Primitive and Definitive Neural Precursor Cells Are Present in Human Cerebral Organoids
Abstract
:1. Introduction
2. Results
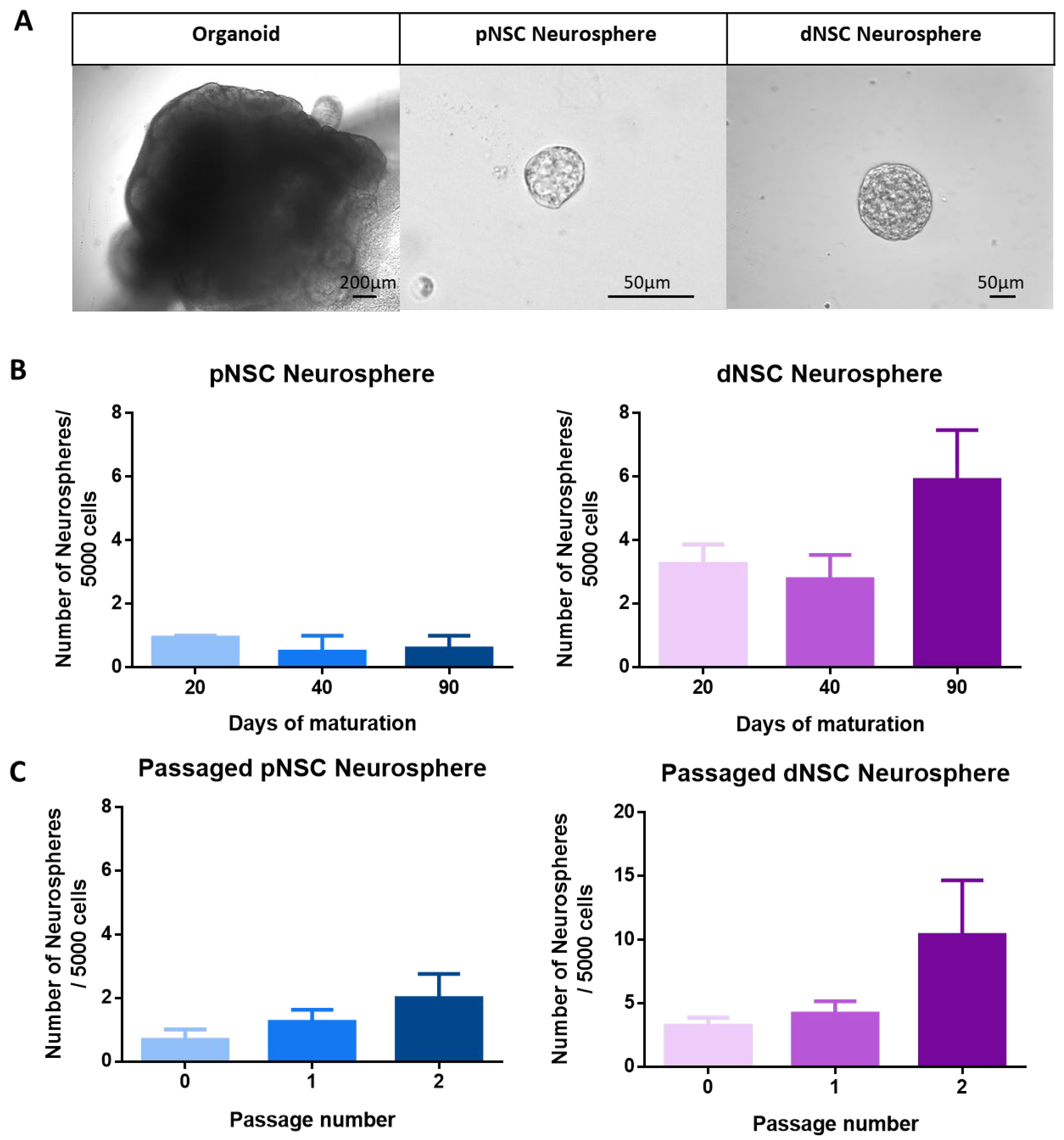
2.1. pNSCs and dNSCs Can Be Isolated from Human Cerebral Organoids
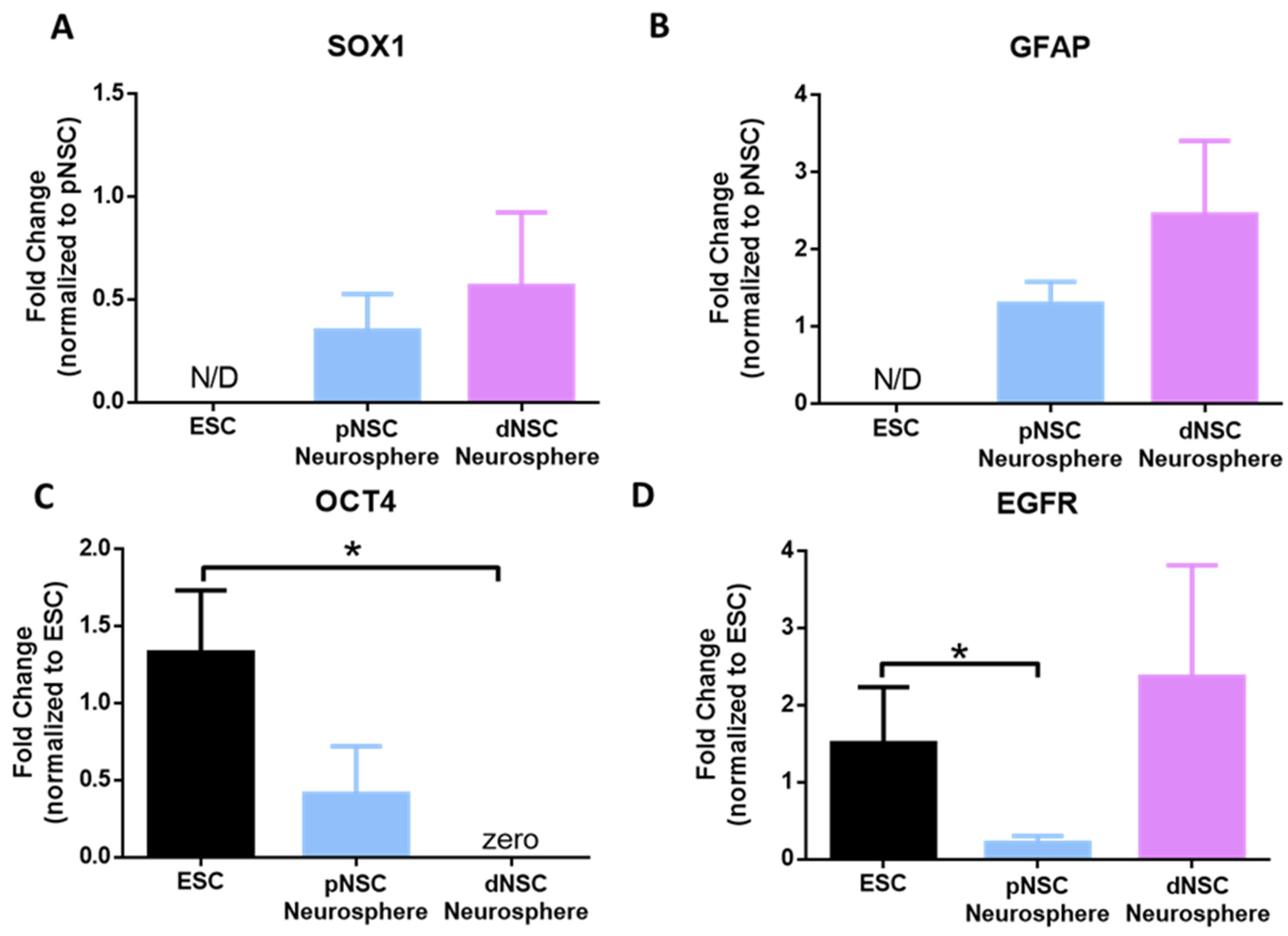
2.2. Human pNSC and dNSC Neurospheres Have Distinct Genetic Expression Profiles
2.3. Human pNSC and dNSC Neurospheres Are Multipotent
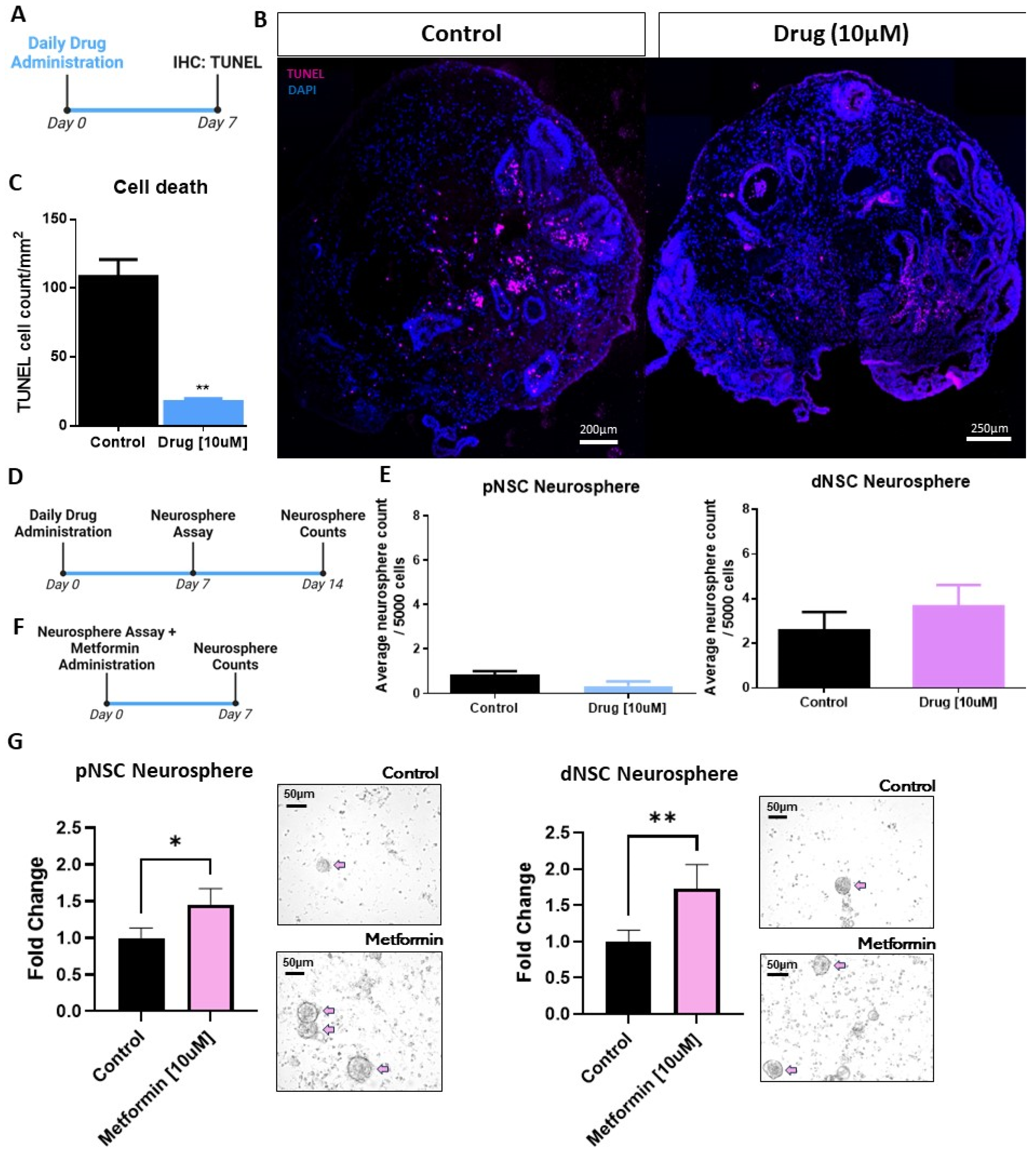
2.4. Human CO-Derived Neural Precursor Cells Are Responsive to Pro-Survival and Proliferation Factors Identified in Mice
3. Discussion
4. Materials and Methods
4.1. Embryonic Stem Cell Culture
4.2. Cerebral Organoid Culture
4.3. Neurosphere Assay
4.4. NWL283 Administration
4.5. Metformin Administration
4.6. TUNEL Labeling
4.7. qPCR
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hitoshi, S.; Seaberg, R.M.; Koscik, C.; Alexson, T.; Kusunoki, S.; Kanazawa, I.; Tsuji, S.; Van Der Kooy, D. Primitive Neural Stem Cells from the Mammalian Epiblast Differentiate to Definitive Neural Stem Cells under the Control of Notch Signaling. Genes Dev. 2004, 18, 1806. [Google Scholar] [CrossRef] [PubMed]
- Tropepe, V.; Hitoshi, S.; Sirard, C.; Mak, T.W.; Rossant, J.; Van Der Kooy, D. Direct Neural Fate Specification from Embryonic Stem Cells: A Primitive Mammalian Neural Stem Cell Stage Acquired through a Default Mechanism. Neuron 2001, 30, 65–78. [Google Scholar] [CrossRef]
- Reeve, R.L.; Yammine, S.Z.; Deveale, B.; van der Kooy, D. Targeted Activation of Primitive Neural Stem Cells in the Mouse Brain. Eur. J. Neurosci. 2016, 43, 1474–1485. [Google Scholar] [CrossRef]
- Reeve, R.L.; Yammine, S.Z.; Morshead, C.M.; van der Kooy, D. Quiescent Oct4+ Neural Stem Cells (NSCs) Repopulate Ablated Glial Fibrillary Acidic Protein+ NSCs in the Adult Mouse Brain. Stem Cells 2017, 35, 2071–2082. [Google Scholar] [CrossRef]
- Sachewsky, N.; Leeder, R.; Xu, W.; Rose, K.L.; Yu, F.; van der Kooy, D.; Morshead, C.M. Primitive Neural Stem Cells in the Adult Mammalian Brain Give Rise to GFAP-Expressing Neural Stem Cells. Stem Cell Rep. 2014, 2, 810–824. [Google Scholar] [CrossRef] [PubMed]
- Imura, T.; Kornblum, H.I.; Sofroniew, M.V. The Predominant Neural Stem Cell Isolated from Postnatal and Adult Forebrain But Not Early Embryonic Forebrain Expresses GFAP. J. Neurosci. 2003, 23, 2824–2832. [Google Scholar] [CrossRef]
- Reynolds, B.A.; Weiss, S. Generation of Neurons and Astrocytes from Isolated Cells of the Adult Mammalian Central Nervous System. Science 1992, 255, 1707–1710. [Google Scholar] [CrossRef]
- Sachewsky, N.; Xu, W.; Fuehrmann, T.; van der Kooy, D.; Morshead, C.M. Lineage Tracing Reveals the Hierarchical Relationship between Neural Stem Cell Populations in the Mouse Forebrain. Sci. Rep. 2019, 9, 17730. [Google Scholar] [CrossRef]
- Yammine, S.Z.; Burns, I.; Gosio, J.; Peluso, A.; Merritt, D.M.; Innes, B.; Coles, B.L.K.; Yan, W.R.; Bader, G.D.; Morshead, C.M.; et al. Fate Specification of GFAP-Negative Primitive Neural Stem Cells and Their Progeny at Clonal Resolution. Stem Cells Dev. 2023, 32, 606–621. [Google Scholar] [CrossRef] [PubMed]
- Coles-Takabe, B.L.K.; Brain, I.; Purpura, K.A.; Karpowicz, P.; Zandstra, P.W.; Morshead, C.M.; van der Kooy, D. Don’t Look: Growing Clonal versus Nonclonal Neural Stem Cell Colonies. Stem Cells Dayt. Ohio 2008, 26, 2938–2944. [Google Scholar] [CrossRef]
- Xu, W.; Sachewsky, N.; Azimi, A.; Hung, M.; Gappasov, A.; Morshead, C.M. Myelin Basic Protein Regulates Primitive and Definitive Neural Stem Cell Proliferation from the Adult Spinal Cord. Stem Cells Dayt. Ohio 2017, 35, 485–496. [Google Scholar] [CrossRef]
- Christie, K.; Turnley, A. Regulation of Endogenous Neural Stem/Progenitor Cells for Neural Repair—Factors That Promote Neurogenesis and Gliogenesis in the Normal and Damaged Brain. Front. Cell. Neurosci. 2013, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.F.; Larpthaveesarp, A.; McQuillen, P.; Derugin, N.; Wendland, M.; Spadafora, R.; Ferriero, D.M. Erythropoietin Increases Neurogenesis and Oligodendrogliosis of Subventricular Zone Precursor Cells After Neonatal Stroke. Stroke 2013, 44, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Kolb, B.; Morshead, C.; Gonzalez, C.; Kim, M.; Gregg, C.; Shingo, T.; Weiss, S. Growth Factor-Stimulated Generation of New Cortical Tissue and Functional Recovery after Stroke Damage to the Motor Cortex of Rats. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2007, 27, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Z.; Wang, Y.; Zhang, R.; Chopp, M. Treatment of Stroke with Erythropoietin Enhances Neurogenesis and Angiogenesis and Improves Neurological Function in Rats. Stroke 2004, 35, 1732–1737. [Google Scholar] [CrossRef] [PubMed]
- Erlandsson, A.; Lin, C.-H.A.; Yu, F.; Morshead, C.M. Immunosuppression Promotes Endogenous Neural Stem and Progenitor Cell Migration and Tissue Regeneration after Ischemic Injury. Exp. Neurol. 2011, 230, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Sachewsky, N.; Hunt, J.; Cooke, M.J.; Azimi, A.; Zarin, T.; Miu, C.; Shoichet, M.S.; Morshead, C.M. Cyclosporin A Enhances Neural Precursor Cell Survival in Mice through a Calcineurin-Independent Pathway. Dis. Model. Mech. 2014, 7, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Dadwal, P.; Mahmud, N.; Sinai, L.; Azimi, A.; Fatt, M.; Wondisford, F.E.; Miller, F.D.; Morshead, C.M. Activating Endogenous Neural Precursor Cells Using Metformin Leads to Neural Repair and Functional Recovery in a Model of Childhood Brain Injury. Stem Cell Rep. 2015, 5, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Derkach, D.; Kehtari, T.; Renaud, M.; Heidari, M.; Lakshman, N.; Morshead, C.M. Metformin Pretreatment Rescues Olfactory Memory Associated with Subependymal Zone Neurogenesis in a Juvenile Model of Cranial Irradiation. Cell Rep. Med. 2021, 2, 100231. [Google Scholar] [CrossRef]
- Wang, J.; Gallagher, D.; DeVito, L.M.; Cancino, G.I.; Tsui, D.; He, L.; Keller, G.M.; Frankland, P.W.; Kaplan, D.R.; Miller, F.D. Metformin Activates an Atypical PKC-CBP Pathway to Promote Neurogenesis and Enhance Spatial Memory Formation. Cell Stem Cell 2012, 11, 23–35. [Google Scholar] [CrossRef]
- Ruddy, R.M.; Adams, K.V.; Morshead, C.M. Age- and Sex-Dependent Effects of Metformin on Neural Precursor Cells and Cognitive Recovery in a Model of Neonatal Stroke. Sci. Adv. 2019, 5, eaax1912. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.; Ahlfors, J.-E.; Siu, R.; Noman, H.; Akbary, R.; Morshead, C.M. Inhibition of Apoptosis in a Model of Ischemic Stroke Leads to Enhanced Cell Survival, Endogenous Neural Precursor Cell Activation and Improved Functional Outcomes. Int. J. Mol. Sci. 2024, 25, 1786. [Google Scholar] [CrossRef] [PubMed]
- Tropepe, V.; Sibilia, M.; Ciruna, B.G.; Rossant, J.; Wagner, E.F.; Van Der Kooy, D. Distinct Neural Stem Cells Proliferate in Response to EGF and FGF in the Developing Mouse Telencephalon. Dev. Biol. 1999, 208, 166–188. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Renner, M.; Martin, C.A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral Organoids Model Human Brain Development and Microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Saberi, A.; Aldenkamp, A.P.; Kurniawan, N.A.; Bouten, C.V.C. In-Vitro Engineered Human Cerebral Tissues Mimic Pathological Circuit Disturbances in 3D. Commun. Biol. 2022, 5, 254. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, S.; Mukherjee, A.; Sepulveda, S.E.; Gherardelli, C.; Becerra-Calixto, A.; Bravo-Vasquez, N.; Soto, C. Protocol for Controlled Cortical Impact in Human Cerebral Organoids to Model Traumatic Brain Injury. STAR Protoc. 2021, 2, 100987. [Google Scholar] [CrossRef] [PubMed]
- Chiaradia, I.; Imaz-Rosshandler, I.; Nilges, B.S.; Boulanger, J.; Pellegrini, L.; Das, R.; Kashikar, N.D.; Lancaster, M.A. Tissue Morphology Influences the Temporal Program of Human Brain Organoid Development. Cell Stem Cell 2023, 30, 1351–1367.e10. [Google Scholar] [CrossRef] [PubMed]
- Camp, J.G.; Badsha, F.; Florio, M.; Kanton, S.; Gerber, T.; Wilsch-Bräuninger, M.; Lewitus, E.; Sykes, A.; Hevers, W.; Lancaster, M.; et al. Human Cerebral Organoids Recapitulate Gene Expression Programs of Fetal Neocortex Development. Proc. Natl. Acad. Sci. USA 2015, 112, 15672–15677. [Google Scholar] [CrossRef] [PubMed]
- Schuldiner, M.; Yanuka, O.; Itskovitz-Eldor, J.; Melton, D.A.; Benvenisty, N. Effects of Eight Growth Factors on the Differentiation of Cells Derived from Human Embryonic Stem Cells. Proc. Natl. Acad. Sci. USA 2000, 97, 11307–11312. [Google Scholar] [CrossRef]
- Yu, M.; Wei, Y.; Xu, K.; Liu, S.; Ma, L.; Pei, Y.; Hu, Y.; Liu, Z.; Zhang, X.; Wang, B.; et al. EGFR Deficiency Leads to Impaired Self-Renewal and Pluripotency of Mouse Embryonic Stem Cells. PeerJ 2019, 7, e6314. [Google Scholar] [CrossRef]
- Kook, M.G.; Lee, S.-E.; Shin, N.; Kong, D.; Kim, D.-H.; Kim, M.-S.; Kang, H.K.; Choi, S.W.; Kang, K.-S. Generation of Cortical Brain Organoid with Vascularization by Assembling with Vascular Spheroid. Int. J. Stem Cells 2022, 15, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Clarke, L.; van der Kooy, D. Low Oxygen Enhances Primitive and Definitive Neural Stem Cell Colony Formation by Inhibiting Distinct Cell Death Pathways. Stem Cells Dayt. Ohio 2009, 27, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- Eichmüller, O.L.; Knoblich, J.A. Human Cerebral Organoids—A New Tool for Clinical Neurology Research. Nat. Rev. Neurol. 2022, 18, 661–680. [Google Scholar] [CrossRef] [PubMed]
- Williamson, M.R.; Le, S.P.; Franzen, R.L.; Donlan, N.A.; Rosow, J.L.; Nicot-Cartsonis, M.S.; Cervantes, A.; Deneen, B.; Dunn, A.K.; Jones, T.A.; et al. Subventricular Zone Cytogenesis Provides Trophic Support for Neural Repair in a Mouse Model of Stroke. Nat. Commun. 2023, 14, 6341. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.G.; Tropepe, V.; Morshead, C.M.; Reynolds, B.A.; Weiss, S.; van der Kooy, D. In Vivo Growth Factor Expansion of Endogenous Subependymal Neural Precursor Cell Populations in the Adult Mouse Brain. J. Neurosci. Off. J. Soc. Neurosci. 1996, 16, 2649–2658. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Cai, J.; Kenyon, L.; Iozzo, R.; Rosenwasser, R.; Iacovitti, L. Systemic Factors Trigger Vasculature Cells to Drive Notch Signaling and Neurogenesis in Neural Stem Cells in the Adult Brain. Stem Cells 2019, 37, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Sefton, E.; Iwasa, S.N.; Morrison, T.; Naguib, H.E.; Popovic, M.R.; Morshead, C.M. Electric Field Application In Vivo Regulates Neural Precursor Cell Behavior in the Adult Mammalian Forebrain. eNeuro 2020, 7, ENEURO.0273-20.2020. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Choe, S.; Woo, H.; Jeong, H.; An, H.-K.; Moon, H.; Ryu, H.Y.; Yeo, B.K.; Lee, Y.W.; Choi, H.; et al. Autophagic Death of Neural Stem Cells Mediates Chronic Stress-Induced Decline of Adult Hippocampal Neurogenesis and Cognitive Deficits. Autophagy 2020, 16, 512–530. [Google Scholar] [CrossRef]
- Molnár, T.; Pallagi, P.; Tél, B.; Király, R.; Csoma, E.; Jenei, V.; Varga, Z.; Gogolák, P.; Odile Hueber, A.; Máté, Z.; et al. Caspase-9 Acts as a Regulator of Necroptotic Cell Death. FEBS J. 2021, 288, 6476–6491. [Google Scholar] [CrossRef] [PubMed]
- Cakir, B.; Park, I.-H. Getting the Right Cells. eLife 2022, 11, e80373. [Google Scholar] [CrossRef]
- Sato, Y.; Asahi, T.; Kataoka, K. Integrative Single-Cell RNA-Seq Analysis of Vascularized Cerebral Organoids. BMC Biol. 2023, 21, 245. [Google Scholar] [CrossRef] [PubMed]
- Acarin, L.; Villapol, S.; Faiz, M.; Rohn, T.T.; Castellano, B.; González, B. Caspase-3 Activation in Astrocytes Following Postnatal Excitotoxic Damage Correlates with Cytoskeletal Remodeling but Not with Cell Death or Proliferation. Glia 2007, 55, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Burguillos, M.A.; Deierborg, T.; Kavanagh, E.; Persson, A.; Hajji, N.; Garcia-Quintanilla, A.; Cano, J.; Brundin, P.; Englund, E.; Venero, J.L.; et al. Caspase Signalling Controls Microglia Activation and Neurotoxicity. Nature 2011, 472, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Boreland, A.J.; Li, X.; Erickson, C.; Jin, M.; Atkins, C.; Pang, Z.P.; Daniels, B.P.; Jiang, P. Developing Human Pluripotent Stem Cell-Based Cerebral Organoids with a Controllable Microglia Ratio for Modeling Brain Development and Pathology. Stem Cell Rep. 2021, 16, 1923–1937. [Google Scholar] [CrossRef] [PubMed]
- Fatt, M.; Hsu, K.; He, L.; Wondisford, F.; Miller, F.D.; Kaplan, D.R.; Wang, J. Metformin Acts on Two Different Molecular Pathways to Enhance Adult Neural Precursor Proliferation/Self-Renewal and Differentiation. Stem Cell Rep. 2015, 5, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Syal, C.; Kosaraju, J.; Hamilton, L.; Aumont, A.; Chu, A.; Sarma, S.N.; Thomas, J.; Seegobin, M.; Dilworth, F.J.; He, L.; et al. Dysregulated Expression of Monoacylglycerol Lipase Is a Marker for Anti-Diabetic Drug Metformin-Targeted Therapy to Correct Impaired Neurogenesis and Spatial Memory in Alzheimer’s Disease. Theranostics 2020, 10, 6337–6360. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Cheng, J.; Liu, Y.; Wu, J.; Wang, X.; Wei, S.; Zhou, X.; Qin, Z.; Jia, J.; Zhen, X. Improvement of Functional Recovery by Chronic Metformin Treatment Is Associated with Enhanced Alternative Activation of Microglia/Macrophages and Increased Angiogenesis and Neurogenesis Following Experimental Stroke. Brain. Behav. Immun. 2014, 40, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Venna, V.R.; Li, J.; Hammond, M.D.; Mancini, N.S.; McCullough, L.D. Chronic Metformin Treatment Improves Post-Stroke Angiogenesis and Recovery after Experimental Stroke. Eur. J. Neurosci. 2014, 39, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tang, G.; Zhang, Z.; Wang, Y.; Yang, G.-Y. Metformin Promotes Focal Angiogenesis and Neurogenesis in Mice Following Middle Cerebral Artery Occlusion. Neurosci. Lett. 2014, 579, 46–51. [Google Scholar] [CrossRef]
- Bourget, C.; Adams, K.V.; Morshead, C.M. Reduced Microglia Activation Following Metformin Administration or Microglia Ablation Is Sufficient to Prevent Functional Deficits in a Mouse Model of Neonatal Stroke. J. Neuroinflamm. 2022, 19, 146. [Google Scholar] [CrossRef]
- Du, M.-R.; Gao, Q.-Y.; Liu, C.-L.; Bai, L.-Y.; Li, T.; Wei, F.-L. Exploring the Pharmacological Potential of Metformin for Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 838173. [Google Scholar] [CrossRef]
- Dziedzic, A.; Saluk-Bijak, J.; Miller, E.; Bijak, M. Metformin as a Potential Agent in the Treatment of Multiple Sclerosis. Int. J. Mol. Sci. 2020, 21, 5957. [Google Scholar] [CrossRef]
- Islam, R.; Drecun, S.; Varga, B.V.; Vonderwalde, I.; Siu, R.; Nagy, A.; Morshead, C.M. Transplantation of Human Cortically-Specified Neuroepithelial Progenitor Cells Leads to Improved Functional Outcomes in a Mouse Model of Stroke. Front. Cell. Neurosci. 2021, 15, 654290. [Google Scholar] [CrossRef]
- Giandomenico, S.L.; Sutcliffe, M.; Lancaster, M.A. Generation and Long-Term Culture of Advanced Cerebral Organoids for Studying Later Stages of Neural Development. Nat. Protoc. 2020, 16, 579–602. [Google Scholar] [CrossRef]
- Qian, X.; Nguyen, H.N.; Song, M.M.; Hadiono, C.; Ogden, S.C.; Hammack, C.; Yao, B.; Hamersky, G.; Jacob, F.; Zhong, C.; et al. Brain Region-Specific Organoids Using Mini-Bioreactors for Modeling ZIKV Exposure. Cell 2016, 165, 1238–1254. [Google Scholar] [CrossRef]
- Qian, X.; Jacob, F.; Song, M.M.; Nguyen, H.N.; Song, H.; Ming, G.-L. Generation of Human Brain Region-Specific Organoids Using a Miniaturized Spinning Bioreactor. Nat. Protoc. 2018, 13, 565–580. [Google Scholar] [CrossRef]
- Vonderwalde, I.; Azimi, A.; Rolvink, G.; Ahlfors, J.-E.; Shoichet, M.S.; Morshead, C.M. Transplantation of Directly Reprogrammed Human Neural Precursor Cells Following Stroke Promotes Synaptogenesis and Functional Recovery. Transl. Stroke Res. 2020, 11, 93–107. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open Source Software for Digital Pathology Image Analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, R.; Noman, H.; Azimi, A.; Siu, R.; Chinchalongporn, V.; Schuurmans, C.; Morshead, C.M. Primitive and Definitive Neural Precursor Cells Are Present in Human Cerebral Organoids. Int. J. Mol. Sci. 2024, 25, 6549. https://doi.org/10.3390/ijms25126549
Islam R, Noman H, Azimi A, Siu R, Chinchalongporn V, Schuurmans C, Morshead CM. Primitive and Definitive Neural Precursor Cells Are Present in Human Cerebral Organoids. International Journal of Molecular Sciences. 2024; 25(12):6549. https://doi.org/10.3390/ijms25126549
Chicago/Turabian StyleIslam, Rehnuma, Humna Noman, Ashkan Azimi, Ricky Siu, Vorapin Chinchalongporn, Carol Schuurmans, and Cindi M. Morshead. 2024. "Primitive and Definitive Neural Precursor Cells Are Present in Human Cerebral Organoids" International Journal of Molecular Sciences 25, no. 12: 6549. https://doi.org/10.3390/ijms25126549





