Hair Thickness Growth Effect of Adenosine Complex in Male-/Female-Patterned Hair Loss via Inhibition of Androgen Receptor Signaling
Abstract
:1. Introduction
2. Results
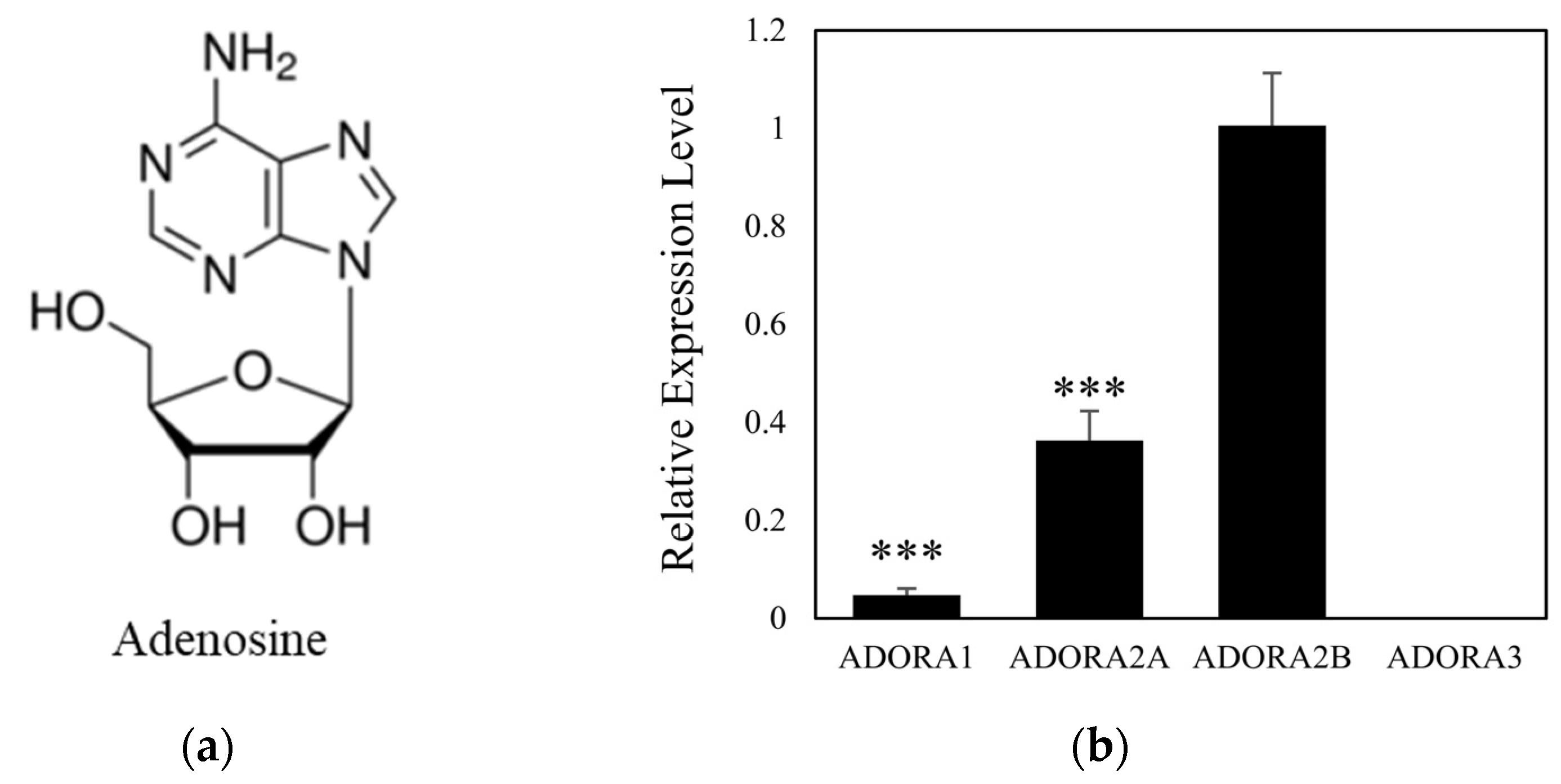
2.1. Adenosine Receptor A2B Is a Dominant Subtype in Cultured Human Dermal Papilla
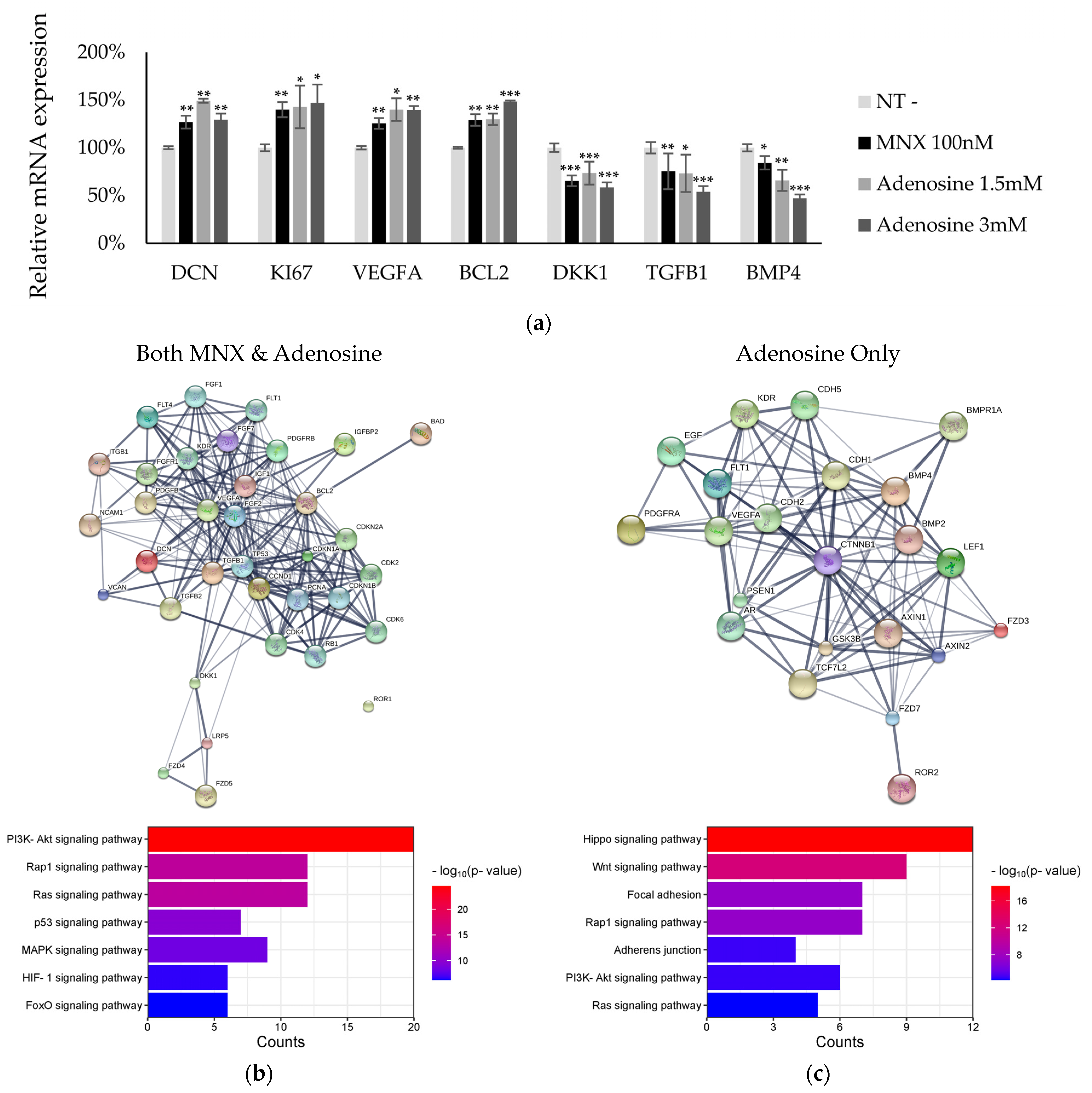
2.2. Activation of Signaling Pathways via MNX and Adenosine
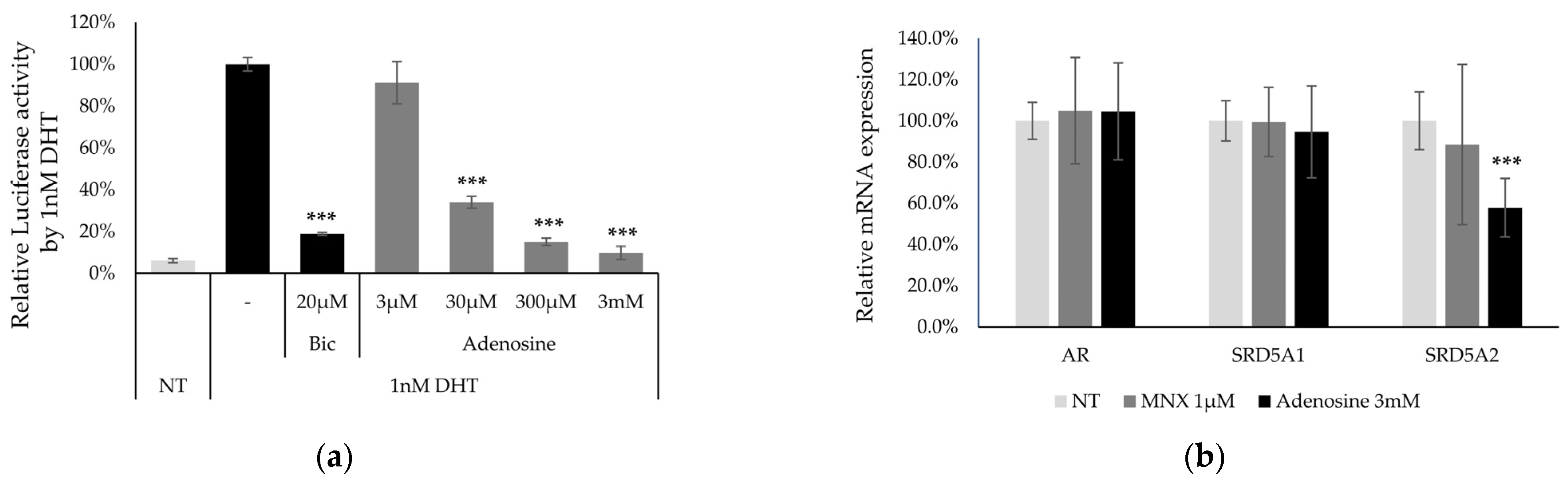
2.3. Anti-Androgenic Effect of Adenosine
2.4. Adenosine Elongated Anagen Stages in an Ex Vivo Hair Follicle Organ Culture
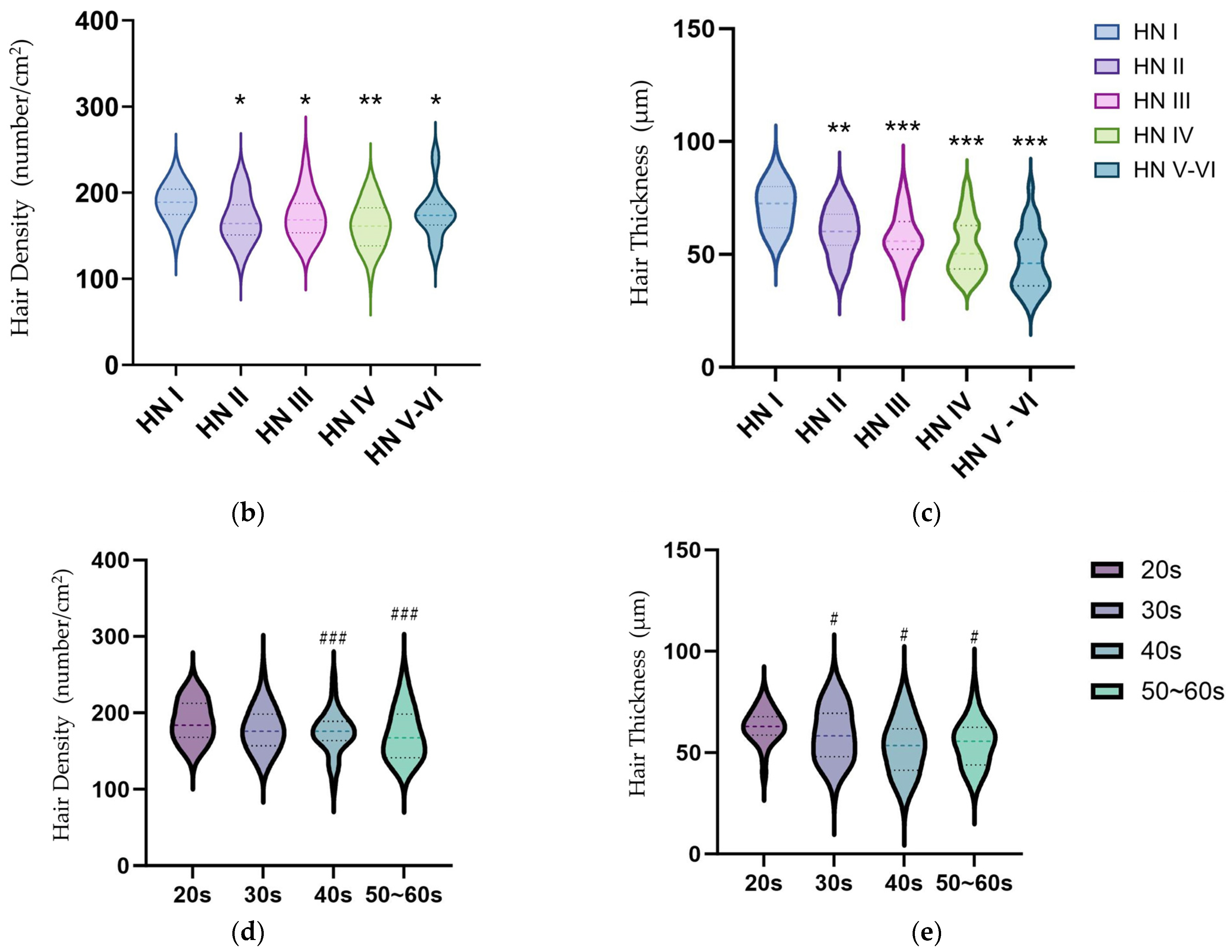
2.5. Hair Thickness Analysis following Alopecia Scale and Senescence
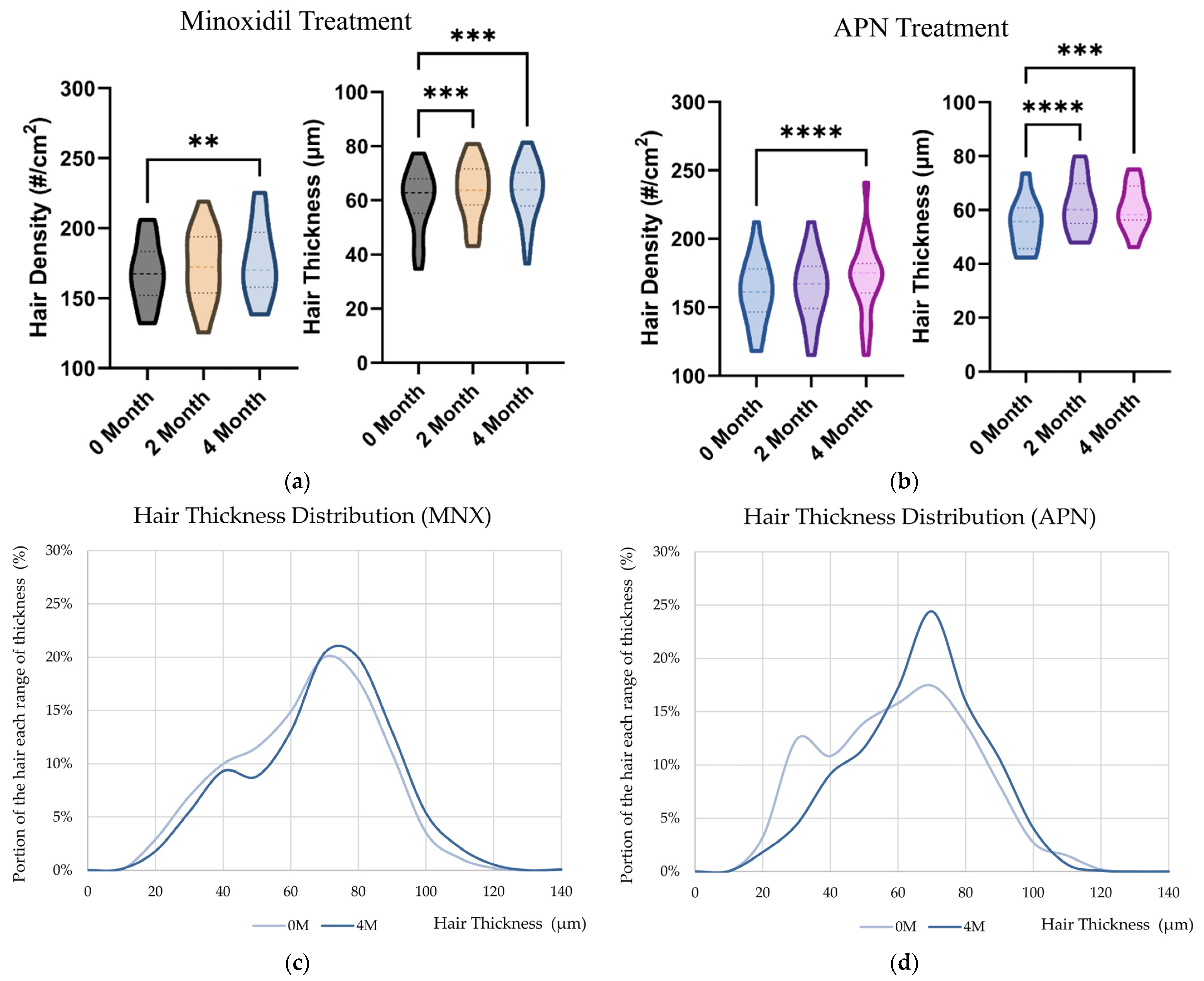
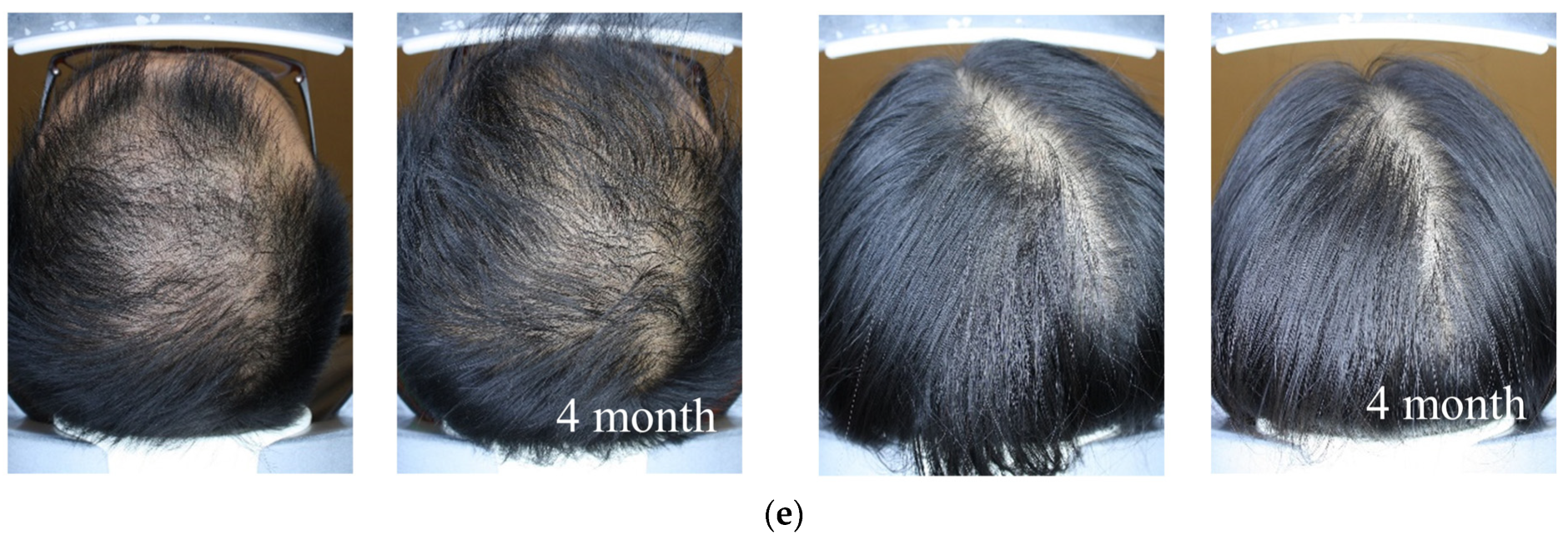
2.6. Hair Thickness Enhancement with MNX and APN Administration In Vivo
3. Discussion
4. Materials and Methods
4.1. Dermal Papilla Cell Culture
4.2. Quantitative Real-Time PCR
4.3. Reporter Assay Using 22Rv1-F5-MMTV/Luc Stable Cell Line
4.4. Protein Dot Blot Analysis for Human MAPK Phosphorylation
4.5. Human Hair Follicle Organ Culture and Hair Cycle Scoring
4.6. Immunohistochemistry of the Hair Follicle Organ
4.7. Participants
4.8. Evaluation of Scalp Parameters
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, R.; Pawlus, A.D.; Thornton, M.J. Getting under the skin of hair aging: The impact of the hair follicle environment. Exp. Dermatol. 2020, 29, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Maymone, M.B.; Laughter, M.; Pollock, S.; Khan, I.; Marques, T.; Abdat, R.; Goldberg, L.J.; Vashi, N.A. Hair aging in different races and ethnicities. J. Clin. Aesthetic Dermatol. 2021, 14, 38. [Google Scholar]
- Nagase, S.; Kajiura, Y.; Mamada, A.; Abe, H.; Shibuichi, S.; Satoh, N.; Itou, T.; Shinohara, Y.; Amemiya, Y. Changes in structure and geometric properties of human hair by aging. J. Cosmet. Sci. 2009, 60, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, Y.; Christiano, A.M. Biology and Genetics of Hair. Annu. Rev. Genom. Hum. Genet. 2010, 11, 109–132. [Google Scholar] [CrossRef] [PubMed]
- Price, V.H. Treatment of hair loss. N. Engl. J. Med. 1999, 341, 964–973. [Google Scholar] [CrossRef] [PubMed]
- King, B.; Ohyama, M.; Kwon, O.; Zlotogorski, A.; Ko, J.; Mesinkovska, N.A.; Hordinsky, M.; Dutronc, Y.; Wu, W.S.; McCollam, J.; et al. Two Phase 3 Trials of Baricitinib for Alopecia Areata. N. Engl. J. Med. 2022, 386, 1687–1699. [Google Scholar] [CrossRef] [PubMed]
- Olsen, E.A.; Dunlap, F.E.; Funicella, T.; Koperski, J.A.; Swinehart, J.M.; Tschen, E.H.; Trancik, R.J. A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and placebo in the treatment of androgenetic alopecia in men. J. Am. Acad. Dermatol. 2002, 47, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.R.; Choi, U.; Gao, J.-L.; Thompson, R.D.; Rodman, L.E.; Malech, H.L.; Kang, E.M. A novel method for screening adenosine receptor specific agonists for use in adenosine drug development. Sci. Rep. 2017, 7, 44816. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, L.; Blandizzi, C.; Pacher, P.; Haskó, G. Immunity, inflammation and cancer: A leading role for adenosine. Nat. Rev. Cancer 2013, 13, 842–857. [Google Scholar] [CrossRef]
- Valls, M.D.; Cronstein, B.N.; Montesinos, M.C. Adenosine receptor agonists for promotion of dermal wound healing. Biochem. Pharmacol. 2009, 77, 1117–1124. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Tosh, D.K.; Jain, S.; Gao, Z.-G. Historical and current adenosine receptor agonists in preclinical and clinical development. Front. Cell. Neurosci. 2019, 13, 124. [Google Scholar] [CrossRef] [PubMed]
- Marubayashi, A.; Nakaya, Y.; Fukui, K.; Li, M.; Arase, S. Minoxidil-induced hair growth is mediated by adenosine in cultured dermal papilla cells: Possible involvement of sulfonylurea receptor 2B as a target of minoxidil. J. Investig. Dermatol. 2001, 117, 1594–1600. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Shin, J.Y.; Choi, Y.-H.; Lee, S.Y.; Jin, M.H.; Kim, C.D.; Kang, N.-G.; Lee, S. Adenosine and Cordycepin Accelerate Tissue Remodeling Process through Adenosine Receptor Mediated Wnt/β-Catenin Pathway Stimulation by Regulating GSK3b Activity. Int. J. Mol. Sci. 2021, 22, 5571. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Shin, J.Y.; Choi, Y.H.; Kang, N.G.; Lee, S. Anti-Hair Loss Effect of Adenosine Is Exerted by cAMP Mediated Wnt/β-Catenin Pathway Stimulation via Modulation of Gsk3β Activity in Cultured Human Dermal Papilla Cells. Molecules 2022, 27, 2184. [Google Scholar] [CrossRef] [PubMed]
- Lemasters, J.J.; Ramshesh, V.K.; Lovelace, G.L.; Lim, J.; Wright, G.D.; Harland, D.; Dawson, T.L., Jr. Compartmentation of Mitochondrial and Oxidative Metabolism in Growing Hair Follicles: A Ring of Fire. J. Investig. Dermatol. 2017, 137, 1434–1444. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Shin, J.Y.; Choi, Y.H.; Jang, M.; Nam, Y.J.; Lee, S.Y.; Jeon, J.; Jin, M.H.; Lee, S. Hair Growth Promoting Effect of Hottuynia cordata Extract in Cultured Human Hair Follicle Dermal Papilla Cells. Biol. Pharm. Bull. 2019, 42, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.Y.; Choi, Y.-H.; Kim, J.; Park, S.Y.; Nam, Y.J.; Lee, S.Y.; Jeon, J.H.; Jin, M.H.; Lee, S. Polygonum multiflorum extract support hair growth by elongating anagen phase and abrogating the effect of androgen in cultured human dermal papilla cells. BMC Complement. Med. Ther. 2020, 20, 144. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, S.R.; Choi, Y.H.; Shin, J.Y.; Kim, C.D.; Kang, N.G.; Park, B.C.; Lee, S. Quercitrin Stimulates Hair Growth with Enhanced Expression of Growth Factors via Activation of MAPK/CREB Signaling Pathway. Molecules 2020, 25, 4004. [Google Scholar] [CrossRef] [PubMed]
- Foitzik, K.; Hoting, E.; Forster, T.; Pertile, P.; Paus, R. L-carnitine-L-tartrate promotes human hair growth in vitro. Exp. Dermatol. 2007, 16, 936–945. [Google Scholar] [CrossRef]
- Foitzik, K.; Hoting, E.; Heinrich, U.; Tronnier, H.; Paus, R. Indications that topical L-carnitin-L-tartrate promotes human hair growth in vivo. J. Dermatol. Sci. 2007, 48, 141–144. [Google Scholar] [CrossRef]
- Shin, J.Y.; Kim, J.; Choi, Y.H.; Kang, N.G.; Lee, S. Dexpanthenol Promotes Cell Growth by Preventing Cell Senescence and Apoptosis in Cultured Human Hair Follicle Cells. Curr. Issues Mol. Biol. 2021, 43, 1361–1373. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Shin, J.Y.; Kim, J.; Kang, N.G.; Lee, S. Niacinamide Down-Regulates the Expression of DKK-1 and Protects Cells from Oxidative Stress in Cultured Human Dermal Papilla Cells. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Kim, J.; Shin, J.Y.; Kang, N.G.; Lee, S. Antiandrogenic activity of Riboflavin 5′-phosphate (FMN) in 22Rv1 and LNCaP human prostate cancer cell lines. Eur. J. Pharmacol. 2022, 917, 174743. [Google Scholar] [CrossRef] [PubMed]
- Courtois, M.; Loussouarn, G.; Hourseau, C.; Grollier, J.F. Hair cycle and alopecia. Ski. Pharmacol. Physiol. 1994, 7, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Randolph, M.; Tosti, A. Oral minoxidil treatment for hair loss: A review of efficacy and safety. J. Am. Acad. Dermatol. 2021, 84, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, R. TrichoScan: Combining epiluminescence microscopy with digital image analysis for the measurement of hair growth in vivo. Eur. J. Dermatol. 2001, 11, 362–368. [Google Scholar] [PubMed]
- Majd, A.; AlJasser, M.; Mirzaalian, H.; Shapiro, J.; Hamarneh, G.; Lui, H.; Santos, L.D.N.; Chu, T.; Lee, T.K. A novel automated approach to rapid and precise in vivo measurement of hair morphometrics using a smartphone. Ski. Res. Technol. 2021, 27, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Goulding, J.; May, L.T.; Hill, S.J. Characterisation of endogenous A2A and A2B receptor-mediated cyclic AMP responses in HEK 293 cells using the GloSensor™ biosensor: Evidence for an allosteric mechanism of action for the A2B-selective antagonist PSB 603. Biochem. Pharmacol. 2018, 147, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, M.; Szklarczyk, D.; Pletscher-Frankild, S.; Blicher, T.H.; Von Mering, C.; Jensen, L.J.; Bork, P. STITCH 4: Integration of protein–chemical interactions with user data. Nucleic Acids Res. 2014, 42, D401–D407. [Google Scholar] [CrossRef]
- Diani, A.R.; Mulholland, M.J.; Shull, K.L.; Kubicek, M.F.; Johnson, G.A.; Schostarez, H.J.; Brunden, M.N.; Buhl, A.E. Hair growth effects of oral administration of finasteride, a steroid 5 alpha-reductase inhibitor, alone and in combination with topical minoxidil in the balding stumptail macaque. J. Clin. Endocrinol. Metab. 1992, 74, 345–350. [Google Scholar]
- Shah, K.; Bradbury, N.A. Kinase modulation of androgen receptor signaling: Implications for prostate cancer. Cancer Cell Microenviron. 2015, 2, e123. [Google Scholar] [PubMed]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Structural characteristics of the aging skin: A review. Cutan. Ocul. Toxicol. 2007, 26, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Aslani, F.S.; Dastgheib, L.; Banihashemi, B.M. Hair counts in scalp biopsy of males and females with androgenetic alopecia compared with normal subjects. J. Cutan. Pathol. 2009, 36, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Yazdabadi, A.; Magee, J.; Harrison, S.; Sinclair, R. The Ludwig pattern of androgenetic alopecia is due to a hierarchy of androgen sensitivity within follicular units that leads to selective miniaturization and a reduction in the number of terminal hairs per follicular unit. Br. J. Dermatol. 2008, 159, 1300–1302. [Google Scholar] [CrossRef]
- Ramos, P.M.; Sinclair, R.D.; Kasprzak, M.; Miot, H.A. Minoxidil 1 mg oral versus minoxidil 5% topical solution for the treatment of female-pattern hair loss: A randomized clinical trial. J. Am. Acad. Dermatol. 2020, 82, 252–253. [Google Scholar] [CrossRef]
- Rishikaysh, P.; Dev, K.; Diaz, D.; Qureshi, W.M.S.; Filip, S.; Mokry, J. Signaling Involved in Hair Follicle Morphogenesis and Development. Int. J. Mol. Sci. 2014, 15, 1647–1670. [Google Scholar] [CrossRef]
- Iwabuchi, T.; Ideta, R.; Ehama, R.; Yamanishi, H.; Iino, M.; Nakazawa, Y.; Kobayashi, T.; Ohyama, M.; Kishimoto, J. Topical adenosine increases the proportion of thick hair in Caucasian men with androgenetic alopecia. J. Dermatol. 2016, 43, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Rocha, E.M.; Wickham, L.A.; da Silveira, L.A.; Krenzer, K.L.; Yu, F.-S.; Toda, I.; Sullivan, B.D.; Sullivan, D.A. Identification of androgen receptor protein and 5α-reductase mRNA in human ocular tissues. Br. J. Ophthalmol. 2000, 84, 76–84. [Google Scholar] [CrossRef]
- Alimirah, F.; Panchanathan, R.; Chen, J.; Zhang, X.; Ho, S.-M.; Choubey, D. Expression of androgen receptor is negatively regulated by p53. Neoplasia 2007, 9, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Gioeli, D.; Black, B.E.; Gordon, V.; Spencer, A.; Kesler, C.T.; Eblen, S.T.; Paschal, B.M.; Weber, M.J. Stress kinase signaling regulates androgen receptor phosphorylation, transcription, and localization. Mol. Endocrinol. 2006, 20, 503–515. [Google Scholar] [CrossRef]
- Huang, P.-H.; Wang, D.; Chuang, H.-C.; Wei, S.; Kulp, S.K.; Chen, C.-S. α-Tocopheryl succinate and derivatives mediate the transcriptional repression of androgen receptor in prostate cancer cells by targeting the PP2A-JNK-Sp1-signaling axis. Carcinogenesis 2009, 30, 1125–1131. [Google Scholar] [CrossRef]
- Xu, R.; Hu, J. The role of JNK in prostate cancer progression and therapeutic strategies. Biomed. Pharmacother. 2020, 121, 109679. [Google Scholar] [CrossRef]
- Zoubeidi, A.; Zardan, A.; Beraldi, E.; Fazli, L.; Sowery, R.; Rennie, P.; Nelson, C.; Gleave, M. Cooperative interactions between androgen receptor (AR) and heat-shock protein 27 facilitate AR transcriptional activity. Cancer Res. 2007, 67, 10455–10465. [Google Scholar] [CrossRef] [PubMed]
- Kwack, M.H.; Sung, Y.K.; Chung, E.J.; Im, S.U.; Ahn, J.S.; Kim, M.K.; Kim, J.C. Dihydrotestosterone-inducible dickkopf 1 from balding dermal papilla cells causes apoptosis in follicular keratinocytes. J. Investig. Dermatol. 2008, 128, 262–269. [Google Scholar] [CrossRef]
- Montano, M.; Dinnon, K.H.; Jacobs, L.; Xiang, W.; Iozzo, R.V.; Bushman, W. Dual regulation of decorin by androgen and Hedgehog signaling during prostate morphogenesis. Dev. Dyn. 2018, 247, 679–685. [Google Scholar] [CrossRef]
- Iino, M.; Ehama, R.; Nakazawa, Y.; Iwabuchi, T.; Ogo, M.; Tajima, M.; Arase, S. Adenosine stimulates fibroblast growth factor-7 gene expression via adenosine A2b receptor signaling in dermal papilla cells. J. Investig. Dermatol. 2007, 127, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.A.; Hwang, Y.L.; Lee, M.H.; Kim, N.R.; Roh, S.S.; Lee, Y.; Kim, C.D.; Lee, J.H.; Choi, K.C. Adenosine stimulates growth of dermal papilla and lengthens the anagen phase by increasing the cysteine level via fibroblast growth factors 2 and 7 in an organ culture of mouse vibrissae hair follicles. Int. J. Mol. Med. 2012, 29, 195–201. [Google Scholar]
- Huelsken, J.; Vogel, R.; Erdmann, B.; Cotsarelis, G.; Birchmeier, W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 2001, 105, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Morgan, B.A. Wnt signaling through the beta-catenin pathway is sufficient to maintain, but not restore, anagen-phase characteristics of dermal papilla cells. J. Investig. Dermatol. 2004, 122, 239–245. [Google Scholar] [CrossRef]
- Hawkshaw, N.; Hardman, J.; Alam, M.; Jimenez, F.; Paus, R. Deciphering the molecular morphology of the human hair cycle: Wnt signalling during the telogen–anagen transformation. Br. J. Dermatol. 2020, 182, 1184–1193. [Google Scholar] [CrossRef]
- Oura, H.; Iino, M.; Nakazawa, Y.; Tajima, M.; Ideta, R.; Nakaya, Y.; Arase, S.; Kishimoto, J. Adenosine increases anagen hair growth and thick hairs in Japanese women with female pattern hair loss: A pilot, double-blind, randomized, placebo-controlled trial. J. Dermatol. 2008, 35, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Nagashima, T.; Hanzawa, N.; Ishino, A.; Nakazawa, Y.; Ogo, M.; Iwabuchi, T.; Tajima, M. Topical adenosine increases thick hair ratio in J apanese men with androgenetic alopecia. Int. J. Cosmet. Sci. 2015, 37, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Lolli, F.; Pallotti, F.; Rossi, A.; Fortuna, M.C.; Caro, G.; Lenzi, A.; Sansone, A.; Lombardo, F. Androgenetic alopecia: A review. Endocrine 2017, 57, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, K.D.; Olsen, E.A.; Whiting, D.; Savin, R.; DeVillez, R.; Bergfeld, W.; Price, V.H.; Van Neste, D.; Roberts, J.L.; Hordinsky, M.; et al. Finasteride in the treatment of men with androgenetic alopecia. J. Am. Acad. Dermatol. 1998, 39, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Van Neste, D.; Fuh, V.; Sanchez-Pedreno, P.; Lopez-Bran, E.; Wolff, H.; Whiting, D.; Roberts, J.; Kopera, D.; Stene, J.J.; Calvieri, S.; et al. Finasteride increases anagen hair in men with androgenetic alopecia. Br. J. Dermatol. 2000, 143, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Walth, C.B.; Wessman, L.L.; Wipf, A.; Carina, A.; Hordinsky, M.K.; Farah, R.S. Response to: “Rethinking biotin therapy for hair, nail, and skin disorders”. J. Am. Acad. Dermatol. 2018, 79, e121–e124. [Google Scholar] [CrossRef] [PubMed]
- Aksu Cerman, A.; Sarikaya Solak, S.; Kivanc Altunay, I. Vitamin D deficiency in alopecia areata. Br. J. Dermatol. 2014, 170, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Kligman, A.M. The human hair cycle. J. Investig. Dermatol. 1959, 33, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Peters, E.M.; Hansen, M.G.; Overall, R.W.; Nakamura, M.; Pertile, P.; Klapp, B.F.; Arck, P.C.; Paus, R. Control of human hair growth by neurotrophins: Brain-derived neurotrophic factor inhibits hair shaft elongation, induces catagen, and stimulates follicular transforming growth factor beta2 expression. J. Investig. Dermatol. 2005, 124, 675–685. [Google Scholar] [CrossRef]
- Lee, W.-S.; Ro, B.I.; Hong, S.P.; Bak, H.; Sim, W.-Y.; Park, J.K.; Ihm, C.-W.; Eun, H.C.; Kwon, O.S.; Choi, G.S. A new classification of pattern hair loss that is universal for men and women: Basic and specific (BASP) classification. J. Am. Acad. Dermatol. 2007, 57, 37–46. [Google Scholar] [CrossRef]
- Oh, J.W.; Kloepper, J.; Langan, E.A.; Kim, Y.; Yeo, J.; Kim, M.J.; Hsi, T.-C.; Rose, C.; Yoon, G.S.; Lee, S.-J. A guide to studying human hair follicle cycling in vivo. J. Investig. Dermatol. 2016, 136, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Pouradier, F.; Céline, C.; Marie-Florence, D.A.; Frederic, F.; Segolene, P.; Stephane, D.; Genevieve, L. Functional and structural age-related changes in the scalp skin of Caucasian women. Ski. Res. Technol. 2013, 19, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Jemec, G.B.; Selvaag, E.; Ågren, M.; Wulf, H.C. Measurement of the mechanical properties of skin with ballistometer and suction cup. Ski. Res. Technol. 2001, 7, 122–126. [Google Scholar] [CrossRef]
- Woo, M.; Moon, K.; Jung, H.; Park, S.; Moon, T.; Kim, N.; Lee, B. Comparison of skin elasticity test results from the Ballistometer® and Cutometer®. Ski. Res. Technol. 2014, 20, 422–428. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s), not of the MDPI and/or editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |



| Node1 | Node2 | Combined Score |
|---|---|---|
| AR | CTNNB1 | 0.999 |
| GSK3B | 0.987 | |
| CDH1 | 0.913 | |
| CDH1 | BMP4 | 0.903 |
| CDH2 | AXIN1 | 0.996 |
| CDH1 | 0.908 | |
| CDH5 | KDR | 0.999 |
| CDH2 | 0.921 | |
| CDH1 | 0.919 | |
| PSEN1 | CDH1 | 0.999 |
| GSK3B | 0.996 | |
| CDH2 | 0.903 | |
| TCF7L2 | CTNNB1 | 0.999 |
| AXIN2 | 0.991 | |
| LEF1 | 0.956 | |
| AR | 0.912 |
| Scalp Parameters | Ages | |||
|---|---|---|---|---|
| 20s | 30s | 40s | 50~60s | |
| Alopecia Scale (HN Scale) | 1.73 (±1.05) (a) | 2.20 (±1.30) (b) ns | 3.70 (±1.11) (c) *** | 3.97 (±0.72) (d) *** |
| Hair Density (number/cm2) | 189.4 (±27.0) (a) | 178.9 (±28.1) (b) * | 173.7 (±27.4) (c) * | 172.0 (±31.6) (d) * |
| Hair Thickness (μm) | 63.0 (±5.4) (e) | 59.2 (±13.2) (f) *** | 53.1 (±13.1) (c) *** | 60.8 (±11.8) (d) *** |
| Scalp Skin Barrier (TEWL) (g/m2h) | 33.5 (±4.4) (g) | 34.6 (±5.8) (h) * | 34.8 (±6.2) (i) * | |
| Scalp Elasticity (Area) | 21.2 (±4.1) (j) | 22.2 (±3.4) (k) ns | 24.5 (±2.9) (l) * | |
| Hair Density [Number/cm2] | Hair Thickness [μm] | |||||
|---|---|---|---|---|---|---|
| 0 Month | 2 Month | 4 Month | 0 Month | 2 Month | 4 Month | |
| MNX | 168.1 ± 21.8 | 172.6 ± 24.0 | 176.5 ± 25.9 ** | 60.2 ± 11.1 | 63.4 ± 10.4 *** | 63.3 ± 10.6 *** |
| APN | 161.3 ± 22.7 | 164.2 ± 23.1 | 171.3 ± 27.0 *** | 54.9 ± 8.9 | 61.3 ± 9.6 *** | 60.5 ± 7.8 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Shin, J.y.; Choi, Y.-H.; Joo, J.H.; Kwack, M.H.; Sung, Y.K.; Kang, N.G. Hair Thickness Growth Effect of Adenosine Complex in Male-/Female-Patterned Hair Loss via Inhibition of Androgen Receptor Signaling. Int. J. Mol. Sci. 2024, 25, 6534. https://doi.org/10.3390/ijms25126534
Kim J, Shin Jy, Choi Y-H, Joo JH, Kwack MH, Sung YK, Kang NG. Hair Thickness Growth Effect of Adenosine Complex in Male-/Female-Patterned Hair Loss via Inhibition of Androgen Receptor Signaling. International Journal of Molecular Sciences. 2024; 25(12):6534. https://doi.org/10.3390/ijms25126534
Chicago/Turabian StyleKim, Jaeyoon, Jae young Shin, Yun-Ho Choi, Jang Ho Joo, Mi Hee Kwack, Young Kwan Sung, and Nae Gyu Kang. 2024. "Hair Thickness Growth Effect of Adenosine Complex in Male-/Female-Patterned Hair Loss via Inhibition of Androgen Receptor Signaling" International Journal of Molecular Sciences 25, no. 12: 6534. https://doi.org/10.3390/ijms25126534






