Correlations between Gut Microbiota and Hematological, Inflammatory, Biochemical and Oxidative Stress Parameters in Treatment-Naïve Psoriasis Patients
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. Study Group
3.2. Ethical Aspects
3.3. Protocol
3.3.1. Assessing Oxidant Status through Free Oxygen Radical Test (FORT) Evaluation
3.3.2. Assessing Antioxidant Status through FORD Evaluation
3.3.3. Assessing Psoriasis Severity and the Impact on the Quality of Life of Psoriasis Patients
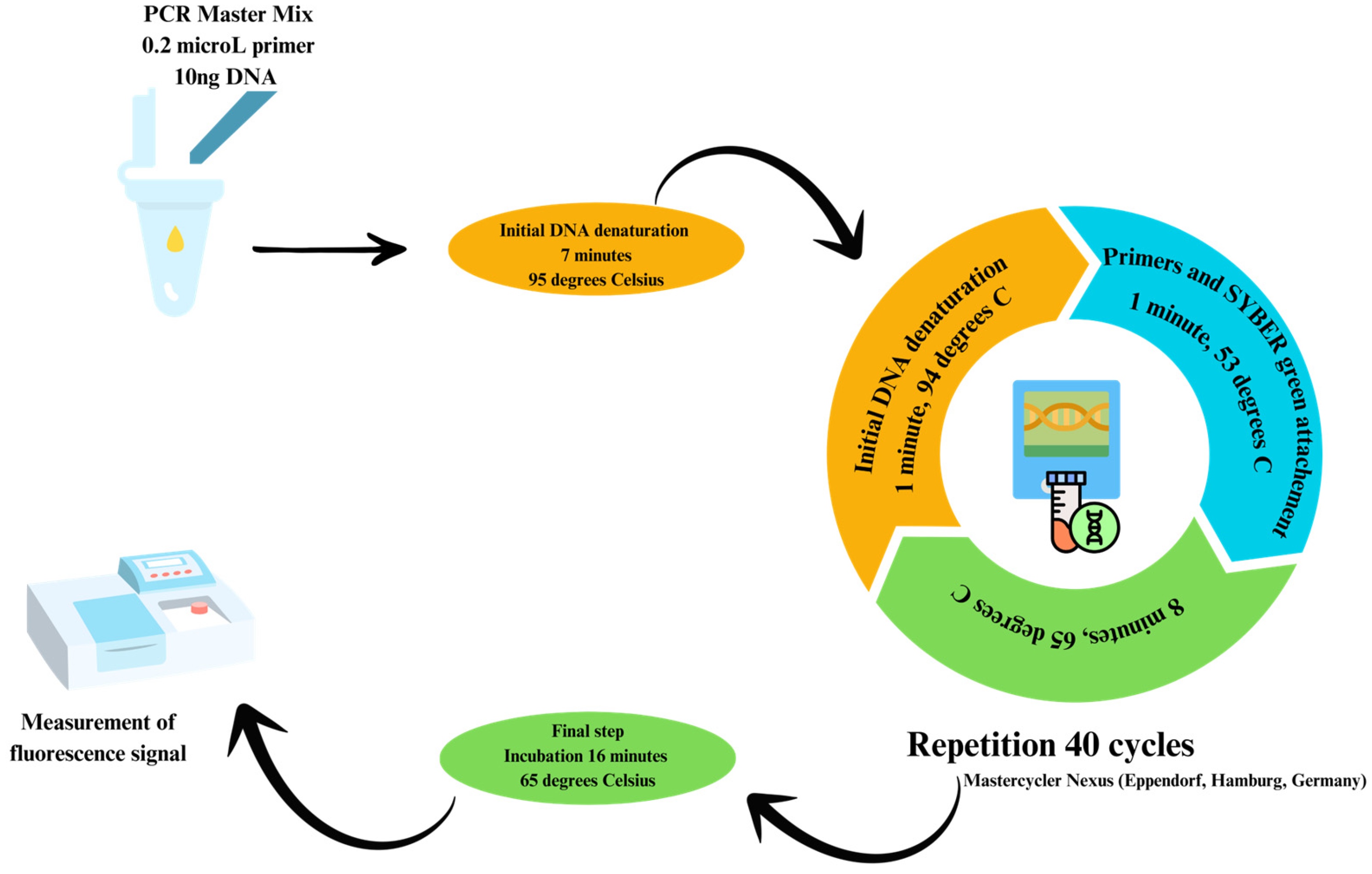
3.3.4. Assessing Gut Microbiota Composition
3.3.5. Assessing Hematological Parameters and Inflammatory Markers
3.4. Statistical Analysis
4. Discussions
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meneguin, S.; de Godoy, N.A.; Pollo, C.F.; Miot, H.A.; de Oliveira, C. Quality of Life of Patients Living with Psoriasis: A Qualitative Study. BMC Dermatol. 2020, 20, 22. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting Oxidative Stress in Disease: Promise and Limitations of Antioxidant Therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Vijay, A.; Valdes, A.M. Role of the Gut Microbiome in Chronic Diseases: A Narrative Review. Eur. J. Clin. Nutr. 2022, 76, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Durack, J.; Lynch, S.V. The Gut Microbiome: Relationships with Disease and Opportunities for Therapy. J. Exp. Med. 2019, 216, 20–40. [Google Scholar] [CrossRef] [PubMed]
- Dobrică, E.-C.; Cozma, M.-A.; Găman, M.-A.; Voiculescu, V.-M.; Găman, A.M. The Involvement of Oxidative Stress in Psoriasis: A Systematic Review. Antioxidants 2022, 11, 282. [Google Scholar] [CrossRef] [PubMed]
- Kadam, D.P.; Suryakar, A.N.; Ankush, R.D.; Kadam, C.Y.; Deshpande, K.H. Role of Oxidative Stress in Various Stages of Psoriasis. Indian J. Clin. Biochem. 2010, 25, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Vamanu, E.; Ene, M.; Biță, B.; Ionescu, C.; Crăciun, L.; Sârbu, I. In Vitro Human Microbiota Response to Exposure to Silver Nanoparticles Biosynthesized with Mushroom Extract. Nutrients 2018, 10, 607. [Google Scholar] [CrossRef]
- Avram, I.; Pelinescu, D.; Gatea, F.; Ionescu, R.; Barcan, A.; Rosca, R.; Zanfirescu, A.; Vamanu, E. Boletus Edulis Extract—A New Modulator of Dysbiotic Microbiota. Life 2023, 13, 1481. [Google Scholar] [CrossRef]
- Cozma, E.C.; Găman, M.-A.; Orzan, O.; Hamed, K.-V.; Voiculescu, V.M.; Găman, A.-M. Oxidative Stress and Inflammation Levels in a Population of Eastern European Naïve versus Treated Psoriasis Patients. Cureus 2023, 15, e48177. [Google Scholar] [CrossRef]
- Sikora, M.; Stec, A.; Chrabaszcz, M.; Knot, A.; Waskiel-Burnat, A.; Rakowska, A.; Olszewska, M.; Rudnicka, L. Gut Microbiome in Psoriasis: An Updated Review. Pathogens 2020, 9, 463. [Google Scholar] [CrossRef] [PubMed]
- Dei-Cas, I.; Giliberto, F.; Luce, L.; Dopazo, H.; Penas-Steinhardt, A. Metagenomic Analysis of Gut Microbiota in Non-Treated Plaque Psoriasis Patients Stratified by Disease Severity: Development of a New Psoriasis-Microbiome Index. Sci. Rep. 2020, 10, 12754. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Cantabrana, C.; Gómez, J.; Delgado, S.; Requena-López, S.; Queiro-Silva, R.; Margolles, A.; Coto, E.; Sánchez, B.; Coto-Segura, P. Gut Microbiota Dysbiosis in a Cohort of Patients with Psoriasis. Br. J. Dermatol. 2019, 181, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Gao, R.; Yu, N.; Zhu, Y.; Ding, Y.; Qin, H. Dysbiosis of Gut Microbiota Was Closely Associated with Psoriasis. Sci. China Life Sci. 2019, 62, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Loh, G.; Blaut, M. Role of Commensal Gut Bacteria in Inflammatory Bowel Diseases. Gut Microbes 2012, 3, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.-J.; Tsai, W.-C.; Hung, W.-C.; Hung, W.-W.; Chang, C.-C.; Dai, C.-Y.; Tsai, Y.-C. Gut Microbiota and Subclinical Cardiovascular Disease in Patients with Type 2 Diabetes Mellitus. Nutrients 2021, 13, 2679. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Meenatchi, R.; Ahmed, Z.H.T.; Thacharodi, A.; Rohinth; Kumar, R.R.S.; Varthan, M.K.H.; Hassan, S. Implications of the Gut Microbiome in Cardiovascular Diseases: Association of Gut Microbiome with Cardiovascular Diseases, Therapeutic Interventions and Multi-Omics Approach for Precision Medicine. Med. Microecol. 2024, 19, 100096. [Google Scholar] [CrossRef]
- Maciel-Fiuza, M.F.; Muller, G.C.; Campos, D.M.S.; do Socorro Silva Costa, P.; Peruzzo, J.; Bonamigo, R.R.; Veit, T.; Vianna, F.S.L. Role of Gut Microbiota in Infectious and Inflammatory Diseases. Front. Microbiol. 2023, 14, 1098386. [Google Scholar] [CrossRef]
- van den Munckhof, I.C.L.; Kurilshikov, A.; ter Horst, R.; Riksen, N.P.; Joosten, L.A.B.; Zhernakova, A.; Fu, J.; Keating, S.T.; Netea, M.G.; de Graaf, J.; et al. Role of Gut Microbiota in Chronic Low-grade Inflammation as Potential Driver for Atherosclerotic Cardiovascular Disease: A Systematic Review of Human Studies. Obes. Rev. 2018, 19, 1719–1734. [Google Scholar] [CrossRef]
- Samaddar, A.; van Nispen, J.; Armstrong, A.; Song, E.; Voigt, M.; Murali, V.; Krebs, J.; Manithody, C.; Denton, C.; Ericsson, A.C.; et al. Lower Systemic Inflammation Is Associated with Gut Firmicutes Dominance and Reduced Liver Injury in a Novel Ambulatory Model of Parenteral Nutrition. Ann. Med. 2022, 54, 1701–1713. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, W.-D.; Wang, Y.-D. The Relationship between Gut Microbiota and Inflammatory Diseases: The Role of Macrophages. Front. Microbiol. 2020, 11, 1065. [Google Scholar] [CrossRef] [PubMed]
- Appleton, J. The Gut-Brain Axis: Influence of Microbiota on Mood and Mental Health. Integr. Med. A Clin. J. 2018, 17, 28. [Google Scholar]
- Rieder, E.; Tausk, F. Psoriasis, a Model of Dermatologic Psychosomatic Disease: Psychiatric Implications and Treatments. Int. J. Dermatol. 2012, 51, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Hunter, H.J.A.; Griffiths, C.E.M.; Kleyn, C.E. Does Psychosocial Stress Play a Role in the Exacerbation of Psoriasis? Br. J. Dermatol. 2013, 169, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Heller, M.M.; Lee, E.S.; Koo, J.Y. Stress as an Influencing Factor in Psoriasis. Skin Ther. Lett. 2011, 16, 1–4. [Google Scholar]
- Huang, Y.; Shi, X.; Li, Z.; Shen, Y.; Shi, X.; Wang, L.; Li, G.; Yuan, Y.; Wang, J.; Zhang, Y.; et al. Possible Association of Firmicutes in the Gut Microbiota of Patients with Major Depressive Disorder. Neuropsychiatr. Dis. Treat. 2018, 14, 3329–3337. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered Fecal Microbiota Composition in Patients with Major Depressive Disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.M.; Johns, N.; Salek, S.; Finlay, A.Y. Correlating the Dermatology Life Quality Index with Psychiatric Measures: A Systematic Review. Clin. Dermatol. 2018, 36, 691–697. [Google Scholar] [CrossRef]
- Jiang, H.-Y.; Zhang, X.; Yu, Z.-H.; Zhang, Z.; Deng, M.; Zhao, J.-H.; Ruan, B. Altered Gut Microbiota Profile in Patients with Generalized Anxiety Disorder. J. Psychiatr. Res. 2018, 104, 130–136. [Google Scholar] [CrossRef]
- Zeng, M.Y.; Inohara, N.; Nuñez, G. Mechanisms of Inflammation-Driven Bacterial Dysbiosis in the Gut. Mucosal Immunol. 2017, 10, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Ng, S.C. The Gut Microbiota in the Pathogenesis and Therapeutics of Inflammatory Bowel Disease. Front. Microbiol. 2018, 9, 2247. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wu, F.; Zhou, Q.; Wei, W.; Yue, J.; Xiao, B.; Luo, Z. Lactobacillus and Intestinal Diseases: Mechanisms of Action and Clinical Applications. Microbiol. Res. 2022, 260, 127019. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Olejar, K.J.; On, S.L.W.; Chelikani, V. The Potential of Lactobacillus Spp. For Modulating Oxidative Stress in the Gastrointestinal Tract. Antioxidants 2020, 9, 610. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.; Singh, A. Gut Microbiome and Human Health: Exploring How the Probiotic Genus Lactobacillus Modulate Immune Responses. Front. Pharmacol. 2022, 13, 1042189. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, J.; Zhao, L.-L.; Yao, T.-T.; Chen, Y.; Ma, J.; Zhang, X.; Wang, J.-X.; Wang, Y.; Cui, Z.; et al. Gut Lactobacillus Level Is a Predictive Marker for Coronary Atherosclerotic Lesions Progress and Prognosis in Patients with Acute Coronary Syndrome. Front. Cell. Infect. Microbiol. 2021, 11, 687827. [Google Scholar] [CrossRef] [PubMed]
- Zang, C.; Liu, J.; Mao, M.; Zhu, W.; Chen, W.; Wei, B. Causal Associations between Gut Microbiota and Psoriasis: A Mendelian Randomization Study. Dermatol. Ther. 2023, 13, 2331–2343. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Niu, M.; Bi, J.; Du, N.; Liu, S.; Yang, K.; Li, H.; Yao, J.; Du, Y.; Duan, Y. Protective Effects of a New Generation of Probiotic Bacteroides Fragilis against Colitis in Vivo and in Vitro. Sci. Rep. 2023, 13, 15842. [Google Scholar] [CrossRef]
- Million, M.; Armstrong, N.; Khelaifia, S.; Guilhot, E.; Richez, M.; Lagier, J.-C.; Dubourg, G.; Chabriere, E.; Raoult, D. The Antioxidants Glutathione, Ascorbic Acid and Uric Acid Maintain Butyrate Production by Human Gut Clostridia in the Presence of Oxygen in Vitro. Sci. Rep. 2020, 10, 7705. [Google Scholar] [CrossRef]
- Buonacera, A.; Stancanelli, B.; Colaci, M.; Malatino, L. Neutrophil to Lymphocyte Ratio: An Emerging Marker of the Relationships between the Immune System and Diseases. Int. J. Mol. Sci. 2022, 23, 3636. [Google Scholar] [CrossRef] [PubMed]

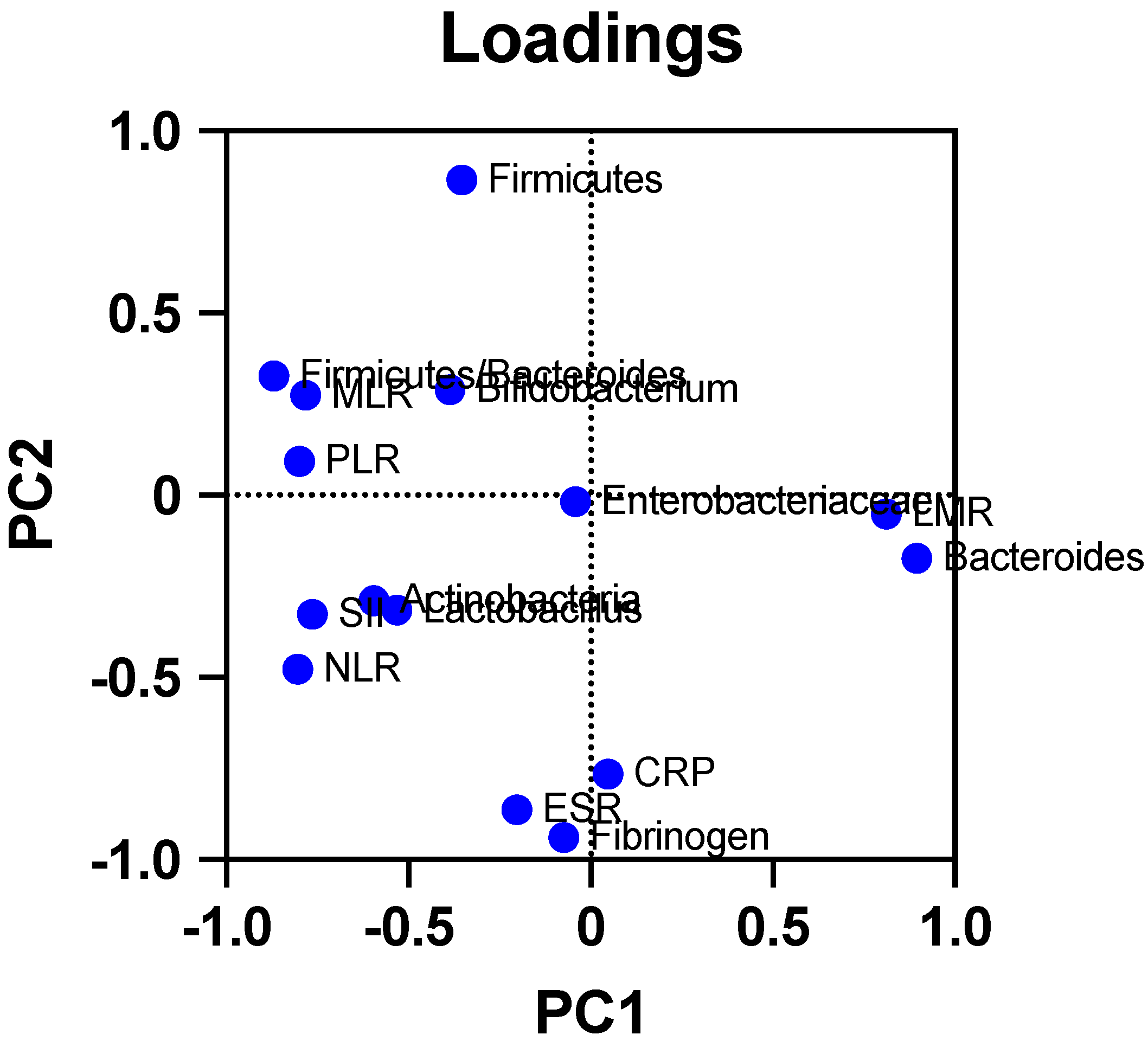

| Parameter | Mean Value ± SD 20 | Unit | Normal Range |
|---|---|---|---|
| Sex | 50% | % Female | - |
| Age | 47.9 ± 12.59 | Years | - |
| Disease duration time | 4.55 ± 3.43 | Years | - |
| Hematological parameters | |||
| WBC 1 | 7.23 ± 2.31 | ×103/μL | 4.6–10.2 |
| Neutrophils | 4.52 ± 2.03 | ×103/μL | 1.5–6.9 |
| Lymphocytes | 1.95 ± 0.56 | ×103/μL | 0.6–3.4 |
| Monocytes | 0.53 ± 0.15 | ×103/μL | 0.0–0.9 |
| Eosinophils | 0.19 ± 0.11 | ×103/μL | 0.0–0.7 |
| Basophils | 0.03 ± 0.01 | ×103/μL | 0.0–0.2 |
| RBC 2 | 5.02 ± 0.44 | ×103/μL | 3.8–5.3 |
| Hb 3 | 14.59 ± 1.23 | g/dL | 11.7–15.9 |
| HTC 4 | 44.02 ± 1.23 | % | 35.0–47.0 |
| Platelets | 297.9 ± 106.5 | ×103/μL | 150–400 |
| Inflammation parameters | |||
| ESR 5 | 15 ± 12.16 | mm/1 h | 0–15 |
| CRP 6 | 7.23 ± 6.46 | mg/dL | <10 |
| Fibrinogen | 362.7 ± 145.3 | mg/dL | 238–498 |
| MLR 7 | 0.29 ± 0.14 | - | - |
| LMR 8 | 3.93 ± 1.4 | - | - |
| PLR 9 | 160.7 ± 56.28 | - | - |
| NLR 10 | 2.50 ± 1.25 | - | - |
| SII 11 | 760.9 ± 448.6 | - | - |
| MPV/PLT 12 | 0.039 ± 0.013 | - | - |
| Biochemical parameters | |||
| Glucose | 96.6 ± 27.3 | mg/dL | 70–115 |
| Urea | 28.9 ± 11.61 | mg/dL | 19–43 |
| Uric Acid | 5.65 ± 2.15 | mg/dL | 2.0–6.2 |
| Creatinine | 0.78 ± 0.14 | mg/dL | 0.5–1.2 |
| Triglycerides | 109 ± 61.53 | mg/dL | 35–150 |
| Total Cholesterol | 210 ± 44.85 | mg/dL | 140–200 |
| ALT 13 | 29.5 ± 16.6 | U/L | 6–55 |
| AST 14 | 23.9 ± 9.27 | U/L | 11–34 |
| GGT 15 | 30.5 ± 16.43 | U/L | 0–30 |
| Total Bilirubin | 0.65 ± 0.21 | mg/dL | 0.2–1.2 |
| Oxidative status parameters | |||
| FORT 16 | 273.3 ± 132 | FORT units | <310 |
| FORD 17 | 0.34 ± 0.14 | Trolox units | 1.07–1.53 |
| Disease severity parameters | |||
| PASI 18 | 12.65 ± 8.37 | points | 0 |
| DLQI 19 | 12.9 ± 2.33 | points | 0 |
| Microorganism | Inflammatory Markers | p-Value | Pearson r Coefficient |
|---|---|---|---|
| Actinobacteria | LMR | 0.04 | −0.655 |
| Bacteroides | SII | 0.05 | −0.617 |
| MLR | 0.02 | −0.697 | |
| PLR | 0.03 | −0.694 | |
| NLR | 0.02 | −0.709 | |
| Firmicutes | Fibrinogen | 0.02 | −0.701 |
| CRP | 0.005 | −0.803 | |
| Firmicutes/Bacteroides | MLR | 0.01 | 0.767 |
| PLR | 0.02 | 0.680 |
| Microorganism | Hematological Parameter | p-Value | Pearson r Coefficient |
|---|---|---|---|
| Actinobacteria | Monocytes | 0.01 | 0.730 |
| Bacteroides | Eosinophils | 0.05 | −0.632 |
| Lactobacillus | Basophils | 0.02 | 0.706 |
| Bifidobacterium | Monocytes | 0.01 | 0.765 |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Histopathological confirmation of psoriasis diagnosis | Chronic alcohol consumption and smoking |
| Moderate to severe psoriasis: - DLQI > 10; - 1 of 3 criteria (PASI ≥ 11 or BSA ≥ 10 or sPGA ≥ 3) and DLQI ≥ 5; or - 2 of 3 criteria (PASI ≥ 11 or BSA ≥ 10 or sPGA ≥ 3). | Chronic diseases that may cause an increase in oxidative stress levels (cardiovascular diseases, autoimmune diseases, cancer, hepatic and renal failure, inflammatory diseases, type 2 diabetes mellitus, obesity, cancer, or atherosclerosis) |
| Patients who have not received treatment (topical, conventional systemic, or biological targeted treatment) for at least 3 months before enrollment | Other chronic diseases (except psoriasis) that may cause an alteration of the gut microbiota (inflammatory bowel diseases, type 2 diabetes mellitus, obesity, or cardiovascular diseases) |
| Patients older than 18 years old | Patients younger than 18 years old |
| Patients who have not received systemic antibiotic therapy for at least 6 months prior to enrollment | Pregnant patients |
| Patients who agreed to take part in the study and signed the informed consent form | Chronic psychiatric diseases that prevent the patient from understanding the purpose of the study and signing the ethical agreement form |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cozma, E.C.; Avram, I.; Voiculescu, V.M.; Mihai, M.M.; Găman, A.M. Correlations between Gut Microbiota and Hematological, Inflammatory, Biochemical and Oxidative Stress Parameters in Treatment-Naïve Psoriasis Patients. Int. J. Mol. Sci. 2024, 25, 6649. https://doi.org/10.3390/ijms25126649
Cozma EC, Avram I, Voiculescu VM, Mihai MM, Găman AM. Correlations between Gut Microbiota and Hematological, Inflammatory, Biochemical and Oxidative Stress Parameters in Treatment-Naïve Psoriasis Patients. International Journal of Molecular Sciences. 2024; 25(12):6649. https://doi.org/10.3390/ijms25126649
Chicago/Turabian StyleCozma, Elena Codruța, Ionela Avram, Vlad Mihai Voiculescu, Mara Mădălina Mihai, and Amelia Maria Găman. 2024. "Correlations between Gut Microbiota and Hematological, Inflammatory, Biochemical and Oxidative Stress Parameters in Treatment-Naïve Psoriasis Patients" International Journal of Molecular Sciences 25, no. 12: 6649. https://doi.org/10.3390/ijms25126649






