Expression Profiles of Dopamine-Related Genes and miRNAs Regulating Their Expression in Breast Cancer
Abstract
1. Introduction
2. Results
2.1. Microarray and qRT-PCR Profile of Dopamine-Related Gene Breast Cancer Samples in Comparison with Control Tissue
2.2. Prediction of Dopamine-Related Gene Expression Regulation by miRNAs
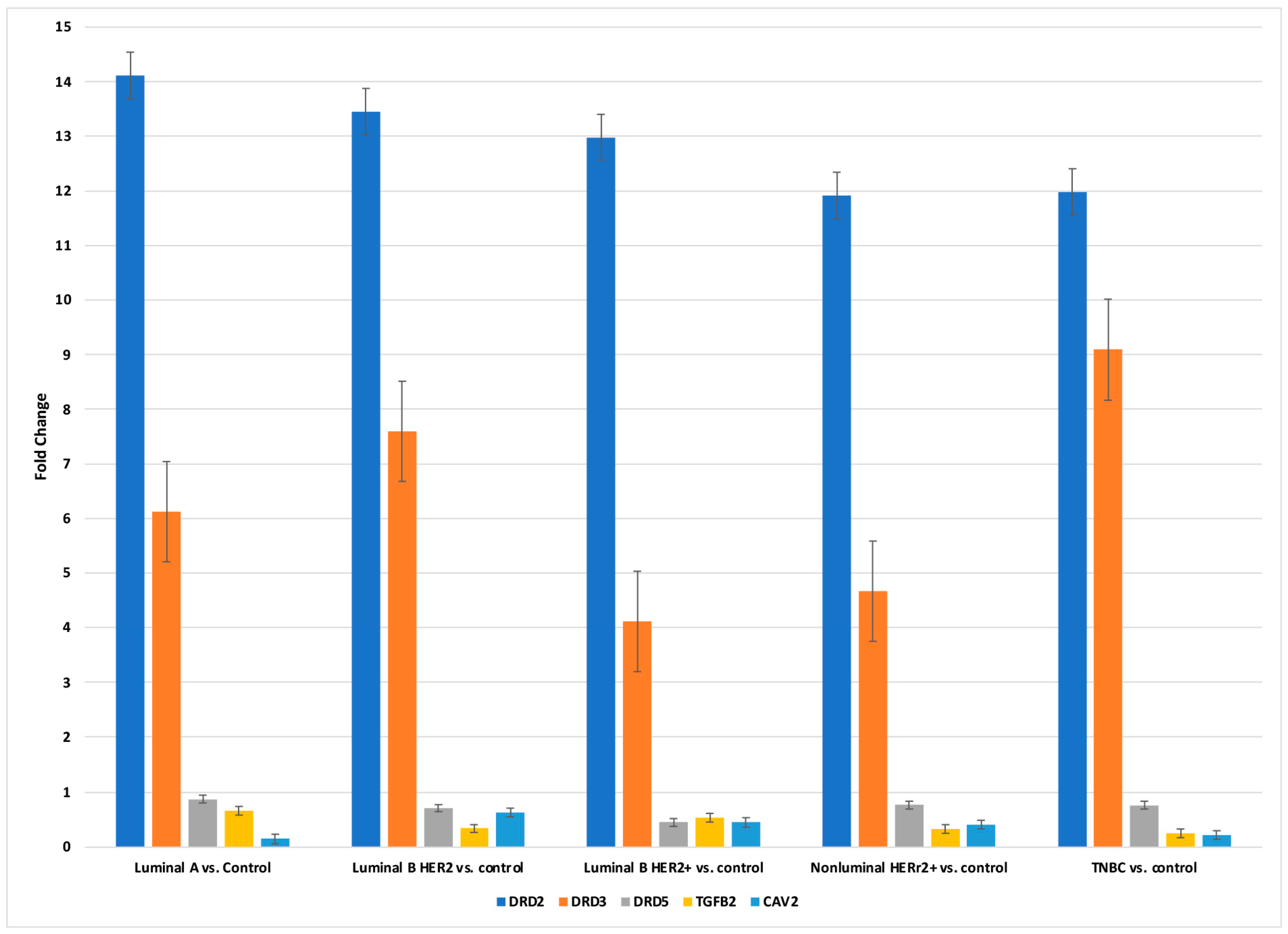
2.3. Concentration of DRD2, DRD3, DRD5, TGF-β-2, and CAV2 in Breast Cancer Tissues and Control at the Protein Level
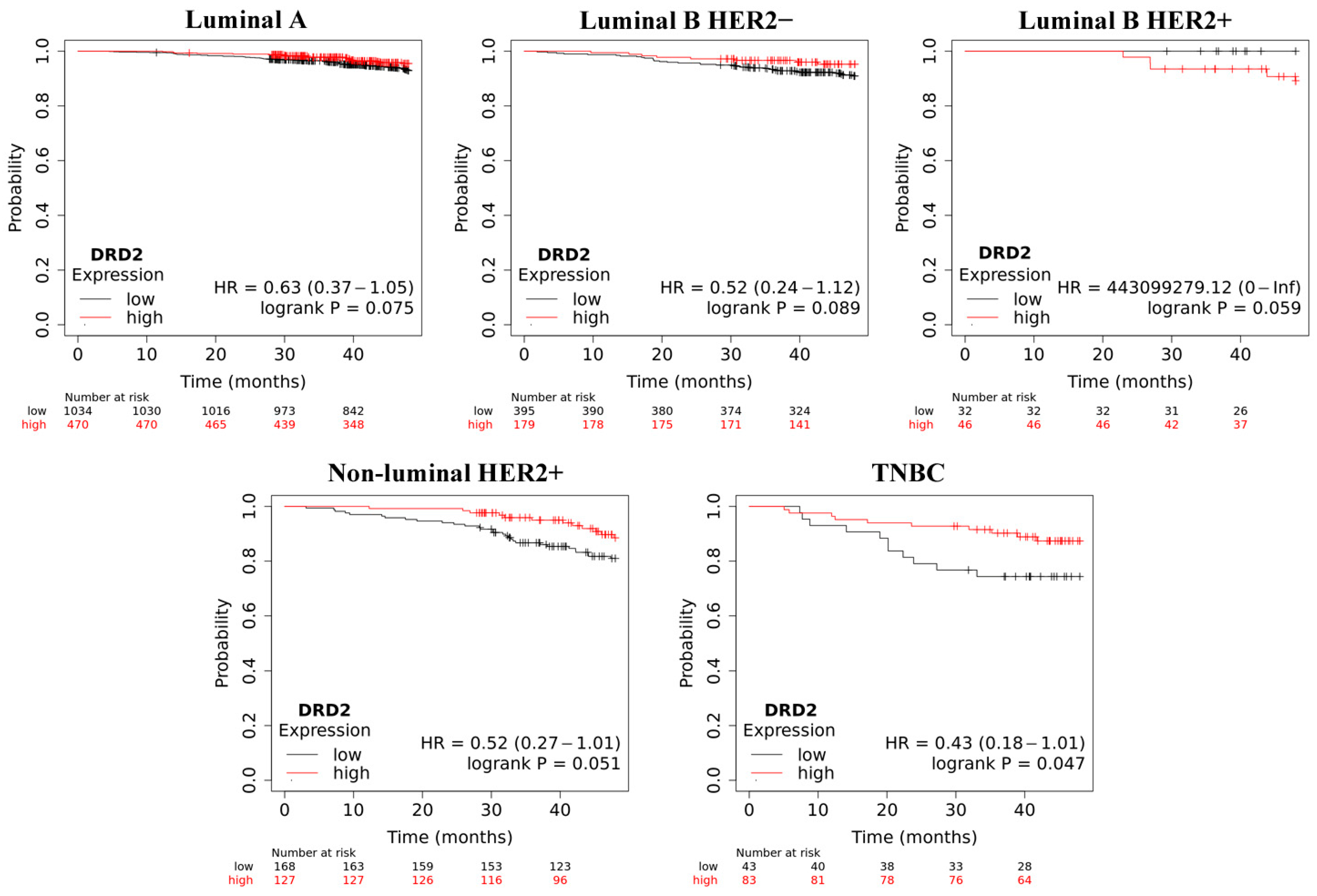
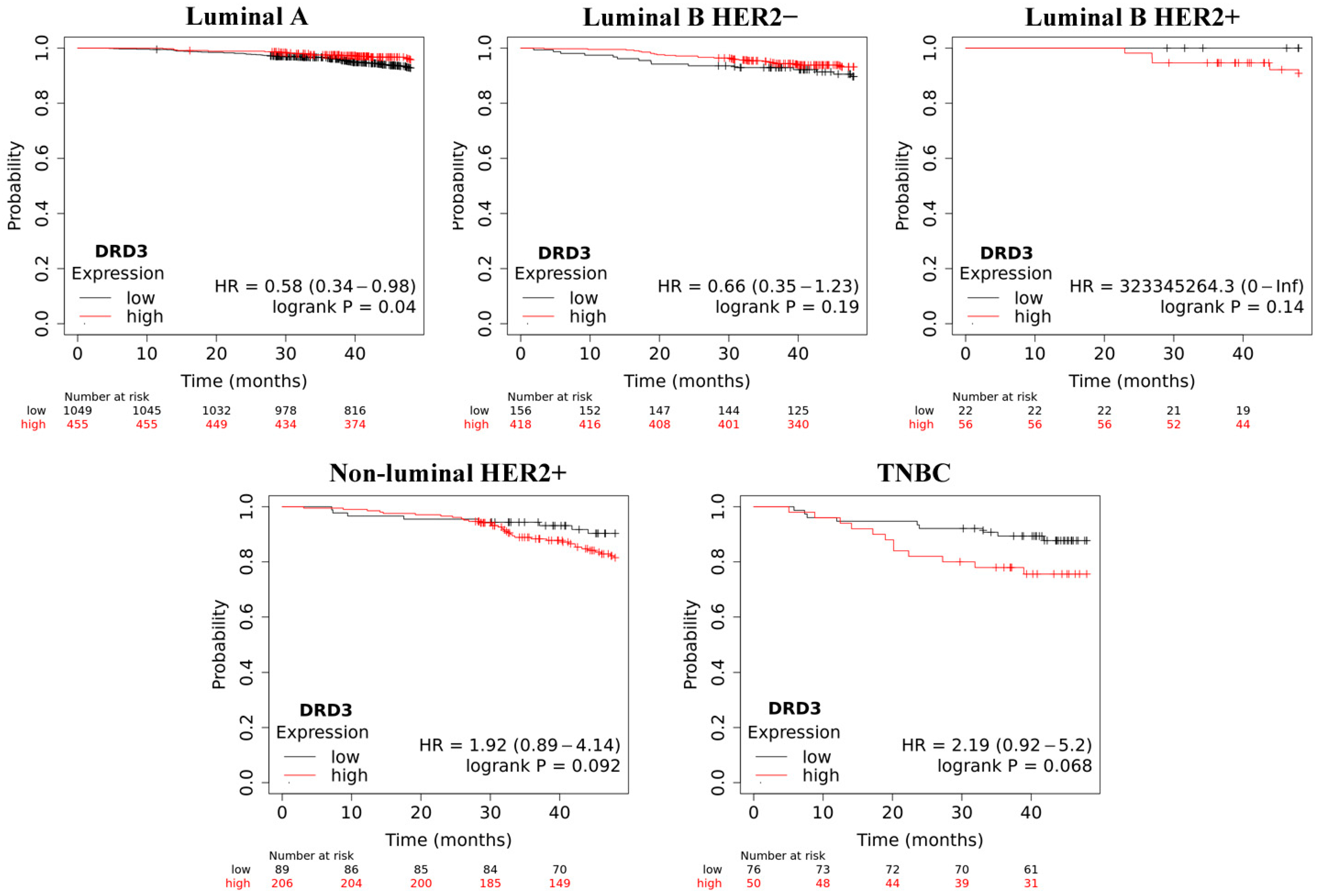
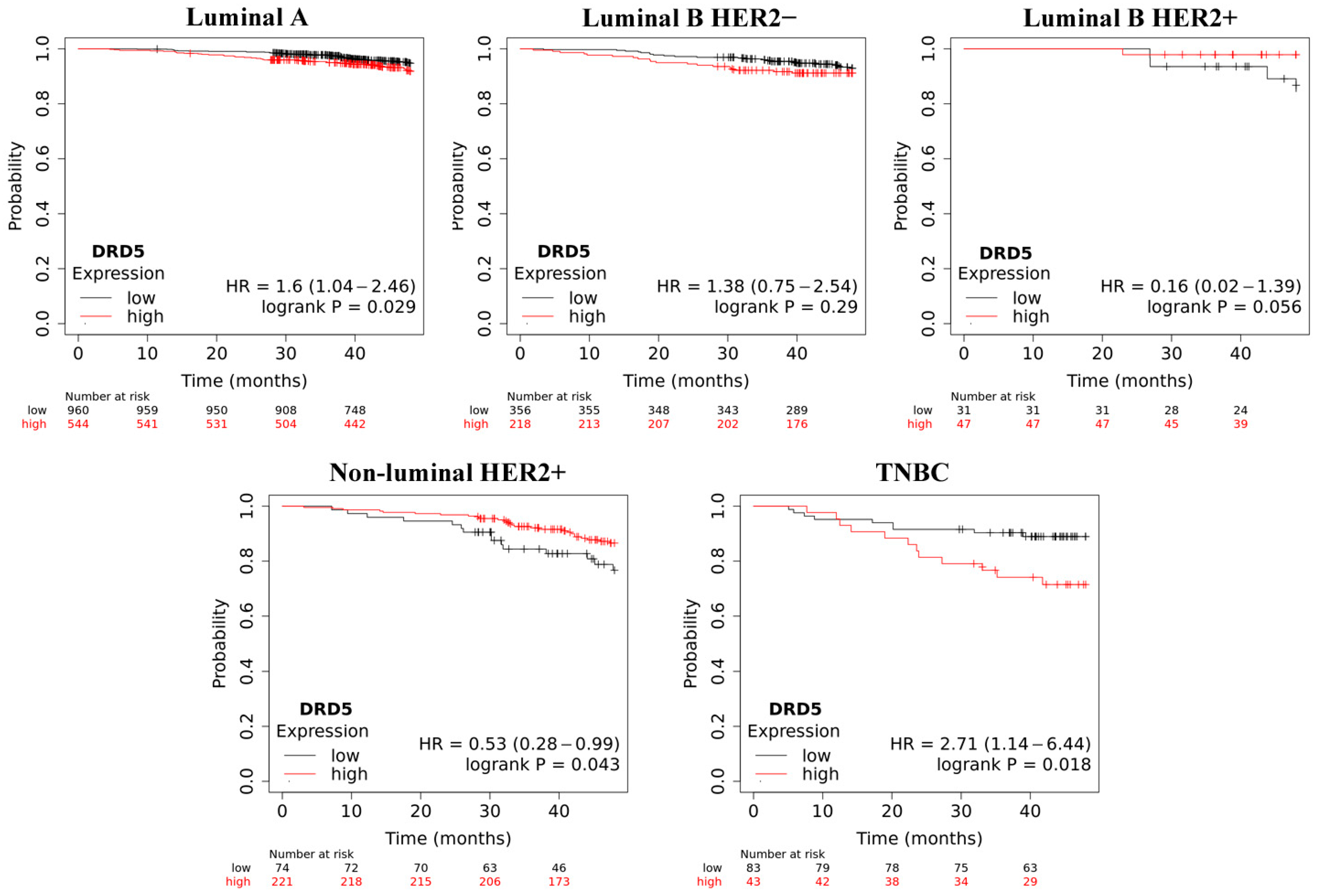
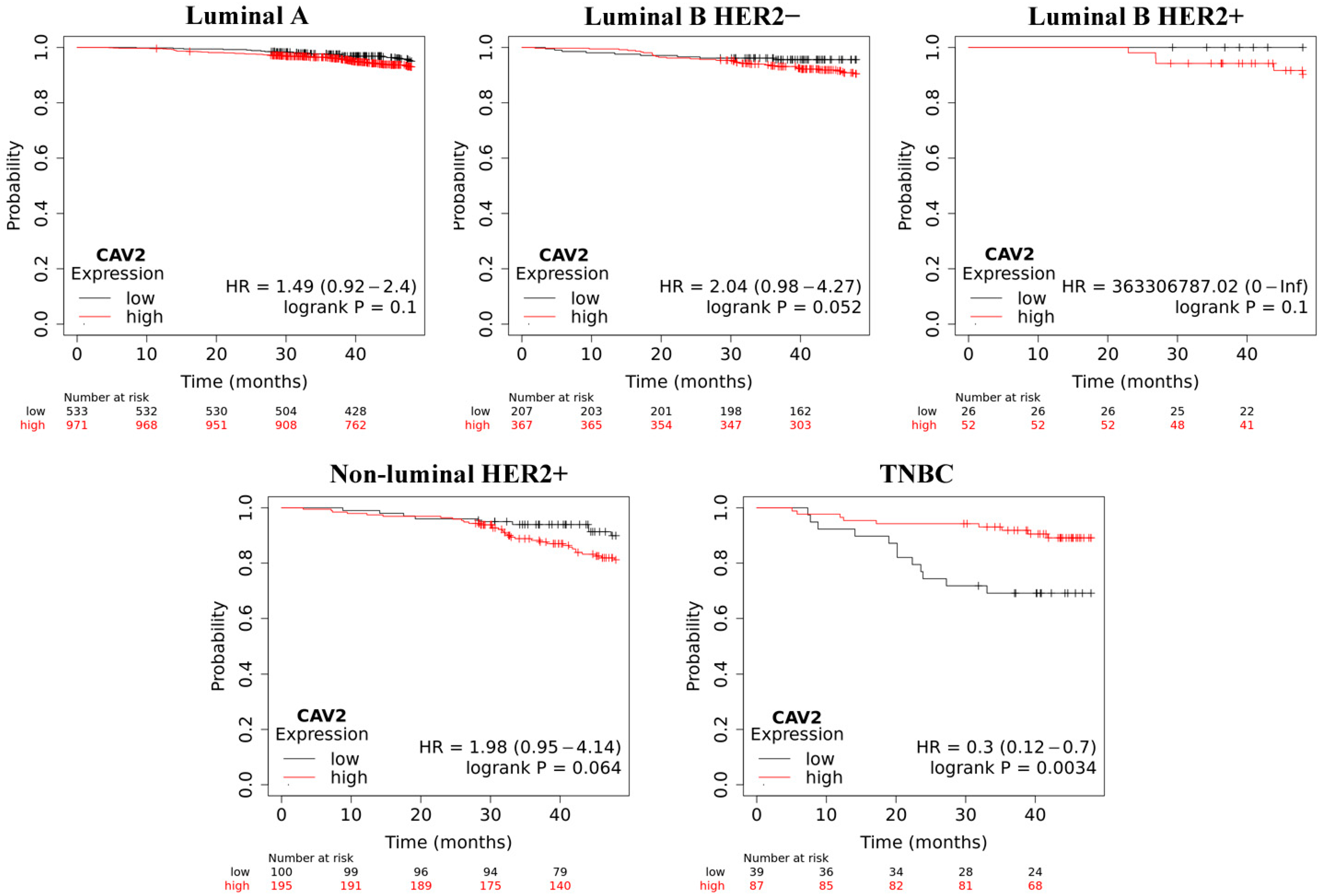
2.4. Overall Survive Analysis
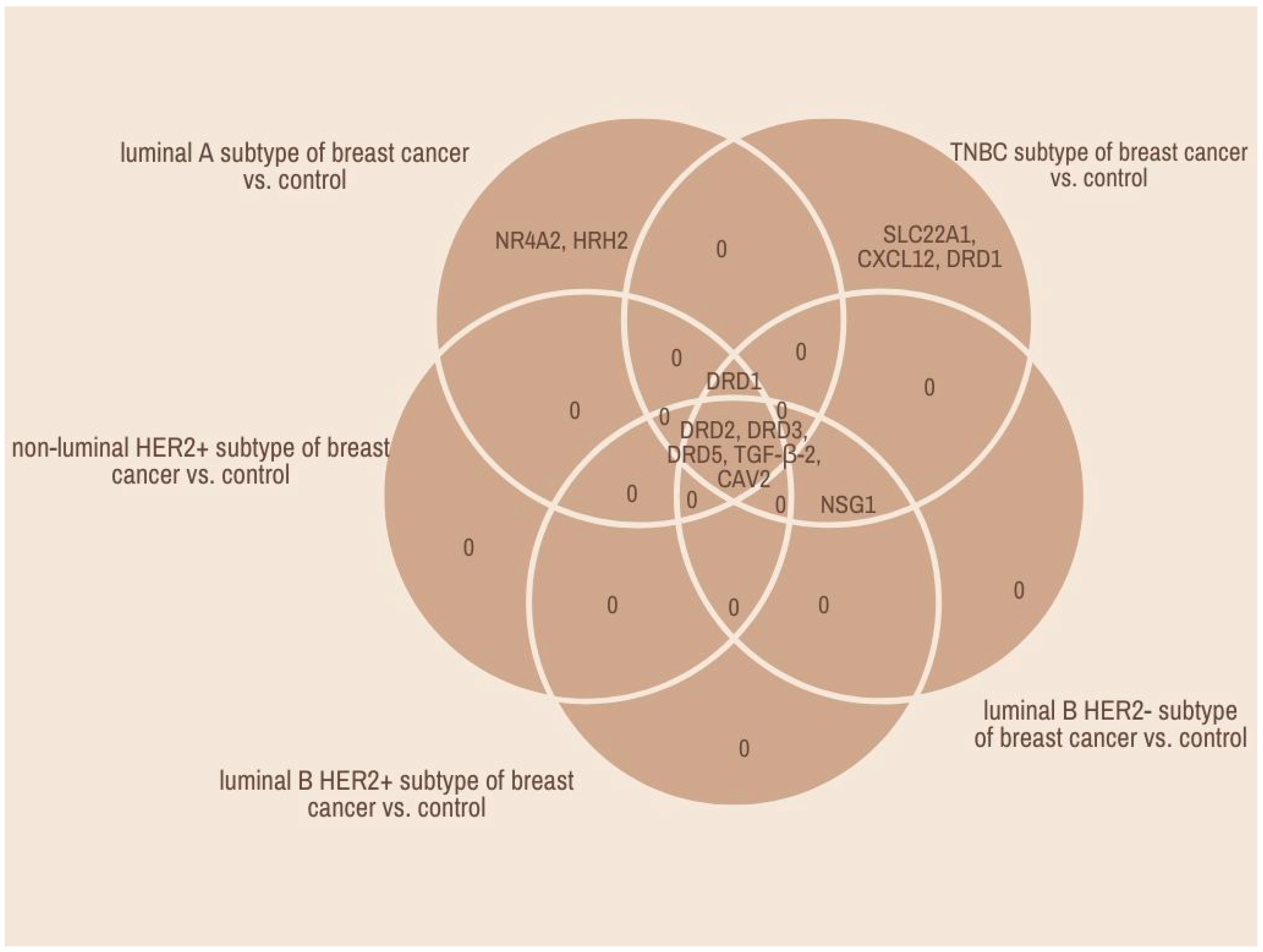
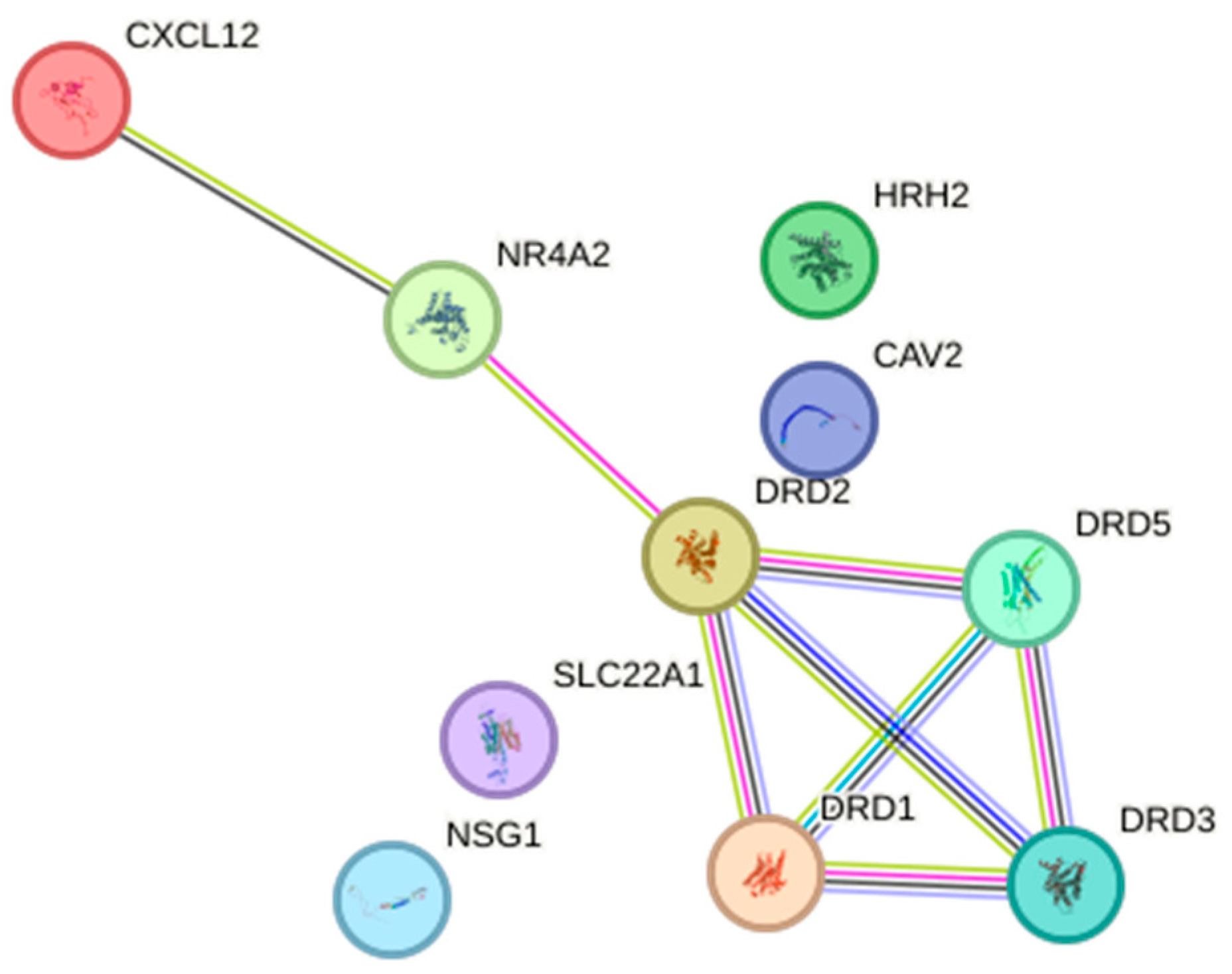
2.5. Relationship Network for the Selected Dopamine Pathway Differentiation Genes
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Isolation of Total Ribonucleic Acid (RNA)
4.3. Microarray Profiling of Dopamine-Related Genes
4.4. Comprehensive Microarray Profiling of Dopamine-Related miRNAs and Their Potential Impact on Gene Expression
4.5. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) Analysis
4.6. Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Statistical Analysis
Sample Size Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowska, U.; Barańska, K.; Miklewska, M.; Didkowska, J.A. Cancer Incidence and Mortality in Poland in 2020. Biul. Pol. Tow. Onkol. Nowotw. 2023, 8, 167–183. [Google Scholar] [CrossRef]
- Riihimäki, M.; Thomsen, H.; Sundquist, K.; Sundquist, J.; Hemminki, K. Clinical Landscape of Cancer Metastases. Cancer Med. 2018, 7, 5534–5542. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.R.; Stoltzfus, K.C.; Lehrer, E.J.; Dawson, L.A.; Tchelebi, L.; Gusani, N.J.; Sharma, N.K.; Chen, H.; Trifiletti, D.M.; Zaorsky, N.G. Epidemiology of Liver Metastases. Cancer Epidemiol. 2020, 67, 101760. [Google Scholar] [CrossRef] [PubMed]
- Testa, U.; Castelli, G.; Pelosi, E. Breast Cancer: A Molecularly Heterogenous Disease Needing Subtype-Specific Treatments. Med. Sci. 2020, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Rakha, E.A.; Tse, G.M.; Quinn, C.M. An Update on the Pathological Classification of Breast Cancer. Histopathology 2023, 82, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Tsang, J.Y.; Gary, M.T. Molecular Classification of Breast Cancer. Adv. Anat. Pathol. 2020, 27, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Gann, P.H.; McGregor, S.M.; Sethi, A. Quantification of Subtype Purity in Luminal A Breast Cancer Predicts Clinical Characteristics and Survival. Breast Cancer Res. Treat. 2023, 200, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Lafcı, O.; Celepli, P.; Öztekin, P.S.; Koşar, P.N. DCE-MRI Radiomics Analysis in Differentiating Luminal a and Luminal B Breast Cancer Molecular Subtypes. Acad. Radiol. 2023, 30, 22–29. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Y.; Huang, Y.; Chen, Z.; Zhang, H.; Yu, Y.; Wang, X.; Cao, X. The Regrouping of Luminal B ( HER2 Negative), a Better Discriminator of Outcome and Recurrence Score. Cancer Med. 2023, 12, 2493–2504. [Google Scholar] [CrossRef]
- Falato, C.; Schettini, F.; Pascual, T.; Brasó-Maristany, F.; Prat, A. Clinical Implications of the Intrinsic Molecular Subtypes in Hormone Receptor-Positive and HER2-Negative Metastatic Breast Cancer. Cancer Treat. Rev. 2023, 112, 102496. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Reis-Filho, J.S.; Geyer, C.E., Jr.; Wen, H.Y. Rare Subtypes of Triple Negative Breast Cancer: Current Understanding and Future Directions. NPJ Breast Cancer 2023, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ma, F.; Shan, Q.; Gao, D.; Jin, Y.; Sun, P. Melatonin Modulates Lactation by Regulating Prolactin Secretion via Tuberoinfundibular Dopaminergic Neurons in the Hypothalamus-Pituitary System. Curr. Protein Pept. Sci. 2020, 21, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Chen, Q.; Cai, J.; Xiao, L.; Zhang, J. Three Lactation-Related Hormones: Regulation of Hypothalamus-Pituitary Axis and Function on Lactation. Mol. Cell. Endocrinol. 2021, 520, 111084. [Google Scholar] [CrossRef] [PubMed]
- Toledo, I.M.; Zhao, X.; Lacasse, P. Effects of Milking Frequency and Domperidone Injections on Milk Production and Prolactin Signaling in the Mammary Gland of Dairy Cows. J. Dairy Sci. 2020, 103, 1969–1981. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Imaoka, T.; Vomachka, A.J.; Gudelsky, G.A.; Hou, Z.; Mistry, M.; Bailey, J.P.; Nieport, K.M.; Walther, D.J.; Bader, M. Serotonin Regulates Mammary Gland Development via an Autocrine-Paracrine Loop. Dev. Cell 2004, 6, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Martel, J.C.; Gatti McArthur, S. Dopamine Receptor Subtypes, Physiology and Pharmacology: New Ligands and Concepts in Schizophrenia. Front. Pharmacol. 2020, 11, 1003. [Google Scholar] [CrossRef]
- Zhuang, Y.; Xu, P.; Mao, C.; Wang, L.; Krumm, B.; Zhou, X.E.; Huang, S.; Liu, H.; Cheng, X.; Huang, X.-P. Structural Insights into the Human D1 and D2 Dopamine Receptor Signaling Complexes. Cell 2021, 184, 931–942. [Google Scholar] [CrossRef] [PubMed]
- Minami, K.; Liu, S.; Liu, Y.; Chen, A.; Wan, Q.; Na, S.; Li, B.-Y.; Matsuura, N.; Koizumi, M.; Yin, Y. Inhibitory Effects of Dopamine Receptor D1 Agonist on Mammary Tumor and Bone Metastasis. Sci. Rep. 2017, 7, 45686. [Google Scholar] [CrossRef]
- Yang, L.; Yao, Y.; Yong, L.; Feng, Y.; Su, H.; Yao, Q.; Xue, J.; Lu, W.; Zhou, T. Dopamine D1 Receptor Agonists Inhibit Lung Metastasis of Breast Cancer Reducing Cancer Stemness. Eur. J. Pharmacol. 2019, 859, 172499. [Google Scholar] [CrossRef]
- Li, J.; Yao, Q.; Xue, J.; Wang, L.; Yuan, Y.; Tian, X.; Su, H.; Wang, S.; Chen, W.; Lu, W. Dopamine D2 Receptor Antagonist Sulpiride Enhances Dexamethasone Responses in the Treatment of Drug-Resistant and Metastatic Breast Cancer. Acta Pharmacol. Sin. 2017, 38, 1282–1296. [Google Scholar] [CrossRef] [PubMed]
- Mirzaghasab, A.; Rigi, G. Differential Expression Analysis of Dopamine Receptor Genes Drd2, Drd3 and Drd4 in the Tumoral and Tumor Margin Samples of Breast Cancer Patients. Biomacromolecular J. 2020, 6, 56–63. [Google Scholar]
- Sakhteman, A.; Pasdaran, A.; Afifi, M.; Hamedi, A. An Assay on the Possible Effect of Essential Oil Constituents on Receptors Involved in Women’s Hormonal Health and Reproductive System Diseases. J. Evid. Based Complement. Altern. Med. 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Pierce, S.R.; Fang, Z.; Yin, Y.; West, L.; Asher, M.; Hao, T.; Zhang, X.; Tucker, K.; Staley, A.; Fan, Y.; et al. Targeting Dopamine Receptor D2 as a Novel Therapeutic Strategy in Endometrial Cancer. J. Exp. Clin. Cancer Res. 2021, 40, 61. [Google Scholar] [CrossRef] [PubMed]
- Borcherding, D.C.; Tong, W.; Hugo, E.R.; Barnard, D.F.; Fox, S.; LaSance, K.; Shaughnessy, E.; Ben-Jonathan, N. Expression and Therapeutic Targeting of Dopamine Receptor-1 (D1R) in Breast Cancer. Oncogene 2016, 35, 3103–3113. [Google Scholar] [CrossRef] [PubMed]
- Pornour, M.; Ahangari, G.; H Hejazi, S.; Deezagi, A. New Perspective Therapy of Breast Cancer Based on Selective Dopamine Receptor D2 Agonist and Antagonist Effects on MCF-7 Cell Line. Recent. Pat. Anti-Cancer Drug Discov. 2015, 10, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.B. MicroRNA (miRNA) in Cancer. Cancer Cell Int. 2015, 15, 38. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Xu, M.; Tian, X.; Cai, S.; Zeng, S. Research Advances in the Detection of miRNA. J. Pharm. Anal. 2019, 9, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, Z. The Emerging Role of microRNAs in Breast Cancer. J. Oncol. 2020, 2020. [Google Scholar] [CrossRef]
- Petri, B.J.; Klinge, C.M. Regulation of Breast Cancer Metastasis Signaling by miRNAs. Cancer Metastasis Rev. 2020, 39, 837–886. [Google Scholar] [CrossRef]
- Zabaleta, M.E.; Forbes-Hernández, T.Y.; Simal-Gandara, J.; Quiles, J.L.; Cianciosi, D.; Bullon, B.; Giampieri, F.; Battino, M. Effect of Polyphenols on HER2-Positive Breast Cancer and Related miRNAs: Epigenomic Regulation. Food Res. Int. 2020, 137, 109623. [Google Scholar] [CrossRef] [PubMed]
- Kalinina, T.; Kononchuk, V.; Alekseenok, E.; Obukhova, D.; Sidorov, S.; Strunkin, D.; Gulyaeva, L. Expression of Estrogen Receptor-and Progesterone Receptor-Regulating microRNAs in Breast Cancer. Genes 2021, 12, 582. [Google Scholar] [CrossRef] [PubMed]
- Kalinina, T.; Kononchuk, V.; Alekseenok, E.; Abdullin, G.; Sidorov, S.; Ovchinnikov, V.; Gulyaeva, L. Associations between the Levels of Estradiol-, Progesterone-, and Testosterone-Sensitive MiRNAs and Main Clinicopathologic Features of Breast Cancer. J. Pers. Med. 2021, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jia, Y.; Peng, H.; Li, J. Recent Developments in Dopamine-Based Materials for Cancer Diagnosis and Therapy. Adv. Colloid. Interface Sci. 2018, 252, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, C.; Chakroborty, D.; Chowdhury, U.R.; Dasgupta, P.S.; Basu, S. Dopamine Increases the Efficacy of Anticancer Drugs in Breast and Colon Cancer Preclinical Models. Clin. Cancer Res. 2008, 14, 2502–2510. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Cruz, A.; Salinas-Jazmín, N.; Velázquez, M.A.V. Dopamine Receptors in Cancer: Are They Valid Therapeutic Targets? Technol. Cancer Res. Treat. 2021, 20. [Google Scholar] [CrossRef] [PubMed]
- Weissenrieder, J.S.; Neighbors, J.D.; Mailman, R.B.; Hohl, R.J. Cancer and the Dopamine D2 Receptor: A Pharmacological Perspective. J. Pharmacol. Exp. Ther. 2019, 370, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Roney, M.S.I.; Park, S.-K. Antipsychotic Dopamine Receptor Antagonists, Cancer, and Cancer Stem Cells. Arch. Pharm. Res. 2018, 41, 384–408. [Google Scholar] [CrossRef]
- Pornour, M.; Ahangari, G.; Hejazi, S.H.; Ahmadkhaniha, H.R.; Akbari, M.E. Dopamine Receptor Gene (DRD1-DRD5) Expression Changes as Stress Factors Associated with Breast Cancer. Asian Pac. J. Cancer Prev. 2015, 15, 10339–10343. [Google Scholar] [CrossRef]
- Prabhu, V.V.; Madhukar, N.S.; Gilvary, C.; Kline, C.L.B.; Oster, S.; El-Deiry, W.S.; Elemento, O.; Doherty, F.; VanEngelenburg, A.; Durrant, J.; et al. Dopamine Receptor D5 Is a Modulator of Tumor Response to Dopamine Receptor D2 Antagonism. Clin. Cancer Res. 2019, 25, 2305–2313. [Google Scholar] [CrossRef]
- Williford, S.E.; Libby, C.J.; Ayokanmbi, A.; Otamias, A.; Gordillo, J.J.; Gordon, E.R.; Cooper, S.J.; Redmann, M.; Li, Y.; Griguer, C.; et al. Novel Dopamine Receptor 3 Antagonists Inhibit the Growth of Primary and Temozolomide Resistant Glioblastoma Cells. PLoS ONE 2021, 16, e0250649. [Google Scholar] [CrossRef] [PubMed]
- Leng, Z.G.; Lin, S.J.; Wu, Z.R.; Guo, Y.H.; Cai, L.; Shang, H.B.; Tang, H.; Xue, Y.J.; Lou, M.Q.; Zhao, W.; et al. Activation of DRD5 (Dopamine Receptor D5) Inhibits Tumor Growth by Autophagic Cell Death. Autophagy 2017, 13, 1404–1419. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Mei, J.; Hu, W. Hypermethylation of DRD5 Promoter Is a Biomarker Across 12 Cancer Types. DNA Cell Biol. 2020, 39, 2052–2058. [Google Scholar] [CrossRef] [PubMed]
- Rinnerthaler, G.; Hackl, H.; Gampenrieder, S.P.; Hamacher, F.; Hufnagl, C.; Hauser-Kronberger, C.; Zehentmayr, F.; Fastner, G.; Sedlmayer, F.; Mlineritsch, B.; et al. miR-16-5p Is a Stably-Expressed Housekeeping MicroRNA in Breast Cancer Tissues from Primary Tumors and from Metastatic Sites. Int. J. Mol. Sci. 2016, 17, 156. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Khoshbakht, T.; Hussen, B.M.; Abdullah, S.T.; Taheri, M.; Samadian, M. A review on the role of mir-16-5p in the carcinogenesis. Cancer Cell Int. 2022, 22, 342. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, C.; Zhao, K.-N.; Masci, P.P.; Lakhani, S.R.; Antonsson, A.; Simpson, P.T.; Vitetta, L. TGFβ Isoforms and Receptors mRNA Expression in Breast Tumours: Prognostic Value and Clinical Implications. BMC Cancer 2015, 15, 1010. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, H.; Arteaga, C.L.; Jensen, R.A.; Simpson, J.F.; Dupont, W.D.; Olson, S.J.; Schuyler, P.A.; Plummer, W.D.; Page, D.L. Loss of Expression of Transforming Growth Factor Beta Type II Receptor Correlates with High Tumour Grade in Human Breast In-Situ and Invasive Carcinomas. Histopathology 2000, 36, 168–177. [Google Scholar] [CrossRef]
- Hao, Y.; Baker, D.; Ten Dijke, P. TGF-β-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int. J. Mol. Sci. 2019, 20, 2767. [Google Scholar] [CrossRef]
- Lamouille, S.; Subramanyam, D.; Blelloch, R.; Derynck, R. Regulation of Epithelial–Mesenchymal and Mesenchymal–Epithelial Transitions by microRNAs. Curr. Opin. Cell Biol. 2013, 25, 200–207. [Google Scholar] [CrossRef]
- Derynck, R.; Turley, S.J.; Akhurst, R.J. TGFβ Biology in Cancer Progression and Immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 9–34. [Google Scholar] [CrossRef]
- Stavast, C.J.; Erkeland, S.J. The Non-Canonical Aspects of microRNAs: Many Roads to Gene Regulation. Cells 2019, 8, 1465. [Google Scholar] [CrossRef] [PubMed]
- Dexheimer, P.J.; Cochella, L. MicroRNAs: From Mechanism to Organism. Front. Cell Dev. Biol. 2020, 8, 409. [Google Scholar] [CrossRef]
- Dragomir, M.P.; Knutsen, E.; Calin, G.A. Classical and Noncanonical Functions of miRNAs in Cancers. Trends Genet. 2022, 38, 379–394. [Google Scholar] [CrossRef]
- Song, W.; Wu, S.; Wu, Q.; Zhou, L.; Yu, L.; Zhu, B.; Gong, X. The microRNA-141-3p/ CDK8 Pathway Regulates the Chemosensitivity of Breast Cancer Cells to Trastuzumab. J. Cell. Biochem. 2019, 120, 14095–14106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, J.; Jia, S.; Wang, Y.; Kang, Y.; Zhang, W. Down-Regulation of lncRNA-ATB Inhibits Epithelial–Mesenchymal Transition of Breast Cancer Cells by Increasing miR-141-3p Expression. Biochem. Cell Biol. 2019, 97, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Ma, M.; Li, M.; Guo, Y.; Zuo, X.; Gu, X.; Zhang, M.; Shi, Y. LncRNA MEG3 Regulates Breast Cancer Proliferation and Apoptosis through miR-141-3p/RBMS3 Axis. Genomics 2021, 113, 1689–1704. [Google Scholar] [CrossRef]
- Elsheikh, S.E.; Green, A.R.; Rakha, E.A.; Samaka, R.M.; Ammar, A.A.; Powe, D.; Reis-Filho, J.S.; Ellis, I.O. Caveolin 1 and Caveolin 2 Are Associated with Breast Cancer Basal-like and Triple-Negative Immunophenotype. Br. J. Cancer 2008, 99, 327–334. [Google Scholar] [CrossRef]
- Williams, T.M.; Schneeweiss, A.; Jackisch, C.; Shen, C.; Weber, K.E.; Fasching, P.A.; Denkert, C.; Furlanetto, J.; Heinmöller, E.; Schmatloch, S.; et al. Caveolin Gene Expression Predicts Clinical Outcomes for Early-Stage HER2-Negative Breast Cancer Treated with Paclitaxel-Based Chemotherapy in the GeparSepto Trial. Clin. Cancer Res. 2023, 29, 3384–3394. [Google Scholar] [CrossRef]
- Ozturk, S.; Papageorgis, P.; Wong, C.K.; Lambert, A.W.; Abdolmaleky, H.M.; Thiagalingam, A.; Cohen, H.T.; Thiagalingam, S. SDPR Functions as a Metastasis Suppressor in Breast Cancer by Promoting Apoptosis. Proc. Natl. Acad. Sci. USA 2016, 113, 638–643. [Google Scholar] [CrossRef]
- Tian, Y.; Yu, Y.; Hou, L.; Chi, J.; Mao, J.; Xia, L.; Wang, X.; Wang, P.; Cao, X. Serum Deprivation Response Inhibits Breast Cancer Progression by Blocking Transforming Growth Factor-β Signaling. Cancer Sci. 2016, 107, 274–280. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, X.; Hu, J.; Zhang, H.; Wang, B.; Li, Y.; Fu, L.; Su, R.; Yu, Y. Integrated Bioinformatic Analysis of the Expression and Prognosis of Caveolae-Related Genes in Human Breast Cancer. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, B.; Brierley, J.; Byrd, D.; Bosman, F.; Kehoe, S.; Kossary, C.; Piñeros, M.; Van Eycken, E.; Weir, H.K.; Gospodarowicz, M. The TNM Classification of Malignant Tumours—towards Common Understanding and Reasonable Expectations. Lancet Oncol. 2017, 18, 849–851. [Google Scholar] [CrossRef]
- Agarwal, V.; Bell, G.W.; Nam, J.-W.; Bartel, D.P. Predicting Effective microRNA Target Sites in Mammalian mRNAs. eLife 2015, 4, e05005. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X. miRDB: An Online Database for Prediction of Functional microRNA Targets. Nucleic Acids Res. 2020, 48, D127–D131. [Google Scholar] [CrossRef]
- Liu, W.; Wang, X. Prediction of Functional microRNA Targets by Integrative Modeling of microRNA Binding and Target Expression Data. Genome Biol. 2019, 20, 18. [Google Scholar] [CrossRef]
- Győrffy, B. Integrated Analysis of Public Datasets for the Discovery and Validation of Survival-Associated Genes in Solid Tumors. Innovation (Camb) 2024, 5, 100625. [Google Scholar] [CrossRef] [PubMed]
- Győrffy, B. Transcriptome-Level Discovery of Survival-Associated Biomarkers and Therapy Targets in Non-Small-Cell Lung Cancer. Br. J. Pharmacol. 2024, 181, 362–374. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING Database in 2023: Protein-Protein Association Networks and Functional Enrichment Analyses for Any Sequenced Genome of Interest. Nucleic Acids Res 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- Kalkulator Doboru Próby. Available online: https://www.naukowiec.org/dobor.html (accessed on 19 April 2022).
- Krajowy Rejestr Nowotworów. Nowotwór Piersi- 2019. Available online: https://onkologia.org.pl/sites/default/files/Pier%C5%9B.pdf (accessed on 1 February 2024).
- Dai, X.; Li, T.; Bai, Z.; Yang, Y.; Liu, X.; Zhan, J.; Shi, B. Breast Cancer Intrinsic Subtype Classification, Clinical Use and Future Trends. Am. J. Cancer Res. 2015, 5, 2929–2943. [Google Scholar]
- Jassem, E.J.; Krzakowski, M.; Jassem, A.J.; Krzakowski, M.; Bobek-Billewicz, B.; Duchnowska, R.; Jeziorski, A.; Olszewski, W.; Senkus-Konefka, E.; Tchórzewska-Korba, H.; et al. Breast Cancer. Oncol. Clin. Pract. 2020, 16, 207–260. [Google Scholar]

| ID | mRNA | Luminal A vs. Control | Luminal B HER2− vs. Control | Luminal B HER2+ vs. Control | Non-Luminal HER2+ vs. Control | TNBC vs. Control |
|---|---|---|---|---|---|---|
| 214652_at | DRD1 | 6.78 ± 0.18 | 3.45 ± 0.12 | 3.54 ± 0.88 | 3.98 ± 0.12 | 7.87 ± 1.09 |
| 216924_s_at | DRD2 | 14.56 ± 0.91 | 14.78 ± 0.91 | 15.98 ± 1.34 | 11.99 ± 2.01 | 10.98 ± 0.91 |
| 216938_x_at | 14.65 ± 2.11 | 15.01 ± 0.91 | 14.98 ± 1.45 | 10.98 ± 1.98 | 10.54 ± 1.76 | |
| 206590_x_at | 14.43 ± 1.98 | 14.56 ± 0.76 | 15.56 ± 1.67 | 11.65 ± 2.11 | 10.19 ± 2.10 | |
| 211625_s_at | DRD3 | 6.98 ± 0.87 | 7.51 ± 0.16 | 4.56 ± 0.43 | 5.67 ± 0.56 | 9.87 ± 0.42 |
| 214559_at | 4.56 ± 0.78 | 5.67 ± 0.19 | 7.12 ± 0.54 | 3.09 ± 0.13 | 5.67 ± 0.56 | |
| 208486_at | DRD5 | −4.57 ± 0.91 | −11.98 ± 1.23 | −6.98 ± 1.87 | −13.01 ± 0.81 | −4.56 ± 0.19 |
| 209908_s_at | TGF-β-2 | −5.66 ± 0.81 | −12.09 ± 0.18 | −11.09 ± 0.23 | −8.78 ± 0.76 | −9.98 ± 0.98 |
| 209909_s_at | −5.45 ± 0.44 | −12.76 ± 0.87 | −10.98 ± 0.65 | −8.99 ± 0.34 | −8.98 ± 0.65 | |
| 220406_at | −5.69 ± 0.23 | −11.23 ± 0.81 | −10.11 ± 0.41 | −9.09 ± 0.65 | −9.99 ± 0.17 | |
| 220407_s_at | −5.98 ± 0.87 | −12.98 ± 0.14 | −11.34 ± 0.56 | −10.34 ± 0.98 | −10.09 ± 0.19 | |
| 203323_at | CAV2 | −3.45 ± 0.23 | −4.56 ± 0.65 | −4.09 ± 0.34 | −3.41 ± 0.76 | −5.67 ± 0.18 |
| 203324_s_at | −3.78 ± 0.54 | −4.09 ± 0.76 | −4.15 ± 0.77 | −3.98 ± 0.11 | −5.19 ± 0.19 | |
| 213426_s_at | −3.87 ± 0.45 | −4.13 ± 0.18 | −4.78 ± 0.66 | −4.01 ± 0.19 | −5.13 ± 0.54 | |
| 207201_s_at | SLC22A1 | 3.98 ± 0.12 | 4.01 ± 0.14 | 3.78 ± 0.19 | 5.87 ± 0.24 | 5.11 ± 0.98 |
| 209687_at | CXCL12 | 8.98 ± 1.01 | 6.19 ± 0.98 | 3.01 ± 0.24 | 5.01 ± 0.23 | 6.17 ± 0.51 |
| 203666_at | 8.17 ± 0.87 | 6.22 ± 0.87 | 4.56 ± 0.43 | 5.07 ± 0.67 | 6.23 ± 0.78 | |
| 204621_s_at | NR4A2 | 4.56 ± 0.18 | 4.71 ± 0.18 | 4.99 ± 0.91 | 4.76 ± 0.34 | 7.09 ± 0.32 |
| 204622_x_at | 4.16 ± 0.71 | 4.71 ± 0.23 | 4.89 ± 0.12 | 5.09 ± 0.67 | 7.12 ± 0.14 | |
| 216248_s_at | 4.76 ± 0.56 | 4.55 ± 0.44 | 4.51 ± 0.54 | 5.19 ± 0.13 | 7.56 ± 0.71 | |
| 220805_at | HRH2 | 4.15 ± 0.23 | 4.76 ± 0.24 | 4.15 ± 0.81 | 5.12 ± 0.45 | 7.18 ± 0.65 |
| 209569_x_at | NSG1 | 3.45 ± 0.19 | 3.09 ± 0.18 | 3.18 ± 0.34 | 3.98 ± 0.19 | 3.91 ± 0.13 |
| 209570_s_at | 3.21 ± 0.22 | 3.12 ± 0.14 | 3.19 ± 0.33 | 3.31 ± 0.22 | 3.78 ± 0.17 | |
| 213533_at | 3.56 ± 0.24 | 3.14 ± 0.23 | 3.29 ± 0.31 | 3.11 ± 0.21 | 3.81 ± 0.42 |
| mRNA | miRNA | Target Score | Luminal A vs. Control (FC) | Luminal B HER2− vs. Control (FC) | Luminal B HER2+ vs. Control (FC) | Non-Luminal Her2+ vs. Control (FC) | TNBC vs. Control (FC) |
|---|---|---|---|---|---|---|---|
| DRD2 TGF-β-2 | hsa-miR-141-3p | 94 95 | −3.98 ± 0.19 | −3.45 ± 0.12 | −3.12 ± 0.23 | −7.87 ± 0.18 | −3.01 ± 0.32 |
| DRD3 DRD5 | hsa-miR-4441 | 90 93 | −6.13 ± 0.19 | −4.56 ± 0.76 | −5.66 ± 0.21 | −3.45 ± 0.18 | −3.98 ± 0.87 |
| DRD5 | hsa-miR-16-5p | 89 | −4.10 ± 0.98 | −4.87 ± 0.19 | −4.12 ± 0.98 | −3.65 ± 0.98 | −5.03 ± 0.81 |
| Protein | Control Tissue | Luminal A | Luminal B HER2− | Luminal B HER2+ | Non-Luminal HER2+ | TNBC |
|---|---|---|---|---|---|---|
| DRD2 [ng/mL] | 3.13 ± 0.98 | 10.91 ± 0.98 * | 6.87 ± 1.12 * | 6.17 ± 1.91 * | 9.99 ± 1.67 * | 10.98 ± 2.12 * |
| DRD3 [ng/mL] | 6.71 ± 4.56 | 10.98 ± 2.10 * | 9.10 ± 0.98 * | 12.87 ± 2.45 * | 11.88 ± 2.65 * | 13.12 ± 1.45 * |
| DRD5 [ng/mL] | 22.98 ± 5.06 | 16.81 ± 2.18 * | 18.81 ± 2.34 * | 16.87 ± 2.19 * | 17.09 ± 0.51 * | 5.98 ± 0.43 * |
| TGF-β-2 [pg/mL] | 4.15 ± 2.18 | 1.09 ± 0.13 * | below detection threshold * | below detection threshold * | below detection threshold * | below detection threshold * |
| CAV2 [ng/mL] | 2.34 ± 0.19 | 0.76 ± 0.18 * | 0.65 ± 0.04 * | 0.98 ± 0.09 * | 0.34 ± 0.02 * | 0.14 ± 0.06 * |
| Molecular Type | Degree of Histological Malignancy | Age | BMI [kg/m2] | |||
|---|---|---|---|---|---|---|
| G1 | G2 | G3 | <50 Years | >50 Years | ||
| Luminal A | 23 (18%) | 48 (37%) | 59 (45%) | 43 (33%) | 87 (67%) | 30.78 ± 2.76 |
| Luminal B HER2− | 31 (31%) | 57 (57%) | 12 (12%) | 32 (32%) | 68 (68%) | 30.18 ± 4.56 |
| Luminal B HER+ | 23 (24%) | 57 (59%) | 16 (17%) | 19 (20%) | 77 (80%) | 32.09 ± 6.19 |
| Non-luminal HER2+ | 9 (25%) | 12 (33%) | 15 (42%) | 9 (25%) | 27 (75%) | 33.18 ± 5.67 |
| TNBC | 14 (32%) | 21 (49%) | 8 (19%) | 10 (23%) | 33 (77%) | 34.67 ± 2.98 |
| mRNA | Nucleotide Sequence | |
|---|---|---|
| DRD2 | Forward | 5′-GATTTGGAGAGGTAGAATTGGAGT-3′ |
| Reverse | 5′-CAACCCAAAACATAACCAATATAAC-3′ | |
| DRD3 | Forward | 5′-GGTTTTTATTGTTTGTTGGTTGTTT-3′ |
| Reverse | 5′-ATATTCCCTCTTCTACTCCCTCAAC-3′ | |
| DRD5 | Forward | 5′-TAGTTTAATTGGTATAGGGATTAGG-3′ |
| Reverse | 5′-TAAAATCACAATTCTCTACATTCAC-3′ | |
| TGF-β-2 | Forward | 5′-TACTACGCCAAGGAGGTTTACAAA-3 ′ |
| Reverse | 5′-TTGTTCAGGCACTCTGGCTTT-3 ′ | |
| CAV2 | Forward | 5′-TGTTTTTGGTTATTTTTTTGGTTTT-3′ |
| Reverse | 5′-TCCAAATATTCAATCCTAACTCAATTAC-3′ | |
| ACTB | Forward | 5′-TCACCCACACTGTGCC CATCTACGA-3′ |
| Reverse | 5′-CAGCGGAACCGCTCATTGCCAATGG-3′ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirek, T.; Sirek, A.; Borawski, P.; Ryguła, I.; Król-Jatręga, K.; Opławski, M.; Boroń, D.; Chalcarz, M.; Ossowski, P.; Dziobek, K.; et al. Expression Profiles of Dopamine-Related Genes and miRNAs Regulating Their Expression in Breast Cancer. Int. J. Mol. Sci. 2024, 25, 6546. https://doi.org/10.3390/ijms25126546
Sirek T, Sirek A, Borawski P, Ryguła I, Król-Jatręga K, Opławski M, Boroń D, Chalcarz M, Ossowski P, Dziobek K, et al. Expression Profiles of Dopamine-Related Genes and miRNAs Regulating Their Expression in Breast Cancer. International Journal of Molecular Sciences. 2024; 25(12):6546. https://doi.org/10.3390/ijms25126546
Chicago/Turabian StyleSirek, Tomasz, Agata Sirek, Przemysław Borawski, Izabella Ryguła, Katarzyna Król-Jatręga, Marcin Opławski, Dariusz Boroń, Michał Chalcarz, Piotr Ossowski, Konrad Dziobek, and et al. 2024. "Expression Profiles of Dopamine-Related Genes and miRNAs Regulating Their Expression in Breast Cancer" International Journal of Molecular Sciences 25, no. 12: 6546. https://doi.org/10.3390/ijms25126546
APA StyleSirek, T., Sirek, A., Borawski, P., Ryguła, I., Król-Jatręga, K., Opławski, M., Boroń, D., Chalcarz, M., Ossowski, P., Dziobek, K., Zmarzły, N., Boroń, K., Mickiewicz, P., & Grabarek, B. O. (2024). Expression Profiles of Dopamine-Related Genes and miRNAs Regulating Their Expression in Breast Cancer. International Journal of Molecular Sciences, 25(12), 6546. https://doi.org/10.3390/ijms25126546









