The Waxy Gene Has Pleiotropic Effects on Hot Water-Soluble and -Insoluble Amylose Contents in Rice (Oryza sativa) Grains
Abstract
1. Introduction
2. Results
2.1. Variation in the TAC, SAC, and IAC in Two Sets of CSSLs
2.2. Mapping and Identifying QTLs for TAC, SAC, and IAC
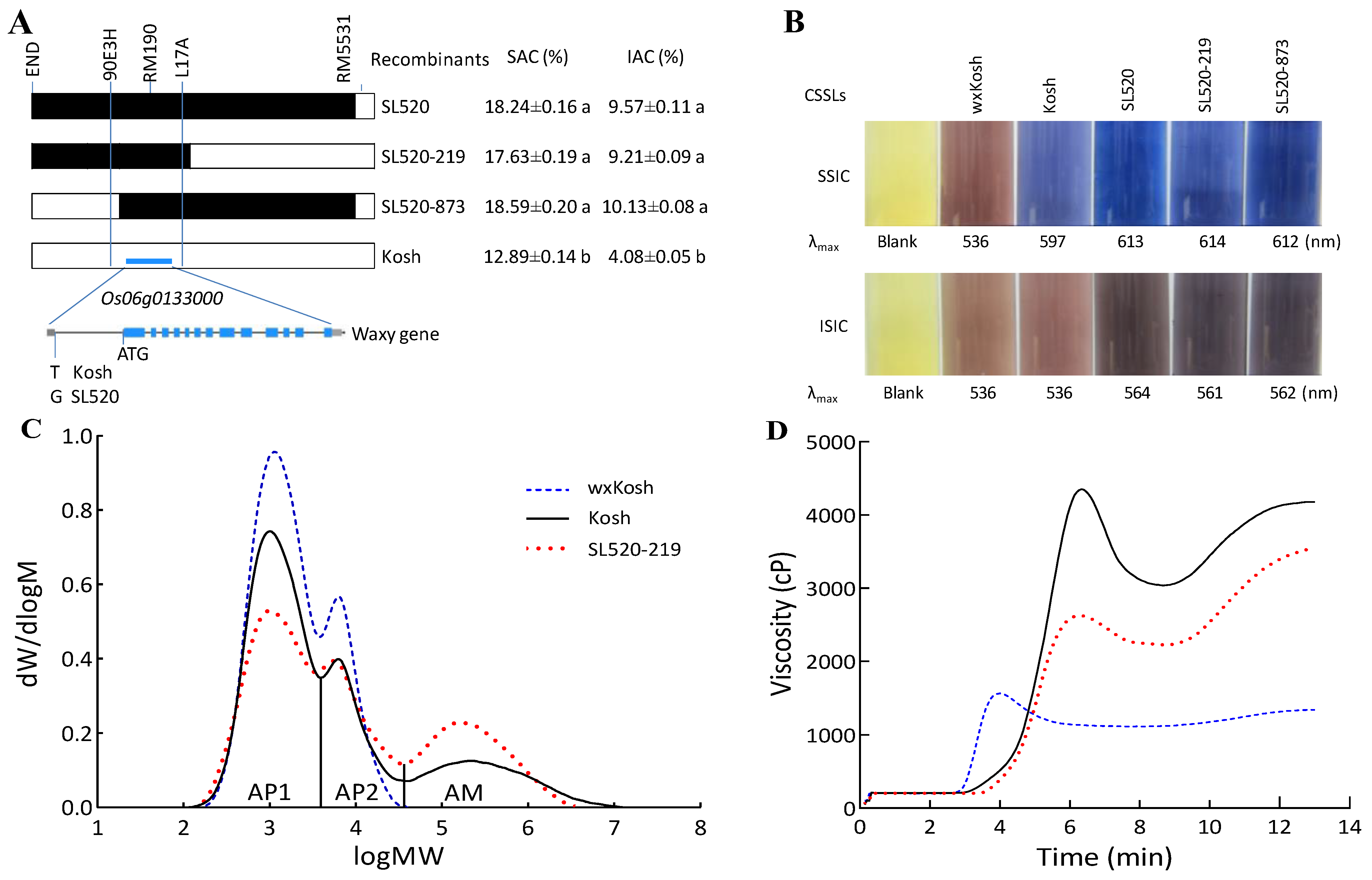
2.3. Compositions of the HWS and HWI Fractions of Starch
2.4. Relationship between SAC or IAC and Quality
3. Discussion
3.1. Amylose Content (AC) and Its Relationship with Rice Quality
3.2. AP Fine Structure and Its Relationship to Quality
3.3. Pleiotropy of Wx
4. Materials and Methods
4.1. Plant Materials
4.2. Separation of the HWS and HWI Fractions
4.3. Determination of the TAC, SAC, and IAC
4.4. QTL Analysis
4.5. Molecular Weight Distribution of Starch
4.6. Pasting Properties of Rice Flour
4.7. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramesh, M.; Bhattacharya, K.R.; Mitchell, J.R. Developments in Understanding the Basis of Cooked-Rice Texture. Crit. Rev. Food Sci. Nutr. 2000, 40, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Kong, X. Fine Structure of Amylose and Amylopectin. In Starch Structure, Functionality and Application in Foods; Wang, S., Ed.; Springer Nature Singapore Pte Ltd.: Singapore, 2020; pp. 29–39. [Google Scholar]
- Bhattacharya, K.R. Eating quality of rice. In Rice Quality; Bhattacharya, K.R., Ed.; Woodhead Publishing Limited: Sawston, UK, 2011; pp. 193–246. [Google Scholar]
- Bhattacharya, K.R.; Sowbhagya, C.M.; Swamy, Y.M.I. Interrelationship between Certain Physicochemical Properties of Rice. J. Food Sci. 1972, 37, 733–735. [Google Scholar] [CrossRef]
- Bhattacharya, K.R.; Sowbhagya, C.M.; Swamy, Y.M.I. Quality Profiles of Rice: A Tentative Scheme for Classification. J. Food Sci. 1982, 47, 564–569. [Google Scholar] [CrossRef]
- Sowbhagya, C.M.; Ramesh, B.S.; Bhattacharya, K.R. The relationship between cooked-rice texture and the physicochemical characteristics of rice. J. Cereal Sci. 1987, 5, 287–297. [Google Scholar] [CrossRef]
- Juliano, B.O.; Perdon, A.A. Gel and Molecular Properties of Waxy Rice Starch. Starch-Stärke 1975, 27, 69–71. [Google Scholar] [CrossRef]
- Kongseree, N.; Juliano, B.O. Physicochemical properties of rice grain and starch from lines differing in amylose content and gelatinization temperature. J. Agric. Food Chem. 1972, 20, 714–718. [Google Scholar] [CrossRef]
- Kumar, B.M.; Upadhyay, J.K.; Bhattacharya, K.R. Objective Tests for the Stickiness of Cooked Rice. J. Texture Stud. 1976, 7, 271–278. [Google Scholar] [CrossRef]
- Indudhara Swamy, Y.M.; Sowbhagya, C.M.; Bhattacharya, K.R. Changes in the physicochemical properties of rice with ageing. J. Sci. Food Agric. 1978, 29, 627–639. [Google Scholar] [CrossRef]
- Bhattacharya, K.R.; Sowbhagya, C.M.; Indudhara Swamy, Y.M. Importance of insoluble amylose as a determinant of rice quality. J. Sci. Food Agric. 1978, 29, 359–364. [Google Scholar] [CrossRef]
- Iturriaga, L.B.; Lopez de Mishima, B.; Añon, M.C. A study of the retrogradation process in five argentine rice starches. LWT—Food Sci. Technol. 2010, 43, 670–674. [Google Scholar] [CrossRef]
- He, P.; Li, S.G.; Qian, Q.; Ma, Y.Q.; Li, J.Z.; Wang, W.M.; Chen, Y.; Zhu, L.H. Genetic analysis of rice grain quality. Theor. Appl. Genet. 1999, 98, 502–508. [Google Scholar] [CrossRef]
- Tan, Y.F.; Li, J.X.; Yu, S.B.; Xing, Y.Z.; Xu, C.G.; Zhang, Q. The three important traits for cooking and eating quality of rice grains are controlled by a single locus in an elite rice hybrid, Shanyou 63. Theor. Appl. Genet. 1999, 99, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Zheng, F.Q.; Shen, G.Z.; Gao, J.P.; Snustad, D.P.; Li, M.G.; Zhang, J.L.; Hong, M.M. The amylose content in rice endosperm is related to the post-transcriptional regulation of the waxy gene. Plant J. 1995, 7, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Isshiki, M.; Nakajima, M.; Satoh, H.; Shimamoto, K. dull: Rice mutants with tissue-specific effects on the splicing of the waxy pre-mRNA. Plant J. 2001, 23, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Yan, M.; Wang, Y.; Liu, X.; Qian, Q.; Li, J. Du1, encoding a novel Prp1 protein, regulates starch biosynthesis through affecting the splicing of Wx b pre-mRNAs in rice (Oryza sativa L.). Plant Mol. Biol. 2007, 65, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Isshiki, M.; Matsuda, Y.; Takasaki, A.; Wong, H.L.; Satoh, H.; Shimamoto, K. Du3, a mRNA cap-binding protein gene, regulates amylose content in Japonica rice seeds. Plant Biotechnol. 2008, 25, 483–487. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, W.; Fu, Y.; Shan, Z.; Xu, J.; Wang, P.; Kong, F.; Jin, J.; Yan, H.; Ge, X.; et al. Du13 encodes a C2H2 zinc-finger protein that regulates Wxb pre-mRNA splicing and microRNA biogenesis in rice endosperm. Plant Biotechnol. J. 2022, 20, 1387–1401. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.L.; Wang, Z.Y.; Xing, Y.Y.; Zhang, J.L.; Hong, M.M. Aberrant splicing of intron 1 leads to the heterogeneous 5′ UTR and decreased expression of waxy gene in rice cultivars of intermediate amylose content. Plant J. 1998, 14, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Isshiki, M.; Morino, K.; Nakajima, M.; Okagaki, R.J.; Wessler, S.R.; Izawa, T.; Shimamoto, K. A naturally occurring functional allele of the rice waxy locus has a GT to TT mutation at the 5′ splice site of the first intron. Plant J. 1998, 15, 133–138. [Google Scholar] [CrossRef]
- Cai, X.L.; Liu, Q.Q.; Tang, S.Z.; Gu, M.H.; Wang, Z.Y. Development of a molecular marker for screening the rice cultivars with intermediate amylose content in Oryza sativa subsp. indica. J. Plant Physiol. Mol. Biol. 2002, 28, 137–144. [Google Scholar]
- Zhang, C.; Zhu, J.; Chen, S.; Fan, X.; Li, Q.; Lu, Y.; Wang, M.; Yu, H.; Yi, C.; Tang, S.; et al. Wx, the Ancestral Allele of Rice Waxy Gene. Mol. Plant 2019, 12, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Hizukuri, S.; Juliano, B.O. Purification and structure of amylose from rice starch. Carbohydr. Res. 1986, 148, 299–308. [Google Scholar] [CrossRef]
- Takeda, Y.; Hizukuri, S.; Juliano, B.O. Structures of rice amylopectins with low and high affinities for iodine. Carbohydr. Res. 1987, 168, 79–88. [Google Scholar] [CrossRef]
- Zhu, L.-J.; Liu, Q.-Q.; Sang, Y.; Gu, M.-H.; Shi, Y.-C. Underlying reasons for waxy rice flours having different pasting properties. Food Chem. 2010, 120, 94–100. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, S.; Ren, X.; Lu, Y.; Liu, D.; Cai, X.; Li, Q.; Gao, J.; Liu, Q. Molecular Structure and Physicochemical Properties of Starches from Rice with Different Amylose Contents Resulting from Modification of OsGBSSI Activity. J. Agric. Food Chem. 2017, 65, 2222–2232. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, L.; Shao, K.; Gu, M.; Liu, Q. Toward underlying reasons for rice starches having low viscosity and high amylose: Physiochemical and structural characteristics. J. Sci. Food Agric. 2012, 93, 1543–1551. [Google Scholar] [CrossRef]
- Mizukami, H.; Takeda, Y.; Hizukuri, S. The structure of the hot-water soluble components in the starch granules of new Japanese rice cultivars. Carbohydr. Polym. 1999, 38, 329–336. [Google Scholar] [CrossRef]
- Hanashiro, I.; Itoh, K.; Kuratomi, Y.; Yamazaki, M.; Igarashi, T.; Matsugasako, J.i.; Takeda, Y. Granule-Bound Starch Synthase I is Responsible for Biosynthesis of Extra-Long Unit Chains of Amylopectin in Rice. Plant Cell Physiol. 2008, 49, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, M.; Zakiuddin Ali, S.; Bhattacharya, K.R. Structure of rice starch and its relation to cooked-rice texture. Carbohydr. Polym. 1999, 38, 337–347. [Google Scholar] [CrossRef]
- Takeda, Y.; Maruta, N.; Hizukuri, S.; Juliano, B.O. Structures of indica rice starches (IR48 and IR64) having intermediate affinities for iodine. Carbohydr. Res. 1989, 187, 287–294. [Google Scholar] [CrossRef]
- Radhika Reddy, K.; Zakiuddin Ali, S.; Bhattacharya, K.R. The fine structure of rice-starch amylopectin and its relation to the texture of cooked rice. Carbohydr. Polym. 1993, 22, 267–275. [Google Scholar] [CrossRef]
- Hanashiro, I.; Matsugasako, J.-i.; Egashira, T.; Takeda, Y. Structural Characterization of Long Unit-Chains of Amylopectin. J. Appl. Glycosci. 2005, 52, 233–237. [Google Scholar] [CrossRef]
- Butardo, V.M.; Sreenivasulu, N.; Juliano, B.O. Improving Rice Grain Quality: State-of-the-Art and Future Prospects. In Rice Grain Quality: Methods and Protocols; Sreenivasulu, N., Ed.; Springer Science+Business Media, LLC; Springer Nature: New York, NY, USA, 2019; Volume 1892, pp. 19–55. [Google Scholar]
- Cuevas, R.P.; Gilbert, R.G.; Fitzgerald, M.A. Structural differences between hot-water-soluble and hot-water-insoluble fractions of starch in waxy rice (Oryza sativa L.). Carbohydr. Polym. 2010, 81, 524–532. [Google Scholar] [CrossRef]
- Chen, M.-H.; Bergman, C.J.; Pinson, S.R.M.; Fjellstrom, R.G. Waxy gene haplotypes: Associations with pasting properties in an international rice germplasm collection. J. Cereal Sci. 2008, 48, 781–788. [Google Scholar] [CrossRef]
- Williams, V.R.; Wu, W.-T.; Tsai, H.Y.; Bates, H.G. Rice Starch, Varietal Differences in Amylose Content of Rice Starch. J. Agric. Food Chem. 1958, 6, 47–48. [Google Scholar] [CrossRef]
- Kumar, I.; Khush, G.S. Genetics of amylose content in rice (Oryza sativa L). J. Genet. 1986, 65, 1–11. [Google Scholar] [CrossRef]
- Ong, M.H.; Blanshard, J.M.V. Texture determinants in cooked, parboiled rice. I: Rice starch amylose and the fine stucture of amylopectin. J. Cereal Sci. 1995, 21, 251–260. [Google Scholar] [CrossRef]
- James, M.G.; Denyer, K.; Myers, A.M. Starch synthesis in the cereal endosperm. Curr. Opin. Plant Biol. 2003, 6, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Hannah, L.C.; Greene, T. The Complexities of Starch Biosynthesis in Cereal Endosperms. In Molecular Genetic Approaches to Maize Improvement; Springer: Berlin/Heidelberg, Germany, 2009; Volume 63, pp. 287–301. [Google Scholar] [CrossRef]
- Tian, Z.; Qian, Q.; Liu, Q.; Yan, M.; Liu, X.; Yan, C.; Liu, G.; Gao, Z.; Tang, S.; Zeng, D.; et al. Allelic diversities in rice starch biosynthesis lead to a diverse array of rice eating and cooking qualities. Proc. Natl. Acad. Sci. USA 2009, 106, 21760–21765. [Google Scholar] [CrossRef]
- Fujita, N.; Yoshida, M.; Kondo, T.; Saito, K.; Utsumi, Y.; Tokunaga, T.; Nishi, A.; Satoh, H.; Park, J.-H.; Jane, J.-L.; et al. Characterization of SSIIIa-Deficient Mutants of Rice: The Function of SSIIIa and Pleiotropic Effects by SSIIIa Deficiency in the Rice Endosperm. Plant Physiol. 2007, 144, 2009–2023. [Google Scholar] [CrossRef]
- Nishi, A.; Nakamura, Y.; Tanaka, N.; Satoh, H. Biochemical and Genetic Analysis of the Effects ofAmylose-Extender Mutation in Rice Endosperm. Plant Physiol. 2001, 127, 459–472. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, C.; Zhang, Y.; Wang, C.; Liu, Q. Genetic manipulation of endosperm amylose for designing superior quality rice to meet the demands in the 21st century. J. Cereal Sci. 2022, 105, 103481. [Google Scholar] [CrossRef]
- Shanthy, A.P.; Sowbhagya, C.M.; Bhattacharya, K.R. Simplified Determination of Water-Insoluble Amylose Content of Rice. Starch-Stärke 1980, 32, 409–411. [Google Scholar] [CrossRef]
- EN ISO 6647-1:2020; Rice—Determination of Amylose Content—Part 1: Spectrophotometric Method with a Defatting Procedure by Methanol and with Calibration Solutions of Potato Amylose and Waxy Rice Amylopectin. European Standards Coordinating Committee: Brussels, Belgium, 2020.
- Meng, L.; Li, H.; Zhang, L.; Wang, J. QTL IciMapping: Integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. Crop J. 2015, 3, 269–283. [Google Scholar] [CrossRef]
- Xu, S.; Fei, Y.; Wang, Y.; Zhao, W.; Hou, L.; Cao, Y.; Wu, M.; Wu, H. Identification of a Seed Vigor–Related QTL Cluster Associated with Weed Competitive Ability in Direct–Seeded Rice (Oryza sativa L.). Rice 2023, 16, 45. [Google Scholar] [CrossRef]
- Feng, L.; Lu, C.; Yang, Y.; Lu, Y.; Li, Q.; Huang, L.; Fan, X.; Liu, Q.; Zhang, C. The Physicochemical Properties of Starch Are Affected by Wxlv in Indica Rice. Foods 2021, 10, 3089. [Google Scholar] [CrossRef]
- Wu, M.; Xu, S.; Fei, Y.; Cao, Y.; Wu, H.; Yamamoto, T. The rice (Oryza sativa) Rc gene improves resistance to preharvest sprouting and retains seed and milled rice quality. Plant Breed. 2020, 140, 53–64. [Google Scholar] [CrossRef]

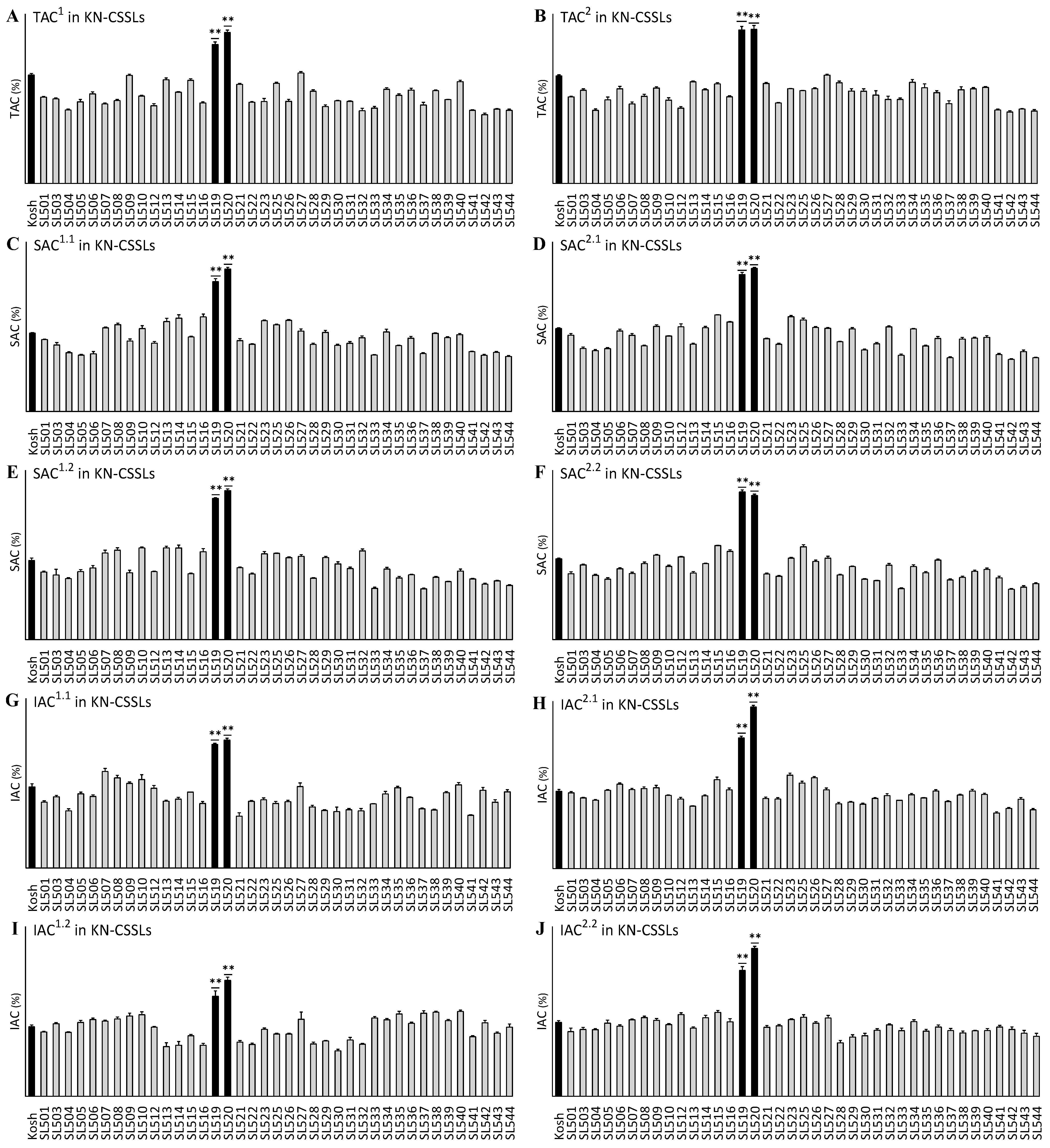
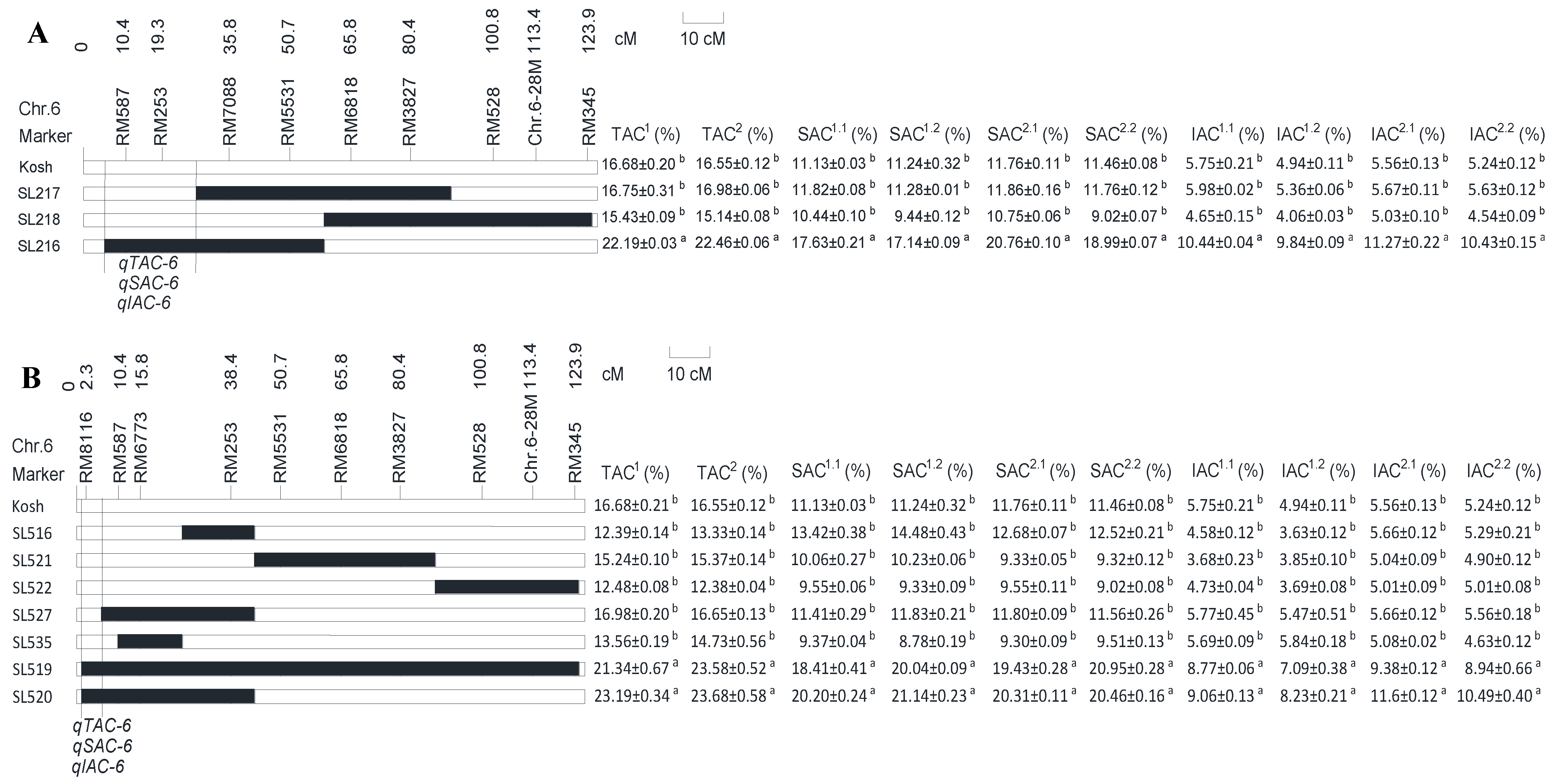
| Populations | Traits | Years | QTLs | Chr. | Intervals | LOD | M(QQ) (%) | M(qq) (%) | ADD (%) | PVE (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| KK-CSSL | TAC | 2016 | qTAC-6-1 | 6 | end-G200 | 3.04 | 22.19 | 14.04 | 4.07 | 41.7 |
| 2017 | qTAC-6-1 | 6 | end-G200 | 3.78 | 22.46 | 13.70 | 4.38 | 48.8 | ||
| SAC | 2016.1 | qSAC-6-1 | 6 | end-G200 | 3.61 | 17.63 | 9.95 | 3.84 | 47.2 | |
| 2016.2 | qSAC-6-1 | 6 | end-G200 | 3.15 | 17.14 | 9.74 | 3.70 | 42.7 | ||
| 2017.1 | qSAC-6-1 | 6 | end-G200 | 4.69 | 20.77 | 10.59 | 5.09 | 45.1 | ||
| 2017.2 | qSAC-6-1 | 6 | end-G200 | 3.71 | 18.99 | 10.07 | 4.46 | 37.8 | ||
| IAC | 2016.1 | qIAC-6-1 | 6 | end-G200 | 4.77 | 10.65 | 5.74 | 2.45 | 41.0 | |
| 2016.2 | qIAC-6-1 | 6 | end-G200 | 3.80 | 9.84 | 4.60 | 2.62 | 49.0 | ||
| 2017.1 | qIAC-6-1 | 6 | end-G200 | 10.57 | 11.98 | 5.91 | 3.03 | 66.0 | ||
| 2017.2 | qIAC-6-1 | 6 | end-G200 | 14.35 | 10.43 | 4.91 | 2.76 | 84.1 | ||
| KN-CSSL | TAC | 2016 | qTAC-6-2 | 6 | end-RM5963 | 6.37 | 22.27 | 13.02 | 4.62 | 57.8 |
| 2017 | qTAC-6-2 | 6 | end-RM5963 | 8.64 | 23.63 | 13.99 | 4.82 | 70.1 | ||
| SAC | 2016.1 | qSAC-6-2 | 6 | end-RM5963 | 4.32 | 19.30 | 10.18 | 4.56 | 44.3 | |
| 2016.2 | qSAC-6-2 | 6 | end-RM5963 | 4.56 | 20.59 | 10.02 | 5.29 | 46.1 | ||
| 2017.2 | qSAC-6-2 | 6 | end-RM5963 | 7.00 | 20.71 | 9.45 | 5.63 | 56.3 | ||
| 2017.1 | qSAC-6-2 | 6 | end-RM5963 | 5.34 | 19.87 | 9.93 | 4.97 | 46.8 | ||
| IAC | 2016.1 | qIAC-6-2 | 6 | end-RM5963 | 5.33 | 8.92 | 4.89 | 2.01 | 44.1 | |
| 2016.2 | qIAC-6-2 | 6 | end-RM5963 | 3.57 | 7.66 | 4.82 | 1.42 | 38.5 | ||
| 2017.1 | qIAC-6-2 | 6 | end-RM5963 | 14.28 | 11.15 | 5.80 | 2.67 | 75.5 | ||
| 2017.2 | qIAC-6-2 | 6 | end-RM5963 | 14.70 | 9.72 | 4.93 | 2.39 | 82.4 |
| NILs | AP1 | AP2 | AM | AP2/AP1 |
|---|---|---|---|---|
| wxKosh | 72.44 ± 0.48 a | 27.56 ± 0.48 a | 0 | 0.3804 ± 0.009 b |
| Kosh | 59.68 ± 0.37 b | 22.61 ± 0.43 c | 17.71 ± 0.06 b | 0.3788 ± 0.010 b |
| SL520-219 | 45.52 ± 0.46 c | 26.40 ± 0.20 b | 28.08 ± 0.96 a | 0.5799 ± 0.005 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Wang, S.; Wu, M. The Waxy Gene Has Pleiotropic Effects on Hot Water-Soluble and -Insoluble Amylose Contents in Rice (Oryza sativa) Grains. Int. J. Mol. Sci. 2024, 25, 6561. https://doi.org/10.3390/ijms25126561
Wu H, Wang S, Wu M. The Waxy Gene Has Pleiotropic Effects on Hot Water-Soluble and -Insoluble Amylose Contents in Rice (Oryza sativa) Grains. International Journal of Molecular Sciences. 2024; 25(12):6561. https://doi.org/10.3390/ijms25126561
Chicago/Turabian StyleWu, Hongkai, Siyuan Wang, and Min Wu. 2024. "The Waxy Gene Has Pleiotropic Effects on Hot Water-Soluble and -Insoluble Amylose Contents in Rice (Oryza sativa) Grains" International Journal of Molecular Sciences 25, no. 12: 6561. https://doi.org/10.3390/ijms25126561
APA StyleWu, H., Wang, S., & Wu, M. (2024). The Waxy Gene Has Pleiotropic Effects on Hot Water-Soluble and -Insoluble Amylose Contents in Rice (Oryza sativa) Grains. International Journal of Molecular Sciences, 25(12), 6561. https://doi.org/10.3390/ijms25126561






