Gas6 and Protein S Ligands Cooperate to Regulate MerTK Rhythmic Activity Required for Circadian Retinal Phagocytosis
Abstract
1. Introduction
2. Results
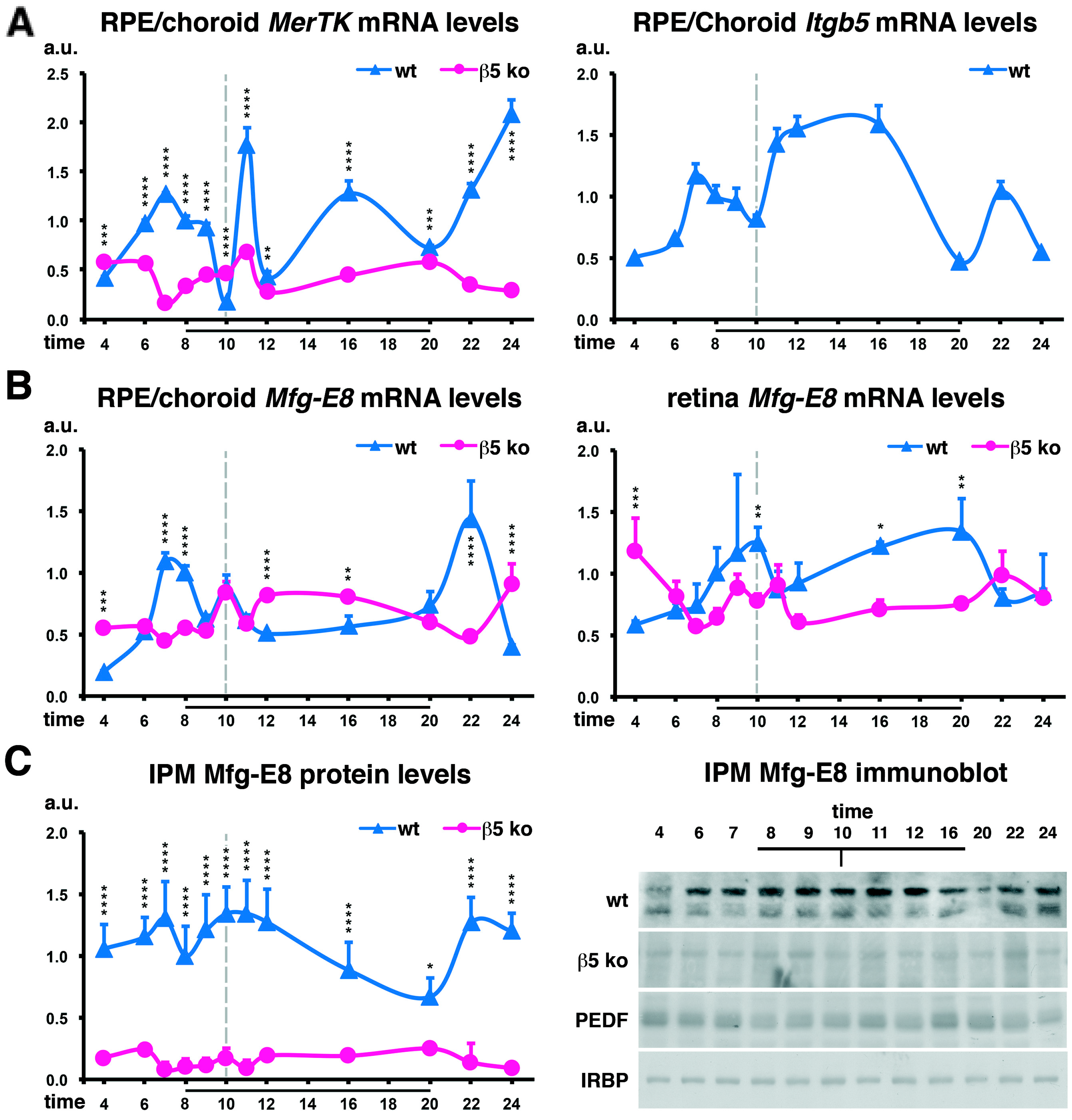
2.1. Diminished MerTK and Mfg-E8 Expression in β5−/− Mice Devoid of the Phagocytic Peak
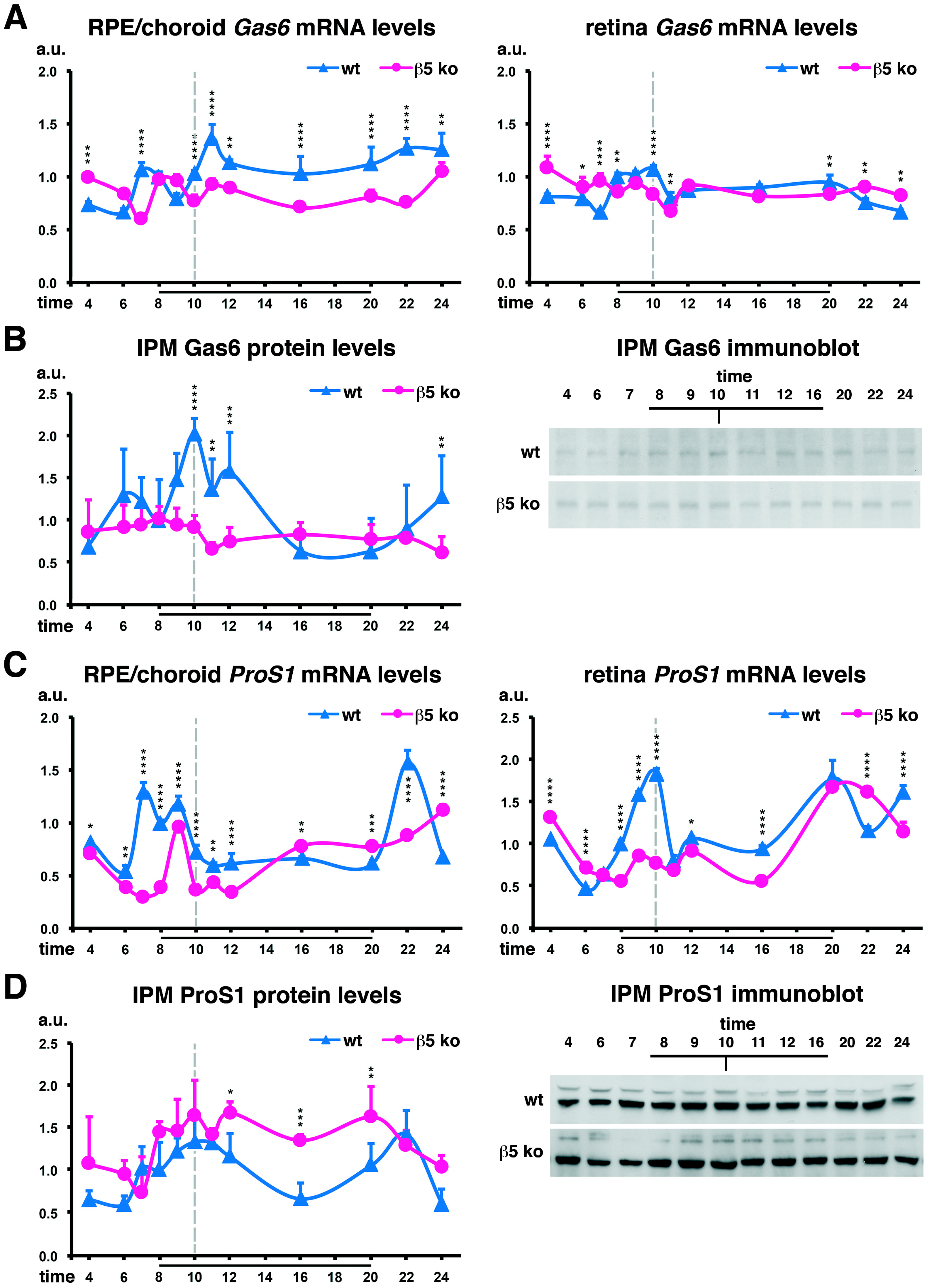
2.2. Complementary Gas6 and Protein S Expression Profiles
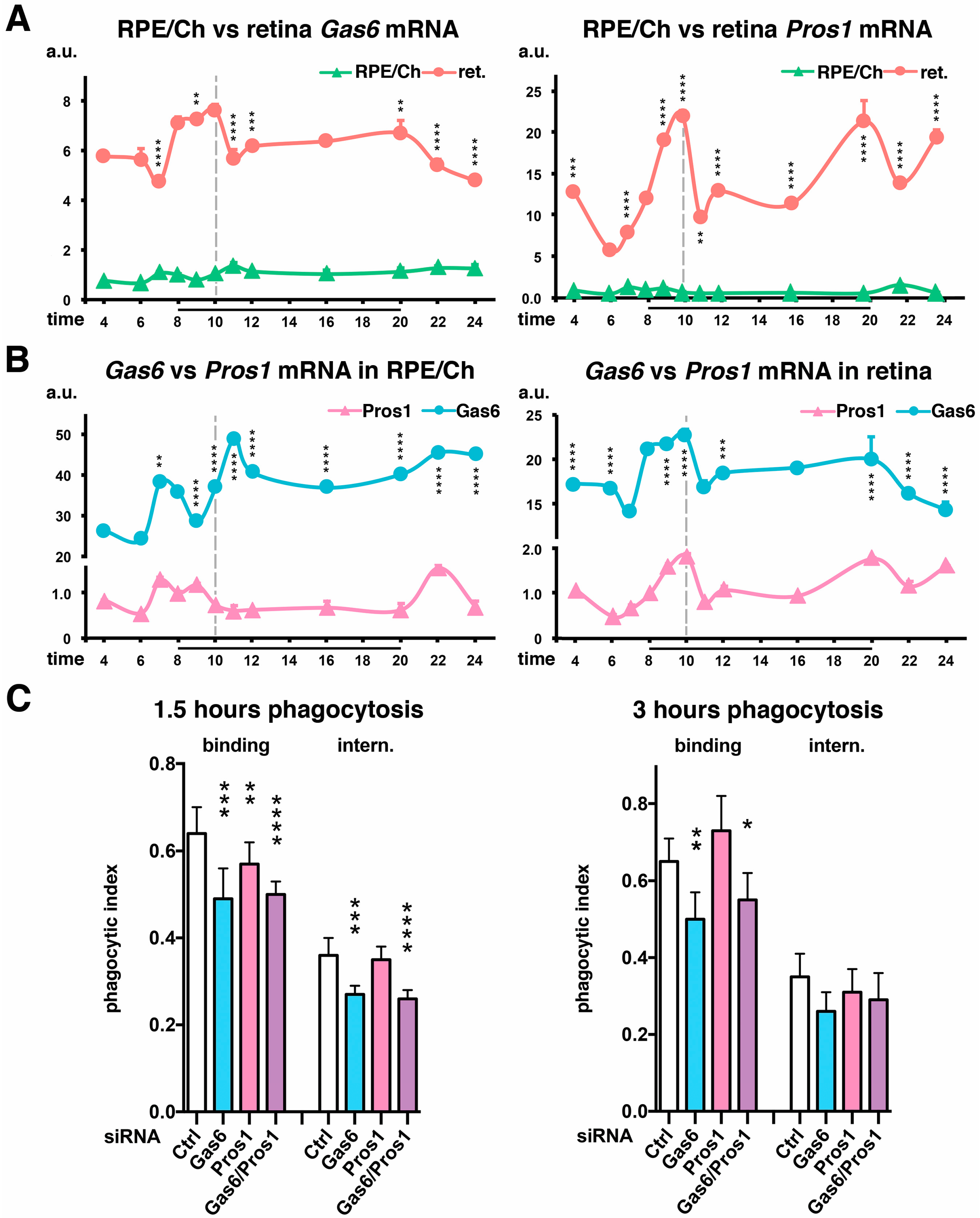
2.3. Importance of the Relative Expression Levels of Each Ligand
2.4. Gas6 and Protein S Recognize Different Amino Acids of MerTK Ig-like Domains
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Animals
4.3. RNA Extraction, Reverse Transcription, and Real-Time Quantitative PC
4.4. Retrieval of Soluble Proteins, Sample Lysis, and Immunoblotting
4.5. MerTK cDNA Mutagenesis
4.6. Cell Culture and Transfection
4.7. POS Isolation and Phagocytosis
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Young, R.W.; Bok, D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J. Cell Biol. 1969, 42, 392–403. [Google Scholar] [CrossRef] [PubMed]
- LaVail, M.M. Rod outer segment disk shedding in rat retina: Relationship to cyclic lighting. Science 1976, 194, 1071–1074. [Google Scholar] [CrossRef] [PubMed]
- Bok, D.; Hall, M.O. The role of the pigment epithelium in the etiology of inherited retinal dystrophy in the rat. J. Cell Biol. 1971, 49, 664–682. [Google Scholar] [CrossRef] [PubMed]
- Nandrot, E.F.; Kim, Y.; Brodie, S.E.; Huang, X.; Sheppard, D.; Finnemann, S.C. Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking alphavbeta5 integrin. J. Exp. Med. 2004, 200, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Savill, J.; Dransfield, I.; Hogg, N.; Haslett, C. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature 1990, 343, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Finnemann, S.C.; Bonilha, V.L.; Marmorstein, A.D.; Rodriguez-Boulan, E. Phagocytosis of rod outer segments by retinal pigment epithelial cells requires alpha(v)beta5 integrin for binding but not for internalization. Proc. Natl. Acad. Sci. USA 1997, 94, 12932–12937. [Google Scholar] [CrossRef] [PubMed]
- Hanayama, R.; Tanaka, M.; Miwa, K.; Shinohara, A.; Iwamatsu, A.; Nagata, S. Identification of a factor that links apoptotic cells to phagocytes. Nature 2002, 417, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Nandrot, E.F.; Anand, M.; Almeida, D.; Atabai, K.; Sheppard, D.; Finnemann, S.C. Essential role for MFG-E8 as ligand for alphavbeta5 integrin in diurnal retinal phagocytosis. Proc. Natl. Acad. Sci. USA 2007, 104, 12005–12010. [Google Scholar] [CrossRef] [PubMed]
- Lemke, G. Phosphatidylserine Is the Signal for TAM Receptors and Their Ligands. Trends Biochem. Sci. 2017, 42, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, L.; Connor, M.P.; Chen, J.; Langen, R.; Finnemann, S.C. Diurnal, localized exposure of phosphatidylserine by rod outer segment tips in wild-type but not Itgb5−/− or Mfge8−/− mouse retina. Proc. Natl. Acad. Sci. USA 2012, 109, 8145–8148. [Google Scholar] [CrossRef]
- Scott, R.S.; McMahon, E.J.; Pop, S.M.; Reap, E.A.; Caricchio, R.; Cohen, P.L.; Earp, H.S.; Matsushima, G.K. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature 2001, 411, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Nandrot, E.; Dufour, E.M.; Provost, A.C.; Péquignot, M.O.; Bonnel, S.; Gogat, K.; Marchant, D.; Rouillac, C.; Sépulchre de Condé, B.; Bihoreau, M.T.; et al. Homozygous deletion in the coding sequence of the c-mer gene in RCS rats unravels general mechanisms of physiological cell adhesion and apoptosis. Neurobiol. Dis. 2000, 7, 586–599. [Google Scholar] [CrossRef]
- D’Cruz, P.M.; Yasumura, D.; Weir, J.; Matthes, M.T.; Abderrahim, H.; LaVail, M.M.; Vollrath, D. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum. Mol. Genet. 2000, 9, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Gal, A.; Li, Y.; Thompson, D.A.; Weir, J.; Orth, U.; Jacobson, S.G.; Apfelstedt-Sylla, E.; Vollrath, D. Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat. Genet. 2000, 26, 270–271. [Google Scholar] [CrossRef] [PubMed]
- Tschernutter, M.; Jenkins, S.A.; Waseem, N.H.; Saihan, Z.; Holder, G.E.; Bird, A.C.; Bhattacharya, S.S.; Ali, R.R.; Webster, A.R. Clinical characterisation of a family with retinal dystrophy caused by mutation in the Mertk gene. Br. J. Ophthalmol. 2006, 90, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Cheong, H.S.; Lee, S.O.; Choi, C.B.; Sung, Y.K.; Shin, H.D.; Bae, S.C. MERTK polymorphisms associated with risk of haematological disorders among Korean SLE patients. Rheumatology 2007, 46, 209–214. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dickhout, J.G.; Basseri, S.; Austin, R.C. Macrophage function and its impact on atherosclerotic lesion composition, progression, and stability: The good, the bad, and the ugly. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1413–1415. [Google Scholar] [CrossRef] [PubMed]
- Cummings, C.T.; Deryckere, D.; Earp, H.S.; Graham, D.K. Molecular pathways: MERTK signaling in cancer. Clin. Cancer Res. 2013, 19, 5275–5280. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Singh, S.; Georgescu, M.M.; Birge, R.B. A role for Mer tyrosine kinase in alphavbeta5 integrin-mediated phagocytosis of apoptotic cells. J. Cell Sci. 2005, 118, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Nandrot, E.F.; Silva, K.E.; Scelfo, C.; Finnemann, S.C. Retinal pigment epithelial cells use a MerTK-dependent mechanism to limit the phagocytic particle binding activity of αvβ5 integrin. Biol. Cell 2012, 104, 326–341. [Google Scholar] [CrossRef] [PubMed]
- Hafizi, S.; Dahlbäck, B. Signalling and functional diversity within the Axl subfamily of receptor tyrosine kinases. Cytokine Growth Factor Rev. 2006, 17, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Lemke, G. Biology of the TAM receptors. Cold Spring Harb. Perspect. Biol. 2013, 5, a009076. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-García, L.; Mayer, C.; Burrola, P.G.; Huang, Y.; Shokhirev, M.N.; Lemke, G. The TAM receptor tyrosine kinases Axl and Mer drive the maintenance of highly phagocytic macrophages. Front. Immunol. 2022, 13, 960401. [Google Scholar] [CrossRef] [PubMed]
- Zagórska, A.; Través, P.G.; Lew, E.D.; Dransfield, I.; Lemke, G. Diversification of TAM receptor tyrosine kinase function. Nat. Immunol. 2014, 15, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Prasad, D.; Rothlin, C.V.; Burrola, P.; Burstyn-Cohen, T.; Lu, Q.; Garcia de Frutos, P.; Lemke, G. TAM receptor function in the retinal pigment epithelium. Mol. Cell. Neurosci. 2006, 33, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Stitt, T.N.; Conn, G.; Goret, M.; Lai, C.; Bruno, J.; Radziejewski, C.; Mattsson, K.; Fisher, J.; Gies, D.R.; Jones, P.F.; et al. The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell 1995, 80, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Varnum, B.C.; Young, C.; Elliott, G.; Garcia, A.; Bartley, T.D.; Fridell, Y.W.; Hunt, R.W.; Trail, G.; Clogston, C.; Toso, R.J.; et al. Axl receptor tyrosine kinase stimulated by the vitamin K-dependent protein encoded by growth-arrest-specific gene 6. Nature 1995, 373, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Hafizi, S.; Dahlbäck, B. Gas6 and protein S. Vitamin K-dependent ligands for the Axl receptor tyrosine kinase subfamily. FEBS J. 2006, 273, 5231–5244. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, L.; Négrier, C.; Boukerche, H. Protein S: A multifunctional anticoagulant vitamin K-dependent protein at the crossroads of coagulation, inflammation, angiogenesis, and cancer. Crit. Rev. Oncol. Hematol. 2013, 88, 637–654. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.A.; Maylock, C.A.; Williams, J.A.; Paweletz, C.P.; Shu, H.; Shacter, E. Serum-derived protein S binds to phosphatidylserine and stimulates the phagocytosis of apoptotic cells. Nat. Immunol. 2003, 4, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Uehara, H.; Shacter, E. Auto-oxidation and oligomerization of protein S on the apoptotic cell surface is required for Mer tyrosine kinase-mediated phagocytosis of apoptotic cells. J. Immunol. 2008, 180, 2522–2530. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Ishimoto, Y.; Kishino, J.; Umeda, M.; Inoue, K.; Nagata, K.; Ohashi, K.; Mizuno, K.; Arita, H. Cell adhesion to phosphatidylserine mediated by a product of growth arrest-specific gene 6. J. Biol. Chem. 1997, 272, 29411–29414. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.O.; Obin, M.S.; Prieto, A.L.; Burgess, B.L.; Abrams, T.A. Gas6 binding to photoreceptor outer segments requires gamma-carboxyglutamic acid (Gla) and Ca(2+) and is required for OS phagocytosis by RPE cells in vitro. Exp. Eye Res. 2002, 75, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Wanke, F.; Gutbier, S.; Rümmelin, A.; Steinberg, M.; Hughes, L.D.; Koenen, M.; Komuczki, J.; Regan-Komito, D.; Wagage, S.; Hesselmann, J.; et al. Ligand-dependent kinase activity of MERTK drives efferocytosis in human iPSC-derived macrophages. Cell Death Dis. 2021, 12, 538. [Google Scholar] [CrossRef] [PubMed]
- Lew, E.D.; Oh, J.; Burrola, P.G.; Lax, I.; Zagórska, A.; Través, P.G.; Schlessinger, J.; Lemke, G. Differential TAM receptor-ligand-phospholipid interactions delimit differential TAM bioactivities. Elife 2014, 3, e03385. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.O.; Obin, M.S.; Heeb, M.J.; Burgess, B.L.; Abrams, T.A. Both protein S and Gas6 stimulate outer segment phagocytosis by cultured rat retinal pigment epithelial cells. Exp. Eye Res. 2005, 81, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Burstyn-Cohen, T.; Lew, E.D.; Través, P.G.; Burrola, P.G.; Hash, J.C.; Lemke, G. Genetic dissection of TAM receptor-ligand interaction in retinal pigment epithelial cell phagocytosis. Neuron 2012, 76, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Law, A.L.; Parinot, C.; Chatagnon, J.; Gravez, B.; Sahel, J.A.; Bhattacharya, S.S.; Nandrot, E.F. Cleavage of Mer tyrosine kinase (MerTK) from the cell surface contributes to the regulation of retinal phagocytosis. J. Biol. Chem. 2015, 290, 4941–4952. [Google Scholar] [CrossRef] [PubMed]
- Enderlin, J.; Rieu, Q.; Réty, S.; Vanoni, E.M.; Roux, S.; Dégardin, J.; César, Q.; Augustin, S.; Nous, C.; Cai, B.; et al. Retinal atrophy, inflammation, phagocytic and metabolic disruptions develop in the MerTK-cleavage-resistant mouse model. Front. Neurosci. 2024, 18, 1256522. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.H.; Fisher, S.K.; Erickson, P.A.; Tabor, G.A. Rod and cone disc shedding in the rhesus monkey retina: A quantitative study. Exp. Eye Res. 1980, 30, 559–574. [Google Scholar] [CrossRef] [PubMed]
- Finnemann, S.C. Focal adhesion kinase signaling promotes phagocytosis of integrin-bound photoreceptors. EMBO J. 2003, 22, 4143–4154. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Knyazev, P.G.; Clout, N.J.; Cheburkin, Y.; Göhring, W.; Ullrich, A.; Timpl, R.; Hohenester, E. Structural basis for Gas6-Axl signalling. EMBO J. 2006, 25, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Finnemann, S.C.; Rodriguez-Boulan, E. Macrophage and retinal pigment epithelium phagocytosis: Apoptotic cells and photoreceptors compete for alphavbeta3 and alphavbeta5 integrins, and protein kinase C regulates alphavbeta5 binding and cytoskeletal linkage. J. Exp. Med. 1999, 190, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Vargas, J.A.; Finnemann, S.C. Differences in diurnal rhythm of rod outer segment renewal between 129T2/SvEmsJ and C57BL/6J mice. Int. J. Mol. Sci. 2022, 23, 9466. [Google Scholar] [CrossRef] [PubMed]
- Goyal, V.; DeVera, C.; Laurent, V.; Sellers, J.; Chrenek, M.A.; Hicks, D.; Baba, K.; Iuvone, P.M.; Tosini, G. Dopamine 2 receptor signaling controls the daily burst in phagocytic activity in the mouse retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2020, 61, 10. [Google Scholar] [CrossRef] [PubMed]
- DeVera, C.; Dixon, J.; Chrenek, M.A.; Baba, K.; Le, Y.Z.; Iuvone, P.M.; Tosini, G. The circadian clock in the retinal pigment epithelium controls the diurnal rhythm of phagocytic activity. Int. J. Mol. Sci. 2022, 23, 5302. [Google Scholar] [CrossRef] [PubMed]
- Kociok, N.; Joussen, A.M. Varied expression of functionally important genes of RPE and choroid in the macula and in the periphery of normal human eyes. Graefe’s Arch. Clin. Exp. Ophthalmol. 2007, 245, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Tsou, W.I.; Nguyen, K.Q.; Calarese, D.A.; Garforth, S.J.; Antes, A.L.; Smirnov, S.V.; Almo, S.C.; Birge, R.B.; Kotenko, S.V. Receptor tyrosine kinases, TYRO3, AXL, and MER, demonstrate distinct patterns and complex regulation of ligand-induced activation. J. Biol. Chem. 2014, 289, 25750–25763. [Google Scholar] [CrossRef] [PubMed]
- Yanagihashi, Y.; Segawa, K.; Maeda, R.; Nabeshima, Y.I.; Nagata, S. Mouse macrophages show different requirements for phosphatidylserine receptor Tim4 in efferocytosis. Proc. Natl. Acad. Sci. USA 2017, 114, 8800–8805. [Google Scholar] [CrossRef] [PubMed]
- Pilli, V.S.; Datta, A.; Dorsey, A.; Liu, B.; Majumber, R. Modulation of protein S and growth arrest specific 6 protein signaling inhibits pancreatic cancer cell survival and proliferation. Oncol. Rep. 2020, 44, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Griffiths, M.; Wu, J.; Farese, R.V., Jr.; Sheppard, D. Normal development, wound healing, and adenovirus susceptibility in beta5-deficient mice. Mol. Cell. Biol. 2000, 20, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Parinot, C.; Rieu, Q.; Chatagnon, J.; Finnemann, S.C.; Nandrot, E.F. Large-scale purification of porcine or bovine photoreceptor outer segments for phagocytosis assays on retinal pigment epithelial cells. J. Vis. Exp. 2014, 94, 52100. [Google Scholar]


| Domain | Amino Acid Change | Sequence Change |
|---|---|---|
| Ig-like 1 | p.Gly122Arg | c.364G>C |
| p.Thr140Ala | c.418A>G | |
| p.Phe142Val | c.424T>G | |
| Ig-like 2 | p.Lys263Ile | c.788A>T |
| p.Lys269Leu | c.805A>C, c.806A>T |
| Mouse Gene Accession # | Forward Primer | Reverse Primer |
|---|---|---|
| Rplp0 NM_007475.5 | CCTGAAGTGCTCGACATCAC | TGCCAGGACGCGCTTGTAC |
| MerTK NM_008587 | CGTCTGTCCTAACCGTACCT | GTACTGTTGAGGATATGGACT |
| Itgb5 NM_001145884 | GGTTTCGGGTCTTTTGTTGAC | ACTCTGTCTGTGAGAGGCAG |
| Mfg-E8 NM_008594.2 | GCCTGAAGGTTAACATGTTCA | GTGTTATTCTTCAGGCCCAG |
| Gas6 NM_019521.2 | ATCAACCACGGCATGTGGC | CGGTGAGATTCAGGTGATAG |
| Pros1 NM_011173.2 | GCAGGAGTTGTCTTATATCTG | CACGAAGCGCAATCAGGAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parinot, C.; Chatagnon, J.; Rieu, Q.; Roux, S.; Néel, D.; Hamieh, F.; Nandrot, E.F. Gas6 and Protein S Ligands Cooperate to Regulate MerTK Rhythmic Activity Required for Circadian Retinal Phagocytosis. Int. J. Mol. Sci. 2024, 25, 6630. https://doi.org/10.3390/ijms25126630
Parinot C, Chatagnon J, Rieu Q, Roux S, Néel D, Hamieh F, Nandrot EF. Gas6 and Protein S Ligands Cooperate to Regulate MerTK Rhythmic Activity Required for Circadian Retinal Phagocytosis. International Journal of Molecular Sciences. 2024; 25(12):6630. https://doi.org/10.3390/ijms25126630
Chicago/Turabian StyleParinot, Célia, Jonathan Chatagnon, Quentin Rieu, Solène Roux, Dorine Néel, Florian Hamieh, and Emeline F. Nandrot. 2024. "Gas6 and Protein S Ligands Cooperate to Regulate MerTK Rhythmic Activity Required for Circadian Retinal Phagocytosis" International Journal of Molecular Sciences 25, no. 12: 6630. https://doi.org/10.3390/ijms25126630
APA StyleParinot, C., Chatagnon, J., Rieu, Q., Roux, S., Néel, D., Hamieh, F., & Nandrot, E. F. (2024). Gas6 and Protein S Ligands Cooperate to Regulate MerTK Rhythmic Activity Required for Circadian Retinal Phagocytosis. International Journal of Molecular Sciences, 25(12), 6630. https://doi.org/10.3390/ijms25126630





