Copper, Iron, Cadmium, and Arsenic, All Generated in the Universe: Elucidating Their Environmental Impact Risk on Human Health Including Clinical Liver Injury
Abstract
1. Introduction
2. Basic Considerations
2.1. Laboratory Criteria of Liver Injury
2.2. Types of Liver Injury
2.3. Diagnostic Approaches and Causality Assessment
2.4. Exclusion of Alternative Causes
2.5. Heavy Metals in Plants and Horizontal Transfer
2.6. Issue of Experimental Studies
3. Copper
3.1. Physiology
3.2. Copper as Pollutant and the Issue of Health Hazards
3.2.1. Sources of Copper as an Environmental Pollutant
3.2.2. Elucidating Health Hazards of Environmental Copper
3.3. Acute Human Liver Injury by Exogenous Copper
3.4. Chronic Human Liver Injury by Exogenous Copper
3.5. Wilson Disease
3.5.1. Natural Course
3.5.2. Clinical Characteristics
3.5.3. Diagnostic Approach
3.5.4. Therapy
3.5.5. Prognosis
3.5.6. Cascade of Molecular Events Leading to Copper Liver Injury
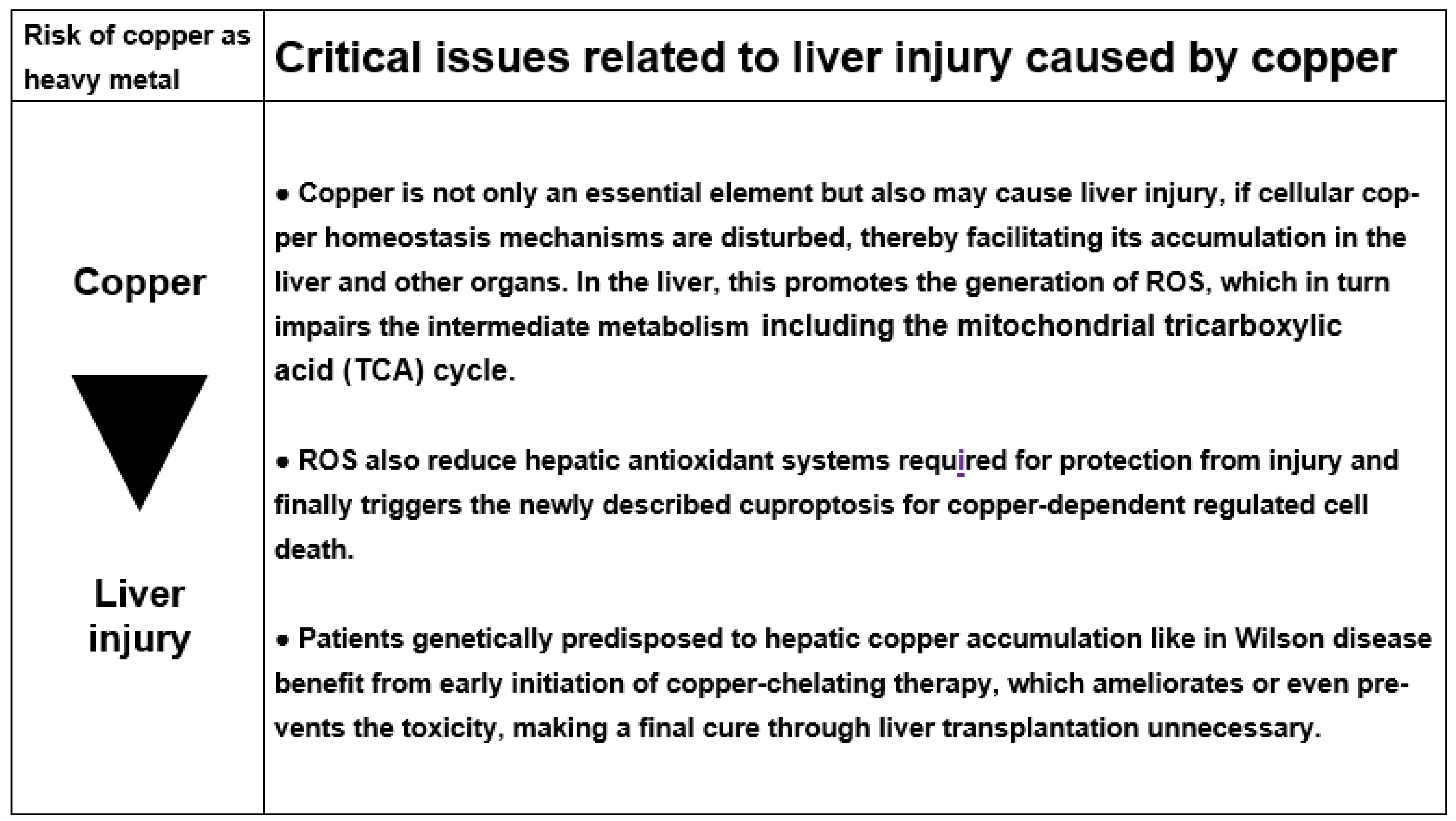
4. Iron
4.1. Physiology
4.2. Iron as Pollutant and the Issue of Health Hazards
4.2.1. Sources of Iron as an Environmental Pollutant
4.2.2. Elucidating Health Hazards of Environmental Iron
4.3. Acute human Liver Injury by Exogenous Iron
4.4. Chronic Human Liver Injury by Exogenous Iron
4.5. Hemochromatosis
- Type 2a (mutations of hemojuvelin gene) and type 2b (mutations of the hepcidin gene): Autosomal recessive disorder that is seen both in whites and non-whites. Its onset is usually at 15–20 years [189].
- Type 3 (mutations of transferrin receptor-2 gene): Autosomal recessive disorder that is seen both in whites and non-whites. Its onset is at 30–40 years [224].
- Type 4 (mutations of the ferroportin gene): Autosomal dominant disease that is seen both in whites and non-whites. Its onset is at 10–80 years [189].
4.5.1. Natural Course
4.5.2. Clinical Characteristics
4.5.3. Diagnostic Approach
4.5.4. Therapy
4.5.5. Prognosis
4.5.6. Cascade of Molecular Events Leading to Iron Liver Injury
5. Cadmium
5.1. Physiology
5.2. Cadmium as Pollutant and the Issue of Health Hazards
5.2.1. Sources of Cadmium as an Environmental Pollutant
5.2.2. Elucidating Health Hazards of Environmental Cadmium
5.3. Acute Human Liver Injury by Cadmium
5.4. Chronic Human Liver Injury by Cadmium
5.5. Cascade of Molecular Events Leading to Cadmium Liver Injury
6. Arsenic
6.1. Physiology
6.2. Arsenic as Pollutant and the Issue of Health Hazards
6.2.1. Sources of Arsenic as an Environmental Pollutant
6.2.2. Elucidating Health Hazards of Environmental Arsenic
6.3. Acute Human Liver Injury by Arsenic
6.4. Chronic Human Liver Injury by Arsenic
6.5. Cascade of Molecular Events Leading to Arsenic Liver Injury
7. Conclusions
Funding
Conflicts of Interest
References
- Belford, R. The Origin of the Elements. Available online: https://chem.libretexts.org/Courses/University_of_Arkansas_Little_Rock/Chem_1403%3A_General_Chemistry_2/Text/21%3A_Nuclear_Chemistry/21.06%3A_The_Origin_of_the_Elements (accessed on 19 March 2024).
- Chaudhry, H.S.; Anilkumar, A.C. Wilson Disease. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441990/ (accessed on 5 March 2024).
- Clery, D. Some of the Universe’s Heavier Elements are Created by Neutron Star Collisions. Available online: https://e.org/content/article/some-universe-s-heavier-elements-are-created-neutron-star-collisions (accessed on 19 March 2024).
- Frebel, A.; Beers, T.C. Some of the universe’s heavier elements are created by neutron star collisions. Phys. Today 2018, 71, 30–37. [Google Scholar] [CrossRef]
- Sheftel, A.D.; Mason, A.B.; Ponka, P. The long history of iron in the Universe and in health and disease. Biochim. Biophys. Acta 2012, 1820, 161–187. [Google Scholar] [CrossRef] [PubMed]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects in humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Angon, P.B.; Islam, M.S.; Kc, S.; Das, A.; Anjum, N.; Poudel, A.; Suchi, S.A. Sources, effects and present perspectives of heavy metals contamination: Soil, plants and human food chain. Heliyon 2024, 10, e28357. [Google Scholar] [CrossRef] [PubMed]
- Trefts, E.; Gannon, M.; Wasserman, D.H. The liver. Curr. Biol. 2017, 27, R1147–R1151. [Google Scholar] [CrossRef] [PubMed]
- Kalra, A.; Yetiskul, E.; Wehrle, C.J.; Tuma, F. Physiology, Liver. 1 May 2023. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK535438/ (accessed on 18 March 2024).
- França, M.; Amorim, J.P. Liver increased iron deposition and storage diseases. In Imaging of the Liver and Intra-Hepatic Biliary Tract. Medical Radiology; Quaia, E., Ed.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Vaja, R.; Rana, M. Drugs and the liver. Anaesth. Intensiv. Care Med. 2020, 21, 517–523. [Google Scholar] [CrossRef]
- Wen, Y.; Lambrecht, J.; Ju, C.; Tacke, F. Hepatic macrophages in liver homeostasis and diseases-diversity, plasticity and therapeutic opportunities. Cell Mol. Immunol. 2021, 18, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Judge, A.; Dodd, M.S. Metabolism. Essays Biochem. 2020, 64, 607–647. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R.; Danan, G. Liver injury by drugs metabolized via cytochrome P450. J. Mod. Med. Chem. 2020, 8, 93–98. [Google Scholar] [CrossRef]
- Teschke, R.; Danan, G. Idiosyncratic drug induced liver injury, cytochrome P450, metabolic risk factors, and lipophilicity: Highlights and controversies. Int. J. Mol. Sci. 2021, 22, 3441. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R.; Uetrecht, J. Mechanism of idiosyncratic drug induced liver injury (DILI): Unresolved basic issues. In special issue: Unresolved basic issues in hepatology, guest editors Ralf Weiskirchen and Wolfgang Stremmel. Ann. Transl. Med. 2021, 9, 730. [Google Scholar]
- Teschke, R. Alcoholic steatohepatitis (ASH) and acute alcoholic hepatitis (AH): Cascade of events, clinical features, and pharmacotherapy options. Exp. Opin. Pharmacother. 2018, 19, 779–793. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R. Alcoholic liver disease: Alcohol metabolism, cascade of molecular mechanisms, cellular targets, and clinical aspects. Biomedicines 2018, 6, 106. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R. Alcoholic liver disease: Current mechanistic aspects with focus on their clinical relevance. Biomedicines 2019, 7, 68. [Google Scholar]
- Teschke, R. Biochemical aspects of the hepatic microsomal ethanol-oxidizing system (MEOS). Resolved initial controversies and updated molecular views. Biochem. Pharmacol. 2019, 8, 267. [Google Scholar] [CrossRef][Green Version]
- Teschke, R. Aliphatic halogenated hydrocarbons: Liver injury in 60 patients. J. Clin. Trans. Hepatol. 2018, 6, 1–12. [Google Scholar]
- Teschke, R. Liver injury by carbon tetrachloride intoxication in 16 patients treated with forced ventilation to accelerate toxin removal via the lungs: A clinical report. Toxics 2018, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R. Intoxications by aliphatic halogenated hydrocarbons: Hepatotoxic risks for patients and clinical issues including role of CO2-induced hyperventilation as therapy option. J. Clin. Exp. Toxicol. 2018, 2, 25–29. [Google Scholar]
- Vongdala, N.; Tran, H.D.; Xuan, T.D.; Teschke, R.; Khanh, T.D. Heavy metal accumulation in water, soil, and plants of municipal solid waste landfill in Vientiane, Laos. Int. J. Environ. Res. Public. Health 2019, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- Quan, N.V.; Xuan, T.D.; Teschke, R. Potential hepatotoxins found in herbal medicinal products: A systematic review. Int. J. Mol. Sci. 2020, 21, 5011. [Google Scholar] [CrossRef]
- Teschke, R.; Xuan, T.D. Heavy metals, halogenated hydrocarbons, phthalates, glyphosate, cordycepin, alcohol, drugs, and herbs, assessed for liver injury and mechanistic steps. Special Issue: Hepatotoxicity: Molecular Mechanisms and Pathophysiology. Front. Biosci. Landmark 2022, 27, 314. [Google Scholar] [CrossRef]
- Teschke, R. Aluminum, arsenic, beryllium, cadmium, chromium, cobalt, copper, iron, lead, mercury, molybdenum, nickel, platinum, thallium, titanium, vanadium, and zinc: Molecular aspects in experimental liver injury. Int. J. Mol. Sci. 2022, 23, 12213. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.C.; Jeffrey, G.; Ryan, J. Haemochromatosis. Lancet 2023, 401, 10390. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.; Roberts, S.M.; Saab, I.N. Review of regulatory reference values and background levels for heavy metals in the human diet. Regul. Toxicol. Pharmacol. 2022, 130, 105122. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. In: Luch, A. (eds) Molecular, Clinical and Environmental Toxicology. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [CrossRef] [PubMed]
- Aldridge, J.E.; Gibbons, J.A.; Flaherty, M.M.; Kreider, M.L.; Romano, J.A.; Levin, E.D. Heterogeneity of toxicant response: Sources of human variability. Toxicol. Sci. 2003, 76, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Danan, G.; Bénichou, C. Causality assessment of adverse reactions to drugs—I. A novel method based on the conclusions of international consensus meetings: Application to drug-induced liver injuries. J. Clin. Epidemiol. 1993, 46, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Danan, G.; Teschke, R. RUCAM in drug and herb induced liver injury: The update. Int. J. Mol. Sci. 2016, 17, 14. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Guo, D.; Xu, Y.; Zhu, M.; Yao, C.; Chen, C.; Jia, W. Comparison of different liver test thresholds for drug-induced liver injury: Updated RUCAM versus other methods. Front. Pharmacol. 2019, 10, 816. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.; Guraka, A.; Shawa, I.T.; Tripathi, G.; Moritz, W.; Kermanizadeh, A. Drug induced liver injury—A 2023 update. J. Toxicol. Environ. Health Part B 2023, 12, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Hosack, T.; Damry, D.; Biswas, S. Drug-induced liver injury: A comprehensive review. Ther. Adv. Gastroenterol. 2023, 16, 17562848231163410. [Google Scholar]
- Kobayashi, T.; Iwaki, M.; Nogami, A.; Yoneda, M. Epidemiology and management of drug-induced liver injury: Importance of the updated RUCAM. J. Clin. Transl. Hepatol. 2023, 11, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Kamal, S.; Abdelhakam, S.; Ghoraba, D.; Massoud, Y.; Aziz, K.A.; Hassan, H.; Hafez, T.; Abdel Sallam, A. The frequency, clinical course, and health related quality of life in adults with Gilbert’s syndrome: A longitudinal study. BMC Gastroenterol. 2019, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R. Idiosyncratic DILI: Analysis of 46,266 cases assessed for causality by RUCAM and published from 2014 to early 2019. Special issue. Front. Pharmacol. 2019, 10, 730. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.M.; Chen, C.L.; Chang, C.H.; Lee, M.R.; Wang, J.Y.; Hu, R.H.; Lee, P.H. Circulatory inflammatory mediators in the prediction of anti-tuberculous drug-induced liver injury using RUCAM for causality assessment. Biomedicines 2021, 9, 891. [Google Scholar] [CrossRef]
- Teschke, R. DILI, HILI, RUCAM algorithm, and AI, the Artificial Intelligence: Provocative issues, progress, and proposals. Arch. Gastroenterol. Res. 2020, 1, 4–11. [Google Scholar]
- Teschke, R.; Danan, G. Worldwide use of RUCAM for causality assessment in 81,856 idiosyncratic DILI and 14,029 HILI cases published 1993—Mid 2020: A comprehensive analysis. Medicines 2020, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Sivasailam, B.; Kumar, A.; Marciniak, E.; Deepak, J. Acute liver failure induced by Joss paper ingestion. Med. Case Rep. 2019, 5, 9. [Google Scholar]
- Witkowska, D.; Słowik, J.; Chilicka, K. Heavy metals and human health: Possible exposure pathways and the competition for protein binding sites. Molecules 2021, 26, 6060. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R.; Vongdala, N.; Quan, N.V.; Quy, T.N.; Xuan, T.D. Metabolic toxification of 1,2-unsaturated pyrrolizidine alkaloids causes human hepatic sinusoidal obstruction syndrome: The update. Int. J. Mol. Sci. 2021, 22, 10419. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.; Wittke, C.; Lederer, I.; Klier, B.; Kleinwächter, M.; Selmar, D. Interspecific transfer of pyrrolizidine alkaloids: An unconsidered source of contaminations of phytopharmaceuticals and plant derived commodities. Food Chem. 2016, 213, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Selmar, D.; Wittke, C.; Beck von Wolffersdorff, I.; Klier, B.; Lewerenz, L.; Kleinwächter, M.; Nowak, M. Transfer of pyrrolizidine alkaloids between living plants: A disregarded source of contaminations. Environ. Pollut. 2019, 248, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Lv, L.; Liu, Y.; Ji, M.; Zang, E.; Liu, Q.; Zhang, M.; Li, M. Applied analytical methods for detecting heavy metals in medicinal plants. Crit. Rev. Analyt. Chem. 2023, 53, 339–359. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Wang, B.; Jiang, J.; Fitzgerald, M.; Huang, Q.; Yu, Z.; Li, H.; Zhang, J.; Wei, J.; Yang, C.; et al. Heavy metal contaminations in herbal medicines: Determination, comprehensive risk assessments, and solutions. Front. Pharmacol. 2021, 11, 595335. [Google Scholar] [CrossRef] [PubMed]
- Philips, A.B.; Ahamed, R.; Abduljaleel, J.K.; Rajesh, S.; Theruvath, A.H.; Raveendran, R.; Augustine, R. Ayurvedic treatment induced severe alcoholic hepatitis and non-cirrhotic portal hypertension in a 14-year-old girl. Oxf. Med. Case Rep. 2022, 2022, omac113. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R. Molecular idiosyncratic toxicology of drugs in the human liver compared with animals: Basic considerations. Int. J. Mol. Sci. 2023, 24, 6663. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.F. Copper nutrition and biochemistry and human (patho)physiology. Adv. Food Nutr. Res. 2021, 96, 311–364. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. Trace elements in human physiology and pathology. Copper. Biomed. Pharmacother. 2003, 57, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zheng, L.; Ma, S.R.; Li, J.; Yang, S. Cuproptosis: Emerging biomarkers and potential therapeutics in cancer. Front. Oncol. 2023, 13, 1288504. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yang, Y.; Gao, Y.; He, J. Cuproptosis: Mechanisms and links with cancers. Mol. Cancer 2023, 22, 46. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Xiao, P.; Qiu, B.; Yu, H.F.; Teng, C.B. Copper chaperone antioxidant 1: Multiple roles and a potential therapeutic target. J. Mol. Med. 2023, 101, 527–542. [Google Scholar] [PubMed]
- Bost, M.; Houdart, S.; Oberli, M.; Kalonji, E.; Huneau, J.F.; Margaritis, I. Dietary copper and human health: Current evidence and unresolved issues. J. Trace Elem. Med. Biol. 2016, 35, 107–115. [Google Scholar] [CrossRef]
- Murray, L.; Arteche, A.; Fearon, P.; Halligan, S.; Goodyer, I.; Cooper, P. Maternal postnatal depression and the development of depression in offspring up to 16 years of age. J. Am. Acad. Child. Adolesc. Psychiatry 2011, 50, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.A.; Sarkar, B. Liver as a key organ in the supply, storage, and excretion of copper. Am. J. Clin. Nutr. 2008, 88, 851S–854S. [Google Scholar] [CrossRef] [PubMed]
- Chanpong, A.; Dhawan, A. Wilson disease in children and young adults—State of the art. Saudi J. Gastroenterol. 2022, 28, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Gaetke, L.M.; Chow-Johnson, H.S.; Chow, C.K. Copper: Toxicological relevance and mechanisms. Arch. Toxicol. 2014, 88, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, E.; Jankowski, K. Copper and zinc concentrations in medicinal herbs and Soil surrounding ponds on agricultural land. Landsc. Ecol. Eng. 2017, 13, 183–188. [Google Scholar] [CrossRef]
- Nayak, N.C.; Chitale, A.R. Indian childhood cirrhosis (ICC) and ICC-like diseases: The changing scenario of facts versus notions. Indian. J. Med. Res. 2013, 137, 1029–1042. [Google Scholar] [PubMed]
- Yruela, I. Copper in plants: Acquisition, transport and interactions. Funct. Plant Biol. 2009, 36, 409–430. [Google Scholar] [CrossRef]
- Nawrot, N.; Wojciechowska, E.; Rezania, S.; Walkusz-Miotk, J.; Pazdro, K. The effects of urban vehicle traffic on heavy metal contamination in road sweeping waste and bottom sediments of retention tanks. Sci. Total Environ. 2020, 749, 141511. [Google Scholar] [CrossRef] [PubMed]
- Stremmel, W.; Weiskirchen, R. Therapeutic strategies in Wilson disease: Pathophysiology and mode of action. Ann. Transl. Med. 2021, 9, 732. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.; Solioz, M. Evaluation of chocolate as a source of dietary copper. Eur. Food Technol. 2014, 238, 1063–1066. [Google Scholar] [CrossRef][Green Version]
- Adeyeye, E.I.; Arogundade, L.A.; Asaolu, S.S.; Olaofe, O. Fungicide-derived copper content in soil and vegetation component, Owena cocoa (Theobroma cacao L.) plantations in Nigeria. Bangladesh J. Sci. Ind. Res. 2006, 41, 129–140. [Google Scholar] [CrossRef]
- Flemming, C.A.; Trevors, J.T. Copper toxicity and chemistry in the environment: A review. Water Air Soil. Pollut. 1989, 44, 143–158. [Google Scholar] [CrossRef]
- Kovačič, G.R.; Lešnik, M.; Vršič, S. An overview of the copper situation and usage in viticulture. Bulg. J. Agric. Sci. 2013, 19, 50–59. [Google Scholar]
- Gaberšek, M.; Watts, M.J.; Gosar, M. Attic dust: An archive of historical air contamination of the urban environment and potential hazard to health? J. Hazard. Mat. 2022, 432, 128745. [Google Scholar] [CrossRef] [PubMed]
- Brüssow, H. What is health? Microb. Biotechnol. 2013, 6, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Bircher, J.; Kuruvilla, S. Defining health by addressing individual, social, and environmental determinants: New opportunities for health care and public health. J. Public Health Policy 2014, 35, 363–386. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.; Liu, L.; Wang, Q.; Saleem, M.H.; Bashir, S.; Ullah, S.; Peng, D. Copper environmental toxicology, recent advances, and future outlook: A review. Environ. Sci. Pollut. Res. Int. 2019, 26, 18003–18016. [Google Scholar] [CrossRef] [PubMed]
- Covre, W.P.; Ramos, S.J.; Pereira, W.V.D.S.; Souza, E.S.; Martins, G.C.; Teixeira, O.M.M.; Amarante, C.B.D.; Dias, Y.N.; Fernandes, A.R. Impact of copper mining wastes in the Amazon: Properties and risks to environment and human health. J. Hazard. Mater. 2022, 421, 126688. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, Z.; Sardar, A.; Shabbir, A.; Abbas, G.; Shamshad, S.; Khalid, S.; Natasha Murtaza, G.; Dumat, C.; Shahid, M. Copper uptake, essentiality, toxicity, detoxification and risk assessment in soil-plant environment. Chemosphere 2020, 259, 127436. [Google Scholar] [CrossRef] [PubMed]
- Georgopoulos, P.G.; Roy, A.; Yonone-Lioy, M.J.; Opiekun, R.E.; Lioy, P.J. Environmental copper: Its dynamics and human exposure issues. J. Toxicol. Environ. Health B Crit. Rev. 2001, 4, 341–394. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.W.; Bondy, S.C.; Kitazawa, M. Environmental and dietary exposure to copper and its cellular mechanisms linking to Alzheimer’s disease. Toxicol. Sci. 2018, 163, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Pandita, S.; Singh Sidhu, G.P.; Sharma, A.; Khanna, K.; Kaur, P.; Bali, A.S.; Setia, R. Copper bioavailability, uptake, toxicity and tolerance in plants: A comprehensive review. Chemosphere 2021, 262, 127810. [Google Scholar] [CrossRef] [PubMed]
- Civardi, C.; Schwarze, F.W.; Wick, P. Micronized copper wood preservatives: An efficiency and potential health risk assessment for copper-based nanoparticles. Environ. Pollut. 2015, 200, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Xu, D.M.; Chen, T.; Yan, Z.A.; Li, L.L.; Wang, M.H. Leachability characteristic of heavy metals and associated health risk study in typical copper mining-impacted sediments. Chemosphere 2020, 239, 124748. [Google Scholar] [CrossRef] [PubMed]
- Stern, B.R. Essentiality and toxicity in copper health risk assessment: Overview, update and regulatory considerations. J. Toxicol. Environ. Health A 2010, 73, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Filimon, M.N.; Caraba, I.V.; Popescu, R.; Dumitrescu, G.; Verdes, D.; Petculescu Ciochina, L.; Sinitean, A. Potential ecological and human health risks of heavy metals in soils in selected copper mining areas-a case study: The Bor area. Int. J. Environ. Res. Public. Health 2021, 18, 1516. [Google Scholar] [CrossRef] [PubMed]
- Ameh, T.; Sayes, C.M. The potential exposure and hazards of copper nanoparticles: A review. Environ. Toxicol. Pharmacol. 2019, 71, 10322. [Google Scholar] [CrossRef]
- Chowdhury, R.; Ramond, A.; O′Keeffe, L.M.; Shahzad, S.; Kunutsor, S.K.; Muka, T.; Gregson, J.; Willeit, P.; Warnakula, S.; Khan, H.; et al. Environmental toxic metal contaminants and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2018, 362, k3310. [Google Scholar] [CrossRef] [PubMed]
- Gujre, N.; Rangan, L.; Mitra, S. Occurrence, geochemical fraction, ecological and health risk assessment of cadmium, copper and nickel in soils contaminated with municipal solid wastes. Chemosphere 2021, 271, 129573. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.A.; Tsuji, J.S.; Garry, M.R.; McArdle, M.E.; Goodfellow, W.L., Jr.; Adams, W.J.; Menzie, C.A. Critical review of exposure and effects: Implications for setting regulatory health criteria for ingested copper. Environ. Manag. 2020, 65, 131–159. [Google Scholar] [CrossRef] [PubMed]
- Nematollahi, M.J.; Keshavarzi, B.; Zaremoaiedi, F.; Rajabzadeh, M.A.; Moore, F. Ecological-health risk assessment and bioavailability of potentially toxic elements (PTEs) in soil and plant around a copper smelter. Environ. Monit. Assess. 2020, 192, 639. [Google Scholar] [CrossRef]
- Wang, M.; Lv, Y.; Lv, X.; Wang, Q.; Li, Y.; Lu, P.; Yu, H.; Wei, P.; Cao, Z.; An, T. Distribution, sources and health risks of heavy metals in indoor dust across China. Chemosphere 2023, 313, 137595. [Google Scholar] [CrossRef] [PubMed]
- Lerner, C.A.; Sundar, I.K.; Watson, R.M.; Elder, A.; Jones, R.; Done, D.; Kurtzman, R.; Ossip, D.J.; Robinson, R.; McIntosh, S.; et al. Environmental health hazards of e-cigarettes and their components: Oxidants and copper in e-cigarette aerosols. Environ. Pollut. 2015, 198, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, Q. Association between serum copper levels and lung cancer risk: A meta-analysis. J. Int. Med. Res. 2018, 46, 4863–4873. [Google Scholar] [CrossRef] [PubMed]
- Royer, A.; Sharman, T. Copper Toxicity. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557456/ (accessed on 18 March 2024).
- Carvalho, J.A.; Boavida, L.; Ferreira, R.; Favas, C.; Delgado Alves, J. Copper-induced haemolytic anaemia. Eur. J. Case Rep. Intern. Med. 2021, 8, 002785. [Google Scholar] [CrossRef]
- Gamakaranage, C.S.; Rodrigo, C.; Weerasinghe, S.; Gnanathasan, A.; Puvanaraj, V.; Fernando, H. Complications and management of acute copper sulphate poisoning; a case discussion. J. Occup. Med. Toxicol. 2011, 6, 34. [Google Scholar] [CrossRef]
- Jantsch, W.; Kulig, K.; Rumack, B.H. Massive copper sulfate ingestion resulting in hepatotoxicity. J. Toxicol. Clin. Toxicol. 1985, 22, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Sinkovic, A.; Striding, A.; Svensek, F. Severe acute copper sulphate poisoning: A case report. Arh. Hig. Rada Toksikol. 2008, 59, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Sonal Sekhar, M.; Rao, M. Clinical toxicology of copper: Source, toxidrome, mechanism of toxicity, and management. In Metal Toxicology Handbook; Bagchi, D., Bagchi, M., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 199–217. [Google Scholar]
- Berentsen, S.; Barcellini, W. Autoimmune hemolytic anemias. N. Engl. J. Med. 2021, 385, 1407–1419. [Google Scholar] [CrossRef] [PubMed]
- Lizaola-Mayo, B.C.; Dickson, R.C.; Lam-Himlin, D.M.; Chascsa, D.M. Exogenous copper exposure causing clinical Wilson disease in a patient with copper deficiency. BMC Gastroenterol. 2021, 21, 278. [Google Scholar] [CrossRef] [PubMed]
- Priya, S. Acute copper sulphate poisoning. Ind. J. Med. Spec. 2018, 9, 140–142. [Google Scholar] [CrossRef]
- Chugh, K.S.; Sharma, B.K.; Singhal, P.C.; Das, K.C.; Datta, B.N. Acute renal failure following copper sulphate intoxication. Postgrad. Med. J. 1977, 53, 18–23. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Araya, M.; Kelleher, S.L.; Arredondo, M.A.; Sierralta, W.; Vial, M.T.; Uauy, R.; Lönnerdal, B. Effects of chronic copper exposure during early life in rhesus monkeys. Am. J. Clin. Nutr. 2005, 81, 1065–1071. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Araya, M.; Núñez, H.; Pavez, L.; Arredondo, M.; Méndez, M.; Cisternas, F.; Pizarro, F.; Sierralta, W.; Uauy, R.; González, M. Administration of high doses of copper to Capuchin Monkeys does not cause liver damage but induces transcriptional activation of hepatic proliferative responses. J. Nutr. 2012, 142, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Xu, M.; Luo, J.; Zhao, L.; Ye, G.; Shi, F.; Lv, C.; Chen, H.; Wang, Y.; Li, Y. Liver toxicity assessments in rats following sub-chronic oral exposure to copper nanoparticles. Environ. Sci. Eur. 2019, 31, 30. [Google Scholar] [CrossRef]
- Vogel, F.S. The deposition of exogenous copper under experimental conditions with observations on its neurotoxic and nephrotoxic properties in relation to Wilson’s disease. J. Exp. Med. 1959, 110, 801–810. [Google Scholar] [CrossRef]
- Stremmel, W.; Merle, U.; Weiskirchen, R. Clinical features of Wilson disease. Ann. Transl. Med. 2019, 7 (Suppl. S2), S61. [Google Scholar] [CrossRef] [PubMed]
- Garrido, I.; Marques, M.; Liberal, R.; Cardoso, H.; Lopes, S.; Macedo, G. Wilson disease in Northern Portugal: A long-term follow-up study. Orphanet J. Rare Dis. 2022, 17, 82. [Google Scholar] [CrossRef] [PubMed]
- Moini, M.; To, U.; Schilsky, M.K. Recent advances in Wilson disease. Trans. Gastroenterol. Hepatol. 2021, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Pandit, A.; Bhave, S. Present interpretation of the role of copper in Indian childhood cirrhosis. Am. J. Clin. Nutr. 1996, 63, 830S–835S. [Google Scholar] [CrossRef] [PubMed]
- Hamza, I.; Gitlin, J.D. Hepatic copper transport. In Madame Curie Bioscience Database; Landes Bioscience: Austin, TX, USA, 2000–2013. Available online: https://www.ncbi.nlm.nih.gov/books/NBK6381/ (accessed on 8 March 2024).
- Müller-Höcker, J.; Weiß, M.; Meyer, U.; Schramel, P.; Wiebecke, B.; Belohradsky, B.H.; Hübner, G. Fatal copper storage disease of the liver in a German infant resembling Indian childhood cirrhosis. Virchows Arch. A 1987, 411, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Feichtinger, H.; Berger, H.; Müller, W. Endemic Tyrolean infantile cirrhosis: An ecogenetic disorder. Lancet 1996, 347, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Müller, W.; Feichtinger, H. Idiopathic copper toxicosis. Am. J. Clin. Nutr. 1998, 67, 1082S–1086S. [Google Scholar] [CrossRef] [PubMed]
- Horslen, S.P.; Tanner, M.S.; Lyon, T.D.; Fell, G.S.; Lowry, M.F. Copper associated childhood cirrhosis. Gut 1994, 35, 1497–1500. [Google Scholar] [CrossRef] [PubMed]
- Scheinberg, I.H.; Sternlieb, I. Wilson disease and idiopathic copper toxicosis. Am. J. Clin. Nutr. 1996, 63, 842S–845S. [Google Scholar] [CrossRef]
- Sandahl, T.D.; Laursen, T.L.; Munk, D.E.; Vilstrup, H.; Weiss, K.H.; Ott, P. The prevalence of Wilson’s disease: An update. Hepatology 2020, 71, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Merle, U.; Schaefer, M.; Ferenci, P.; Stremmel, W. Clinical presentation, diagnosis and long-term outcome of Wilson’s disease: A cohort study. Gut 2007, 56, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Watanabe, K.; Inui, A.; Kato, A.; Tatsumi, Y.; Okumura, A.; Fujisawa, T.; Kato, K. Alanine aminotransferase as the first test parameter for Wilson’s disease. J. Clin. Transl. Hepatol. 2019, 7, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, J.; Hammer, N.; Magini, G.; Ponte, B.; Ongaro, M.; Rougemont, A.L.; Goossens, N.; Frossard, J.L.; Spahr, L. Management of acute Wilsonian hepatitis with severe hemolysis: A successful combination of chelation and MARS dialysis. Case Rep. Hepatol. 2021, 2021, 5583654. [Google Scholar] [CrossRef] [PubMed]
- Kasztelan-Szczerbinska, B.; Cichoz-Lach, H. Wilson’s disease: An update on the diagnostic workup and management. J. Clin. Med. 2021, 10, 5097. [Google Scholar] [CrossRef] [PubMed]
- Mohr, I.; Weiss, K.H. Current anti-copper therapies in management of Wilson disease. Ann. Transl. Med. 2019, 7 (Suppl. S2), S69. [Google Scholar] [CrossRef]
- Alkhouri, N.; Gonzalez-Peralta, R.P.; Medici, V. Wilson disease: A summary of the updated AASLD Practice Guidance. Hepatol. Commun. 2023, 7, e0150. [Google Scholar] [CrossRef] [PubMed]
- Korman, J.D.; Volenberg, I.; Balko, J.; Webster, J.; Schiodt, F.V.; Squires, R.H., Jr.; Fontana, R.J.; Lee, W.M.; Schilsky, M.L.; Pediatric and Adult Acute Liver Failure Study Groups. Screening for Wilson disease in acute liver failure: A comparison of currently available diagnostic tests. Hepatology 2008, 48, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Mbala, J.; Belmalih, A.; Guillaud, O.; Lachaux, A.; Couchonnal Bedoya, E. Evaluation of vitamin B6 supplementation in Wilson’s disease patients treated with D-penicillamine. BMJ Open Gastroenterol. 2023, 10, e001211. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Dong, J.; Cheng, N.; Yang, R.; Han, Y.; Han, Y. Inflammatory cytokines expression in Wilson’s disease. Neurol. Sci. 2019, 40, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Antczak-Kowalska, M.; Członkowska, A.; Eyileten, C.; Palejko, A.; Cudna, A.; Wolska, M.; Piechal, A.; Litwin, T. Autoantibodies in Wilson disease: Impact on clinical course. JIMD Rep. 2022, 63, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Hennes, E.M.; Zeniya, M.; Czaja, A.J.; Parés, A.; Dalekos, G.N.; Krawitt, E.L.; Bittencourt, P.L.; Porta, G.; Boberg, K.M.; Hofer, H.; et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology 2008, 48, 169–176. [Google Scholar] [CrossRef]
- Ferenci, P.; Caca, K.; Loudianos, G.; Mieli-Vergani, G.; Tanner, S.; Sternlieb, I.; Schilsky, M.; Cox, D.; Berr, F. Diagnosis and phenotypic classification of Wilson disease. Liver Int. 2003, 23, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Nagral, A.; Sarma, M.S.; Matthai, J.; Kukkle, P.L.; Devarbhavi, H.; Sinha, S.; Alam, S.; Bavdekar, A.; Dhiman, R.K.; Eapen, C.E.; et al. Wilson’s Disease: Clinical Practice Guidelines of the Indian National Association for Study of the Liver, the Indian Society of Pediatric Gastroenterology, Hepatology and Nutrition, and the Movement Disorders Society of India. J. Clin. Exp. Hepatol. 2019, 9, 74–98, Erratum in J. Clin. Exp. Hepatol. 2020, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Sood, V.; Rawat, D.; Khanna, R.; Alam, S. Cholestatic liver disease masquerading as Wilson disease. Indian J. Gastroenterol. 2015, 34, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, S.M.; Matsukuma, K.E.; Medici, V. Wilson disease and the differential diagnosis of its hepatic manifestations: A narrative review of clinical, laboratory, and liver histological features. Ann. Transl. Med. 2021, 9, 1394. [Google Scholar] [CrossRef] [PubMed]
- Emanuele, P.; Goodman, Z.D. A simple and rapid stain for copper in liver tissue. Ann. Diagn. Pathol. 1998, 2, 125–126. [Google Scholar] [CrossRef]
- EASL. EASL clinical practice guidelines: Wilson’s disease. J. Hepatol. 2012, 56, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Sternlieb, I. Mitochondrial and fatty changes in hepatocytes of patients with Wilson’s disease. Gastroenterology 1968, 55, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Weiss, K.; Stremmel, W. Evolving perspectives in Wilson disease: Diagnosis, treatment and monitoring. Curr. Gastroenterol. Rep. 2012, 14, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kolbaum, A.E.; Sarvan, I.; Bakhiya, N.; Spolders, M.; Pieper, R.; Schubert, J.; Jung, C.; Hackethal, C.; Sieke, C.; Grünewald, K.H.; et al. Long-term dietary exposure to copper in the population in Germany—Results from the BfR MEAL study. Food Chem. Toxicol. 2023, 176, 113759. [Google Scholar] [CrossRef] [PubMed]
- Ferenci, P.; Stremmel, W.; Członkowska, A.; Szalay, F.; Viveiros, A.; Stättermayer, A.F.; Bruha, R.; Houwen, R.; Pop, T.L.; Stauber, R.; et al. Age and sex but not ATP7B genotype effectively influence the clinical phenotype of Wilson disease. Hepatology 2019, 69, 1464–1476. [Google Scholar] [CrossRef] [PubMed]
- Woimant, F.; Debray, D.; Morvan, E.; Obadia, M.A.; Poujois, A. Efficacy and safety of two salts of trientine in the treatment of Wilson’s disease. J. Clin. Med. 2022, 11, 3975. [Google Scholar] [CrossRef] [PubMed]
- Siegemund, R.; Lössner, J.; Günther, K.; Kühn, H.J.; Bachmann, H. Mode of action of triethylenetetramine dihydrochloride on copper metabolism in Wilson’s disease. Acta Neurol. Scand. 1991, 83, 364–366. [Google Scholar] [CrossRef] [PubMed]
- Ranucci, G.; Di Dato, F.; Spagnuolo, M.I.; Vajro, P.; Iorio, R. Zinc monotherapy is effective in Wilson’s disease patients with mild liver disease diagnosed in childhood: A retrospective study. Orphanet J. Rare Dis. 2014, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Avan, A.; Członkowska, A.; Gaskin, S.; Granzotto, A.; Sensi, S.L.; Hoogenraad, T.U. The role of zinc in the treatment of Wilson’s disease. Int. J. Mol. Sci. 2022, 23, 9316. [Google Scholar] [CrossRef] [PubMed]
- Soriot, P. AstraZeneca Cuts Alexion’s PhIII Wilson Disease Drug, Takes $244M Write-Down. Available online: https://endpts.com/astrazeneca-cuts-alexions-phiii-wilson-disease-drug-takes-244m-writedown/ (accessed on 27 April 2023).
- Kim, P.; Zhang, C.C.; Thoröe-Boveleth, S.; Buhl, E.M.; Weiskirchen, S.; Stremmel, W.; Merle, U.; Weiskirchen, R. Analyzing the therapeutic efficacy of Bis-Choline-Tetrathiomolybdate in the Atp7b-/- copper overload mouse model. Biomedicines 2021, 9, 1861. [Google Scholar] [CrossRef] [PubMed]
- Stremmel, W. Bis-choline tetrathiomolybdate as old drug in a new design for Wilson’s disease: Good for brain and liver? Hepatology 2019, 69, 901–903. [Google Scholar] [CrossRef] [PubMed]
- Weiss, K.H.; Askari, F.K.; Czlonkowska, A.; Ferenci, P.; Bronstein, J.M.; Bega, D.; Ala, A.; Nicholl, D.; Flint, S.; Olsson, L.; et al. Bis-choline tetrathiomolybdate in patients with Wilson’s disease: An open-label, multicentre, phase 2 study. Lancet Gastroenterol. Hepatol. 2017, 2, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Catana, A.M.; Medici, V. Liver transplantation for Wilson disease. World J. Hepatol. 2012, 4, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Litwin, T.; Bembenek, J.P.; Antos, A.; Przybyłkowski, A.; Skowrońska, M.; Kurkowska-Jastrzębska, I.; Członkowska, A. Liver transplantation as a treatment for Wilson’s disease with neurological presentation: A systematic literature review. Acta Neurol. Belg. 2022, 122, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Roy-Chowdhury, N.; Roy-Chowdhury, J. Hepatocyte Transplantation. Available online: https://www.uptodate.com/contents/hepatocyte-transplantation (accessed on 3 November 2023).
- Chen, X.; Xing, S.; Feng, Y.; Chen, S.; Pei, Z.; Wang, C.; Liang, X. Early stage transplantation of bone marrow cells markedly ameliorates copper metabolism and restores liver function in a mouse model of Wilson disease. BMC Gastroenterol. 2011, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R.; Eickhoff, A. Wilson disease: Copper-mediated cuproptosis, iron-related ferroptosis, and clinical issues, with comprehensive and critical analysis update Special issue: Heavy metals. Int. J. Mol. Sci. 2024, 25, 4753. [Google Scholar] [CrossRef] [PubMed]
- Gioilli, B.D.; Kidane, T.Z.; Fieten, H.; Tellez, M.; Dalphin, M.; Nguyen, A.; Nguyen, K.; Linder, M.C. Secretion and uptake of copper via a small copper carrier in blood fluid. Metallomics 2022, 14, mfac006. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.F.; Latorre-Muro, P.; Puigserver, P. Mechanisms of mitochondrial respiratory adaptation. Nat. Rev. Mol. Cell Biol. 2022, 23, 817–835. [Google Scholar] [CrossRef] [PubMed]
- Morio, B.; Panthu, B.; Bassot, A.; Rieusset, J. Role of mitochondria in metabolic health and diseases. Cell Calcium 2021, 94, 102336. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wang, J.; Pu, C.; Qiao, L.; Jiang, C. Wilson’s disease: A comprehensive review of the molecular mechanisms. Int. J. Mol. Sci. 2015, 16, 6419–6431. [Google Scholar] [CrossRef] [PubMed]
- Dalgiç, B.; Sönmez, N.; Biberoğlu, G.; Hasanoğlu, A.; Erbaş, D. Evaluation of oxidant stress in Wilson’s disease and non-Wilsonian chronic liver disease in childhood. Turk. J. Gastroenterol. 2005, 16, 7–11. [Google Scholar] [PubMed]
- Kalita, J.; Kumar, V.; Misra, U.K.; Kumar, S. Movement disorder in Wilson disease: Correlation with MRI and biomarkers of cell injury. J. Mol. Neurosci. 2021, 71, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Barber, R.G.; Grenier, Z.A.; Burkhead, J.L. Copper toxicity is not just oxidative damage: Zinc systems and insight from Wilson disease. Biomedicines 2021, 9, 316. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Deng, L.; Ma, X.; Guo, Y.; Feng, Z.; Liu, M.; Guan, Y.; Huang, Y.; Deng, J.; Li, H.; et al. Altered diversity and composition of gut microbiota in Wilson’s disease. Sci. Rep. 2020, 10, 21825. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.S.; Daudu, P.A.; Taylor, P.C.; Mackey, B.E.; Turnlund, J.R. Effects of low-copper diets on human immune response. Am. J. Clin. Nutr. 1995, 62, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, R.G.; Failla, M.L. Copper deficiency reduces interleukin-2 (IL-2) production and IL-2 mRNA in human T-lymphocytes. J. Nutr. 1997, 127, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, R.G.; Failla, M.L. Transcriptional regulation of interkeukin-2 gene expression is impaired by copper deficiency in Jurkat T lymphocytes. J. Nutr. 1999, 129, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Kisseleva, T.; Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Fuentealba, I.C.; Aburto, E.M. Animal models of copper-associated liver disease. Comp. Hepatol. 2003, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S.; Okada, S.; Hamazaki, S.; Fujioka, M.; Li, J.L.; Midorikawa, O. Cirrhosis of the liver induced by cupric nitrilotriacetate in Wistar rats. An experimental model of copper toxicosis. Am. J. Pathol. 1989, 134, 1263–1274. [Google Scholar]
- Zhao, L.; Xia, Z.; Wang, F. Zebrafish in the sea of mineral (iron, zinc, and copper) metabolism. Front. Pharmacol. 2014, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Ems, T.; St Lucia, K.; Huecker, M.R. Biochemistry, Iron Absorption. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK448204/ (accessed on 12 June 2024).
- Vogt, A.S.; Arsiwala, T.; Mohsen, M.; Vogel, M.; Manolova, V.; Bachmann, M.F. On iron metabolism and its regulation. Int. J. Mol. Sci. 2021, 22, 4591. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xiong, Z.; Wu, W.; Ling, H.Q.; Kong, D. Iron in the symbiosis of plants and microorganisms. Plants 2023, 12, 1958. [Google Scholar] [CrossRef] [PubMed]
- McLaren, G.D.; Nathanson, M.H.; Jacobs, A.; Trevett, D.; Thomson, W. Regulation of intestinal iron absorption and mucosal iron kinetics in hereditary hemochromatosis. J. Lab. Clin. Med. 1991, 117, 390–401. [Google Scholar] [PubMed]
- Teichmann, R.; Stremmel, W. Iron uptake by human upper small intestine microvillous membrane vesicles. Indication for a facilitated transport mechanism mediated by a membrane iron-binding protein. J. Clin. Investig. 1990, 86, 2145–2153. [Google Scholar] [CrossRef] [PubMed]
- Stremmel, W.; Schneider, M.; Lotz, G.; Niederau, C.; Teschke, R.; Strohmeyer, G. Iron uptake by rat duodenal microvillous membrane vesicles. Gastroenterology 1985, 88, A1602. [Google Scholar]
- Stremmel, W.; Schneider, M.; Lotz, G.; Niederau, C.; Teschke, R.; Strohmeyer, G. Iron uptake by rat duodenal microvillous membrane vesicles: Evidence for a carrier-mediated process. Eur. J. Clin. Investig. 1987, 17, 136–145. [Google Scholar] [CrossRef]
- Piskin, E.; Cianciosi, D.; Gulec, S.; Tomas, M.; Capanoglu, E. Iron absorption: Factors, limitations, and Improvement methods. ACS Omega 2022, 7, 20441–20456. [Google Scholar] [CrossRef] [PubMed]
- Sukhbaatar, N.; Weichhart, T. Iron regulation: Macrophages in control. Pharmaceuticals 2018, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Li, J.; Cao, F.; Yin, H.L.; Huang, Z.J.; Lin, Z.T.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef]
- Gilardi, G.; Di Nardo, G. Heme iron centers in cytochrome P450: Structure and catalytic activity. Rend. Fis. Acc. Lincei 2017, 28, 159–167. [Google Scholar] [CrossRef]
- Rishi, G.; Subramaniam, V.N. The liver in regulation of iron homeostasis. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 313, G157–G165. [Google Scholar] [CrossRef] [PubMed]
- Fleming, R.E.; Ponka, P. Iron overload in human disease. N. Engl. J. Med. 2012, 366, 348–359, Erratum in N. Engl. J. Med. 2012, 366, 771. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.J.; Frazer, D.M. Hepatic iron metabolism. Semin. Liver Dis. 2005, 25, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Qiao, B.; Sugianto, P.; Fung, E.; Del-Castillo-Rueda, A.; Moran-Jimenez, M.J.; Ganz, T.; Nemeth, E. Hepcidin-induced endocytosis of ferroportin is dependent on ferroportin ubiquitination. Cell Metab. 2012, 15, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Daher, R.; Karim, Z. Iron metabolism: State of the art. Transfus. Clin. Biol. 2017, 24, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar] [PubMed]
- Pfeiffer, C.M.; Looker, A.C. Laboratory methodologies for indicators of iron status: Strengths, limitations, and analytical challenges. Am. J. Clin. Nutr. 2017, 106 (Suppl. S6), 1606S–1614S. [Google Scholar] [CrossRef] [PubMed]
- Daru, J.; Colman, K.; Stanworth, S.J.; De La Salle, B.; Wood, E.M.; Pasricha, S.R. Serum ferritin as an indicator of iron status: What do we need to know? Am. J. Clin. Nutr. 2017, 106 (Suppl. S6), 1634S–1639S. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.C.; Lam, J.M.; Wong, A.H.C.; Hon, K.L.; Li, X. Iron Deficiency Anemia: An Updated Review. Curr. Pediatr. Rev. 2024, 20, 339–356. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Wimbley, T.D.; Graham, D.Y. Diagnosis and management of iron deficiency anemia in the 21st century. Therap Adv. Gastroenterol. 2011, 4, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Lugović-Mihić, L.; Pilipović, K.; Crnarić, I.; Šitum, M.; Duvančić, T. Differential diagnosis of cheilitis—How to classify cheilitis? Acta Clin. Croat. 2018, 57, 342–351. [Google Scholar] [CrossRef]
- Porter, J.L.; Rawla, P. Hemochromatosis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430862/ (accessed on 12 June 2024).
- Salomao, M.A. Pathology of hepatic iron overload. Clin. Liver Dis. 2021, 17, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Kamble, R.K.; Thakare, M.G.; Ingle, A.B. Iron in the environment. Indian J. Environ. Prot. 2013, 33, 881–888. [Google Scholar]
- Koné, W.M.; Koffi, A.G.; Bomisso, E.L.; Tra Bi, F.H. Ethnomedical study and iron content of some medicinal herbs used in traditional medicine in Cote d‘Ivoire for the treatment of anaemia. Afr. J. Tradit. Complement. Altern. Med. 2011, 9, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhang, J.; Li, W.; Xu, M.; Liu, S. Disruption of iron homeostasis and resultant health effects upon exposure to various environmental pollutants: A critical review. J. Environ. Sci. 2015, 34, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Schreinemachers, D.M.; Ghio, A.J. Effects of environmental pollutants on cellular iron homeostasis and ultimate links to human disease. Environ. Health Insights 2016, 10, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, G.C.; Khan, M.J.H.; Chakraborty, T.K.; Zaman, S.; Kabir, A.H.M.E.; Tanaka, H. Human health Risk assessment of elevated and variable iron and manganese intake with arsenic-safe groundwater in Jashore, Bangladesh. Sci. Rep. 2020, 10, 5206. [Google Scholar] [CrossRef] [PubMed]
- Lal, A. Iron in health and disease: An update. Indian J. Pediatr. 2020, 87, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Schildroth, S.; Kordas, K.; White, R.F.; Friedman, A.; Placidi, D.; Smith, D.; Lucchini, R.G.; Wright, R.O.; Horton, M.; Claus Henn, B. An industry-relevant metal mixture, iron status, and reported attention-related behaviors in Italian adolescents. Environ. Health Perspect. 2024, 132, 27008. [Google Scholar] [CrossRef] [PubMed]
- Maher, B.A.; González-Maciel, A.; Reynoso-Robles, R.; Torres-Jardón, R.; Calderón-Garcidueñas, L. Iron-rich air pollution nanoparticles: An unrecognised environmental risk factor for myocardial mitochondrial dysfunction and cardiac oxidative stress. Environ. Res. 2020, 188, 109816. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, E.D. The hazards of iron loading. Metallomics 2010, 2, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Diami, S.M.; Kusin, F.M.; Madzin, Z. Potential ecological and human health risks of heavy metals in surface soils associated with iron ore mining in Pahang, Malaysia. Environ. Sci. Pollut. Res. Int. 2016, 23, 21086–21097. [Google Scholar] [CrossRef] [PubMed]
- de Mello Gabriel, G.V.; Pitombo, L.M.; Rosa, L.M.T.; Navarrete, A.A.; Botero, W.G.; do Carmo, J.B.; de Oliveira, L.C. The environmental importance of iron speciation in soils: Evaluation of classic methodologies. Environ. Monit. Assess. 2021, 193, 63. [Google Scholar] [CrossRef]
- Roemhild, K.; von Maltzahn, F.; Weiskirchen, R.; Knüchel, R.; von Stillfried, S.; Lammers, T. Iron metabolism: Pathophysiology and pharmacology. Trends Pharmacol. Sci. 2021, 42, 640–656. [Google Scholar] [CrossRef]
- Gulec, S.; Anderson, G.J.; Collins, J.F. Mechanistic and regulatory aspects of intestinal iron absorption. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G397–G409. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Jones, A.; Waite, T.D.; Chen, Y.; Huang, X.; Rosso, K.M.; Kappler, A.; Mansor, M.; Tratnyek, P.G.; Zhang, H. Fe(II) redox chemistry in the environment. Chem. Rev. 2021, 121, 8161–8233. [Google Scholar] [CrossRef] [PubMed]
- Biel, D.; Steiger, T.K.; Bunzeck, N. Age-related iron accumulation and demyelination in the basal ganglia are closely related to verbal memory and executive functioning. Sci. Rep. 2021, 11, 9438. [Google Scholar] [CrossRef] [PubMed]
- Bateman, D.N.; Eagling, V.; Sandilands, E.A.; Jackson, G.; Crawford, C.; Hawkins, L.; Cheung, T.; Cooper, G.; Bradberry, S.M.; Thompson, J.P.; et al. Iron overdose epidemiology, clinical features and iron concentration-effect relationships: The UK experience 2008–2017. Clin. Toxicol. 2018, 56, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Verma, S. Long-term follow-up of overdose of iron tablets in adult successfully managed without liver transplant: A case report. J. Gstro. Hepato. 2022, V9, 1–3. [Google Scholar]
- Nishikawa, Y.; Matsuo, Y.; Watanabe, R.; Miyazato, M.; Matsuo, M.; Nagahama, Y.; Tanaka, H.; Ooshio, T.; Goto, M.; Okada, Y.; et al. Hepatocyte-specific damage in acute toxicity of sodium ferrous citrate: Presentation of a human autopsy case and experimental results in mice. Toxicol. Rep. 2023, 10, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Yuen, H.W.; Becker, W. Iron Toxicity. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459224/ (accessed on 12 June 2024).
- Eça, R.; Ferreira, S.; Gandara, J.; Pessegueiro, H.; Daniel, J. Liver transplantation for acute hepatic failure following intentional iron overdose. Cureus 2023, 15, e48392. [Google Scholar] [CrossRef] [PubMed]
- Morales-Cruz, M.J.; MejiasMorales, D.; Hunsaker, P.; Butler, E.; Tassone, M. Ingestion of toxic iron dose with benign outcome. Cureus 2023, 15, e40103. [Google Scholar] [CrossRef] [PubMed]
- Abhilash, K.P.; Arul, J.J.; Bala, D. Fatal overdose of iron tablets in adults. Indian J. Crit. Care Med. 2013, 17, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Sane, M.R.; Malukani, K.; Kulkarni, R.; Varun, A. Fatal iron toxicity in an adult: Clinical profile and review. Indian J. Crit. Care Med. 2018, 22, 801–803. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.W.; Tse, M.L.; Lau, F.L.; Chu, W. Endoscopic removal of iron bezoar following acute overdose. Clin. Toxicol. 2008, 46, 913–915. [Google Scholar] [CrossRef] [PubMed]
- Majdanik, S.; Potocka-Banas, B.; Glowinski, S.; Borowiak, K. Suicide by intoxication with iron (III) chloride. Forensic Toxicol. 2012, 39, 513–517. [Google Scholar] [CrossRef]
- Lands, R.; Isang, E. Secondary hemochromatosis due to chronic oral iron supplementation. Case Rep. Hematol. 2017, 2017, 2494167. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.G.; Warner, C.G. Chronic exposure to iron oxide, chromium oxide, and nickel oxide fumes of metal dressers in a steelworks. Br. J. Ind. Med. 1972, 29, 169–177. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bala, S.; Tabaku, A. Chronic obstructive pulmonary disease in iron-steel and ferrochrome industry workers. Cent. Eur. J. Public Health 2010, 18, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, S.A.; Brown, K.E. Iron-induced liver injury: A critical reappraisal. Int. J. Mol. Sci. 2019, 20, 2132. [Google Scholar] [CrossRef] [PubMed]
- Feder, J.N.; Gnirke, A.; Thomas, W.; Tsuchihashi, Z.; Ruddy, D.A.; Basava, A.; Dormishian, F.; Domingo, R.; Ellis, M.C.; Fullan, A.; et al. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat. Genet. 1996, 13, 399–408. [Google Scholar] [CrossRef]
- Girelli, D.; Busti, F.; Brissot, P.; Cabantchik, I.; Muckenthaler, M.U.; Porto G; on behalf of the Nomenclature Committee of the International Society for the Study of Iron in Biology and Medicine (BIOIRON Society). Hemochromatosis classification: Update and recommendations by the BIOIRON Society. Blood 2022, 139, 3018–3029. [Google Scholar] [CrossRef] [PubMed]
- Bardou-Jacquet, E.; Brissot, P. Diagnostic evaluation of hereditary hemochromatosis (HFE and non-HFE). Hematol. Oncol. Clin. N. Am. 2014, 28, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.; Vincelette, N.D. Update on iron metabolism and molecular perspective of common genetic and acquired disorder, hemochromatosis. Crit. Rev. Oncol. Hematol. 2015, 95, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Shvartsman, M.; Morán, E.; Lois, S.; Aranda, J.; Barqué, A.; de la Cruz, X.; Bruguera, M.; Vagace, J.M.; Gervasini, G.; et al. Functional consequences of transferrin receptor-2 mutations causing hereditary hemochromatosis type 3. Mol. Genet. Genom. Med. 2015, 3, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, Y.; Yano, M.; Wakusawa, S.; Miyajima, H.; Ishikawa, T.; Imashuku, S.; Takano, A.; Nihei, W.; Kato, A.; Kato, K.; et al. A Revised classification of primary iron overload syndromes. J. Clin. Transl. Hepatol. 2024, 12, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Milman, N.T. Managing Genetic Hemochromatosis: An overview of dietary measures, which may reduce intestinal iron absorption in persons with iron overload. Gastroenterol. Res. 2021, 14, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Brissot, P.; Thibault Cavey, T.; Martine Ropert, M.; Pascal Guggenbuhl, P.; Loréal, O. Clinical management of hemochromatosis: Current perspectives. Int. J. Clin. Transfus. Med. 2017, 5, 1–7. [Google Scholar] [CrossRef]
- Barton, J.C.; McLaren, C.E.; Chen, W.P.; Ramm, G.A.; Anderson, G.J.; Powell, L.W.; Subramaniam, V.N.; Adams, P.C.; Phatak, P.D.; Gurrin, L.C.; et al. Cirrhosis in hemochromatosis: Independent risk factors in 368 HFE p.C282Y homozygotes. Ann. Hepatol. 2018, 17, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, A.L.; Filho, R.; Cruz, S.; Franco, R.; Tavella, M.; Secaf, M.; Ramalho, L.; Zucoloto, S.; Rodrigues, S.; Zago, M. Hereditary hemochromatosis in a Brazilian university hospital in São Paulo State (1990–2000). Genet. Mol. Res. 2005, 4, 31–38. [Google Scholar] [PubMed]
- Nash, S.; Marconi, S.; Sikorska, K.; Naeem, R.; Nash, G. Role of liver biopsy in the diagnosis of hepatic iron overload in the era of genetic testing. Am. J. Clin. Pathol. 2002, 118, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Golfeyz, S.; Lewis, S.; Weisberg, I.S. Hemochromatosis: Pathophysiology, evaluation, and management of hepatic iron overload with a focus on MRI. Expert. Rev. Gastroenterol. Hepatol. 2018, 12, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Stål, P.; Glaumann, H.; Hultcrantz, R. Liver cell damage and lysosomal iron storage in patients with idiopathic hemochromatosis. A light and electron microscopic study. J. Hepatol. 1990, 11, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.; Adams, P.C. Biochemical liver profile in hemochromatosis. A survey of 100 patients. J. Clin. Gastroenterol. 1991, 13, 316–320. [Google Scholar] [CrossRef]
- Tavill, A.S.; Adams, P.C. A diagnostic approach to hemochromatosis. Can. J. Gastroenterol. 2006, 20, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Strohmeyer, G.; Niederau, C.; Stremmel, W. Survival and causes of death in hemochromatosis. Observations in 163 patients. Ann. N. Y. Acad. Sci. 1988, 526, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Niederau, C.; Fischer, R.; Sonnenberg, A.; Stremmel, W.; Trampisch, H.J.; Strohmeyer, G. Survival and causes of death in cirrhotic and in noncirrhotic patients with primary hemochromatosis. N. Engl. J. Med. 1985, 313, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Niederau, C.; Fischer, R.; Pürschel, A.; Stremmel, W.; Häussinger, D.; Strohmeyer, G. Long-term survival in patients with hereditary hemochromatosis. Gastroenterology 1996, 110, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Parmanand, B.; Watson, M.; Boland, K.J.; Ramamurthy, N.; Wharton, V.; Morovat, A.; Lund, E.K.; Collier, J.; Le Gall, G.; Kellingray, L.; et al. Systemic iron reduction by venesection alters the gut microbiome in patients with haemochromatosis. JHEP Rep. 2020, 2, 100154. [Google Scholar] [CrossRef] [PubMed]
- Rombout-Sestrienkova, E.; Winkens, B.; Essers, B.A.; Nieman, F.H.; Noord, P.A.; Janssen, M.C.; van Deursen, C.T.; Boonen, A.; Reuser-Kaasenbrood, E.P.; Heeremans, J.; et al. Erythrocytapheresis versus phlebotomy in the maintenance treatment of HFE hemochromatosis patients: Results from a randomized crossover trial. Transfusion 2016, 56, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Phatak, P.; Brissot, P.; Wurster, M.; Adams, P.C.; Bonkovsky, H.L.; Gross, J.; Malfertheiner, P.; McLaren, G.D.; Niederau, C.; Piperno, A.; et al. A phase I-II dose-escalation trial of deferasirox for the treatment of iron overload in HFE-related hereditary hemochromatosis. Hepatology 2010, 52, 1671–1679. [Google Scholar] [CrossRef] [PubMed]
- Cançado, R.; Melo, M.R.; de Moraves Bastos, R.; Santos, P.C.; Guerra-Shinohara, E.M.; Chiattone, C.; Ballas, S.K. Deferasirox in patients with iron overload secondary to hereditary hemochromatosis: Results of a 1-yr Phase 2 study. Eur. J. Haematol. 2015, 95, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Babitt, J.L.; Lin, H.Y. The molecular pathogenesis of hereditary hemochromatosis. Semin. Liver Dis. 2011, 31, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Niederau, C.; Strohmeyer, G. Strategies for early diagnosis of haemochromatosis. Eur. J. Gastroenterol. Hepatol. 2002, 14, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Pantopoulos, K. Inherited disorders of iron overload. Front. Nutr. 2018, 5, 103. [Google Scholar] [CrossRef] [PubMed]
- Grønlien, H.K.; Christoffersen, T.E.; Nystrand, C.F.; Garabet, L.; Syvertsen, T.; Moe, M.K.; Olstad, O.K.; Jonassen, C.M. Cytokine and gene expression profiling in patients with HFE-associated hereditary hemochromatosis according to genetic profile. Acta Haematol. 2021, 144, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Schmidtke, J. Twenty-Five years of contemplating genotype-based hereditary hemochromatosis population screening. Genes 2022, 13, 1622. [Google Scholar] [CrossRef] [PubMed]
- Faruqi, A.; Mukkamalla, S.K.R. Iron binding capacity. [Updated 2 January 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Mehta, K.J.; Farnaud, S.J.; Sharp, P.A. Iron and liver fibrosis: Mechanistic and clinical aspects. World J. Gastroenterol. 2019, 25, 521–538. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. Toxicity of iron and hydrogen peroxide: The Fenton reaction. Toxicol. Lett. 1995, 82–83, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Houglum, K.; Ramm, G.A.; Crawford, D.H.; Witztum, J.L.; Powell, L.W.; Chojkier, M. Excess iron induces hepatic oxidative stress and transforming growth factor beta1 in genetic hemochromatosis. Hepatology 1997, 26, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Shizukuda, Y.; Tripodi, D.J.; Rosing, D.R. Iron Overload or Oxidative Stress? Insight into a mechanism of early cardiac manifestations of asymptomatic hereditary hemochromatosis subjects with C282Y homozygosity. J. Cardiovasc. Transl. Res. 2016, 9, 400–401. [Google Scholar] [CrossRef] [PubMed]
- Niemelä, O.; Parkkila, S.; Britton, R.S.; Brunt, E.; Janney, C.; Bacon, B. Hepatic lipid peroxidation in hereditary hemochromatosis and alcoholic liver injury. J. Lab. Clin. Med. 1999, 133, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Chung, F.L. Oxidative stress and hepatocarcinogenesis. Hepatoma Res. 2018, 4, 39. [Google Scholar] [CrossRef] [PubMed]
- Macías-Rodríguez, R.U.; Inzaugarat, M.E.; Ruiz-Margáin, A.; Nelson, L.J.; Trautwein, C.; Cubero, F.J. Reclassifying hepatic cell death during liver damage: Ferroptosis—A novel form of non-apoptotic cell death? Int. J. Mol. Sci. 2020, 21, 1651. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Zhao, T.; Song, Y.; Lin, L.; Fan, X.; Cui, B.; Feng, H.; Wang, X.; Yu, Q.; Zhang, J.; et al. The emerging role of ferroptosis in non-cancer liver diseases: Hype or increasing hope? Cell Death Dis. 2020, 11, 518. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R. Hemochromatosis: Ferroptosis, ROS, gut microbiome, and clinical challenges with alcohol as variable. Int. J. Mol. Sci. 2024, 25, 2668. [Google Scholar] [CrossRef] [PubMed]
- Lawless, M.W.; Mankan, A.K.; White, M.; O′Dwyer, M.J.; Norris, S. Expression of hereditary hemochromatosis C282Y HFE protein in HEK293 cells activates specific endoplasmic reticulum stress responses. BMC Cell Biol. 2007, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Hedges, J.C.; Singer, C.A.; Gerthoffer, W.T. Mitogen-activated protein kinases regulate cytokine gene expression in human airway myocytes. Am. J. Respir. Cell Mol. Biol. 2000, 23, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Arcones, A.; Thielemann, F.K. Origin of the elements. Astron. Astrophys. Rev. 2023, 31, 1. [Google Scholar] [CrossRef]
- Johnson, J.A.; Fields, B.D.; Thompson, T.A. The origin of the elements: A century of progress. Phil. Trans. R. Soc. 2020, 378, 2019030120190301. [Google Scholar] [CrossRef] [PubMed]
- Roederer, I.U.; Karakas, A.I.; Pignatari, M.; Herwig, F. The diverse origins of neutron-capture elements in the metal-poor Star HD 94028: Possible detection of products of I-process nucleosynthesis. Astrophys. J 2016, 821, 37. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The effects of cadmium toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- Thévenod, F.; Fels, J.; Lee, W.K.; Zarbock, R. Channels, transporters and receptors for cadmium and cadmium complexes in eukaryotic cells: Myths and facts. Biometals 2019, 32, 469–489. [Google Scholar] [CrossRef]
- Chandravanshi, L.; Shiv, K.; Kumar, S. Developmental toxicity of cadmium in infants and children: A review. Environ. Anal. Health Toxicol. 2021, 36, e2021003. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Li, H.; Zhang, B.; Zheng, T.; Li, Y.; Zhou, A.; Du, X.; Pan, X.; Yang, J.; Wu, C.; et al. Prenatal cadmium exposure and preterm low birth weight in China. J. Exp. Sci. Environ. Epidemiol. 2017, 27, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Branca, J.J.V.; Morucci, G.; Pacini, A. Cadmium-induced neurotoxicity: Still much ado. Neural Regen. Res. 2018, 13, 1879–1882. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Garrett, S.H.; Sens, M.A.; Sens, D.A. Cadmium, environmental exposure, and health outcomes. Cien Saude Colet. 2011, 16, 2587–2602. [Google Scholar] [CrossRef] [PubMed]
- Koons, A.L.; Rajasurya, V. Cadmium Toxicity. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK536966/ (accessed on 19 March 2024).
- Adnan, M.; Xiao, B.; Xiao, P.; Zhao, P.; Bibi, S. Heavy metal, waste, COVID-19, and rapid industrialization in this modern era—Fit for sustainable future. Sustainability 2022, 14, 4746. [Google Scholar] [CrossRef]
- Niture, S.; Lin, M.; Qi, Q.; Moore, J.T.; Levine, K.E.; Fernando, R.A.; Kumar, D. Role of autophagy in cadmium-induced hepatotoxicity and liver diseases. J. Toxicol. 2021, 2021, 9564297. [Google Scholar] [CrossRef] [PubMed]
- Renu, K.; Chakraborty, R.; Myakala, H.; Koti, R.; Famurewa, A.C.; Madhyastha, H.; Vellingiri, B.; George, A.; Valsala Gopalakrishnan, A. Molecular mechanism of heavy metals (lead, chromium, arsenic, mercury, nickel and cadmium)—Induced hepatotoxicity—A review. Chemosphere 2021, 271, 129735. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.M.; Jahan, F.; Brune, M.N.; Gorman, J.F.; Rahman, M.J.; Carpenter, D.; Islam, Z.; Rahman, M.; Aich, N.; Knibbs, L.D.; et al. Health consequences of exposure to e-waste: An updated systematic review. Lancet Planet. Health 2021, 5, e905–e920. [Google Scholar] [CrossRef] [PubMed]
- WHO. Preventing Disease through Healthy Environments. Exposure to Cadmium: A Major Public Health Concern. Available online: https://iris.who.int/handle/10665/329480 (accessed on 22 March 2024).
- Charkiewicz, A.E.; Omeljaniuk, W.J.; Nowak, K.; Garley, M.; Nikliński, J. Cadmium toxicity and health effects—A brief summary. Molecules 2023, 28, 6620. [Google Scholar] [CrossRef] [PubMed]
- Godt, J.; Scheidig, F.; Grosse-Siestrup, C.; Esche, V.; Brandenburg, P.; Reich, A.; Groneberg, D.A. The toxicity of cadmium and resulting hazards for human health. J. Occup. Med. Toxicol. 2006, 1, 22. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, M.; Kortenkamp, A. Cadmium exposures and deteriorations of cognitive abilities: Estimation of a reference dose for mixture risk assessments based on a systematic review and confidence rating. Environ. Health 2022, 21, 6. [Google Scholar] [CrossRef] [PubMed]
- Rafati Rahimzadeh, M.; Rafati Rahimzadeh, M.; Kazemi, S.; Moghadamnia, A.A. Cadmium toxicity and treatment: An update. Caspian J. Intern. Med. 2017, 8, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Min, J.Y.; Min, K.B. Association between cadmium exposure and liver function in adults in the United States: A cross-sectional study. J. Prev. Med. Public. Health 2021, 54, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Watanabe, T.; Zhang, Z.W.; Moon, C.S.; Shimbo, S. The integrity of the liver among people environmentally exposed to cadmium at various levels. Int. Arch. Occup. Environ. Health 1997, 69, 379–385. [Google Scholar] [CrossRef]
- Moon, S.; Lee, J.; Yu, J.M.; Choi, H.; Choi, S.; Park, J.; Choi, K.; Kim, E.; Kim, H.; Kim, M.J.; et al. Association between environmental cadmium exposure and increased mortality in the U.S. National Health and Nutrition Examination Survey (1999–2018). J. Exp. Sci. Environ. Epidemiol. 2023, 33, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Ohshiro, H.; Nose, T.; Sugiyama, K.; Meshitsuka, S.; Kurozawa, Y.; Kuranobu, M.; Funakawa, K.; Yamasaki, H.; Kinosita, K. [A case study of acute cadmium poisoning by welding work]. Sangyo Igaku 1988, 30, 210–211. (In Japanese) [Google Scholar] [CrossRef][Green Version]
- Raval, G.; Straughen, J.E.; McMillin, G.A.; Bornhorst, J.A. Unexplained hemolytic anemia with multiorgan failure. Clin. Chem. 2011, 57, 1485–1488. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Townshend, R.H. Acute cadmium pneumonitis: A 17-year follow-up. Br. J. Ind. Med. 1982, 39, 411–412. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cantilena, L.R., Jr.; Klaassen, C.D. Comparison of the effectiveness of several chelators after single administration on the toxicity, excretion, and distribution of cadmium. Toxicol. Appl. Pharmacol. 1981, 58, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, X.; Zeng, Z.; Lin, X.; Qin, Q.; Huo, X. Blood lead and cadmium levels associated with hematological and hepatic functions in patients from an e-waste-polluted area. Chemosphere 2019, 220, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Sung, G.; Lee, S.; Han, K.J.; Han, H. Serum cadmium is associated with hepatic steatosis and fibrosis. Medicine 2022, 101, e28559. [Google Scholar] [CrossRef] [PubMed]
- Hyder, O.; Chung, M.; Cosgrove, D.; Herman, J.M.; Li, Z.; Firoozmand, A.; Gurakar, A.; Koteish, A.; Pawlik, T.M. Cadmium exposure and liver disease among US adults. J. Gastrointest. Surg. 2013, 17, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Ock, J.; Moon, K.W.; Park, C.H. Association between Pb, Cd, and Hg exposure and liver injury among Korean adults. Int. J. Environ. Res. Publ. Health 2021, 18, 6783. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Lian, I.B.; Kor, C.T.; Chang, C.C.; Su, P.Y.; Chang, W.T.; Liang, Y.F.; Su, W.W.; Soon, M.S. Association between soil heavy metals and fatty liver disease in men in Taiwan: A cross sectional study. BMJ Open 2017, 7, e014215. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Sánchez, N.; Bugianesi, E.; Gish, R.G.; Lammert, F.; Tilg, H.; Nguyen, M.H.; Sarin, S.K.; Fabrellas, N.; Zelber-Sagi, S.; Fan, J.G.; et al. Global multi-stakeholder endorsement of the MAFLD definition. Lancet Gastroenterol. Hepatol. 2022, 7, 388–390. [Google Scholar] [CrossRef] [PubMed]
- Baba, H.; Tsuneyama, K.; Yazaki, M.; Nagata, K.; Minamisaka, T.; Tsuda, T.; Nomoto, K.; Hayashi, S.; Miwa, S.; Nakajima, T.; et al. The liver in itai-itai disease (chronic cadmium poisoning): Pathological features and metallothionein expression. Mod. Pathol. 2013, 26, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Go, Y.M.; Sutliff, R.L.; Chandler, J.D.; Khalidur, R.; Kang, B.Y.; Anania, F.A.; Orr, M.; Hao, L.; Fowler, B.A.; Jones, D.P. Low-dose cadmium causes metabolic and genetic dysregulation associated with fatty liver disease in mice. Toxicol. Sci. 2015, 147, 524–534. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Gao, J.; Hou, H.; Qi, Z.; Chen, H.; Zhang, X. Inhibition of mitochondrial fatty acid oxidation contributes to development of nonalcoholic fatty liver disease induced by environmental cadmium exposure. Environ. Sci. Technol. 2019, 53, 13992–14000. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Sun, J.; Wu, B.; Yuan, Y.; Gu, J.; Bian, J.; Liu, X.; Liu, Z. Effects of cadmium and/or lead on autophagy and liver injury in rats. Biol. Trace Elem. Res. 2020, 198, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Rana, K.; Verma, Y.; Rana, S.V.S. Possible mechanisms of liver injury induced by cadmium sulfide nanoparticles in rat. Biol. Trace Elem. Res. 2021, 199, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Kuester, R.K.; Waalkes, M.P.; Goering, P.L.; Fisher, B.L.; McCuskey, R.S.; Sipes, I.G. Differential Hepatotoxicity Induced by Cadmium in Fischer 344 and Sprague-Dawley Rats. Toxicol. Sci. 2002, 65, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhu, Y.; Lu, Z.; Guo, W.; Tumen, B.; He, Y.; Chen, C.; Hu, S.; Xu, K.; Wang, Y.; et al. Cadmium Induces acute liver injury by inhibiting Nrf2 and the role of NF-κB, NLRP3, and MAPKs signaling pathway. Int. J. Environ. Res. Public Health 2020, 17, 138. [Google Scholar] [CrossRef] [PubMed]
- Rikans, L.E.; Yamano, T. Mechanisms of cadmium-mediated acute hepatotoxicity. J. Biochem. Mol. Toxicol. 2000, 14, 110–117. [Google Scholar] [CrossRef]
- Ikediobi, C.O.; Badisa, V.L.; Ayuk-Takem, L.T.; Latinwo, L.M.; West, J. Response of antioxidant enzymes and redox metabolites to cadmium-induced oxidative stress in CRL-1439 normal rat liver cells. Int. J. Mol. Med. 2004, 14, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Tinkov, A.A.; Gritsenko, V.A.; Skalnaya, M.G.; Cherkasov, S.V.; Aaseth J Skalny, A.V. Gut as a target for cadmium toxicity. Environ. Pollut. 2018, 235, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Souza-Arroyo, V.; Fabián, J.J.; Bucio-Ortiz, L.; Miranda-Labra, R.U.; Gomez-Quiroz, L.E.; Gutiérrez-Ruiz, M.C. The mechanism of the cadmium-induced toxicity and cellular response in the liver. Toxicology 2022, 480, 153339. [Google Scholar] [CrossRef]
- Yamano, T.; DeCicco, L.A.; Rikans, L.E. Attenuation of cadmium-induced liver injury in senescent male fischer 344 rats: Role of Kupffer cells and inflammatory cytokines. Toxicol. Appl. Pharmacol. 2000, 162, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Li, T.; Zhuo, W.; Zhu, Y. Elevated serum and hair levels of cadmium as a risk factor for liver carcinoma: A meta-analysis. Nutr. Cancer 2023, 75, 1438–1447. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Pan, Z.; Zhu, M.; Gao, R.; Wang, Y.; Cheng, Y.; Zhang, N. Exposure to essential and non-essential trace elements and risks of congenital heart defects: A narrative review. Front. Nutr. 2023, 10, 1121826. [Google Scholar] [CrossRef] [PubMed]
- Rosen, B.P.; Liu, Z. Transport pathways for arsenic and selenium: A minireview. Environ. Int. 2009, 35, 512–515. [Google Scholar] [CrossRef] [PubMed]
- Calatayud, M.; Laparra Llopis, J.M. Arsenic through the gastrointestinal tract. In Handbook of Arsenic Toxicology; Academic Press: Cambridge, MA, USA, 2015; pp. 281–299. [Google Scholar] [CrossRef]
- Bjørklund, G.; Oliinyk, P.; Lysiuk, R.; Rahaman, M.S.; Antonyak, H.; Lozynska, I.; Lenchyk, L.; Peana, M. Arsenic intoxication: General aspects and chelating agents. Arch. Toxicol. 2020, 94, 1879–1897. [Google Scholar] [CrossRef] [PubMed]
- Guha Mazumder, D.N. Arsenic and liver disease. J. Indian. Med. Assoc. 2001, 311, 314–315. [Google Scholar]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic mechanisms of five heavy metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [PubMed]
- Kuivenhoven, M.; Mason, K. Arsenic Toxicity. [Updated 12 June 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK541125/ (accessed on 18 March 2024).
- Muzaffar, S.; Khan, J.; Srivastava, R.; Gorbatyuk, M.S.; Athar, M. Mechanistic understanding of the toxic effects of arsenic and warfare arsenicals on human health and environment. Cell Biol. Toxicol. 2023, 39, 85–110. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Jiménez, E.; Esteban, E.; Peñalosa, J.M. The fate of arsenic in soil-plant systems. Rev. Environ. Contam. Toxicol. 2012, 215, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Naghibi, S.A.; Motevalli, A.; Hashemi, H. Human-induced arsenic pollution modeling in surface waters—An integrated approach using machine learning algorithms and environmental factors. J. Environ. Manag. 2022, 305, 114347. [Google Scholar] [CrossRef] [PubMed]
- Guha Mazumder, D.N.; Chakraborty, A.K.; Ghose, A.; Gupta, J.D.; Chakraborty, D.P.; Dey, S.B.; Chattopadhyay, N. Chronic arsenic toxicity from drinking tubewell water in rural West Bengal. Bull. World Health Organ. 1988, 66, 499–506. [Google Scholar] [PubMed]
- Gorby, M.S. Arsenic poisoning. West. J. Med. 1988, 149, 308–315. [Google Scholar] [PubMed]
- LiverTox. Arsenic. 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548490/?report=reader (accessed on 18 March 2024).
- Theruvath, A.H.; Raveendran, R.; Philips, C.A. Dangerous placebo during the COVID-19 pandemic: A series of homoeopathic arsenicum album-induced liver injury. Cureus 2022, 14, e26062. [Google Scholar] [CrossRef] [PubMed]
- Theruvath, A.H.; Raveendran, R.; Philips, C.A.; Ahamed, R.; Abduljaleel, J.K.; Tharakan, A.; Rajesh, S.; Augustine, P. A series of homeopathic remedies-related severe drug-induced liver injury from South India. Hepatol. Commun. 2023, 7, e0064. [Google Scholar] [CrossRef] [PubMed]
- Upshaw, C.B.; Claiborne, T.S. Medicinal arsenic poisoning: 27-year follow-up. South. Med. J. 1995, 88, 892–893. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xi, S.; Liu, Z.; Yang, Y.; Zheng, Q.; Wang, F.; Xu, Y.; Wang, Y.; Zheng, Y.; Sun, G. Arsenic methylation metabolism and liver injury of acute promyelocytic leukemia patients undergoing arsenic trioxide treatment. Environ. Toxicol. 2013, 28, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Devarbhavi, H. Ayurvedic and herbal medicine-induced liver injury: It is time to wake up and take notice. Indian. J. Gastroenterol. 2018, 37, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Hardin, J.; Seltzer, J.; Suhandynata, R.; Spiegel, B.; Silver, R.; Thomas, D.; Galust, H.; Friedman, N.; Clark, R.; Momper, J. Severe arsenic poisoning due to Ayurvedic supplements. Clin. Case Rep. 2023, 11, e7733. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Chara, M.K. Letter to the Editor: Homeopathic drug-induced liver injury—An example of biases pertaining to Roussel Uclaf causality assessment method. Hepatol. Commun. 2023, 7, e00177. [Google Scholar] [CrossRef] [PubMed]
- Philips, C.A.; Paramaguru, R.; Joy, A.K.; Antony, K.L.; Augustine, P. Clinical outcomes, histopathologic patterns and chemical analysis of ayurveda and herbal medicine associated with severe liver injury—A single center experience from South India. Indian J. Gastroenterol. 2018, 37, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Pinto, B.; Goyal, P.; Flora, S.J.; Gill, K.D.; Singh, S. Chronic arsenic poisoning following ayurvedic medication. J. Med. Toxicol. 2014, 10, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, Y.; Yun, Z.; He, B.; Zhang, Q.; Hu, L.; Jiang, G. Speciation and bioaccessibility of arsenic in traditional Chinese medicines and assessment of its potential health risk. Sci. Total Environ. 2018, 619–620, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Inada, I.; Kiuchi, F.; Urushihara, H. Comparison of regulations for arsenic and heavy metals in herbal medicines using pharmacopoeias of nine counties/regions. Ther. Innov. Regul. Sci. 2023, 57, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Spilchuk, V.; Thompson, A. Chronic arsenic poisoning from traditional Chinese medicine. CMAJ 2019, 191, E424. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yan, R.; Guan, R.; Du, Y.; Liu, Y.; Wu, S.; Zhu, S.; Song, M.; Hang, T. Arsenic-related health risk assessment of realgar-containing NiuHuangJieDu tablets in healthy volunteers po administration. Front. Pharmacol. 2022, 12, 761801. [Google Scholar] [CrossRef] [PubMed]
- Islam, K.; Haque, A.; Karim, R.; Fajol, A.; Hossain, E.; Salam, K.A.; Ali, N.; Saud, Z.A.; Rahman, M.; Rahman, M.; et al. Dose-response relationship between arsenic exposure and the serum enzymes for liver function tests in the individuals exposed to arsenic: A cross sectional study in Bangladesh. Environ. Health 2011, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Abernathy, C.O.; Liu, Y.P.; Longfellow, D.; Aposhian, H.V.; Beck, B.; Fowler, B.; Goyer, R.; Menzer, R.; Rossman, T.; Thompson, C.; et al. Arsenic: Health effects, mechanisms of actions, and research issues. Environ. Health Perspect. 1999, 107, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Abernathy, C.O.; Thomas, D.J.; Calderon, R.L. Health effects and risk assessment of arsenic. J. Nutr. 2003, 133, 1536S–1538S. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.C.; Wang, J.; Shraim, A. A global health problem caused by arsenic from natural sources. Chemosphere 2003, 52, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Patlolla, A.K.; Centeno, J.A. Carcinogenic and systemic health effects associated with arsenic exposure--a critical review. Toxicol. Pathol. 2003, 31, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.C.; Saha, K.C.; Pati, S.; Dutta, R.N.; Rahman, M.M.; Sengupta, M.K.; Ahamed, S.; Lodh, D.; Das, B.; Hossain, M.A.; et al. Murshidabad—One of the nine groundwater arsenic-affected districts of West Bengal, India. Part II: Dermatological, neurological, and obstetric findings. Clin. Toxicol. 2005, 43, 835–848. [Google Scholar] [CrossRef] [PubMed]
- Kapaj, S.; Peterson, H.; Liber, K.; Bhattacharya, P. Human health effects from chronic arsenic poisoning—A review. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2006, 41, 2399–2428. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Kumar, D.; Sahu, A.P. Arsenic in the environment: Effects on human health and possible prevention. J. Environ. Biol. 2007, 28, 359–365. [Google Scholar]
- Vahter, M. Health effects of early life exposure to arsenic. Basic. Clin. Pharmacol. Toxicol. 2008, 102, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Fatmi, Z.; Abbasi, I.N.; Ahmed, M.; Kazi, A.; Kayama, F. Burden of skin lesions of arsenicosis at higher exposure through groundwater of taluka Gambat district Khairpur, Pakistan: A cross-sectional survey. Environ. Geochem. Health 2013, 35, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Naujokas, M.F.; Anderson, B.; Ahsan, H.; Aposhian, H.V.; Graziano, J.H.; Thompson, C.; Suk, W.A. The broad scope of health effects from chronic arsenic exposure: Update on a worldwide public health problem. Environ. Health Perspect. 2013, 121, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J. Health hazards and mitigation of chronic poisoning from arsenic in drinking water: Taiwan experiences. Rev. Environ. Health 2014, 29, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Palma-Lara, I.; Martínez-Castillo, M.; Quintana-Pérez, J.C.; Arellano-Mendoza, M.G.; Tamay-Cach, F.; Valenzuela-Limón, O.L.; García-Montalvo, E.A.; Hernández-Zavala, A. Arsenic exposure: A public health problem leading to several cancers. Regul. Toxicol. Pharmacol. 2020, 110, 104539. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Ahmad, M.F.; Ahmad, I.; Ashfaq, F.; Wahab, S.; Alsayegh, A.A.; Kumar, S.; Hakeem, K.R. Arsenic exposure through dietary intake and associated health hazards in the Middle East. Nutrients 2022, 14, 2136. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, M.; Metin, M.; Altay, V.; Bhat, R.A.; Ejaz, M.; Gul, A.; Unal, B.T.; Hasanuzzaman, M.; Nibir, L.; Nahar, K.; et al. Arsenic and human health: Genotoxicity, epigenomic effects, and cancer signaling. Biol. Trace Elem. Res. 2022, 200, 988–1001. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Ali, M.; Raj, V.; Kumari, A.; Rachamalla, M.; Niyogi, S.; Kumar, D.; Sharma, A.; Saxena, A.; Panjawani, G.; et al. Arsenic causing gallbladder cancer disease in Bihar. Sci. Rep. 2023, 13, 4259. [Google Scholar] [CrossRef] [PubMed]
- Aryan, Y.; Pon, T.; Panneerselvam, B.; Dikshit, A.K. A comprehensive review of human health risks of arsenic and fluoride contamination of groundwater in the South Asia region. J. Water Health 2024, 22, 235–267. [Google Scholar] [CrossRef] [PubMed]
- Demissie, S.; Mekonen, S.; Awoke, T.; Teshome, B.; Mengistie, B. Examining carcinogenic and noncarcinogenic health risks related to arsenic exposure in Ethiopia: A longitudinal study. Toxicol. Rep. 2024, 12, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Dou, D.C.; Qi, R.; Xiao, S.M.; Su, G.X.; Guo, Y.X. Distribution Characteristics of Arsenic in Drinking Water in China and Its Health Risk Based on Disability-adjusted Life Years. Huan Jing Ke Xue 2024, 45, 131–139. (In Chinese) [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; Del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.R.; Leblanc, J.C.; et al. Update of the risk assessment of inorganic arsenic in food. EFSA J. 2024, 22, e8488. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Gao, T.; Li, X.; Wāng, Y. Didactical approaches and insights into environmental processes and cardiovascular hazards of arsenic contaminants. Chemosphere 2024, 352, 141381. [Google Scholar] [CrossRef] [PubMed]
- Issanov, A.; Adewusi, B.; Saint-Jacques, N.; Dummer, T.J.B. Arsenic in drinking water and lung cancer: A systematic review of 35 years of evidence. Toxicol. Appl. Pharmacol. 2024, 483, 116808. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.J.; Cao, Y.R.; Lee, D.Y. Assessment of health risks associated with prediction of vegetable inorganic arsenic concentrations given different soil properties. Environ. Geochem. Health 2024, 46, 71. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, W.; Zhang, J.; Yan, Y.; Zhou, Q.; Liu, Q.; Guan, Y.; Zhao, Z.; An, J.; Cheng, X.; et al. Associations of arsenic exposure and arsenic metabolism with the risk of non-alcoholic fatty liver disease. Int. J. Hyg. Environ. Health 2024, 257, 114342. [Google Scholar] [CrossRef] [PubMed]
- Sevak, P.; Pushkar, B. Arsenic pollution cycle, toxicity and sustainable remediation technologies: A comprehensive review and bibliometric analysis. J. Environ. Manag. 2024, 349, 119504. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Paul, S.; Chatterjee, D.; Banerjee, N.; Majumder, N.S.; Sarma, N.; Sau, T.J.; Basu, S.; Banerjee, S.; Majumder, P.; et al. Arsenic exposure through drinking water increases the risk of liver and cardiovascular diseases in the population of West Bengal, India. BMC Public Health 2012, 12, 639. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jiang, X.; Qian, H.; Li, X.; Su, J.; Zhang, G.; Li, X. Associations of arsenic exposure with liver injury in US adults: NHANES 2003–2018. Environ. Sci. Pollut. Res. Int. 2023, 30, 48260–48269. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Zeng, Q.; Luo, P.; Sun, B.; Liang, B.; Wei, S.; Xu, Y.; Wang, Q.; Liu, Q.; Zhang, A. Assessing the risk of coal-burning arsenic-induced liver damage: A population-based study on hair arsenic and cumulative arsenic. Environ. Sci. Pollut. Res. Int. 2021, 28, 50489–50499. [Google Scholar] [CrossRef] [PubMed]
- Benramdane, L.; Accominotti, M.; Fanton, L.; Malicier, D.; Vallon, J.J. Arsenic speciation in human organs following fatal arsenic trioxide poisoning—A case report. Clin. Chem. 1999, 45, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Lech, T.; Trela, F. Massive acute arsenic poisonings. Forensic Sci. Int. 2005, 151, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Ma, J.Q.; Li, F.; Xu, G.H.; Guo, W.; Zhou, H.M. A Fatal case of acute arsenic poisoning. J. Forensic Sci. 2019, 64, 1271–1273. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tong, X.; Zhao, S.; Yu, Z.; Zhang, J.; Ma, L.; Shi, Q.; Zhou, Y. Four cases of fatal acute arsenic poisoning: Histopathology, toxicology, and new trends. Forensic. Sci. Med. Pathol. 2023; in press. [Google Scholar] [CrossRef]
- Kim, L.H.; Abel, S.J. Survival after a massive overdose of arsenic trioxide. Crit. Care Resusc. 2009, 11, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Fowler, B.A.; Selene, C.H.; Chou, R.J.; Jones, D.L.; Sullivan, W., Jr.; Chen, C.J. Chapter 28—Arsenic. In Handbook on the Toxicology of Metals, 4th ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 581–624. [Google Scholar]
- Guha Mazumder, D.N. Effect of chronic intake of arsenic-contaminated water on liver. Toxicol. Appl. Pharmacol. 2005, 206, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Philips, C.A.; Theruvath, A.H. Homeopathic remedies and liver injury: Authors’ reply to central council for research in homeopathy. Hepatol. Commun. 2023, 7, e0170. [Google Scholar] [CrossRef] [PubMed]
- Datta, D.V.; Mitra, S.K.; Chhuttani, P.N.; Chakravarti, R.N. Chronic oral arsenic intoxication as a possible aetiological factor in idiopathic portal hypertension (non-cirrhotic portal fibrosis) in India. Gut 1979, 20, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.S.; Schmid, M.; Newman, S.; Scheuer, P.J.; Sherlock, S. Arsenic and noncirrhotic portal hypertension. Gastroenterology 1974, 66, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Neale, G.; Azzopardi, G. Chronic arsenical poisoning and non-cirrhotic portal hypertension. A case for diagnosis. Br. Med. J. 1971, 4, 725–730. [Google Scholar]
- Villeneuve, J.P.; Huet, P.M.; Joly, J.G.; Lafortune, M.; Lavoie, P.; Viallet, A. Idiopathic portal hypertension. Am. J. Med. 1976, 61, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Goering, P.L.; Barber, D.S. Hepatotoxicity of copper, Iron, cadmium, and arsenic. Compr. Toxicol. 2010, 9, 501–526. [Google Scholar] [CrossRef]
- Akhand, A.A.; Du, J.; Liu, W.; Hossain, K.; Miyata, T.; Nagase, F.; Kato, M.; Suzuki, H.; Nakashima, I. Redox-linked cell surface-oriented signaling for T-cell death. Antiox. Redox Signal. 2002, 4, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Hossain, K.; Akhand, A.A.; Kato, M.; Du, J.; Takeda, K.; Wu, J.; Takeuchi, K.; Liu, W.; Suzuki, H.; Nakashima, I. Arsenite induces apoptosis of murine T lymphocytes through membrane Raft-linked signaling for activation of c-Jun amino-terminal kinase. J. Immunol. 2000, 165, 4290–4297. [Google Scholar] [CrossRef] [PubMed]
- Rossman, T. Mechanism of arsenic carcinogenesis: An integrated approach. Mut. Res. 2003, 533, 37–65. [Google Scholar] [CrossRef]
- Li, C.; Li, P.; Tan, Y.M.; Lam, S.H.; Chan, E.C.Y.; Gong, Z. Metabolomic characterization of liver injury caused by acute arsenic toxicity in Zebrafish. PLoS ONE 2016, 11, e0151225. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Raveendran, V.A.; Kundu, S.; Samanta, T.; Shunmugam, R.; Pal, D.; Sarma, J.D. Mechanisms of Arsenic-Induced Toxicity with Special Emphasis on Arsenic-Binding Proteins; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- Miltonprabu, S.; Sumedha, N.C. Arsenic-induced hepatic mitochondrial toxicity in rats and its amelioration by diallyl trisulfide. Toxicol. Mech. Methods 2014, 24, 124–135. [Google Scholar] [CrossRef]
- Hrycay, E.G.; Bandiera, S.M. Involvement of cytochrome P450 in reactive oxygen species formation and cancer. Adv. Pharmacol. 2015, 74, 35–84. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, J.; Lou, B.; Wu, R.; Wang, G.; Lu, C.; Wang, H.; Pi, J.; Xu, Y. The role of reactive oxygen species in arsenic toxicity. Biomolecules 2020, 10, 240. [Google Scholar] [CrossRef]
- Shi, H.; Shi, X.; Liu, K.J. Oxidative mechanism of arsenic toxicity and carcinogenesis. Mol. Cell Biochem. 2004, 255, 67–78. [Google Scholar] [CrossRef]
- Zhang, H.; Jin, B.; Liu, L.; Li, H.; Zheng, X.; Li, M.; He, R.; Wang, K. Glutathione might attenuate arsenic-induced liver injury by modulating the Foxa2-XIAP Axis to reduce oxidative stress and mitochondrial apoptosis. Biol. Trace Elem. Res. 2023, 20, 5201–5212. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Liu, Y.; Wang, D.; Zhu, K.; Zou, Z.; Zhang, A. Imbalanced inflammatory response in subchronic arsenic-induced liver injury and the protective effects of Ginkgo biloba extract in rats: Potential role of cytokines mediated cell-cell interactions. Environ. Toxicol. 2021, 36, 2073–2092. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Pi, R.; Luo, J.; Liu, J.; Zhang, A.; Sun, B. Association between arsenic exposure and inflammatory cytokines and C-reaction protein: A systematic review and meta-analysis. Medicine 2022, 101, e32352. [Google Scholar] [CrossRef] [PubMed]
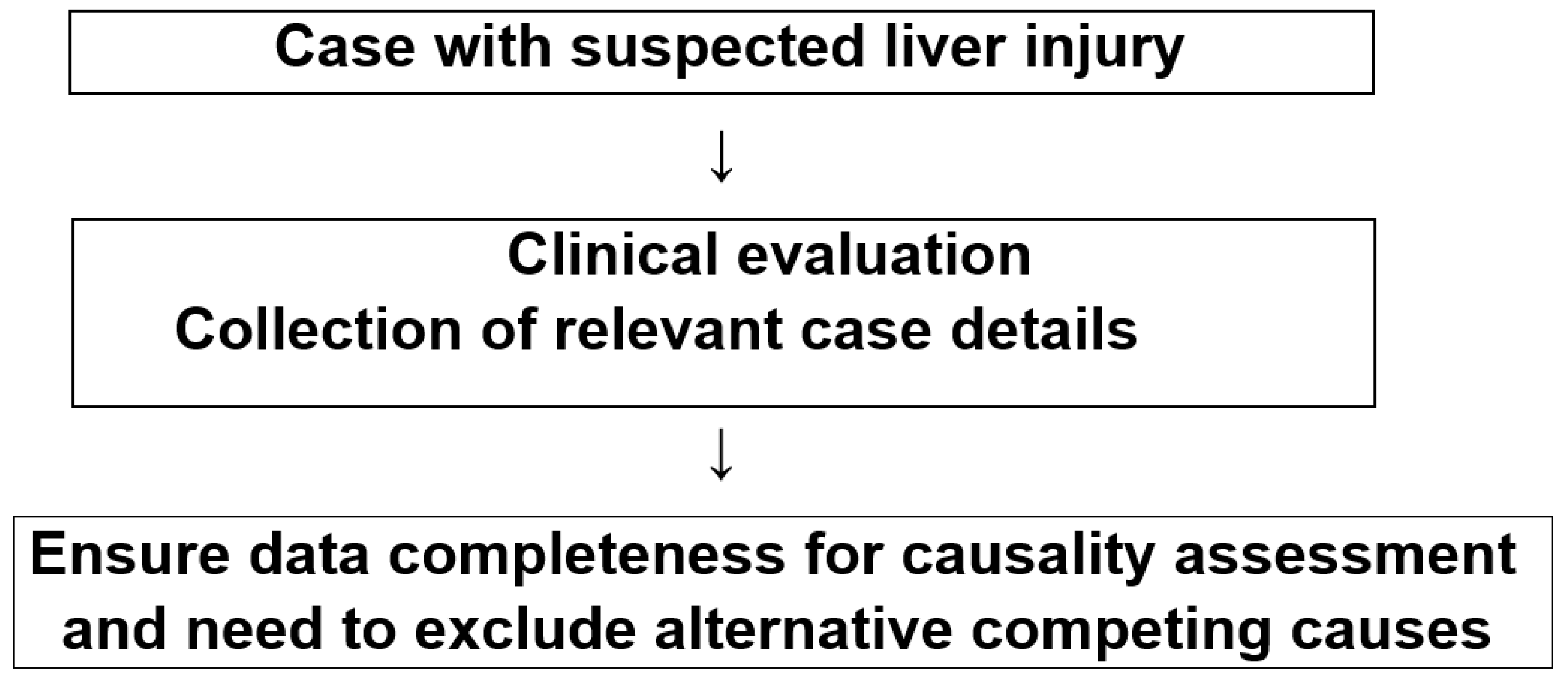
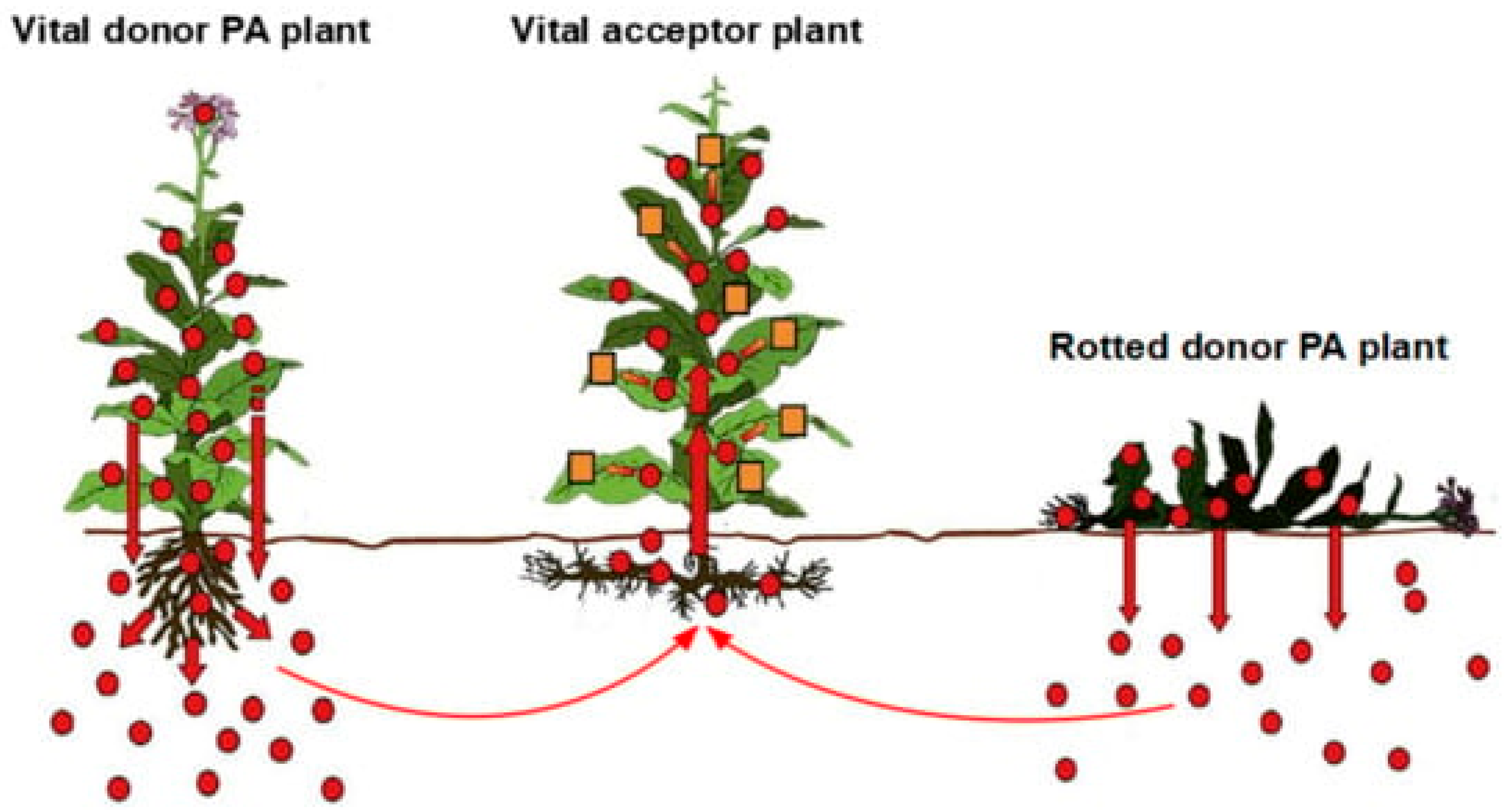
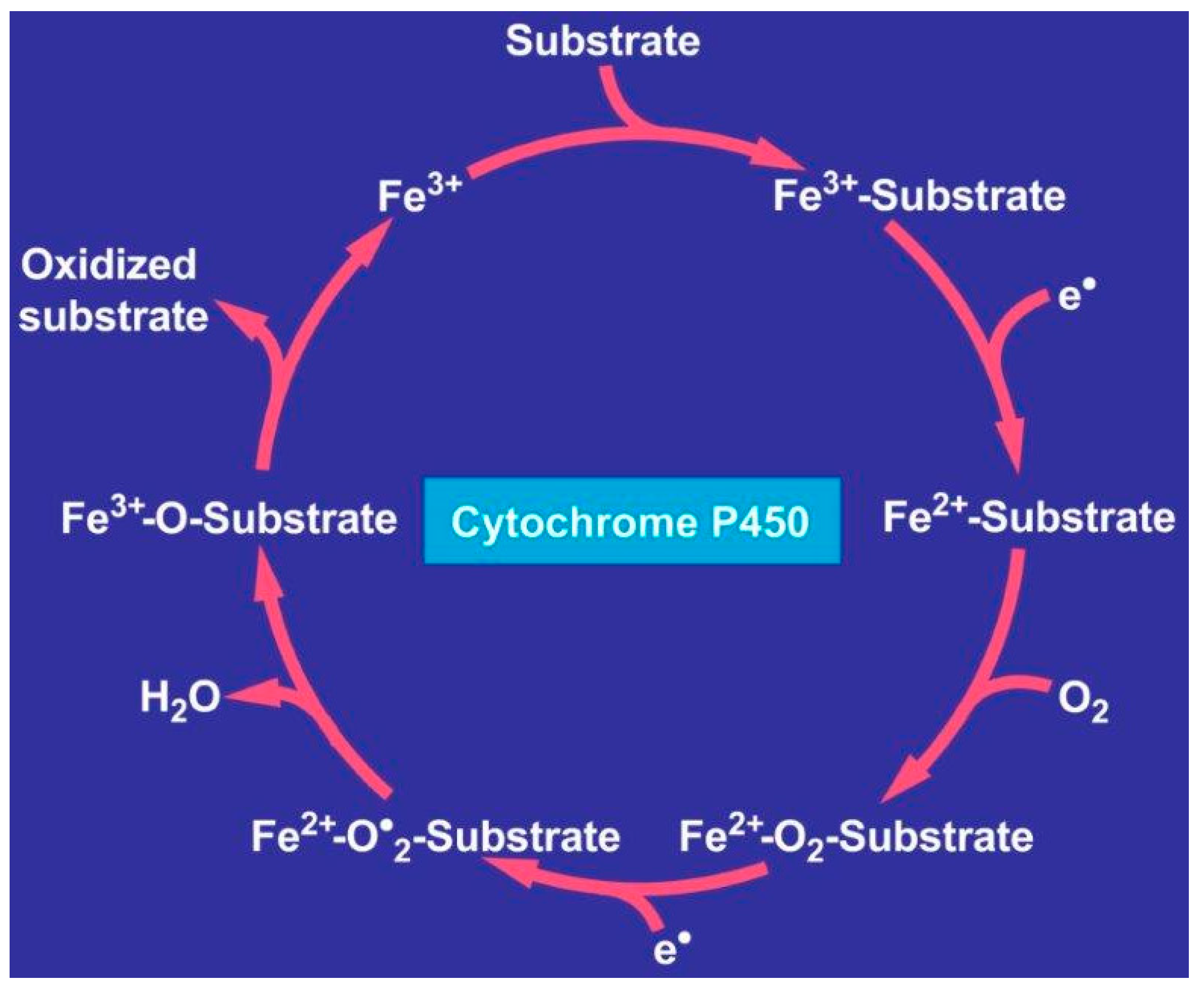

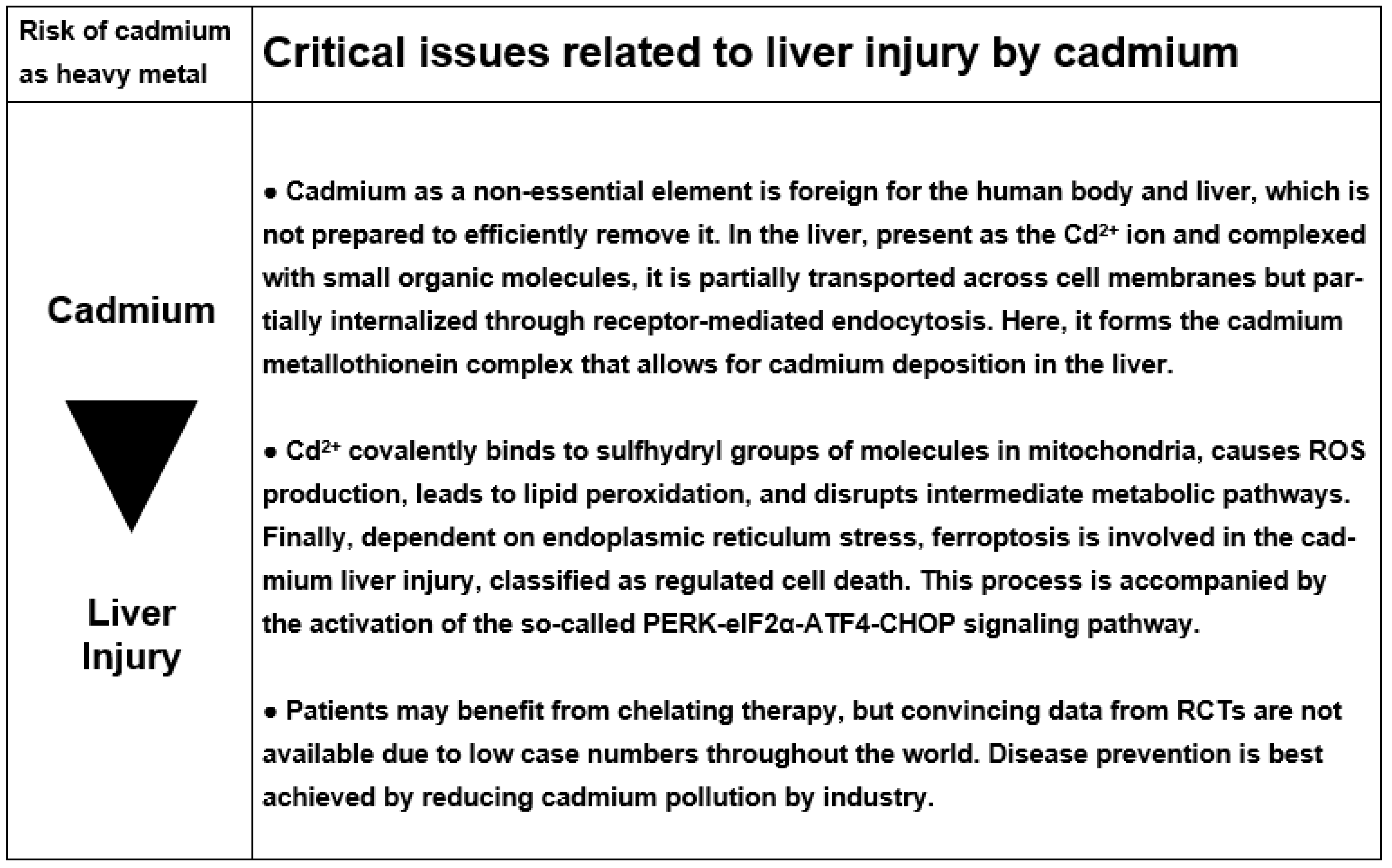
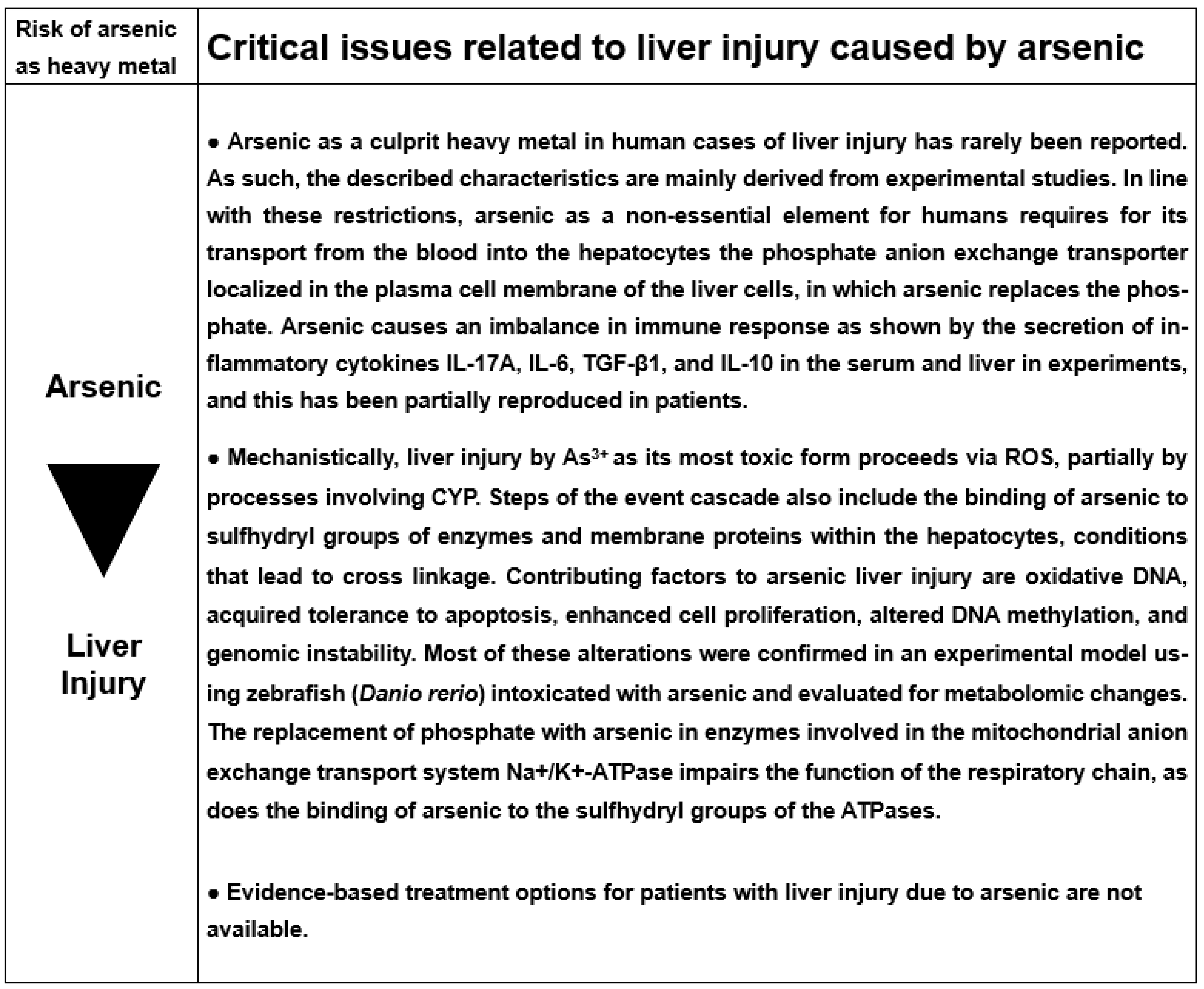
| Differential Diagnosis | Diagnostic Parameters |
|---|---|
| Hepatitis A virus (HAV) | Anti-HAV IgM |
| Hepatitis B virus (HBV) | HBV DNA, anti-HBc IgM |
| Hepatitis C virus (HCV) | HCV RNA, anti-HCV |
| Hepatitis E virus (HEV) | HEV RNA, titer change for anti-HEV IgM/anti-HEV IgG |
| Cytomegalovirus (CMV) | CMV PCR, titer change for anti-CMV IgM/anti-CMV IgG |
| Epstein Barr virus (EBV) | EBV PCR, titer change for anti-EBV IgM/anti-EBV IgG |
| Herpes simplex virus (HSV) | HSV PCR, titer change for anti-HSV IgM/anti-HSV IgG |
| Varicella zoster virus (VZV) | VZV PCR, titer change for anti-VZV IgM/anti-VZV IgG |
| Other viral infections according to the clinical context | Specific serology of Adenovirus, Coxsackie-B-Virus, Echovirus, Measles virus, Rubella virus, Flavivirus, Arenavirus, Filovirus, Parvovirus, HIV, and others |
| Other infectious diseases | Specific assessment of bacteria, fungi, parasites, and others |
| Autoimmune hepatitis (AIH) type I | Gamma globulins, ANA, SMA, AAA, SLA/LP, anti-LSP, and anti-ASGPR. Simplified criteria for the diagnosis of autoimmune hepatitis. |
| Autoimmune hepatitis (AIH) type II | Gamma globulins, anti-LKM-1 (CYP 2D6), anti-LKM-2 (CYP 2C9), and anti-LKM-3. Simplified criteria for the diagnosis of autoimmune hepatitis. |
| Primary biliary cholangitis (PBC) | AMA, anti-PDH-E2 |
| Primary sclerosing cholangitis (PSC) | p-ANCA, MRC |
| Autoimmune cholangitis (AIC) | ANA, SMA |
| Overlap syndromes | See AIH, PBC, PSC, and AIC |
| Non-alcoholic steatohepatitis (NASH) | BMI, insulin resistance, hepatomegaly, echogenicity of the liver |
| Alcoholic liver disease (ALD) | Patient’s history, clinical and laboratory assessment, other features of alcoholic disease |
| Cocaine, ecstasy and other amphetamines | Toxin screening |
| Rare intoxications | Toxin screening for household and occupational toxins |
| Drug-induced liver injury (DILI) and herb-induced liver injury (HILI) | Diagnostic algorithm of the updated Roussel Uclaf Causality Assessment Method (RUCAM) for DILI and HILI |
| Hemochromatosis | Serum ferritin, total iron-binding capacity, genotyping for C2824 and H63D mutation, hepatic iron content |
| Wilson disease | Copper excretion (24-h urine), ceruloplasmin in serum, free copper in serum, Coombs-negative hemolytic anemia, hepatic copper content, Kayser–Fleischer ring, neurologic-psychiatric work-up, and genotyping. Modified Leipzig Scoring System for diagnosis of Wilson disease. |
| Porphyria | Porphobilinogen in urine, total porphyrins in urine |
| α1-Antitrypsin deficiency | α1-Antitrypsin in serum |
| Biliary diseases | Clinical and laboratory assessment, hepatobiliary sonography, and other imaging (CT, MRC) |
| Pancreatic diseases | Clinical and laboratory assessment, sonography, CT, MRT |
| Celiac disease | TTG antibodies, endomysium antibodies, duodenal biopsy |
| Anorexia nervosa | Clinical context |
| Parenteral nutrition | Clinical context |
| Cardiopulmonary diseases | Cardiopulmonary assessment of congestive heart disease, myocardial infarction, cardiomyopathy, cardiac valvular dysfunction, pulmonary embolism, pericardial diseases, arrhythmia, hemorrhagic shock, and various other conditions |
| Addison disease | Plasma cortisol |
| Thyroid diseases | TSH basal, T4, T3 |
| Grand mal seizures | Clinical context of epileptic seizure (duration >30 min) |
| Heat stroke | Shock, hyperthermia |
| Polytrauma | Shock, liver injury |
| Systemic diseases | Specific assessment of sarcoidosis, amyloidosis, metastatic tumor, sepsis, and others |
| Other diseases | Clinical context |
| Copper Source | Human Health Risk Issues | Comments | References |
|---|---|---|---|
| Terrestrial and aquatic environment due to anthropogenetic activity are considered as important. | Copper and other heavy metals as environmental pollutants with their toxicological effects in humans mentioned. | Copper with detrimental effects on human health is discussed but lacking quantitative health risk evaluation. | Briffa, 2020 [6] |
| Municipal solid waste landfill comprising household, healthcare, and industrial waste. | Copper and other heavy metal accumulation found in water, soil, and plants of municipal solid waste landfill in Vientiane, Laos. | Leaching from landfills, many compounds can harmfully influence human health but lacking quantitative risk data. | Vongdala, 2019 [23] |
| Plants used as herbal medicines and grown on soils contaminated with heavy metals. | Heavy metals found as potential hepatotoxins in herbal medicinal products may impair human health. | It remains to be clarified how often contaminated herbal medicinal products can trigger HILI. | Quan, 2020 [24] |
| Heavy metals with wide distribution in the environment due to multiple industrial, technological, medical, agricultural, and domestic applications. Because even low levels of exposure may already be hazardous to humans, global public health concern exists. | Concern of heavy metal toxicity and their potential effects on human and public health is discussed. As systemic toxicants, they are known to cause multiple organ damage, considering variability of molecular mechanisms of toxicity, genotoxicity, and specifically carcinogenicity. | Metallic elements are classified as known or probable human carcinogens according to the US Environmental Protection Agency, and the International Agency for Research on Cancer. Carcinogenicity involves many mechanistic steps, some of which are not clearly elucidated or understood. | Tchounwou, 2012 [29] |
| Copper–chromium–arsenic (CCA), which is a preservative substance used in various countries to treat timber. This leads to the presence of copper pollution in the soil. | Copper and other heavy metals are widely found and distributed in the environment, raising concerns over their potential effects on human health with impact that may depend on possible routes of heavy metal exposure. | Several studies have shown that toxic metal exposure causes long-term health problems in humans. Little is known about the health impact of mixtures of these toxic elements with additive or synergistic effects. | Witkowska, 2021 [44] |
| Copper and various other heavy metals are found in herbal medicinal plants due to environmental heavy metal pollution of the soil where the plants grow. | New analytical methods may help detect heavy metals in medicinal plants. Using medicinal plants polluted by copper significantly threatens the health of consumers. | Health hazards are not specified in detail, clearly a difficult approach due to plant contamination by many other heavy metals apart from copper. | Guo, 2023 [48] |
| Critical evaluation of human health issues by dietary copper. Gaps prevail as well as unresolved issues on health risks due to copper intake. | Results from observation and intervention studies do not support a link between copper and a risk of cardiovascular disease, cognitive decline, arthritis, or cancer. | High amounts of ingested copper seemingly show no causal relationship for various diseases, a discussion that certainly needs further studies for confirmation. | Bost, 2016 [57] |
| Sources of copper in the environment may include copper water pipes, drinking water, copper cookware, birth control pills, copper intrauterine devices, and fungicides. | Occupational exposure to machinists, plumbers, welders, and others who work with copper are at risk for copper toxicity. Due to tissue injury, disrupted homeostasis is associated with a number of diseases. | By overwhelming body antioxidant systems and inducing DNA damage, ROS finally may lead to degenerative diseases, cancer, cardiovascular disease, and chronic inflammation. | Gaetke, 2014 [61] |
| Copper found in soil and medicinal herbs surrounding ponds on agricultural land. | Contaminated with heavy metals, herbs growing on such soils can become harmful to living organisms. | Specific risk issues of human health due to contaminated plants were not studied. | Malinowska, 2017 [62] |
| Urban vehicle traffic contamination by copper and various other heavy metals was studied in road sweeping and waste bottom sediments of retention tanks. | Copper is among the most documented elements on the surface of the street. Human health risk and non-carcinogenetic and carcinogenetic risk was calculated. | Whether data obtained following calculation represent real world conditions remains to be established. Health risk of environmental copper was discussed. | Nawrot, 2020 [65] |
| High fungicide-derived copper levels detected in soil and vegetation in Owena cocoa (Theobroma cacao L.) plantations in Nigeria raise health concern. | High contaminating copper content in Owena cocoa explains high copper values in commercial chocolate. Local conditions may not be safe for human health. | Due to increased copper levels, use of chocolate is not recommended in patients with copper-related Wilson disease. Local concern for health was not further detailed. | Adeyeye, 2006 [68] |
| Copper is widely used in the industry and agriculture. | Elevated copper levels are risky as commonly found in the biosphere. | Specific human health concerns are not detailed. | Flemming, 1989 [69] |
| Overview of the copper situation and broad usage in viticulture areas. | Copper-containing products are used as fungicides and increase environmental intake of copper. | Exposed organisms are at higher risk of suffering from developmental and reproductive disorders. | Kovačič, 2013 [70] |
| Attic dust studies show historical air pollution by copper and other heavy metals. | Question was raised on potential health hazard posed by copper and other heavy metals as urban pollutants. | Specific risks of human health were not studied in this otherwise pioneering work. | Gaberšek, 2022 [71] |
| Copper environmental toxicology is of major concern for humans, animals, and plants. | Worldwide contamination of agricultural lands by copper and other heavy metals is viewed as risky. | It has become a serious threat to humans and animals via their entry in the food web. | Rehman, 2019 [74] |
| Currently, Brazil is among the largest copper ore producers. | Impact of copper mining waste in the Amazon on risks to human health. | Human health risks were not provided in sufficient detail. | Covre, 2022 [75] |
| Copper uptake by edible plants from soil contaminated with this heavy metal determines potential health risk associated with the ingestion of contaminated food. | Health risk assessment is essential due to copper build-up in plant food and consumption of foods rich in copper. Concern has been expressed that copper can induce serious health disorders. | Environmental copper as part of the soil–plant transfer within the food chain that potentially reaches humans and may lead to major health risks without delineating details of possible diseases. | Shabbir, 2020 [76] |
| Overview of various environmental patterns and dynamics due to copper that helps examine current human exposures. | High levels of copper can be detrimental to life. More specifically, human health issues related to copper intoxications as exemplified by Wilson disease. | Environmental copper polluting the food chain will impair human health in susceptible individuals due to genetic aberration like in Wilson disease. | Georgopou-los, 2001 [77] |
| Environmental copper partially may be linked to Alzheimer’s disease development through the food chain. | Dyshomeostasis of copper and its valency in the body, instead of its accumulation, are major determinants for Alzheimer’s disease. | Copper is incriminated as potential major causative factor in the development of Alzheimer’s disease. | Hsu, 2018 [78] |
| Copper bioavailability, uptake, toxicity aspects and tolerance in plants are reviewed in detail and seen as connected to human health. | Copper in excess has detrimental impact on human health and the prescribed dose–response curve of copper in humans is U-shaped. | Excessive copper levels are viewed as risks to human health and may lead to liver and Alzheimer’s disorders. | Kumar, 2021 [79] |
| Micronized copper as wood preservatives are potential health risks but need further studies for confirmation. | During decomposition of treated wood, copper-based nanoparticles may be inhaled and can cause harm to human health. | Nanoparticles based on copper could become a potential risk for human health secondary to their inhalation. | Civardi, 2015 [80] |
| Leachability features of copper and related heavy metals cause health risk in copper mining-impacted sediments. | The hazard index and carcinogenetic risk indices showed significant risks of human exposure, but additional information was not provided. | The carcinogenetic risk of copper must be evaluated under real-world conditions among individuals confronted with copper exposure. | Yan, 2020 [81] |
| Essentiality and toxicity due to copper was assessed with focus on human health risk including diseases. | A conceptual framework for this type of risk evaluation was applied for analysis of the potential human toxicity by high copper intake. | Human health risks attributable to excess copper intake were discussed and require confirmation. | Stern, 2010 [82] |
| Potential human health risks of heavy metals in soils exist in copper mining areas. | Copper may promote the formation of amyloid plaques characteristic for Alzheimer’s disease. | Copper together with iron causes atherosclerosis and neurodegenerative diseases. | Filimon, 2021 [83] |
| The potential exposure and hazards of copper nanoparticles were critically reviewed. Copper nanoparticles are increasingly used, including in cancer chemotherapy. | Copper nanoparticles deposit in both the upper and lower respiratory tract. Prolonged persistence leads to enhanced oxidative stress and inflammatory response due to local irritation. | Current evidence that copper nanoparticles may harm human health is not available, but their toxicology risk is assumed to be ten times lower compared with other copper forms. | Ameh, 2019 [84] |
| A systematic review and meta-analysis evaluated toxic metal contaminants of the environment and their risk of cardiovascular disease and coronary heart disease. | Exposure to environmental copper in high amounts is significantly associated with an increased relative risk of both cardiovascular disease and coronary heart disease through increased systolic blood pressure. | The increased risk also was attributed to oxidative stress by generation of ROS and a copper–homocysteine complex responsible for vascular injury and endothelial dysfunction. | Chowdhury, 2018 [85] |
| Health risk assessment of copper was done in soils contaminated with municipal wastes. | Copper with elevated levels can cause respiratory problems, dizziness, nausea, and diarrhea. | Copper is considered in the present study as not a potentially toxic element. | Gujre, 2021 [86] |
| Implications for setting regulatory health criteria for ingested copper. | Human health risks due to exposure to copper are described and proposals for its safe use are given. | There is good news, as copper is not viewed as a carcinogenetic element, important for clinicians. | Taylor, 2020 [87] |
| Human health risk of copper was assessed in soil and plants near to a copper smelter, providing high copper values. | Soil samples display a significant level of copper enrichment but the hazard index for non-carcinogenic copper is low in children and adults. | Health risks of copper contaminating the soil near copper smelters are seemingly low despite high copper values in local soil. | Nematollahi, 2020 [88] |
| Distribution, sources and health risks of heavy metals were assessed in indoor dust across China. | Copper was identified as a contaminant in indoor dust, an important finding as most people spend up to 90% of their times indoors. | No considerable non-carcinogenic risk was found for copper that contaminated indoor dust in Chinese households. | Wang, 2023 [89] |
| Environmental health hazards of e-cigarettes and their components: oxidants and copper in e-cigarette aerosols. | Copper is among other elemental constituents identified in aerosol volumes from disposable e-cigarettes. | E-cigarette aerosols have a lower health risk than conventional cigarette smoke as copper amount is smaller. | Lerner, 2015 [90] |
| Association between serum copper levels and lung cancer risk was studied in a meta-analysis | Serum copper levels are higher in patients with lung cancer in comparison with a control group lacking lung cancer. | Such correlation between serum copper levels and cancer does not allow for assuming a priori any firm causality association. | Zhang, 2018 [91] |
| Parameter | Score |
|---|---|
Kayser–Fleischer rings
| 2 0 |
Serum ceruloplasmin
| 0 3 2 1 |
24 h urinary copper (in the absence of chronic cholestatic liver disease)
| 2 1 0 |
Coombs-negative hemolytic anemia with liver disease
| 1 0 |
Mutational analysis
| 4 1 0 |
Liver biopsy for histology suggestive of Wilson disease with
| 1 |
Neurobehavioral symptoms
| 2 0 |
Typical features on magnetic resonance imaging of the brain
| 1 0 |
History of Wilson disease in a family member
| 1 |
Evaluation
| Total score ≥4 3 ≤2 |
| Iron Source | Human Health Risk Issues | Comments | References |
|---|---|---|---|
| Iron overload is possibly related to human neurodegenerative disorders. | Evidence-based data in support of this assumption were not provided and need to be supplemented. | It is certainly challenging for future studies to close up the gap between this disease and excess iron. | Abbaspour, 2014 [183] |
| Iron homeostasis is viewed as disrupted upon exposure to various environmental pollutants and may have health effects. | Environmental pollutants may give rise to disordered iron homeostasis, which could lead to cytotoxicity and increased cancer risk in exposed humans. | Firm evidence that this proposal is functioning under normal field conditions and is lacking requiring additional studies for verification. | Guo, 2015 [193] |
| Iron in humans with disturbed cellular iron homeostasis caused by pollutants in the environment led to increased intestinal iron uptake from ingested food. | This ultimately was linked to human diseases like diabetes mellitus, cancer, and renal, cardiovascular, respiratory, autoimmune, neurodegenerative (like Alzheimer’s disease), and cerebrovascular diseases. | Challenging proposal that disturbed iron homeostasis may increase the risk cancer incidence and cancer mortality, but further studies are required to prove this concept. | Schreinema-chers, 2016, [194] |
| Human health hazard assessment of iron intake with arsenic-safe ground water in Jashore, Bangladesh. | The assessment revealed that the non-carcinogenetic risks due to ingestion of iron were 1.446 for adults and 0.590 for children. | Regretfully, iron content in exposed individuals was not measured, though viewed as a risk for heart disease and diabetes. | Gosh, 2020 [195] |
| Iron status and iron homeostasis are often environmentally altered and are risk factors for human health. | Tissue-specific brain iron overload is observed in degenerative neurological diseases without an increase in systemic iron. | A gap in understanding exists for brain overload despite a lack of increase in systemic iron stores, more evidence is needed. | Lal, 2020, [196] |
| Industry-relevant iron and iron status have been associated with attention deficits. | Higher iron concentrations and ferritin were jointly associated with worse attention behaviors. | Using data on ferritin as a diagnostic parameter of iron body stores remains problematic as it is not specific. | Schildroth, 2024 [197] |
| Presence of abundant iron-rich air pollution nanoparticles, emitted from industry and sources related to traffic likely represents a major health risk for cardiac disease with co-associated metals. | Exogenous nanoparticles (with diameter around 15–40 nm) within myocardial mitochondria of young, highly exposed individuals are dominantly iron-rich, eventually causing cardiovascular disease. | A cautionary note with focus on confounding variables is warranted and may include other trace exogenous metal species like aluminum and titanium as co-associated with these iron-rich nanoparticles. | Maher, 2020 [198] |
| Iron loading may be associated with various health hazards as exemplified by a high number of assumed diseases. Iron may be inhaled by industrial workers: iron miners, foundry workers, or welders. | Limited data exist on the role of ferriferous materials inhaled by industrial workers as a potential risk factor in causing pulmonary tract neoplasms and the dissemination of inhaled iron via macrophages to other body tissues to result in systemic iron loading. | Assuming the exposed workers were healthy before and have an iron homeostatic system that is not impaired, it should be no problem for these workers to limit iron uptake and remove excess iron. | Weinberg, 2010 [199] |
| Iron ore mining in Pahang, Malaysia, with potential human health risks of this special heavy metal detected in some surface soils. | For iron, no significant potential health risk was found to children and adults as the hazards indices of non-carcinogenic risks all were lower than one. | Data are as expected. If heathy individuals did incorporate excessive iron, they should have a system allowing for iron homeostasis. | Diami, 2016 [200] |
| Cadmium Source | Human Health Risk Issues | Comments | References |
|---|---|---|---|
| Derived from industrial as well as agricultural sources, exposure to cadmium primarily occurs though the ingestion of food and water contaminated with cadmium. | Epidemiological data relate both environmental and occupational cadmium exposure to various types of cancer, including breast, nasopharynx, lung, kidney, prostate, and pancreas. | The liver and kidneys are extremely sensitive to the toxic effects of cadmium, likely due to the ability of these organs to form metallothioneins that bind tightly the toxic cadmium ions. | Genchi, 2020, [263] |
| High rates of soil to plant transfer renders food contaminated with cadmium as the main source of exposure among non-smoking and non-occupationally exposed individuals. | In prospective studies in Japan and the US an excess of cancer mortality was found to be associated with environmental cadmium exposure. In addition, cadmium may cause endometrial cancer. | A very large health risk is seemingly associated with cadmium exposure at levels experienced by many populations in the world. No escape is possible as cadmium is found to be ubiquitous. | Satarug, 2011 [268] |
| Cadmium derived from the environment exerts toxic effects on various organs and in addition, is classified as a human carcinogen. | Toxicity primarily relates to kidneys, bones, and the respiratory system, while cancer may develop in lungs and prostate due to alteration of DNA repair. | A variety of risk mitigation recommendations have been presented but their efficiency remains unclear due to multiple cadmium sources and its ubiquity. | WHO, 2024 [274] |
| Cadmium is naturally found in soil, minerals, and water and enters the human body via the lungs and the digestive tract. Smokers are especially at risk as tobacco leaves are often contaminated by cadmium derived from contaminated soil. | Cadmium is described by both its toxicity and health effects. Emphasis is given to high blood pressure and lung diseases like asthma and emphysema. It may play a crucial role in the development of Alzheimer’s disease, multiple sclerosis, Parkinson’s disease, and Huntington’s disease. | A cautionary statement is required with respect to the lung diseases also triggered by smoking itself, which must be viewed as a confounding variable. Regarding the mentioned neurology diseases, there are likely further studies required for confirmation of a causal attribution. | Charkiewicz, 2023 [275] |
| Various environmental sources of cadmium are mentioned. The blood concentration of cadmium serves as a reliable indicator for a recent exposition as opposed to the urinary concentration, which reflects past exposure. | Several studies showed cadmium association with bone injury. Cadmium was also implicated in the Itai-Itai disease, characterized by osteoporosis and a high rate of fractures, caused by use of rice contaminated with cadmium following cadmium irrigation. | Also under discussion is the role of cadmium in causing cancer, including renal cancer, if cadmium specifically enters the human body via the respiratory system, but molecular mechanisms of carcinogenesis by cadmium are unknown. | Godt, 2006 [276] |
| Cadmium exposure is widespread among non-occupationally exposed populations and non-smokers. | A recent systematic review revealed that cadmium exposure potentially leads to deteriorations of cognitive abilities. | This topic is still under discussion as only 10/15 eligible studies supported such association while 5/10 studies did not. | Chatterjee, 2022 [277] |
| Cadmium exists in the environment due to use of fossil fuels, metal ore combustion, and waste burning. | Cadmium is a health risk. Cadmium only injures cells which cannot synthesize metallothioneins capable of scavenging free radicals. | Instead, metallothioneins formed in cells prevent cellular cadmium toxicity because toxic radicals are effectively scavenged. | Rafati Rahimzadeh, 2017 [278] |
| Cadmium is regularly found with other heavy metals such as zinc, copper, and lead in the polluted environment. | Neurologic dysfunction can occur as parkinsonism, impaired higher cortical functioning, and olfactory disturbances. | Divergent statements: Liver injury after exposure to cadmium was not reported by some [269,279], but was reported by others [280]. | Koons, 2023 [269]; Hong, 2021 [279]; Ikeda, 1997 [280] |
| Cadmium present in the environment and causing health hazards in the US population was examined in a survey from 1999 to 2018. | Cadmium is toxic to human health, and upon exposure, it significantly increases overall mortality including cancer deaths and deaths from causes related to cardiovascular disease. | The mediating effect of smoking was estimated at 32%, as opposed to a large proportion of 68%, for which a direct effect of cadmium remained. | Moon, 2023 [281] |
| Arsenic Source | Human Health Risk Issues | Comments | References |
|---|---|---|---|
| Exposure to toxic arsenic in tap water worldwide. | Cancers of the skin and various internal organs as well as noncarcinogenic effects were reported. | This early report described major health hazards. | Abernathy, 1999 [333] |
| Humans are at risk of exposure to arsenic through air, food, or water. | Health hazards include vascular changes, diabetes, and cancers of organs such as the bladder, lung, liver, kidney, and prostate. | This, again, is one of the earlier reports on health issues. | Abernathy, 1999 [334] |
| Arsenic from natural sources causes global health problems. | Arsenic causes cancers of the skin, lung, and bladder and many disorders of various organs and tissues unrelated to cancer. They all may impair health. | Summary of health hazards due to prolonged arsenic uptake. | Ng, 2003 [335] |
| Arsenic exposure may occur near factories that produce metals. | General health hazards include higher mortality rates due to cancers of the skin, lung, liver, urinary bladder, kidney, and colon. | Arsenic causes a bundle of health hazards including cancer. | Tchounwou, 2003 [336] |
| Arsenic contaminating drinking water was assessed for health effects on some liver tests and histology. | Patients (93/193) with hepatomegaly showed elevated ALT (>40 U/L), AST (>40 U/L), and ALP (>400 U/L). Liver histology showed portal fibrosis in 91.3% of cases, cirrhosis in 2.9% of cases, and a normal picture in 5.8% of cases. | Such high values of increased liver test incidence have not been reported in other previous reports on this topic. | Guha Mazumder, 2005 [310] |
| Groundwater polluted by arsenic in India. | Skin lesions like cancer, gangrene, and melanosis. Spontaneous abortion. | All depended on arsenic levels or exposure lengths. | Mukherjee, 2005 [337] |
| Arsenic sources are water, air, and food. | Health hazards include lung cancer and fetal loss. | Data were obtained prior to regulatory restrictions. | Kapaj, 2006 [338] |
| Arsenic is a major environmental pollutant as a contaminant of drinking water. | Chronic arsenic exposure in humans results in malignant, degenerative, and inflammatory changes. | Health hazards affecting many organs and tissues were reported and analyzed in detail. | Singh, 2007 [339] |
| Inorganic arsenic is a human carcinogen in adults and also a general toxicant even for fetuses. | Inorganic arsenic and its several metabolites pass through the placenta and may increase the risk of fetal loss and retard fetal growth. | Arsenic is a major health hazard for adults as well as for fetuses, and a challenging health issue. | Vahter, 2008 [340] |
| Arsenic found in wells along river Indus in Pakistan leads to health risks. | Prevalence of arsenicosis skin lesions overall was 13.5%, as assessed in 72/534 cases. | Prevalence rates were highest at 100–199 ppb arsenic levels. | Fatmi, 2013 [341] |
| More than 200 million persons worldwide might be chronically exposed to arsenic tap water. | Substantial health issues of prolonged exposures to arsenic generally relate to skin and neurological diseases, in addition to malignant tumors. | For some cancer types, the risk is increased in a dose-dependent linear trend. | Naujokas, 2013 [342] |
| Two consecutive endemic long-term exposures to arsenic from drinking water in Taiwan. | Several diseases have been well documented to be associated with chronic consumption of drinking water that contained arsenic as pollutant contaminant. | Illnesses related to the consumed arsenic partially showed a dose–response relation. | Chen, 2014 [343] |
| Arsenic polluted water through natural and anthropogenic sources like mining, industrial processes, and the production and use of pesticides. | Arsenic exposure is known to be associated with the development of vascular diseases including stroke, ischemic heart disease, and peripheral vascular disease. Similarly, and according to the International Agency for Research on Cancer and the US FDA, there is an increased risk of tumors of the bladder, lungs, kidneys, and liver. | A critical comprehensive overview was provided on health hazards due to arsenic exposure. | Palma-Lara, 2020 [344] |
| Contamination of food by arsenic is seen as a public health issue in the Middle East, where the food supply relies on its import. | Exposure to arsenic leads to an increased risk of diseases such as dysbiosis, obesity, metabolic syndrome, diabetes, chronic kidney disease, chronic heart disease, cancer, and maternal as well as fetal complications. | An informative overview on many diseases caused by prolonged exposure to arsenic was provided. | Khan, 2022 [345] |
| Humans generally become exposed to arsenic by contaminated tap water. | Exposure to arsenic can result in many health problems, ranging from cancer to skin diseases. It may cause genotoxicity and lead to life-threatening abnormalities of the inflammatory and immune system. | The focus was on new insights on genotoxicity, and epigenomic changes and other alterations. | Ozturk, 3022 [346] |
| Exposure to arsenic may occur via use of contaminated drinking water and food. | In patients from India with gallbladder carcinoma, a high arsenic concentration was found in blood and bile samples, as well as in biological gallbladder tissue, gallbladder stones, and hair samples. | New insights on a high gallbladder cancer risk due to arsenic intake were reported. | Kumar, 2023 [347] |
| Around 80 million people in India consume arsenic contained in groundwater. | In India, more than 0.1 million deaths and 0.3 million cases of illness are due to arsenic-contaminated groundwater. Arsenic in utero is associated with impaired cognitive development. | Arsenic damages chromosomal deoxyribonucleic acid (DNA). | Aryan 2024 [348] |
| Arsenic contaminating the drinking water in Ethiopia was studied. | The mean of arsenic concentration in the groundwater samples was 11.15 ± 9.38 µg/L. The cancer risk for children was 1.15 × 10−2 and the risk for adults 4.95 × 10−3. | The cancer risks exceed commonly accepted threshold values. | Demissie, 2024 [349] |
| Long-term use of arsenic in water is potentially life-threatening. | The estimated health risk of arsenic in drinking water was up to 1.63 × 10−6 disability-adjusted life years (DALYs) in some parts of Northern China. | Prolonged arsenic uptake in drinking water can cause increased DALYs. | Dou, 2024 [350] |
| EFSA updated its risk assessment on arsenic in food. | Epidemiological studies show that chronic intake is associated with increased risk of cancers of the skin, bladder, and lung. | Epidemiology, again, ascertained increased cancer risks. | EFSA, 2024 [351] |
| Arsenic has the ability to move in environmental media and has become a major public and environmental concern. | Hypertension and atherosclerosis are the most extensively studied topics, with redox imbalance, apoptosis, and methylation being the primary mechanistic clues. Cardiovascular damage caused by arsenic includes arrhythmia, cardiac remodeling, vascular leakage, and abnormal angiogenesis. | This article offers a comprehensive overview of current research and actual data on cardiovascular hazards caused by arsenic. | Han, 2024 [352] |
| The association between higher but not lower arsenic levels in drinking water and lung cancer is well described. | Individuals exposed to low to moderate levels of arsenic (<150 μg/L) were at an elevated risk of developing or dying from lung cancer. For lung cancer incidence, the predicted posterior mean relative risks (RRs) at arsenic concentrations of 150 μg/L were 1.11 (0.86–1.43). | Information was presented on the effect of exposure to low and moderate arsenic levels on outcomes of lung cancer. | Issanov, 2024 [353] |
| Vegetables can accumulate high arsenic amounts and are widely consumed. | Health risk assessments associated with arsenic exposure through consumption of water spinach and amaranth were conducted using prediction models and soil samples collected in Taiwan. | Total contents of arsenic in soil were predictors of arsenic amounts in water spinach. | Liao, 2024 [354] |
| Though findings were still vague, some evidence supported that arsenic exposure contributes to non-alcoholic fatty liver disease (NAFLD) risk. | NAFLD was diagnosed by liver ultrasound, and logistic regression was used to evaluate the associations. The results suggested that inorganic arsenic exposure is positively associated with NAFLD risk, whereby efficiency of arsenic methylation plays a role in the NAFLD. | Clues were presented to explore potential interventions for the prevention of NAFLD. | Liu, 2024 [355] |
| Arsenic pollution causing severe health issues is widely reported and has gained global attention in the last few decades. | Bibliometric analysis of arsenic pollution and its health hazards has revealed that arsenic pollution is primarily caused by anthropogenic sources, and the key sources of arsenic exposure are drinking water, sea food, and agricultural products. Arsenic pollution is related to major health hazards such as cancer. | Arsenic has a biogeochemical cycle that, with its complexity, plays a significant role in pollution and thus in emerging health problems. | Sevak, 2024 [356] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teschke, R. Copper, Iron, Cadmium, and Arsenic, All Generated in the Universe: Elucidating Their Environmental Impact Risk on Human Health Including Clinical Liver Injury. Int. J. Mol. Sci. 2024, 25, 6662. https://doi.org/10.3390/ijms25126662
Teschke R. Copper, Iron, Cadmium, and Arsenic, All Generated in the Universe: Elucidating Their Environmental Impact Risk on Human Health Including Clinical Liver Injury. International Journal of Molecular Sciences. 2024; 25(12):6662. https://doi.org/10.3390/ijms25126662
Chicago/Turabian StyleTeschke, Rolf. 2024. "Copper, Iron, Cadmium, and Arsenic, All Generated in the Universe: Elucidating Their Environmental Impact Risk on Human Health Including Clinical Liver Injury" International Journal of Molecular Sciences 25, no. 12: 6662. https://doi.org/10.3390/ijms25126662
APA StyleTeschke, R. (2024). Copper, Iron, Cadmium, and Arsenic, All Generated in the Universe: Elucidating Their Environmental Impact Risk on Human Health Including Clinical Liver Injury. International Journal of Molecular Sciences, 25(12), 6662. https://doi.org/10.3390/ijms25126662






