Substance P Promotes Leukocyte Infiltration in the Liver and Lungs of Mice with Sepsis: A Key Role for Adhesion Molecules on Vascular Endothelial Cells
Abstract
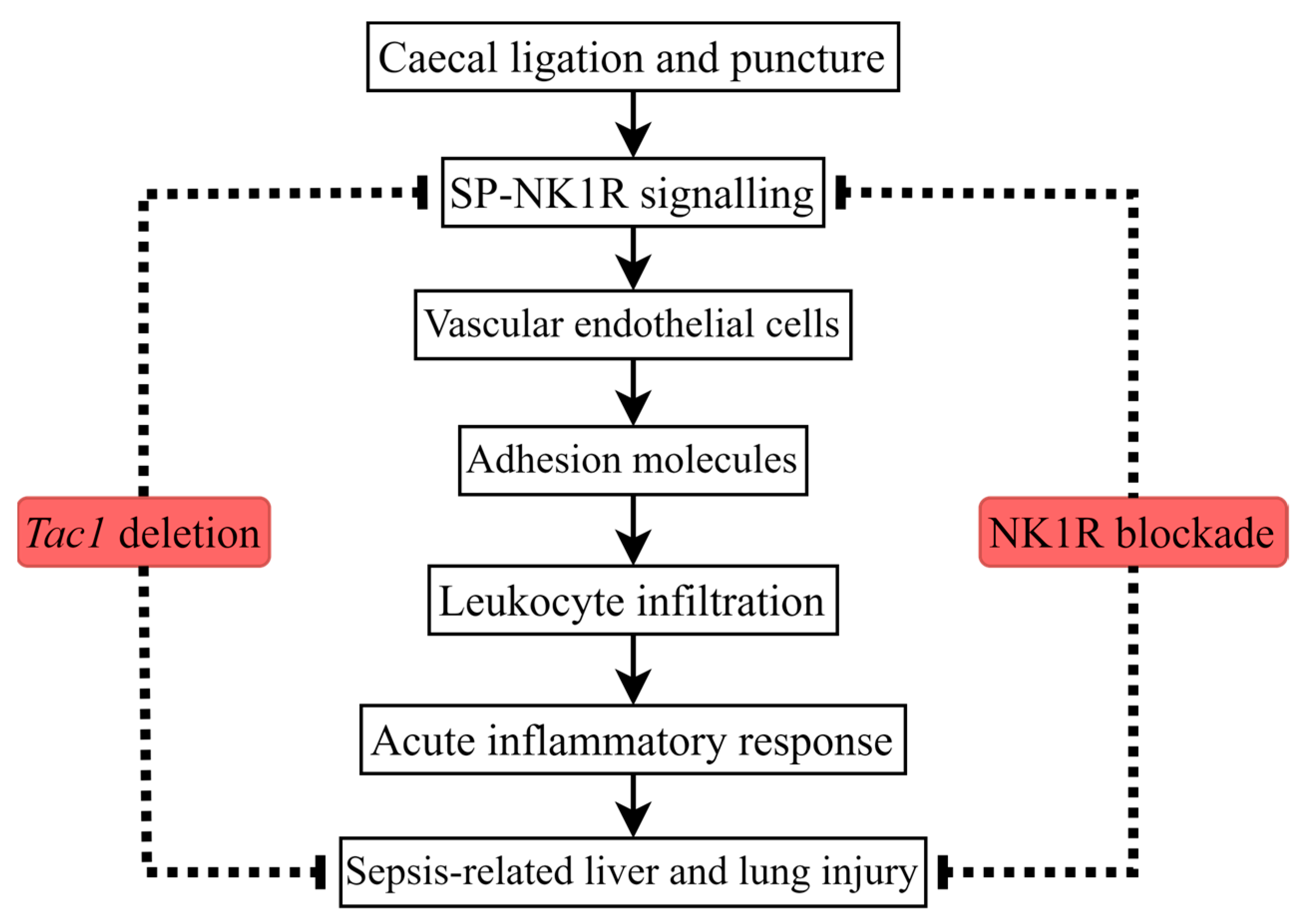
1. Introduction
2. Results
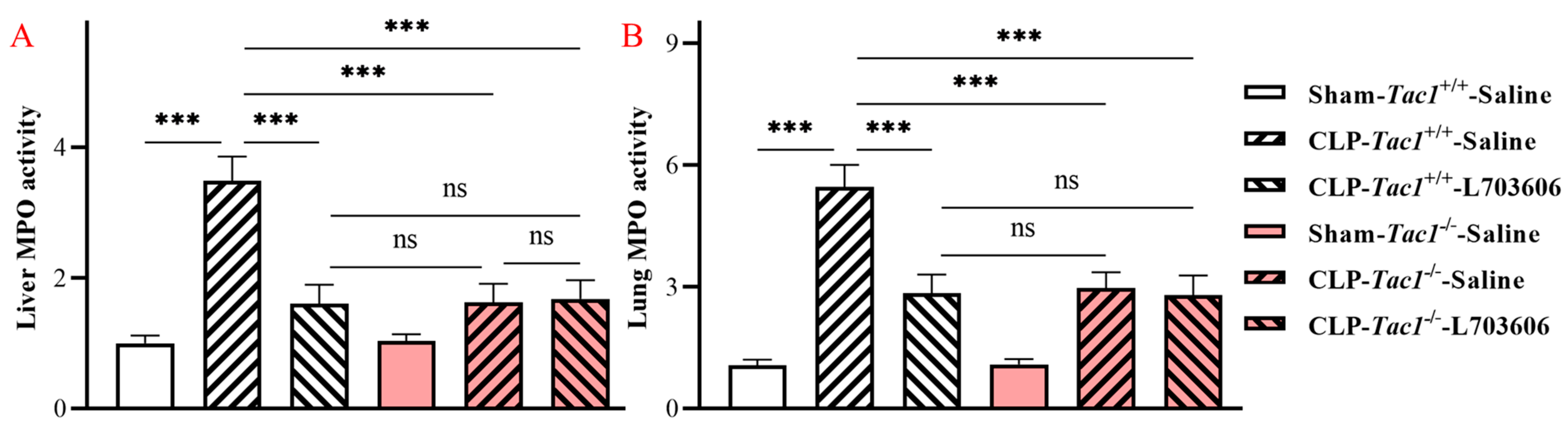
2.1. Inhibiting the Biosynthesis of SP or Blocking NK1R Mitigated the Elevation in the Activity of MPO in the Liver of Mice with Sepsis Induced by CLP Surgery
2.2. Inhibiting the Biosynthesis of SP or Blocking NK1R Attenuated the Increase in the Activity of MPO in the Lungs of Mice with Sepsis Induced by CLP Surgery
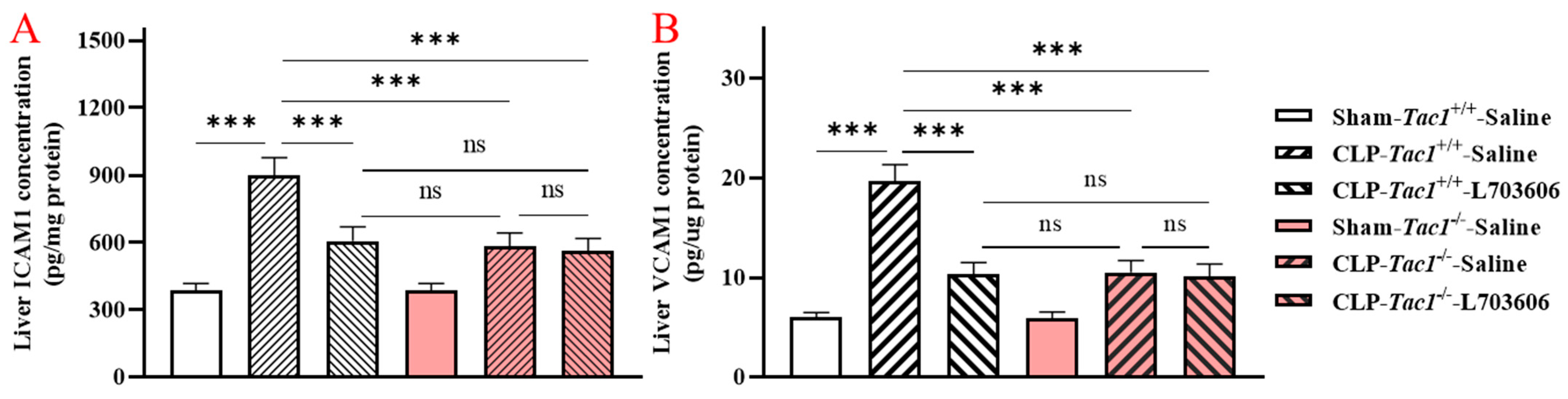
2.3. Inhibiting the Biosynthesis of SP or Blocking NK1R Attenuated the Increased Concentrations of ICAM1 and VCAM1 in the Liver of Mice with Sepsis Induced by CLP Surgery
2.4. Inhibiting the Biosynthesis of SP or Blocking NK1R Attenuated the Increases in the Concentrations of ICAM1 and VCAM1 in the Lungs of Mice with Sepsis Induced by CLP Surgery
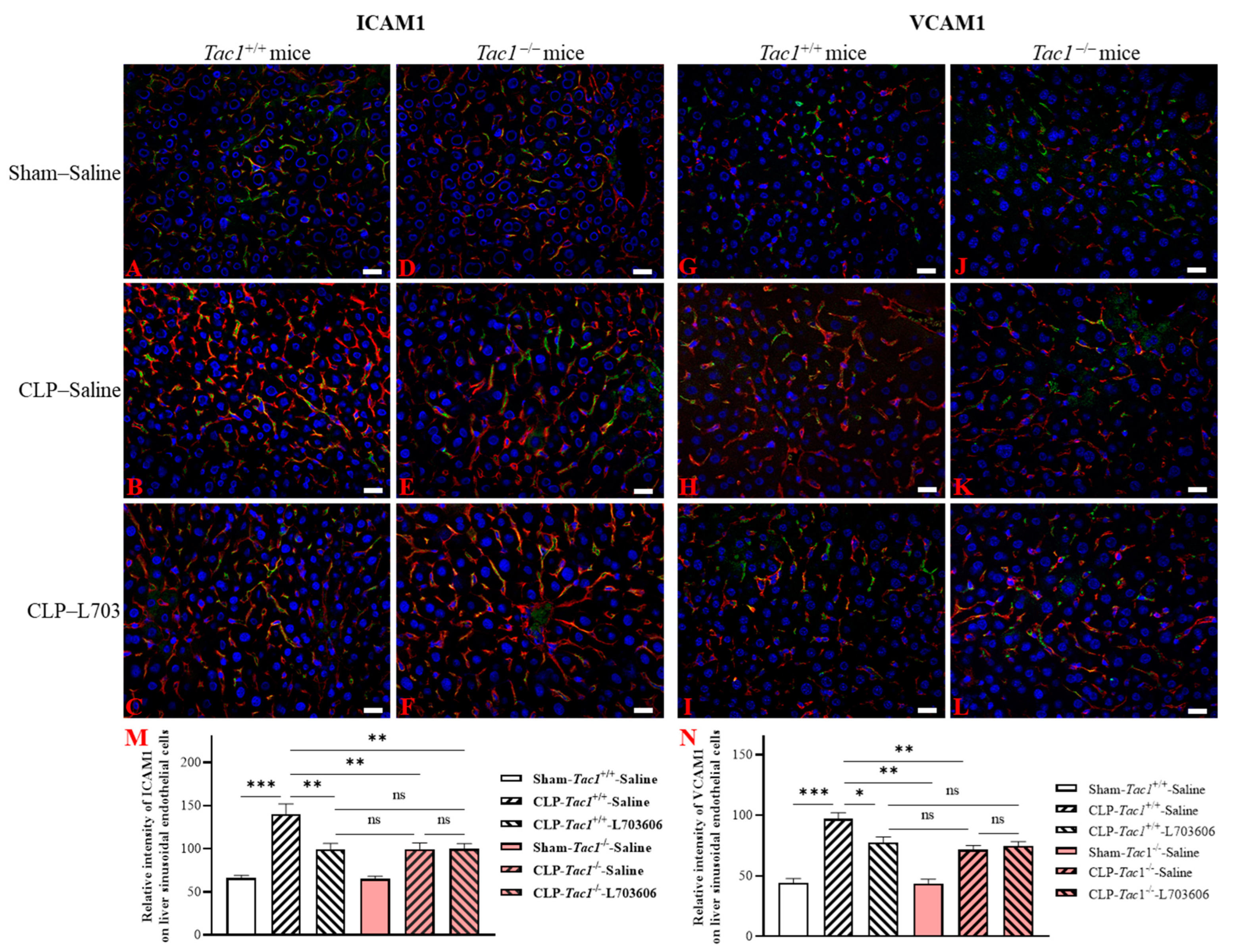
2.5. Inhibiting the Biosynthesis of SP or Blocking NK1R Attenuated the Upregulation of the Expression of ICAM1 and VCAM1 on the Liver Sinusoidal Endothelial Cells of Mice with Sepsis Induced by CLP Surgery
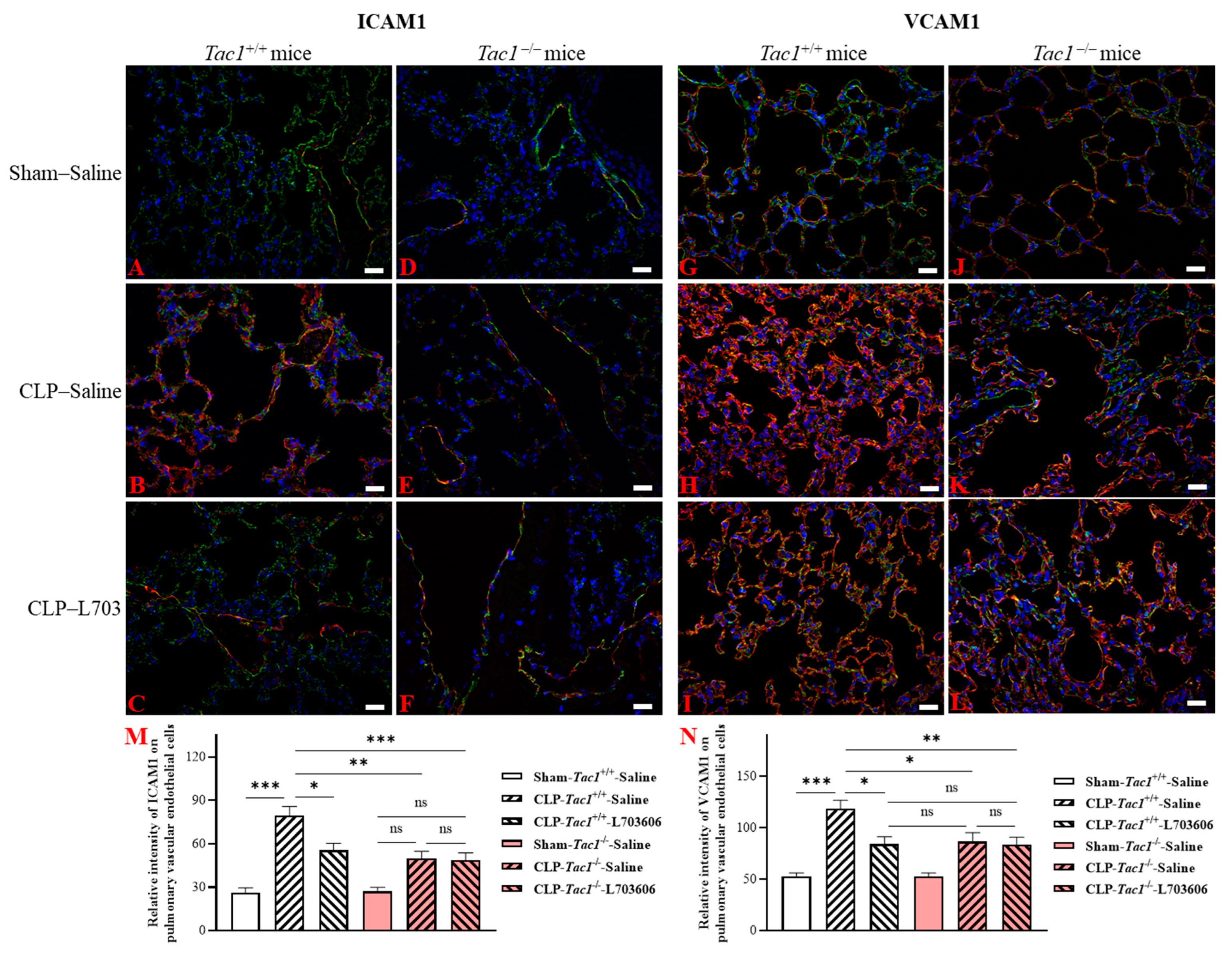
2.6. Inhibiting the Biosynthesis of SP or Blocking NK1R Attenuated the Upregulation of the Expression of ICAM1 and VCAM1 on the Pulmonary Endothelial Cells of Mice with Sepsis Induced by CLP Surgery
3. Discussion
4. Materials and Methods
4.1. Group Setting
4.2. Sepsis Establishment, Manipulations, and Sample Collection from Mice
4.3. Measurement of the Activity of MPO in Tissues
4.4. Measurement of the Concentrations of ICAM1 and VCAM1 in Tissues
4.5. Measurement of the Expression of ICAM1 and VCAM1 on Vascular Endothelial Cells in Tissues by Double Immunofluorescence Staining
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lelubre, C.; Vincent, J.L. Mechanisms and treatment of organ failure in sepsis. Nat. Rev. Nephrol. 2018, 14, 417–427. [Google Scholar] [PubMed]
- Pool, R.; Gomez, H.; Kellum, J.A. Mechanisms of Organ Dysfunction in Sepsis. Crit. Care Clin. 2018, 34, 63–80. [Google Scholar] [PubMed]
- Caraballo, C.; Jaimes, F. Organ Dysfunction in Sepsis: An Ominous Trajectory from Infection to Death. Yale J. Biol. Med. 2019, 92, 629–640. [Google Scholar] [PubMed]
- Huang, M.; Cai, S.; Su, J. The Pathogenesis of Sepsis and Potential Therapeutic Targets. Int. J. Mol. Sci. 2019, 20, 5376. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, M.S.; von Mentzer, B.; Geppetti, P.; Pothoulakis, C.; Bunnett, N.W. Tachykinins and their receptors: Contributions to physiological control and the mechanisms of disease. Physiol. Rev. 2014, 94, 265–301. [Google Scholar]
- Zhu, Z.; Chambers, S.; Bhatia, M. Suppressing the Substance P-NK1R Signalling Protects Mice against Sepsis-Associated Acute Inflammatory Injury and Ferroptosis in the Liver and Lungs. Antioxidants 2024, 13, 300. [Google Scholar] [CrossRef]
- Kumar, V.; Abbas, A.K.; Aster, J.C. Inflammation and Repair. In Robbins Basic Pathology, 10th ed.; Saunders: Philadephia, PA, USA, 2017; pp. 57–93. [Google Scholar]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Kelly, M.; Hwang, J.M.; Kubes, P. Modulating leukocyte recruitment in inflammation. J. Allergy Clin. Immunol. 2007, 120, 3–10. [Google Scholar]
- Vestweber, D. How leukocytes cross the vascular endothelium. Nat. Rev. Immunol. 2015, 15, 692–704. [Google Scholar]
- Langer, H.F.; Chavakis, T. Leukocyte-endothelial interactions in inflammation. J. Cell. Mol. Med. 2009, 13, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Pober, J.S.; Sessa, W.C. Inflammation and the blood microvascular system. Cold Spring Harb. Perspect. Biol. 2014, 7, a016345. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, Y.; Wang, N. New paradigms in inflammatory signaling in vascular endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H317–H325. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Saredy, J.; Yang, W.Y.; Sun, Y.; Lu, Y.; Saaoud, F.; Drummer, C.T.; Johnson, C.; Xu, K.; Jiang, X.; et al. Vascular Endothelial Cells and Innate Immunity. Arterioscler. Thromb. Vasc. Biol. 2020, 40, e138–e152. [Google Scholar] [CrossRef]
- Pober, J.S.; Sessa, W.C. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 2007, 7, 803–815. [Google Scholar]
- Hack, C.E.; Zeerleder, S. The endothelium in sepsis: Source of and a target for inflammation. Crit. Care Med. 2001, 29, S21–S27. [Google Scholar] [CrossRef]
- Joffre, J.; Hellman, J.; Ince, C.; Ait-Oufella, H. Endothelial Responses in Sepsis. Am. J. Respir. Crit. Care Med. 2020, 202, 361–370. [Google Scholar] [CrossRef]
- Dolmatova, E.V.; Wang, K.; Mandavilli, R.; Griendling, K.K. The effects of sepsis on endothelium and clinical implications. Cardiovasc. Res. 2021, 117, 60–73. [Google Scholar]
- Ince, C.; Mayeux, P.R.; Nguyen, T.; Gomez, H.; Kellum, J.A.; Ospina-Tascón, G.A.; Hernandez, G.; Murray, P.; De Backer, D. The endothelium in sepsis. Shock 2016, 45, 259–270. [Google Scholar] [CrossRef]
- Sørensen, K.K.; Simon-Santamaria, J.; McCuskey, R.S.; Smedsrød, B. Liver Sinusoidal Endothelial Cells. Comp. Physiol. 2015, 5, 1751–1774. [Google Scholar]
- Shetty, S.; Lalor, P.F.; Adams, D.H. Liver sinusoidal endothelial cells—Gatekeepers of hepatic immunity. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 555–567. [Google Scholar]
- Madokoro, Y.; Kamikokuryo, C.; Niiyama, S.; Ito, T.; Hara, S.; Ichinose, H.; Kakihana, Y. Early ascorbic acid administration prevents vascular endothelial cell damage in septic mice. Front. Pharmacol. 2022, 13, 929448. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, D.; Kong, L.; Li, M.; Feng, Z.; Shuai, B.; Wang, L.; Wei, Y.; Li, H.; Wu, S.; et al. Intermedin protects against sepsis by concurrently re-establishing the endothelial barrier and alleviating inflammatory responses. Nat. Commun. 2018, 9, 2644. [Google Scholar] [PubMed]
- Jiang, J.; Huang, K.; Xu, S.; Garcia, J.G.N.; Wang, C.; Cai, H. Targeting NOX4 alleviates sepsis-induced acute lung injury via attenuation of redox-sensitive activation of CaMKII/ERK1/2/MLCK and endothelial cell barrier dysfunction. Redox Biol. 2020, 36, 101638. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.F.; Wang, Y.C.; Feng, Q.R.; Zhang, Y.S.; Zhuang, Y.F.; Xie, Z.X.; Bai, X.J. Inhibition of the C3a receptor attenuates sepsis-induced acute lung injury by suppressing pyroptosis of the pulmonary vascular endothelial cells. Free Radic. Biol. Med. 2022, 184, 208–217. [Google Scholar] [PubMed]
- Gaddam, R.R.; Chambers, S.; Fraser, R.; Cogger, V.C.; Le Couteur, D.G.; Ishii, I.; Bhatia, M. Cystathionine-Gamma-Lyase-Derived Hydrogen Sulfide-Regulated Substance P Modulates Liver Sieve Fenestrations in Caecal Ligation and Puncture-Induced Sepsis. Int. J. Mol. Sci. 2019, 20, 3191. [Google Scholar] [CrossRef]
- Manandhar, S.; Chambers, S.; Miller, A.; Ishii, I.; Bhatia, M. Pharmacological Inhibition and Genetic Deletion of Cystathionine Gamma-Lyase in Mice Protects against Organ Injury in Sepsis: A Key Role of Adhesion Molecules on Endothelial Cells. Int. J. Mol. Sci. 2023, 24, 13650. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hegde, A.; Ng, S.W.; Adhikari, S.; Moochhala, S.M.; Bhatia, M. Hydrogen sulfide up-regulates substance P in polymicrobial sepsis-associated lung injury. J. Immunol. 2007, 179, 4153–4160. [Google Scholar] [CrossRef]
- Xia, Y.; Zweier, J.L. Measurement of myeloperoxidase in leukocyte-containing tissues. Anal. Biochem. 1997, 245, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Gaddam, R.R.; Fraser, R.; Badiei, A.; Chambers, S.; Cogger, V.C.; Le Couteur, D.G.; Ishii, I.; Bhatia, M. Cystathionine-Gamma-Lyase Gene Deletion Protects Mice against Inflammation and Liver Sieve Injury following Polymicrobial Sepsis. PLoS ONE 2016, 11, e0160521. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M.; Zhi, L.; Zhang, H.; Ng, S.W.; Moore, P.K. Role of substance P in hydrogen sulfide-induced pulmonary inflammation in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 291, L896–L904. [Google Scholar] [CrossRef]
- Hegde, A.; Koh, Y.H.; Moochhala, S.M.; Bhatia, M. Neurokinin-1 receptor antagonist treatment in polymicrobial sepsis: Molecular insights. Int. J. Inflamm. 2010, 2010, 601098. [Google Scholar] [CrossRef]
- Laudes, I.J.; Guo, R.F.; Riedemann, N.C.; Speyer, C.; Craig, R.; Sarma, J.V.; Ward, P.A. Disturbed homeostasis of lung intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 during sepsis. Am. J. Pathol. 2004, 164, 1435–1445. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.Q.; Mantyh, P.W.; Carlson, E.J.; Gillespie, A.M.; Epstein, C.J.; Basbaum, A.I. Primary afferent tachykinins are required to experience moderate to intense pain. Nature 1998, 392, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Snider, R.M.; Constantine, J.W.; Lowe, J.A., 3rd; Longo, K.P.; Lebel, W.S.; Woody, H.A.; Drozda, S.E.; Desai, M.C.; Vinick, F.J.; Spencer, R.W.; et al. A potent nonpeptide antagonist of the substance P (NK1) receptor. Science 1991, 251, 435–437. [Google Scholar] [CrossRef]
- Brougham-Cook, A.; Kimmel, H.R.C.; Monckton, C.P.; Owen, D.; Khetani, S.R.; Underhill, G.H. Engineered matrix microenvironments reveal the heterogeneity of liver sinusoidal endothelial cell phenotypic responses. APL Bioeng. 2022, 6, 046102. [Google Scholar] [CrossRef]
- Wong, E.; Nguyen, N.; Hellman, J. Isolation of Primary Mouse Lung Endothelial Cells. J. Vis. Exp. 2021, 177, e63253. [Google Scholar]
- Shihan, M.H.; Novo, S.G.; Le Marchand, S.J.; Wang, Y.; Duncan, M.K. A simple method for quantitating confocal fluorescent images. Biochem. Biophys. Rep. 2021, 25, 100916. [Google Scholar] [CrossRef]


| Antibody | Dilution | Source, Catalogue No. |
|---|---|---|
| ICAM1 | 1:1000 | R&D Systems, Minneapolis, MN, USA |
| VCAM1 | 1:200 | R&D Systems, Minneapolis, MN, USA |
| LYVE1 | 1:200 | Abcam, Cambridge, UK |
| CD31 | 1:100 | Abcam, Cambridge, UK |
| FITC-conjugated secondary antibody | 1:500 | Abcam, Cambridge, UK |
| Texas red-conjugated secondary antibody | 1:500 | Abcam, Cambridge, UK |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Chambers, S.; Bhatia, M. Substance P Promotes Leukocyte Infiltration in the Liver and Lungs of Mice with Sepsis: A Key Role for Adhesion Molecules on Vascular Endothelial Cells. Int. J. Mol. Sci. 2024, 25, 6500. https://doi.org/10.3390/ijms25126500
Zhu Z, Chambers S, Bhatia M. Substance P Promotes Leukocyte Infiltration in the Liver and Lungs of Mice with Sepsis: A Key Role for Adhesion Molecules on Vascular Endothelial Cells. International Journal of Molecular Sciences. 2024; 25(12):6500. https://doi.org/10.3390/ijms25126500
Chicago/Turabian StyleZhu, Zhixing, Stephen Chambers, and Madhav Bhatia. 2024. "Substance P Promotes Leukocyte Infiltration in the Liver and Lungs of Mice with Sepsis: A Key Role for Adhesion Molecules on Vascular Endothelial Cells" International Journal of Molecular Sciences 25, no. 12: 6500. https://doi.org/10.3390/ijms25126500
APA StyleZhu, Z., Chambers, S., & Bhatia, M. (2024). Substance P Promotes Leukocyte Infiltration in the Liver and Lungs of Mice with Sepsis: A Key Role for Adhesion Molecules on Vascular Endothelial Cells. International Journal of Molecular Sciences, 25(12), 6500. https://doi.org/10.3390/ijms25126500






