Matrine Ameliorates DSS-Induced Colitis by Suppressing Inflammation, Modulating Oxidative Stress and Remodeling the Gut Microbiota
Abstract
:1. Introduction
2. Results
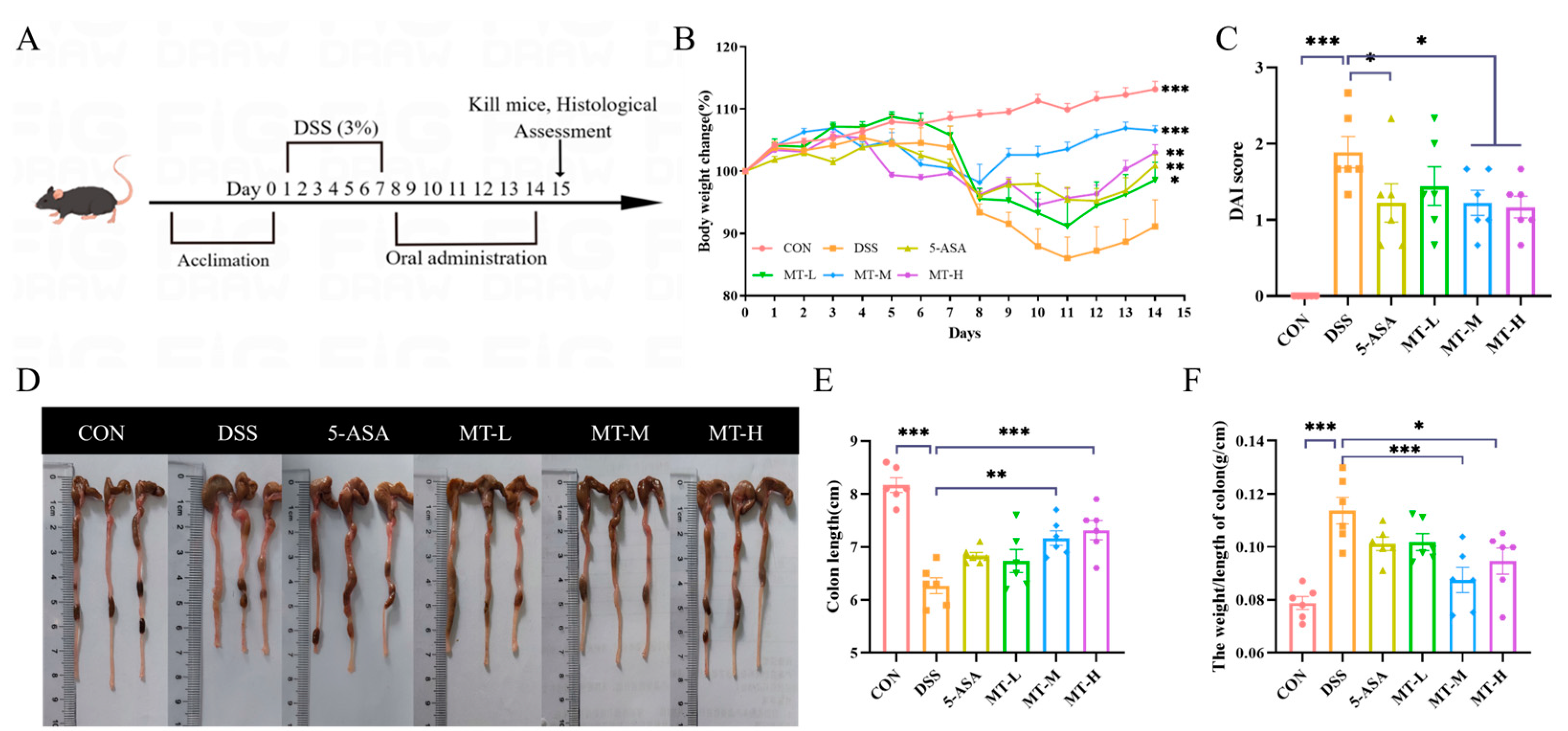
2.1. Matrine Alleviated DSS-Induced Colitic Symptoms
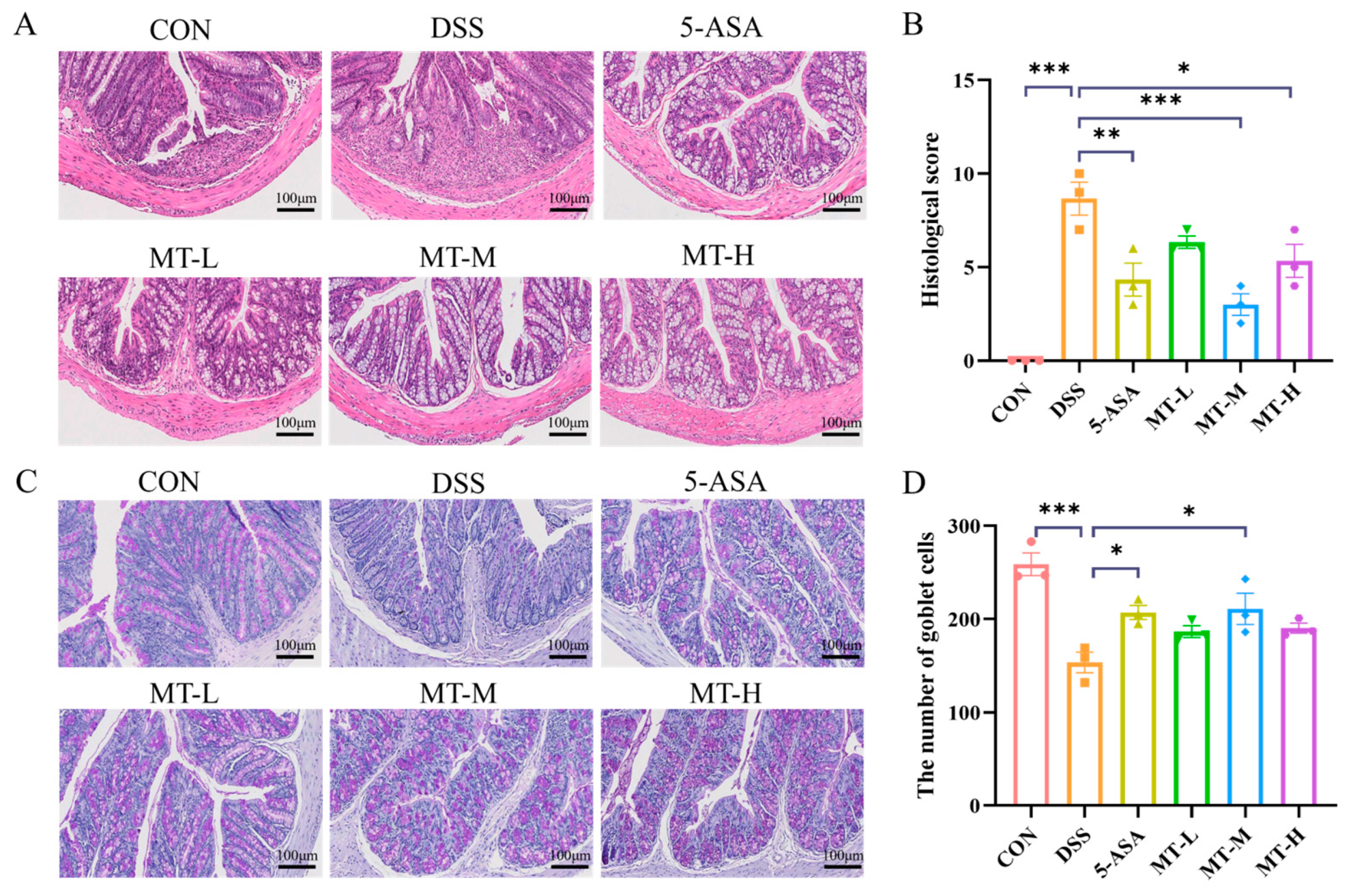
2.2. Histopathologic Analysis of the Colon
2.3. Matrine Alleviated DSS-Induced Colonic Inflammation
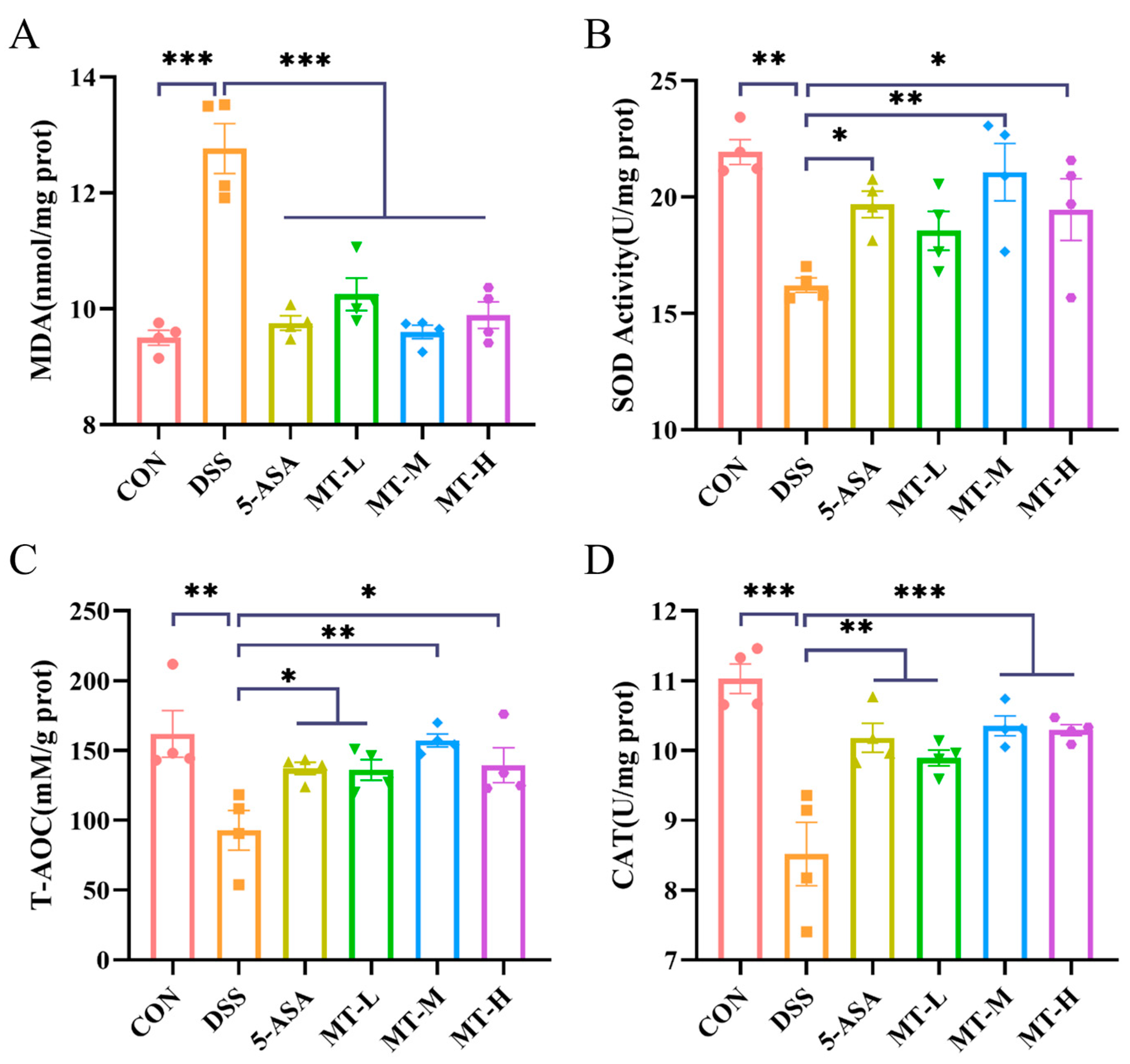
2.4. Matrine Alleviated DSS-Induced Colonic Oxidative Stress
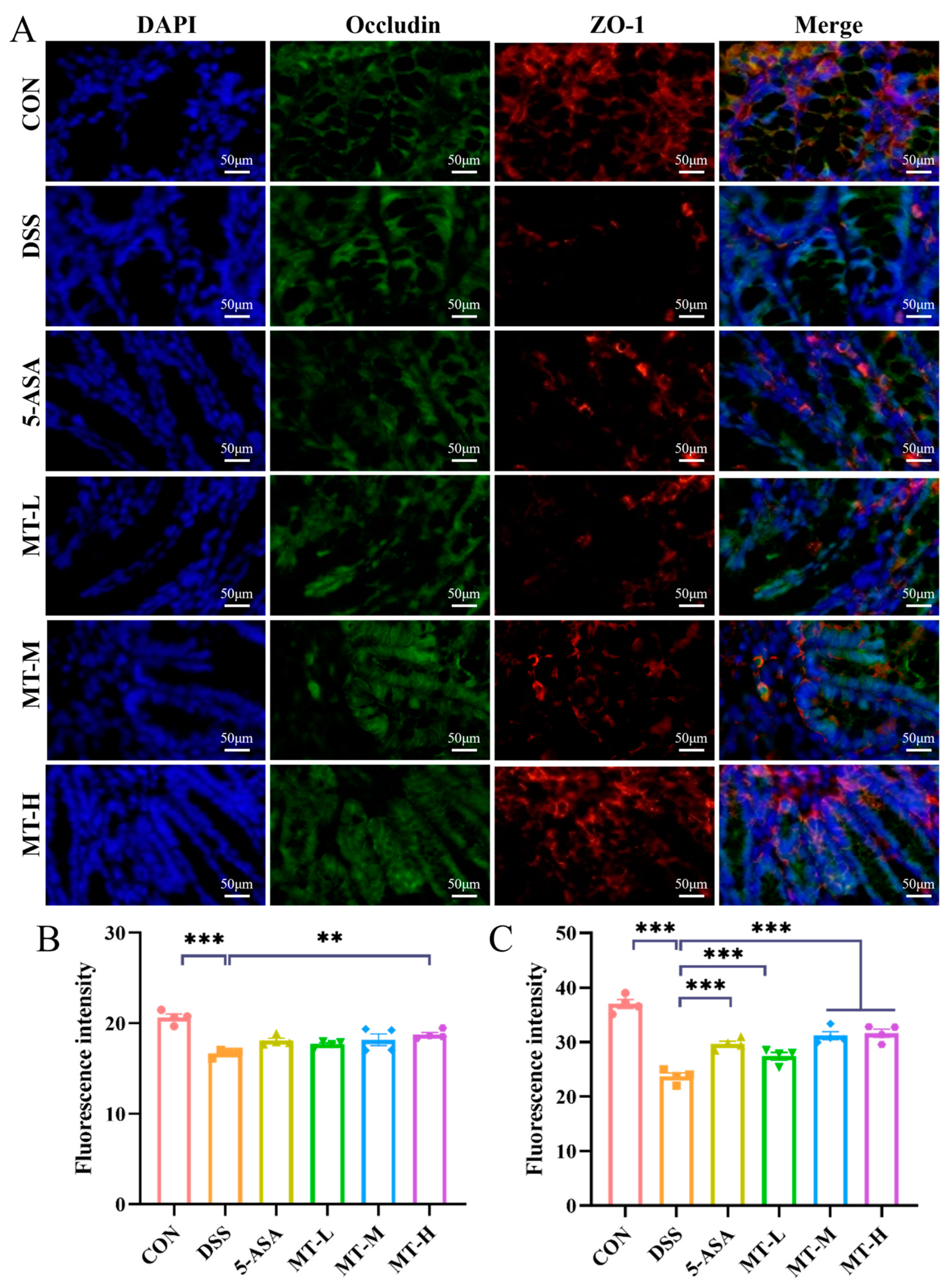
2.5. Matrine Protected against DSS-Induced Intestinal Barrier Damage
2.6. Matrine Regulated the Balance of Treg/Th17 Cells in Colitis Mice
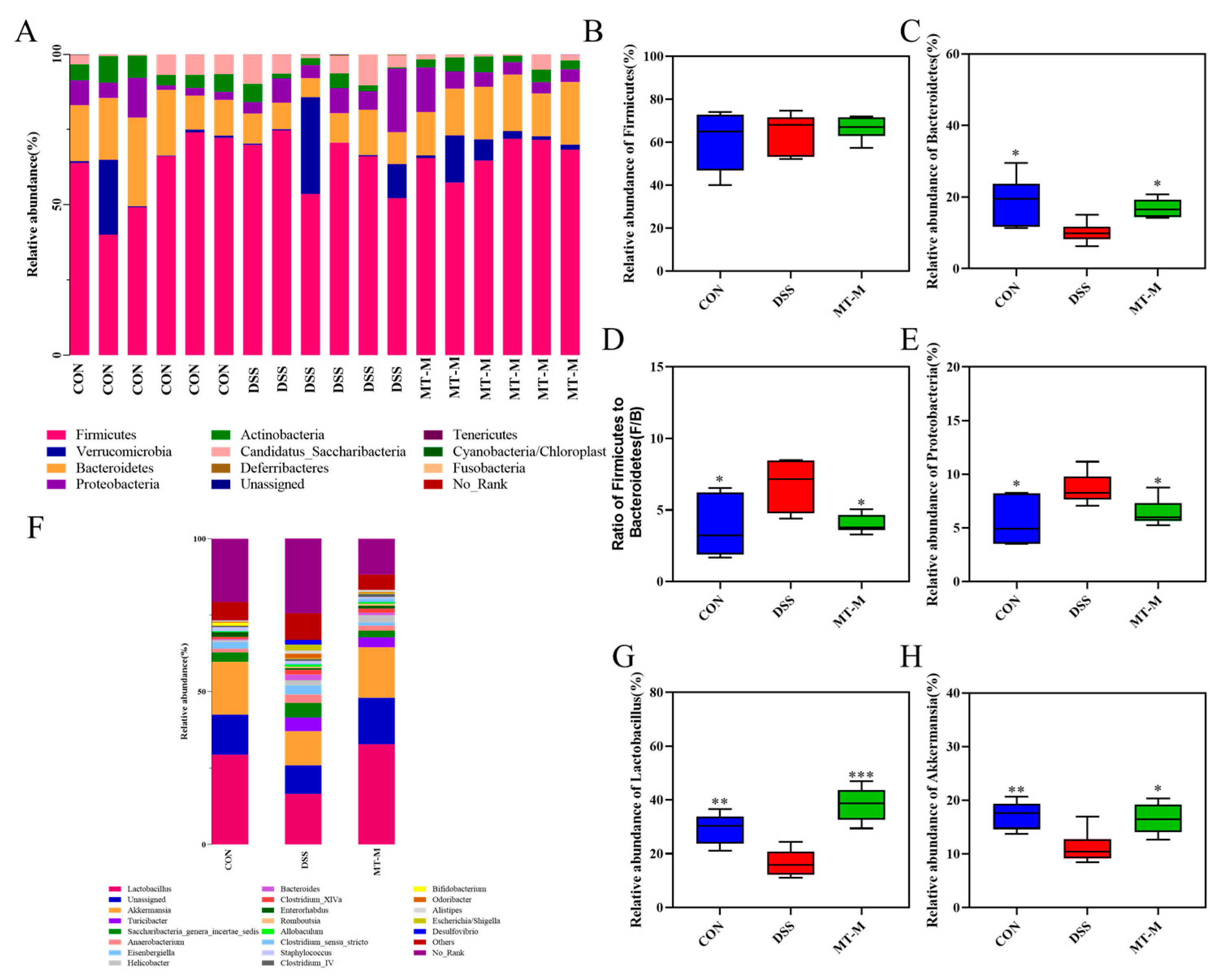
2.7. Matrine Modulated the Intestinal Flora in Colitis Mice
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Mice
4.3. Animal Experiment Design
4.4. Colon Histology
4.5. Alcian Blue–Periodic Acid Schiff (AB-PAS) Staining
4.6. Cytokine Analysis of Colonic Tissues
4.7. Quantitative Real-Time PCR Analysis
4.8. Evaluation of Oxidative Stress
4.9. Immunofluorescence Staining
4.10. Flow Cytometry
4.11. 16S rRNA Sequencing
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baumgart, D.C.; Sandborn, W.J. Inflammatory bowel disease: Clinical aspects and established and evolving therapies. Lancet 2007, 369, 1641–1657. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Loftus, E.V.; Colombel, J.-F.; Sandborn, W.J. Long-term complications, extraintestinal manifestations, and mortality in adult Crohn’s disease in population-based cohorts. Inflamm. Bowel Dis. 2011, 17, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Altobelli, E.; Latella, G.; Morroni, M.; Licini, C.; Tossetta, G.; Mazzucchelli, R.; Profeta, V.F.; Coletti, G.; Leocata, P.; Castellucci, M.; et al. Low HtrA1 expression in patients with long-standing ulcerative colitis and colorectal cancer. Oncol. Rep. 2017, 38, 418–426. [Google Scholar] [CrossRef]
- Loftus, E.V. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology 2004, 126, 1504–1517. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef]
- Ramos, G.P.; Papadakis, K.A. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin. Proc. 2019, 94, 155–165. [Google Scholar] [CrossRef]
- Jeong, D.Y.; Kim, S.; Son, M.J.; Son, C.Y.; Kim, J.Y.; Kronbichler, A.; Lee, K.H.; Shin, J.I. Induction and maintenance treatment of inflammatory bowel disease: A comprehensive review. Autoimmun. Rev. 2019, 18, 439–454. [Google Scholar] [CrossRef] [PubMed]
- Breitrück, A.; Weigel, M.; Hofrichter, J.; Sempert, K.; Kerkhoff, C.; Mohebali, N.; Mitzner, S.; Hain, T.; Kreikemeyer, B. Smectite as a Preventive Oral Treatment to Reduce Clinical Symptoms of DSS Induced Colitis in Balb/c Mice. Int. J. Mol. Sci. 2021, 22, 8699. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Xue, X.; Liu, J.; Li, Z.-S.; Yang, G.-Y.; Song, Y.; Pan, Y.; Ma, Y.; Hu, S.; et al. A Phase I Trial of Berberine in Chinese with Ulcerative Colitis. Cancer Prev. Res. 2020, 13, 117–126. [Google Scholar] [CrossRef]
- Genua, F.; Raghunathan, V.; Jenab, M.; Gallagher, W.M.; Hughes, D.J. The Role of Gut Barrier Dysfunction and Microbiome Dysbiosis in Colorectal Cancer Development. Front. Oncol. 2021, 11, 626349. [Google Scholar] [CrossRef]
- Odenwald, M.A.; Turner, J.R. The intestinal epithelial barrier: A therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 9–21. [Google Scholar] [CrossRef]
- Liu, B.; Ye, D.; Yang, H.; Song, J.; Sun, X.; Mao, Y.; He, Z. Two-Sample Mendelian Randomization Analysis Investigates Causal Associations Between Gut Microbial Genera and Inflammatory Bowel Disease, and Specificity Causal Associations in Ulcerative Colitis or Crohn’s Disease. Front. Immunol. 2022, 13, 921546. [Google Scholar] [CrossRef]
- Li, M.; Cheng, D.; Peng, C.; Huang, Y.; Geng, J.; Huang, G.; Wang, T.; Xu, A. Therapeutic mechanisms of the medicine and food homology formula Xiao-Ke-Yin on glucolipid metabolic dysfunction revealed by transcriptomics, metabolomics and microbiomics in mice. Chin. Med. 2023, 18, 57. [Google Scholar] [CrossRef]
- Sun, X.-Y.; Jia, L.-Y.; Rong, Z.; Zhou, X.; Cao, L.-Q.; Li, A.-H.; Guo, M.; Jin, J.; Wang, Y.-D.; Huang, L.; et al. Research Advances on Matrine. Front. Chem. 2022, 10, 867318. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sun, Z.; Zhang, B.; Lv, M.; Xu, H. Non-food bioactive products: Semisynthesis, biological activities, and mechanisms of action of oximinoether derivatives of matrine from Sophora flavescens. Ind. Crops Prod. 2019, 131, 134–141. [Google Scholar] [CrossRef]
- Rashid, H.u.; Xu, Y.; Muhammad, Y.; Wang, L.; Jiang, J. Research advances on anticancer activities of matrine and its derivatives: An updated overview. Eur. J. Med. Chem. 2019, 161, 205–238. [Google Scholar] [CrossRef]
- Pu, J.; Fang, F.-F.; Li, X.-Q.; Shu, Z.-H.; Jiang, Y.-P.; Han, T.; Peng, W.; Zheng, C.-J. Matrine Exerts a Strong Anti-Arthritic Effect on Type II Collagen-Induced Arthritis in Rats by Inhibiting Inflammatory Responses. Int. J. Mol. Sci. 2016, 17, 1410. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, F.-P.; Huang, Y.-R.; Li, H.-D.; Cao, X.-Y.; You, Y.; Meng, Z.-F.; Sun, K.-Y.; Shen, X.-Y. Matrine suppresses NLRP3 inflammasome activation via regulating PTPN2/JNK/SREBP2 pathway in sepsis. Phytomedicine 2023, 109, 154574. [Google Scholar] [CrossRef]
- Li, P.; Lei, J.; Hu, G.; Chen, X.; Liu, Z.; Yang, J. Matrine Mediates Inflammatory Response via Gut Microbiota in TNBS-Induced Murine Colitis. Front. Physiol. 2019, 10, 28. [Google Scholar] [CrossRef]
- Wirtz, S.; Popp, V.; Kindermann, M.; Gerlach, K.; Weigmann, B.; Fichtner-Feigl, S.; Neurath, M.F. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat. Protoc. 2017, 12, 1295–1309. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wei, S.; Marabada, D.; Wang, Z.; Huang, Q. Research Progress of Natural Matrine Compounds and Synthetic Matrine Derivatives. Molecules 2023, 28, 5780. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Yang, Y.; Zhu, Y.; Chen, Q.; Zhao, T.; Xiao, Z.; Wang, M.; Song, X.; Jiang, Y.; Yang, Y.; et al. Oral Metal-Free Melanin Nanozymes for Natural and Durable Targeted Treatment of Inflammatory Bowel Disease (IBD). Small 2023, 19, 2207350. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Cho, K.A.; Kang, J.L.; Kim, K.H.; Woo, S.Y. Comparison of experimental mouse models of inflammatory bowel disease. Int. J. Mol. Med. 2014, 33, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Y.; Thakur, K.; Feng, J.Y.; Zhang, J.G.; Hu, F.; Cespedes-Acuna, C.L.; Liao, C.; Wei, Z.J. Riboflavin Bioenriched Soymilk Alleviates Oxidative Stress Mediated Liver Injury, Intestinal Inflammation, and Gut Microbiota Modification in B(2) Depletion-Repletion Mice. J. Agric. Food Chem. 2022, 70, 3818–3831. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.A.; Choi, H.I.; Hong, S.W.; Kang, S.; Jegal, H.Y.; Choi, E.W.; Park, B.S.; Kim, J.S. Extracellular Vesicles Derived from Kefir Grain Lactobacillus Ameliorate Intestinal Inflammation via Regulation of Proinflammatory Pathway and Tight Junction Integrity. Biomedicines 2020, 8, 522. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gong, Y.; Xie, Y.; Sun, Q.; Li, Y. Clostridium butyricum protects the epithelial barrier by maintaining tight junction protein expression and regulating microflora in a murine model of dextran sodium sulfate-induced colitis. Scand. J. Gastroenterol. 2018, 53, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, X.; Liu, J.; Chen, M.; Huang, M.; Huang, G.; Chen, X.; Du, Q.; Su, J.; Lin, R. Ethanol extract of Centella asiatica alleviated dextran sulfate sodium-induced colitis: Restoration on mucosa barrier and gut microbiota homeostasis. J. Ethnopharmacol. 2021, 267, 113445. [Google Scholar] [CrossRef] [PubMed]
- Noack, M.; Miossec, P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun. Rev. 2014, 13, 668–677. [Google Scholar] [CrossRef]
- Wilson, D.C.; Inflammatory Bowel Disease, W.G. S1154 A Comprehensive Review of the English Language Evidence Base for the Management of Paediatric Inflammatory Bowel Disease (IBD) Illustrates the Difficulties for Evidence-Based Clinical Guideline Formation. Gastroenterology 2008, 134, A-189. [Google Scholar] [CrossRef]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54 e42; quiz e30. [Google Scholar] [CrossRef] [PubMed]
- Laroui, H.; Ingersoll, S.A.; Liu, H.C.; Baker, M.T.; Ayyadurai, S.; Charania, M.A.; Laroui, F.; Yan, Y.; Sitaraman, S.V.; Merlin, D. Dextran sodium sulfate (DSS) induces colitis in mice by forming nano-lipocomplexes with medium-chain-length fatty acids in the colon. PLoS ONE 2012, 7, e32084. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zuo, S.; Tan, H.; Hu, J.; Cheng, J.; Wu, Q.; Nie, S. Preventive effects of pectin with various degrees of esterification on ulcerative colitis in mice. Food Funct. 2020, 11, 2886–2897. [Google Scholar] [CrossRef]
- Neurath, M.F. Targeting cytokines in inflammatory bowel disease. Sci. Transl. Med. 2022, 14, eabq4473. [Google Scholar] [CrossRef]
- Chen, B.; Luo, J.; Han, Y.; Du, H.; Liu, J.; He, W.; Zhu, J.; Xiao, J.; Wang, J.; Cao, Y.; et al. Dietary Tangeretin Alleviated Dextran Sulfate Sodium-Induced Colitis in Mice via Inhibiting Inflammatory Response, Restoring Intestinal Barrier Function, and Modulating Gut Microbiota. J. Agric. Food Chem. 2021, 69, 7663–7674. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Du, X.; Chen, S.; Liang, J.; Huang, S.; Hou, S.; Gao, J.; Ding, P. Effect of vitexin on alleviating liver inflammation in a dextran sulfate sodium (DSS)-induced colitis model. Biomed. Pharmacother. 2020, 121, 109683. [Google Scholar] [CrossRef]
- Garbers, C.; Hermanns, H.M.; Schaper, F.; Muller-Newen, G.; Grotzinger, J.; Rose-John, S.; Scheller, J. Plasticity and cross-talk of interleukin 6-type cytokines. Cytokine Growth Factor Rev. 2012, 23, 85–97. [Google Scholar] [CrossRef]
- Li, R.; Chen, Y.; Shi, M.; Xu, X.; Zhao, Y.; Wu, X.; Zhang, Y. Gegen Qinlian decoction alleviates experimental colitis via suppressing TLR4/NF-kappaB signaling and enhancing antioxidant effect. Phytomedicine 2016, 23, 1012–1020. [Google Scholar] [CrossRef]
- Stevenson, B.R.; Siliciano, J.D.; Mooseker, M.S.; Goodenough, D.A. Identification of ZO-1: A high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J. Cell Biol. 1986, 103, 755–766. [Google Scholar] [CrossRef]
- Furuse, M.; Itoh, M.; Hirase, T.; Nagafuchi, A.; Yonemura, S.; Tsukita, S.; Tsukita, S. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J. Cell Biol. 1994, 127, 1617–1626. [Google Scholar] [CrossRef]
- Sheng, K.; Xu, Y.; Kong, X.; Wang, J.; Zha, X.; Wang, Y. Probiotic Bacillus cereus Alleviates Dextran Sulfate Sodium-Induced Colitis in Mice through Improvement of the Intestinal Barrier Function, Anti-Inflammation, and Gut Microbiota Modulation. J. Agric. Food Chem. 2021, 69, 14810–14823. [Google Scholar] [CrossRef] [PubMed]
- Schoultz, I.; Keita, Å.V. Cellular and Molecular Therapeutic Targets in Inflammatory Bowel Disease—Focusing on Intestinal Barrier Function. Cells 2019, 8, 193. [Google Scholar] [CrossRef] [PubMed]
- Mottet, C.; Uhlig, H.H.; Powrie, F. Cutting Edge: Cure of Colitis by CD4+CD25+ Regulatory T Cells1. J. Immunol. 2003, 170, 3939–3943. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, Z.; Dardalhon, V.; Xiao, S.; Thalhamer, T.; Liao, M.; Madi, A.; Franca, R.F.; Han, T.; Oukka, M.; et al. The transcription factor musculin promotes the unidirectional development of peripheral T(reg) cells by suppressing the T(H)2 transcriptional program. Nat. Immunol. 2017, 18, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.B.; Kang, Z.P.; Zhou, B.G.; Wang, H.Y.; Long, J.; Zhou, W.; Zhao, H.M.; Liu, D.Y. Curcumin Regulated the Homeostasis of Memory T Cell and Ameliorated Dextran Sulfate Sodium-Induced Experimental Colitis. Front. Pharmacol. 2020, 11, 630244. [Google Scholar] [CrossRef] [PubMed]
- Mehandru, S.; Colombel, J.F. The intestinal barrier, an arbitrator turned provocateur in IBD. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 83–84. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Qi, Y.; Qu, S.; Chen, X.; Li, A.; Hendi, M.; Xu, C.; Wang, L.; Hou, T.; Si, J.; et al. B. adolescentis ameliorates chronic colitis by regulating Treg/Th2 response and gut microbiota remodeling. Gut Microbes 2021, 13, 1826746. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cao, W.; Xie, J.; Che, H.; Liu, L.; Dong, X.; Song, L.; Xie, W. alpha-D-1,6-glucan from Castanea mollissima Blume alleviates dextran sulfate sodium-induced colitis in vivo. Carbohydr. Polym. 2022, 289, 119410. [Google Scholar] [CrossRef]
- Zhang, T.; Ji, X.; Lu, G.; Zhang, F. The potential of Akkermansia muciniphila in inflammatory bowel disease. Appl. Microbiol. Biotechnol. 2021, 105, 5785–5794. [Google Scholar] [CrossRef]
- Gibson-Corley, K.N.; Olivier, A.K.; Meyerholz, D.K. Principles for valid histopathologic scoring in research. Vet. Pathol. 2013, 50, 1007–1015. [Google Scholar] [CrossRef]
- Hu, J.; Huang, H.; Che, Y.; Ding, C.; Zhang, L.; Wang, Y.; Hao, H.; Shen, H.; Cao, L. Qingchang Huashi Formula attenuates DSS-induced colitis in mice by restoring gut microbiota-metabolism homeostasis and goblet cell function. J. Ethnopharmacol. 2021, 266, 113394. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Zhu, M.; Wang, K.; Zhao, X.; Hu, L.; Jing, W.; Lu, H.; Wang, S. Dihydromyricetin improves DSS-induced colitis in mice via modulation of fecal-bacteria-related bile acid metabolism. Pharmacol. Res. 2021, 171, 105767. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Yu, F.; Li, D.; Chen, Y.; Zhang, M.; Lu, K.; Wang, N.; Hu, S.; Zhao, Y.; Xu, H. Polydopamine-cladded montmorillonite micro-sheets as therapeutic platform repair the gut mucosal barrier of murine colitis through inhibiting oxidative stress. Mater. Today Bio 2023, 20, 100654. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Wusiman, A.; Gu, P.; Mao, N.; Xu, S.; Zhu, T.; He, J.; Liu, Z.; Wang, D. Supplementation of Alhagi honey polysaccharides contributes to the improvement of the intestinal immunity regulating the structure of intestinal flora in mice. Food Funct. 2021, 12, 9693–9707. [Google Scholar] [CrossRef] [PubMed]
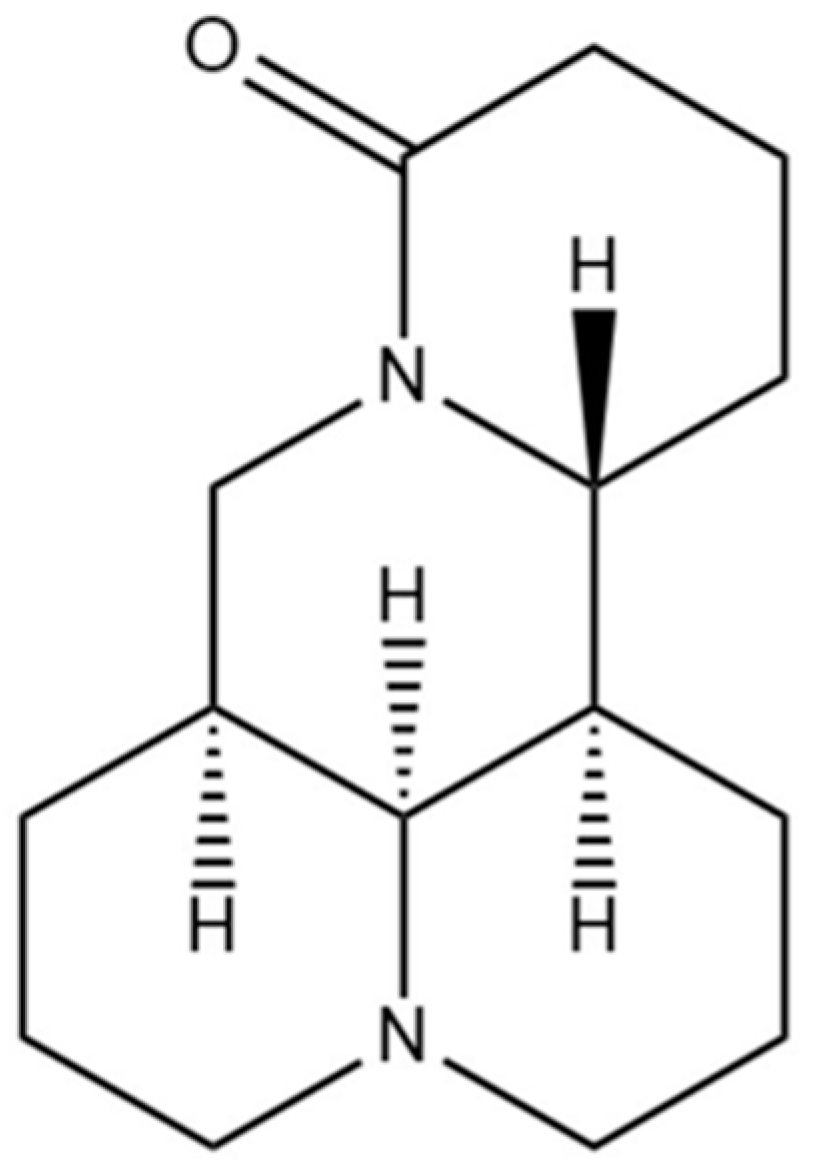



| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| β-actin | GGGCCGTATTCCCGAGTATC | TTTTGGACTGCGCCTCATCT |
| IL-1ß | TGCCACCTTTTGACAGTGATG | TTCTTGTGACCCTGAGCGAC |
| iNOs | CATTCAGATCCCGAAACGCT | TGTAGGACAATCCACAACTCGC |
| TNF-α | CAGGCGGTGCCTATGTCTC | CGATCACCCCGAAGTTCAGTAG |
| IL-6 | CCAAGAGGTGAGTGCTTCCC | CTGTTGTTCAGACTCTCTCCCT |
| IL-10 | ATGCTGCCTGCTCTTACTGACTG | CCCAAGTAACCCTTAAAGTCCTGC |
| IL-17A | TTTAACTCCCTTGGCGCAAAA | CTTTCCCTCCGCATTGACAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, N.; Yu, Y.; He, J.; Yang, Y.; Liu, Z.; Lu, Y.; Wang, D. Matrine Ameliorates DSS-Induced Colitis by Suppressing Inflammation, Modulating Oxidative Stress and Remodeling the Gut Microbiota. Int. J. Mol. Sci. 2024, 25, 6613. https://doi.org/10.3390/ijms25126613
Mao N, Yu Y, He J, Yang Y, Liu Z, Lu Y, Wang D. Matrine Ameliorates DSS-Induced Colitis by Suppressing Inflammation, Modulating Oxidative Stress and Remodeling the Gut Microbiota. International Journal of Molecular Sciences. 2024; 25(12):6613. https://doi.org/10.3390/ijms25126613
Chicago/Turabian StyleMao, Ningning, Yaming Yu, Jin He, Yang Yang, Zhenguang Liu, Yu Lu, and Deyun Wang. 2024. "Matrine Ameliorates DSS-Induced Colitis by Suppressing Inflammation, Modulating Oxidative Stress and Remodeling the Gut Microbiota" International Journal of Molecular Sciences 25, no. 12: 6613. https://doi.org/10.3390/ijms25126613
APA StyleMao, N., Yu, Y., He, J., Yang, Y., Liu, Z., Lu, Y., & Wang, D. (2024). Matrine Ameliorates DSS-Induced Colitis by Suppressing Inflammation, Modulating Oxidative Stress and Remodeling the Gut Microbiota. International Journal of Molecular Sciences, 25(12), 6613. https://doi.org/10.3390/ijms25126613






