Relationship between Hypoxia and Hypercapnia Tolerance and Life Expectancy
Abstract
1. Introduction
2. Hypoxia Resistance in Some “Long-Lived” Species
3. The Role of Carbon Dioxide in the Gero-Protective Mechanism in Mammals
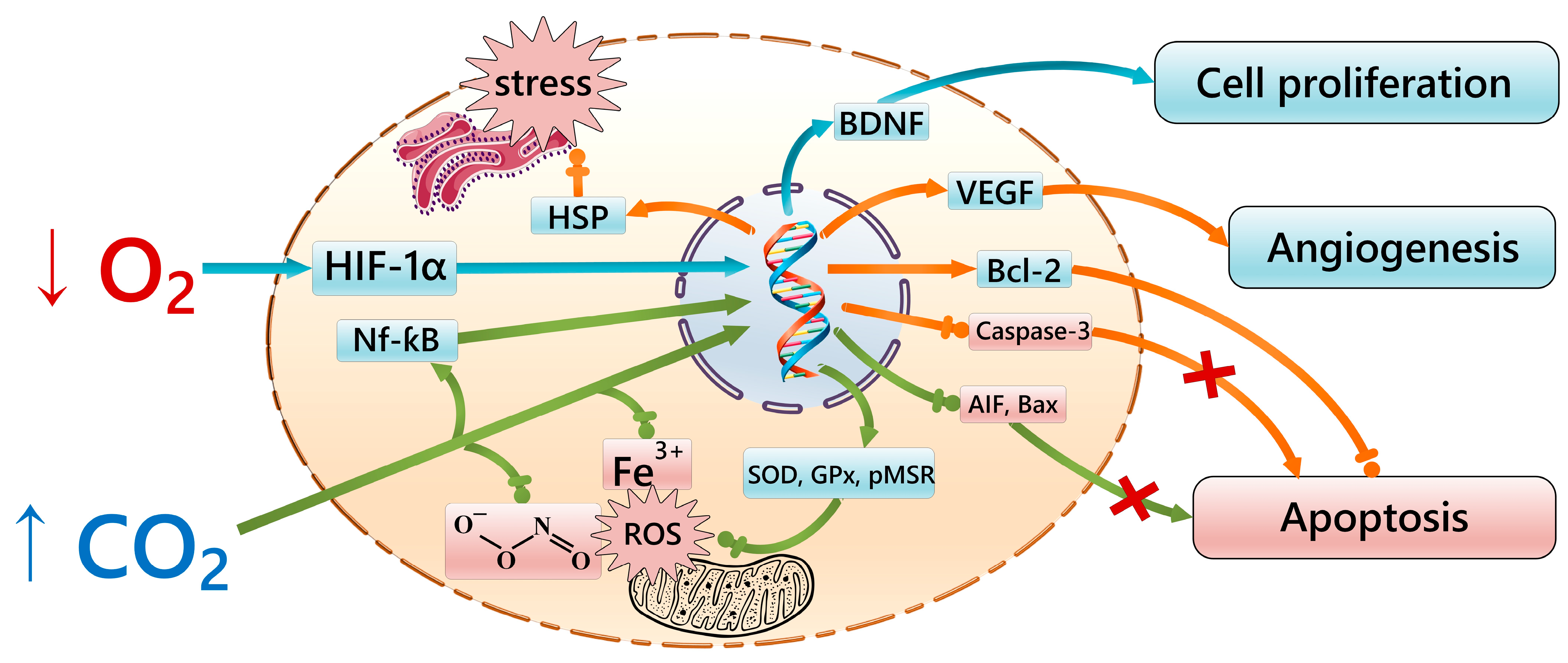
4. Signaling Pathways of the Gero-Protective Potential of Intermittent Hypercapnic–Hypoxic Exposures
5. Influence of the Protective Effects of Hypercapnia and Hypoxia on the Mechanisms of Nervous System Aging
6. Perspectives on Translational Research
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BDNF | brain-derived neurotrophic factor |
| HIF | hypoxia-inducible factor |
| ANKH | Progressive ankylosis protein homolog |
| Arnt2 | Aryl Hydrocarbon Receptor Nuclear Translocator 2 |
| Ca1–3/4/12–14 | Carbonic anhydrase 1–3/4/12–14 |
| COL4A4 | Collagen, type IV alpha 4 |
| COVID-19 | COronaVIrus Disease 2019 |
| CYGB | Cytoglobin |
| CYP17A1 | Cytochrome P450 family 17 subfamily A member 1 |
| DNA | Deoxyribonucleic Acid |
| EGLN1 | Egl-9 family hypoxia inducible factor 1 |
| EPAS1 | Endothelial PAS domain protein 1 |
| FiCO2 | CO2 concentration at the end of inhalation |
| FiO2 | O2 concentration at the end of inhalation |
| HBA | Hemoglobin subunit alpha |
| HBB | Hemoglobin subunit beta |
| HBG2 | Hemoglobin subunit gamma 2 |
| HFE | Homeostatic iron regulator |
| HLA-DPB1 | Major histocompatibility complex, class II, DP beta 1 |
| HLA-DQB1 | Major histocompatibility complex, class II, DQ beta 1 |
| HMOX2 | Heme oxygenase 2 |
| Hnf-4 | Hepatocyte nuclear factor 4 |
| HSP-90 | Heat shock protein 90 |
| iNOS | Inducible nitric oxide synthase |
| MCI | mild cognitive impairment |
| mTOR | mammalian target of rapamycin |
| NcoA1 | Nuclear receptor coactivator 1 |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NGB | Neuroglobin |
| PCO2 | Partial pressure CO2 |
| PKLR | Pyruvate kinase L/R |
| PO2 | Partial pressure O2 |
| Ppar | Peroxisome proliferator activated receptor |
| PPARA | Peroxisome proliferator activated receptor alpha |
| PaCO2 | Partial pressure of CO2 in arterial blood |
| Rora | RAR-related orphan receptor alpha |
| Rpaa | Rhythm of phycobilisome associated |
| S100/A8/A9/B/P | S100 calcium binding protein A8/A9/B/P |
| SARS-CoV-2 | Severe acute respiratory syndrome-related coronavirus 2 |
| SKN-1 | Skin antigen 1 |
| VEGF | vascular endothelial growth factor |
| VHL | Von-Hippel-Lindau |
| ZNF532 | Zinc finger protein 532 |
References
- Agadzhanian, N.A.; Radysh, I.V.; Severin, A.E.; Ermakova, N.V. Ecology, adaptation and biorhythms. Aviakosm. Ekolog. Med. 1995, 29, 16–19. (In Russian) [Google Scholar] [PubMed]
- Matsubayashi, K.; Okumiya, K. Field Medicine: A New Paradigm of Geriatric Medicine. Geriatr. Gerontol. Int. 2012, 12, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Boretto, J.M.; Cabezas-Cartes, F.; Ibargüengoytía, N.R. Slow Life Histories in Lizards Living in the Highlands of the Andes Mountains. J. Comp. Physiol. B 2018, 188, 491–503. [Google Scholar] [CrossRef]
- Pamenter, M.E.; Hall, J.E.; Tanabe, Y.; Simonson, T.S. Cross-Species Insights Into Genomic Adaptations to Hypoxia. Front. Genet. 2020, 11, 743. [Google Scholar] [CrossRef] [PubMed]
- Omotoso, O.; Gladyshev, V.N.; Zhou, X. Lifespan Extension in Long-Lived Vertebrates Rooted in Ecological Adaptation. Front. Cell. Dev. Biol. 2021, 9, 704966. [Google Scholar] [CrossRef] [PubMed]
- Keane, M.; Semeiks, J.; Webb, A.E.; Li, Y.I.; Quesada, V.; Craig, T.; Madsen, L.B.; van Dam, S.; Brawand, D.; Marques, P.I.; et al. Insights into the Evolution of Longevity from the Bowhead Whale Genome. Cell Rep. 2015, 10, 112–122. [Google Scholar] [CrossRef]
- Fang, X.; Seim, I.; Huang, Z.; Gerashchenko, M.V.; Xiong, Z.; Turanov, A.A.; Zhu, Y.; Lobanov, A.V.; Fan, D.; Yim, S.H.; et al. Adaptations to a Subterranean Environment and Longevity Revealed by the Analysis of Mole Rat Genomes. Cell Rep. 2014, 8, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Lukyanova, L.D.; Germanova, E.L.; Kopaladze, R.A. Development of Resistance of an Organism under Various Conditions of Hypoxic Preconditioning: Role of the Hypoxic Period and Reoxygenation. Bull. Exp. Biol. Med. 2009, 147, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Lin, L.C.; Wu, M.S.; Chien, C.T.; Lai, M.K. Repetitive Hypoxic Preconditioning Attenuates Renal Ischemia/Reperfusion Induced Oxidative Injury via Upregulating HIF-1 Alpha-Dependent Bcl-2 Signaling. Transplantation 2009, 88, 1251–1260. [Google Scholar] [CrossRef]
- Li, G.; Zhang, T.; Chen, X.; Shang, C.; Wang, Y. Effect of Intermittent Hypoxic Training on Hypoxia Tolerance Based on Brain Functional Connectivity. Physiol. Meas. 2016, 37, 2299–2316. [Google Scholar] [CrossRef]
- Rybnikova, E.A.; Nalivaeva, N.N.; Zenko, M.Y.; Baranova, K.A. Intermittent Hypoxic Training as an Effective Tool for Increasing the Adaptive Potential, Endurance and Working Capacity of the Brain. Front. Neurosci. 2022, 16, 941740. [Google Scholar] [CrossRef] [PubMed]
- Neckář, J.; Papoušek, F.; Nováková, O.; Ošt’ádal, B.; Kolář, F. Cardioprotective Effects of Chronic Hypoxia and Ischaemic Preconditioning Are Not Additive. Basic Res. Cardiol. 2002, 97, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Shatilo, V.B.; Korkushko, O.V.; Ischuk, V.A.; Downey, H.F.; Serebrovskaya, T.V. Effects of Intermittent Hypoxia Training on Exercise Performance, Hemodynamics, and Ventilation in Healthy Senior Men. High Alt. Med. Biol. 2008, 9, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Obrenovitch, T.P. Molecular physiology of preconditioning-induced brain tolerance to ischemia. Physiol. Rev. 2008, 88, 211–247. [Google Scholar] [CrossRef] [PubMed]
- Sharp, F.R.; Ran, R.; Lu, A.; Tang, Y.; Strauss, K.I.; Glass, T.; Ardizzone, T.; Bernaudin, M. Hypoxic preconditioning protects against ischemic brain injury. NeuroRx 2004, 1, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.J.; Wang, C.T.; Niu, K.C.; Gao, C.; Li, Z.; Lin, M.T.; Chang, C.P. Hypobaric hypoxia preconditioning attenuates acute lung injury during high-altitude exposure in rats via up-regulating heat-shock protein 70. Clin. Sci. 2011, 121, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Kulikov, V.P.; Bespalov, A.G.; Yakushev, N.N. The state of cerebral hemodynamics in conditions of prolonged adaptation to hypercapnic hypoxia. Neurosci. Behav. Physiol. 2009, 39, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Kulikov, V.P.; Tregub, P.P.; Bespalov, A.G.; Vvedenskiy, A.J. Comparative efficacy of hypoxia, hypercapnia and hypercapnic hypoxia increases body resistance to acute hypoxia in rats. Patol. Fiziol. Eksp. Ter. 2013, 3, 59–61. (In Russian) [Google Scholar]
- Tregub, P.P.; Kulikov, V.P.; Bespalov, A.G. Tolerance to acute hypoxia maximally increases in case of joint effect of normobaric hypoxia and permissive hypercapnia in rats. Pathophysiology 2013, 20, 165–170. [Google Scholar] [CrossRef]
- Simonson, T.S. Altitude Adaptation: A Glimpse Through Various Lenses. High Alt. Med. Biol. 2015, 16, 125–137. [Google Scholar] [CrossRef]
- Ding, D.; Liu, G.; Hou, L.; Gui, W.; Chen, B.; Kang, L. Genetic Variation in PTPN1 Contributes to Metabolic Adaptation to High-Altitude Hypoxia in Tibetan Migratory Locusts. Nat. Commun. 2018, 9, 4991. [Google Scholar] [CrossRef]
- Yépez, Y.; Marcano-Ruiz, M.; Bortolini, M.C. Adaptive Strategies of Aquatic Mammals: Exploring the Role of the HIF Pathway and Hypoxia Tolerance. Genet. Mol. Biol. 2024, 46, e20230140. [Google Scholar] [CrossRef] [PubMed]
- Beall, C.M.; Cavalleri, G.L.; Deng, L.; Elston, R.C.; Gao, Y.; Knight, J.; Li, C.; Li, J.C.; Liang, Y.; McCormack, M.; et al. Natural Selection on EPAS1 (HIF2alpha) Associated with Low Hemoglobin Concentration in Tibetan Highlanders. Proc. Natl. Acad. Sci. USA 2010, 107, 11459–11464. [Google Scholar] [CrossRef]
- Li, Y.; Wang, M.S.; Otecko, N.O.; Wang, W.; Shi, P.; Wu, D.D.; Zhang, Y.P. Hypoxia Potentially Promotes Tibetan Longevity. Cell Res. 2017, 27, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Basang, Z.; Wang, B.; Li, L.; Yang, L.; Liu, L.; Cui, C.; Lanzi, G.; Yuzhen, N.; Duo, J.; Zheng, H.; et al. HIF2A Variants Were Associated with Different Levels of High-Altitude Hypoxia among Native Tibetans. PLoS ONE 2015, 10, e0137956. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Wang, Z.; Niu, X.; Zhou, K.; Xu, S.; Yang, G. Evolutionary Genetics of Hypoxia Tolerance in Cetaceans during Diving. Genome Biol. Evol. 2016, 8, 827–839. [Google Scholar] [CrossRef]
- Leiser, S.F.; Fletcher, M.; Begun, A.; Kaeberlein, M. Life-Span Extension from Hypoxia in Caenorhabditis Elegans Requires Both HIF-1 and DAF-16 and Is Antagonized by SKN-1. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Steinkraus, K.A.; Sutphin, G.L.; Ramos, F.J.; Shamieh, L.S.; Huh, A.; Davis, C.; Chandler-Brown, D.; Kaeberlein, M. Proteasomal Regulation of the Hypoxic Response Modulates Aging in C. elegans. Science 2009, 324, 1196–1198. [Google Scholar] [CrossRef] [PubMed]
- Tyshkovskiy, A.; Bozaykut, P.; Borodinova, A.A.; Gerashchenko, M.V.; Ables, G.P.; Garratt, M.; Khaitovich, P.; Clish, C.B.; Miller, R.A.; Gladyshev, V.N. Identification and Application of Gene Expression Signatures Associated with Lifespan Extension. Cell Metab. 2019, 30, 573–593.e8. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.B.; Fang, X.; Fushan, A.A.; Huang, Z.; Lobanov, A.V.; Han, L.; Marino, S.M.; Sun, X.; Turanov, A.A.; Yang, P.; et al. Genome Sequencing Reveals Insights into Physiology and Longevity of the Naked Mole Rat. Nature 2011, 479, 223–227. [Google Scholar] [CrossRef]
- Lavinka, P.C.; Brand, A.; Landau, V.J.; Wirtshafter, D.; Park, T.J. Extreme Tolerance to Ammonia Fumes in African Naked Mole-Rats: Animals That Naturally Lack Neuropeptides from Trigeminal Chemosensory Nerve Fibers. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 2009, 195, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Nevo, E.; Han, L.; Levanon, E.Y.; Zhao, J.; Avivi, A.; Larkin, D.; Jiang, X.; Feranchuk, S.; Zhu, Y.; et al. Genome-Wide Adaptive Complexes to Underground Stresses in Blind Mole Rats Spalax. Nat. Commun. 2014, 5, 3966. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.N.; Rubinstein, N.D.; Buffenstein, R.A. Window into Extreme Longevity; the Circulating Metabolomic Signature of the Naked Mole-Rat, a Mammal That Shows Negligible Senescence. Geroscience 2018, 40, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Conti, B.; Sanchez-Alavez, M.; Winsky-Sommerer, R.; Morale, M.C.; Lucero, J.; Brownell, S.; Fabre, V.; Huitron-Resendiz, S.; Henriksen, S.; Zorrilla, E.P.; et al. Transgenic Mice with a Reduced Core Body Temperature Have an Increased Life Span. Science 2006, 314, 825–828. [Google Scholar] [CrossRef]
- Tolstun, D.A.; Knyazer, A.; Tushynska, T.V.; Dubiley, T.A.; Bezrukov, V.V.; Fraifeld, V.E.; Muradian, K.K. Metabolic Remodelling of Mice by Hypoxic-Hypercapnic Environment: Imitating the Naked Mole-Rat. Biogerontology 2020, 21, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, P.J.; Hart, D.W.; Merchant, H.N.; Voigt, C.; Bennett, N.C. The Evolution and Ecology of Oxidative and Antioxidant Status: A Comparative Approach in African Mole-Rats. Antioxidants 2023, 12, 1486. [Google Scholar] [CrossRef] [PubMed]
- Snell, T.W.; Johnston, R.K.; Jones, B.L. Hypoxia Extends Lifespan of Brachionus Manjavacas (Rotifera). Limnetica 2019, 38, 159–166. [Google Scholar] [CrossRef]
- Rascón, B.; Harrison, J.F. Lifespan and Oxidative Stress Show a Non-Linear Response to Atmospheric Oxygen in Drosophila. J. Exp. Biol. 2010, 213, 3441–3448. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.; Zhao, M.; Yang, W.; Bo, Y.; Li, W. Neuroprotective Effects of Therapeutic Hypercapnia on Spatial Memory and Sensorimotor Impairment via Anti-Apoptotic Mechanisms after Focal Cerebral Ischemia/Reperfusion. Neurosci. Lett. 2014, 573, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pruimboom, L.; Muskiet, F.A.J. Intermittent Living; the Use of Ancient Challenges as a Vaccine against the Deleterious Effects of Modern Life-A Hypothesis. Med. Hypotheses 2018, 120, 28–42. [Google Scholar] [CrossRef]
- Tregub, P.P.; Kulikov, V.P.; Motin, Y.G.; Bespalov, A.G.; Osipov, I.S. Combined Exposure to Hypercapnia and Hypoxia Provides Its Maximum Neuroprotective Effect during Focal Ischemic Injury in the Brain. J. Stroke Cerebrovasc. Dis. 2015, 24, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Kulikov, V.P.; Tregub, P.P.; Osipov, I.S.; Trukhanov, A.I. Hypercapnic Hypoxia as a Potential Means to Extend Life Expectancy and Improve Physiological Activity in Mice. Biogerontology 2019, 20, 677–686. [Google Scholar] [CrossRef]
- Krivoruchko, A.; Storey, K.B. Forever Young: Mechanisms of Natural Anoxia Tolerance and Potential Links to Longevity. Oxid. Med. Cell. Longev. 2010, 3, 186–198. [Google Scholar] [CrossRef]
- Muradian, K. “Pull and Push Back” Concepts of Longevity and Life Span Extension. Biogerontology 2013, 14, 687–691. [Google Scholar] [CrossRef]
- Chung, D.; Dzal, Y.A.; Seow, A.; Milsom, W.K.; Pamenter, M.E. Naked Mole Rats Exhibit Metabolic but Not Ventilatory Plasticity Following Chronic Sustained Hypoxia. Proc. Biol. Sci. 2016, 283, 20160216. [Google Scholar] [CrossRef] [PubMed]
- Park, T.J.; Reznick, J. Extreme Physiology Extreme Tolerance to Hypoxia, Hypercapnia, and Pain in the Naked Mole-Rat. J. Muscle Res. Cell Motil. 2023, 44, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Park, T.J.; Reznick, J.; Peterson, B.L.; Blass, G.; Omerbašić, D.; Bennett, N.C.; Kuich, P.H.J.L.; Zasada, C.; Browe, B.M.; Hamann, W.; et al. Fructose-Driven Glycolysis Supports Anoxia Resistance in the Naked Mole-Rat. Science 2017, 356, 307–311. [Google Scholar] [CrossRef]
- Tregub, P.P.; Kulikov, V.P.; Ibrahimli, I.; Tregub, O.F.; Volodkin, A.V.; Ignatyuk, M.A.; Kostin, A.A.; Atiakshin, D.A. Molecular Mechanisms of Neuroprotection after the Intermittent Exposures of Hypercapnic Hypoxia. Int. J. Mol. Sci. 2024, 25, 3665. [Google Scholar] [CrossRef]
- Mas-Bargues, C.; Borrás, C.; Viña, J. The Multimodal Action of Genistein in Alzheimer’s and Other Age-Related Diseases. Free Radic. Biol. Med. 2022, 183, 127–137. [Google Scholar] [CrossRef]
- Sharma, D.; Khan, H.; Kumar, A.; Grewal, A.K.; Dua, K.; Singh, T.G. Pharmacological Modulation of HIF-1 in the Treatment of Neuropsychiatric Disorders. J. Neural Transm. 2023, 130, 1523–1535. [Google Scholar] [CrossRef]
- Sun, S.; Meng, Y.; Li, M.; Tang, X.; Hu, W.; Wu, W.; Li, G.; Pang, Q.; Wang, W.; Liu, B. CD133+ Endothelial-like Stem Cells Restore Neovascularization and Promote Longevity in Progeroid and Naturally Aged Mice. Nat. Aging 2023, 3, 1401–1414. [Google Scholar] [CrossRef] [PubMed]
- Bespalov, A.G.; Tregub, P.P.; Kulikov, V.P.; Pijanzin, A.I.; Belousov, A.A. The role of VEGF, HSP-70 and protein S-100B in the potentiation effect of the neuroprotective effect of hypercapnic hypoxia. Patol. Fiziol. Eksp. Ter. 2014, 2, 24–27. (In Russian) [Google Scholar]
- Song, B.X.; Azhar, L.; Koo, G.K.Y.; Marzolini, S.; Gallagher, D.; Swardfager, W.; Chen, C.; Ba, J.; Herrmann, N.; Lanctôt, K.L. The Effect of Exercise on Blood Concentrations of Angiogenesis Markers in Older Adults: A Systematic Review and Meta-Analysis. Neurobiol. Aging 2024, 135, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Lu, Y.Y.; Zhao, J. Challenges and strategies in the treatment of neovascular age-related macular degeneration. Zhonghua Yan Ke Za Zhi 2024, 60, 215–219. (In Chinese) [Google Scholar] [CrossRef]
- Wang, L.; Wan, L.; Zhang, T.; Guan, C.; Hu, J.; Xu, D.; Lu, H. A Combined Treatment of BMP2 and Soluble VEGFR1 for the Enhancement of Tendon-Bone Healing by Regulating Injury-Activated Skeletal Stem Cell Lineage. Am. J. Sports Med. 2024, 52, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Barth, A.; Bauer, R.; Gedrange, T.; Walter, B.; Klinger, W.; Zwiener, U. Influence of hypoxia and hypoxia/hypercapnia upon brain and blood peroxidative and glutathione status in normal weight and growth-restricted newborn piglets. Exp. Toxicol. Pathol. 1998, 50, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Veselá, A.; Wilhelm, J. The role of carbon dioxide in free radical reactions of the organism. Physiol. Res. 2002, 51, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Goss, S.P.A.; Singh, R.J.; Kalyanaraman, B. Bicarbonate Enhances the Peroxidase Activity of Cu,Zn-Superoxide Dismutase. Role of Carbonate Anion Radical. J. Biol. Chem. 1999, 274, 28233–28239. [Google Scholar] [CrossRef] [PubMed]
- Serezhenkov, V.A.; Kalinina, E.V.; Glazunova, V.A.; Saprin, A.N.; Vanin, A.F. Why does iron abrogate the cytotoxic effect of S-nitrosothiols on human and animal cultured cells? Biofizika 2007, 52, 869–875. [Google Scholar]
- Conrado, A.B.; D’Angelantonio, M.; Torreggiani, A.; Pecci, L.; Fontana, M. Reactivity of Hypotaurine and Cysteine Sulfinic Acid toward Carbonate Radical Anion and Nitrogen Dioxide as Explored by the Peroxidase Activity of Cu, Zn Superoxide Dismutase and by Pulse Radiolysis. Free Radic. Res. 2014, 48, 1300–1310. [Google Scholar] [CrossRef]
- Augusto, O.; Goldstein, S.; Hurst, J.K.; Lind, J.; Lymar, S.V.; Merenyi, G.; Radi, R. Carbon Dioxide-Catalyzed Peroxynitrite Reactivity-The Resilience of the Radical Mechanism after Two Decades of Research. Free Radic. Biol. Med. 2019, 135, 210–215. [Google Scholar] [CrossRef]
- Kniffin, C.D.; Burnett, L.E.; Burnett, K.G. Recovery from Hypoxia and Hypercapnic Hypoxia: Impacts on the Transcription of Key Antioxidants in the Shrimp Litopenaeus vannamei. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2014, 170, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Fantini, C.; Corinaldesi, C.; Lenzi, A.; Migliaccio, S.; Crescioli, C. Vitamin D as a Shield against Aging. Int. J. Mol. Sci. 2023, 24, 4546. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Solé, C.; Pinilla-González, V.; Lillo-Moya, J.; González-Fernández, T.; Saso, L.; Rodrigo, R. Integrated Approach to Reducing Polypharmacy in Older People: Exploring the Role of Oxidative Stress and Antioxidant Potential Therapy. Redox Rep. 2024, 29, 2289740. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, P.J.; Hart, D.W.; Bennett, N.C. Plasma Oxidative Stress in Reproduction of Two Eusocial African Mole-Rat Species, the Naked Mole-Rat and the Damaraland Mole-Rat. Front. Zool. 2021, 18, 45. [Google Scholar] [CrossRef] [PubMed]
- Tregub, P.P.; Malinovskaya, N.A.; Hilazheva, E.D.; Morgun, A.V.; Kulikov, V.P. Permissive Hypercapnia and Hypercapnic Hypoxia Inhibit Signaling Pathways of Neuronal Apoptosis in Ischemic/Hypoxic Rats. Mol. Biol. Rep. 2023, 50, 2317–2333. [Google Scholar] [CrossRef] [PubMed]
- Tregub, P.P.; Morgun, A.V.; Osipova, E.D.; Kulikov, V.P.; Malinovskaya, N.A.; Kuzovkov, D.A. Hypercapnia and Hypoxia Stimulate Proliferation of Astrocytes and Neurons In Vitro. Bull. Exp. Biol. Med. 2020, 169, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zeng, Z.; Luo, H.; Xiao, H.; Zeng, Y. The Effects of CypA on Apoptosis: Potential Target for the Treatment of Diseases. Appl. Microbiol. Biotechnol. 2024, 108, 28. [Google Scholar] [CrossRef]
- Hong, J.M.; Munna, A.N.; Moon, J.H.; Seol, J.W.; Park, S.Y. Melatonin-Mediated Calcineurin Inactivation Attenuates Amyloid Beta-Induced Apoptosis. IBRO Neurosci. Rep. 2024, 16, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Yang, J.; Qi, Z.; Wu, H.; Wang, B.; Zou, F.; Mei, H.; Liu, J.; Wang, W.; Liu, Q. Heat Shock Proteins: Biological Functions, Pathological Roles, and Therapeutic Opportunities. MedComm 2022, 3, e161. [Google Scholar] [CrossRef]
- Deocaris, C.C.; Takano, S.; Priyandoko, D.; Kaul, Z.; Yaguchi, T.; Kraft, D.C.; Yamasaki, K.; Kaul, S.C.; Wadhwa, R. Glycerol Stimulates Innate Chaperoning, Proteasomal and Stress-Resistance Functions: Implications for Geronto-Manipulation. Biogerontology 2008, 9, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Magon, N.; Chopra, S.; Kumar, P. Geroprotection: A Promising Future. J. Midlife Health 2012, 3, 56. [Google Scholar] [CrossRef] [PubMed]
- Lippi, A.; Krisko, A. CORE at the Boundary of Stress Resistance and Longevity. Int. J. Biochem. Cell Biol. 2022, 151, 106277. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, C.; Perrotta, R.; Graziano, A.; Calabrese, E.J.; Calabrese, V. Stress Responses, Vitagenes and Hormesis as Critical Determinants in Aging and Longevity: Mitochondria as a “Chi”. Immun. Ageing 2013, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Scapagnini, G.; Davinelli, S.; Koverech, G.; Koverech, A.; De Pasquale, C.; Salinaro, A.T.; Scuto, M.; Calabrese, E.J.; Genazzani, A.R. Sex Hormonal Regulation and Hormesis in Aging and Longevity: Role of Vitagenes. J. Cell Commun. Signal. 2014, 8, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Tregub, P.P.; Kulikov, V.P.; Motin, Y.G.; Nagibaeva, M.E.; Zabrodina, A.S. Stress of the Endoplasmic Reticulum of Neurons in Stroke Can Be Maximally Limited by Combined Exposure to Hypercapnia and Hypoxia. Bull. Exp. Biol. Med. 2016, 161, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Benderro, G.F.; Tsipis, C.P.; Sun, X.; Kuang, Y.; Lamanna, J.C. Increased HIF-1α and HIF-2α Accumulation, but Decreased Microvascular Density, in Chronic Hyperoxia and Hypercapnia in the Mouse Cerebral Cortex. Adv. Exp. Med. Biol. 2013, 789, 29–35. [Google Scholar] [CrossRef]
- Tregub, P.P.; Malinovskaya, N.A.; Morgun, A.V.; Osipova, E.D.; Kulikov, V.P.; Kuzovkov, D.A.; Kovzelev, P.D. Hypercapnia Potentiates HIF-1α Activation in the Brain of Rats Exposed to Intermittent Hypoxia. Respir. Physiol. Neurobiol. 2020, 278, 103442. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Xu, Y.; Sun, L.; Hashimoto, M.; Wei, J. Microglial Response to Aging and Neuroinflammation in the Development of Neurodegenerative Diseases. Neural. Regen. Res. 2024, 19, 1241–1248. [Google Scholar] [CrossRef]
- Richardson, B.; Goedert, T.; Quraishe, S.; Deinhardt, K.; Mudher, A. How Do Neurons Age? A Focused Review on the Aging of the Microtubular Cytoskeleton. Neural Regen. Res. 2024, 19, 1899–1907. [Google Scholar] [CrossRef]
- Dallaire-Théroux, C.; Smith, C.; Duchesne, S. Clinical Predictors of Postmortem Amyloid and Nonamyloid Cerebral Small Vessel Disease in Middle-Aged to Older Adults. Neurol. Clin. Pract. 2024, 14, e200271. [Google Scholar] [CrossRef]
- Burtscher, J.; Mallet, R.T.; Burtscher, M.; Millet, G.P. Hypoxia and Brain Aging: Neurodegeneration or Neuroprotection? Ageing Res. Rev. 2021, 68, 101343. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-Aging. An Evolutionary Perspective on Immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Komleva, Y.; Chernykh, A.; Lopatina, O.; Gorina, Y.; Lokteva, I.; Salmina, A.; Gollasch, M. Inflamm-Aging and Brain Insulin Resistance: New Insights and Role of Life-Style Strategies on Cognitive and Social Determinants in Aging and Neurodegeneration. Front. Neurosci. 2021, 14, 618395. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, I.; Williams, S.C.R.; Morrella, M.J. The Impact of Sleep and Hypoxia on the Brain: Potential Mechanisms for the Effects of Obstructive Sleep Apnea. Curr. Opin. Pulm. Med. 2014, 20, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Boroujerdi, A.; Milner, R. Defining the Critical Hypoxic Threshold That Promotes Vascular Remodeling in the Brain. Exp. Neurol. 2015, 263, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Snyder, B.; Shell, B.; Cunningham, J.T.; Cunningham, R.L. Chronic Intermittent Hypoxia Induces Oxidative Stress and Inflammation in Brain Regions Associated with Early-Stage Neurodegeneration. Physiol. Rep. 2017, 5, e13258. [Google Scholar] [CrossRef] [PubMed]
- Parmar, J.; Jones, N.M. Hypoxic Preconditioning Can Reduce Injury-Induced Inflammatory Processes in the Neonatal Rat Brain. Int. J. Dev. Neurosci. 2015, 43, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz Avci, A.; Avci, S.; Lakadamyali, H.; Can, U. Hypoxia and Inflammation Indicate Significant Differences in the Severity of Obstructive Sleep Apnea within Similar Apnea-Hypopnea Index Groups. Sleep Breath. 2017, 21, 703–711. [Google Scholar] [CrossRef]
- Coronel-Oliveros, C.; Medel, V.; Whitaker, G.A.; Astudillo, A.; Gallagher, D.; Z-Rivera, L.; Prado, P.; El-Deredy, W.; Orio, P.; Weinstein, A. Elevating Understanding: Linking High-Altitude Hypoxia to Brain Aging through EEG Functional Connectivity and Spectral Analyses. Netw. Neurosci. 2024, 8, 275–292. [Google Scholar] [CrossRef]
- Kim, K.S.; Kwak, J.W.; Lim, S.J.; Park, Y.K.; Yang, H.S.; Kim, H.J. Oxidative Stress-Induced Telomere Length Shortening of Circulating Leukocyte in Patients with Obstructive Sleep Apnea. Aging Dis. 2016, 7, 604–613. [Google Scholar] [CrossRef]
- Sivagurunathan, N.; Calivarathan, L. SARS-CoV-2 Infection to Premature Neuronal Aging and Neurodegenerative Diseases: Is There Any Connection with Hypoxia? CNS Neurol. Disord. Drug Targets 2024, 23, 431–448. [Google Scholar] [CrossRef] [PubMed]
- Hwang, A.B.; Lee, S.J. Regulation of Life Span by Mitochondrial Respiration: The HIF-1 and ROS Connection. Aging 2011, 3, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Ristow, M.; Schmeisser, S. Extending Life Span by Increasing Oxidative Stress. Free Radic. Biol. Med. 2011, 51, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Di Giulio, C.; Bianchi, G.; Cacchio, M.; Artese, L.; Rapino, C.; Macrì, M.A.; Di Ilio, C. Oxygen and Life Span: Chronic Hypoxia as a Model for Studying HIF-1alpha, VEGF and NOS during Aging. Respir. Physiol. Neurobiol. 2005, 147, 31–38. [Google Scholar] [CrossRef]
- Glazachev, O.S.; Dudnik, E.N.; Zapara, M.A.; Samarceva, V.G.; Kofler, W.W. Adaptation to Dosed Hypoxia-Hyperoxia as a Factor in the Improvement of Quality of Life for Elderly Patients with Cardiac Pathology. Adv. Gerontol. 2019, 9, 453–458. [Google Scholar] [CrossRef]
- Wang, H.; Shi, X.; Schenck, H.; Hall, J.R.; Ross, S.E.; Kline, G.P.; Chen, S.; Mallet, R.T.; Chen, P. Intermittent Hypoxia Training for Treating Mild Cognitive Impairment: A Pilot Study. Am. J. Alzheimers Dis. Other Demen. 2020, 35, 1533317519896725. [Google Scholar] [CrossRef]
- Kuznetsova, D.V.; Kulikov, V.P. Cerebrovascular and Systemic Hemodynamic Response to Carbon Dioxide in Humans. Blood Press. Monit. 2014, 19, 81–89. [Google Scholar] [CrossRef]
- Fried, R. The hyperventilation syndrome-research and clinical treatment. J. Neurol. Neurosurg. Psychiatry 1988, 51, 1600–1601. [Google Scholar] [CrossRef]
- Nardi, A.; Valença, A.; Lopes, F.; Nascimento, I.; Mezzasalma, M.; Zin, W. Clinical features of panic patients sensitive to hyperventilation or breath-holding methods for inducing panic attacks. Braz. J. Med Biol. Res. 2004, 37, 251–257. [Google Scholar] [CrossRef]
- Xie, A.; Skatrud, J.B.; Khayat, R.; Dempsey, J.A.; Morgan, B.; Russell, D. Cerebrovascular response to carbon dioxide in patientswith congestive heart failure. Am. J. Respir. Crit. Care Med. 2005, 172, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Burgess, K.R.; Fan, J.L.; Peebles, K.C.; Thomas, K.N.; Lucas, S.; Lucas, R.; Dawson, A.; Swart, M.; Shepherd, K.; Ainslie, P. Exacerbation of obstructive sleep apnea by oral indomethacin. Chest 2010, 137, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Kulikov, V.P.; Kuznetsova, D.V.; Zarya, A.N. Role of Cerebrovascular and Cardiovascular CO2-Reactivity in the Pathogenesis of Arterial Hypertension. Arter. Hypertens. 2017, 23, 433–446. [Google Scholar] [CrossRef]
- Flück, D.; Braz, I.D.; Keiser, S.; Hüppin, F.; Haider, T.; Hilty, M.P.; Fisher, J.P.; Lundby, C. Age, aerobic fitness, and cerebral perfusion during exercise: Role of carbon dioxide. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H515–H523. [Google Scholar] [CrossRef]
- Kumari, P.; Wadhwa, M.; Chauhan, G.; Alam, S.; Roy, K.; Kumar Jha, P.; Kishore, K.; Ray, K.; Kumar, S.; Chandra Nag, T.; et al. Hypobaric Hypoxia Induced Fear and Extinction Memory Impairment and Effect of Ginkgo Biloba in Its Amelioration: Behavioral, Neurochemical and Molecular Correlates. Behav. Brain Res. 2020, 387, 112595. [Google Scholar] [CrossRef]
- Meng, S.X.; Wang, B.; Li, W.T. Intermittent hypoxia improves cognition and reduces anxiety-related behavior in APP/PS1 mice. Brain Behav. 2020, 10, e01513. [Google Scholar] [CrossRef]
- Navarrete-Opazo, A.; Mitchell, G.S. Therapeutic Potential of Intermittent Hypoxia: A Matter of Dose. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, 1181–1197. [Google Scholar] [CrossRef]
- Iwamoto, E.; Hanson, B.E.; Bock, J.M.; Casey, D.P. Intermittent Hypoxia Enhances Shear-Mediated Dilation of the Internal Carotid Artery in Young Adults. J. Appl. Physiol. 2020, 129, 603–611. [Google Scholar] [CrossRef]
- Ackert-Bicknell, C.L.; Anderson, L.C.; Sheehan, S.; Warren, G.H.; Bo, C.; Gary, A.C.; Elissa, J.C.; Ron, K.; Luanne, L.P. Aging Research Using Mouse Models. Curr. Protoc. Mouse Biol. 2015, 5, 95–133. [Google Scholar] [CrossRef] [PubMed]
- Bernardes de Jesus, B.; Vera, E.; Schneeberger, K.; Tejera, A.M.; Ayuso, E.; Bosch, F.; Blasco, M.A. Telomerase Gene Therapy in Adult and Old Mice Delays Aging and Increases Longevity without Increasing Cancer. EMBO Mol. Med. 2012, 4, 691–704. [Google Scholar] [CrossRef]
- Harrison, D.E.; Strong, R.; Sharp, Z.D.; Nelson, J.F.; Astle, C.M.; Flurkey, K.; Nadon, N.L.; Wilkinson, J.E.; Frenkel, K.; Carter, C.S.; et al. Rapamycin Fed Late in Life Extends Lifespan in Genetically Heterogeneous Mice. Nature 2009, 460, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, A.G.; Ouellette, M.; Frolkis, M.; Holt, S.E.; Chiu, C.P.; Morin, G.B.; Harley, C.B.; Shay, J.W.; Lichtsteiner, S.; Wright, W.E. Extension of Life-Span by Introduction of Telomerase into Normal Human Cells. Science 1998, 279, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Anisimov, V.N.; Popovich, I.G.; Zabezhinski, M.A. Methods of Evaluating the Effect of Pharmacological Drugs on Aging and Life Span in Mice. Methods Mol. Biol. 2007, 371, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Caulin, A.F.; Maley, C.C. Peto’s Paradox: Evolution’s Prescription for Cancer Prevention. Trends Ecol. Evol. 2011, 26, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Delaney, M.A.; Imai, D.M.; Buffenstein, R. Spontaneous Disease and Pathology of Naked Mole-Rats. Adv. Exp. Med. Biol. 2021, 1319, 353–380. [Google Scholar] [CrossRef]
- Finch, C.E. Update on Slow Aging and Negligible Senescence—A Mini-Review. Gerontology 2009, 55, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M. Healthspan Pharmacology. Rejuvenation Res. 2015, 18, 573–580. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tregub, P.P.; Komleva, Y.K.; Kulikov, V.P.; Chekulaev, P.A.; Tregub, O.F.; Maltseva, L.D.; Manasova, Z.S.; Popova, I.A.; Andriutsa, N.S.; Samburova, N.V.; et al. Relationship between Hypoxia and Hypercapnia Tolerance and Life Expectancy. Int. J. Mol. Sci. 2024, 25, 6512. https://doi.org/10.3390/ijms25126512
Tregub PP, Komleva YK, Kulikov VP, Chekulaev PA, Tregub OF, Maltseva LD, Manasova ZS, Popova IA, Andriutsa NS, Samburova NV, et al. Relationship between Hypoxia and Hypercapnia Tolerance and Life Expectancy. International Journal of Molecular Sciences. 2024; 25(12):6512. https://doi.org/10.3390/ijms25126512
Chicago/Turabian StyleTregub, Pavel P., Yulia K. Komleva, Vladimir P. Kulikov, Pavel A. Chekulaev, Oksana F. Tregub, Larisa D. Maltseva, Zaripat Sh. Manasova, Inga A. Popova, Natalia S. Andriutsa, Natalia V. Samburova, and et al. 2024. "Relationship between Hypoxia and Hypercapnia Tolerance and Life Expectancy" International Journal of Molecular Sciences 25, no. 12: 6512. https://doi.org/10.3390/ijms25126512
APA StyleTregub, P. P., Komleva, Y. K., Kulikov, V. P., Chekulaev, P. A., Tregub, O. F., Maltseva, L. D., Manasova, Z. S., Popova, I. A., Andriutsa, N. S., Samburova, N. V., Salmina, A. B., & Litvitskiy, P. F. (2024). Relationship between Hypoxia and Hypercapnia Tolerance and Life Expectancy. International Journal of Molecular Sciences, 25(12), 6512. https://doi.org/10.3390/ijms25126512







