Characterising Biological and Physiological Drought Signals in Diverse Parents of a Wheat Mapping Population
Abstract
:1. Introduction
2. Results
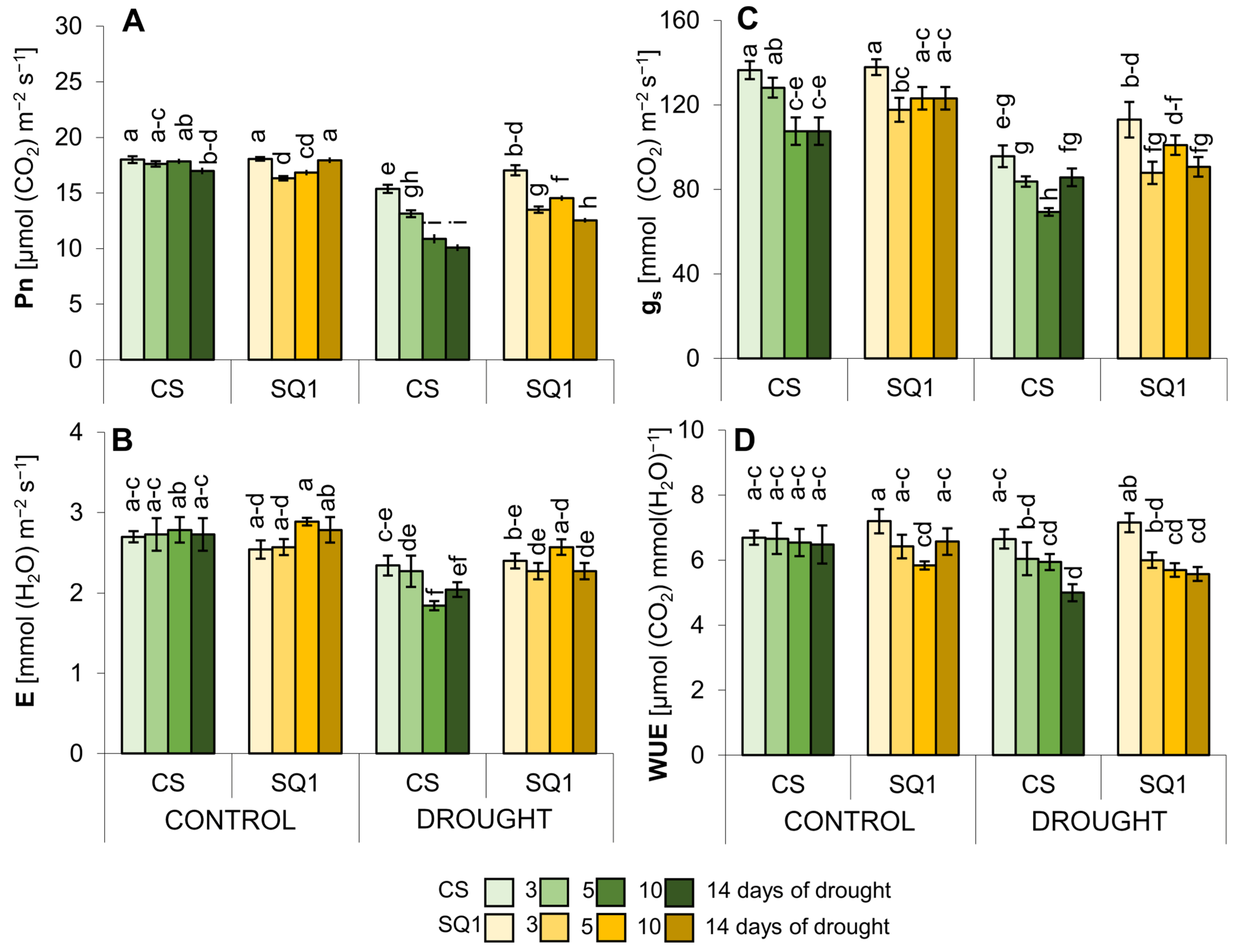
2.1. Physiological Changes
2.2. Biochemical Changes
3. Discussion
4. Materials and Methods
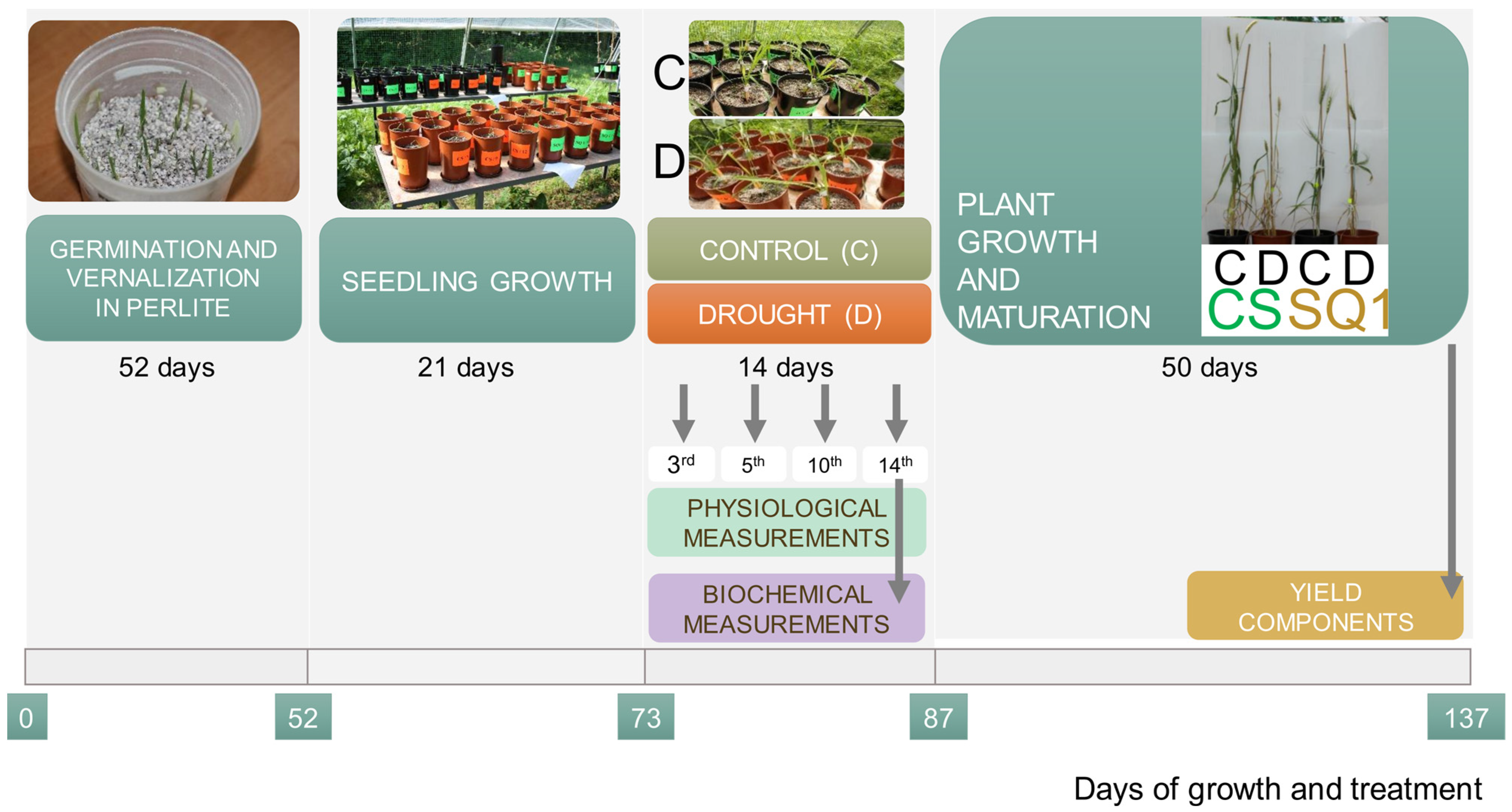
4.1. Plant Material
4.2. Growth Conditions
4.3. Measurements and Analysis
4.4. Physiological Parameters
4.5. Biochemical Parameters
4.6. Yield Components and Biomass
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ray, D.K.; Gerber, J.S.; Macdonald, G.K.; West, P.C. Climate variation explains a third of global crop yield variability. Nat. Commun. 2015, 6, 5989. [Google Scholar] [CrossRef] [PubMed]
- Piniewski, M.; Marcinkowski, P.; O’Keeffe, J.; Szcześniak, M.; Nieróbca, A.; Kozyra, J.; Kundzewicz, Z.W.; Okruszko, T. Model-based reconstruction and projections of soil moisture anomalies and crop losses in Poland. Theor. Appl. Climatol. 2020, 140, 691–708. [Google Scholar] [CrossRef]
- Savelli, E.; Rusca, M.; Cloke, H.; Di Baldassarre, G. Drought and society: Scientific progress, blind spots, and future prospects. WIREs Clim. Chang. 2022, 13, e761. [Google Scholar] [CrossRef]
- Zhang, X.; Hao, Z.; Singh, V.P.; Zhang, Y.; Feng, S.; Xu, Y.; Hao, F. Drought propagation under global warming: Characteristics, approaches, processes, and controlling factors. Sci. Total Environ. 2022, 838, 156021. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Cheng, M.; Jiang, H.; Zhang, X.; Peng, C.; Lu, X.; Zhang, M.; Jin, J. Effect of Drought on Agronomic Traits of Rice and Wheat: A Meta-Analysis. Int. J. Environ. Res. Public Health 2018, 15, 839. [Google Scholar] [CrossRef]
- Cohen, I.; Zandalinas, S.I.; Huck, C.; Fritschi, F.B.; Mittler, R. Meta-analysis of drought and heat stress combination impact on crop yield and yield components. Physiol. Plant. 2021, 171, 66–76. [Google Scholar] [CrossRef]
- Daryanto, S.; Wang, L.; Jacinthe, P. Global Synthesis of Drought Effects on Food Legume Production. PLoS ONE 2015, 10, e0127401. [Google Scholar] [CrossRef] [PubMed]
- Demirevska, K.; Zasheva, D.; Dimitrov, R.; Simova-Stoilova, L.; Stamenova, M.; Feller, U. Drought stress effects on Rubisco in wheat: Changes in the Rubisco large subunit. Acta Physiol. Plant. 2009, 31, 1129–1138. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Ahmad, Z.; Waraich, E.A.; Akhtar, S.; Anjum, S.; Ahmad, T.; Mahboob, W.; Hafeez, O.B.A.; Tapera, T.; Labuschagne, M.; Rizwan, M. Physiological responses of wheat to drought stress and its mitigation approaches. Acta Physiol. Plant. 2018, 40, 80. [Google Scholar] [CrossRef]
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant. 2021, 172, 1321–1335. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Lam, H.-M.; Zhang, J. Responses in gas exchange and water status between drought-tolerant and -susceptible soybean genotypes with ABA application. Crop J. 2015, 3, 500–506. [Google Scholar] [CrossRef]
- Rao, D.E.; Chaitanya, K. V Photosynthesis and antioxidative defense mechanisms in deciphering drought stress tolerance of crop plants. Biol. Plant. 2016, 60, 201–218. [Google Scholar] [CrossRef]
- Lachman, J.; Miholová, D.; Pivec, V.; Jírů, K.; Janovská, D. Content of phenolic antioxidants and selenium in grain of einkorn (Triticum monococcum), emmer (Triticum dicoccum) and spring wheat (Triticum aestivum) varieties. Plant Soil Environ. 2011, 57, 235–243. [Google Scholar] [CrossRef]
- Lachman, J.; Orsák, M.; Pivec, V.; Jírů, K. Antioxidant activity of grain of einkorn (Triticum monococcum L.), emmer (Triticum dicoccum Schuebl [Schrank]) and spring wheat (Triticum aestivum L.) varieties. Plant Soil Environ. 2012, 58, 15–21. [Google Scholar] [CrossRef]
- Hura, T.; Grzesiak, S.; Hura, K.; Thiemt, E.; Tokarz, K.; Wȩdzony, M. Physiological and biochemical tools useful in drought-tolerance detection in genotypes of winter triticale: Accumulation of ferulic acid correlates with drought tolerance. Ann. Bot. 2007, 100, 767–775. [Google Scholar] [CrossRef]
- Hura, T.; Hura, K.; Grzesiak, S. Leaf dehydration induces different content of phenolics and ferulic acid in drought-resistant and -sensitive genotypes of spring triticale. Z. Naturforsch Sect. C J. Biosci. 2009, 64, 85–95. [Google Scholar] [CrossRef]
- Hura, T.; Hura, K.; Dziurka, K.; Ostrowska, A.; Ba̧czek, R.; Grzesiak, M. An increase in the content of cell wall-bound phenolics correlates with the productivity of triticale under soil drought. J. Plant Physiol. 2012, 169, 1728–1736. [Google Scholar] [CrossRef]
- Hura, K.; Ostrowska, A.; Dziurka, K.; Hura, T. Photosynthetic apparatus activity in relation to high and low contents of cell wall-bound phenolics in triticale under drought stress. Photosynthetica 2017, 55, 698–704. [Google Scholar] [CrossRef]
- Hura, T.; Hura, K.; Ostrowska, A.; Urban, K. Toward resilient agriculture and environmental protection: The role of cell wall-bound phenolics. J. Plant Physiol. 2023, 287, 154020. [Google Scholar] [CrossRef] [PubMed]
- Hura, T.; Hura, K.; Ostrowska, A.; Grzesiak, M.; Dziurka, K. The cell wall-bound phenolics as a biochemical indicator of soil drought resistance in winter triticale. Plant Soil Environ. 2013, 59, 189–195. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant Drought Stress: Effects, Mechanisms and Management. In Sustainable Agriculture; Springer: Dordrecht, The Netherlands, 2009; pp. 153–188. ISBN 9789048126651. [Google Scholar]
- Zhang, J.; Jia, W.; Yang, J.; Ismail, A.M. Role of ABA in integrating plant responses to drought and salt stresses. Field Crop. Res. 2006, 97, 111–119. [Google Scholar] [CrossRef]
- Anjum, S.; Xie, X.; Wang, L. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar] [CrossRef]
- Nazir, Y.; Halim, H.; Prabhakaran, P.; Ren, X.; Naz, T.; Mohamed, H.; Nosheen, S.; Mustafa, K.; Yang, W.; Abdul Hamid, A.; et al. Different classes of phytohormones act synergistically to enhance the growth, lipid and DHA biosynthetic capacity of Aurantiochytrium sp. SW1. Biomolecules 2020, 10, 755. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.; Munné-Bosch, S. Malondialdehyde: Facts and artifacts. Plant Physiol. 2019, 180, 1246–1250. [Google Scholar] [CrossRef] [PubMed]
- Pospíšilová, J. Participation of phytohormones in the stomatal regulation of gas exchange during water stress. Biol. Plant. 2003, 46, 491–506. [Google Scholar] [CrossRef]
- Wilkinson, S.; Davies, W.J. ABA-based chemical signalling: The coordination of responses to stress in plants. Plant. Cell Environ. 2002, 25, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Daszkowska-Golec, A. The Role of Abscisic Acid in Drought Stress: How ABA Helps Plants to Cope with Drought Stress. In Drought Stress Tolerance in Plants; Hossain, M.A., Wani, S.H., Bhattacharjee, S., Burritt, D.J., Tran, L.-S.P., Eds.; Springer: Cham, Switzerland, 2016; Volume 2, pp. 123–151. ISBN 978-3-319-32421-0. [Google Scholar]
- Muhammad Aslam, M.; Waseem, M.; Jakada, B.H.; Okal, E.J.; Lei, Z.; Saqib, H.S.A.; Yuan, W.; Xu, W.; Zhang, Q. Mechanisms of Abscisic Acid-Mediated Drought Stress Responses in Plants. Int. J. Mol. Sci. 2022, 23, 1084. [Google Scholar] [CrossRef]
- Sarkar, A.K.; Lahiri, A. Specificity determinants for the abscisic acid response element. FEBS Open Bio 2013, 3, 101–105. [Google Scholar] [CrossRef]
- Najafian, S.; Khoshkhui, M.; Tavallali, V.; Saharkhiz, M.J. Effect of salicylic acid and salinity in thyme (Thymus vulgaris L.): Investigation on changes in gas exchange, water relations, and membrane stabilization and biomass accumulation. Aust. J. Basic Appl. Sci. 2009, 3, 2620–2626. [Google Scholar]
- Sahu, G.K.; Sabat, S.C. Changes in growth, pigment content and antioxidants in the root and leaf tissues of wheat plants under the influence of exogenous salicylic acid. Braz. J. Plant Physiol. 2011, 23, 209–218. [Google Scholar] [CrossRef]
- Okamoto, M.; Tsuboi, Y.; Chikayama, E.; Kikuchi, J.; Hirayama, T. Metabolic movement upon abscisic acid and salicylic acid combined treatments. Plant Biotechnol. 2009, 26, 551–560. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Waraich, E.A.; Ahmad, R.; Saifullah; Ahmad, A. Water stress and nitrogen management effects on gas exchange, water relations, and water use efficiency in wheat. J. Plant Nutr. 2011, 34, 1867–1882. [Google Scholar] [CrossRef]
- Budak, H.; Kantar, M.; Yucebilgili Kurtoglu, K. Drought tolerance in modern and wild wheat. Sci. World J. 2013, 2013, 548246. [Google Scholar] [CrossRef] [PubMed]
- Pál, M.; Tajti, J.; Szalai, G.; Peeva, V.; Végh, B.; Janda, T. Interaction of polyamines, abscisic acid and proline under osmotic stress in the leaves of wheat plants. Sci. Rep. 2018, 8, 12839. [Google Scholar] [CrossRef]
- Maiale, S.; Sánchez, D.H.; Guirado, A.; Vidal, A.; Ruiz, O.A. Spermine accumulation under salt stress. J. Plant Physiol. 2004, 161, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Huang, Z. Effects of endogenous abscisic acid, jasmonic acid, polyamines, and polyamine oxidase activity in tomato seedlings under drought stress. Sci. Hortic. 2013, 159, 172–177. [Google Scholar] [CrossRef]
- Capell, T.; Bassie, L.; Christou, P. Modulation of the polyamine biosynthetic pathway in transgenic rice confers tolerance to drought stress. Proc. Natl. Acad. Sci. USA 2004, 101, 9909–9914. [Google Scholar] [CrossRef]
- Alcázar, R.; Marco, F.; Cuevas, J.C.; Patron, M.; Ferrando, A.; Carrasco, P.; Tiburcio, A.F.; Altabella, T. Involvement of polyamines in plant response to abiotic stress. Biotechnol. Lett. 2006, 28, 1867–1876. [Google Scholar] [CrossRef]
- Liu, J.-H. Polyamine biosynthesis of apple callus under salt stress: Importance of the arginine decarboxylase pathway in stress response. J. Exp. Bot. 2006, 57, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Ponappa, T.; Scheerens, J.C.; Miller, A.R. Vacuum infiltration of polyamines increases firmness of strawberry slices under various storage conditions. J. Food Sci. 1993, 58, 361–364. [Google Scholar] [CrossRef]
- Cohen, S.S. Introduction to the Polyamines; Prentice-Hall: Englewood Cliffs, NJ, USA, 1971. [Google Scholar]
- Moschou, P.N.; Wu, J.; Cona, A.; Tavladoraki, P.; Angelini, R.; Roubelakis-Angelakis, K.A. The polyamines and their catabolic products are significant players in the turnover of nitrogenous molecules in plants. J. Exp. Bot. 2012, 63, 5003–5015. [Google Scholar] [CrossRef]
- Duan, B.; Yang, Y.; Lu, Y.; Korpelainen, H.; Berninger, F.; Li, C. Interactions between water deficit, ABA, and provenances in Picea asperata. J. Exp. Bot. 2007, 58, 3025–3036. [Google Scholar] [CrossRef]
- Lizana, C.; Wentworth, M.; Martinez, J.P.; Villegas, D.; Meneses, R.; Murchie, E.H.; Pastenes, C.; Lercari, B.; Vernieri, P.; Horton, P.; et al. Differential adaptation of two varieties of common bean to abiotic stress. J. Exp. Bot. 2006, 57, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Quarrie, S.A.; Steed, A.; Calestani, C.; Semikhodskii, A.; Lebreton, C.; Chinoy, C.; Steele, N.; Pljevljakusić, D.; Waterman, E.; Weyen, J.; et al. A high-density genetic map of hexaploid wheat (Triticum aestivum L.) from the cross Chinese Spring x SQ1 and its use to compare QTLs for grain yield across a range of environments. Theor. Appl. Genet. 2005, 110, 865–880. [Google Scholar] [CrossRef]
- Quarrie, S.; Pekic Quarrie, S.; Radosevic, R.; Rancic, D.; Kaminska, A.; Barnes, J.; Leverington, M.; Ceoloni, C.; Dodig, D. Dissecting a wheat QTL for yield present in a range of environments: From the QTL to candidate genes. J. Exp. Bot. 2006, 57, 2627–2637. [Google Scholar] [CrossRef] [PubMed]
- Czyczyło-Mysza, I.; Marcińska, I.; Skrzypek, E.; Chrupek, M.; Grzesiak, S.; Hura, T.; Stojałowski, S.; Myśków, B.; Milczarski, P.; Quarrie, S. Mapping QTLs for yield components and chlorophyll a fluorescence parameters in wheat under three levels of water availability. Plant Genet. Resour. 2011, 9, 291–295. [Google Scholar] [CrossRef]
- Czyczyło-Mysza, I.M.; Cyganek, K.; Dziurka, K.; Quarrie, S.; Skrzypek, E.; Marcińska, I.; Myśków, B.; Dziurka, M.; Warchoł, M.; Kapłoniak, K.; et al. Genetic parameters and QTLs for total phenolic content and yield of wheat mapping populationof CSDH lines under drought stress. Int. J. Mol. Sci. 2019, 20, 6064. [Google Scholar] [CrossRef]
- Appels, R.; Eversole, K.; Stein, N.; Feuillet, C.; Keller, B.; Rogers, J.; Pozniak, C.J.; Choulet, F.; Distelfeld, A.; Poland, J.; et al. Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 2018, 361, eaar7191. [Google Scholar] [CrossRef] [PubMed]
- Quarrie, S.A. Genetic variability and heritability of drought-induced abscisic acid accumulation in spring wheat. Plant. Cell Environ. 1981, 4, 147–151. [Google Scholar] [CrossRef]
- Semikhodskii, A.G. Mapping quantitative traits for salinity responses in wheat (Triticum aestivum L.). Ph.D. Thesis, University of East Anglia, Norwich, UK, 1997. Available online: https://ethos.bl.uk/OrderDetails.do?uin=uk.bl.ethos.302054 (accessed on 9 April 2024).
- Quarrie, S.A. Implications of genetic differences in ABA accumulation for crop production. In Abscisic Acid: Physiology and Biochemistry; Davies, W., Jones, H., Eds.; BIOS Scientific Publishers Ltd.: Oxford, UK, 1991; pp. 227–243. [Google Scholar]
- Nergui, K.; Jin, S.; Zhao, L.; Liu, X.; Xu, T.; Wei, J.; Chen, X.; Yang, Y.; Li, H.; Liu, Y.; et al. Comparative analysis of physiological, agronomic and transcriptional responses to drought stress in wheat local varieties from Mongolia and Northern China. Plant Physiol. Biochem. 2022, 170, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Marcińska, I.; Czyczyło-Mysza, I.; Skrzypek, E.; Filek, M.; Grzesiak, S.; Grzesiak, M.T.; Janowiak, F.; Hura, T.; Dziurka, M.; Dziurka, K.; et al. Impact of osmotic stress on physiological and biochemical characteristics in drought-susceptible and drought-resistant wheat genotypes. Acta Physiol. Plant. 2013, 35, 451–461. [Google Scholar] [CrossRef]
- Innes, P.; Blackwell, R.D.; Quarrie, S.A. Some effects of genetic variation in drought-induced abscisic acid accumulation on the yield and water use of spring wheat. J. Agric. Sci. 1984, 102, 341–351. [Google Scholar] [CrossRef]
- Schurr, U.; Walter, A.; Rascher, U. Functional dynamics of plant growth and photosynthesis—From steady-state to dynamics—From homogeneity to heterogeneity. Plant. Cell Environ. 2006, 29, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhou, G. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J. Exp. Bot. 2008, 59, 3317–3325. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.V.V.; Pisipati, S.R.; Momčilović, I.; Ristic, Z. Independent and combined effects of high temperature and drought stress during grain filling on plant yield and chloroplast EF-Tu expression in spring wheat. J. Agron. Crop Sci. 2011, 197, 430–441. [Google Scholar] [CrossRef]
- Cruz De Carvalho, M.H. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef]
- Xiong, L.; Yang, J.; Jiang, Y.; Lu, B.; Hu, Y.; Zhou, F.; Mao, S.; Shen, C. Phenolic compounds and antioxidant capacities of 10 common edible flowers from China. J. Food Sci. 2014, 79, C517–C525. [Google Scholar] [CrossRef]
- Nounjan, N.; Chansongkrow, P.; Charoensawan, V.; Siangliw, J.L.; Toojinda, T.; Chadchawan, S.; Theerakulpisut, P. High performance of photosynthesis and osmotic adjustment are associated with salt tolerance ability in rice carrying drought tolerance QTL: Physiological and co-expression network analysis. Front. Plant Sci. 2018, 9, 1135. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Du, G.; Li, X.; Zhang, C.; Guo, J. A major locus controlling malondialdehyde content under water stress is associated with Fusarium crown rot resistance in wheat. Mol. Genet. Genom. 2015, 290, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Khoubnasabjafari, M.; Ansarin, K.; Jouyban, A. Reliability of malondialdehyde as a biomarker of oxidative stress in psychological disorders. BioImpacts 2015, 5, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; King, R.W. Abscisic acid is not the only stomatal inhibitor in the transpiration stream of wheat plants. Plant Physiol. 1988, 88, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.-L.; Wang, Z.-Y.; Fan, J.-W.; Turner, N.C.; He, J.; Wang, T.; Li, F.-M. Exogenous abscisic acid reduces water loss and improves antioxidant defence, desiccation tolerance and transpiration efficiency in two spring wheat cultivars subjected to a soil water deficit. Funct. Plant Biol. 2013, 40, 494. [Google Scholar] [CrossRef] [PubMed]
- Quarrie, S.A.; Jones, H.G. Effects of abscisic acid and water stress on development and morphology of mheat. J. Exp. Bot. 1977, 28, 192–203. [Google Scholar] [CrossRef]
- Rosyara, U.R.; Ghimire, A.A.; Subedi, S.; Sharma, R.C. Variation in south Asian wheat germplasm for seedling drought tolerance traits. Plant Genet. Resour. 2009, 7, 88–93. [Google Scholar] [CrossRef]
- Munns, R.; Brady, C.; Barlow, E. Solute accumulation in the apex and leaves of wheat during water stress. Funct. Plant Biol. 1979, 6, 379. [Google Scholar] [CrossRef]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savouré, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef]
- Bouchereau, A.; Aziz, A.; Larher, F.; Martin-Tanguy, J. Polyamines and environmental challenges: Recent development. Plant Sci. 1999, 140, 103–125. [Google Scholar] [CrossRef]
- Ebeed, H.T.; Hassan, N.M.; Aljarani, A.M. Exogenous applications of Polyamines modulate drought responses in wheat through osmolytes accumulation, increasing free polyamine levels and regulation of polyamine biosynthetic genes. Plant Physiol. Biochem. 2017, 118, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Pang, J.; Meng, F.; Li, Y.; Xu, N.; Yang, C.; Liu, J. Putrescine, spermidine, and spermine play distinct roles in rice salt tolerance. J. Integr. Agric. 2020, 19, 643–655. [Google Scholar] [CrossRef]
- Hassan, N.; Ebeed, H.; Aljaarany, A. Exogenous application of spermine and putrescine mitigate adversities of drought stress in wheat by protecting membranes and chloroplast ultra-structure. Physiol. Mol. Biol. Plants 2020, 26, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline mechanisms of stress survival. Antioxid. Redox Signal. 2013, 19, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Treichel, S.; Brinckmann, E.; Scheitler, B.; von Willert, D.J. Occurrence and changes of proline content in plants in the southern Namib Desert in relations to increasing and decreasing drought. Planta 1984, 162, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Batanouny, K.H.; Hassan, A.H.; Zayed, K.M. Proline Accumulation In Plants Of Different Ecological Groups As A Response To Water Deficit. Qatar Univ. Sci. J. 1985, 5, 131–143. [Google Scholar]
- Abreu, M.E.; Munné-Bosch, S. Salicylic acid may be involved in the regulation of drought-induced leaf senescence in perennials: A case study in field-grown Salvia officinalis L. plants. Environ. Exp. Bot. 2008, 64, 105–112. [Google Scholar] [CrossRef]
- Malaga, S.; Janeczko, A.; Janowiak, F.; Waligórski, P.; Oklestkova, J.; Dubas, E.; Krzewska, M.; Nowicka, A.; Surówka, E.; Rapacz, M.; et al. Involvement of homocastasterone, salicylic and abscisic acids in the regulation of drought and freezing tolerance in doubled haploid lines of winter barley. Plant Growth Regul. 2020, 90, 173–188. [Google Scholar] [CrossRef]
- Hsu, P.; Dubeaux, G.; Takahashi, Y.; Schroeder, J.I. Signaling mechanisms in abscisic acid-mediated stomatal closure. Plant J. 2021, 105, 307–321. [Google Scholar] [CrossRef]
- Liu, W.J.; Yuan, S.; Zhang, N.H.; Lei, T.; Duan, H.G.; Liang, H.G.; Lin, H.H. Effect of water stress on photosystem 2 in two wheat cultivars. Biol. Plant. 2006, 50, 597–602. [Google Scholar] [CrossRef]
- Boyer, J.S. Grain yields with limited water. J. Exp. Bot. 2004, 55, 2385–2394. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Shiran, B.; Wan, J.; Lewis, D.C.; Jenkins, C.L.D.; Condon, A.G.; Richards, R.A.; Dolferus, R. Importance of pre-anthesis anther sink strength for maintenance of grain number during reproductive stage water stress in wheat. Plant Cell Environ. 2010, 33, 926–942. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Huang, X.; Sun, W.; Du, C.; Wang, C.; Xie, Y.; Ma, Y.; Ma, D. Accumulation of water-soluble carbohydrates and gene expression in wheat stems correlates with drought resistance. J. Plant Physiol. 2018, 231, 182–191. [Google Scholar] [CrossRef]
- Monneveux, P.; Jing, R.; Misra, S.C. Phenotyping for drought adaptation in wheat using physiological traits. Front. Physiol. 2012, 3, 429. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1938, 347, 32. [Google Scholar]
- Marcińska, I.; Dziurka, K.; Waligórski, P.; Janowiak, F.; Skrzypek, E.; Warchoł, M.; Juzoń, K.; Kapłoniak, K.; Czyczyło-Mysza, I.M. Exogenous polyamines only indirectly induce stress tolerance in wheat growing in hydroponic culture under polyethylene glycol-induced osmotic stress. Life 2020, 10, 151. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, P. A colorimetric method for the determination of sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Wilbert, S.M.; Ericsson, L.H.; Gordon, M.P. Quantification of jasmonic acid, methyl jasmonate, and salicylic acid in plants by capillary liquid chromatography electrospray tandem mass spectrometry. Anal. Biochem. 1998, 257, 186–194. [Google Scholar] [CrossRef]
- Dziurka, M.; Janeczko, A.; Juhász, C.; Gullner, G.; Oklestková, J.; Novák, O.; Saja, D.; Skoczowski, A.; Tóbiás, I.; Barna, B. Local and systemic hormonal responses in pepper leaves during compatible and incompatible pepper-tobamovirus interactions. Plant Physiol. Biochem. 2016, 109, 355–364. [Google Scholar] [CrossRef]
- Walker-Simmos, M.K.; Abrams, S.R. Use of ABA immunoassays. In Abscisic Acid, Physiology and Biochemistry; Davies, W.J., Jones, H., Eds.; Bios Scientific Publishers: Oxford, UK, 1991; pp. 53–63. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Laskoś, K.; Pisulewska, E.; Waligórski, P.; Janowiak, F.; Janeczko, A.; Sadura, I.; Polaszczyk, S.; Czyczyło-Mysza, I.M. Herbal Additives Substantially Modify Antioxidant Properties and Tocopherol Content of Cold-Pressed Oils. Antioxidants 2021, 10, 781. [Google Scholar] [CrossRef]
- Marcińska, I.; Czyczyło-Mysza, I.; Skrzypek, E.; Grzesiak, M.T.; Janowiak, F.; Filek, M.; Dziurka, M.; Dziurka, K.; Waligórski, P.; Juzoń, K.; et al. Alleviation of osmotic stress effects by exogenous application of salicylic or abscisic acid on wheat seedlings. Int. J. Mol. Sci. 2013, 14, 13171–13193. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laskoś, K.; Czyczyło-Mysza, I.M.; Waligórski, P.; Dziurka, K.; Skrzypek, E.; Warchoł, M.; Juzoń-Sikora, K.; Janowiak, F.; Dziurka, M.; Grzesiak, M.T.; et al. Characterising Biological and Physiological Drought Signals in Diverse Parents of a Wheat Mapping Population. Int. J. Mol. Sci. 2024, 25, 6573. https://doi.org/10.3390/ijms25126573
Laskoś K, Czyczyło-Mysza IM, Waligórski P, Dziurka K, Skrzypek E, Warchoł M, Juzoń-Sikora K, Janowiak F, Dziurka M, Grzesiak MT, et al. Characterising Biological and Physiological Drought Signals in Diverse Parents of a Wheat Mapping Population. International Journal of Molecular Sciences. 2024; 25(12):6573. https://doi.org/10.3390/ijms25126573
Chicago/Turabian StyleLaskoś, Kamila, Ilona Mieczysława Czyczyło-Mysza, Piotr Waligórski, Kinga Dziurka, Edyta Skrzypek, Marzena Warchoł, Katarzyna Juzoń-Sikora, Franciszek Janowiak, Michał Dziurka, Maciej T. Grzesiak, and et al. 2024. "Characterising Biological and Physiological Drought Signals in Diverse Parents of a Wheat Mapping Population" International Journal of Molecular Sciences 25, no. 12: 6573. https://doi.org/10.3390/ijms25126573








