The Modulation of Respiratory Epithelial Cell Differentiation by the Thickness of an Electrospun Poly-ε-Carprolactone Mesh Mimicking the Basement Membrane
Abstract
1. Introduction
2. Results
2.1. Characteristics of Electrospun PCL Mesh
2.2. Viability and Barrier Function of Differentiated NHBE Cells during ALI
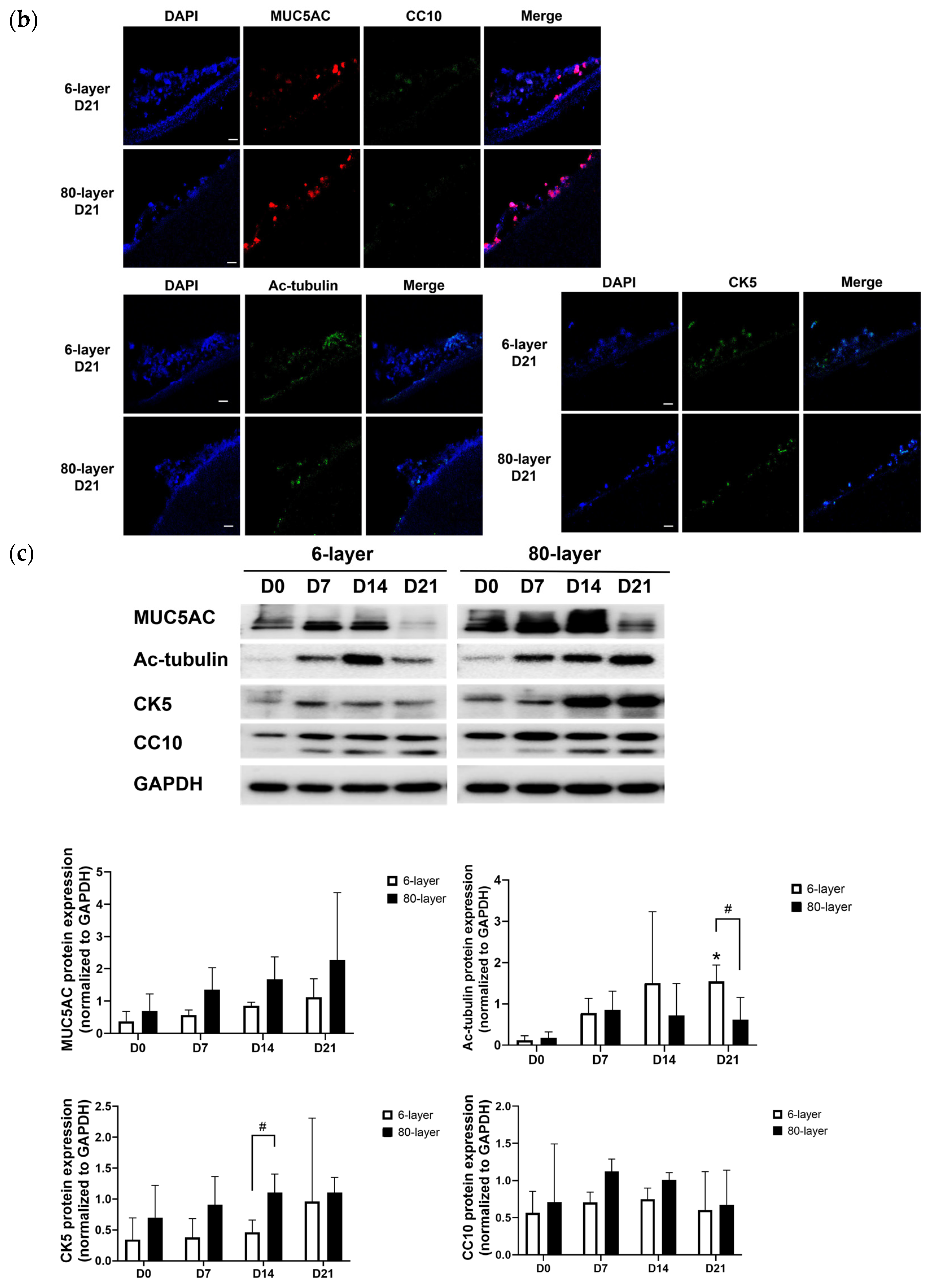
2.3. Generation of Airway Epithelium on the 6- and 80-Layer PCL Mesh
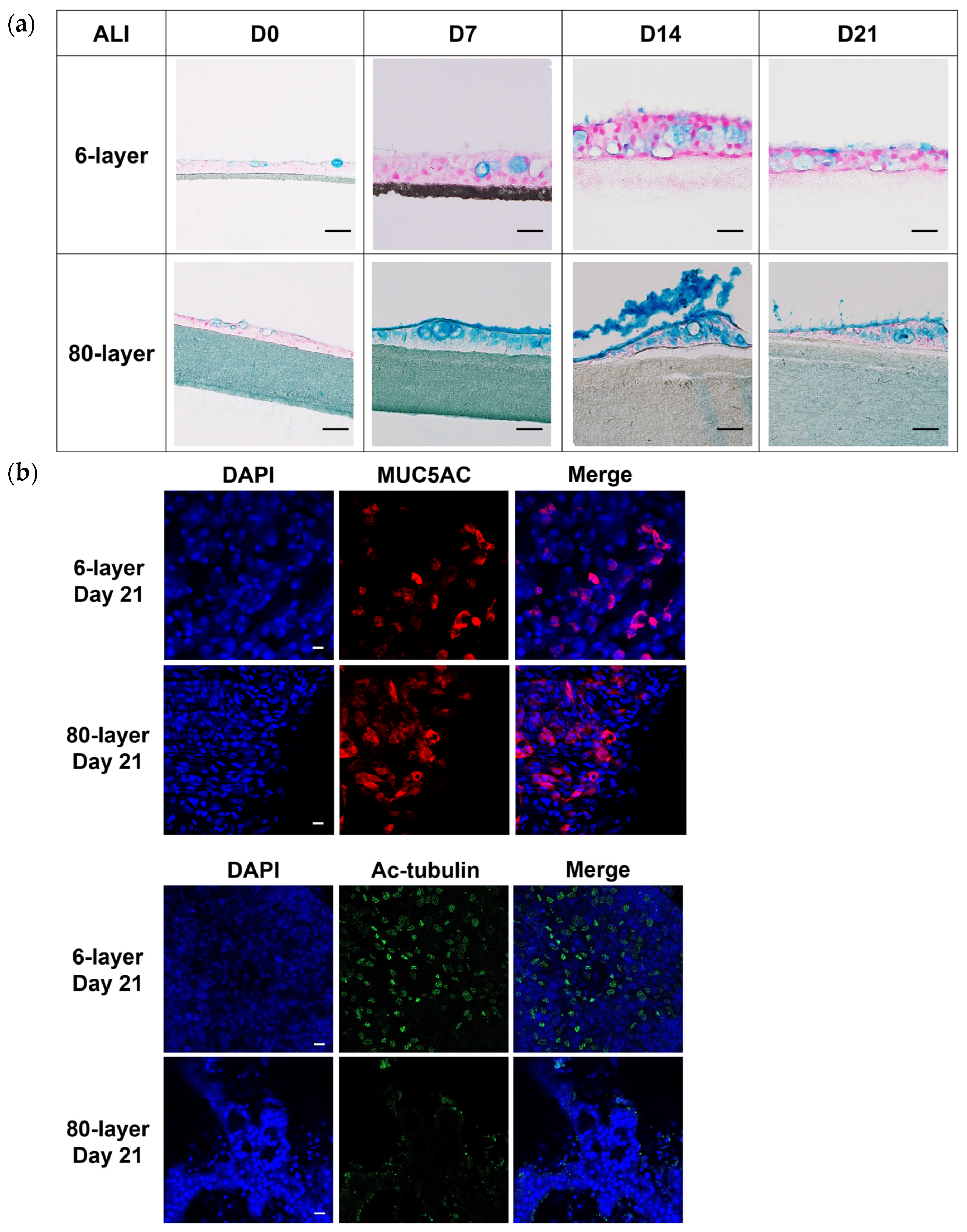
2.4. Hyperplastic Goblet Cells on the 80-Layer PCL Mesh Compared to on the 6-Layer PCL Mesh
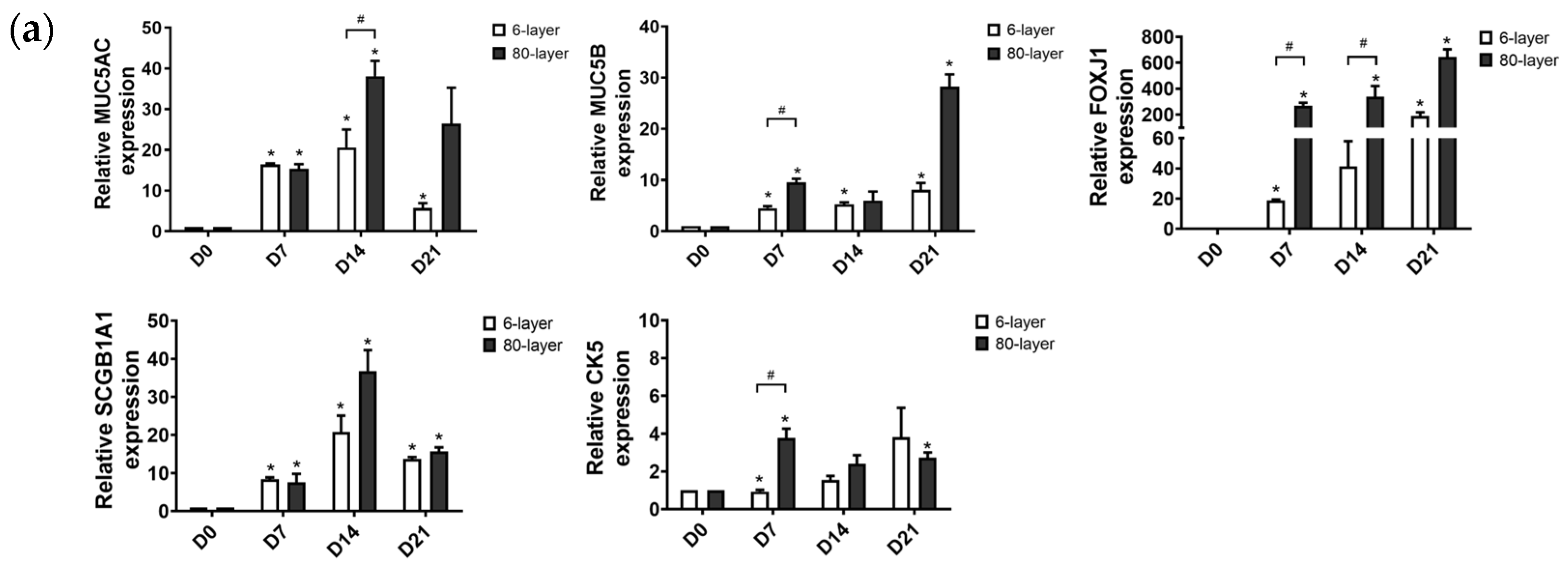
2.5. The Expression of EMT Markers in Cells Differentiated on the 6- and 80-Layer PCL Meshes
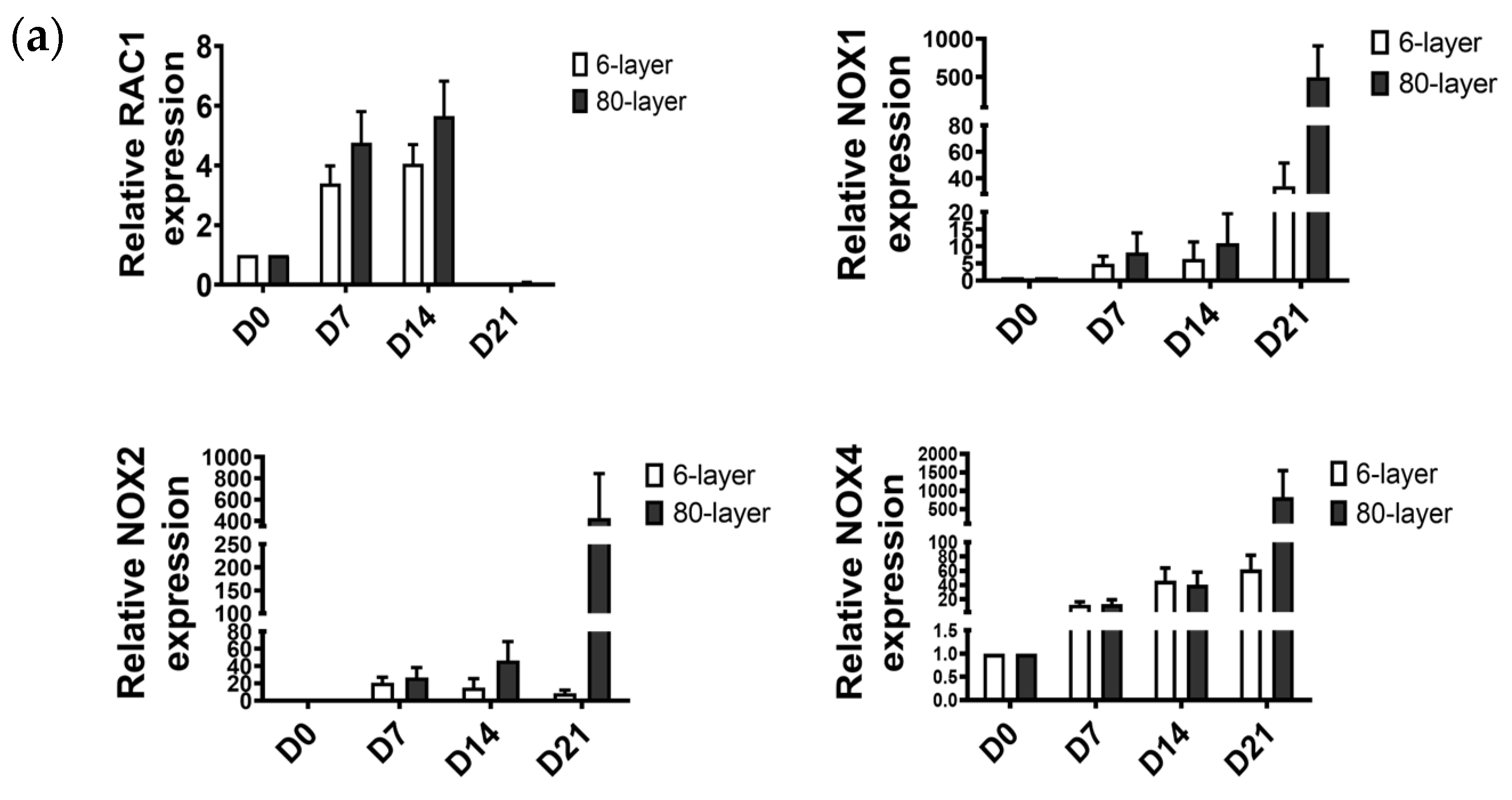
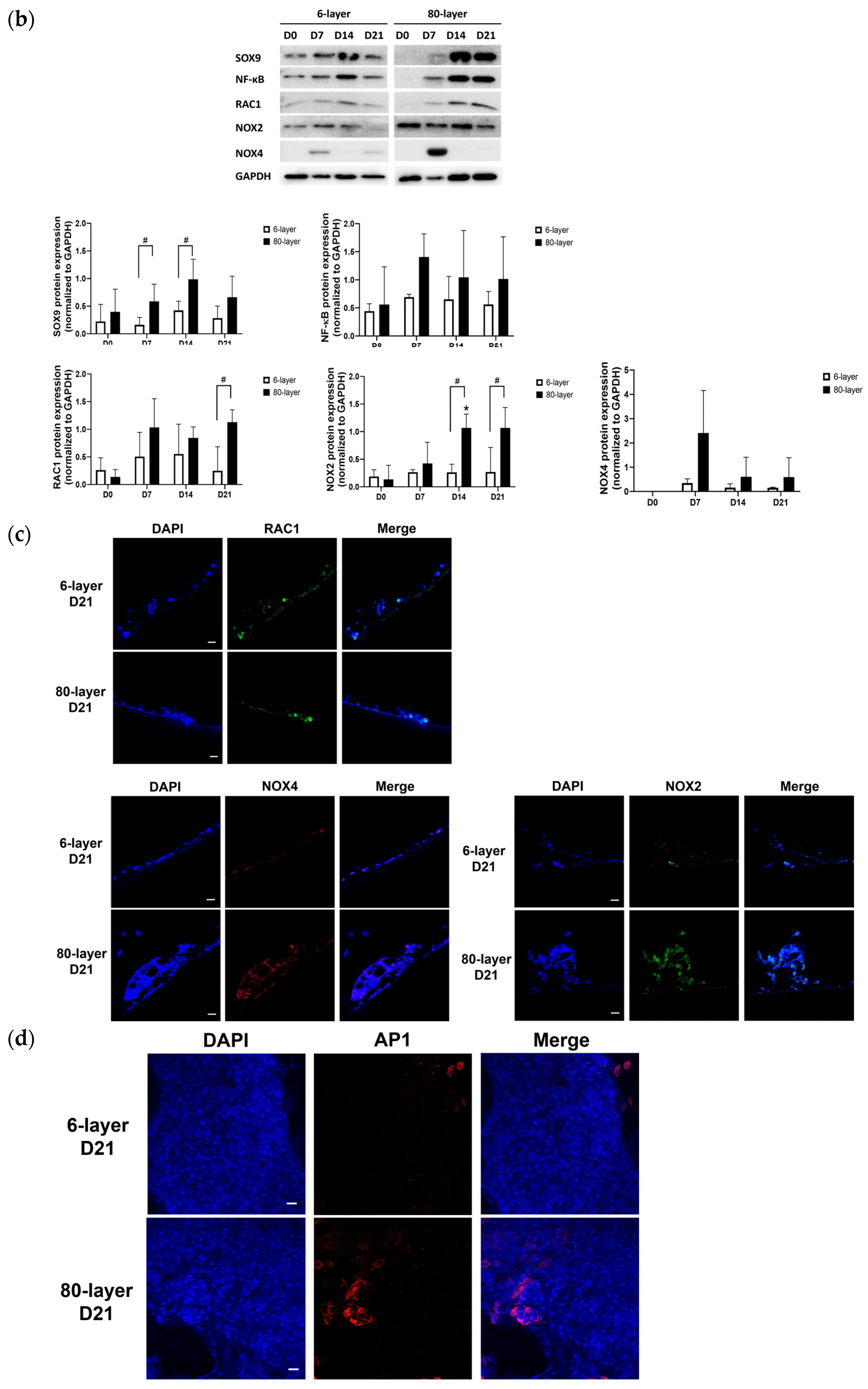
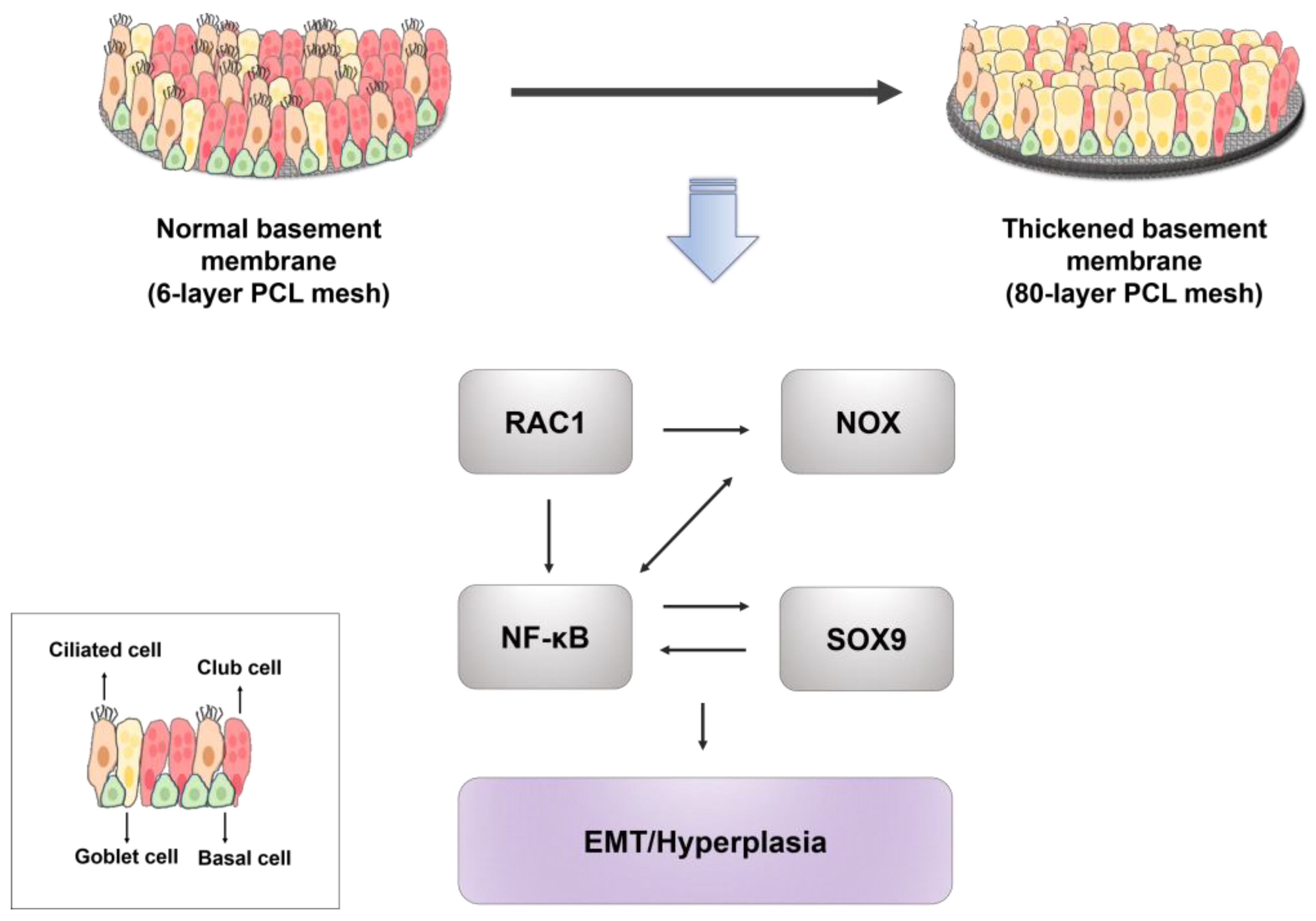
2.6. Oxidative Stress-Related Signaling Pathway in NHBE Cells Differentiated on the 80-Layer PCL Mesh
3. Discussion
4. Materials and Methods
4.1. Fabrication of Electrospun PCL Mesh
4.2. Electrospun PCL Mesh Characterization and Tensile Strength Evaluation
4.3. Preperation of PCL Mesh and Expansion of Primary Airway Epithelial Cells
4.4. Differentiation of NHBE Cells by ALI
4.5. Cell Viability Assay
- At is the absorbance of NHBE cells on the membrane under ALI conditions;
- Ab is the absorbance of the membrane only under ALI conditions;
- At0 is the absorbance of NHBE cells on the membrane under ALI day 0;
- Ab0 is the absorbance of the membrane only under ALI day 0.
4.6. Trans-Epithelial Electrical Resistance (TEER)
4.7. Histological and Immunochemical Analysis
4.8. Alcian Blue Staining
4.9. Whole-Mount Immunostaining
4.10. Western Blot Analysis
4.11. Quantitative Real-Time Polymerase Chain Reaction (PCR)
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jain, P.; Rauer, S.B.; Moller, M.; Singh, S. Mimicking the Natural Basement Membrane for Advanced Tissue Engineering. Biomacromolecules 2022, 23, 3081–3103. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.J.; Van Winkle, L.S.; Fanucchi, M.V.; Plopper, C.G. Cellular and molecular characteristics of basal cells in airway epithelium. Exp. Lung Res. 2001, 27, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ibrahim, Z.; Marchand, M.F.; Piolot, T.; Kamboj, S.; Carreiras, F.; Yamada, A.; Schanne-Klein, M.C.; Chen, Y.; Lambert, A.; et al. Three-Dimensional Collagen Topology Shapes Cell Morphology, beyond Stiffness. ACS Biomater. Sci. Eng. 2022, 8, 5284–5294. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Rimal, R.; Moller, M.; Singh, S. Topographical influence of electrospun basement membrane mimics on formation of cellular monolayer. Sci. Rep. 2023, 13, 8382. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, K.; Gu, X.; Leong, K.W. Biophysical Regulation of Cell Behavior-Cross Talk between Substrate Stiffness and Nanotopography. Engineering 2017, 3, 36–54. [Google Scholar] [CrossRef] [PubMed]
- Khalilgharibi, N.; Mao, Y. To form and function: On the role of basement membrane mechanics in tissue development, homeostasis and disease. Open Biol. 2021, 11, 200360. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Giordani, P.; Zuccari, G. Synthesis and Physicochemical Characterization of Gelatine-Based Biodegradable Aerogel-like Composites as Possible Scaffolds for Regenerative Medicine. Int. J. Mol. Sci. 2024, 25, 5009. [Google Scholar] [CrossRef]
- Berube, K.; Prytherch, Z.; Job, C.; Hughes, T. Human primary bronchial lung cell constructs: The new respiratory models. Toxicology 2010, 278, 311–318. [Google Scholar] [CrossRef]
- Rayner, R.E.; Makena, P.; Prasad, G.L.; Cormet-Boyaka, E. Optimization of Normal Human Bronchial Epithelial (NHBE) Cell 3D Cultures for in vitro Lung Model Studies. Sci. Rep. 2019, 9, 500. [Google Scholar] [CrossRef]
- Jiang, D.; Schaefer, N.; Chu, H.W. Air-Liquid Interface Culture of Human and Mouse Airway Epithelial Cells. Methods Mol. Biol. 2018, 1809, 91–109. [Google Scholar]
- Leclech, C.; Natale, C.F.; Barakat, A.I. The basement membrane as a structured surface—Role in vascular health and disease. J. Cell Sci. 2020, 133, jcs239889. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, C.F.; Gasperini, L.; Marques, A.P.; Reis, R.L. The stiffness of living tissues and its implications for tissue engineering. Nat. Rev. Mater. 2020, 5, 351–370. [Google Scholar] [CrossRef]
- Lu, H.; Menary, G. Determination of Young’s Modulus of PET Sheets from Lamb Wave Velocity Measurement. Exp. Mech. 2024, 64, 377–391. [Google Scholar] [CrossRef]
- Polio, S.R.; Kundu, A.N.; Dougan, C.E.; Birch, N.P.; Aurian-Blajeni, D.E.; Schiffman, J.D.; Crosby, A.J.; Peyton, S.R. Cross-platform mechanical characterization of lung tissue. PLoS ONE 2018, 13, e0204765. [Google Scholar] [CrossRef] [PubMed]
- Doryab, A.; Taskin, M.B.; Stahlhut, P.; Schröppel, A.; Wagner, D.E.; Groll, J.; Schmid, O. A Biomimetic, Copolymeric Membrane for Cell-Stretch Experiments with Pulmonary Epithelial Cells at the Air-Liquid Interface. Adv. Funct. Mater. 2021, 31, 2004707. [Google Scholar] [CrossRef]
- Wu, H.; Yu, Y.; Huang, H.; Hu, Y.; Fu, S.; Wang, Z.; Shi, M.; Zhao, X.; Yuan, J.; Li, J.; et al. Progressive Pulmonary Fibrosis Is Caused by Elevated Mechanical Tension on Alveolar Stem Cells. Cell 2020, 180, 107–121.e17. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; He, C.; Venado, A.; Zhou, Y. Extracellular Matrix Stiffness in Lung Health and Disease. Compr. Physiol. 2022, 12, 3523–3558. [Google Scholar] [PubMed]
- Dekkers, B.G.J.; Saad, S.I.; van Spelde, L.J.; Burgess, J.K. Basement membranes in obstructive pulmonary diseases. Matrix Biol. Plus 2021, 12, 100092. [Google Scholar] [CrossRef]
- Boucher, R.C. Muco-Obstructive Lung Diseases. N. Engl. J. Med. 2019, 380, 1941–1953. [Google Scholar] [CrossRef]
- Augustine, R.; Nethi, S.K.; Kalarikkal, N.; Thomas, S.; Patra, C.R. Electrospun polycaprolactone (PCL) scaffolds embedded with europium hydroxide nanorods (EHNs) with enhanced vascularization and cell proliferation for tissue engineering applications. J. Mater. Chem. B 2017, 5, 4660–4672. [Google Scholar] [CrossRef]
- Amokrane, G.; Humblot, V.; Jubeli, E.; Yagoubi, N.; Ramtani, S.; Migonney, V.; Falentin-Daudre, C. Electrospun Poly(epsilon-caprolactone) Fiber Scaffolds Functionalized by the Covalent Grafting of a Bioactive Polymer: Surface Characterization and Influence on in Vitro Biological Response. ACS Omega 2019, 4, 17194–17208. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, N.; Kuang, L.; Del Ponte, M.; Chain, C.; Deng, M. 1—Design and fabrication of nanocomposites for musculoskeletal tissue regeneration. In Nanocomposites for Musculoskeletal Tissue Regeneration; Liu, H., Ed.; Woodhead Publishing: Oxford, UK, 2016; pp. 3–29. [Google Scholar]
- Tang, X.; Thankappan, S.K.; Lee, P.; Fard, S.E.; Harmon, M.D.; Tran, K.; Yu, X. Chapter 21—Polymeric Biomaterials in Tissue Engineering and Regenerative Medicine. In Natural and Synthetic Biomedical Polymers; Kumbar, S.G., Laurencin, C.T., Deng, M., Eds.; Elsevier: Oxford, UK, 2014; pp. 351–371. [Google Scholar]
- Bliley, J.M.; Marra, K.G. Chapter 11—Polymeric Biomaterials as Tissue Scaffolds. In Stem Cell Biology and Tissue Engineering in Dental Sciences; Vishwakarma, A., Sharpe, P., Shi, S., Ramalingam, M., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 149–161. [Google Scholar]
- Dias, J.R.; Sousa, A.; Augusto, A.; Bartolo, P.J.; Granja, P.L. Electrospun Polycaprolactone (PCL) Degradation: An In Vitro and In Vivo Study. Polymers 2022, 14, 3397. [Google Scholar] [CrossRef] [PubMed]
- Bosworth, L.A.; Downes, S. Physicochemical characterisation of degrading polycaprolactone scaffolds. Polym. Degrad. Stab. 2010, 95, 2269–2276. [Google Scholar] [CrossRef]
- Nishiguchi, A.; Singh, S.; Wessling, M.; Kirkpatrick, C.J.; Moller, M. Basement Membrane Mimics of Biofunctionalized Nanofibers for a Bipolar-Cultured Human Primary Alveolar-Capillary Barrier Model. Biomacromolecules 2017, 18, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Nishiguchi, A.; Linz, G.; Wessling, M.; Ludwig, A.; Rossaint, R.; Moller, M.; Singh, S. Reconstruction of Ultra-thin Alveolar-capillary Basement Membrane Mimics. Adv. Biol. 2021, 5, e2000427. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Doshi, U.; Wolz, R.L.; Kosachevsky, P.; Oldham, M.J.; Gillman, I.G.; Lee, K.M. Fit-for-purpose characterization of air-liquid-interface (ALI) in vitro exposure systems for e-vapor aerosol. Toxicol. Vitr. 2022, 82, 105352. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, G.; Koch, W.; Ritter, D.; Gutleb, A.C.; Larsen, S.T.; Loret, T.; Zanetti, F.; Constant, S.; Chortarea, S.; Rothen-Rutishauser, B.; et al. Air-Liquid Interface In Vitro Models for Respiratory Toxicology Research: Consensus Workshop and Recommendations. Appl. Vitr. Toxicol. 2018, 4, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Yim, E.K.; Pang, S.W.; Leong, K.W. Synthetic nanostructures inducing differentiation of human mesenchymal stem cells into neuronal lineage. Exp. Cell Res. 2007, 313, 1820–1829. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.R.; Kwon, K.W.; Jung, H.; Kim, H.N.; Suh, K.Y.; Kim, K.; Kim, K.S. Direct differentiation of human embryonic stem cells into selective neurons on nanoscale ridge/groove pattern arrays. Biomaterials 2010, 31, 4360–4366. [Google Scholar] [CrossRef]
- Metwally, S.; Stachewicz, U. Surface potential and charges impact on cell responses on biomaterials interfaces for medical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109883. [Google Scholar] [CrossRef]
- Mohammadalizadeh, Z.; Bahremandi-Toloue, E.; Karbasi, S. Recent advances in modification strategies of pre- and post-electrospinning of nanofiber scaffolds in tissue engineering. React. Funct. Polym. 2022, 172, 105202. [Google Scholar] [CrossRef]
- Yoo, H.S.; Kim, T.G.; Park, T.G. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv. Drug Deliv. Rev. 2009, 61, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Puddicombe, S.M.; Field, S.; Haywood, J.; Broughton-Head, V.; Puxeddu, I.; Haitchi, H.M.; Vernon-Wilson, E.; Sammut, D.; Bedke, N.; et al. Defective epithelial barrier function in asthma. J. Allergy Clin. Immunol. 2011, 128, 549–556.e12. [Google Scholar] [CrossRef] [PubMed]
- DiGuilio, K.M.; Del Rio, E.A.; Harty, R.N.; Mullin, J.M. Micronutrients at Supplemental Levels, Tight Junctions and Epithelial Barrier Function: A Narrative Review. Int. J. Mol. Sci. 2024, 25, 3452. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.; Wadsworth, S.J.; Yang, S.J.; Dorscheid, D.R. Structural and functional variations in human bronchial epithelial cells cultured in air-liquid interface using different growth media. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, L1063–L1073. [Google Scholar] [CrossRef]
- Cao, X.; Coyle, J.P.; Xiong, R.; Wang, Y.; Heflich, R.H.; Ren, B.; Gwinn, W.M.; Hayden, P.; Rojanasakul, L. Invited review: Human air-liquid-interface organotypic airway tissue models derived from primary tracheobronchial epithelial cells-overview and perspectives. Vitr. Cell. Dev. Biol. Anim. 2021, 57, 104–132. [Google Scholar] [CrossRef] [PubMed]
- Rojanasakul, Y.; Wang, L.Y.; Bhat, M.; Glover, D.D.; Malanga, C.J.; Ma, J.K. The transport barrier of epithelia: A comparative study on membrane permeability and charge selectivity in the rabbit. Pharm. Res. 1992, 9, 1029–1034. [Google Scholar] [CrossRef]
- Schamberger, A.C.; Staab-Weijnitz, C.A.; Mise-Racek, N.; Eickelberg, O. Cigarette smoke alters primary human bronchial epithelial cell differentiation at the air-liquid interface. Sci. Rep. 2015, 5, 8163. [Google Scholar] [CrossRef] [PubMed]
- Fahy, J.V.; Dickey, B.F. Airway mucus function and dysfunction. N. Engl. J. Med. 2010, 363, 2233–2247. [Google Scholar] [CrossRef]
- Kesimer, M. Mucins MUC5AC and MUC5B in the Airways: MUCing around Together. Am. J. Respir. Crit. Care Med. 2022, 206, 1055–1057. [Google Scholar] [CrossRef]
- Song, D.; Iverson, E.; Kaler, L.; Boboltz, A.; Scull, M.A.; Duncan, G.A. MUC5B mobilizes and MUC5AC spatially aligns mucociliary transport on human airway epithelium. Sci. Adv. 2022, 8, eabq5049. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, S.; Bertuccio, F.R.; del Frate, L.; Perrotta, F.; Corsico, A.G.; Stella, G.M. The Plastic Interplay between Lung Regeneration Phenomena and Fibrotic Evolution: Current Challenges and Novel Therapeutic Perspectives. Int. J. Mol. Sci. 2024, 25, 547. [Google Scholar] [CrossRef] [PubMed]
- Curran, D.R.; Cohn, L. Advances in mucous cell metaplasia: A plug for mucus as a therapeutic focus in chronic airway disease. Am. J. Respir. Cell Mol. Biol. 2010, 42, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Conti, C.; Montero-Fernandez, A.; Borg, E.; Osadolor, T.; Viola, P.; De Lauretis, A.; Stock, C.J.; Bonifazi, M.; Bonini, M.; Caramori, G.; et al. Mucins MUC5B and MUC5AC in Distal Airways and Honeycomb Spaces: Comparison among Idiopathic Pulmonary Fibrosis/Usual Interstitial Pneumonia, Fibrotic Nonspecific Interstitial Pneumonitis, and Control Lungs. Am. J. Respir. Crit. Care Med. 2016, 193, 462–464. [Google Scholar] [CrossRef] [PubMed]
- Arason, A.J.; Jonsdottir, H.R.; Halldorsson, S.; Benediktsdottir, B.E.; Bergthorsson, J.T.; Ingthorsson, S.; Baldursson, O.; Sinha, S.; Gudjonsson, T.; Magnusson, M.K. deltaNp63 has a role in maintaining epithelial integrity in airway epithelium. PLoS ONE 2014, 9, e88683. [Google Scholar] [CrossRef] [PubMed]
- Eapen, M.S.; Sharma, P.; Gaikwad, A.V.; Lu, W.; Myers, S.; Hansbro, P.M.; Sohal, S.S. Epithelial-mesenchymal transition is driven by transcriptional and post transcriptional modulations in COPD: Implications for disease progression and new therapeutics. Int. J. Chron. Obstruct. Pulmon. Dis. 2019, 14, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N. Transforming growth factor-beta in tissue fibrosis. J. Exp. Med. 2020, 217, e20190103. [Google Scholar] [CrossRef] [PubMed]
- Tarantal, A.F.; Chen, H.; Shi, T.T.; Lu, C.H.; Fang, A.B.; Buckley, S.; Kolb, M.; Gauldie, J.; Warburton, D.; Shi, W. Overexpression of transforming growth factor-beta1 in fetal monkey lung results in prenatal pulmonary fibrosis. Eur. Respir. J. 2010, 36, 907–914. [Google Scholar] [CrossRef]
- Malaviya, R.; Laskin, J.D.; Laskin, D.L. Anti-TNFalpha therapy in inflammatory lung diseases. Pharmacol. Ther. 2017, 180, 90–98. [Google Scholar] [CrossRef]
- Rockich, B.E.; Hrycaj, S.M.; Shih, H.P.; Nagy, M.S.; Ferguson, M.A.; Kopp, J.L.; Sander, M.; Wellik, D.M.; Spence, J.R. Sox9 plays multiple roles in the lung epithelium during branching morphogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, E4456–E4464. [Google Scholar] [CrossRef] [PubMed]
- Gajjala, P.R.; Kasam, R.K.; Soundararajan, D.; Sinner, D.; Huang, S.K.; Jegga, A.G.; Madala, S.K. Dysregulated overexpression of Sox9 induces fibroblast activation in pulmonary fibrosis. JCI Insight 2021, 6, e152503. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Tam, W.L.; Shibue, T.; Kaygusuz, Y.; Reinhardt, F.; Ng Eaton, E.; Weinberg, R.A. Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature 2015, 525, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Fang, Y.; Li, Y.; Huang, H.; Wei, Z.; Gao, X.; Sung, H.K.; Hu, J.; Qiang, L.; Ruan, J.; et al. VEGF receptor 2 (KDR) protects airways from mucus metaplasia through a Sox9-dependent pathway. Dev. Cell 2021, 56, 1646–1660.e5. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Duan, J.; Liu, W.; Ji, J.; Liu, B.; Zhang, M. Feedback activation of NF-KB signaling leads to adaptive resistance to EZH2 inhibitors in prostate cancer cells. Cancer Cell Int. 2021, 21, 191. [Google Scholar] [CrossRef]
- Saldanha, J.F.; Leal Vde, O.; Stenvinkel, P.; Carraro-Eduardo, J.C.; Mafra, D. Resveratrol: Why is it a promising therapy for chronic kidney disease patients? Oxid. Med. Cell. Longev. 2013, 2013, 963217. [Google Scholar] [CrossRef]
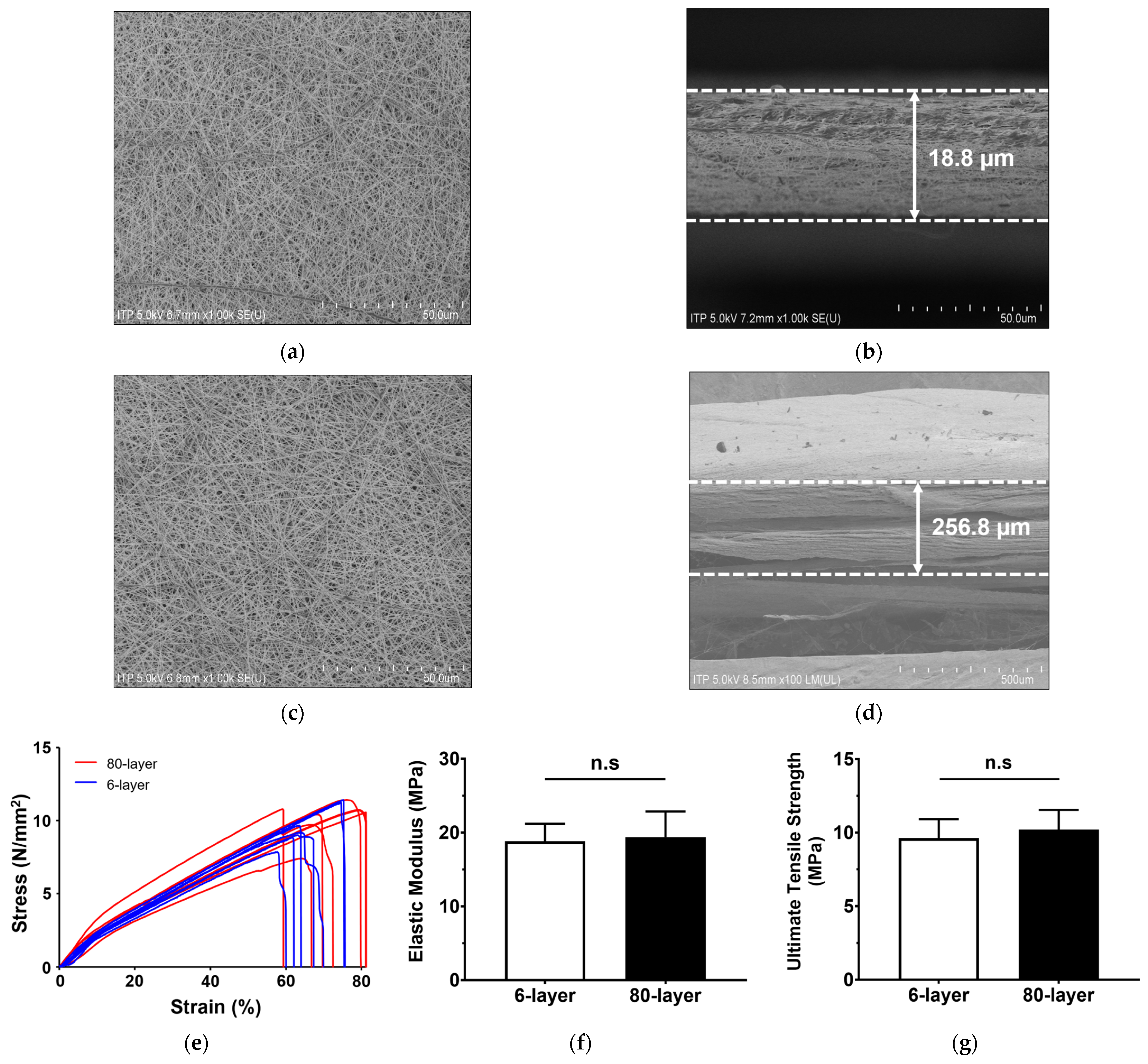


| Unit: µm | ||
|---|---|---|
| Mean ± SD (Min, Max) | ||
| 6-Layer | 80-Layer | |
| Fiber diameter | 0.23 ± 0.04 (0.18, 0.39) | 0.25 ± 0.02 (0.20, 0.28) |
| Pore size | 0.59 ± 0.22 (0.27, 1.15) | 0.64 ± 0.41 (0.17, 1.81) |
| Membrane thickness | 18.75 ± 1.92 (16.83, 20.67) | 256.83 ± 21.64 ** (235.19, 278.47) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.Y.; Kim, H.J.; Hwang, S.; Park, J.; Park, J.; Lee, J.W.; Son, K.H. The Modulation of Respiratory Epithelial Cell Differentiation by the Thickness of an Electrospun Poly-ε-Carprolactone Mesh Mimicking the Basement Membrane. Int. J. Mol. Sci. 2024, 25, 6650. https://doi.org/10.3390/ijms25126650
Choi SY, Kim HJ, Hwang S, Park J, Park J, Lee JW, Son KH. The Modulation of Respiratory Epithelial Cell Differentiation by the Thickness of an Electrospun Poly-ε-Carprolactone Mesh Mimicking the Basement Membrane. International Journal of Molecular Sciences. 2024; 25(12):6650. https://doi.org/10.3390/ijms25126650
Chicago/Turabian StyleChoi, Seon Young, Hyun Joo Kim, Soyoung Hwang, Jangho Park, Jungkyu Park, Jin Woo Lee, and Kuk Hui Son. 2024. "The Modulation of Respiratory Epithelial Cell Differentiation by the Thickness of an Electrospun Poly-ε-Carprolactone Mesh Mimicking the Basement Membrane" International Journal of Molecular Sciences 25, no. 12: 6650. https://doi.org/10.3390/ijms25126650
APA StyleChoi, S. Y., Kim, H. J., Hwang, S., Park, J., Park, J., Lee, J. W., & Son, K. H. (2024). The Modulation of Respiratory Epithelial Cell Differentiation by the Thickness of an Electrospun Poly-ε-Carprolactone Mesh Mimicking the Basement Membrane. International Journal of Molecular Sciences, 25(12), 6650. https://doi.org/10.3390/ijms25126650







