Pseudolaric Acid B Targets CD147 to Selectively Kill Acute Myeloid Leukemia Cells
Abstract
:1. Introduction
2. Results
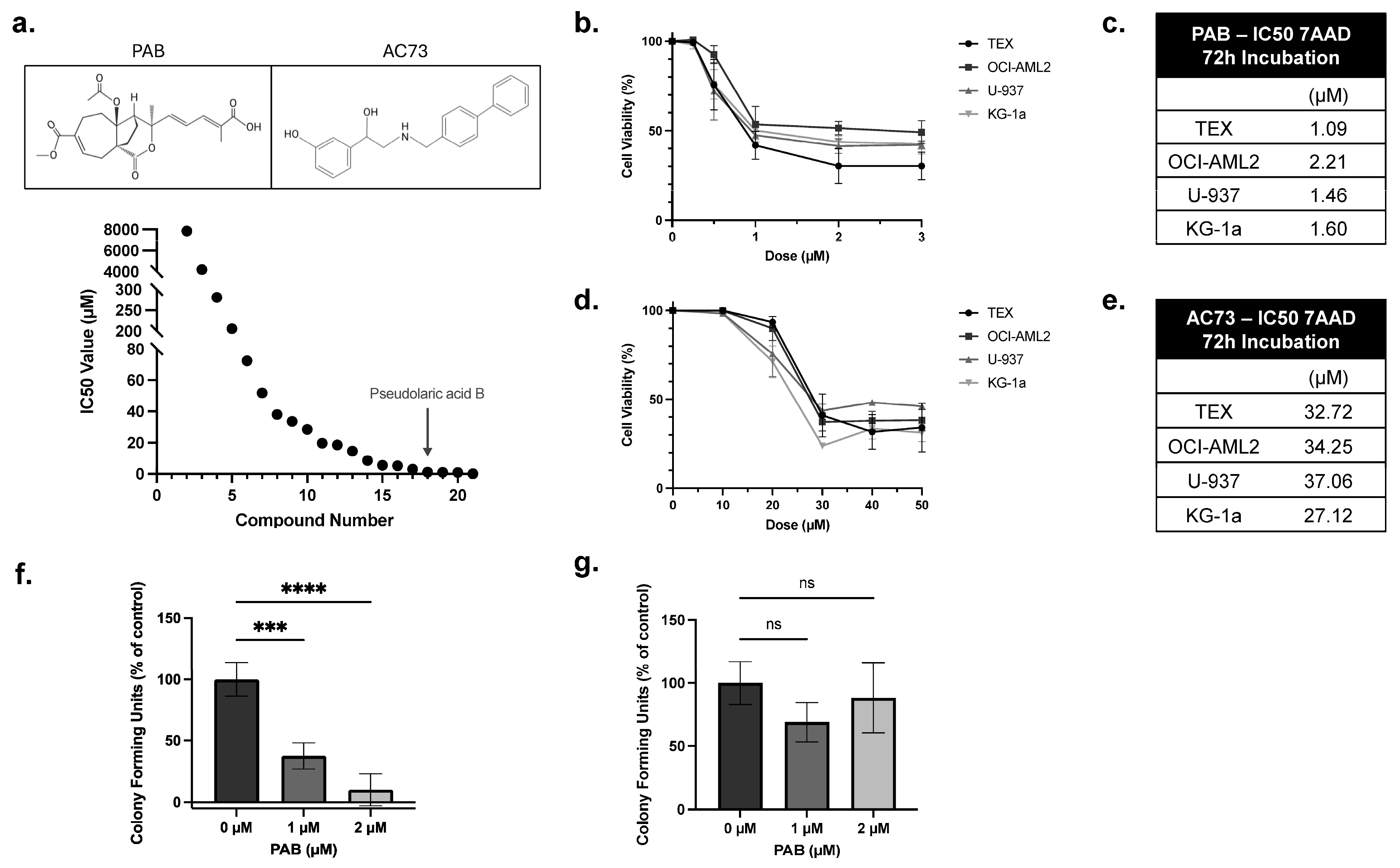
2.1. PAB Exhibits Anti-Leukemia Activities In Vitro
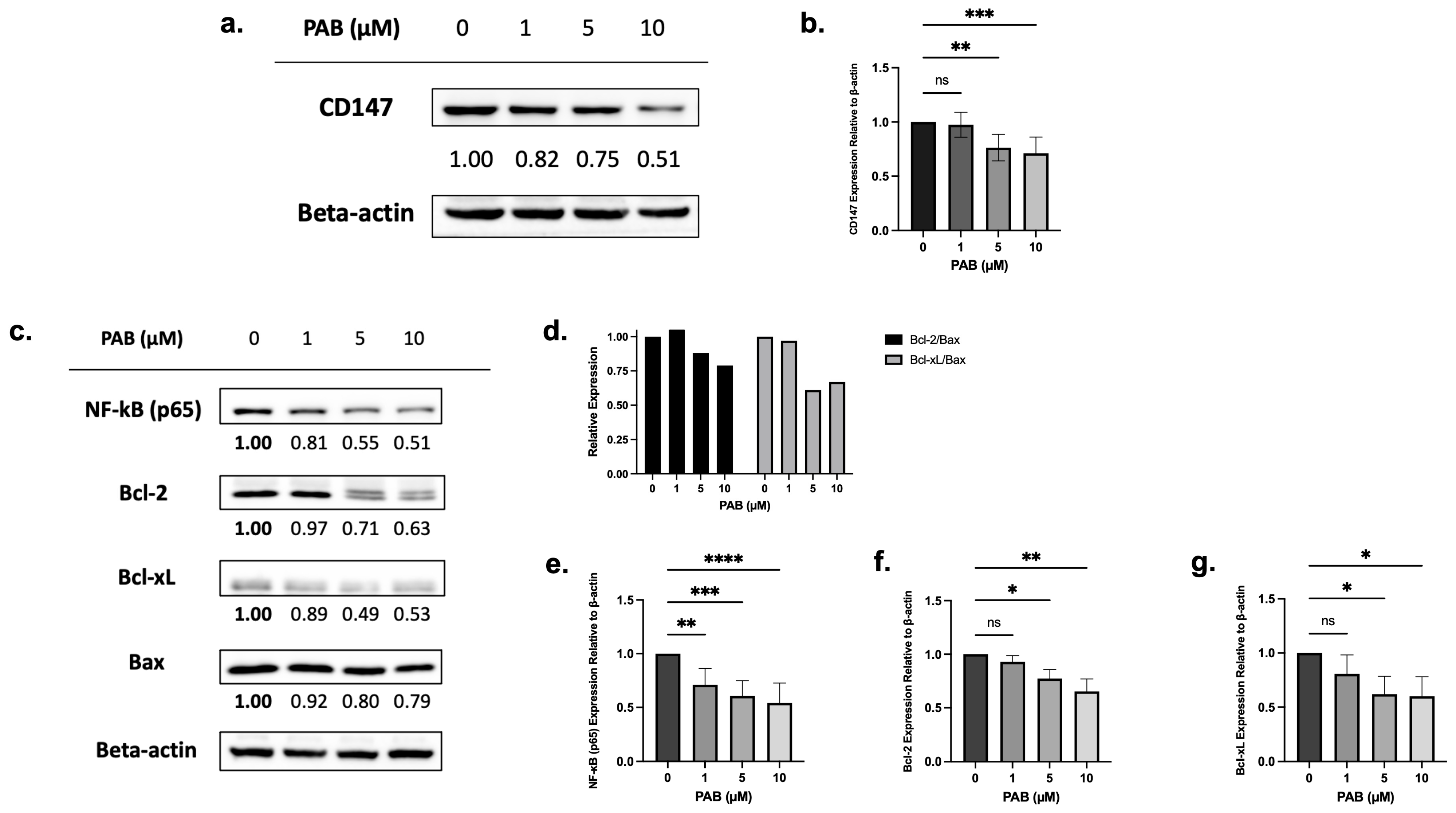
2.2. PAB Regulates CD147, NF-κB, and Bcl-2 Expression in AML Cell Lines
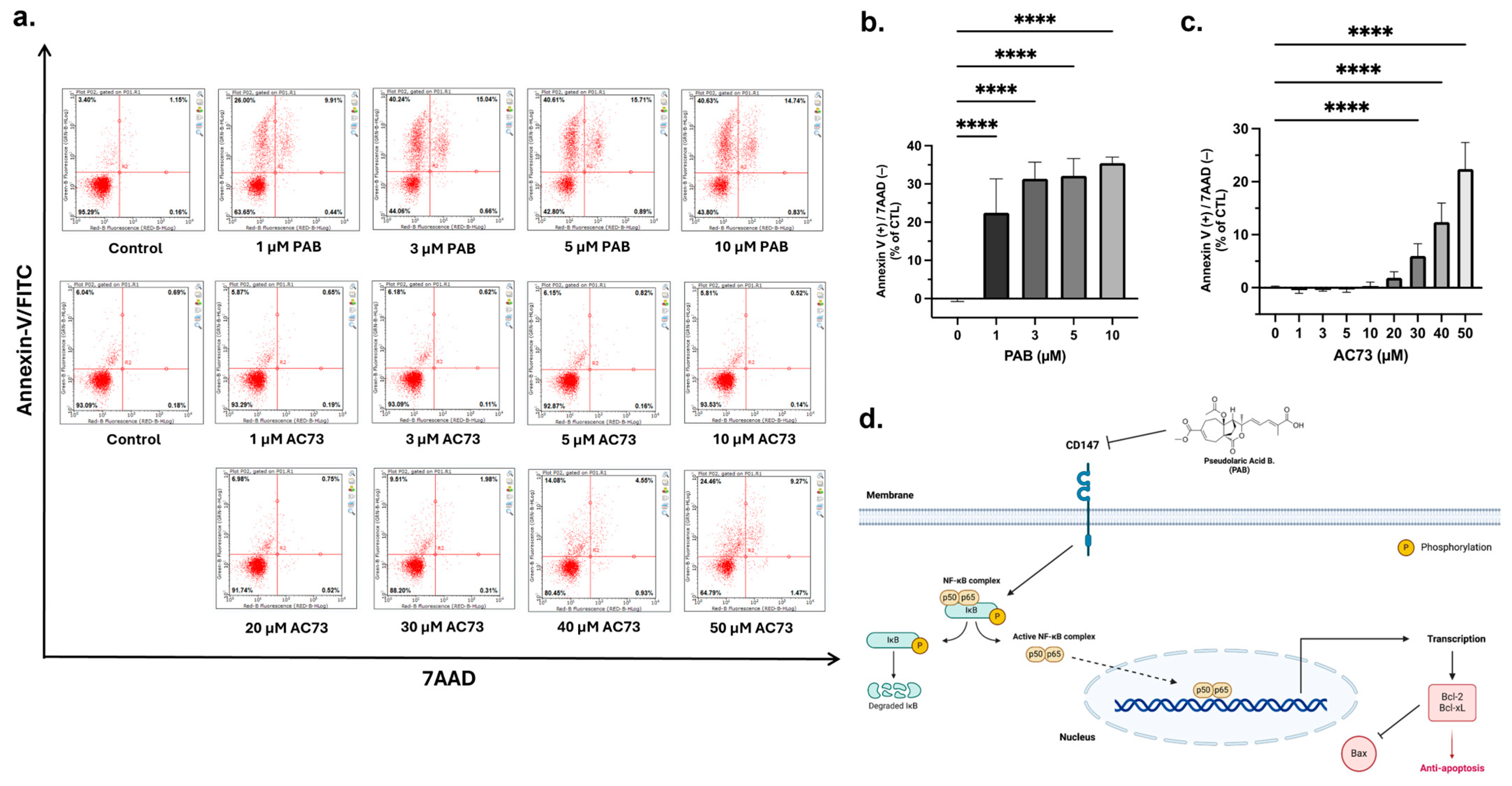
2.3. PAB Induces Apoptosis in AML Cells
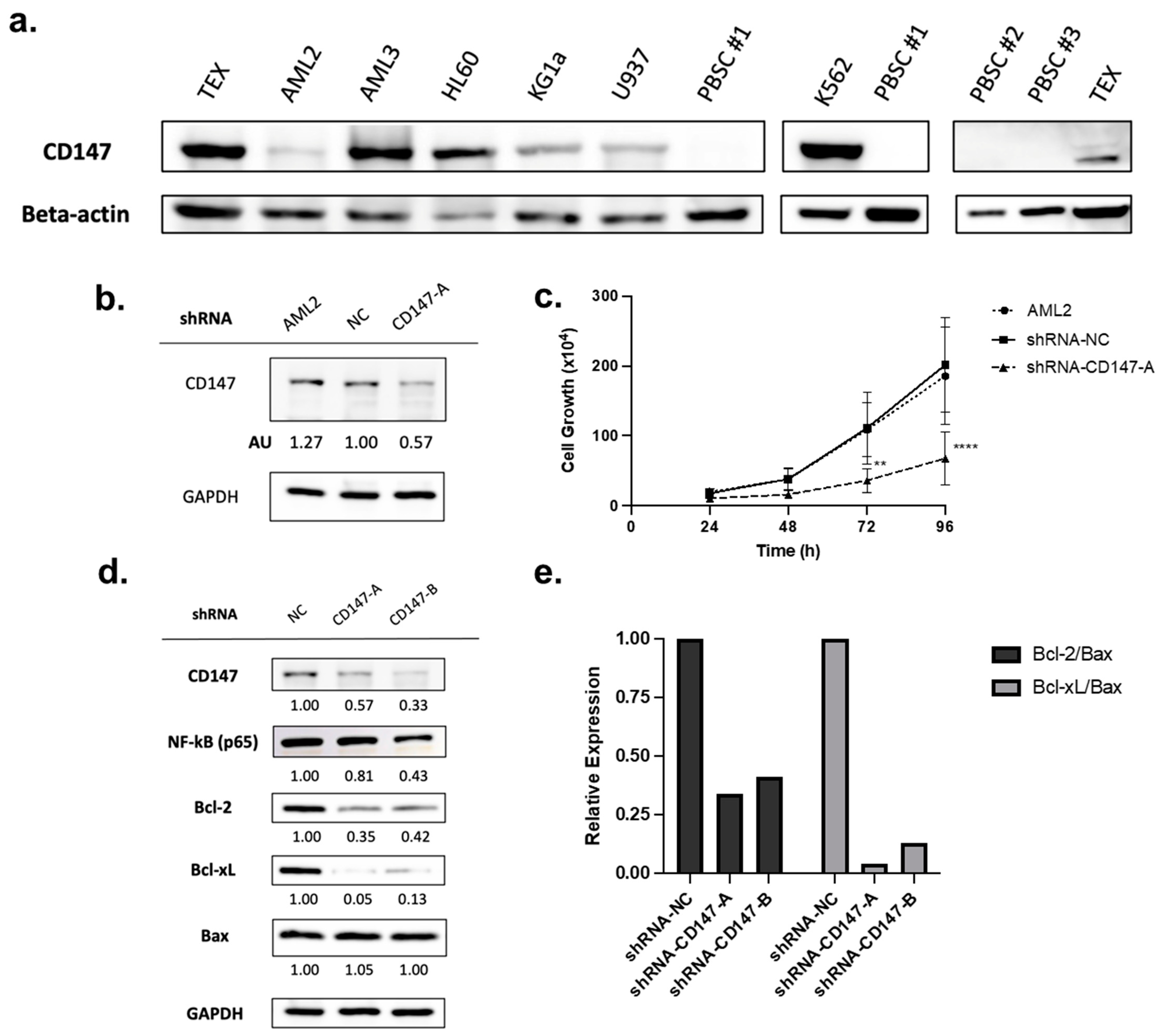
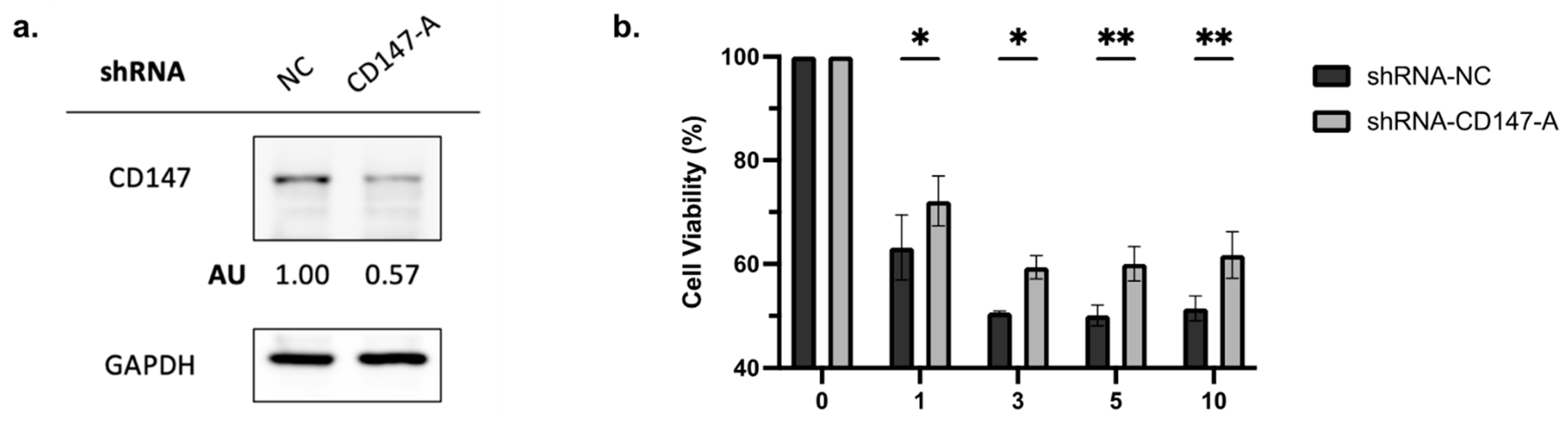
2.4. Overexpressed in AML Cells, CD147 Regulates AML Cell Growth and Alters NF-κB and Bcl-2 Family Protein Expression
2.5. Knockdown of CD147 Reduces PAB-Mediated Cytotoxicity in AML Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Compounds
4.3. Cell Viability Assay
4.4. Apoptosis Assay
4.5. Colony Formation Assays
4.6. Retroviral Mediated Knockdown of CD147
4.7. Immunoblot Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chennamadhavuni, A.; Lyengar, V.; Mukkamalla, S.K.R.; Shimanovsky, A. Leukemia. In StatPearls [Internet]; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Sabath, D.E. Leukemia. In Brenner’s Encyclopedia of Genetics, 2nd ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 226–227. [Google Scholar] [CrossRef]
- Statistics Canada Table 13-10-0158-01 Age-Specific Five-Year Net Survival Estimates for Primary Sites of Cancer, by Sex, Three Years Combined. Available online: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310015801&pickMembers%5B0%5D=1.1&pickMembers%5B1%5D=3.1&pickMembers%5B2%5D=4.51&cubeTimeFrame.startYear=2006+%2F+2008&cubeTimeFrame.endYear=2010+%2F+2012&referencePeriods=20060101%2C20100101 (accessed on 16 April 2024).
- Avvisati, G. AML: Treatment-Related Toxicity or Not? Blood 2007, 110, 3491. [Google Scholar] [CrossRef]
- Kayser, S.; Döhner, K.; Krauter, J.; Köhne, C.H.; Horst, H.A.; Held, G.; Von Lilienfeld-Toal, M.; Wilhelm, S.; Kündgen, A.; Götze, K.; et al. The Impact of Therapy-Related Acute Myeloid Leukemia (AML) on Outcome in 2853 Adult Patients with Newly Diagnosed AML. Blood 2011, 117, 2137–2145. [Google Scholar] [CrossRef] [PubMed]
- Kimby, E.; Nygren, P.; Glimelius, B. A Systematic Overview of Chemotherapy Effects in Acute Myeloid Leukaemia. Acta Oncol. 2001, 40, 231–252. [Google Scholar] [CrossRef] [PubMed]
- Stentoft, J. The Toxicity of Cytarabine. Drug Saf. 1990, 5, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Veatch, J.R.; Sandhu, R.K.; Shannon-Dorcy, K.; Pagel, J.M.; Becker, P.S.; Petersdorf, S.; Appelbaum, F.R.; Estey, E.H. NCI Common Toxicity Criteria and Mortality After Chemotherapy for Acute Myeloid Leukemia (AML). Blood 2012, 120, 1479. [Google Scholar] [CrossRef]
- Qi, M.; Yao, G.; Fan, S.; Cheng, W.; Tashiro, S.I.; Onodera, S.; Ikejima, T. Pseudolaric Acid B Induces Mitotic Catastrophe Followed by Apoptotic Cell Death in Murine Fibrosarcoma L929 Cells. Eur. J. Pharmacol. 2012, 683, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Waser, J.; Meyer, A. Total Synthesis of (-)-Pseudolaric Acid B. J. Am. Chem. Soc. 2007, 129, 14556–14557. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Du, R.; Wang, W.; An, F.; Ye, L.; Chen, H.; Jiang, T.; Li, T.; Zhao, J. The Anti-Inflammatory Effects of Pseudorlaric Acid D on Atherosclerosis. Biomed. Pharmacother. 2020, 125, 109993. [Google Scholar] [CrossRef]
- Li, E.; Clark, A.M.; Hufford, C.D. Antifungal Evaluation of Pseudolaric Acid b, a Major Constituent of Pseudolarix Kaempferi. J. Nat. Prod. 1995, 58, 57–67. [Google Scholar] [CrossRef]
- Li, M.H.; Miao, Z.H.; Tan, W.F.; Yue, J.M.; Zhang, C.; Lin, L.P.; Zhang, X.W.; Ding, J. Pseudolaric Acid B Inhibits Angiogenesis and Reduces Hypoxia-Inducible Factor 1alpha by Promoting Proteasome-Mediated Degradation. Clin. Cancer Res. 2004, 10, 8266–8274. [Google Scholar] [CrossRef]
- Tong, Y.G.; Zhang, X.W.; Geng, M.Y.; Yue, J.M.; Xin, X.L.; Tian, F.; Shen, X.; Tong, L.J.; Li, M.H.; Zhang, C.; et al. Pseudolarix Acid B, a New Tubulin-Binding Agent, Inhibits Angiogenesis by Interacting with a Novel Binding Site on Tubulin. Mol. Pharmacol. 2006, 69, 1226–1233. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Luo, H.; Zhao, L.; Zhang, Z.; Shen, X. Pseudolaric Acid B Exhibits Anti-Cancer Activity on Human Hepatocellular Carcinoma through Inhibition of Multiple Carcinogenic Signaling Pathways. Phytomedicine 2019, 59, 152759. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wen, C.; He, Q.; Sun, Y.; Wang, J.; Lan, X.; Rohondia, S.; Dou, Q.P.; Shi, X.; Liu, J. Pseudolaric Acid B Induces Mitotic Arrest and Apoptosis in Both Imatinib-Sensitive and -Resistant Chronic Myeloid Leukaemia Cells. Eur. J. Pharmacol. 2020, 876, 173064. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Chong, L.; Li, X.C.; Khan, I.A.; Walker, L.A.; Khan, S.I. Selective Inhibition of Human Leukemia Cell Growth and Induction of Cell Cycle Arrest and Apoptosis by Pseudolaric Acid B. J. Cancer Res. Clin. Oncol. 2010, 136, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Kan, L.; Shu, L.H.; Wang, N.; Li, N.J.; Zhang, M. Pseudolaric Acid B Induces Apoptosis in U937 Human Leukemia Cells via Caspase-9-Mediated Activation of the Mitochondrial Death Pathway. Mol. Med. Rep. 2013, 8, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Di, Z.; Li, X.; Shan, Y.; Li, W.; Zhang, H.; Xiao, Y. Chemical Proteomics Reveal CD147 as a Functional Target of Pseudolaric Acid B in Human Cancer Cells. Chem. Commun. 2017, 53, 8671–8674. [Google Scholar] [CrossRef] [PubMed]
- Landras, A.; Reger De Moura, C.; Jouenne, F.; Lebbe, C.; Menashi, S.; Mourah, S. CD147 Is a Promising Target of Tumor Progression and a Prognostic Biomarker. Cancers 2019, 11, 1803. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, J.; Chen, L.; Zhong, W.D.; Zhang, Z.; Mi, L.; Zhang, Y.; Liao, C.G.; Bian, H.J.; Jiang, J.L.; et al. HAb18G (CD147), a Cancer-Associated Biomarker and Its Role in Cancer Detection. Histopathology 2009, 54, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Spinello, I.; Saulle, E.; Quaranta, M.T.; Pasquini, L.; Pelosi, E.; Castelli, G.; Ottone, T.; Voso, M.T.; Testa, U.; Labbaye, C. The Small-Molecule Compound AC-73 Targeting CD147 Inhibits Leukemic Cell Proliferation, Induces Autophagy and Increases the Chemotherapeutic Sensitivity of Acute Myeloid Leukemia Cells. Haematologica 2019, 104, 973–985. [Google Scholar] [CrossRef]
- Xiong, L.; Edwards, C.K.; Zhou, L. The Biological Function and Clinical Utilization of CD147 in Human Diseases: A Review of the Current Scientific Literature. Int. J. Mol. Sci. 2014, 15, 17411–17441. [Google Scholar] [CrossRef]
- Asgari, R.; Vaisi-Raygani, A.; Aleagha, M.S.E.; Mohammadi, P.; Bakhtiari, M.; Arghiani, N. CD147 and MMPs as Key Factors in Physiological and Pathological Processes. Biomed. Pharmacother. 2023, 157, 113983. [Google Scholar] [CrossRef]
- Grass, G.D.; Toole, B.P. How, with Whom and When: An Overview of CD147-Mediated Regulatory Networks Influencing Matrix Metalloproteinase Activity. Biosci. Rep. 2016, 36, e00283. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Bültmann, A.; Fischel, S.; Gillitzer, A.; Cullen, P.; Walch, A.; Jost, P.; Ungerer, M.; Tolley, N.D.; Lindemann, S.; et al. Extracellular Matrix Metalloproteinase Inducer (CD147) Is a Novel Receptor on Platelets, Activates Platelets, and Augments Nuclear Factor ΚB–Dependent Inflammation in Monocytes. Circ. Res. 2008, 102, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Hemler, M.E. Regulation of MMP-1 and MMP-2 Production through CD147/Extracellular Matrix Metalloproteinase Inducer Interactions. Canser Res. 2001, 61, 2276–2281. [Google Scholar]
- Tang, W.; Chang, S.B.; Hemler, M.E. Links between CD147 Function, Glycosylation, and Caveolin-1. Mol. Biol. Cell 2004, 15, 4043–4050. [Google Scholar] [CrossRef]
- Wang, C.; Jin, R.; Zhu, X.; Yan, J.; Li, G. Function of CD147 in Atherosclerosis and Atherothrombosis. J. Cardiovasc. Transl. Res. 2015, 8, 59. [Google Scholar] [CrossRef]
- Zhai, Y.; Wu, B.; Li, J.; Yao, X.; Zhu, P.; Chen, Z. CD147 Promotes IKK/IκB/NF-ΚB Pathway to Resist TNF-Induced Apoptosis in Rheumatoid Arthritis Synovial Fibroblasts. J. Mol. Med. 2016, 94, 71. [Google Scholar] [CrossRef]
- Park, M.H.; Hong, J.T. Roles of NF-ΚB in Cancer and Inflammatory Diseases and Their Therapeutic Approaches. Cells 2016, 5, 15. [Google Scholar] [CrossRef]
- Kumar, D.; Vetrivel, U.; Parameswaran, S.; Subramanian, K.K. Structural Insights on Druggable Hotspots in CD147: A Bull’s Eye View. Life Sci. 2019, 224, 76–87. [Google Scholar] [CrossRef]
- Fu, Z.G.; Wang, L.; Cuiv, H.Y.; Peng, J.L.; Wangc, S.J.; Gengc, J.J.; Liu, J.D.; Feng, F.; Song, F.; Li, L.; et al. A Novel Small-Molecule Compound Targeting CD147 Inhibits the Motility and Invasion of Hepatocellular Carcinoma Cells. Oncotarget 2016, 7, 9429–9447. [Google Scholar] [CrossRef]
- Fu, Z.G.; Wang, Y.; Wang, S.; Shao, D.; Tian, L.; Li, Y.X.; Jiang, J.L.; Chen, Z.N.; Wen, N. Synthesis and Evaluation of a Novel Small-Molecule Compound as an Anticancer Inhibitor of CD147. Biomed. Environ. Sci. 2019, 32, 673–686. [Google Scholar] [CrossRef]
- Hu, X.; Su, J.; Zhou, Y.; Xie, X.; Peng, C.; Yuan, Z.; Chen, X. Repressing CD147 Is a Novel Therapeutic Strategy for Malignant Melanoma. Oncotarget 2017, 8, 25806. [Google Scholar] [CrossRef]
- Wu, W.Y.; Guo, H.Z.; Qu, G.Q.; Han, J.; Guo, D.A. Mechanisms of Pseudolaric Acid B-Induced Apoptosis in Bel-7402 Cell Lines. Am. J. Chin. Med. 2012, 34, 887–899. [Google Scholar] [CrossRef]
- Nyalali, A.M.K.; Leonard, A.U.; Xu, Y.; Li, H.; Zhou, J.; Zhang, X.; Rugambwa, T.K.; Shi, X.; Li, F. CD147: An Integral and Potential Molecule to Abrogate Hallmarks of Cancer. Front. Oncol. 2023, 13, 1238051. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, B.; Valente, A.J.; Prabhu, S.D.; Shanmugam, P.; Delafontaine, P.; Chandrasekar, B. EMMPRIN Activates Multiple Transcription Factors in Cardiomyocytes, and Induces Interleukin-18 Expression via Rac1-Dependent PI3K/Akt/IKK/NF-ΚB AndMKK7/JNK/AP-1 Signaling. J. Mol. Cell Cardiol. 2010, 49, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Abraham, E.; Luce, J.M.; Yaffe, M.B.; Fink, M.P. NF-ΚB Activation. Crit. Care Med. 2000, 28, N100–N104. [Google Scholar] [CrossRef]
- Pikarsky, E.; Porat, R.M.; Stein, I.; Abramovitch, R.; Amit, S.; Kasem, S.; Gutkovich-Pyest, E.; Uriell-Shoval, S.; Galun, E.; Ben-Neriah, Y. NF-KappaB Functions as a Tumour Promoter in Inflammation-Associated Cancer. Nature 2004, 431, 461–466. [Google Scholar] [CrossRef]
- Karin, M.; Greten, F.R. NF-ΚB: Linking Inflammation and Immunity to Cancer Development and Progression. Nat. Rev. Immunol. 2005, 5, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, J. Series Introduction: The Transcription Factor NF-ΚB and Human Disease. J. Clin. Investig. 2001, 107, 3. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Shared Principles in NF-ΚB Signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. NF-ΚB, the First Quarter-Century: Remarkable Progress and Outstanding Questions. Genes. Dev. 2012, 26, 203. [Google Scholar] [CrossRef]
- Catz, S.D.; Johnson, J.L. Transcriptional Regulation of Bcl-2 by Nuclear Factor ΚB and Its Significance in Prostate Cancer. Oncogene 2001, 20, 7342–7351. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Guttridge, D.C.; Mayo, M.W.; Baldwin, A.S. NF-ΚB Induces Expression of the Bcl-2 Homologue A1/Bfl-1 To Preferentially Suppress Chemotherapy-Induced Apoptosis. Mol. Cell Biol. 1999, 19, 5923–5929. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Edelstein, L.C.; Line, C.É.; Linas, G.É. The Rel/NF-B Family Directly Activates Expression of the Apoptosis Inhibitor Bcl-XL. Mol. Cell Biol. 2000, 20, 2687–2695. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; Strasser, A. The BCL-2 Protein Family: Opposing Activities That Mediate Cell Death. Nat. Rev. Mol. Cell Biol. 2008, 9, 47–59. [Google Scholar] [CrossRef]
- Adams, J.M.; Cory, S. The Bcl-2 Apoptotic Switch in Cancer Development and Therapy. Oncogene 2007, 26, 1324–1337. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; El-Deiry, W.S. Overview of Cell Death Signaling Pathways. Cancer Biol. Ther. 2005, 4, 147–171. [Google Scholar] [CrossRef]
- Li, J.; Huang, Q.; Long, X.; Zhang, J.; Huang, X.; Aa, J.; Yang, H.; Chen, Z.; Xing, J. CD147 Reprograms Fatty Acid Metabolism in Hepatocellular Carcinoma Cells through Akt/MTOR/SREBP1c and P38/PPARa Pathways. J. Hepatol. 2015, 63, 1378–1389. [Google Scholar] [CrossRef]
- Huang, Q.; Li, J.; Xing, J.; Li, W.; Li, H.; Ke, X.; Zhang, J.; Ren, T.; Shang, Y.; Yang, H.; et al. CD147 Promotes Reprogramming of Glucose Metabolism and Cell Proliferation in HCC Cells by Inhibiting the P53-Dependent Signaling Pathway. J. Hepatol. 2014, 61, 859–866. [Google Scholar] [CrossRef]
- Cui, H.Y.; Guo, T.; Wang, S.J.; Zhao, P.; Dong, Z.S.; Zhang, Y.; Jiang, J.L.; Chen, Z.N.; Yu, X.L. Dimerization Is Essential for HAb18G/CD147 Promoting Tumor Invasion via MAPK Pathway. Biochem. Biophys. Res. Commun. 2012, 419, 517–522. [Google Scholar] [CrossRef]
- Łacina, P.; Butrym, A.; Turlej, E.; Stachowicz-Suhs, M.; Wietrzyk, J.; Mazur, G.; Bogunia-Kubik, K. BSG (CD147) Serum Level and Genetic Variants Are Associated with Overall Survival in Acute Myeloid Leukaemia. J. Clin. Med. 2022, 11, 332. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.K.S.; Leung, W.C.; Ho, W.K.; Chiu, P. Herbal Diterpenoids Induce Growth Arrest and Apoptosis in Colon Cancer Cells with Increased Expression of the Nonsteroidal Anti-Inflammatory Drug-Activated Gene. Eur. J. Pharmacol. 2007, 559, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhao, X.; Liu, X.; Xu, P.; Zhang, K.; Lin, X. Antitumor Effects of Traditional Chinese Medicine Targeting the Cellular Apoptotic Pathway. Drug Des. Devel Ther. 2015, 9, 2735. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.L.; Sun, D.; Li, T.; Chen, H. A Systematic Review of the Immune-Regulating and Anticancer Activities of Pseudolaric Acid B. Front. Pharmacol. 2017, 8, 265155. [Google Scholar] [CrossRef]
- Wang, Z.; Ding, Y.; Wang, X.; Lu, S.; Wang, C.; He, C.; Wang, L.; Piao, M.; Chi, G.; Luo, Y.; et al. Pseudolaric Acid B Triggers Ferroptosis in Glioma Cells via Activation of Nox4 and Inhibition of XCT. Cancer Lett. 2018, 428, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Roma, A.; Tcheng, M.; Ahmed, N.; Walker, S.; Jayanth, P.; Minden, M.D.; Hope, K.; Nekkar Rao, P.P.; Luc, J.; Doxey, A.C.; et al. Glutamine Metabolism Mediates Sensitivity to Respiratory Complex II Inhibition in Acute Myeloid Leukemia. Mol. Cancer Res. 2022, 20, 1659–1673. [Google Scholar] [CrossRef]
- Roma, A.; Tcheng, M.; Ahmed, N.; Walker, S.; Jayanth, P.; Minden, M.D.; Reisz, J.A.; D’Alessandro, A.; Rohlena, J.; Spagnuolo, P.A. Shikonin Impairs Mitochondrial Activity to Selectively Target Leukemia Cells. Phytomed. Plus 2022, 2, 100300. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, S.; Parfenova, E.; Vrdoljak, N.; Minden, M.D.; Spagnuolo, P.A. Pseudolaric Acid B Targets CD147 to Selectively Kill Acute Myeloid Leukemia Cells. Int. J. Mol. Sci. 2024, 25, 6517. https://doi.org/10.3390/ijms25126517
Zou S, Parfenova E, Vrdoljak N, Minden MD, Spagnuolo PA. Pseudolaric Acid B Targets CD147 to Selectively Kill Acute Myeloid Leukemia Cells. International Journal of Molecular Sciences. 2024; 25(12):6517. https://doi.org/10.3390/ijms25126517
Chicago/Turabian StyleZou, Sheng, Ekaterina Parfenova, Nikolina Vrdoljak, Mark D. Minden, and Paul A. Spagnuolo. 2024. "Pseudolaric Acid B Targets CD147 to Selectively Kill Acute Myeloid Leukemia Cells" International Journal of Molecular Sciences 25, no. 12: 6517. https://doi.org/10.3390/ijms25126517




