Modeling of Blood–Brain Barrier (BBB) Dysfunction and Immune Cell Migration Using Human BBB-on-a-Chip for Drug Discovery Research
Abstract
:1. Introduction
2. Results
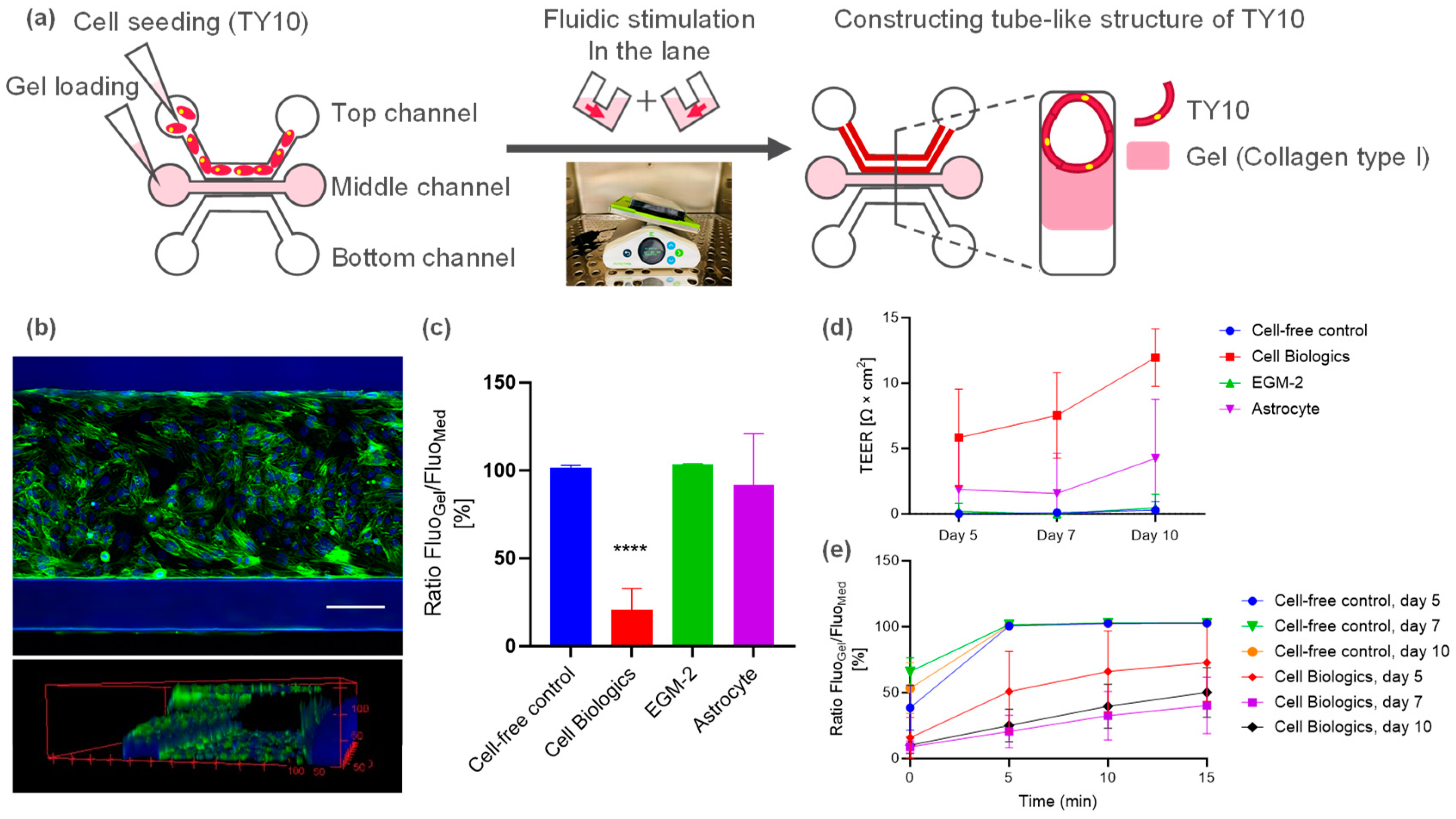
2.1. Microfluidic Culture of Human Brain Endothelial Cell (TY10)
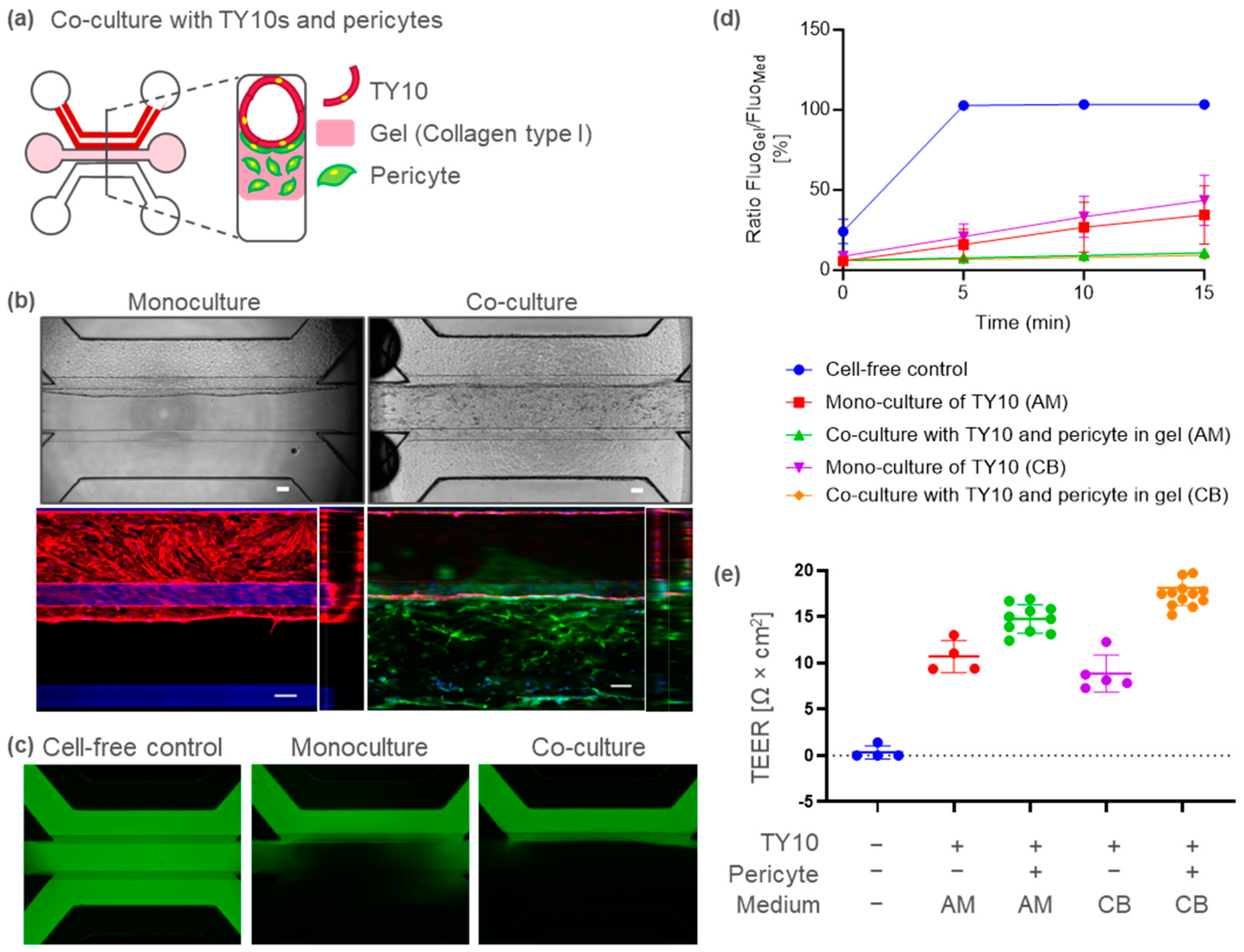
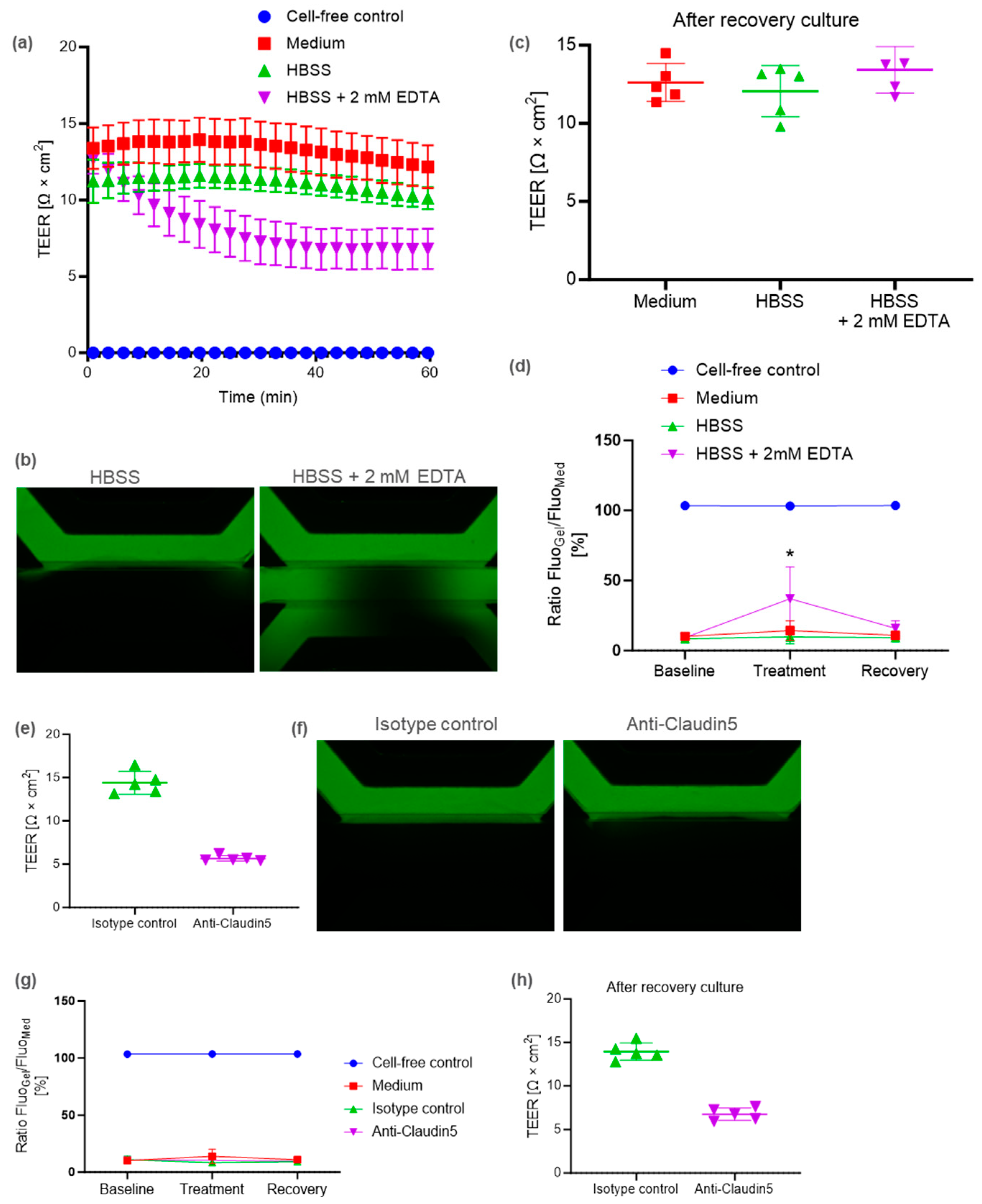
2.2. BBB-on-a-Chip Containing Pericytes to Model Barrier Disruption
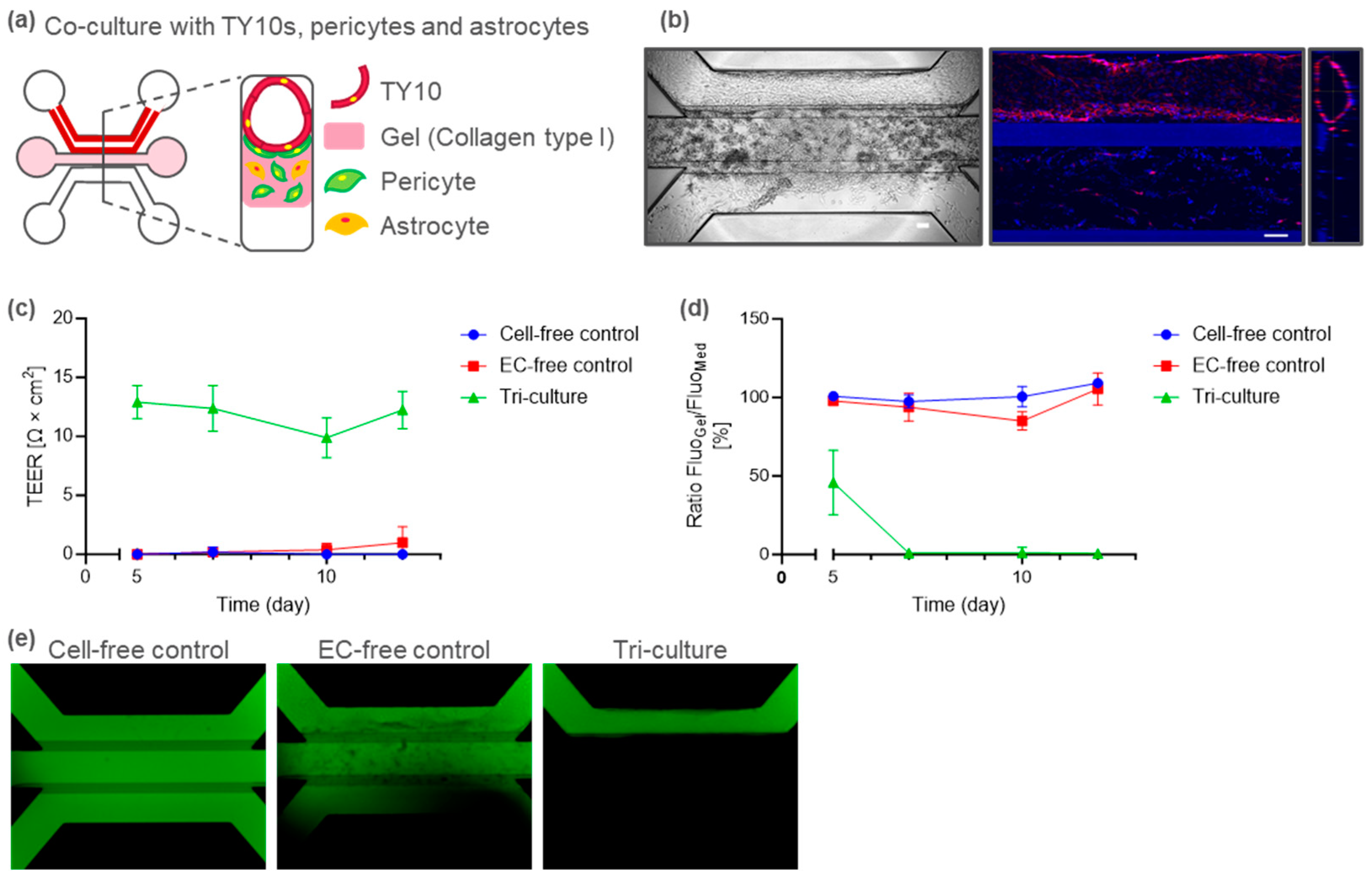
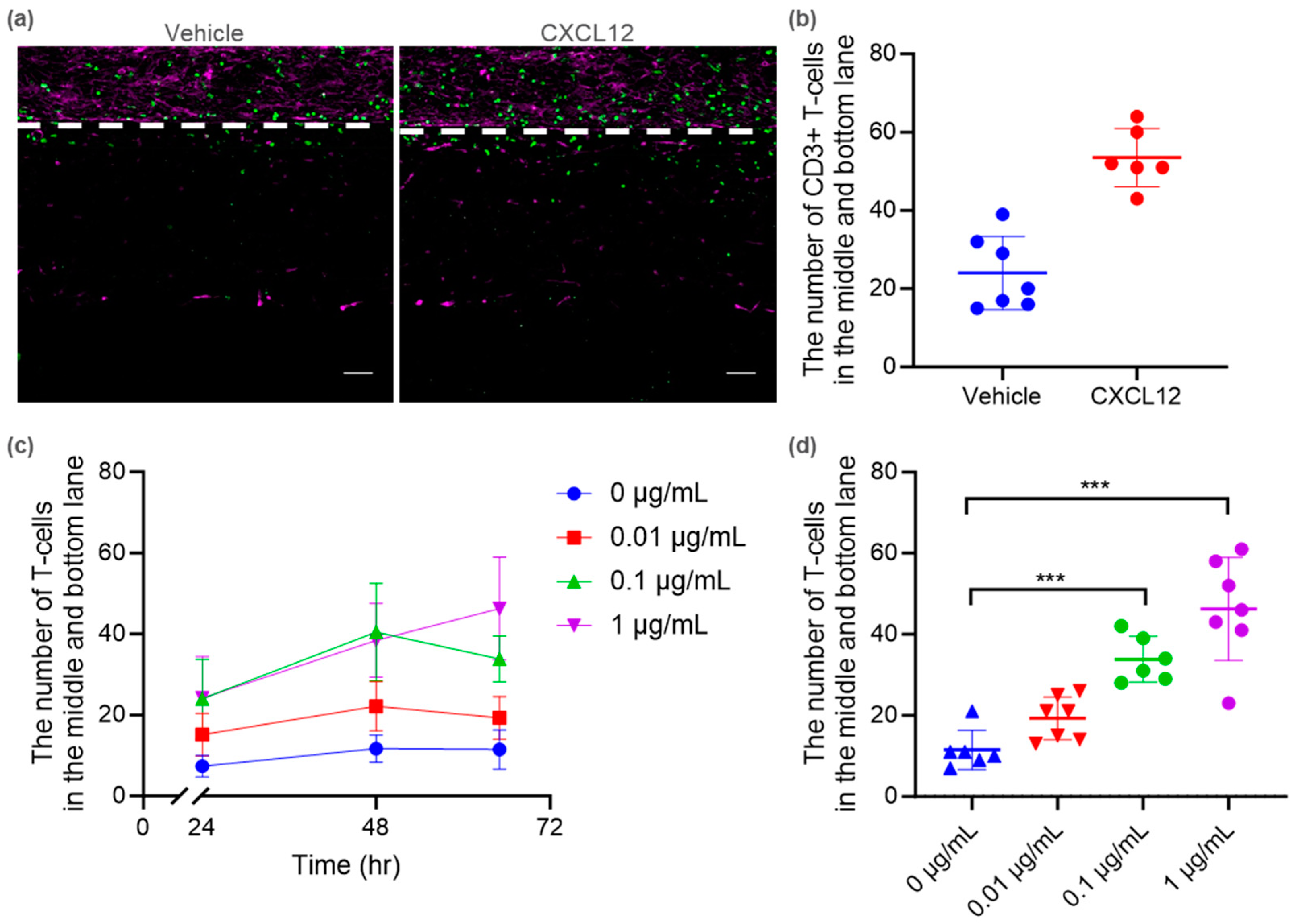
2.3. Tri-Culture BBB-on-a-Chip for Immune Cell Migration
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Culture of TY10 Microvessels in OrganoPlate 3-Lane
4.3. BBB-on-a-Chip Co-/Tri-Cultured in OrganoPlate 3-Lane
4.4. TEER Measurements
4.5. Barrier Integrity Assay
4.6. Barrier Disruption Assay
4.7. T Cell Isolation, Stimulation, and Labelling
4.8. Immune Cell Migration Assay
4.9. Immunocytochemistry
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte-Endothelial Interactions at the Blood-Brain Barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Hawkins, B.T.; Davis, T.P. The Blood-Brain Barrier/Neurovascular Unit in Health and Disease. Pharmacol. Rev. 2005, 57, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, F.; Nishihara, H.; Kanda, T. Blood-Brain Barrier Dysfunction in Immuno-Mediated Neurological Diseases. Immunol. Med. 2018, 41, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Borges, N.; Shi, F.; Azevedo, I.; Audus, K.L. Changes in Brain Microvessel Endothelial Cell Monolayer Permeability Induced by Adrenergic Drugs. Eur. J. Pharmacol. 1994, 269, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.L.; England, T.J.; O’Sullivan, S.E. A Novel Transwell Blood Brain Barrier Model Using Primary Human Cells. Front. Cell. Neurosci. 2019, 13, 230. [Google Scholar] [CrossRef] [PubMed]
- Cucullo, L.; Hossain, M.; Puvenna, V.; Marchi, N.; Janigro, D. The Role of Shear Stress in Blood-Brain Barrier Endothelial Physiology. BMC Neurosci. 2011, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Griep, L.M.; Wolbers, F.; de Wagenaar, B.; ter Braak, P.M.; Weksler, B.B.; Romero, I.A.; Couraud, P.O.; Vermes, I.; van der Meer, A.D.; van den Berg, A. BBB on Chip: Microfluidic Platform to Mechanically and Biochemically Modulate Blood-Brain Barrier Function. Biomed. Microdevices 2013, 15, 145–150. [Google Scholar] [CrossRef] [PubMed]
- van Buul, J.D.; Hordijk, P.L. Signaling in Leukocyte Transendothelial Migration. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 824–833. [Google Scholar] [CrossRef]
- Vestweber, D. How Leukocytes Cross the Vascular Endothelium. Nat. Rev. Immunol. 2015, 15, 692–704. [Google Scholar] [CrossRef]
- Kurosawa, T.; Sako, D.; Tega, Y.; Debori, Y.; Tomihara, Y.; Aoyama, K.; Kubo, Y.; Amano, N.; Deguchi, Y. Construction and Functional Evaluation of a Three-Dimensional Blood-Brain Barrier Model Equipped with Human Induced Pluripotent Stem Cell-Derived Brain Microvascular Endothelial Cells. Pharm. Res. 2022, 39, 1535–1547. [Google Scholar] [CrossRef]
- Park, T.E.; Mustafaoglu, N.; Herland, A.; Hasselkus, R.; Mannix, R.; FitzGerald, E.A.; Prantil-Baun, R.; Watters, A.; Henry, O.; Benz, M.; et al. Hypoxia-Enhanced Blood-Brain Barrier Chip Recapitulates Human Barrier Function and Shuttling of Drugs and Antibodies. Nat. Commun. 2019, 10, 2621. [Google Scholar] [CrossRef] [PubMed]
- Wevers, N.R.; Kasi, D.G.; Gray, T.; Wilschut, K.J.; Smith, B.; van Vught, R.; Shimizu, F.; Sano, Y.; Kanda, T.; Marsh, G.; et al. A Perfused Human Blood-Brain Barrier on-a-Chip for High-Throughput Assessment of Barrier Function and Antibody Transport. Fluids Barriers CNS 2018, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.I.; Sei, Y.J.; Park, H.J.; Kim, J.; Ryu, Y.; Choi, J.J.; Sung, H.J.; MacDonald, T.J.; Levey, A.I.; Kim, Y. Microengineered Human Blood-Brain Barrier Platform for Understanding Nanoparticle Transport Mechanisms. Nat. Commun. 2020, 11, 175. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.L.; Campisi, M.; Osaki, T.; Possenti, L.; Mattu, C.; Adriani, G.; Kamm, R.D.; Chiono, V. Modeling Nanocarrier Transport across a 3D in Vitro Human Blood-Brain-Barrier Microvasculature. Adv. Healthc. Mater. 2020, 9, e1901486. [Google Scholar] [CrossRef] [PubMed]
- Maoz, B.M.; Herland, A.; FitzGerald, E.A.; Grevesse, T.; Vidoudez, C.; Pacheco, A.R.; Sheehy, S.P.; Park, T.E.; Dauth, S.; Mannix, R.; et al. A Linked Organ-on-Chip Model of the Human Neurovascular Unit Reveals the Metabolic Coupling of Endothelial and Neuronal Cells. Nat. Biotechnol. 2018, 36, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.; Hawkins, B.T.; Yun, Y. Three-Dimensional (3D) Tetra-Culture Brain on Chip Platform for Organophosphate Toxicity Screening. Sci. Rep. 2018, 8, 2841. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, Y.B.; Charlebois, C.; Nguyen, T.; Liu, Z.; Bloemberg, D.; Zafer, A.; Baumann, E.; Sodja, C.; Leclerc, S.; et al. Application of Blood Brain Barrier Models in Pre-Clinical Assessment of Glioblastoma-Targeting Car-T Based Immunotherapies. Fluids Barriers CNS 2022, 19, 38. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.A.; Codreanu, S.G.; Shi, M.; Sherrod, S.D.; Markov, D.A.; Neely, M.D.; Britt, C.M.; Hoilett, O.S.; Reiserer, R.S.; Samson, P.C.; et al. Metabolic Consequences of Inflammatory Disruption of the Blood-Brain Barrier in an Organ-on-Chip Model of the Human Neurovascular Unit. J. Neuroinflam. 2016, 13, 306. [Google Scholar] [CrossRef]
- Herland, A.; van der Meer, A.D.; FitzGerald, E.A.; Park, T.E.; Sleeboom, J.J.; Ingber, D.E. Distinct Contributions of Astrocytes and Pericytes to Neuroinflammation Identified in a 3D Human Blood-Brain Barrier on a Chip. PLoS ONE 2016, 11, e0150360. [Google Scholar] [CrossRef]
- Vatine, G.D.; Barrile, R.; Workman, M.J.; Sances, S.; Barriga, B.K.; Rahnama, M.; Barthakur, S.; Kasendra, M.; Lucchesi, C.; Kerns, J.; et al. Human iPSC-Derived Blood-Brain Barrier Chips Enable Disease Modeling and Personalized Medicine Applications. Cell Stem Cell 2019, 24, 995–1005.e1006. [Google Scholar] [CrossRef]
- Nair, A.L.; Groenendijk, L.; Overdevest, R.; Fowke, T.M.; Annida, R.; Mocellin, O.; de Vries, H.E.; Wevers, N.R. Human BBB-on-a-Chip Reveals Barrier Disruption, Endothelial Inflammation, and T Cell Migration under Neuroinflammatory Conditions. Front. Mol. Neurosci. 2023, 16, 1250123. [Google Scholar] [CrossRef] [PubMed]
- Buzhdygan, T.P.; DeOre, B.J.; Baldwin-Leclair, A.; Bullock, T.A.; McGary, H.M.; Khan, J.A.; Razmpour, R.; Hale, J.F.; Galie, P.A.; Potula, R.; et al. The SARS-CoV-2 Spike Protein Alters Barrier Function in 2D Static and 3D Microfluidic In-Vitro Models of the Human Blood-Brain Barrier. Neurobiol. Dis. 2020, 146, 105131. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, K.T.; Lee, J.S.; Shin, J.; Cui, B.; Yang, K.; Choi, Y.S.; Choi, N.; Lee, S.H.; Lee, J.H.; et al. Fungal Brain Infection Modelled in a Human-Neurovascular-Unit-on-a-Chip with a Functional Blood-Brain Barrier. Nat. Biomed. Eng. 2021, 5, 830–846. [Google Scholar] [CrossRef] [PubMed]
- Pediaditakis, I.; Kodella, K.R.; Manatakis, D.V.; Le, C.Y.; Hinojosa, C.D.; Tien-Street, W.; Manolakos, E.S.; Vekrellis, K.; Hamilton, G.A.; Ewart, L.; et al. Modeling Alpha-Synuclein Pathology in a Human Brain-Chip to Assess Blood-Brain Barrier Disruption. Nat. Commun. 2021, 12, 5907. [Google Scholar] [CrossRef] [PubMed]
- van Meer, B.J.; de Vries, H.; Firth, K.S.A.; van Weerd, J.; Tertoolen, L.G.J.; Karperien, H.B.J.; Jonkheijm, P.; Denning, C.; IJzerman, A.P.; Mummery, C.L. Small Molecule Absorption by Pdms in the Context of Drug Response Bioassays. Biochem. Biophys. Res. Commun. 2017, 482, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Delsing, L.; Donnes, P.; Sánchez, J.; Clausen, M.; Voulgaris, D.; Falk, A.; Herland, A.; Brolén, G.; Zetterberg, H.; Hicks, R.; et al. Barrier Properties and Transcriptome Expression in Human iPSC-Derived Models of the Blood-Brain Barrier. Stem Cells 2018, 36, 1816–1827. [Google Scholar] [CrossRef] [PubMed]
- Helms, H.C.; Abbott, N.J.; Burek, M.; Cecchelli, R.; Couraud, P.O.; Deli, M.A.; Forster, C.; Galla, H.J.; Romero, I.A.; Shusta, E.V.; et al. In Vitro Models of the Blood-Brain Barrier: An Overview of Commonly Used Brain Endothelial Cell Culture Models and Guidelines for Their Use. J. Cereb. Blood Flow Metab. 2016, 36, 862–890. [Google Scholar] [CrossRef] [PubMed]
- Gericke, B.; Römermann, K.; Noack, A.; Noack, S.; Kronenberg, J.; Blasig, I.E.; Löscher, W. A Face-to-Face Comparison of Claudin-5 Transduced Human Brain Endothelial (hCMEC/D3) Cells with Porcine Brain Endothelial Cells as Blood-Brain Barrier Models for Drug Transport Studies. Fluids Barriers CNS 2020, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Campisi, M.; Shin, Y.; Osaki, T.; Hajal, C.; Chiono, V.; Kamm, R.D. 3D Self-Organized Microvascular Model of the Human Blood-Brain Barrier with Endothelial Cells, Pericytes and Astrocytes. Biomaterials 2018, 180, 117–129. [Google Scholar] [CrossRef]
- Sano, Y.; Shimizu, F.; Abe, M.; Maeda, T.; Kashiwamura, Y.; Ohtsuki, S.; Terasaki, T.; Obinata, M.; Kajiwara, K.; Fujii, M.; et al. Establishment of a New Conditionally Immortalized Human Brain Microvascular Endothelial Cell Line Retaining an in Vivo Blood-Brain Barrier Function. J. Cell Physiol. 2010, 225, 519–528. [Google Scholar] [CrossRef]
- Maeda, T.; Sano, Y.; Abe, M.; Shimizu, F.; Kashiwamura, Y.; Ohtsuki, S.; Terasaki, T.; Obinata, M.; Ueda, M.; Kanda, T. Establishment and Characterization of Spinal Cord Microvascular Endothelial Cell Lines. Clin. Exp. Neuroimmunol. 2013, 4, 326–338. [Google Scholar] [CrossRef]
- Fengler, S.; Kurkowsky, B.; Kaushalya, S.K.; Roth, W.; Fava, E.; Denner, P. Human iPSC-Derived Brain Endothelial Microvessels in a Multi-Well Format Enable Permeability Screens of Anti-Inflammatory Drugs. Biomaterials 2022, 286, 121525. [Google Scholar] [CrossRef]
- Armulik, A.; Genové, G.; Mäe, M.; Nisancioglu, M.H.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K.; et al. Pericytes Regulate the Blood-Brain Barrier. Nature 2010, 468, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Zhou, L.; Kebede, A.A.; Barres, B.A. Pericytes Are Required for Blood-Brain Barrier Integrity During Embryogenesis. Nature 2010, 468, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Montagne, A.; Barnes, S.R.; Sweeney, M.D.; Halliday, M.R.; Sagare, A.P.; Zhao, Z.; Toga, A.W.; Jacobs, R.E.; Liu, C.Y.; Amezcua, L.; et al. Blood-Brain Barrier Breakdown in the Aging Human Hippocampus. Neuron 2015, 85, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Hajal, C.; Offeddu, G.S.; Shin, Y.; Zhang, S.; Morozova, O.; Hickman, D.; Knutson, C.G.; Kamm, R.D. Engineered Human Blood-Brain Barrier Microfluidic Model for Vascular Permeability Analyses. Nat. Protoc. 2022, 17, 95–128. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Chung, M.; Lee, S.R.; Jeon, N.L. 3D Brain Angiogenesis Model to Reconstitute Functional Human Blood-Brain Barrier in Vitro. Biotechnol. Bioeng. 2020, 117, 748–762. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.I.; Abaci, H.E.; Shuler, M.L. Microfluidic Blood-Brain Barrier Model Provides in Vivo-Like Barrier Properties for Drug Permeability Screening. Biotechnol. Bioeng. 2017, 114, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Stamatovic, S.M.; Johnson, A.M.; Keep, R.F.; Andjelkovic, A.V. Junctional Proteins of the Blood-Brain Barrier: New Insights into Function and Dysfunction. Tissue Barriers 2016, 4, e1154641. [Google Scholar] [CrossRef]
- Greene, C.; Kealy, J.; Humphries, M.M.; Gong, Y.; Hou, J.; Hudson, N.; Cassidy, L.M.; Martiniano, R.; Shashi, V.; Hooper, S.R.; et al. Dose-Dependent Expression of Claudin-5 Is a Modifying Factor in Schizophrenia. Mol. Psychiatry 2018, 23, 2156–2166. [Google Scholar] [CrossRef]
- Greene, C.; Hanley, N.; Campbell, M. Blood-Brain Barrier Associated Tight Junction Disruption Is a Hallmark Feature of Major Psychiatric Disorders. Transl. Psychiatry 2020, 10, 373. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Shirakura, K.; Okada, Y.; Takeda, H.; Endo, K.; Tamura, M.; Watari, A.; Sadamura, Y.; Sawasaki, T.; Doi, T.; et al. Claudin-5-Binders Enhance Permeation of Solutes across the Blood-Brain Barrier in a Mammalian Model. J. Pharmacol. Exp. Ther. 2017, 363, 275–283. [Google Scholar] [CrossRef]
- Nitta, T.; Hata, M.; Gotoh, S.; Seo, Y.; Sasaki, H.; Hashimoto, N.; Furuse, M.; Tsukita, S. Size-Selective Loosening of the Blood-Brain Barrier in Claudin-5-Deficient Mice. J. Cell Biol. 2003, 161, 653–660. [Google Scholar] [CrossRef]
- Gonzalez, H.; Pacheco, R. T-Cell-Mediated Regulation of Neuroinflammation Involved in Neurodegenerative Diseases. J. Neuroinflam. 2014, 11, 201. [Google Scholar] [CrossRef] [PubMed]
- Pilli, D.; Zou, A.; Tea, F.; Dale, R.C.; Brilot, F. Expanding Role of T Cells in Human Autoimmune Diseases of the Central Nervous System. Front. Immunol. 2017, 8, 652. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, H.; Brück, W.; Lucchinetti, C.F. The Immunopathology of Multiple Sclerosis: An Overview. Brain Pathol. 2007, 17, 210–218. [Google Scholar] [CrossRef]
- Calderon, T.M.; Eugenin, E.A.; Lopez, L.; Kumar, S.S.; Hesselgesser, J.; Raine, C.S.; Berman, J.W. A Role for CXCL12 (SDF-1alpha) in the Pathogenesis of Multiple Sclerosis: Regulation of CXCL12 Expression in Astrocytes by Soluble Myelin Basic Protein. J. Neuroimmunol. 2006, 177, 27–39. [Google Scholar] [CrossRef]
- Moll, N.M.; Cossoy, M.B.; Fisher, E.; Staugaitis, S.M.; Tucky, B.H.; Rietsch, A.M.; Chang, A.; Fox, R.J.; Trapp, B.D.; Ransohoff, R.M. Imaging Correlates of Leukocyte Accumulation and CXCR4/CXCL12 in Multiple Sclerosis. Arch. Neurol. 2009, 66, 44–53. [Google Scholar] [CrossRef]
- Brochard, V.; Combadiere, B.; Prigent, A.; Laouar, Y.; Perrin, A.; Beray-Berthat, V.; Bonduelle, O.; Alvarez-Fischer, D.; Callebert, J.; Launay, J.M.; et al. Infiltration of CD4+ Lymphocytes into the Brain Contributes to Neurodegeneration in a Mouse Model of Parkinson Disease. J. Clin. Investig. 2009, 119, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Gate, D.; Tapp, E.; Leventhal, O.; Shahid, M.; Nonninger, T.J.; Yang, A.C.; Strempfl, K.; Unger, M.S.; Fehlmann, T.; Oh, H.; et al. CD4(+) T Cells Contribute to Neurodegeneration in Lewy Body Dementia. Science 2021, 374, 868–874. [Google Scholar] [CrossRef]
- Han, R.T.; Kim, R.D.; Molofsky, A.V.; Liddelow, S.A. Astrocyte-Immune Cell Interactions in Physiology and Pathology. Immunity 2021, 54, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.M. Effect of Inflammatory Agents on Electrical Resistance across the Blood-Brain Barrier in Pial Microvessels of Anaesthetized Rats. Brain Res. 1995, 696, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.M.; Jones, H.C.; Abbott, N.J. Electrical Resistance across the Blood-Brain Barrier in Anaesthetized Rats: A Developmental Study. J. Physiol. 1990, 429, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Marx, U.; Akabane, T.; Andersson, T.B.; Baker, E.; Beilmann, M.; Beken, S.; Brendler-Schwaab, S.; Cirit, M.; David, R.; Dehne, E.M.; et al. Biology-Inspired Microphysiological Systems to Advance Patient Benefit and Animal Welfare in Drug Development. ALTEX 2020, 37, 365–394. [Google Scholar] [CrossRef] [PubMed]
- Han, J.J. Fda Modernization Act 2.0 Allows for Alternatives to Animal Testing. Artif. Organs 2023, 47, 449–450. [Google Scholar] [CrossRef]
- Shimizu, F.; Sano, Y.; Abe, M.A.; Maeda, T.; Ohtsuki, S.; Terasaki, T.; Kanda, T. Peripheral Nerve Pericytes Modify the Blood-Nerve Barrier Function and Tight Junctional Molecules through the Secretion of Various Soluble Factors. J. Cell. Physiol. 2011, 226, 255–266. [Google Scholar] [CrossRef]
- Haruki, H.; Sano, Y.; Shimizu, F.; Omoto, M.; Tasaki, A.; Oishi, M.; Koga, M.; Saito, K.; Takahashi, T.; Nakada, T.; et al. NMO Sera Down-Regulate AQP4 in Human Astrocyte and Induce Cytotoxicity Independent of Complement. J. Neurol. Sci. 2013, 331, 136–144. [Google Scholar] [CrossRef]
- Soragni, C.; Vergroesen, T.; Hettema, N.; Rabussier, G.; Lanz, H.L.; Trietsch, S.J.; de Windt, L.J.; Ng, C.P. Quantify Permeability Using on-a-Chip Models in High-Throughput Applications. STAR Protoc. 2023, 4, 102051. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohbuchi, M.; Shibuta, M.; Tetsuka, K.; Sasaki-Iwaoka, H.; Oishi, M.; Shimizu, F.; Nagasaka, Y. Modeling of Blood–Brain Barrier (BBB) Dysfunction and Immune Cell Migration Using Human BBB-on-a-Chip for Drug Discovery Research. Int. J. Mol. Sci. 2024, 25, 6496. https://doi.org/10.3390/ijms25126496
Ohbuchi M, Shibuta M, Tetsuka K, Sasaki-Iwaoka H, Oishi M, Shimizu F, Nagasaka Y. Modeling of Blood–Brain Barrier (BBB) Dysfunction and Immune Cell Migration Using Human BBB-on-a-Chip for Drug Discovery Research. International Journal of Molecular Sciences. 2024; 25(12):6496. https://doi.org/10.3390/ijms25126496
Chicago/Turabian StyleOhbuchi, Masato, Mayu Shibuta, Kazuhiro Tetsuka, Haruna Sasaki-Iwaoka, Masayo Oishi, Fumitaka Shimizu, and Yasuhisa Nagasaka. 2024. "Modeling of Blood–Brain Barrier (BBB) Dysfunction and Immune Cell Migration Using Human BBB-on-a-Chip for Drug Discovery Research" International Journal of Molecular Sciences 25, no. 12: 6496. https://doi.org/10.3390/ijms25126496
APA StyleOhbuchi, M., Shibuta, M., Tetsuka, K., Sasaki-Iwaoka, H., Oishi, M., Shimizu, F., & Nagasaka, Y. (2024). Modeling of Blood–Brain Barrier (BBB) Dysfunction and Immune Cell Migration Using Human BBB-on-a-Chip for Drug Discovery Research. International Journal of Molecular Sciences, 25(12), 6496. https://doi.org/10.3390/ijms25126496






