The Difference in Serum Metabolomic Profiles between the Good and Poor Outcome Groups at 3 Months in the Early and Late Phases of Aneurysmal Subarachnoid Hemorrhage
Abstract
1. Introduction
2. Results
2.1. Patients’ Characteristics
2.2. Comparison of Serum Metabolomic Profiles According to Early and Late Sampling Times in aSAH Patients
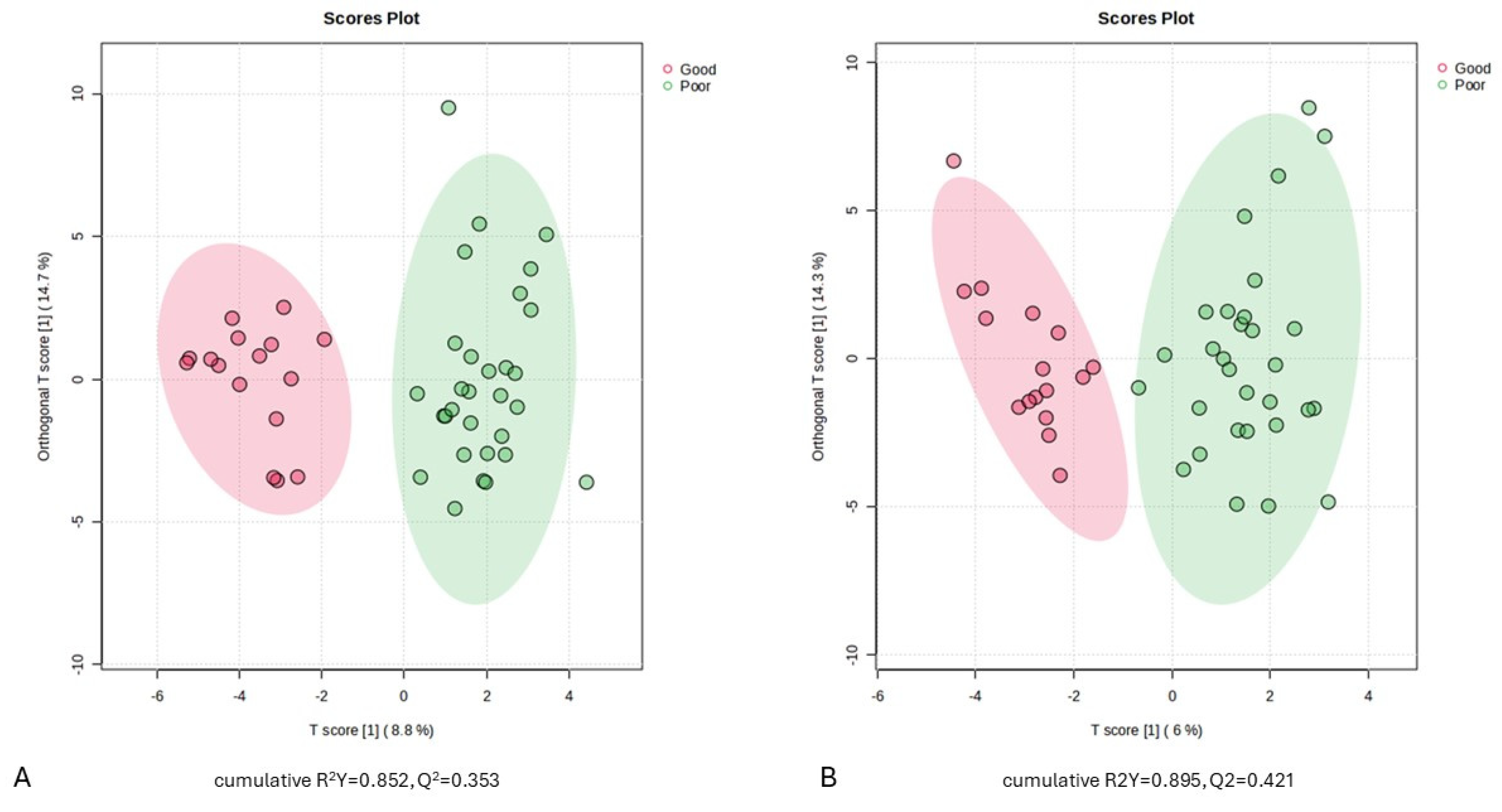
2.3. Serum Metabolic Profiles of aSAH Patients with Good and Poor Outcome
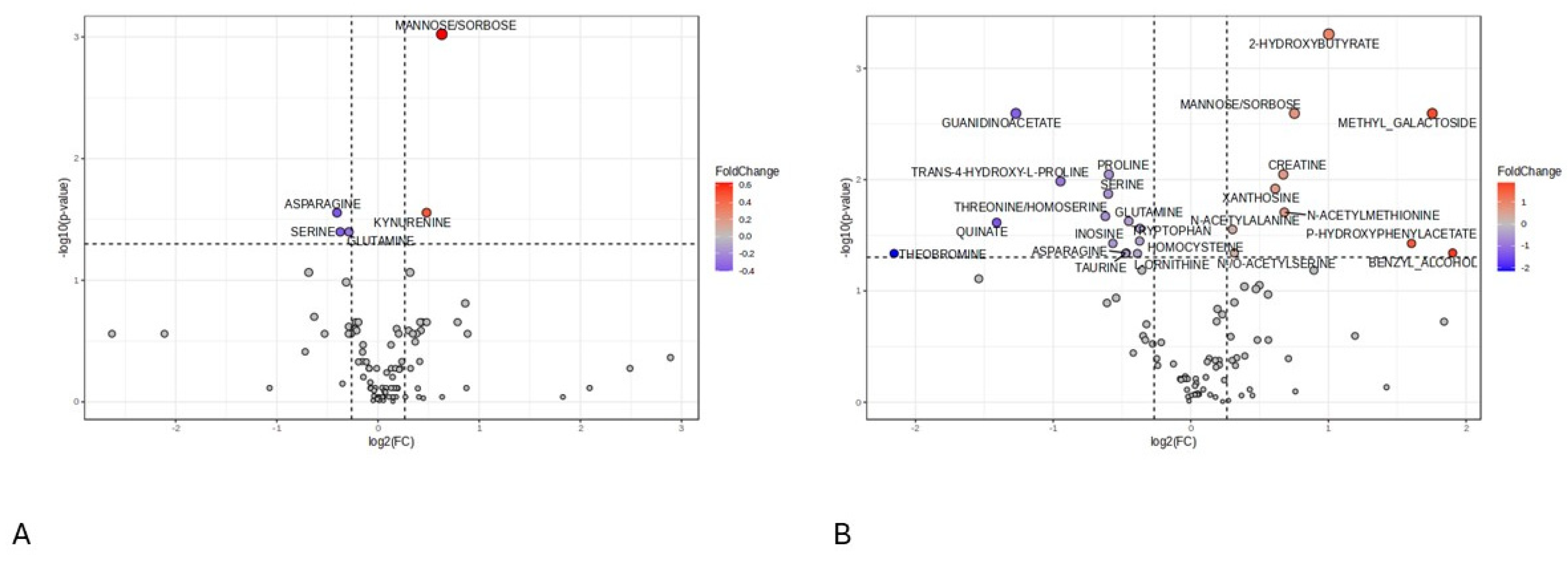
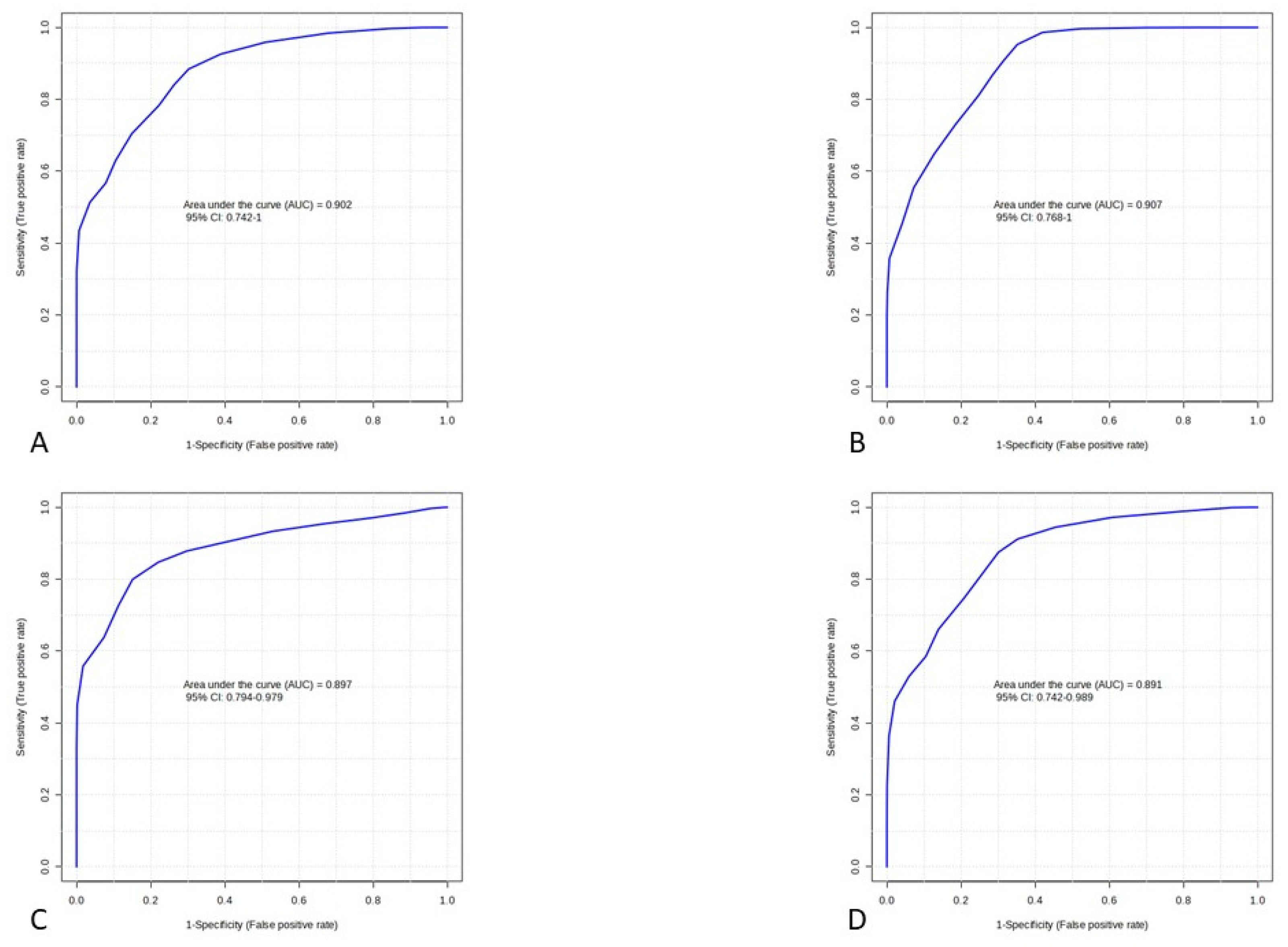
2.4. Screening of Differential Metabolites in aSAH Patients between Outcome Groups
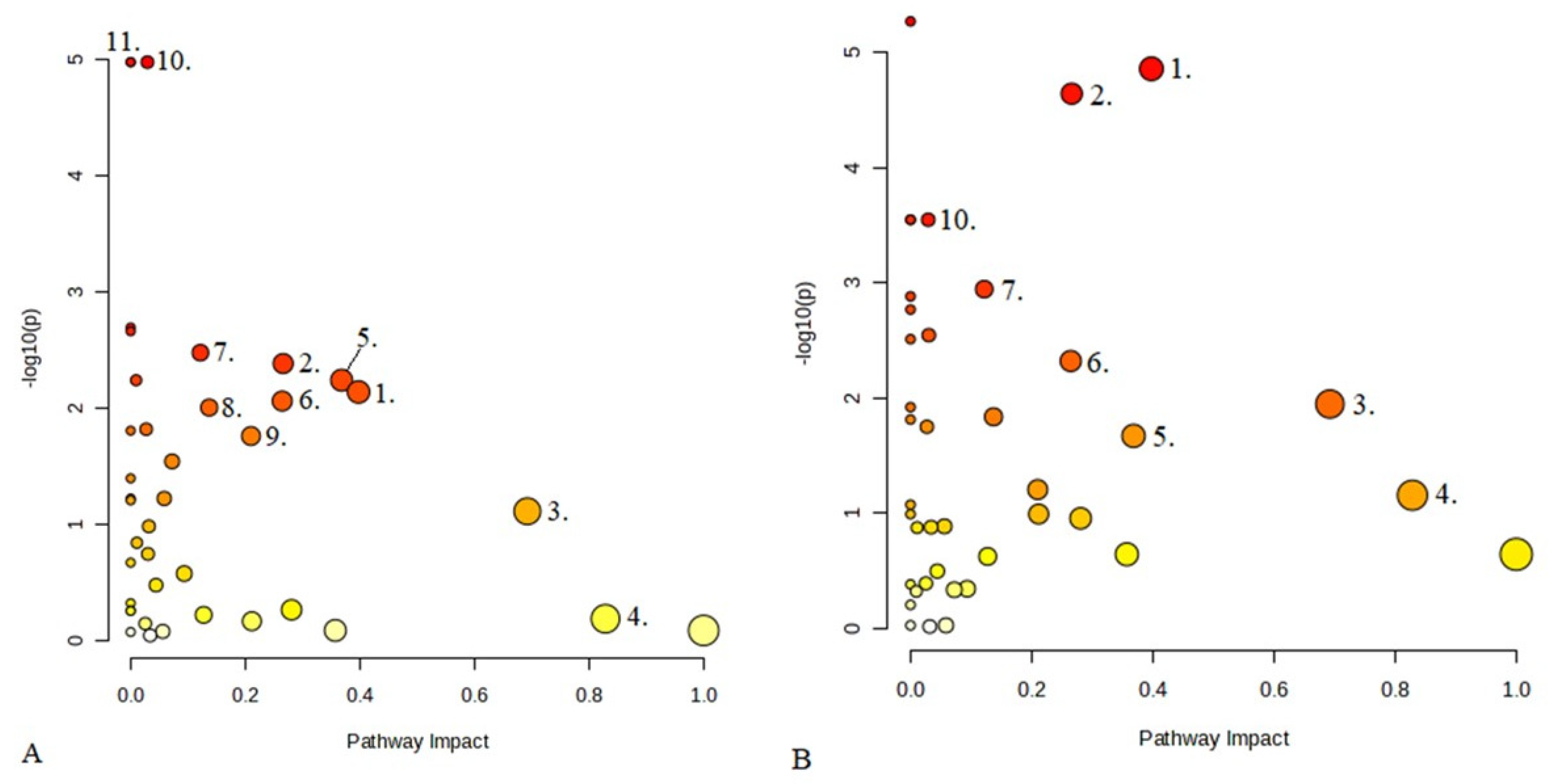
2.5. Analysis of Differential Serum Metabolites and Metabolic Pathways
3. Discussion
- (1)
- At 24 h post-injury, the group with poor 3-month outcomes exhibited significantly elevated levels of mannose/sorbose and kynurenine alongside reduced levels of amino acids.
- (2)
- At 168 h following the injury, significantly higher levels of 2-hydroxybutyrate, methyl galactoside, mannose/sorbose, creatine, xanthosine, N-acetylmethionine, N-acetylalanine, and p-hydroxyphenylacetate were detected in the serum of patients with poor 3-month outcomes.
- (3)
- At 168 h post-aSAH, the poor outcome group exhibited significantly lower levels of guanidinoacetate. Moreover, certain essential amino acids were also found at reduced levels during both the early and late sampling times in this group. Conversely, concentrations of specific N-acetyl amino acid derivatives were elevated in the poor prognosis group at the late sampling time.
- (4)
- Between the two sampling times, homocysteine showed significantly lower serum levels in the late samples compared to the early ones.
4. Materials and Methods
4.1. Subjects and Study Design
4.2. Clinical Definitions Data Collections and Outcome Measures
4.3. Sample Collection, Processing Protocol, and LC/MS Analysis
4.4. Data Processing and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Rooij, N.K.; Linn, F.H.; van der Plas, J.A.; Algra, A.; Rinkel, G.J. Incidence of subarachnoid haemorrhage: A systematic review with emphasis on region, age, gender and time trends. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Roquer, J.; Cuadrado-Godia, E.; Guimaraens, L.; Conesa, G.; Rodríguez-Campello, A.; Capellades, J.; García-Arnillas, M.P.; Fernández-Candil, J.L.; Avellaneda-Gómez, C.; Giralt-Steinhauer, E.; et al. Short- and long-term outcome of patients with aneurysmal subarachnoid hemorrhage. Neurology 2020, 95, 13. [Google Scholar] [CrossRef] [PubMed]
- Lantigua, H.; Ortega-Gutierrez, S.; Schmidt, J.M.; Lee, K.; Badjatia, N.; Agarwal, S.; Claassen, J.; Connolly, E.S.; Mayer, S.A.; Kreiter, K.T. Subarachnoid hemorrhage: Who dies, and why? Crit. Care 2015, 19, 309. [Google Scholar] [CrossRef] [PubMed]
- Helbok, R.; Schiefecker, A.J.; Beer, R.; Dietmann, A.; Antunes, A.P.; Sohm, F.; Rhomberg, P.; Rass, V.; Kofler, M.; Gaasch, M.; et al. Early brain injury after aneurysmal subarachnoid hemorrhage: A multimodal neuromonitoring study. Crit. Care 2015, 19, 75. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, A.; Nielsen, T.H.; Nilsson, O.; Schalen, W.; Nordstrom, C.H. Bedside diagnosis of mitochondrial dysfunction in aneurysmal subarachnoid hemorrhage. Acta Neurol. Scand. 2014, 130, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Treichl, S.A.; Carvi y Nievas, M.H.; Smajda, S.; Schatlo, B.; Heiss, W.D.; Zaro-Weber, O. Cerebral Energy Status and Altered Metabolism in Early Brain Injury after Aneurysmal Subarachnoid Hemorrhage: A Prospective 31P-MRS Pilot Study. Front. Neurol. 2022, 13, 831537. [Google Scholar] [CrossRef] [PubMed]
- Garnett, M.R.; Blamire, A.M.; Rajagopalan, B.; Styles, P.; Cadoux-Hudson, T.A.; Schönlieb, C.B. Altered cellular metabolism following traumatic brain injury: A magnetic resonance spectroscopy study. J. Neurotraum 2001, 18, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Carteron, L.; Helbok, R.; Beseoglu, K.; Schiefecker, A.J.; Kofler, M.; Beer, R.; Pfausler, B.; Weiss, V.; Rhomberg, P.; Schmutzhard, E.; et al. Non-Ischemic Cerebral Energy Dysfunction at the Early Brain Injury Phase following Aneurysmal Subarachnoid Hemorrhage. Front. Neurol. 2017, 8, 325. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Hernandez-Quiceno, S.; Culbreth, M.; Lee, S.; Fitzmaurice, A.G.; O’Callaghan, J.P.; Hu, J.; Lanthier, P.; Pardo, M.; Siman, R.; et al. Development of a translational inflammation panel for the quantification of cerebrospinal fluid Pterin, Tryptophan-Kynurenine and Nitric oxide pathway metabolites. EBioMedicine 2022, 77, 103917. [Google Scholar] [CrossRef]
- Loretz, N.; Pfortmueller, C.A.; Schuepbach, R.A.; Niedermoser, S.; Neylan, C.J.; Exadaktylos, A.K.; Post, K.; Fandino, J.; Takala, J.; Jakob, S.M.; et al. Activation of the kynurenine pathway predicts mortality and neurological outcome in cardiac arrest patients: A validation study. J. Crit. Care 2022, 67, 57–65. [Google Scholar] [CrossRef]
- Darlington, L.G.; Mackay, G.M.; Forrest, C.M.; Stoy, N.; George, C.; Stone, T.W. Altered kynurenine metabolism correlates with infarct volume in stroke. Eur. J. Neurosci. 2007, 26, 2211–2221. [Google Scholar] [CrossRef]
- Dehhaghi, M.; Panizzon, T.; Hall, M.; Moghieb, A.; Guillemin, G.J. The kynurenine pathway in traumatic brain injuries and concussion. Front. Neurol. 2023, 14, 1210453. [Google Scholar] [CrossRef] [PubMed]
- Lauzier, D.C.; Suppa, A.; Scott, C.A.; Owen, E.G.; Platon, A.; Merenda, J.; Allali, G.; Duvernoy, C.; Gaspoz, J.M.; Sarasin, F.P.; et al. Early Brain Injury after Subarachnoid Hemorrhage: Incidence and Mechanisms. Stroke 2023, 54, 1426–1440. [Google Scholar] [CrossRef] [PubMed]
- Rogers, A.J.; McGeachie, M.J.; Baron, R.M.; Gazourian, L.; Haspel, J.A.; Nakahira, K.; Itagaki, K.; Estepar, R.S.J.; Christiani, D.C.; Fredenburgh, L.E. Metabolomic derangements are associated with mortality in critically ill adult patients. PLoS ONE 2014, 9, e87538. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M. Guanidinoacetic acid as a performance-enhancing agent. Amino Acids 2016, 48, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Leuzzi, V.; Bianchi, M.C.; Tosetti, M.; Carducci, C.A.; Cerquiglini, A.; Antonozzi, I.; Battini, R.; Signorini, E.; Della Giustina, E.; Calderini, G.; et al. Brain creatine depletion: Guanidinoacetate methyltransferase deficiency (improving with creatine supplementation). Neurology 2000, 55, 1407–1409. [Google Scholar] [CrossRef]
- Tsubakihara, Y.; Nakayama, M.; Miyazaki, S.; Nakao, T.; Yanagi, H.; Hasegawa, T.; Ohishi, K.; Matsuo, S. Guanidinoacetic acid (GAA) in patients with chronic kidney disease (CKD) and diabetes mellitus (DM). Kidney Res. Clin. Pract. 2012, 31, 309–316. [Google Scholar] [CrossRef]
- Neu, A.; Behie, L.; Ford, B.; Wang, J.; Rashidi, M.; Rivier, C. Activation of GABA(A) receptors by guanidinoacetate: A novel pathophysiological mechanism. Neurobiol. Dis. 2002, 11, 298–307. [Google Scholar] [CrossRef]
- Ostojic, S.M. Advanced physiological roles of guanidinoacetic acid. Eur. J. Nutr. 2015, 54, 1211–1215. [Google Scholar] [CrossRef]
- James, E.; Stombaugh, R.; Thompson, R., Jr. The reaction of phosphagens with ATP: Creatine phosphotransferase. Biochim. Biophys. Acta 1966, 128, 327–336. [Google Scholar] [CrossRef]
- Udenfriend, S.; Titus, E.; Weissbach, H.; Peterson, R.; Brodie, B.B. Inhibitors of norepinephrine metabolism in vivo. Arch. Biochem. Biophys. 1959, 84, 249–251. [Google Scholar] [CrossRef] [PubMed]
- Tousoulis, D.; Kampoli, A.M.; Tentolouris, C.; Papageorgiou, N.; Stefanadis, C. The role of nitric oxide on endothelial function. Curr. Vasc. Pharmacol. 2012, 10, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Feng, H.; Sherchan, P.; Klebe, D.; Zhao, G.; Sun, X.; Zhang, J.H. The harmful effects of subarachnoid hemorrhage on extracerebral organs. BioMed Res. Int. 2014, 2014, 858496. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.; Pitzen, A.; Azami, S.A.; Schreiber, S.J.; Schwab, S.; Werz, O.; Ferreirós, N.; Michalik, M. Hepatic Ketogenesis Induced by Middle Cerebral Artery Occlusion in Mice. J. Am. Heart Assoc. 2017, 6, e005556. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, K.H.; Seifert, T.; Secher, N.H.; Grøndal, T.; van Hall, G. Systemic, cerebral and skeletal muscle ketone body and energy metabolism during acute hyper-d-β-hydroxybutyratemia in post-absorptive healthy males. J. Clin. Endocrinol. Metab. 2015, 100, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Hasselbalch, S.G.; Knudsen, G.M.; Jakobsen, J.; Hageman, L.P.; Holm, S.; Paulson, O.B. Blood-brain barrier permeability of glucose and ketone bodies during short-term starvation in humans. Am. J. Physiol. Endocrinol. Metab. 1995, 268, E1161–E1166. [Google Scholar] [CrossRef] [PubMed]
- Prins, M.L.; Fujima, L.S.; Hovda, D.A. Age-dependent reduction of cortical contusion volume by ketones after traumatic brain injury. J. Neurosci. Res. 2005, 82, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, E.S.; Tenenbein, M. Plasma β-Hydroxybutyrate for the Diagnosis of Diabetic Ketoacidosis in the Emergency Department. Pediatr. Emerg. Care 2021, 37, e1345–e1350. [Google Scholar] [CrossRef] [PubMed]
- Depczynski, B.; Kelly, A.M. The Significance of an Increased Beta-Hydroxybutyrate at Presentation to the Emergency Department in Patients with Diabetes in the Absence of a Hyperglycemic Emergency. J. Diabetes Res. 2019, 2019, 7387128. [Google Scholar] [CrossRef]
- Puchowicz, M.A.; Zechel, J.L.; Valerio, J.; Emancipator, D.S.; Xu, K.; Pundik, S.; LaManna, J.C. Neuroprotection in diet-induced ketotic rat brain after focal ischemia. J. Cereb. Blood Flow. Metab. 2008, 28, 1907–1916. [Google Scholar] [CrossRef]
- Gibson, C.L.; Inglebert, N.; Xu, H. Stroke outcome in the ketogenic state: A systematic review of the animal data. J. Neurochem. 2012, 123, 52–57. [Google Scholar] [CrossRef]
- Kashiwaya, Y.; Takeshima, T.; Mori, N.; Nakashima, K.; Clarke, K.; Veech, R.L. D-β-Hydroxybutyrate protects neurons in models of Alzheimer’s and Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2000, 97, 5440–5444. [Google Scholar] [CrossRef] [PubMed]
- Tieu, K.; Perier, C.; Caspersen, C.; Teismann, P.; Wu, D.C.; Yan, S.D.; Naini, A.; Vila, M.; Jackson-Lewis, V.; Ramasamy, R.; et al. D-β-Hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J. Clin. Investig. 2003, 112, 892–901. [Google Scholar] [CrossRef]
- Puchalska, P.; Crawford, P.A. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef] [PubMed]
- Shimazu, T.; Hirschey, M.D.; Newman, J.; He, W.; Shirakawa, K.; Le Moan, N.; Grueter, C.A.; Lim, H.; Saunders, L.R.; Stevens, R.D.; et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013, 339, 211–214. [Google Scholar] [CrossRef]
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015, 21, 263–273. [Google Scholar] [CrossRef]
- Bazzigaluppi, P.; Niazi, M.; De Blasi, E.; Weisspapir, I.; Stefanovic, B. Imaging the Effects of β-Hydroxybutyrate on Peri-Infarct Neurovascular Function and Metabolism. Stroke 2018, 49, 2173–2181. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, F.; Peuker, A.C.; Kastirr, M.; Ahrenberg, L.; Brenner, T.; Weigand, M.A.; Lother, A.; Uhle, F.; Weigand, M.A. Impaired ketogenesis ties metabolism to T cell dysfunction in COVID-19. Nature 2022, 609, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Sjöberg, R.L.; Marmarou, A.; Rothoerl, R.D.; Vath, A.; Konasiewicz, S.J.; Bullock, R.; Becker, D.P.; Muizelaar, J.P.; Marshall, L.F. Blood Metabolomic Predictors of 1-Year Outcome in Subarachnoid Hemorrhage. Neurocrit. Care 2015, 23, 225–232. [Google Scholar] [CrossRef]
- Sokół, B.; Nowacki, P.; Świercz, A.; Wierzba-Bobrowicz, T.; Droździk, M. Amino Acids in Cerebrospinal Fluid of Patients with Aneurysmal Subarachnoid Haemorrhage: An Observational Study. Front. Neurol. 2017, 8, 438. [Google Scholar] [CrossRef]
- Bobeff, E.J.; Nayak, N.R.; Figueroa, S.A.; Koster, T.; Conner, A.K.; Mahan, M.A.; Westbroek, E.M.; Elwood, L.T.; Sandhu, D.S.; Schwartz, T.H. Plasma Amino Acids May Improve Prediction Accuracy of Cerebral Vasospasm after Aneurysmal Subarachnoid Haemorrhage. J. Clin. Med. 2022, 11, 380. [Google Scholar] [CrossRef] [PubMed]
- Touho, H.; Karasawa, J.; Shishido, H.; Miwa, Y.; Ishikawa, Y.; Sano, K. Hypermetabolism in the acute stage of hemorrhagic cerebrovascular disease. J. Neurosurg. 1990, 72, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Suojaranta-Ylinen, R.; Laakso, J.; Hynninen, M.; Kaukinen, S.; Oja, S.; Paljärvi, L.; Luukkonen, M.; Romppanen, J.; Salmi, J.; Takkunen, O. Hypermetabolism and increased peripheral release of amino acids after subarachnoidal hemorrhage and its operative treatment. Nutrition 1996, 12, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Preiser, J.C.; Ichai, C.; Orban, J.C.; Groeneveld, A.B.J. Metabolic response to the stress of critical illness. Br. J. Anaesth. 2014, 113, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Tappy, L.; Berger, M.M.; Schwarz, J.M.; Schneiter, P.; Kim, S.; Revelly, J.P.; Chiolero, R.; Poletti, P.A.; Babst, R.; Scheidegger, J.R. Effects of isoenergetic glucose-based or lipid-based parenteral nutrition on glucose metabolism, de novo lipogenesis, and respiratory gas exchanges in critically ill patients. Crit. Care Med. 1998, 26, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.; Czapran, A.; Gharbi, M.B.; Hiesmayr, M.; Mayer, K.; et al. ESPEN practical and partially revised guideline: Clinical nutrition in the intensive care unit. Clin. Nutr. 2023, 42, 1671–1689. [Google Scholar] [CrossRef] [PubMed]
- Hoh, B.L.; Ko, N.U.; Amin-Hanjani, S.; Bambakidis, N.C.; Barker, F.G.; Barsan, W.G.; Connolly, E.S.; Coon, A.L.; Derdeyn, C.P.; Dumont, A.S.; et al. Guideline for the Management of Patients with Aneurysmal Subarachnoid Hemorrhage: A Guideline from the American Heart Association/American Stroke Association. Stroke 2023, 54, e314–e370. [Google Scholar] [CrossRef] [PubMed]
- Japur, C.C.; Penaforte, F.R.O.; Chueire, V.B.; Greve, J.M.D.; Monteiro, J.P. Harris-Benedict equation for critically ill patients: Are there differences with indirect calorimetry? J. Crit. Care 2009, 24, 628.e1–628.e5. [Google Scholar] [CrossRef]
- Broadhurst, D.; Goodacre, R.; Reinke, S.N.; Kuligowski, J.; Wilson, I.D.; Lewis, M.R.; Dunn, W.B. Guidelines and considerations for the use of system suitability and quality control samples in mass spectrometry assays applied in untargeted clinical metabolomic studies. Metabolomics 2018, 14, 72. [Google Scholar] [CrossRef]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef]
- Alseekh, S.; Aharoni, A.; Brotman, Y.; Contrepois, K.; D’Auria, J.; Ewald, J.; Chetelat, A.; Fernie, A.R.; Fraser, P.D.; Giavalisco, P.; et al. Mass spectrometry-based metabolomics: A guide for annotation, quantification and best reporting practices. Nat. Methods 2021, 18, 747–756. [Google Scholar] [CrossRef] [PubMed]
| Variable | Good Outcome (N = 16) | Poor Outcome (N = 30) | p-Value |
|---|---|---|---|
| Age, years, mean ± SD | 55 ± 10 | 57 ± 13 | 0.852 |
| Female, N (%) | 16 (70) | 19 (83) | 0.300 |
| Hypertension, N (%) | 9 (39) | 4 (17) | 0.077 |
| NIDDM, N (%) | 0 (0) | 3 (13) | 0.073 |
| COPD, N (%) | 1 (4) | 1 (4) | 1.000 |
| IHD, N (%) | 9 (39) | 14 (61) | 0.140 |
| Current smoking, N (%) | 14 (61) | 11 (48) | 0.375 |
| Aneurysm localization, N (%) | |||
| internal carotid | 0 (0) | 2 (9) | |
| middle cerebral artery | 7 (30) | 7 (30) | |
| a. comm. anterior | 5 (22) | 5 (22) | |
| a. comm. posterior | 4 (18) | 1 (4) | |
| a. cerebri anterior | 1 (4) | 1 (4) | |
| vertebrobasilar | 6 (26) | 7 (30) | |
| mFisher score, median (IQR) | 2 (1–3) | 3 (3–4) | <0.001 |
| WFNS, median (IQR) | 1 (1–2) | 4 (2–5) | <0.001 |
| Creatinine *, µmol/L, median (IQR) | 56 (49–70) | 60 (49–71) | 0.700 |
| CRP *, mg/L, median (IQR) | 5 (2–15) | 27 (2–73) | 0.027 |
| WBC *, G/L, median (IQR) | 11 (8–12) | 10 (9–12) | 0.910 |
| Neutrophile *, G/L, median (IQR) | 7 (6–10) | 8 (7–10) | 0.381 |
| Lymphocyte *, G/L, median (IQR) | 2 (1–2) | 1 (1–2) | 0.023 |
| Extraventricular drainage, N (%) | 2 (9) | 19 (83) | <0.001 |
| Lumbal drainage, N (%) | 11 (48) | 12 (52) | 0.768 |
| Decompressive craniotomy, N (%) | 0 (0) | 2 (9) | 0.148 |
| Mechanical ventilation, N (%) | 2 (9) | 19 (83) | <0.001 |
| Infection, N (%) | 3 (13) | 15 (65) | 0.001 |
| Symptomatic vasospasm, N (%) | 3 (13) | 7 (30) | 0.153 |
| Parenteral nutrition | 0 (0) | 0 (0) | 1.000 |
| Initiation of EN a, hours, mean ± SD | 33.5 ± 2.5 | 32.7 ± 3.5 | 0.465 |
| Enteral nutrition (via NG tube) | 4 (25) | 24 (80) | <0.001 |
| Enteral nutrition (oral diet) | 15 (94) | 9 (30) | <0.001 |
| MRI ischemia, N (%) | 2 (9) | 5 (22) | 0.218 |
| Glucose b, µmol/L, median (IQR) | 7 (6.4–7.2) | 7.3 (7–7.7) | 0.028 |
| Lactate b, µmol/L, median (IQR) | 0.8 (0.6–1) | 0.9 (0.7–1.2) | 0.195 |
| 3-month mRS, median, IQR | 1 (1–3) | 5 (4–5) | <0.001 |
| Metabolites | p-Value a | FDR b | FC c | VIP d | AUC |
|---|---|---|---|---|---|
| Homocysteine | <0.0001 | 0.0000 | 0.54299 | 2.5649 | 0.838 |
| Pantothenate | <0.0001 | 0.0022 | 0.72975 | 2.0931 | 0.736 |
| Theobromine | <0.0001 | 0.0022 | 0.27842 | 2.3236 | 0.732 |
| Homogentisate | <0.0001 | 0.0022 | 1.4076 | 1.3739 | 0.736 |
| Pregnenolone Sulfate | <0.0001 | 0.0023 | 0.27842 | 2.1133 | 0.727 |
| Paraxanthine | 0.0005 | 0.0054 | 0.16176 | 2.1269 | 0.706 |
| Glycocholate | 0.0006 | 0.0054 | 1.9743 | 1.7525 | 0.670 |
| Methyl Galactoside | 0.0014 | 0.0116 | 0.62255 | 1.8354 | 0.691 |
| Quinate | 0.0019 | 0.0149 | 0.72407 | 1.6022 | 0.685 |
| Biliverdin | 0.0032 | 0.0218 | 0.75389 | 1.7025 | 0.677 |
| Xanthurenate | 0.0042 | 0.0257 | 1.4583 | 1.6431 | 0.672 |
| Glycochenodeoxycholate | 0.0046 | 0.0267 | 2.0485 | 1.3549 | 0.670 |
| Bilirubin | 0.0051 | 0.0278 | 0.74985 | 1.6738 | 0.668 |
| Glutamate | 0.0071 | 0.0361 | 0.72695 | 1.4018 | 0.662 |
| Metabolites | p-Value a | FDR b | FC c | VIP d | AUC |
|---|---|---|---|---|---|
| D1 | |||||
| Mannose/Sorbose | <0.0001 | 0.0009 | 1.5782 | 2.2981 | 0.868 |
| Kynurenine | 0.0006 | 0.0278 | 1.3946 | 2.2316 | 0.820 |
| Asparagine | 0.0009 | 0.0278 | 0.7551 | 1.8846 | 0.800 |
| Serine | 0.0020 | 0.0400 | 0.7720 | 1.7817 | 0.758 |
| Glutamine | 0.0022 | 0.0400 | 0.8195 | 1.7818 | 0.768 |
| D7 | |||||
| 2-Hydroxybutyrate | <0.0001 | 0.0014 | 2.0034 | 2.2096 | 0.856 |
| Methyl_Galactoside | 0.0002 | 0.0050 | 3.3747 | 2.0167 | 0.820 |
| Guanidinoacetate | <0.0001 | 0.0000 | 0.4144 | 2.0029 | 0.917 |
| Mannose/Sorbose | 0.0001 | 0.0031 | 1.6853 | 1.9738 | 0.835 |
| Creatine | 0.0004 | 0.0063 | 1.5934 | 1.8054 | 0.808 |
| Proline | 0.0003 | 0.0062 | 0.6618 | 1.7326 | 0.812 |
| Serine | 0.0015 | 0.0142 | 0.6596 | 1.6987 | 0.779 |
| Trans-4-Hydroxy-L-Proline | 0.0012 | 0.0121 | 0.5188 | 1.6620 | 0.785 |
| Threonine/Homoserine | 0.0067 | 0.0327 | 0.6503 | 1.6316 | 0.741 |
| Xanthosine | 0.0022 | 0.0183 | 1.5301 | 1.6144 | 0.770 |
| Glutamine | 0.0046 | 0.0282 | 0.7317 | 1.5320 | 0.752 |
| N-Acetylmethionine | 0.0006 | 0.0073 | 1.6020 | 1.4859 | 0.802 |
| Quinate | 0.0042 | 0.0279 | 0.3762 | 1.4790 | 0.754 |
| P-Hydroxyphenylacetate | 0.0039 | 0.0277 | 3.0389 | 1.4328 | 0.756 |
| Benzyl_Alcohol | 0.0067 | 0.0327 | 3.7385 | 1.3918 | 0.741 |
| Tryptophan | 0.0010 | 0.0113 | 0.7737 | 1.3769 | 0.789 |
| Inosine | 0.0105 | 0.0481 | 0.6753 | 1.3634 | 0.729 |
| N-Acetylalanine | 0.0031 | 0.0235 | 1.2340 | 1.3633 | 0.762 |
| Homocysteine | 0.0063 | 0.0327 | 0.7728 | 1.3288 | 0.743 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orban, B.; Tengölics, R.; Zavori, L.; Simon, D.; Erdo-Bonyar, S.; Molnar, T.; Schwarcz, A.; Csecsei, P. The Difference in Serum Metabolomic Profiles between the Good and Poor Outcome Groups at 3 Months in the Early and Late Phases of Aneurysmal Subarachnoid Hemorrhage. Int. J. Mol. Sci. 2024, 25, 6597. https://doi.org/10.3390/ijms25126597
Orban B, Tengölics R, Zavori L, Simon D, Erdo-Bonyar S, Molnar T, Schwarcz A, Csecsei P. The Difference in Serum Metabolomic Profiles between the Good and Poor Outcome Groups at 3 Months in the Early and Late Phases of Aneurysmal Subarachnoid Hemorrhage. International Journal of Molecular Sciences. 2024; 25(12):6597. https://doi.org/10.3390/ijms25126597
Chicago/Turabian StyleOrban, Brigitta, Roland Tengölics, Laszlo Zavori, Diana Simon, Szabina Erdo-Bonyar, Tihamer Molnar, Attila Schwarcz, and Peter Csecsei. 2024. "The Difference in Serum Metabolomic Profiles between the Good and Poor Outcome Groups at 3 Months in the Early and Late Phases of Aneurysmal Subarachnoid Hemorrhage" International Journal of Molecular Sciences 25, no. 12: 6597. https://doi.org/10.3390/ijms25126597
APA StyleOrban, B., Tengölics, R., Zavori, L., Simon, D., Erdo-Bonyar, S., Molnar, T., Schwarcz, A., & Csecsei, P. (2024). The Difference in Serum Metabolomic Profiles between the Good and Poor Outcome Groups at 3 Months in the Early and Late Phases of Aneurysmal Subarachnoid Hemorrhage. International Journal of Molecular Sciences, 25(12), 6597. https://doi.org/10.3390/ijms25126597







