Purified Clinoptilolite-Tuff as an Efficient Sorbent for Food-Derived Peanut Allergens
Abstract
:1. Introduction
1.1. Allergens Derived from Food
1.1.1. Peanut (Arachis hypogaea)
1.1.2. Gluten
| Peanut (Arachis hypogaea) Protein | Superfamily | Type of Protein |
|---|---|---|
| Ara h 1 | cupins | vicilin |
| Ara h 2 | prolamins | 2S albumin |
| Ara h 3 | cupins | legumin |
| Ara h 4 | cupins | legumin (isoform of Ara h 3) |
| Ara h 5 | profilins | |
| Ara h 6 | prolamins | 2S albumin |
| Ara h 7 | prolamins | 2S albumin |
| Ara h 8 | plant pathogenesis-related proteins PR-10 | |
| Ara h 9 | prolamins | nonspecific lipid transfer proteins |
| Ara h 10 | oleosins | |
| Ara h 11 | oleosin | |
| Ara h 12 | defensins | |
| Ara h 13 | defensins | |
| Ara h 14 | oleosin | |
| Ara h 15 | oleosin | |
| Ara h 16 | prolamins | nonspecific lipid transfer proteins |
| Ara h 17 | prolamins | nonspecific lipid transfer proteins |
| Crop (Genus) | Superfamily | Type of Protein |
| wheat (Triticum aestivum) | prolamins | gliadin |
| barley (Hordeum vulgare) | prolamins | hordein |
| rye (Secale cereale) | prolamins | secalin |
| oat (Avena sativa) | prolamins | avenin |
| corn (Zea mays) | prolamins | zein |
| rice (Oryza sativa) | prolamins | oryzin |
| sorghum (Sorghum bicolor) | prolamins | kafirin |
| wheat (Triticum aestivum) | glutelins | glutenin |
| barley (Hordeum vulgare) | glutelins | hordenin |
| rye (Secale cereale) | glutelins | secalinin |
| oat (Avena sativa) | glutelins | avelanin |
| corn (Zea mays) | glutelins | zeanin |
| rice (Oryza sativa) | glutelins | oryzinin |
| sorghum (Sorghum bicolor) | glutelins | - |
1.2. Zeolite—Structure, Application, and Purification
1.2.1. General Information concerning Structure and Application of Zeolites
1.2.2. Clinoptilolite and Purified Clinoptilolite-Tuff
1.2.3. Purified Clinoptilolite-Tuff as Sorbent of Different Toxic and Harmful Substances
2. Results
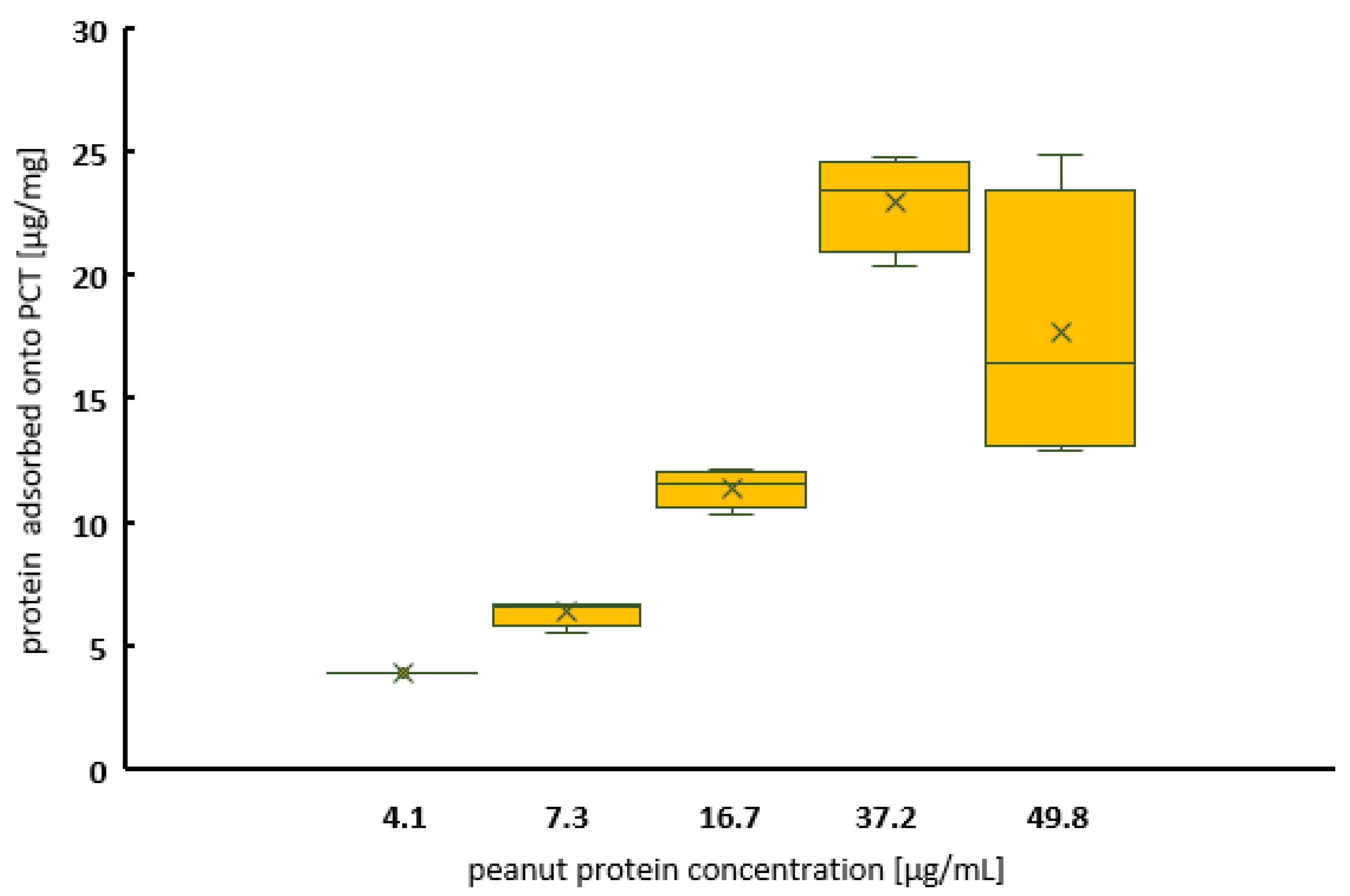
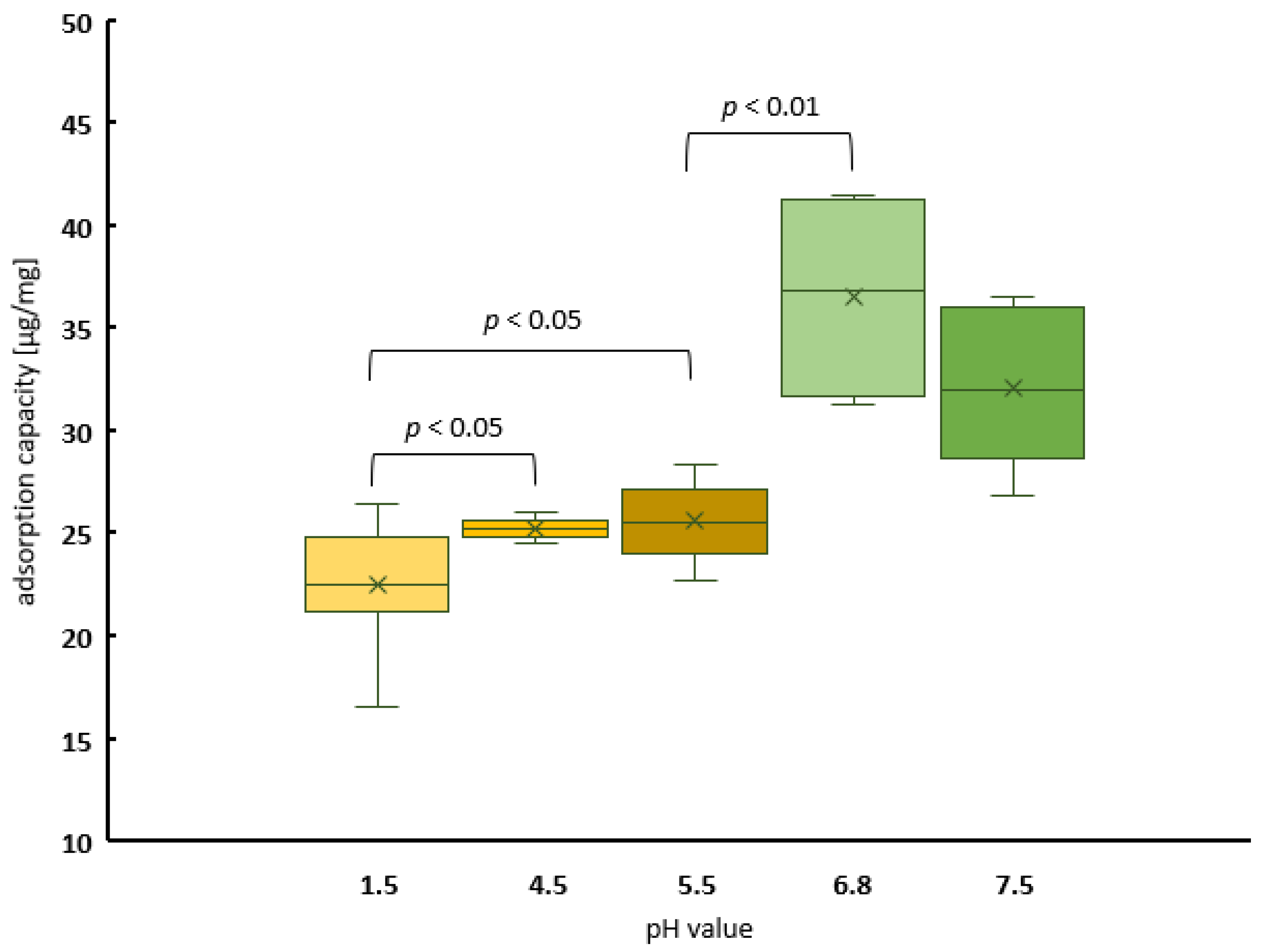
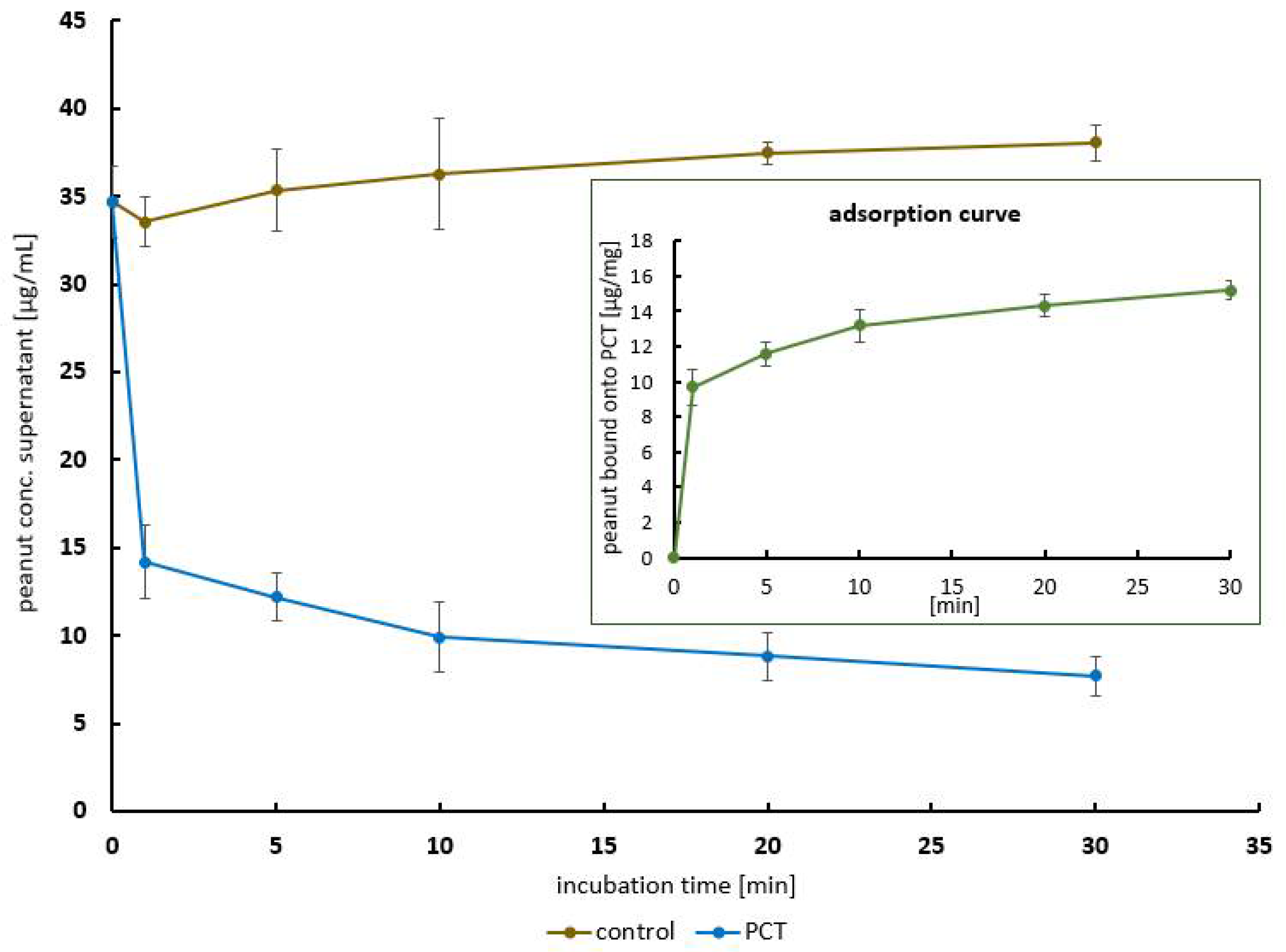
2.1. Adsorption of Peanut Protein on Purified Clinoptilolite-Tuff
2.1.1. The Kinetics of Peanut Protein Adsorption to PCT at Different pH Values in a Concentration-Dependent Manner
2.1.2. The Kinetics of Peanut Protein Adsorption to PCT in a Time-Dependent Manner
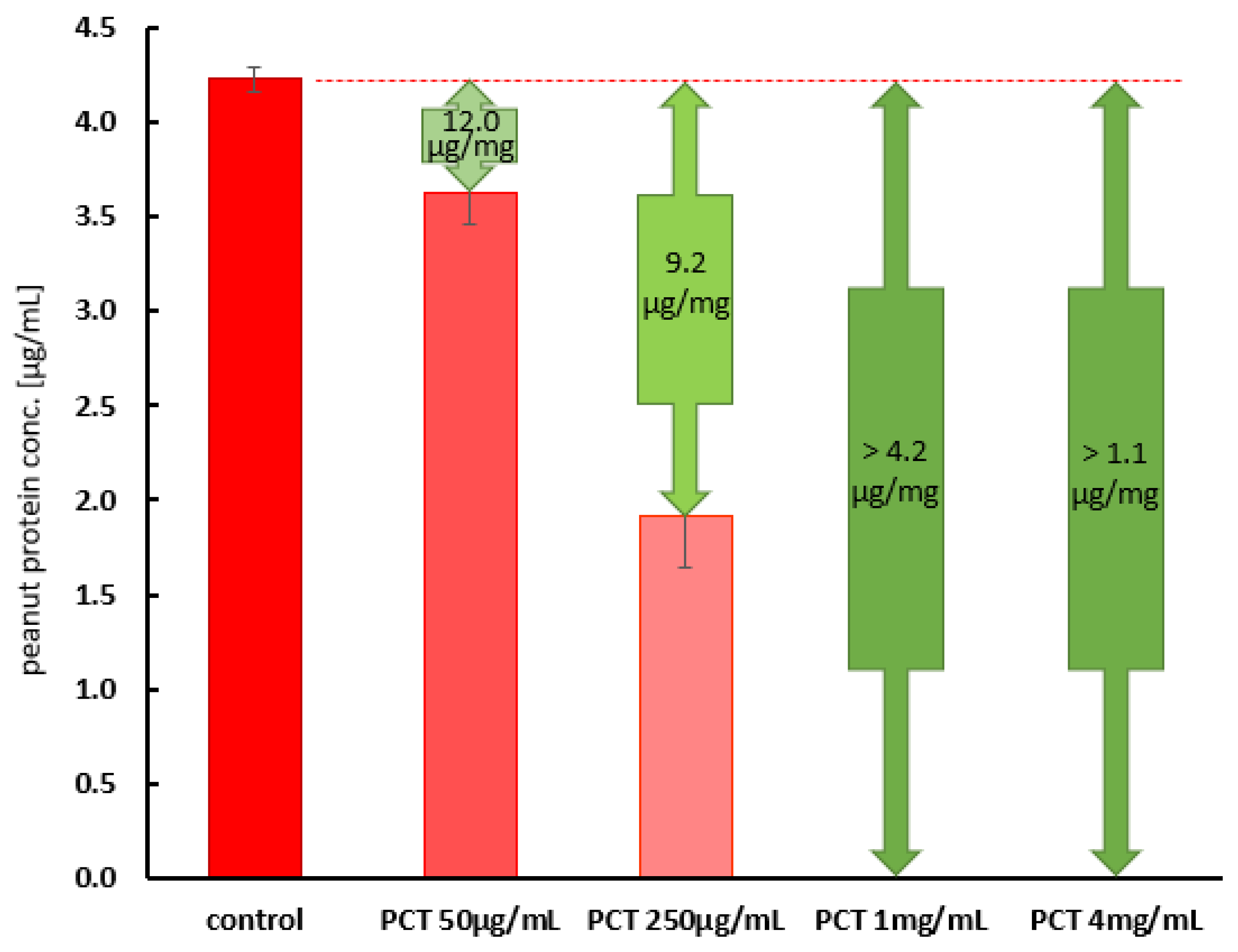
2.2. Neutralization of Peanut Protein by Purified Clinoptilolite-Tuff in an Amount Relevant for Allergic Persons
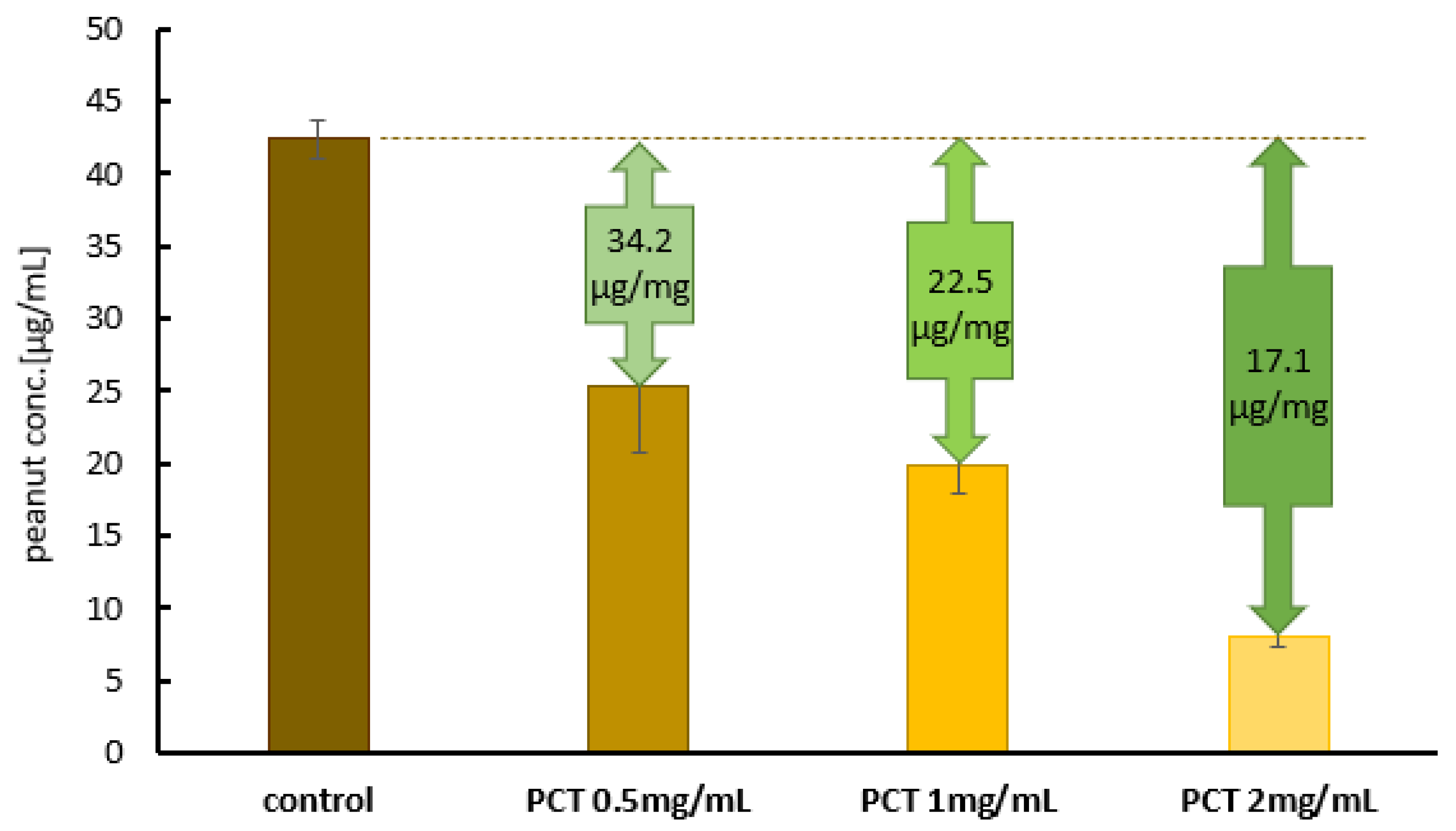
2.2.1. The Adsorption of an Allergy-Relevant Concentration on PCT in Test Solution at pH 6.8
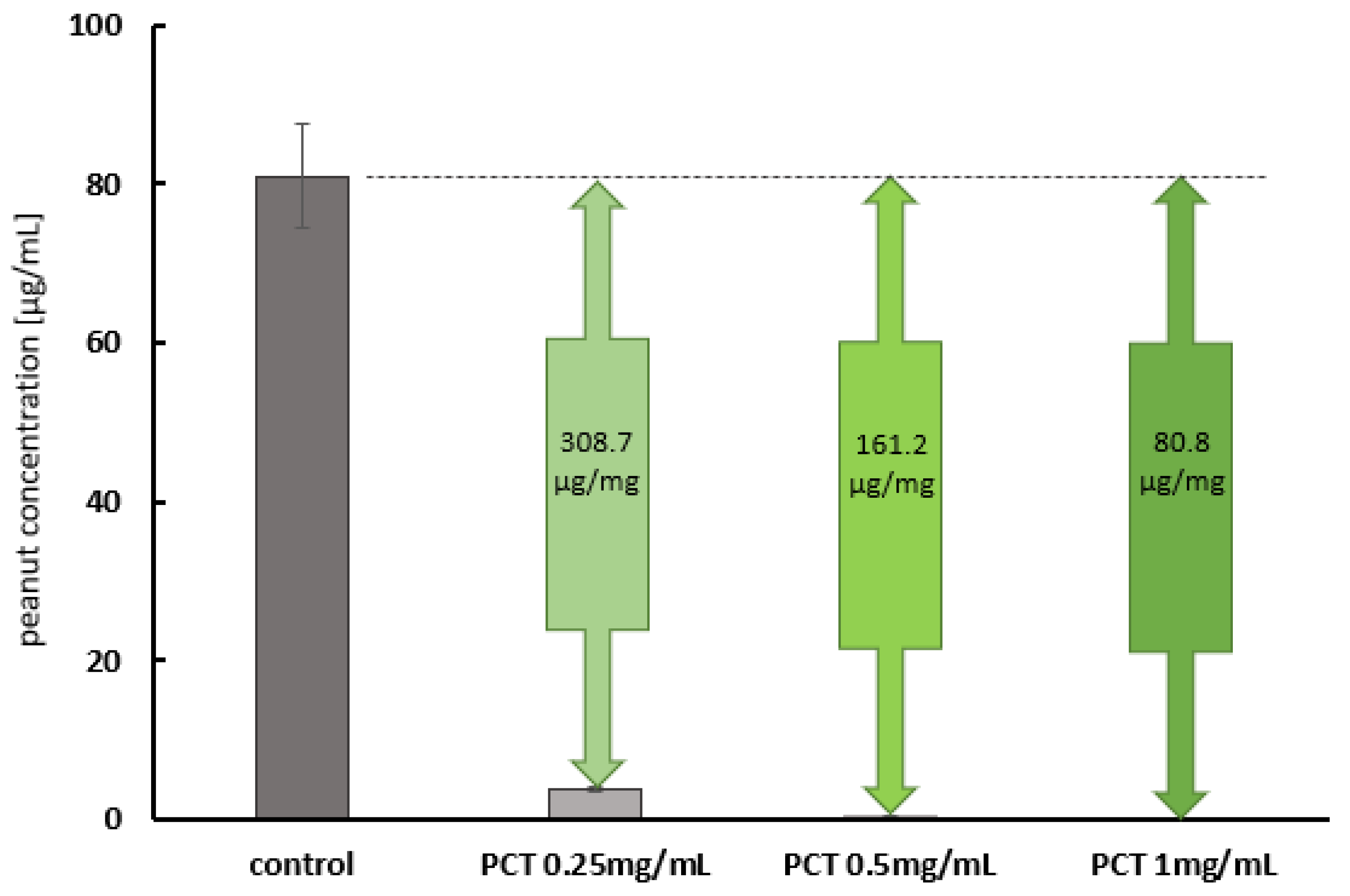
2.2.2. The Adsorption of Allergy-Relevant Concentrations on PCT in Artificial Gastric and Intestinal Fluids
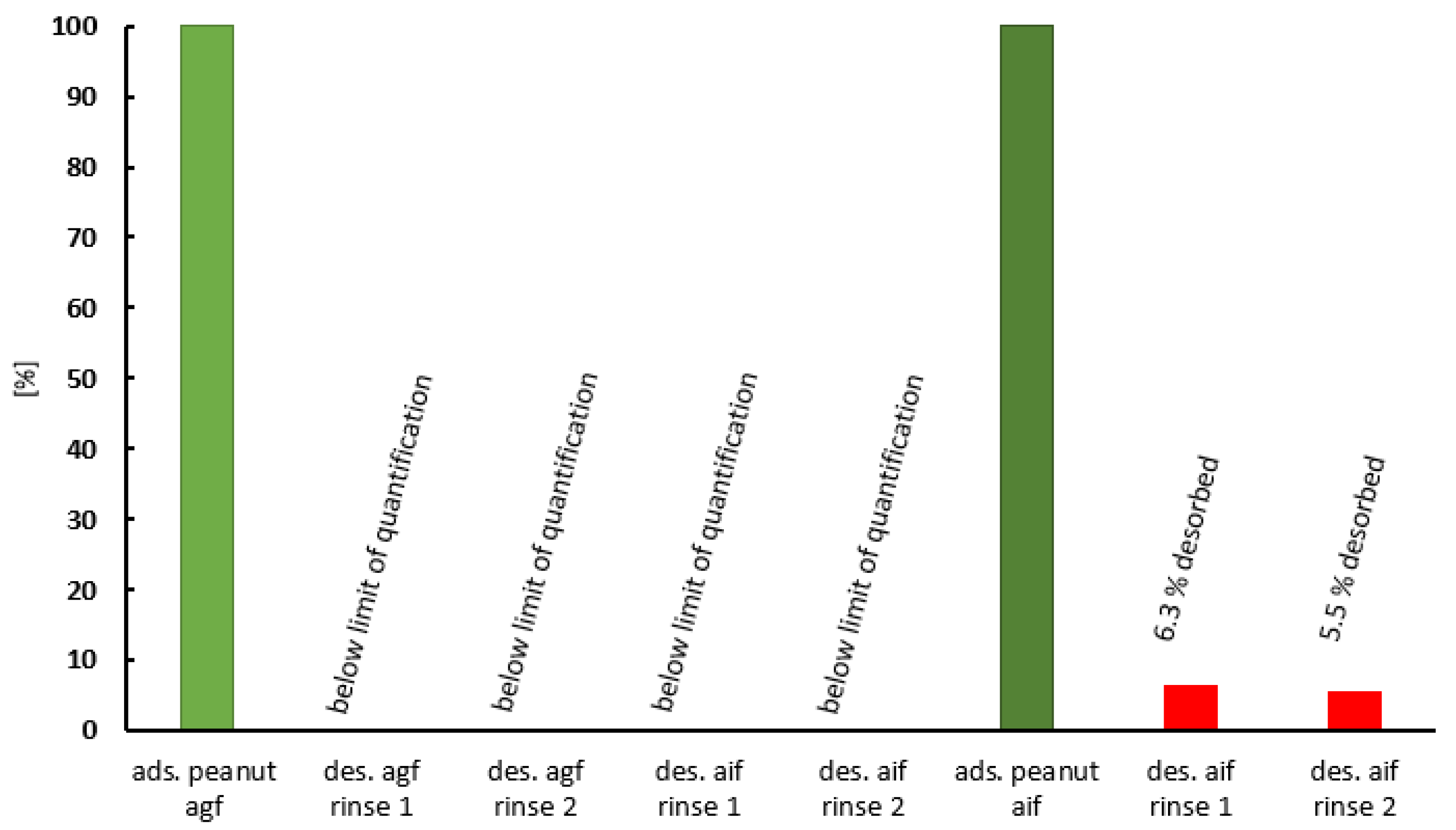
2.3. Desorption of Peanut Protein from Purified Clinoptilolite-Tuff
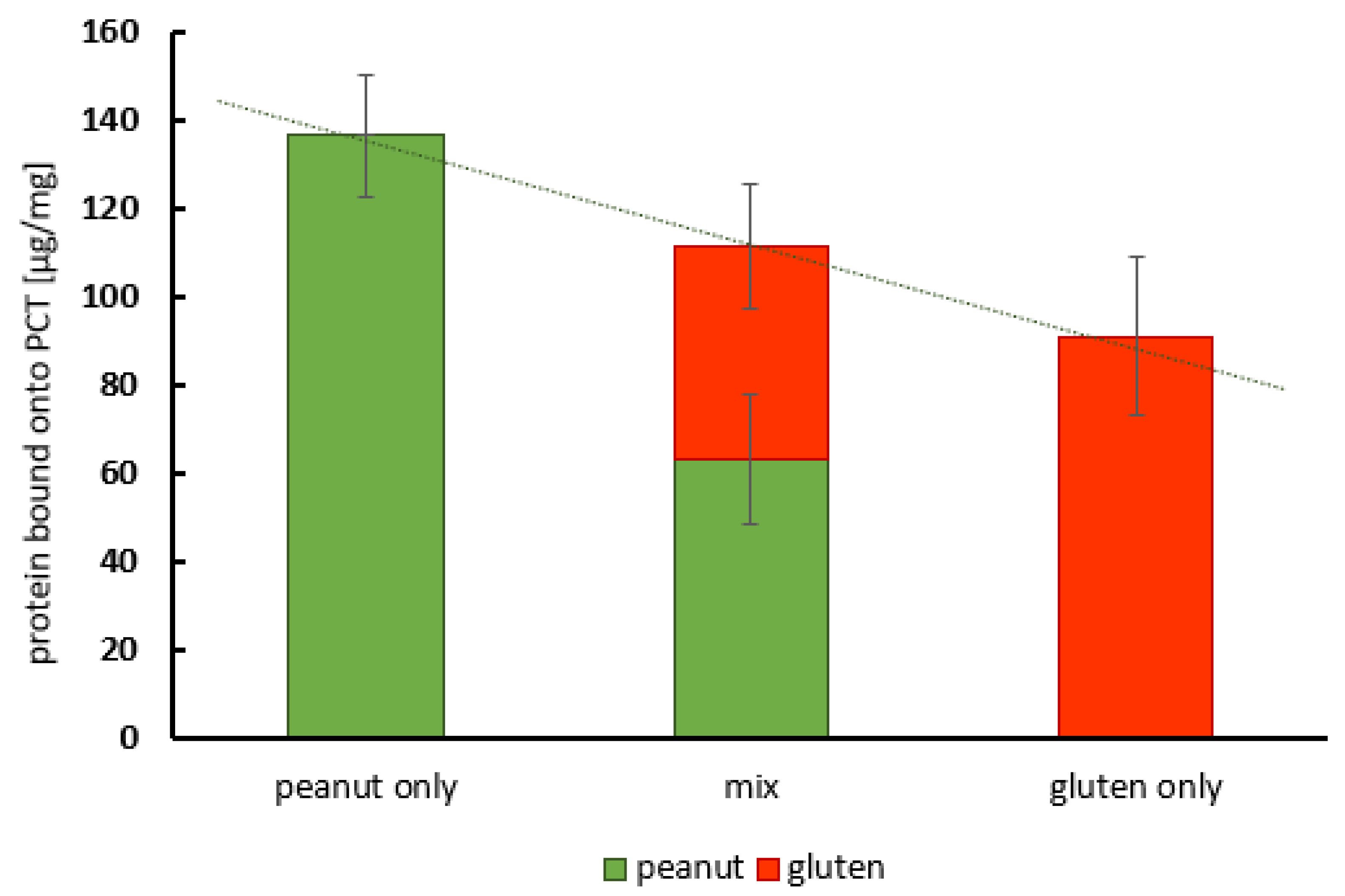
2.4. Competing Adsorption of Peanut Protein and Gliadin onto Purified Clinoptilolite-Tuff
3. Discussion
4. Materials and Methods
4.1. The Chemical Compositions of Buffers Used in the Experiments Performed
4.2. Artificial Fluid Preparation
4.2.1. Preparation of Artificial Gastric Fluid
4.2.2. Preparation of Artificial Intestinal Fluid
4.3. The Extraction of Proteins Derived from Either Peanut Mush or Wheat Flour
4.3.1. Extraction of Peanut Proteins
4.3.2. Extraction of Wheat Proteins
4.4. Preparation of the PCT Suspension
4.5. Adsorption and Desorption of Peanut Proteins and Gluten from Purified Clinoptilolite-Tuff
4.5.1. The Generation of a Saturation Curve
4.5.2. The Influence of pH on Peanut Protein Binding
4.5.3. The Kinetics of Peanut Protein Adsorption to PCT
4.5.4. Adsorption Mimicking in vivo Conditions by Using Artificial Gastric and Intestinal Fluids
4.5.5. Desorption of Peanut Proteins from Purified Clinoptilolite-Tuff
- (a)
- Adsorption in the artificial gastric fluid and desorption in the gastric fluid or pH 1.5 test solution;
- (b)
- Adsorption in the artificial gastric fluid and desorption in the intestinal fluid or pH 6.8 buffer;
- (c)
- Adsorption in the artificial intestinal fluid and desorption in the intestinal fluid or pH 6.8 buffer.
4.5.6. Competing Adsorption in Buffers and Test Solutions between Peanut Proteins and Gluten from Wheat
- (a)
- A mixture of peanut protein and wheat gluten in a ratio of 1:1;
- (b)
- Peanut protein alone;
- (c)
- Wheat gluten alone.
4.6. The Determination of Protein Concentrations with Coomassie Blue Reagent
4.7. Determination of Peanut Protein Content via ELISA
4.8. Determination of Gluten Content via ELISA
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warren, C.M.; Jiang, J.; Gupta, R.S. Epidemiology and Burden of Food Allergy. Curr. Allergy Asthma Rep. 2020, 20, 6. [Google Scholar] [CrossRef]
- Peters, R.L.; Krawiec, M.; Koplin, J.J.; Santos, A.F. Update on food allergy. Pediatr. Allergy Immunol. 2021, 32, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.C. Peanut allergy diagnosis: As simple as Ara h 1, 2, and 3. Consult. Pediatr. 2013, 12, 347–351. [Google Scholar]
- Ozias-Akins, P.; Breiteneder, H. The functional biology of peanut allergens and possible links to their allergenicity. Allergy 2019, 74, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, B. Gluten-related disorders: Celiac disease, wheat allergy, and nonceliac gluten sensitivity. Crit. Rev. Food Sci. Nutr. 2020, 60, 2606–2621. [Google Scholar] [CrossRef] [PubMed]
- Rosell, C.M.; Barro, F.; Sousa, C.; Mena, M.C. Cereals for developing gluten-free products and analytical tools for gluten detection. J. Cereal Sci. 2014, 59, 354–364. [Google Scholar] [CrossRef]
- Leonard, M.M.; Sapone, A.; Catassi, C.; Fasano, A. Celiac Disease and Nonceliac Gluten Sensitivity: A Review. JAMA 2017, 318, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Felber, J.; Bläker, H.; Fischbach, W.; Koletzko, S.; Laaß, M.W.; Lachmann, N.; Lorenz, P.; Lynen, P.; Reese, I.; Scherf, K.; et al. Aktualisierte S2k-Leitlinie Zöliakie der Deutschen Gesellschaft für Gastroenterologie, Verdauungs- und Stoffwechselkrankheiten (DGVS, German Society for Digestive and Metabolic Diseases)—AWMF-Registernummer: 021-021, 790–856. Available online: https://www.awmf.org/uploads/tx_szleitlinien/021-021l_S2k_Zoeliakie_2021-12_1.pdf (accessed on 1 December 2021).
- Joint FAO/WHO Codex Alimentarius Commission. Codex Alimentarius: Standard for Foods for Special Dietary Use for Persons Intolerant to Gluten; CXS 118-1979; Amended; World Health Organization: Geneva, Switzerland, 2015; pp. 1–3. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B118-1979%252FCXS_118e_2015.pdf (accessed on 15 November 2021).
- EU Regulation 828/2014, L 228/5-8. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32014R0828 (accessed on 22 January 2022).
- Association of European Coeliac Societies. AOECS Standard for Gluten-Free Foods. 2016, pp. 1–16. Available online: https://www.aoecs.org/media/5ugmpnoj/aoecs-standard-sept-2016.pdf (accessed on 16 September 2016).
- Shewry, P.R.; Halford, N.G. Cereal seed storage proteins: Structures, properties and role in grain utilization. J. Exp. Bot. 2002, 53, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.H.; Messing, J. Amplification of prolamin storage protein genes in different subfamilies of the Poaceae. Theor. Appl. Genet. 2009, 119, 1397–1412. [Google Scholar] [CrossRef]
- Mumpton, F.A.; Fishman, P.H. The Application of Natural Zeolites in Animal Science and Aquaculture. J. Anim. Sci. 1977, 45, 1188–1203. [Google Scholar] [CrossRef]
- Eroglu, N.; Emekci, M.; Athanassiou, C. Applications of natural zeolites on agriculture and food production. J. Sci. Food Agric. 2017, 97, 3487–3499. [Google Scholar] [CrossRef]
- Mumpton, F.A. La roca magica: Uses of natural zeolites in agriculture and industry. Proc. Natl. Acad. Sci. USA 1999, 96, 3463–3470. [Google Scholar] [CrossRef] [PubMed]
- Tschegg, C.; Rice, A.H.N.; Grasemann, B.; Matiasek, E.; Kobulej, P.; Dzivák, M.; Berger, T. Petrogenesis of a large-scale miocene zeolite tuff in the eastern Slovak Republic: The Nižny Hrabovec open-pit clinoptilolite mine. Econ. Geol. 2019, 114, 1177–1194. [Google Scholar] [CrossRef]
- Samekova, K.; Firbas, C.; Irrgeher, J.; Opper, C.; Prohaska, T.; Retzmann, A.; Tschegg, C.; Meisslitzer, C.; Tchaikovsky, A.; Gouya, G.; et al. Concomitant oral intake of purified clinoptilolite tuff (G-PUR) reduces enteral lead uptake in healthy humans. Sci. Rep. 2021, 11, 14796. [Google Scholar] [CrossRef] [PubMed]
- Haemmerle, M.; Fendrych, J.; Matiasek, E.; Tschegg, C. Adsorption and Release Characteristics of Purified and Non-Purified Clinoptilolite Tuffs towards Health-Relevant Heavy Metals. Crystals 2021, 11, 1343. [Google Scholar] [CrossRef]
- Deinsberger, J.; Marquart, E.; Nizet, S.; Meisslitzer, C.; Tschegg, C.; Uspenska, K.; Gouya, G.; Niederdöckl, J.; Freissmuth, M.; Wolzt, M.; et al. Topically administered purified clinoptilolite-tuff for the treatment of cutaneous wounds: A prospective, randomised phase I clinical trial. Wound Repair Regen. 2022, 30, 198–209. [Google Scholar] [CrossRef]
- Tschegg, C.; Hou, Z.; Rice, A.H.N.; Fendrych, J.; Matiasek, E.; Berger, T.; Grasemann, B. Fault zone structures and strain localization in clinoptilolite-tuff (Nižny Hrabovec, Slovak Republic). J. Struct. Geol. 2020, 138, 104090. [Google Scholar] [CrossRef]
- Glock, G. Method for the Removal of Heavy Metals. EP 2 040 837 B1. 20 June 2012. pp. 1–5. Available online: https://patentimages.storage.googleapis.com/3f/db/58/7f3549f0a6c10a/EP2040837B1.pdf (accessed on 28 April 2022).
- Anderle, K.; Wolzt, M.; Moser, G.; Keip, B.; Peter, J.; Meisslitzer, C.; Gouya, G.; Freissmuth, M.; Tschegg, C. Safety and efficacy of purified clinoptilolite-tuff treatment in patients with irritable bowel syndrome with diarrhea: Randomized controlled trial. World J. Gastroenterol. 2022, 28, 6573–6588. [Google Scholar] [CrossRef]
- Irrgeher, J.; Berger, T.; Tchaikovsky, A.; Tschegg, C.; Gouya, G.; Lechner, P.; Retzmann, A.; Opper, C.; Firbas, C.; Freissmuth, M.; et al. Enriched stable 204Pb as tracer at ultra-low levels in clinical investigations. Anal. Bioanal. Chem. 2023, 415, 255–268. [Google Scholar] [CrossRef]
- Ranftler, C.; Nagl, D.; Sparer, A.; Röhrich, A.; Freissmuth, M.; El-Kasaby, A.; Nasrollahi Shirazi, S.; Koban, F.; Tschegg, C.; Nizet, S. Binding and neutralization of C. difficile toxins A and B by purified clinoptilolite-tuff. PLoS ONE 2021, 16, e0252211. [Google Scholar] [CrossRef] [PubMed]
- Ranftler, C.; Röhrich, A.; Sparer, A.; Tschegg, C.; Nagl, D. Purified Clinoptilolite-Tuff as an Efficient Sorbent for Gluten Derived from Food. Int. J. Mol. Sci. 2022, 23, 5143. [Google Scholar] [CrossRef]
- Nizet, S.; Rieger, J.; Sarabi, A.; Lajtai, G.; Zatloukal, K.; Tschegg, C. Binding and inactivation of human coronaviruses, including SARS-CoV-2, onto purified clinoptilolite-tuff. Sci. Rep. 2023, 13, 4673. [Google Scholar] [CrossRef]
- Jayasena, S.; Smits, M.; Fiechter, D.; de Jong, A.; Nordlee, J.; Baumert, J.; Taylor, S.L.; Pieters, R.H.; Koppelman, S.J. Comparison of six commercial ELISA kits for their specificity and sensitivity in detecting different major peanut allergens. J. Agric. Food Chem. 2015, 63, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Lacorn, M.; Dubois, T.; Gößwein, C.; Kredel, R.; Ferkinghoff, B.; Brunelle, S.; Théolier, J.; Dominguez, S.; Weiss, T. Validation of the RIDASCREEN® Peanut for Determination of Peanut Protein in Cookies, Milk Chocolate, Ice Cream, Trail Mix, Puffed Rice Cereals, and Granola Bar: AOAC Performance Tested MethodSM 112102. J. AOAC Int. 2022, 105, 784–801. [Google Scholar] [CrossRef] [PubMed]
- Council of Europe. European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2019; pp. 1–4370. [Google Scholar]
- Lacorn, M.; Dubois, T.; Weiss, T.; Zimmermann, L.; Schinabeck, T.M.; Loos-Theisen, S.; Scherf, K. Determination of Gliadin as a Measure of Gluten in Food by R5 Sandwich ELISA RIDASCREEN® Gliadin Matrix Extension: Collaborative Study 2012.01. J. AOAC Int. 2022, 105, 442–455. [Google Scholar] [CrossRef]
- Joint FAO/WHO Codex Alimentarius of Practice on Food. Allergen Management for Food Business Operators; CXC 80-2020; FAO: Rome, Italy, 2020; pp. 1–20. [Google Scholar]
- Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers, Amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and Repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004. L 304/18-63. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:304:0018:0063:en:PDF (accessed on 8 February 2024).
- Dean, L.L. Peanut protein-processes and applications: A review. J. Nutr. Food Sci. 2021, 4, 31. Available online: https://www.henrypublishers.com/henry-journal-of-nutrition-and-food-science/jnfs-10028.pdf (accessed on 23 February 2024).
- Zurzolo, G.A.; Allen, K.J.; Taylor, S.L.; Shreffler, W.G.; Baumert, J.L.; Tang, M.L.; Gurrin, L.C.; Mathai, M.L.; Nordlee, J.A.; Dunngalvin, A.; et al. Peanut Allergen Threshold Study (PATS): Validation of eliciting doses using a novel single-dose challenge protocol. Allergy Asthma Clin. Immunol. 2013, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Hourihane, J.O.; Allen, K.J.; Shreffler, W.G.; Dunngalvin, G.; Nordlee, J.A.; Zurzolo, G.A.; Dunngalvin, A.; Gurrin, L.C.; Baumert, J.L.; Taylor, S.L. Peanut Allergen Threshold Study (PATS): Novel single-dose oral food challenge study to validate eliciting doses in children with peanut allergy. J. Allergy Clin. Immunol. 2017, 139, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Wensing, M.; Penninks, A.H.; Hefle, S.L.; Koppelman, S.J.; Bruijnzeel-Koomen, C.A.; Knulst, A.C. The distribution of individual threshold doses eliciting allergic reactions in a population with peanut allergy. J. Allergy Clin. Immunol. 2002, 110, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Daković, A.; Tomašević-Čanović, M.; Dondur, V.; Vujaković, A.; Radošević, P. Kinetics of aflatoxin B1 and G2 adsorption on Ca-clinoptilolite. J. Serbian Chem. Soc. 2000, 65, 715–723. [Google Scholar] [CrossRef]
- Garcia, M.; Blanco, J.L.; Suarez, G. Aflatoxins B1 and G1 solubility in standard solutions and stability during cold storage. Mycotoxin Res. 1994, 10, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. Aflatoxins: Properties, Toxicity and Detoxification. Nutr. Food Sci. Int. J. 2018, 6, 555696. [Google Scholar] [CrossRef]
- Schalk, K.; Lexhaller, B.; Koehler, P.; Scherf, K.A. Isolation and characterization of gluten protein types from wheat, rye, barley and oats for use as reference materials. PLoS ONE 2017, 12, e0172819. [Google Scholar] [CrossRef] [PubMed]
- Haemmerle, M.M.; Tschegg, C. Sorption of Natural Siderophores onto Clinoptilolite-Tuff and Its Controlled-Release Characteristics. Minerals 2023, 13, 611. [Google Scholar] [CrossRef]
- Krejner-Bienias, A.; Grzela, K.; Kulus, M.; Grzela, T. Peanut contamination in food products: A real danger for allergic people? Postep. Dermatol. Alergol. 2023, 40, 625–629. [Google Scholar] [CrossRef]
- Miller, T.A.; Koppelman, S.J.; Bird, J.A.; Hernandez-Trujillo, V.; Thyagarajan, A.; Mack, D.; Chalil, J.M.; Green, T.D.; Baumert, J.L. Peanut cross-contamination in randomly selected baked goods. Ann. Allergy Asthma Immunol. 2022, 128, 439–442. [Google Scholar] [CrossRef]
- McOsker, D.E. The Limiting Amino Acid Sequence in Raw and Roasted Peanut Protein. J. Nutr. 1962, 76, 453–459. [Google Scholar] [CrossRef]
- Koppelman, S.J.; Wensing, M.; Ertmann, M.; Knulst, A.C.; Knol, E.F. Relevance of Ara h1, Ara h2 and Ara h3 in peanut-allergic patients, as determined by immunoglobulin E Western blotting, basophil-histamine release and intracutaneous testing: Ara h2 is the most important peanut allergen. Clin. Exp. Allergy 2004, 34, 583–590. [Google Scholar] [CrossRef]
- Koppelman, S.J.; Hefle, S.L.; Taylor, S.L.; de Jong, G.A. Digestion of peanut allergens Ara h 1, Ara h 2, Ara h 3, and Ara h 6: A comparative in vitro study and partial characterization of digestion-resistant peptides. Mol. Nutr. Food Res. 2010, 54, 1711–1721. [Google Scholar] [CrossRef] [PubMed]
- Di Stasio, L.; Picariello, G.; Mongiello, M.; Nocerino, R.; Berni Canani, R.; Bavaro, S.; Monaci, L.; Ferranti, P.; Mamone, G. Peanut digestome: Identification of digestion resistant IgE binding peptides. Food Chem. Toxicol. 2017, 107 Pt A, 88–98. [Google Scholar] [CrossRef]
- Chung, S.; Reed, S.S.; Zhang, D. Peanut Allergens Attached with p-Aminobenzamidine Are More Resistant to Digestion than Native Allergens. Food Nutr. Sci. 2016, 7, 1352–1363. [Google Scholar] [CrossRef]
- Tian, Y.; Rao, H.; Fu, W.; Tao, S.; Xue, W.-T. Effect of digestion on the immunoreactivity and proinflammatory properties of recombinant peanut allergen Ara h 1. Food Agric. Immunol. 2019, 30, 418–431. [Google Scholar] [CrossRef]
- LaHood, N.A.; Min, J.; Keswani, T.; Richardson, C.M.; Amoako, K.; Zhou, J.; Marini-Rapoport, O.; Bernard, H.; Hazebrouck, S.; Shreffler, W.G.; et al. Immunotherapy-induced neutralizing antibodies disrupt allergen binding and sustain allergen tolerance in peanut allergy. J. Clin. Investig. 2023, 133, e164501. [Google Scholar] [CrossRef] [PubMed]
- Perkin, M.R. Palforzia for peanut allergy: Panacea or predicament. Clin. Exp. Allergy 2022, 52, 729–731. [Google Scholar] [CrossRef] [PubMed]
- Borne, G.E.; Daniel, C.P.; Wagner, M.J.; Plaisance, C.J.; Nolen, A.; Kelkar, R.A.; Ahmadzadeh, S.; Myrcik, D.; Shekoohi, S.; Kaye, A.D.; et al. Palforzia for Peanut Allergy: A Narrative Review and Update on a Novel Immunotherapy. Cureus 2023, 15, e50485. [Google Scholar] [CrossRef]
- van Odijk, J.; Weisheit, A.; Arvidsson, M.; Miron, N.; Nwaru, B.; Ekerljung, L. The Use of DAO as a Marker for Histamine Intolerance: Measurements and Determinants in a Large Random Population-Based Survey. Nutrients 2023, 15, 2887. [Google Scholar] [CrossRef] [PubMed]
- Selvam, T.; Schwieger, W.; Dathe, W. Natural Cuban zeolites for medical use and their histamine binding capacity. Clay Miner. 2014, 49, 501–512. [Google Scholar] [CrossRef]
- Selvam, T.; Schwieger, W.; Dathe, W. Histamine-binding capacities of different natural zeolites: A comparative study. Environ. Geochem. Health 2018, 40, 2657–2665. [Google Scholar] [CrossRef]
- Bustamante, M.Á.; Fernández-Gil, M.P.; Churruca, I.; Miranda, J.; Lasa, A.; Navarro, V.; Simón, E. Evolution of Gluten Content in Cereal-Based Gluten-Free Products: An Overview from 1998 to 2016. Nutrients 2017, 9, 21. [Google Scholar] [CrossRef]
- Valpotić, H.; Gračner, D.; Turk, R.; Đuričić, D.; Vince, S.; Folnožić, I.; Lojkić, M.; Žaja, I.Ž.; Bedrica, L.; Maćešić, N.; et al. Zeolite clinoptilolite nanoporous feed additive for animals of veterinary importance: Potentials and limitations. Period. Biol. 2017, 119, 159–172. [Google Scholar] [CrossRef]
- Minato, H. Characteristics and Uses of Natural Zeolites. Koatsugasu 1968, 5, 536–547. [Google Scholar]
- EURL Evaluation Report on the Analytical Methods Submitted in Connection with the Application for the Authorisation of Feed Additives According to Regulation (EC) No 1831/2003. Clinoptilolite of Volcanic Origin E567, 1–6. Last Updated on 6 June 2016. Available online: https://joint-research-centre.ec.europa.eu/publications/fad-2010-0283_en (accessed on 13 January 2024).
- Gras Notice No AGRN 29, FDA U.S. Food and Drug Administration. 2019; pp. 1–5. Available online: https://www.fda.gov/media/130509/download (accessed on 3 April 2024).
- Pandey, A.K.; Samota, M.K.; Kumar, A.; Silva, A.S.; Dubey, N.K. Fungal mycotoxins in food commodities: Present status and future concerns. Front. Sustain. Food Syst. 2023, 7, 1162595. [Google Scholar] [CrossRef]
- Kihal, A.; Rodríguez-Prado, M.; Calsamiglia, S. The efficacy of mycotoxin binders to control mycotoxins in feeds and the potential risk of interactions with nutrient: A review. J. Anim. Sci. 2022, 100, skac328. [Google Scholar] [CrossRef] [PubMed]

| Test Approach | Protein Sorbed to PCT [µg/mg] | SD [µg/mg] |
|---|---|---|
| Peanut protein only | 136.5 | 13.8 |
| Peanut protein in mix | 63.1 | 14.1 |
| Gluten only | 91.0 | 18.1 |
| Gluten in mix | 48.1 | 14.3 |
| Phosphate Buffer [pH] | KH2PO4 Solution [0.2 M] Volume [mL] | NaOH Solution [0.2 M] Volume [mL] | Final Volume [mL] |
|---|---|---|---|
| 6.8 | 250 | 112 | 1000 |
| 5.5 | 250 | 12 | 1000 |
| Phosphate Buffer [pH] | KH2PO4 [g] | Final Volume [mL] | |
| 7.5 | 44.91 | 1000 | |
| 4.5 | 13.61 | 1000 | |
| Test Solution [pH] | NaCl Solution [0.2 M] Volume [mL] | HCl Solution [0.2 M] Volume [mL] | Final Volume [mL] |
| 1.5 | 250 | 207 | 1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranftler, C.; Zehentner, M.; Pengl, A.; Röhrich, A.; Tschegg, C.; Nagl, D. Purified Clinoptilolite-Tuff as an Efficient Sorbent for Food-Derived Peanut Allergens. Int. J. Mol. Sci. 2024, 25, 6510. https://doi.org/10.3390/ijms25126510
Ranftler C, Zehentner M, Pengl A, Röhrich A, Tschegg C, Nagl D. Purified Clinoptilolite-Tuff as an Efficient Sorbent for Food-Derived Peanut Allergens. International Journal of Molecular Sciences. 2024; 25(12):6510. https://doi.org/10.3390/ijms25126510
Chicago/Turabian StyleRanftler, Carmen, Magdalena Zehentner, Andreas Pengl, Andreas Röhrich, Cornelius Tschegg, and Dietmar Nagl. 2024. "Purified Clinoptilolite-Tuff as an Efficient Sorbent for Food-Derived Peanut Allergens" International Journal of Molecular Sciences 25, no. 12: 6510. https://doi.org/10.3390/ijms25126510






