Connection Failure: Differences in White Matter Microstructure Are Associated with 5-HTTLPR but Not with Risk Seeking for Losses
Abstract
1. Introduction
| Study | Sample | Polymorphism | Tract | Task | Results |
|---|---|---|---|---|---|
| Klucken et al., 2015 [23] | 100 adults (46 females) | Triallelic 5-HTTLPR | Uncinate Fasciculus | Fear conditioning | S-allele carriers showed increased amygdala responses during fear learning and increased amygdala–insula coupling compared to l/l-carriers. S-allele carriers had higher FA values compared to l/l-carriers. |
| Pacheco et al., 2009 [24] | 37 females | Triallelic 5-HTTLPR | Uncinate Fasciculus | n/a | Regression showed reduced FA with an increasing number of S-alleles |
| Jonassen et al., 2012 [25] | 33 females | Triallelic 5-HTTLPR | Uncinate Fasciculus | n/a | ANCOVA revealed lower FA in the left Uncinate for S/S carriers vs L/L carriers as well as a significant linear trend |
| Peper et al., 2013 [27] | 40 adults (20 females) | n/a | Frontostriatal | Delay Discounting | Stronger discounting was associated with higher MD, RD and lower FA |
| Achterberg et al., 2016 [28] | 192 adults (51.2% females | n/a | Frontostriatal | Delay Discounting | Age-related increases in FA were associated with reduced discounting |
| Olson et al., 2009 [29] | 79 adults (53.2% females) | n/a | ATR, CCsp, IFOF, ILF, SLF, UF, CST | Delay Discounting | Lower discounting was associated with higher FA and lower MD |
2. Results
2.1. Sample Information
2.2. Linear Contrast Results for 5-HTTLPR and White Matter Tracts
2.3. Exploratory Nonlinear Contrast Results for 5-HTTLPR and White Matter Tracts
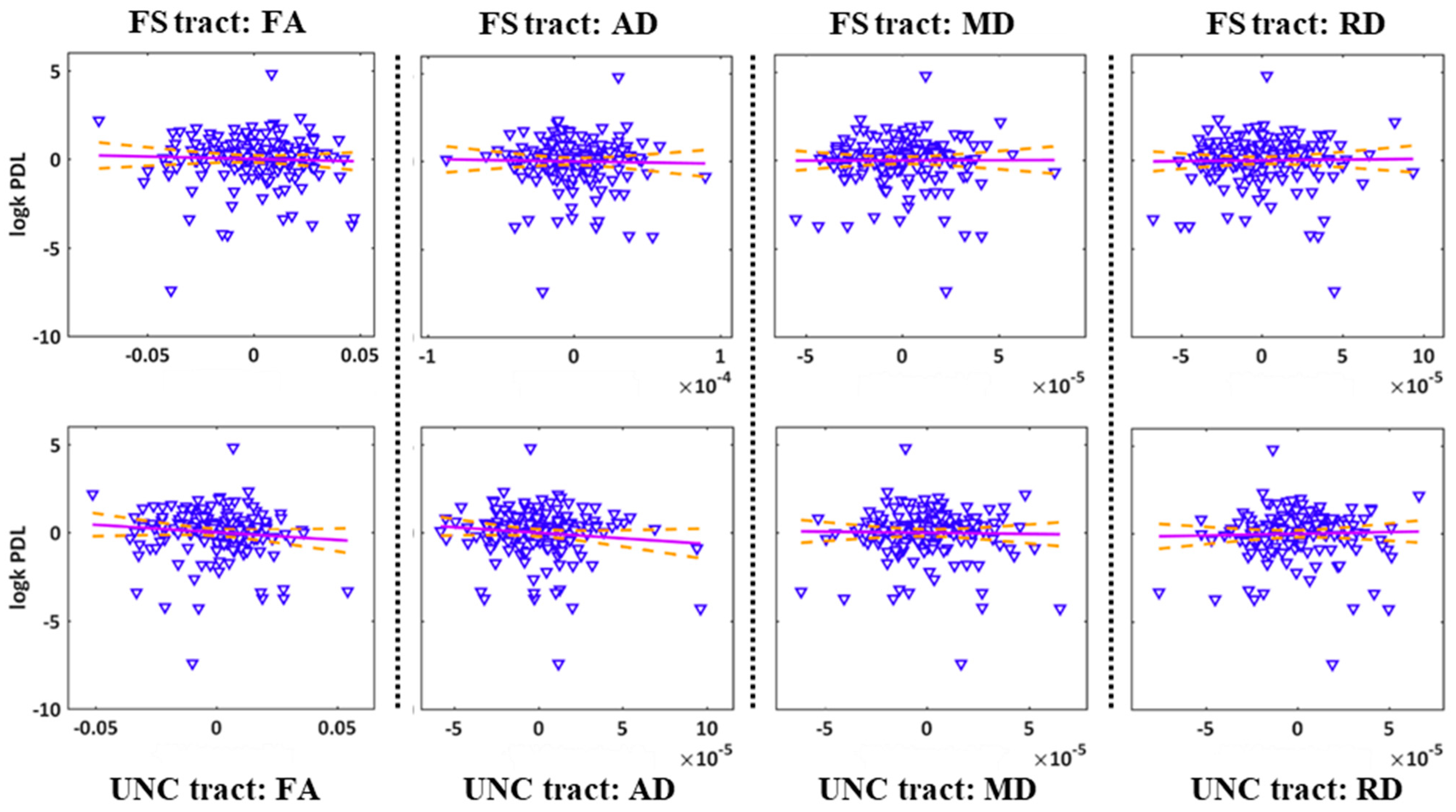
2.4. Correlations between the DTI Parameters of the Frontostriatal/Uncinate Tracts and Risk Seeking for Losses
2.5. Exploratory Whole Brain Results
3. Discussion
3.1. White Matter and Risk Seeking for Losses
3.2. 5-HTTLPR and White Matter
3.3. Limitations
4. Materials and Methods
4.1. Participants
4.2. Probability Discounting for Losses (PDL) Task
4.3. Genotyping
4.4. Imaging
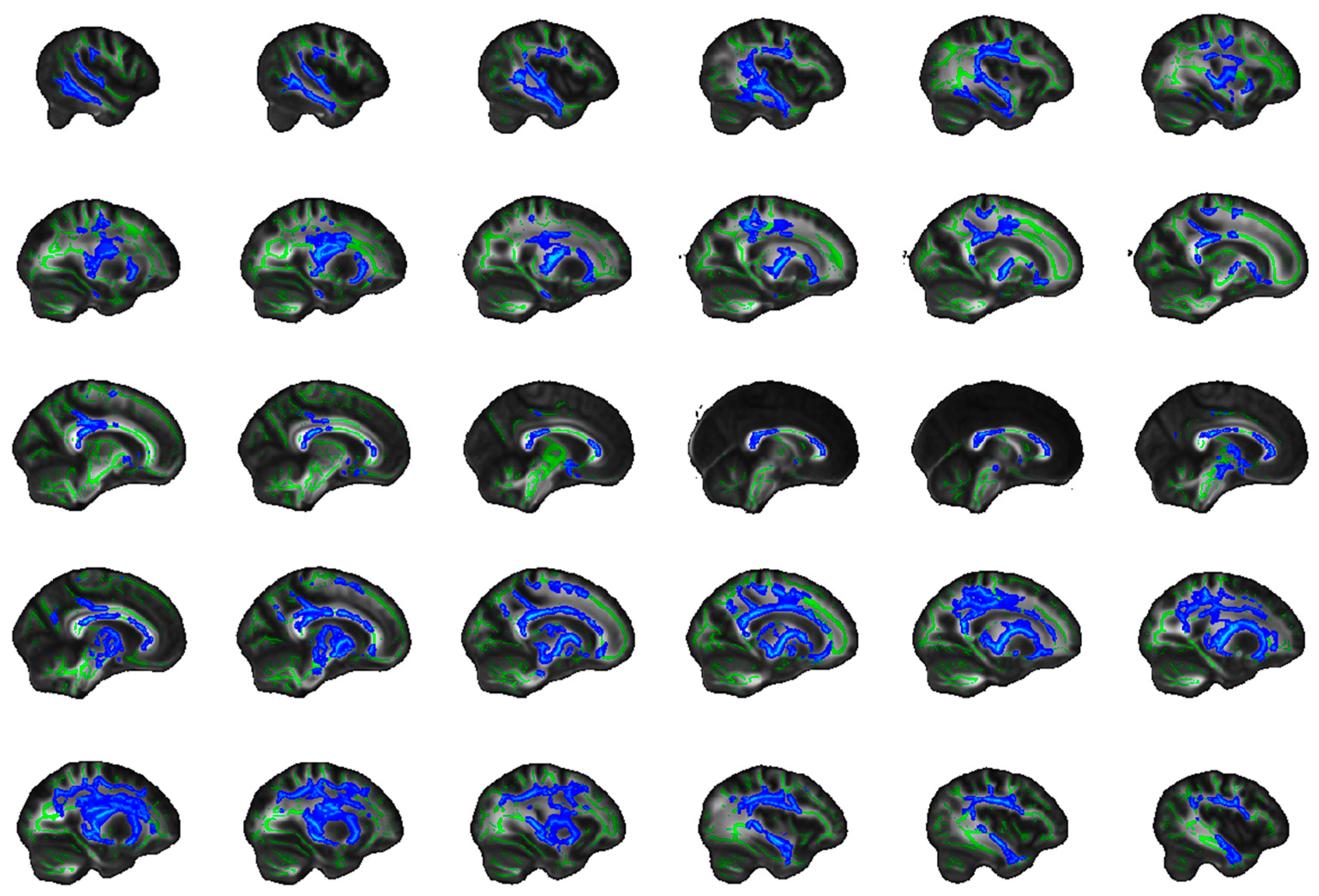
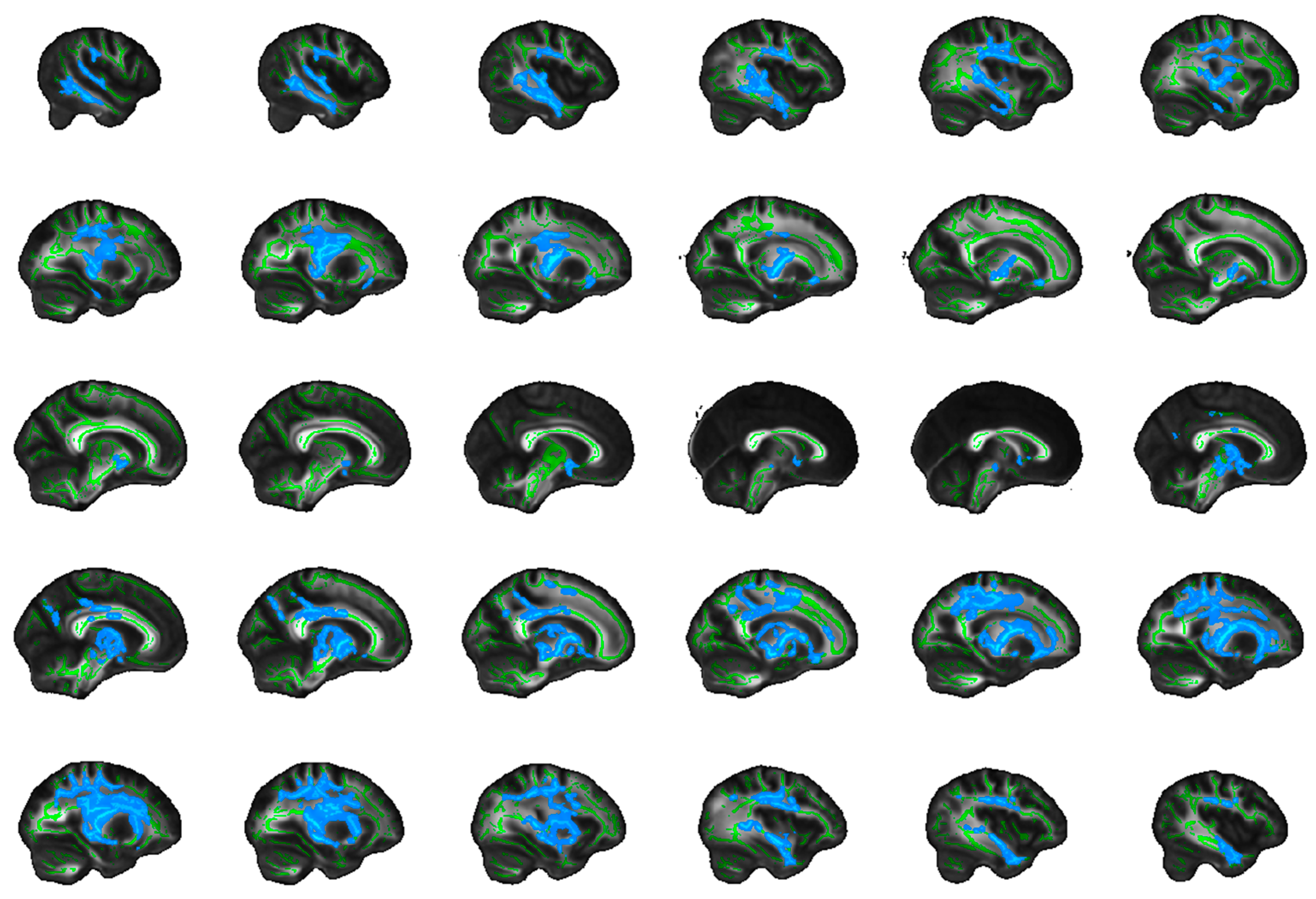
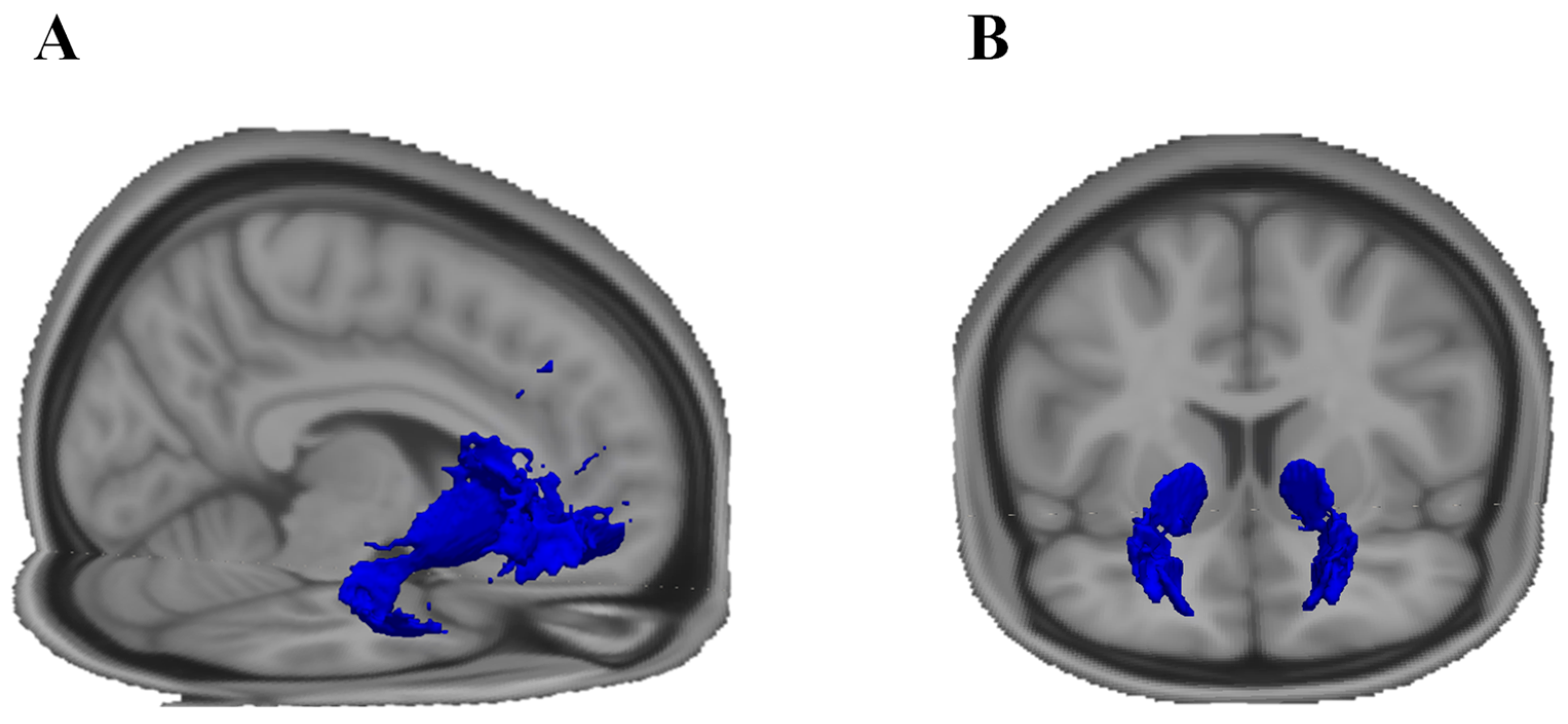
4.5. Frontostriatal and Uncinate Fibre Tract Selection and Volume of Interest (VOI) Generation
4.5.1. Uncinate Fasciculus
4.5.2. Frontostriatal Tract
4.6. 5-HTTLPR and White Matter Tracts Linear Contrast Analysis
4.7. 5-HTTLPR and White Matter Tracts: Exploratory Nonlinear White Matter Tracts Analysis
4.8. Exploratory Whole Brain Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Kahneman, D.; Tversky, A. Prospect Theory: An Analysis of Decision under Risk. Econometrica 1979, 47, 263. [Google Scholar] [CrossRef]
- De Martino, B.; Kumaran, D.; Seymour, B.; Dolan, R.J. Frames, biases, and rational decision-making in the human brain. Science 2006, 313, 684–687. [Google Scholar] [CrossRef] [PubMed]
- Yacubian, J.; Gläscher, J.; Schroeder, K.; Sommer, T.; Braus, D.F.; Büchel, C. Dissociable systems for gain- and loss-related value predictions and errors of prediction in the human brain. J. Neurosci. 2006, 26, 9530–9537. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.; Büchel, C. The neural mechanisms of inter-temporal decision-making: Understanding variability. Trends Cogn. Sci. 2011, 15, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Rangel, A.; Camerer, C.; Montague, P.R. A framework for studying the neurobiology of value-based decision making. Nat. Rev. Neurosci. 2008, 9, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Gottfried, J.A.; O’Doherty, J.; Dolan, R.J. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science 2003, 301, 1104–1107. [Google Scholar] [CrossRef] [PubMed]
- De Martino, B.; Camerer, C.F.; Adolphs, R. Amygdala damage eliminates monetary loss aversion. Proc. Natl. Acad. Sci. USA 2010, 107, 3788–3792. [Google Scholar] [CrossRef] [PubMed]
- Motzkin, J.C.; Philippi, C.L.; Wolf, R.C.; Baskaya, M.K.; Koenigs, M. Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biol. Psychiatry 2015, 77, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Basser, P.J.; Pajevic, S.; Pierpaoli, C.; Duda, J.; Aldroubi, A. In vivo fiber tractography using DT-MRI data. Magn. Reson. Med. 2000, 44, 625–632. [Google Scholar] [CrossRef]
- Jones, D.K.; Knosche, T.R.; Turner, R. White matter integrity, fiber count, and other fallacies: The do’s and don’ts of diffusion MRI. Neuroimage 2013, 73, 239–254. [Google Scholar] [CrossRef]
- Alexander, A.L.; Lee, J.E.; Lazar, M.; Field, A.S. Diffusion tensor imaging of the brain. Neurotherapeutics 2007, 4, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Zhang, J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 2006, 51, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Kranz, G.S.; Kasper, S.; Lanzenberger, R. Reward and the serotonergic system. Neuroscience 2010, 166, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Kobiella, A.; Reimold, M.; Ulshöfer, D.E.; Ikonomidou, V.N.; Vollmert, C.; Vollstädt-Klein, S.; Rietschel, M.; Reischl, G.; Heinz, A.; Smolka, M.N. How the serotonin transporter 5-HTTLPR polymorphism influences amygdala function: The roles of in vivo serotonin transporter expression and amygdala structure. Transl. Psychiatry 2011, 1, e37. [Google Scholar] [CrossRef] [PubMed]
- Hariri, A.R.; Holmes, A. Genetics of emotional regulation: The role of the serotonin transporter in neural function. Trends Cogn. Sci. 2006, 10, 182–191. [Google Scholar] [CrossRef]
- Persico, A.M.; Kalueff, A.V.; LaPorte, J.L. Developmental roles for the serotonin transporter. In Experimental Models in Serotonin Transporter Research, 1st ed.; Kalueff, A.V., LaPorte, J.L., Eds.; University Press: Cambridge, UK, 2010; pp. 78–104. [Google Scholar]
- Kuhnen, C.M.; Chiao, J.Y. Genetic determinants of financial risk taking. PLoS ONE 2009, 4, e4362. [Google Scholar] [CrossRef] [PubMed]
- Kuhnen, C.M.; Samanez-Larkin, G.R.; Knutson, B. Serotonergic genotypes, neuroticism, and financial choices. PLoS ONE 2013, 8, e54632. [Google Scholar] [CrossRef] [PubMed]
- Lesch, K.P.; Bengel, D.; Heils, A.; Sabol, S.Z.; Greenberg, B.D.; Petri, S.; Benjamin, J.; Müller, C.R.; Hamer, D.H.; Murphy, D.L. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 1996, 274, 1527–1531. [Google Scholar] [CrossRef]
- Osher, Y.; Hamer, D.; Benjamin, J. Association and linkage of anxiety-related traits with a functional polymorphism of the serotonin transporter gene regulatory region in Israeli sibling pairs. Mol. Psychiatry 2000, 5, 216–219. [Google Scholar] [CrossRef]
- Anderson, A.; Dreber, A.; Vestman, R. Risk taking, behavioral biases and genes: Results from 149 active investors. J. Behav. Exp. Financ. 2015, 6, 93–100. [Google Scholar] [CrossRef]
- Neukam, P.T.; Kroemer, N.B.; Araujo, Y.I.D.; Hellrung, L.; Pooseh, S.; Rietschel, M.; Witt, S.H.; Schwarzenbolz, U.; Henle, T.; Smolka, M.N. Risk-seeking for losses is associated with 5-HTTLPR, but not with transient changes in 5-HT levels. Psychopharmacology 2018, 235, 2151–2165. [Google Scholar] [CrossRef] [PubMed]
- Klucken, T.; Schweckendiek, J.; Blecker, C.; Walter, B.; Kuepper, Y.; Hennig, J.; Stark, R. The association between the 5-HTTLPR and neural correlates of fear conditioning and connectivity. Soc. Cogn. Affect. Neurosci. 2015, 10, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, J.; Beevers, C.G.; Benavides, C.; McGeary, J.; Stice, E.; Schnyer, D.M. Frontal-limbic white matter pathway associations with the serotonin transporter gene promoter region (5-HTTLPR) polymorphism. J. Neurosci. 2009, 29, 6229–6233. [Google Scholar] [CrossRef]
- Jonassen, R.; Endestad, T.; Neumeister, A.; Foss Haug, K.B.; Berg, J.P.; Landro, N.I. The effects of the serotonin transporter polymorphism and age on frontal white matter integrity in healthy adult women. Front. Hum. Neurosci. 2012, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Rigoard, P.; Buffenoir, K.; Jaafari, N.; Giot, J.P.; Houeto, J.L.; Mertens, P.; Velut, S.; Bataille, B. The accumbofrontal fasciculus in the human brain: A microsurgical anatomical study. Neurosurgery 2011, 68, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Peper, J.S.; Mandl, R.C.; Braams, B.R.; de Water, E.; Heijboer, A.C.; Koolschijn, P.C.M.; Crone, E.A. Delay discounting and frontostriatal fiber tracts: A combined DTI and MTR study on impulsive choices in healthy young adults. Cereb. Cortex 2013, 23, 1695–1702. [Google Scholar] [CrossRef] [PubMed]
- Achterberg, M.; Peper, J.S.; van Duijvenvoorde, A.C.; Mandl, R.C.; Crone, E.A. Frontostriatal White Matter Integrity Predicts Development of Delay of Gratification: A Longitudinal Study. J. Neurosci. 2016, 36, 1954–1961. [Google Scholar] [CrossRef] [PubMed]
- Olson, E.A.; Collins, P.F.; Hooper, C.J.; Muetzel, R.; Lim, K.O.; Luciana, M. White matter integrity predicts delay discounting behavior in 9- to 23-year-olds: A diffusion tensor imaging study. J. Cogn. Neurosci. 2009, 21, 1406–1421. [Google Scholar] [CrossRef]
- van den Bos, W.; Rodriguez, C.A.; Schweitzer, J.B.; McClure, S.M. Connectivity strength of dissociable striatal tracts predict individual differences in temporal discounting. J. Neurosci. 2014, 34, 10298–10310. [Google Scholar] [CrossRef]
- Hampton, W.H.; Alm, K.H.; Venkatraman, V.; Nugiel, T.; Olson, I.R. Dissociable frontostriatal white matter connectivity underlies reward and motor impulsivity. Neuroimage 2017, 150, 336–343. [Google Scholar] [CrossRef]
- Adhikari, A.; Lerner, T.N.; Finkelstein, J.; Pak, S.; Jennings, J.H.; Davidson, T.J.; Ferenczi, E.; Gunaydin, L.A.; Mirzabekov, J.J.; Ye, L.; et al. Basomedial amygdala mediates top-down control of anxiety and fear. Nature 2015, 527, 179–185. [Google Scholar] [CrossRef]
- Karlsgodt, K.H.; John, M.; Ikuta, T.; Rigoard, P.; Peters, B.D.; Derosse, P.; Malhotra, A.K.; Szeszko, P.R. The accumbofrontal tract: Diffusion tensor imaging characterization and developmental change from childhood to adulthood. Hum. Brain Mapp. 2015, 36, 4954–4963. [Google Scholar] [CrossRef]
- Heinz, A.; Smolka, M.N.; Braus, D.F.; Wrase, J.; Beck, A.; Flor, H.; Mann, K.; Schumann, G.; Büchel, C.; Hariri, A.R.; et al. Serotonin transporter genotype (5-HTTLPR): Effects of neutral and undefined conditions on amygdala activation. Biol. Psychiatry 2007, 61, 1011–1014. [Google Scholar] [CrossRef]
- Hariri, A.R.; Mattay, V.S.; Tessitore, A.; Kolachana, B.; Fera, F.; Goldman, D.; Egan, M.F.; Weinberger, D.R. Serotonin transporter genetic variation and the response of the human amygdala. Science 2002, 297, 400–403. [Google Scholar] [CrossRef]
- Pezawas, L.; Meyer-Lindenberg, A.; Drabant, E.M.; Verchinski, B.A.; Munoz, K.E.; Kolachana, B.S.; Egan, M.F.; Mattay, V.S.; Hariri, A.R.; Weinberger, D.R. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: A genetic susceptibility mechanism for depression. Nat. Neurosci. 2005, 8, 828–834. [Google Scholar] [CrossRef]
- Hu, X.Z.; Lipsky, R.H.; Zhu, G.; Akhtar, L.A.; Taubman, J.; Greenberg, B.D.; Xu, K.; Arnold, P.D.; Richter, M.A.; Kennedy, J.L.; et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am. J. Hum. Genet. 2006, 78, 815–826. [Google Scholar] [CrossRef]
- Wendland, J.R.; Moya, P.R.; Kruse, M.R.; Ren-Patterson, R.F.; Jensen, C.L.; Timpano, K.R.; Murphy, D.L. A novel, putative gain-of-function haplotype at SLC6A4 associates with obsessive-compulsive disorder. Hum. Mol. Genet. 2008, 17, 717–723. [Google Scholar] [CrossRef]
- Goldman, N.; Glei, D.A.; Lin, Y.H.; Weinstein, M. The serotonin transporter polymorphism (5-HTTLPR): Allelic variation and links with depressive symptoms. Depress. Anxiety 2010, 27, 260–269. [Google Scholar] [CrossRef]
- Neumeister, A.; Hu, X.-Z.; Luckenbaugh, D.A.; Schwarz, M.; Nugent, A.C.; Bonne, O.; Herscovitch, P.; Goldman, D.; Drevets, W.C.; Charney, D.S. Differential effects of 5-HTTLPR genotypes on the behavioral and neural responses to tryptophan depletion in patients with major depression and controls. Arch. Gen. Psychiatry 2006, 63, 978–986. [Google Scholar] [CrossRef]
- Klucken, T.; Tapia Leon, I.; Blecker, C.; Kruse, O.; Stalder, T.; Stark, R. Failure to Replicate the Association Between Fractional Anisotropy and the Serotonin Transporter Gene (5-HTTLPR, rs25531). Front. Behav. Neurosci. 2018, 12, 80. [Google Scholar] [CrossRef]
- Comings, D.E.; MacMurray, J.P. Molecular heterosis: A review. Mol. Genet. Metab. 2000, 71, 19–31. [Google Scholar] [CrossRef] [PubMed]
- van Dyck, C.H.; Malison, R.T.; Staley, J.K.; Jacobsen, L.K.; Seibyl, J.P.; Laruelle, M.; Baldwin, R.M.; Innis, R.B.; Gelernter, J. Central serotonin transporter availability measured with [123I]beta-CIT SPECT in relation to serotonin transporter genotype. Am. J. Psychiatry 2004, 161, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Little, K.Y.; McLaughlin, D.P.; Zhang, L.; Livermore, C.S.; Dalack, G.W.; McFinton, P.R.; DelProposto, Z.S.; Hill, E.; Cassin, B.J.; Watson, S.J.; et al. Cocaine, ethanol, and genotype effects on human midbrain serotonin transporter binding sites and mRNA levels. Am. J. Psychiatry 1998, 155, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Malmberg, K.; Wargelius, H.L.; Lichtenstein, P.; Oreland, L.; Larsson, J.O. ADHD and Disruptive Behavior scores—Associations with MAO-A and 5-HTT genes and with platelet MAO-B activity in adolescents. BMC Psychiatry 2008, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Steffens, D.C.; Taylor, W.D.; McQuoid, D.R.; Krishnan, K.R. Short/long heterozygotes at 5HTTLPR and white matter lesions in geriatric depression. Int. J. Geriatr. Psychiatry 2008, 23, 244–248. [Google Scholar] [CrossRef]
- Westerhausen, R.; Kreuder, F.; Sequeira, S.D.S.; Walter, C.; Woerner, W.; Wittling, R.A.; Schweiger, E.; Wittling, W. Effects of handedness and gender on macro- and microstructure of the corpus callosum and its subregions: A combined high-resolution and diffusion-tensor MRI study. Brain Res. Cogn. Brain Res. 2004, 21, 418–426. [Google Scholar] [CrossRef] [PubMed]
- van Hemmen, J.; Saris, I.M.J.; Cohen-Kettenis, P.T.; Veltman, D.J.; Pouwels, P.J.W.; Bakker, J. Sex Differences in White Matter Microstructure in the Human Brain Predominantly Reflect Differences in Sex Hormone Exposure. Cereb. Cortex 2017, 27, 2994–3001. [Google Scholar] [CrossRef] [PubMed]
- Menzler, K.; Belke, M.; Wehrmann, E.; Krakow, K.; Lengler, U.; Jansen, A.; Hamer, H.; Oertel, W.; Rosenow, F.; Knake, S. Men and women are different: Diffusion tensor imaging reveals sexual dimorphism in the microstructure of the thalamus, corpus callosum and cingulum. Neuroimage 2011, 54, 2557–2562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Schneider, T.; Wheeler-Kingshott, C.A.; Alexander, D.C. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 2012, 61, 1000–1016. [Google Scholar] [CrossRef] [PubMed]
- Deza-Araujo, Y.I.; Neukam, P.T.; Marxen, M.; Müller, D.K.; Henle, T.; Smolka, M.N. Acute tryptophan loading decreases functional connectivity between the default mode network and emotion-related brain regions. Hum. Brain Mapp. 2019, 40, 1844–1855. [Google Scholar] [CrossRef]
- Kroemer, N.B.; Lee, Y.; Pooseh, S.; Eppinger, B.; Goschke, T.; Smolka, M.N. L-DOPA reduces model-free control of behavior by attenuating the transfer of value to action. Neuroimage 2019, 186, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Neukam, P.T.; Deza-Araujo, Y.I.; Marxen, M.; Pooseh, S.; Rietschel, M.; Schwarzenbolz, U.; Smolka, M.N. No evidence for the involvement of serotonin or the 5-HTTLPR genotype in intertemporal choice in a larger community sample. J. Psychopharmacol. 2019, 33, 1377–1387. [Google Scholar] [CrossRef]
- Petzold, J.; Kienast, A.; Lee, Y.; Pooseh, S.; London, E.D.; Goschke, T.; Smolka, M.N. Baseline impulsivity may moderate L-DOPA effects on value-based decision-making. Sci. Rep. 2019, 9, 5652. [Google Scholar] [CrossRef]
- Petzold, J.; Lee, Y.; Pooseh, S.; Oehme, L.; Beuthien-Baumann, B.; London, E.D.; Goschke, T.; Smolka, M.N. Presynaptic dopamine function measured with [(18)F]fluorodopa and L-DOPA effects on impulsive choice. Sci. Rep. 2019, 9, 17927. [Google Scholar] [CrossRef]
- Pooseh, S.; Bernhardt, N.; Guevara, A.; Huys, Q.J.M.; Smolka, M.N. Value-based decision-making battery: A Bayesian adaptive approach to assess impulsive and risky behavior. Behav. Res. Methods 2018, 50, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Pelli, D.G. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spat. Vis. 1997, 10, 437–442. [Google Scholar] [CrossRef]
- Brainard, D.H. The Psychophysics Toolbox. Spat. Vis. 1997, 10, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Dukal, H.; Frank, J.; Lang, M.; Treutlein, J.; Gilles, M.; Wolf, I.A.; Krumm, B.; Massart, R.; Szyf, M.; Laucht, M.; et al. New-born females show higher stress- and genotype-independent methylation of SLC6A4 than males. Borderline Personal. Disord. Emot. Dysregul. 2015, 2, 8. [Google Scholar] [CrossRef]
- Andersson, J.L.R.; Sotiropoulos, S.N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 2016, 125, 1063–1078. [Google Scholar] [CrossRef]
- Andersson, J.L.R.; Graham, M.S.; Zsoldos, E.; Sotiropoulos, S.N. Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. Neuroimage 2016, 141, 556–572. [Google Scholar] [CrossRef]
- Müller, D.K.; Küttner, R.; Hannig, R.; Frank, T.; Müller, J.; Marxen, M. NICePype: A Web-based pipeline manager for processing neuroimaging data based on Nipype. In Proceedings of the 23rd Annual Meeting of the Proceedings of the International Society for Magnetic Resonance in Medicine Conference, Toronto, ON, Canada, 30 May–5 June 2015. [Google Scholar]
- Leemans, A.; Jeurissen, B.; Sijbers, J.; Jones, D.K. ExploreDTI: A Graphical Toolbox for Processing, Analyzing, and Visualizing Diffusion MR Data; International Society for Magnetic Resonance in Medicine: Honolulu, HI, USA, 2009. [Google Scholar]
- Chang, L.C.; Jones, D.K.; Pierpaoli, C. RESTORE: Robust estimation of tensors by outlier rejection. Magn. Reson. Med. 2005, 53, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, D.J.; Krafft, C.E.; Schwarz, N.F.; Chi, L.; Rodrigue, A.L.; Pierce, J.E.; Allison, J.D.; Yanasak, N.E.; Liu, T.; Davis, C.L.; et al. The relationship between uncinate fasciculus white matter integrity and verbal memory proficiency in children. Neuroreport 2014, 25, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Hua, K.; Zhang, J.; Wakana, S.; Jiang, H.; Li, X.; Reich, D.S.; Calabresi, P.A.; Pekar, J.J.; van Zijl, P.C.; Mori, S. Tract probability maps in stereotaxic spaces: Analyses of white matter anatomy and tract-specific quantification. Neuroimage 2008, 39, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Tzoutio-Mazoyera, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Tzourio-Mazoyer, B.; Joliot, M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002, 15, 273–289. [Google Scholar] [CrossRef]
- TrackVis; Martinos Center for Biomedical Imaging, Massachusetts General Hospital: Boston, MA, USA, 2015; Available online: www.trackvis.org (accessed on 19 May 2017).
- Smith, S.M.; Jenkinson, M.; Johansen-Berg, H.; Rueckert, D.; Nichols, T.E.; Mackay, C.E.; Watkins, K.E.; Ciccarelli, O.; Cader, M.Z.; Matthews, P.M.; et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage 2006, 31, 1487–1505. [Google Scholar] [CrossRef]

| N (Females) | Age (SD) | |
|---|---|---|
| S/S | 39 (14) | 32.3 (5.9) |
| S/L | 56 (12) | 34.8 (4.3) |
| L/L | 80 (35) | 32.7 (5.6) |
| Statistic | χ2 = 7.252, p = 0.027 | F2,169 = 3.027, p = 0.051 |
| S/S | L/L | Contrast Estimate | p-Value | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Frontostriatal | ||||||
| FA | 0.50 | 0.02 | 0.50 | 0.02 | −3.80 × 10−3 | 0.369 |
| AD | 1.23 × 10−3 | 2.22 × 10−5 | 1.23 × 10−3 | 2.22 × 10−5 | −9.40 × 10−6 | 0.060 |
| MD | 7.63 × 10−4 | 2.00 × 10−5 | 7.62 × 10−4 | 2.13 × 10−5 | −2.16 × 10−6 | 0.611 |
| RD | 5.28 × 10−4 | 2.40 × 10−5 | 5.31 × 10−4 | 2.85 × 10−5 | 1.46 × 10−6 | 0.780 |
| Uncinate | ||||||
| FA | 0.47 | 0.01 | 0.46 | 0.02 | −0.004 | 0.278 |
| AD | 1.21 × 10−3 | 2.44 × 10−5 | 1.21 × 10−3 | 2.15 × 10−5 | −1.76 × 10−6 | 0.715 |
| MD | 7.76 × 10−4 | 1.95 × 10−5 | 7.79 × 10−4 | 2.08 × 10−5 | 1.944 × 10−6 | 0.639 |
| RD | 5.59 × 10−4 | 2.08 × 10−5 | 5.63 × 10−4 | 2.50 × 10−5 | 3.797 × 10−6 | 0.407 |
| S/S | S/L | L/L | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | (SD) | N | Mean | (SD) | N | Mean | (SD) | N | |
| Frontostriatal | |||||||||
| Males | |||||||||
| FA | 0.503 | (0.020) | 25 | 0.511 | (0.016) | 44 | 0.503 | 0.021 | 45 |
| AD | 1.23 × 10−3 | (2.53 × 10−5) | 25 | 1.22 × 10−3 | (3.18 × 10−5) | 44 | 1.22 × 10−3 | (2.40 × 10−5) | 45 |
| MD | 7.60 × 10−4 | (2.30 × 10−5) | 25 | 7.48 × 10−4 | (2.00 × 10−5) | 44 | 7.58 × 10−4 | (2.03 × 10−5) | 45 |
| RD | 5.26 × 10−4 | (2.70 × 10−5) | 25 | 5.13 × 10−4 | (1.92 × 10−5) | 44 | 5.26 × 10−4 | (2.58 × 10−5) | 45 |
| Females | |||||||||
| FA | 0.503 | (0.016) | 14 | 0.502 | (0.026) | 12 | 0.494 | (0.026) | 35 |
| AD | 1.24 × 10−3 | (1.20 × 10−5) | 14 | 1.22 × 10−3 | (2.37 × 10−5) | 12 | 1.23 × 10−3 | (1.96 × 10−5) | 35 |
| MD | 7.68 × 10−4 | (1.22 × 10−5) | 14 | 7.53 × 10−4 | (2.95 × 10−5) | 12 | 7.68 × 10−4 | (2.16 × 10−5) | 35 |
| RD | 5.32 × 10−4 | (1.78 × 10−5) | 14 | 5.22 × 10−4 | (3.53 × 10−5) | 12 | 5.37 × 10−4 | (3.08 × 10−5) | 35 |
| Total | |||||||||
| FA | 0.503 | (0.018) | 39 | 0.509 | (0.018) | 56 | 0.499 | (0.024) | 80 |
| AD | 1.23 × 10−3 | (2.22 × 10−5) | 39 | 1.21 × 10−3 | (3.01 × 10−5) | 56 | 1.23 × 10−3 | (2.22 × 10−5) | 80 |
| MD | 7.63 × 10−4 | (2.00 × 10−5) | 39 | 7.49 × 10−4 | (2.22 × 10−5) | 56 | 7.62 × 10−4 | (2.13 × 10−5) | 80 |
| RD | 5.28 × 10−4 | (2.40 × 10−5) | 39 | 5.15 × 10−4 | (2.35 × 10−5) | 56 | 5.31 × 10−4 | (2.85 × 10−5) | 80 |
| Uncinate | |||||||||
| Males | |||||||||
| FA | 0.470 | (0.015) | 25 | 0.471 | (0.010) | 44 | 0.466 | (0.018) | 45 |
| AD | 1.20 × 10−3 | (2.14 × 10−5) | 25 | 1.20 × 10−3 | (3.07 × 10−5) | 44 | 1.21 × 10−3 | (2.28 × 10−5) | 45 |
| MD | 7.68 × 10−4 | (1.85 × 10−5) | 25 | 7.67 × 10−4 | (2.02 × 10−5) | 44 | 7.75 × 10−4 | (1.95 × 10−5) | 45 |
| RD | 5.52 × 10−4 | (2.09 × 10−5) | 25 | 5.51 × 10−4 | (1.72 × 10−5) | 44 | 5.60 × 10−4 | (2.28 × 10−5) | 45 |
| Females | |||||||||
| FA | 0.465 | (0.014) | 14 | 0.468 | (0.023) | 12 | 0.461 | (0.021) | 35 |
| AD | 1.23 × 10−3 | (1.97 × 10−5) | 14 | 1.20 × 10−3 | (2.27 × 10−5) | 12 | 1.21 × 10−3 | (1.97 × 10−5) | 35 |
| MD | 7.90 × 10−4 | (1.21 × 10−5) | 14 | 7.71 × 10−4 | (2.90 × 10−5) | 12 | 7.83 × 10−4 | (2.19 × 10−5) | 35 |
| RD | 5.71 × 10−4 | (1.42 × 10−5) | 14 | 5.55 × 10−4 | (3.35 × 10−5) | 12 | 5.68 × 10−4 | (2.71 × 10−5) | 35 |
| Total | |||||||||
| FA | 0.468 | (0.015) | 39 | 0.471 | (0.013) | 56 | 0.464 | (0.019) | 80 |
| AD | 1.21 × 10−3 | (2.44 × 10−5) | 39 | 1.20 × 10−3 | (2.90 × 10−5) | 56 | 1.21 × 10−3 | (2.15 × 10−5) | 80 |
| MD | 7.76 × 10−4 | (1.95 × 10−5) | 39 | 7.68 × 10−4 | (2.21 × 10−5) | 56 | 7.79 × 10−4 | (2.08 × 10−5) | 80 |
| RD | 5.59 × 10−4 | (2.08 × 10−5) | 39 | 5.52 × 10−4 | (2.14 × 10−5) | 56 | 5.63 × 10−4 | (2.50 × 10−5) | 80 |
| Tracts | Side | Peak Voxel (MNI) | F-Statistic (TFCE) | Cluster Size > 100 (Voxels) | Cluster p-Value | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| FA | |||||||
| SFOF | Left | −22 | −2 | 19 | 19.5 | 10,610 | 0.001 |
| ILF | Right | 45 | −11 | −27 | 14.2 | 6452 | 0.003 |
| UNC, IFOF | Right | 18 | 24 | −12 | 13.8 | 669 | 0.015 |
| AD | |||||||
| Unclassified | Left | −10 | −1 | −14 | 17.3 | 12,958 | 0.001 |
| ILF | Right | 40 | −22 | −21 | 11.5 | 1739 | 0.014 |
| Forceps minor | Right | 12 | 31 | 8 | 10 | 1522 | 0.028 |
| UNC, IFOF | Right | 28 | 14 | −10 | 9.84 | 786 | 0.03 |
| Unclassified | Right | 1 | 10 | 14 | 7.64 | 188 | 0.047 |
| Forceps minor | Left | −12 | 29 | −12 | 9.1 | 157 | 0.042 |
| SLF | Left | −34 | −37 | 21 | 10.3 | 141 | 0.038 |
| ATR, IFOF | Right | 23 | 26 | 23 | 5.94 | 141 | 0.047 |
| MD | |||||||
| ATR | Left | −11 | −17 | −2 | 18.1 | 31,113 | 0.001 |
| RD | |||||||
| ATR | Left | −23 | −2 | 17 | 18.8 | 14,709 | 0.001 |
| ILF | Right | 45 | −10 | −28 | 15.3 | 6641 | 0.004 |
| UNC, IFOF | Right | 18 | 24 | −12 | 13.4 | 209 | 0.039 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neukam, P.T.; Müller, D.K.; Deza-Lougovski, Y.I.; Pooseh, S.; Witt, S.H.; Rietschel, M.; Smolka, M.N. Connection Failure: Differences in White Matter Microstructure Are Associated with 5-HTTLPR but Not with Risk Seeking for Losses. Int. J. Mol. Sci. 2024, 25, 6666. https://doi.org/10.3390/ijms25126666
Neukam PT, Müller DK, Deza-Lougovski YI, Pooseh S, Witt SH, Rietschel M, Smolka MN. Connection Failure: Differences in White Matter Microstructure Are Associated with 5-HTTLPR but Not with Risk Seeking for Losses. International Journal of Molecular Sciences. 2024; 25(12):6666. https://doi.org/10.3390/ijms25126666
Chicago/Turabian StyleNeukam, Philipp T., Dirk K. Müller, Yacila I. Deza-Lougovski, Shakoor Pooseh, Stephanie H. Witt, Marcella Rietschel, and Michael N. Smolka. 2024. "Connection Failure: Differences in White Matter Microstructure Are Associated with 5-HTTLPR but Not with Risk Seeking for Losses" International Journal of Molecular Sciences 25, no. 12: 6666. https://doi.org/10.3390/ijms25126666
APA StyleNeukam, P. T., Müller, D. K., Deza-Lougovski, Y. I., Pooseh, S., Witt, S. H., Rietschel, M., & Smolka, M. N. (2024). Connection Failure: Differences in White Matter Microstructure Are Associated with 5-HTTLPR but Not with Risk Seeking for Losses. International Journal of Molecular Sciences, 25(12), 6666. https://doi.org/10.3390/ijms25126666





