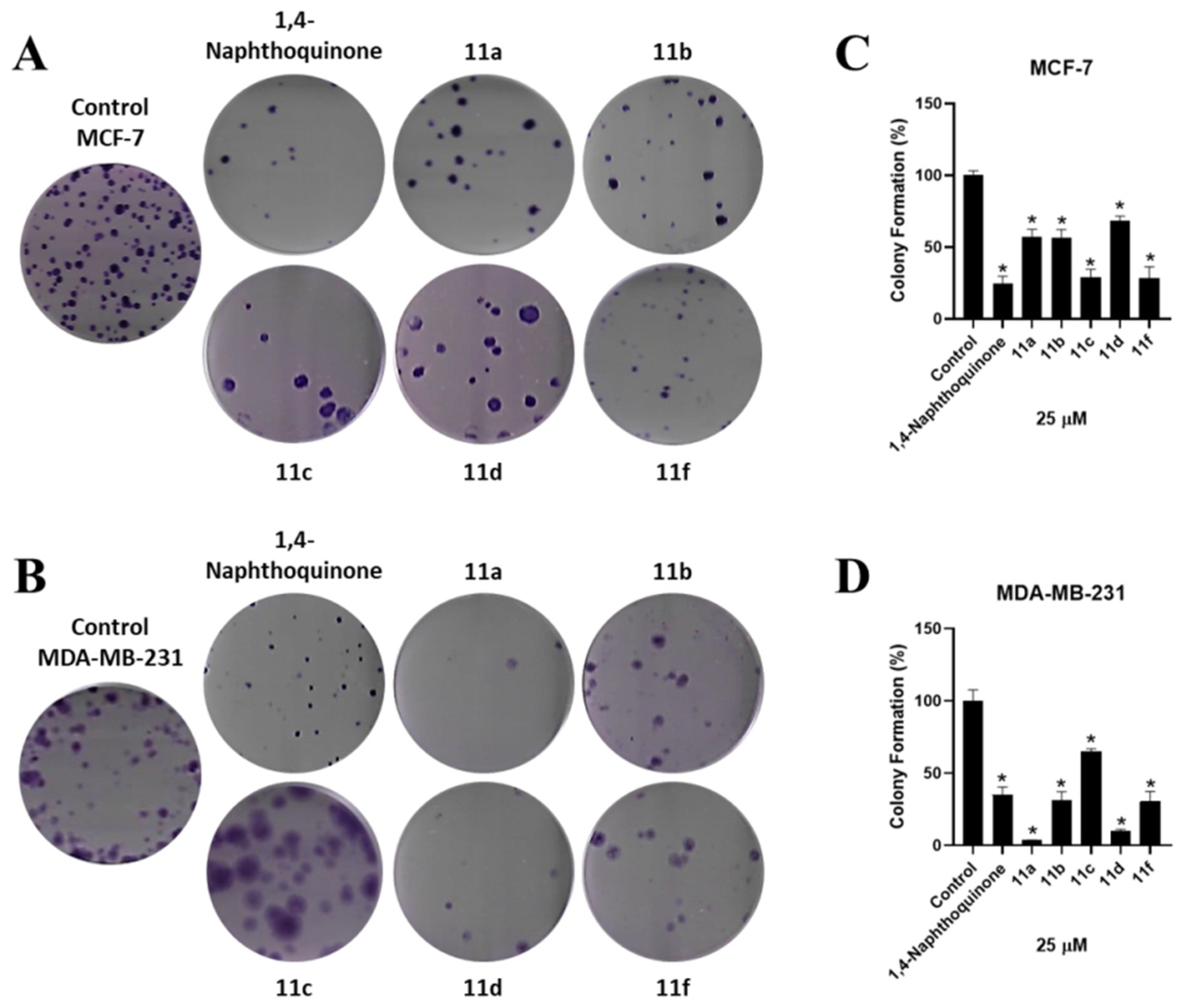
2.3.4. Clonogenic Assay
2.3.5. Duration and Reversibility of the Effects
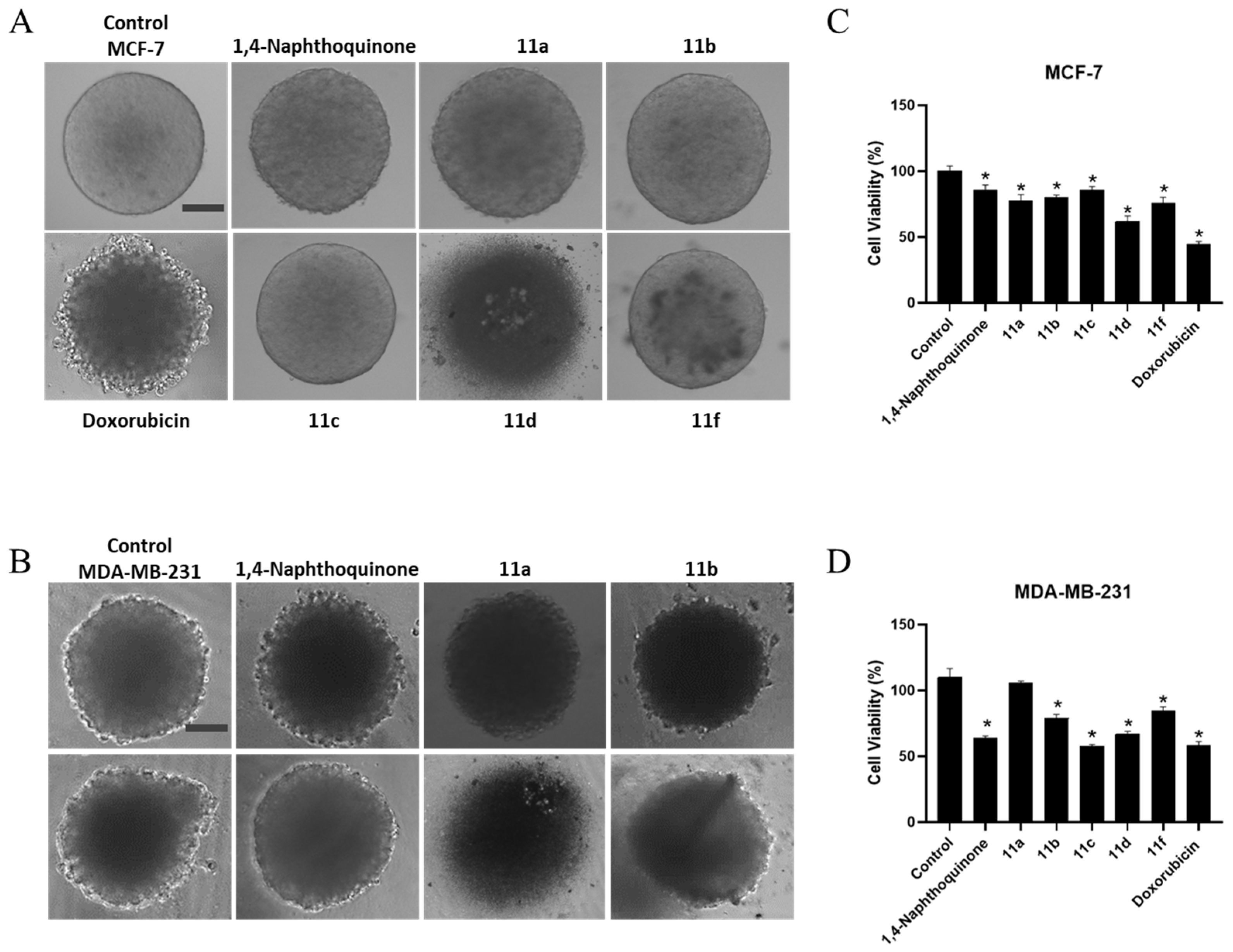
2.3.6. 3D-Cell Culture Viability
2.3.7. In Silico Drug-Likeness and ADME
3. Materials and Methods
3.1. Synthesis
3.2. Biological Assays
3.2.1. Cell Lines
3.2.2. MTT Assay
3.2.3. Clonogenic Assay
3.2.4. Apoptosis Assay
3.2.5. 3D-Cell Culture
3.2.6. Acid Phosphatase Assay
3.2.7. In Silico Drug-Likeness and ADME
3.2.8. Statistical Analyses, IC50, and Selectivity Index Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Patani, N.; Martin, L.A.; Dowsett, M. Biomarkers for the clinical management of breast cancer: International perspective. Int. J. Cancer 2013, 133, 1–13. [Google Scholar] [CrossRef]
- Rawat, P.S.; Jaiswal, A.; Khurana, A.; Bhatti, J.S.; Navik, U. Doxorubicin-induced cardiotoxicity: An update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed. Pharmacother. 2021, 139, 111708. [Google Scholar] [CrossRef] [PubMed]
- Audisio, D.; Messaoudi, S.; Peyrat, J.F.; Brion, J.D.; Alami, M.J. A general copper powder-catalyzed ullmann-type reaction of 3-halo-4(1H)-quinolones with various nitrogen-containing nucleophiles. J. Org. Chem. 2011, 76, 4995–5005. [Google Scholar] [CrossRef] [PubMed]
- Batalha, P.N.; Souza, M.C.B.V.; Penã-Cabrera, E.; Curz, D.C.; Boechat, F.C.S. Quinolones in the search for new anticancer agents. Curr. Pharm. Des. 2016, 22, 6009–6020. [Google Scholar] [CrossRef] [PubMed]
- Hawtin, R.E.; Stockett, D.E.; Byl, J.A.; McDowell, R.S.; Nguyen, T.; Arkin, M.R.; Conroy, A.; Yang, W.; Osheroff, N.; Fox, J.A. Voreloxin is an anticancer quinolone derivative that intercalates DNA and poisons topoisomerase II. PLoS ONE 2010, 5, e10186. [Google Scholar] [CrossRef] [PubMed]
- Forezi, L.S.M.; Tolentino, N.M.C.; Souza, A.M.T.; Castro, H.C.; Montenegro, R.C.; Dantas, R.F.; Oliveira, M.E.I.M.; Silva, F.P., Jr.; Barreto, L.H.; Burbano, R.M.R.; et al. Synthesis, cytotoxicity and mechanistic evaluation of 4-oxoquinoline-3-carboxamides derivatives: Finding new potential anticancer drugs. Molecules 2014, 19, 6651–6670. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, W.; Yang, X. New cytotoxic quinolone alkaloids from fruits of Evodia rutaecarpa. Fitoterapia 2012, 83, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Zan, K.; Shi, S.P.; Zeng, K.W.; Jiang, Y.; Guan, Y.; Xiao, C.L.; Gao, H.Y.; Wu, L.J.; Tu, P.F. Quinolone alkaloids with antibacterial and cytotoxic activities from the fruits of Evodia rutaecarpa. Fitoterapia 2013, 89, 1–7. [Google Scholar] [CrossRef]
- Zhao, N.; Li, Z.L.; Li, D.H.; Sun, Y.T.; Shan, D.T.; Bai, J.; Pei, Y.H.; Jing, Y.K.; Hua, H.M. Quinolone and indole alkaloids from the fruits of Euodia rutaecarpa and their cytotoxicity against two human cancer cell lines. Phytochemistry 2015, 109, 133–139. [Google Scholar] [CrossRef]
- Kawada, M.; Inoue, H.; Ohba, S.; Hatano, M.; Amemiya, M.; Hayashi, C.; Usami, I.; Abe, H.; Watanabe, T.; Kinoshita, N.; et al. Intervenolin, a new antitumor compound with anti-Helicobacter pylori activity, from Nocardia sp. ML96-86F2. J. Antibiot. 2013, 66, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.F.; Ferreira, S.B.; Da Silva, F.C. Strategies for the synthesis of bioactive pyran naphthoquinones. Org. Biomol. Chem. 2010, 8, 4793–4802. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.C.; Ferreira, V.F. Natural naphthoquinones with great importance in medicinal chemistry. Curr. Org. Synth. 2016, 13, 334–371. [Google Scholar] [CrossRef]
- Angulo-Elizari, E.; Henriquez-Figuereo, A.; Mor’an-Serradilla, C.; Plano, D.; Sanmartín, C. Unlocking the potential of 1,4-naphthoquinones: A comprehensive review of their anticancer properties. Eur. J. Med. Chem. 2024, 268, 116249. [Google Scholar] [CrossRef] [PubMed]
- Lemke Thomas, L.; Williams David, A.; Roche Victoria, F.; Zito, S. William. Foyes’s Principles of Medicinal Chemistry, 6th ed.; Thomas L. Lemke: Philadelphia, PA, USA, 2008; p. 1009. [Google Scholar]
- Ferreira, V.F.; Nicoletti, C.D.; Ferreira, P.G.; Futuro, D.O.; da Silva, F.C. Strategies for Increasing the Solubility and Bioavailability of Anticancer Compounds: β-Lapachone and Other Naphthoquinones. Curr. Pharm. Des. 2016, 22, 5899–5914. [Google Scholar] [CrossRef] [PubMed]
- Morrison, R.K.; Brown, D.E.; Oleson, J.J.; Cooney, D.A. Oral toxicology studies with lapachol. Toxicol. Appl. Pharmacol. 1970, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Uysal, S.; Soyer, Z.; Saylam, M.; Tarikogullari, A.H.; Yilmazm, S.; Kirmizibayrak, P.B. Design, synthesis and biological evaluation of novel naphthoquinone-4-aminobenzensulfonamide/carboxamide derivatives as proteasome inhibitors. Eur. J. Med. Chem. 2021, 209, 112890–112905. [Google Scholar] [CrossRef] [PubMed]
- Kayashima, T.; Mori, M.; Yoshida, H.; Mizushina, Y.; Marsubara, K. 1,4-Naphthoquinone is a potent inhibitor of human cancer cell growth and angiogenesis. Cancer Lett. 2009, 278, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.H.; Dreckschmidt, N.E.; Verna, A.K. Plumbagin, a medicinal plant-derived naphthoquinone, is a novel inhibitor of the growth and invasion of hormone-refractory prostate cancer. Cancer Res. 2008, 68, 9024–9032. [Google Scholar] [CrossRef]
- Bansal, Y.; Silakari, O. Multifunctional compounds: Smart molecules for multifactorial diseases. Eur. J. Med. Chem. 2014, 76, 31–42. [Google Scholar] [CrossRef]
- Morphy, R.; Rankovic, Z. Designing multiple ligands-Medicinal chemistry strategies and challenges. Curr. Pharm. Des. 2009, 15, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Branco, J.R.; Oliveira, V.G.; Esteves, A.M.; Chipoline, I.C.; Lima, M.F.O.; Boechat, F.C.S.; Silva, F.C.; Ferreira, V.F.; Sola-Penna, M.; Souza, M.C.B.V.; et al. A novel naphthotriazolyl-4-oxoquinolone derivative that selectively controls breast cancer cells survival through the induction of apoptosis. Curr. Top. Med. Chem. 2018, 18, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Nascimento Mello, A.L.; Sagrillo, F.S.; de Souza, A.G.; Costa, A.R.P.; Campos, V.R.; Cunha, A.C.; Imbroisi Filho, R.; da Costa Santos Boechat, F.; Sola-Penna, M.; de Souza, M.C.B.V.; et al. Selective AMPK activator leads to unfolded protein response downregulation and induces breast cancer cell death and autophagy. Life Sci. 2021, 276, 119470. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.G.; dos Santos Faiões, V.; Gonçalves, G.B.R.; Lima, M.F.O.; Boechat, F.C.S.; Cunha, A.C.; de Andrade-Neto, V.V.; de C da Silva, F.; Torres-Santos, E.C.; de Souza, M.C.B.V. Design, Synthesis and Antileishmanial Activity of Naphthotriazolyl-4-Oxoquinolines. Curr. Top. Med. Chem. 2018, 18, 1454–1464. [Google Scholar] [CrossRef]
- Gould, R.G.; Jacobs, W.A. The Synthesis of Certain Substituted Quinolines and 5,6-Benzoquinolines. J. Am. Chem. Soc. 1939, 61, 2890. [Google Scholar] [CrossRef]
- Duffin, G.F.; Kendall, J.D. The preparation of 4-hydroxyquinoline derivatives from aromatic amines and ethyl ethoxymethylene-malonate. J. Chem. Soc. 1948, 70, 893. [Google Scholar]
- Ruxer, J.M.; Lachoux, C.; Ousset, J.B.; Torregrosa, J.L.; Mattioda, G.J. Synthesis of 1,4-dihydro-4-oxopyrrolo [1,2-b]pyridazine-3-carboxylic acids and 1,4-dihydro-4-oxoimidazo [1,5-b]pyridazine-3-carboxylic acids as potential antibacterial agents. J. Heterocycl. Chem. 1994, 31, 409. [Google Scholar] [CrossRef]
- Lamblin, M.; Naturale, G.; Dessolin, J.; Felpin, F.X. Direct C-H Arylation of Quinones with Anilines. Synlett 2012, 23, 1621–1624. [Google Scholar] [CrossRef]
- Indrayanto, G.; Putra, G.S.; Suhud, F. Validation of in-vitro bioassay methods: Application in herbal drug research. Profiles Drug Subst. Excip. Relat. Methodol. 2021, 46, 273–307. [Google Scholar]
- Kciuk, M.; Gielecińska, A.; Mujwar, S.; Kołat, D.; Kałuzińska-Kołat, Ż.; Celik, I.; Kontek, R. Doxorubicin-An Agent with Multiple Mechanisms of Anticancer Activity. Cells 2023, 12, 659. [Google Scholar] [CrossRef]
- Rajendran, V.; Jain, M.V. In Vitro Tumorigenic Assay: Colony Forming Assay for Cancer Stem Cells. Methods Mol. Biol. 2018, 1692, 89–95. [Google Scholar] [PubMed]
- Baumann, M.; Dubois, W.; Suit, H.D. Response of human squamous cell carcinoma xenografts of different sizes to irradiation: Relationship of clonogenic cells, cellular radiation sensitivity in vivo, and tumor rescuing units. Radiat. Res. 1990, 123, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Zips, D.; Thames, H.D.; Baumann, M. New anticancer agents: In vitro and in vivo evaluation. In Vivo 2005, 19, 1–7. [Google Scholar] [PubMed]
- Decarli, M.C.; Amaral, R.; Santos, D.P.D.; Tofani, L.B.; Katayama, E.; Rezende, R.A.; Silva, J.V.L.D.; Swiech, K.; Suazo, C.A.T.; Mota, C.; et al. Cell spheroids as a versatile research platform: Formation mechanisms, high throughput production, characterization and applications. Biofabrication 2021, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Lin, C.; Cheng, J.; Su, J.; Zhao, H.; Liu, T.; Wen, X.; Zhao, P. Generation of Multicellular Tumor Spheroids with Microwell-Based Agarose Scaffolds for Drug Testing. PLoS ONE 2015, 10, e0130348. [Google Scholar] [CrossRef] [PubMed]
- Benton, G.; DeGray, G.; Kleinman, H.K.; George, J.; Arnaoutova, I. In vitro microtumors provide a physiologically predictive tool for breast cancer therapeutic screening. PLoS ONE 2015, 9, e0123312. [Google Scholar] [CrossRef] [PubMed]
- Imamura, Y.; Mukohara, T.; Shimono, Y.; Funakoshi, Y.; Chayahara, N.; Toyoda, M.; Kiyota, N.; Takao, S.; Kono, S.; Nakatsura, T.; et al. Comparison of 2D- and 3D-culture models as drug-testing platforms in breast cancer. Oncol. Rep. 2015, 33, 1837–1843. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1998, 1, 55. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2012, 64, 4. [Google Scholar] [CrossRef]
- Boechat, F.C.S.; Sacramento, C.Q.; Cunha, A.C.; Sagrillo, F.S.; Nogueira, C.M.; Fintelman-Rodrigues, N.; Santos-Filho, O.; Riscado, C.S.; Forezi, L.S.M.; Faro, L.; et al. 1,2,3-triazolyl-1-4-oxoquinolines: A feasible beginning for promising chemical structures do inhibit oseltamivir-resistant influenza A and B viruses. Bioorg. Med. Chem. 2015, 23, 7777–7784. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.R.P.; Muxfeldt, M.; Boechat, F.; Da, C.S.; De Souza, M.C.B.V.; Silva, J.L.; De Moraes, M.C.; Rangel, L.P.; Vieira, T.C.R.G.; Batalha, P.N. Aminoquinolones and their benzoquinone dimer hybrids as modulators of prion protein conversion. Molecules 2022, 27, 7935. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Paz, M.M.; Ferretti, G.D.S.; Martins-Dinis, M.M.C.; Ferreira, B.I.S.; Faier-Pereira, A.; Barnoud, T.; Moreira, O.C.; Silva, J.L.; Cordeiro, Y.; Rangel, L.P. PRIMA-1 inhibits Y220C p53 amyloid aggregation and synergizes with cisplatin in hepatocellular carcinoma. Front. Mol. Biosci. 2023, 10, 1165132. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, J.; Eder, W.; Castaneda, J.; Doss, M.; Huber, E.; Ebner, R.; Kunz-Schughart, L.A. A reliable tool to determine cell viability in complex 3-d culture: The acid phosphatase assay. SLAS Discov. 2007, 12, 925–937, Erratum in SLAS Discov. 2007, 12, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.; Brünner, N.; Katzenellenbogen, B.S.; Thompson, E.W.; Norman, M.J.; Koppi, C.; Paik, S.; Lippman, M.E.; Dickson, R.B. Progression of human breast cancer cells from hormone-dependent to hormone-independent growth both in vitro and in vivo. Proc. Natl. Acad. Sci. USA 1989, 86, 3649–3653. [Google Scholar] [CrossRef]
- Yin, K.B. The Mesenchymal-Like Phenotype of the MDA-MB-231 Cell Line. In Breast Cancer-Focusing Tumor Microenvironment, Stem Cells and Metastasis; Gunduz, M., Gunduz, E., Eds.; InTech: London, UK, 2011. [Google Scholar]
- Walerych, D.; Napoli, M.; Collavin, L.; Del Sal, G. The rebel angel: Mutant p53 as the driving oncogene in breast cancer. Carcinogenesis 2012, 33, 2007–2017. [Google Scholar] [CrossRef]



| IC50 ± SEM (µM) | SI | ||||
|---|---|---|---|---|---|
| Compound | MCF-7 | MDA-MB-231 | MCF10A | IC50 MCF10A/IC50 MCF-7 | IC50 MCF10A/ IC50 MDA-MB-231 |
| 1,4-Naphthoquinone | 29.52 ± 1.0 | 19.29 ± 1.1 | 8.78 ± 1.1 | 0.30 ± 0.13 | 0.46 ± 0.14 |
| 11a | 18.65 ± 1.1 | 9.16 ± 1.0 | 10.60 ± 1.2 | 0.57 ± 0.12 | 1.15 ± 0.15 |
| 11b | 19.45 ± 1.1 | 15.05 ± 1.0 | 10.71 ± 1.3 | 0.55 ± 0.13 | 0.71 ± 0.14 |
| 11c | 32.84 ± 1.1 | 16.40 ± 1.1 | 10.25 ± 1.1 | 0.31 ± 0.11 | 0.62 ± 0.12 |
| 11d | 25.14 ± 1.2 | 20.12 ± 1.1 | 21.49 ± 1.2 | 0.85 ± 0.07 | 1.07 ± 0.08 |
| 11e | ND * | ND * | ND * | ND * | ND * |
| 11f | 65.70 ± 1.1 | 24.10 ± 1.1 | 6.92 ± 1.1 | 0.11 ± 0.16 | 0.29 ± 0.16 |
| Doxorubicin | 14.22 ± 1.1 | 2.76 ± 1.2 | 1.49 ± 1.3 | 0.10 ± 0.09 | 0.54 ± 0.10 |
| Compound | MW a | nHA b | nAHA c | nRotB d | nHBA e | nHBD f | MR g | TPSA h | MlogP i | ESOL j |
|---|---|---|---|---|---|---|---|---|---|---|
| 11a | 401.41 | 30 | 16 | 5 | 5 | 0 | 113.44 | 82.44 | 1.75 | MS |
| 11b | 449.45 | 34 | 22 | 5 | 5 | 0 | 128.71 | 82.44 | 2.63 | MS |
| 11c | 443.49 | 33 | 16 | 8 | 5 | 0 | 127.86 | 82.44 | 2.37 | MS |
| 11d | 373.36 | 28 | 16 | 3 | 5 | 1 | 104.32 | 93.44 | 1.33 | MS |
| 11e | 435.43 | 33 | 22 | 4 | 5 | 1 | 124.0 | 93.44 | 2.16 | MS |
| 11f | 415.44 | 31 | 16 | 6 | 5 | 1 | 118.74 | 93.44 | 1.96 | MS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Gama Oliveira, V.; Muxfeldt, M.; Muniz da Paz, M.; Silva Coutinho, M.; Eduardo dos Santos, R.; Diniz da Silva Ferretti, G.; Ferraz da Costa, D.C.; Fonseca Regufe, P.; Lelis Gama, I.; da Costa Santos Boechat, F.; et al. Naphthoquinone-Quinolone Hybrids with Antitumor Effects on Breast Cancer Cell Lines—From the Synthesis to 3D-Cell Culture Effects. Int. J. Mol. Sci. 2024, 25, 6490. https://doi.org/10.3390/ijms25126490
da Gama Oliveira V, Muxfeldt M, Muniz da Paz M, Silva Coutinho M, Eduardo dos Santos R, Diniz da Silva Ferretti G, Ferraz da Costa DC, Fonseca Regufe P, Lelis Gama I, da Costa Santos Boechat F, et al. Naphthoquinone-Quinolone Hybrids with Antitumor Effects on Breast Cancer Cell Lines—From the Synthesis to 3D-Cell Culture Effects. International Journal of Molecular Sciences. 2024; 25(12):6490. https://doi.org/10.3390/ijms25126490
Chicago/Turabian Styleda Gama Oliveira, Vanessa, Marcelly Muxfeldt, Mariana Muniz da Paz, Mayra Silva Coutinho, Raissa Eduardo dos Santos, Giulia Diniz da Silva Ferretti, Danielly C. Ferraz da Costa, Pedro Fonseca Regufe, Ivson Lelis Gama, Fernanda da Costa Santos Boechat, and et al. 2024. "Naphthoquinone-Quinolone Hybrids with Antitumor Effects on Breast Cancer Cell Lines—From the Synthesis to 3D-Cell Culture Effects" International Journal of Molecular Sciences 25, no. 12: 6490. https://doi.org/10.3390/ijms25126490
APA Styleda Gama Oliveira, V., Muxfeldt, M., Muniz da Paz, M., Silva Coutinho, M., Eduardo dos Santos, R., Diniz da Silva Ferretti, G., Ferraz da Costa, D. C., Fonseca Regufe, P., Lelis Gama, I., da Costa Santos Boechat, F., Silva Lima, E., Ferreira, V. F., de Moraes, M. C., Bastos Vieira de Souza, M. C., Netto Batalha, P., & Pereira Rangel, L. (2024). Naphthoquinone-Quinolone Hybrids with Antitumor Effects on Breast Cancer Cell Lines—From the Synthesis to 3D-Cell Culture Effects. International Journal of Molecular Sciences, 25(12), 6490. https://doi.org/10.3390/ijms25126490







