Involvement of Reactive Oxygen Species in Prostate Cancer and Its Disparity in African Descendants
Abstract
:1. Introduction
2. Sources of Intracellular ROS Production and Cell Systems That Detoxify ROS
2.1. Enzymes and Organelle That Produce ROS
2.1.1. NADPH Oxidases
2.1.2. Mitochondria
2.1.3. Cytochrome P450
2.1.4. Xanthine Oxidases
2.2. Antioxidants
3. ROS in Prostate Cancer Development, Progression, and Dissemination
4. Prostate Cancer Disparity in African Americans
4.1. Socioeconomic Factors
4.2. Biological Factors
5. ROS and Inflammation Contribute to Prostate Cancer Disparity in African Americans
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hohmann, N.; Schroder, F.; Moreira, B.; Teng, H.; Burhenne, J.; Bruckner, T.; Mueller, S.; Haefeli, W.E.; Seitz, H.K. Effect of Clomethiazole Vs. Clorazepate on Hepatic Fat and Serum Transaminase Activities in Alcohol-Associated Liver Disease: Results from a Randomized, Controlled Phase II Clinical Trial. Alcohol. Alcohol. 2023, 58, 134–141. [Google Scholar] [CrossRef]
- Tavassolifar, M.J.; Vodjgani, M.; Salehi, Z.; Izad, M. The Influence of Reactive Oxygen Species in the Immune System and Pathogenesis of Multiple Sclerosis. Autoimmune Dis. 2020, 2020, 5793817. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Gentile, F.; Arcaro, A.; Pizzimenti, S.; Daga, M.; Cetrangolo, G.P.; Dianzani, C.; Lepore, A.; Graf, M.; Ames, P.R.J.; Barrera, G. DNA damage by lipid peroxidation products: Implications in cancer, inflammation and autoimmunity. AIMS Genet 2017, 4, 103–137. [Google Scholar] [CrossRef]
- Maynard, S.; Schurman, S.H.; Harboe, C.; de Souza-Pinto, N.C.; Bohr, V.A. Base excision repair of oxidative DNA damage and association with cancer and aging. Carcinogenesis 2009, 30, 2–10. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef]
- Poulsen, H.E.; Prieme, H.; Loft, S. Role of oxidative DNA damage in cancer initiation and promotion. Eur. J. Cancer Prev. 1998, 7, 9–16. [Google Scholar] [PubMed]
- Sova, H.; Jukkola-Vuorinen, A.; Puistola, U.; Kauppila, S.; Karihtala, P. 8-Hydroxydeoxyguanosine: A new potential independent prognostic factor in breast cancer. Br. J. Cancer 2010, 102, 1018–1023. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2’-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef]
- Cipriano, A.; Viviano, M.; Feoli, A.; Milite, C.; Sarno, G.; Castellano, S.; Sbardella, G. NADPH Oxidases: From Molecular Mechanisms to Current Inhibitors. J. Med. Chem. 2023, 66, 11632–11655. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.M.; Geng, L.; Cahill-Smith, S.; Liu, F.; Douglas, G.; McKenzie, C.A.; Smith, C.; Brooks, G.; Channon, K.M.; Li, J.M. Nox2 contributes to age-related oxidative damage to neurons and the cerebral vasculature. J. Clin. Investig. 2019, 129, 3374–3386. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.W.; Wang, J.; Zhang, Q.; Wang, R.; Dhandapani, K.M.; Vadlamudi, R.K.; Brann, D.W. NADPH oxidase in brain injury and neurodegenerative disorders. Mol. Neurodegener. 2017, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Guan, B.; Peng, X.; Xu, Y.; Wang, Y.; Wu, J. Analysis on 6 cases with nasal neuroendocrine carcinoma. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2013, 27, 236–238. [Google Scholar] [PubMed]
- Choi, D.H.; Cristovao, A.C.; Guhathakurta, S.; Lee, J.; Joh, T.H.; Beal, M.F.; Kim, Y.S. NADPH oxidase 1-mediated oxidative stress leads to dopamine neuron death in Parkinson’s disease. Antioxid. Redox. Signal 2012, 16, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Cristovao, A.C.; Choi, D.H.; Baltazar, G.; Beal, M.F.; Kim, Y.S. The role of NADPH oxidase 1-derived reactive oxygen species in paraquat-mediated dopaminergic cell death. Antioxid. Redox. Signal 2009, 11, 2105–2118. [Google Scholar] [CrossRef] [PubMed]
- Graham, K.A.; Kulawiec, M.; Owens, K.M.; Li, X.; Desouki, M.M.; Chandra, D.; Singh, K.K. NADPH oxidase 4 is an oncoprotein localized to mitochondria. Cancer Biol. Ther. 2010, 10, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Roy, K.; Wu, Y.; Meitzler, J.L.; Juhasz, A.; Liu, H.; Jiang, G.; Lu, J.; Antony, S.; Doroshow, J.H. NADPH oxidases and cancer. Clin. Sci. 2015, 128, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Arbiser, J.L.; Petros, J.; Klafter, R.; Govindajaran, B.; McLaughlin, E.R.; Brown, L.F.; Cohen, C.; Moses, M.; Kilroy, S.; Arnold, R.S.; et al. Reactive oxygen generated by Nox1 triggers the angiogenic switch. Proc. Natl. Acad. Sci. USA 2002, 99, 715–720. [Google Scholar] [CrossRef]
- Kamata, T. Roles of Nox1 and other Nox isoforms in cancer development. Cancer Sci. 2009, 100, 1382–1388. [Google Scholar] [CrossRef]
- Meitzler, J.L.; Antony, S.; Wu, Y.; Juhasz, A.; Liu, H.; Jiang, G.; Lu, J.; Roy, K.; Doroshow, J.H. NADPH oxidases: A perspective on reactive oxygen species production in tumor biology. Antioxid. Redox. Signal 2014, 20, 2873–2889. [Google Scholar] [CrossRef]
- Carvalho, D.P.; Dupuy, C. Role of the NADPH Oxidases DUOX and NOX4 in Thyroid Oxidative Stress. Eur. Thyroid. J. 2013, 2, 160–167. [Google Scholar] [CrossRef]
- Fortunato, R.S.; Lima de Souza, E.C.; Ameziane-el Hassani, R.; Boufraqech, M.; Weyemi, U.; Talbot, M.; Lagente-Chevallier, O.; de Carvalho, D.P.; Bidart, J.M.; Schlumberger, M.; et al. Functional consequences of dual oxidase-thyroperoxidase interaction at the plasma membrane. J. Clin. Endocrinol. Metab. 2010, 95, 5403–5411. [Google Scholar] [CrossRef]
- Heit, C.; Dong, H.; Chen, Y.; Thompson, D.C.; Deitrich, R.A.; Vasiliou, V.K. The role of CYP2E1 in alcohol metabolism and sensitivity in the central nervous system. Subcell. Biochem. 2013, 67, 235–247. [Google Scholar] [PubMed]
- Veith, A.; Moorthy, B. Role of Cytochrome P450s in the Generation and Metabolism of Reactive Oxygen Species. Curr. Opin. Toxicol. 2018, 7, 44–51. [Google Scholar] [CrossRef]
- Hille, R. Xanthine Oxidase-A Personal History. Molecules 2023, 28, 1921. [Google Scholar] [CrossRef]
- Bortolotti, M.; Polito, L.; Battelli, M.G.; Bolognesi, A. Xanthine oxidoreductase: One enzyme for multiple physiological tasks. Redox. Biol. 2021, 41, 101882. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Zweier, J.L. Characterization of free radical generation by xanthine oxidase. Evidence for hydroxyl radical generation. J. Biol. Chem. 1989, 264, 9880–9884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Pan, C.; Feng, C.; Yan, C.; Yu, Y.; Chen, Z.; Guo, C.; Wang, X. Role of mitochondrial reactive oxygen species in homeostasis regulation. Redox. Rep. 2022, 27, 45–52. [Google Scholar] [CrossRef]
- Younus, H. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. 2018, 12, 88–93. [Google Scholar]
- Camargo, L.L.; Harvey, A.P.; Rios, F.J.; Tsiropoulou, S.; Da Silva, R.N.O.; Cao, Z.; Graham, D.; McMaster, C.; Burchmore, R.J.; Hartley, R.C.; et al. Vascular Nox (NADPH Oxidase) Compartmentalization, Protein Hyperoxidation, and Endoplasmic Reticulum Stress Response in Hypertension. Hypertension 2018, 72, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Kops, G.J.; Dansen, T.B.; Polderman, P.E.; Saarloos, I.; Wirtz, K.W.; Coffer, P.J.; Huang, T.T.; Bos, J.L.; Medema, R.H.; Burgering, B.M. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 2002, 419, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Krafczyk, N.; Klotz, L.O. FOXO transcription factors in antioxidant defense. IUBMB Life 2022, 74, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Zahra, K.F.; Lefter, R.; Ali, A.; Abdellah, E.C.; Trus, C.; Ciobica, A.; Timofte, D. The Involvement of the Oxidative Stress Status in Cancer Pathology: A Double View on the Role of the Antioxidants. Oxid. Med. Cell Longev. 2021, 2021, 9965916. [Google Scholar] [CrossRef] [PubMed]
- Asadi-Samani, M.; Kaffash Farkhad, N.; Reza Mahmoudian-Sani, M.; Shirzad, H. Antioxidants as a Double-Edged Sword in the Treatment of Cancer. IntechOpen 2019. [Google Scholar] [CrossRef]
- Han, C.; Wang, Z.; Xu, Y.; Chen, S.; Han, Y.; Li, L.; Wang, M.; Jin, X. Roles of Reactive Oxygen Species in Biological Behaviors of Prostate Cancer. Biomed. Res. Int. 2020, 2020, 1269624. [Google Scholar] [CrossRef] [PubMed]
- Sekhoacha, M.; Riet, K.; Motloung, P.; Gumenku, L.; Adegoke, A.; Mashele, S. Prostate Cancer Review: Genetics, Diagnosis, Treatment Options, and Alternative Approaches. Molecules 2022, 27, 5730. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury-Paulino, I.M.; Ericsson, C.; Vince, R., Jr.; Spratt, D.E.; George, D.J.; Mucci, L.A. Racial disparities in prostate cancer among black men: Epidemiology and outcomes. Prostate Cancer Prostatic Dis. 2022, 25, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Hinata, N.; Fujisawa, M. Racial Differences in Prostate Cancer Characteristics and Cancer-Specific Mortality: An Overview. World J. Mens. Health 2022, 40, 217–227. [Google Scholar] [CrossRef]
- Zeng, H.; Xu, M.; Xie, Y.; Nawrocki, S.; Morze, J.; Ran, X.; Shan, T.; Xia, C.; Wang, Y.; Lu, L.; et al. Racial/ethnic disparities in the cause of death among patients with prostate cancer in the United States from 1995 to 2019: A population-based retrospective cohort study. EClinicalMedicine 2023, 62, 102138. [Google Scholar] [CrossRef]
- Oczkowski, M.; Dziendzikowska, K.; Pasternak-Winiarska, A.; Wlodarek, D.; Gromadzka-Ostrowska, J. Dietary Factors and Prostate Cancer Development, Progression, and Reduction. Nutrients 2021, 13, 496. [Google Scholar] [CrossRef]
- Eroglu, C.; Avci, E.; Vural, H.; Kurar, E. Anticancer mechanism of Sinapic acid in PC-3 and LNCaP human prostate cancer cell lines. Gene 2018, 671, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Vivarelli, F.; Canistro, D.; Cirillo, S.; Papi, A.; Spisni, E.; Vornoli, A.; Croce, C.M.D.; Longo, V.; Franchi, P.; Filippi, S.; et al. Co-carcinogenic effects of vitamin E in prostate. Sci. Rep. 2019, 9, 11636. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Koul, S.; Khandrika, L.; Meacham, R.B.; Koul, H.K. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008, 68, 1777–1785. [Google Scholar] [CrossRef]
- Lim, S.D.; Sun, C.; Lambeth, J.D.; Marshall, F.; Amin, M.; Chung, L.; Petros, J.A.; Arnold, R.S. Increased Nox1 and hydrogen peroxide in prostate cancer. Prostate 2005, 62, 200–207. [Google Scholar] [CrossRef]
- Holl, M.; Koziel, R.; Schafer, G.; Pircher, H.; Pauck, A.; Hermann, M.; Klocker, H.; Jansen-Durr, P.; Sampson, N. ROS signaling by NADPH oxidase 5 modulates the proliferation and survival of prostate carcinoma cells. Mol. Carcinog. 2016, 55, 27–39. [Google Scholar] [CrossRef]
- Thurner, E.M.; Krenn-Pilko, S.; Langsenlehner, U.; Stojakovic, T.; Pichler, M.; Gerger, A.; Kapp, K.S.; Langsenlehner, T. The elevated C-reactive protein level is associated with poor prognosis in prostate cancer patients treated with radiotherapy. Eur. J. Cancer 2015, 51, 610–619. [Google Scholar] [CrossRef]
- Gomez-Gomez, E.; Carrasco-Valiente, J.; Campos-Hernandez, J.P.; Blanca-Pedregosa, A.M.; Jimenez-Vacas, J.M.; Ruiz-Garcia, J.; Valero-Rosa, J.; Luque, R.M.; Requena-Tapia, M.J. Clinical association of metabolic syndrome, C-reactive protein and testosterone levels with clinically significant prostate cancer. J. Cell Mol. Med. 2019, 23, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Beyaztas, H.; Ersoz, C.; Ozkan, B.N.; Olgun, I.; Polat, H.S.; Dastan, A.I.; Cetinkaya, E.; Guler, E.M. The role of oxidative stress and inflammation biomarkers in pre- and postoperative monitoring of prostate cancer patients. Free Radic. Res. 2024, 58, 98–106. [Google Scholar] [CrossRef]
- Shukla, S.; Srivastava, J.K.; Shankar, E.; Kanwal, R.; Nawab, A.; Sharma, H.; Bhaskaran, N.; Ponsky, L.E.; Fu, P.; MacLennan, G.T.; et al. Oxidative Stress and Antioxidant Status in High-Risk Prostate Cancer Subjects. Diagnostics 2020, 10, 126. [Google Scholar] [CrossRef]
- DeSantis, C.E.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Cancer statistics for African Americans, 2019. CA Cancer J. Clin. 2019, 69, 211–233. [Google Scholar] [CrossRef] [PubMed]
- Lillard, J.W., Jr.; Moses, K.A.; Mahal, B.A.; George, D.J. Racial disparities in Black men with prostate cancer: A literature review. Cancer 2022, 128, 3787–3795. [Google Scholar] [CrossRef] [PubMed]
- Cackowski, F.C.; Mahal, B.; Heath, E.I.; Carthon, B. Evolution of Disparities in Prostate Cancer Treatment: Is This a New Normal? Am. Soc. Clin. Oncol. Educ. Book 2021, 41, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pollack, C.E.; Armstrong, K.A.; Mitra, N.; Chen, X.; Ward, K.R.; Radhakrishnan, A.; Wong, M.S.; Bekelman, J.E.; Branas, C.C.; Rhodes, K.V.; et al. A multidimensional view of racial differences in access to prostate cancer care. Cancer 2017, 123, 4449–4457. [Google Scholar] [CrossRef] [PubMed]
- Ogunsanya, M.E.; Brown, C.M.; Odedina, F.T.; Barner, J.C.; Adedipe, T.B.; Corbell, B. Knowledge of Prostate Cancer and Screening Among Young Multiethnic Black Men. Am. J. Mens. Health 2017, 11, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.S.; Allen, R.S.; Eichorst, M.K.; Mieskowski, L.; Ewell, P.J.; Payne-Foster, P.; Ragin, C. A pilot study of prostate cancer knowledge among African American men and their health care advocates: Implications for screening decisions. Cancer Causes Control 2018, 29, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, S.S. A review of social determinants of prostate cancer risk, stage, and survival. Prostate Int. 2020, 8, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.D.; Perera, M.; Bolton, D.M.; Papa, N. Social determinants of health: Does socioeconomic status affect access to staging imaging for men with prostate cancer. Prostate Cancer Prostatic Dis. 2023, 26, 429–431. [Google Scholar] [CrossRef]
- Lowder, D.; Rizwan, K.; McColl, C.; Paparella, A.; Ittmann, M.; Mitsiades, N.; Kaochar, S. Racial disparities in prostate cancer: A complex interplay between socioeconomic inequities and genomics. Cancer Lett. 2022, 531, 71–82. [Google Scholar] [CrossRef]
- Major, J.M.; Norman Oliver, M.; Doubeni, C.A.; Hollenbeck, A.R.; Graubard, B.I.; Sinha, R. Socioeconomic status, healthcare density, and risk of prostate cancer among African American and Caucasian men in a large prospective study. Cancer Causes Control 2012, 23, 1185–1191. [Google Scholar] [CrossRef]
- Cheng, I.; Witte, J.S.; McClure, L.A.; Shema, S.J.; Cockburn, M.G.; John, E.M.; Clarke, C.A. Socioeconomic status and prostate cancer incidence and mortality rates among the diverse population of California. Cancer Causes Control 2009, 20, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Pichardo, M.S.; Minas, T.Z.; Pichardo, C.M.; Bailey-Whyte, M.; Tang, W.; Dorsey, T.H.; Wooten, W.; Ryan, B.M.; Loffredo, C.A.; Ambs, S. Association of Neighborhood Deprivation With Prostate Cancer and Immune Markers in African American and European American Men. JAMA Netw. Open 2023, 6, e2251745. [Google Scholar] [CrossRef]
- Vidal, A.C.; Oyekunle, T.; Howard, L.E.; Shivappa, N.; De Hoedt, A.; Figueiredo, J.C.; Taioli, E.; Fowke, J.H.; Lin, P.H.; Hebert, J.R.; et al. Dietary inflammatory index (DII) and risk of prostate cancer in a case-control study among Black and White US Veteran men. Prostate Cancer Prostatic Dis. 2019, 22, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, S.A.; Marchese, M.; Cole, A.P.; Mahal, B.A.; Friedlander, D.F.; Krimphove, M.; Kilbridge, K.L.; Lipsitz, S.R.; Nguyen, P.L.; Choueiri, T.K.; et al. Geographic Distribution of Racial Differences in Prostate Cancer Mortality. JAMA Netw. Open 2020, 3, e201839. [Google Scholar] [CrossRef]
- Yamoah, K.; Lee, K.M.; Awasthi, S.; Alba, P.R.; Perez, C.; Anglin-Foote, T.R.; Robison, B.; Gao, A.; DuVall, S.L.; Katsoulakis, E.; et al. Racial and Ethnic Disparities in Prostate Cancer Outcomes in the Veterans Affairs Health Care System. JAMA Netw. Open 2022, 5, e2144027. [Google Scholar] [CrossRef] [PubMed]
- Lofton, H.; Ard, J.D.; Hunt, R.R.; Knight, M.G. Obesity among African American people in the United States: A review. Obesity 2023, 31, 306–315. [Google Scholar] [CrossRef]
- Barrington, W.E.; Schenk, J.M.; Etzioni, R.; Arnold, K.B.; Neuhouser, M.L.; Thompson, I.M., Jr.; Lucia, M.S.; Kristal, A.R. Difference in Association of Obesity With Prostate Cancer Risk Between US African American and Non-Hispanic White Men in the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA Oncol. 2015, 1, 342–349. [Google Scholar] [CrossRef]
- Laurent, V.; Toulet, A.; Attane, C.; Milhas, D.; Dauvillier, S.; Zaidi, F.; Clement, E.; Cinato, M.; Le Gonidec, S.; Guerard, A.; et al. Periprostatic Adipose Tissue Favors Prostate Cancer Cell Invasion in an Obesity-Dependent Manner: Role of Oxidative Stress. Mol. Cancer Res. 2019, 17, 821–835. [Google Scholar] [CrossRef]
- Stikbakke, E.; Richardsen, E.; Knutsen, T.; Wilsgaard, T.; Giovannucci, E.L.; McTiernan, A.; Eggen, A.E.; Haugnes, H.S.; Thune, I. Inflammatory serum markers and risk and severity of prostate cancer: The PROCA-life study. Int. J. Cancer 2020, 147, 84–92. [Google Scholar] [CrossRef]
- Deo, S.H.; Holwerda, S.W.; Keller, D.M.; Fadel, P.J. Elevated peripheral blood mononuclear cell-derived superoxide production in healthy young black men. Am. J. Physiol. Heart. Circ. Physiol. 2015, 308, H548–H552. [Google Scholar] [CrossRef]
- Feairheller, D.L.; Park, J.Y.; Sturgeon, K.M.; Williamson, S.T.; Diaz, K.M.; Veerabhadrappa, P.; Brown, M.D. Racial differences in oxidative stress and inflammation: In vitro and in vivo. Clin. Transl. Sci. 2011, 4, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Rundle, A.G.; Sadasivan, S.M.; Chitale, D.A.; Gupta, N.S.; Williamson, S.R.; Kryvenko, O.N.; Chen, Y.; Bobbitt, K.; Tang, D.; Rybicki, B.A. Racial differences in the systemic inflammatory response to prostate cancer. PLoS ONE 2021, 16, e0252951. [Google Scholar] [CrossRef] [PubMed]
- Singhal, U.; Nallandhighal, S.; Bolton, S.; Farha, M.; Strangl-Kremser, J.; Palapattu, G.; Bollig-Fischer, A.; Udagar, A.; Powell, I.; Salami, S. Molecular profiling of prostate cancer reveals increased inflammatory markers and poor clinical outcomes in African American compared to European American men. J. Urol. 2021, 206, e1042–e1043. [Google Scholar] [CrossRef]
- Awasthi, S.; Berglund, A.; Abraham-Miranda, J.; Rounbehler, R.J.; Kensler, K.; Serna, A.; Vidal, A.; You, S.; Freeman, M.R.; Davicioni, E.; et al. Comparative Genomics Reveals Distinct Immune-oncologic Pathways in African American Men with Prostate Cancer. Clin. Cancer Res. 2021, 27, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Cacciatore, S.; Wium, M.; Licari, C.; Ajayi-Smith, A.; Masieri, L.; Anderson, C.; Salukazana, A.S.; Kaestner, L.; Carini, M.; Carbone, G.M.; et al. Inflammatory metabolic profile of South African patients with prostate cancer. Cancer Metab. 2021, 9, 29. [Google Scholar] [CrossRef] [PubMed]
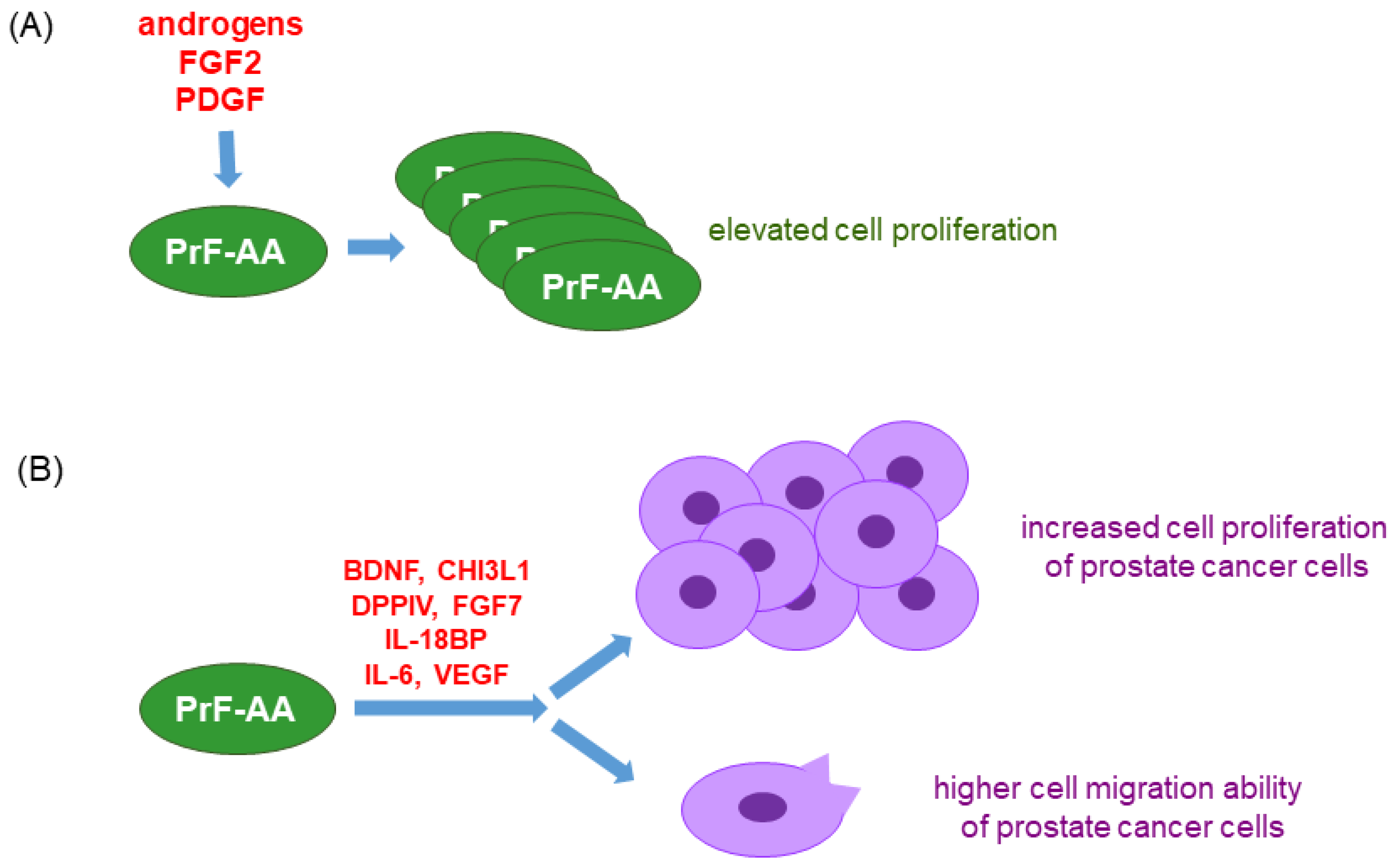
- Gillard, M.; Javier, R.; Ji, Y.; Zheng, S.L.; Xu, J.; Brendler, C.B.; Crawford, S.E.; Pierce, B.L.; Griend, D.J.V.; Franco, O.E. Elevation of Stromal-Derived Mediators of Inflammation Promote Prostate Cancer Progression in African-American Men. Cancer Res. 2018, 78, 6134–6145. [Google Scholar] [CrossRef] [PubMed]
- Maynard, J.P.; Godwin, T.N.; Lu, J.; Vidal, I.; Lotan, T.L.; De Marzo, A.M.; Joshu, C.E.; Sfanos, K.S. Localization of macrophage subtypes and neutrophils in the prostate tumor microenvironment and their association with prostate cancer racial disparities. Prostate 2022, 82, 1505–1519. [Google Scholar] [CrossRef] [PubMed]
- Bassey, I.E.; Emodi, B.A.; Akpan, U.O.; Iyakndue, I.F.A.; Anakebe, E.A.; Icha, B.E.; Efobi, H.A.; Ntinya, A.J.; Udoh, A.E. Impact of Androgen Deprivation on Oxidative Stress and Antioxidant Status in Nigerian Patients With Prostate Cancer Undergoing Androgen Deprivation Therapy. JCO Glob. Oncol. 2020, 6, 1481–1489. [Google Scholar] [CrossRef]
- McCrow, J.P.; Petersen, D.C.; Louw, M.; Chan, E.K.; Harmeyer, K.; Vecchiarelli, S.; Lyons, R.J.; Bornman, M.S.; Hayes, V.M. Spectrum of mitochondrial genomic variation and associated clinical presentation of prostate cancer in South African men. Prostate 2016, 76, 349–358. [Google Scholar] [CrossRef]
| Oxidative Stress Status | Oxidative Stress Indicator | Patient Treatment | Patient Survival | Tumor Stage | Gleason Score/PSA Levels | Reference |
|---|---|---|---|---|---|---|
| high | CRP ≥ 8.6 mg/L | radiotherapy | low | no correlation | no correlation | [45] |
| high | CRP > 2.5 mg/L | N/A | ND | advanced | high | [46] |
| high | Total level of oxidants and antioxidants, IL-10, IL-1β, IL-6 and TNF levels | Patients who received surgeries had reduced oxidative stress levels | ND | ND | ND | [47] |
| high | 8-OHdG, GST * | N/A | ND | ND | high | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liou, G.-Y.; C’lay-Pettis, R.; Kavuri, S. Involvement of Reactive Oxygen Species in Prostate Cancer and Its Disparity in African Descendants. Int. J. Mol. Sci. 2024, 25, 6665. https://doi.org/10.3390/ijms25126665
Liou G-Y, C’lay-Pettis R, Kavuri S. Involvement of Reactive Oxygen Species in Prostate Cancer and Its Disparity in African Descendants. International Journal of Molecular Sciences. 2024; 25(12):6665. https://doi.org/10.3390/ijms25126665
Chicago/Turabian StyleLiou, Geou-Yarh, Reauxqkwuanzyiia C’lay-Pettis, and Sravankumar Kavuri. 2024. "Involvement of Reactive Oxygen Species in Prostate Cancer and Its Disparity in African Descendants" International Journal of Molecular Sciences 25, no. 12: 6665. https://doi.org/10.3390/ijms25126665





