The Stylo Cysteine-Rich Peptide SgSnakin1 Is Involved in Aluminum Tolerance through Enhancing Reactive Oxygen Species Scavenging
Abstract
1. Introduction
2. Results
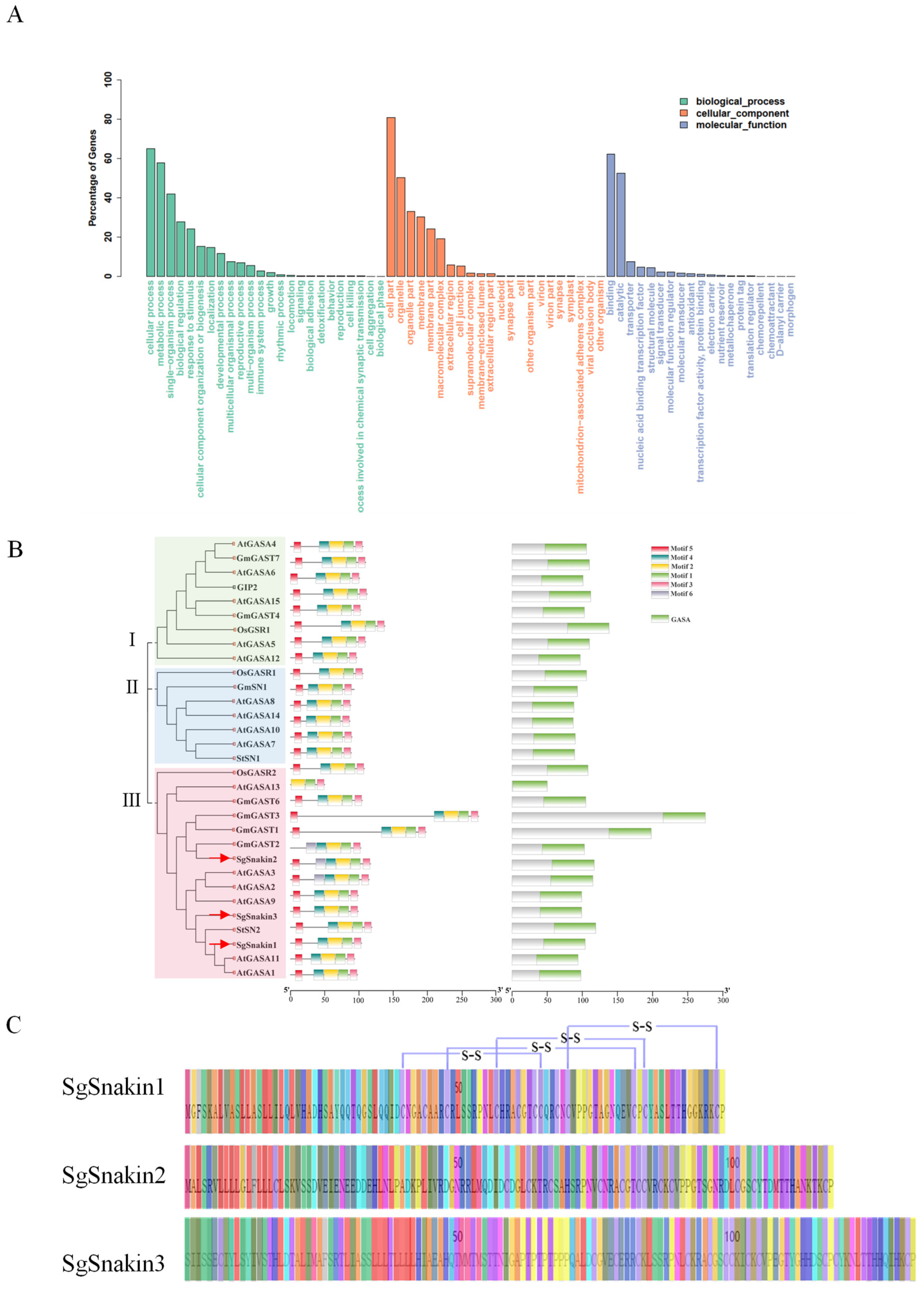
2.1. Full-Length Transcriptome Sequencing for Stylo Root Tips
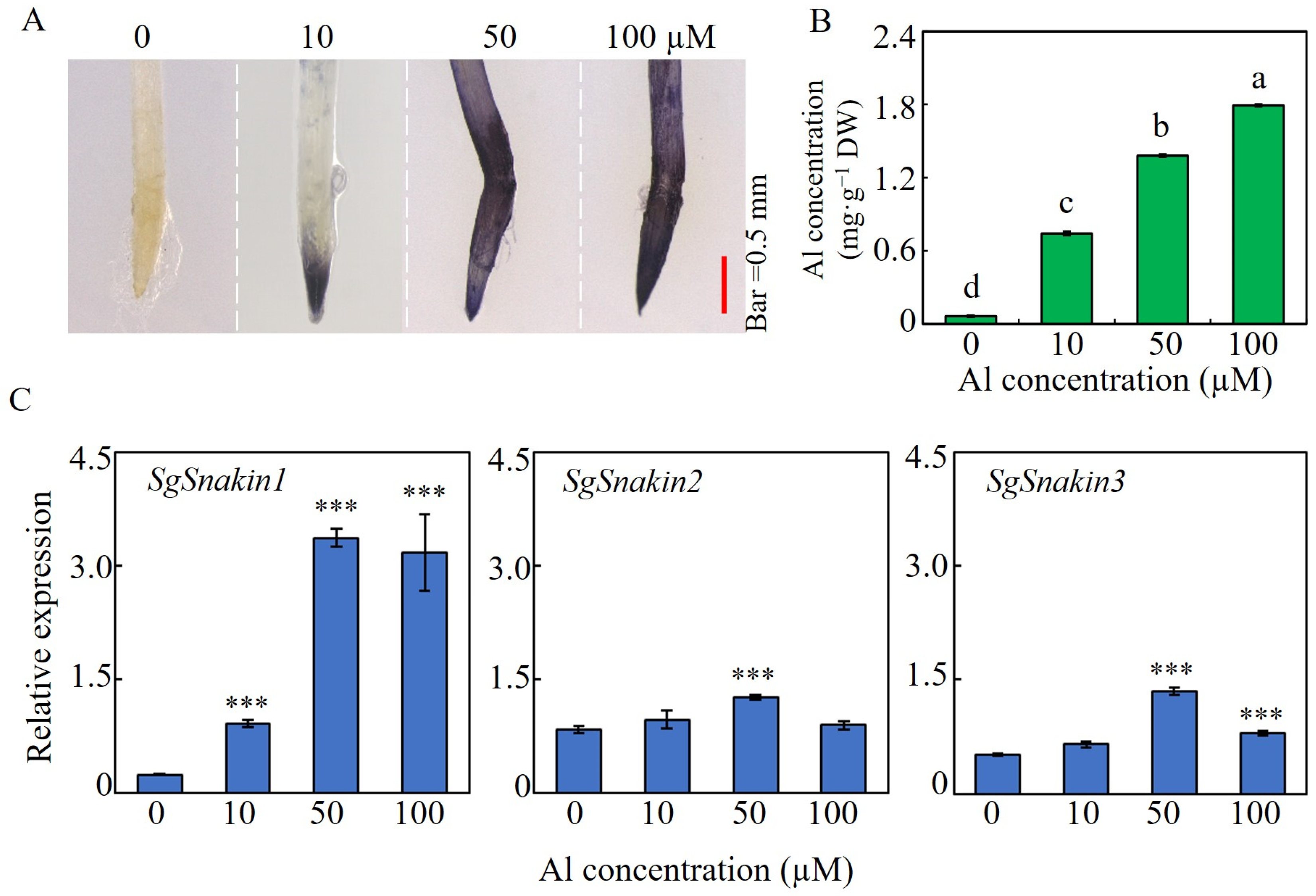
2.2. Duration of Al Treatment Affected Stylo Root Growth, Al Accumulation, and the Expressions of Three SgSnakins
2.3. Root Growth, Al Accumulation, and the Expressions of Three SgSnakins in Response to Al Concentration
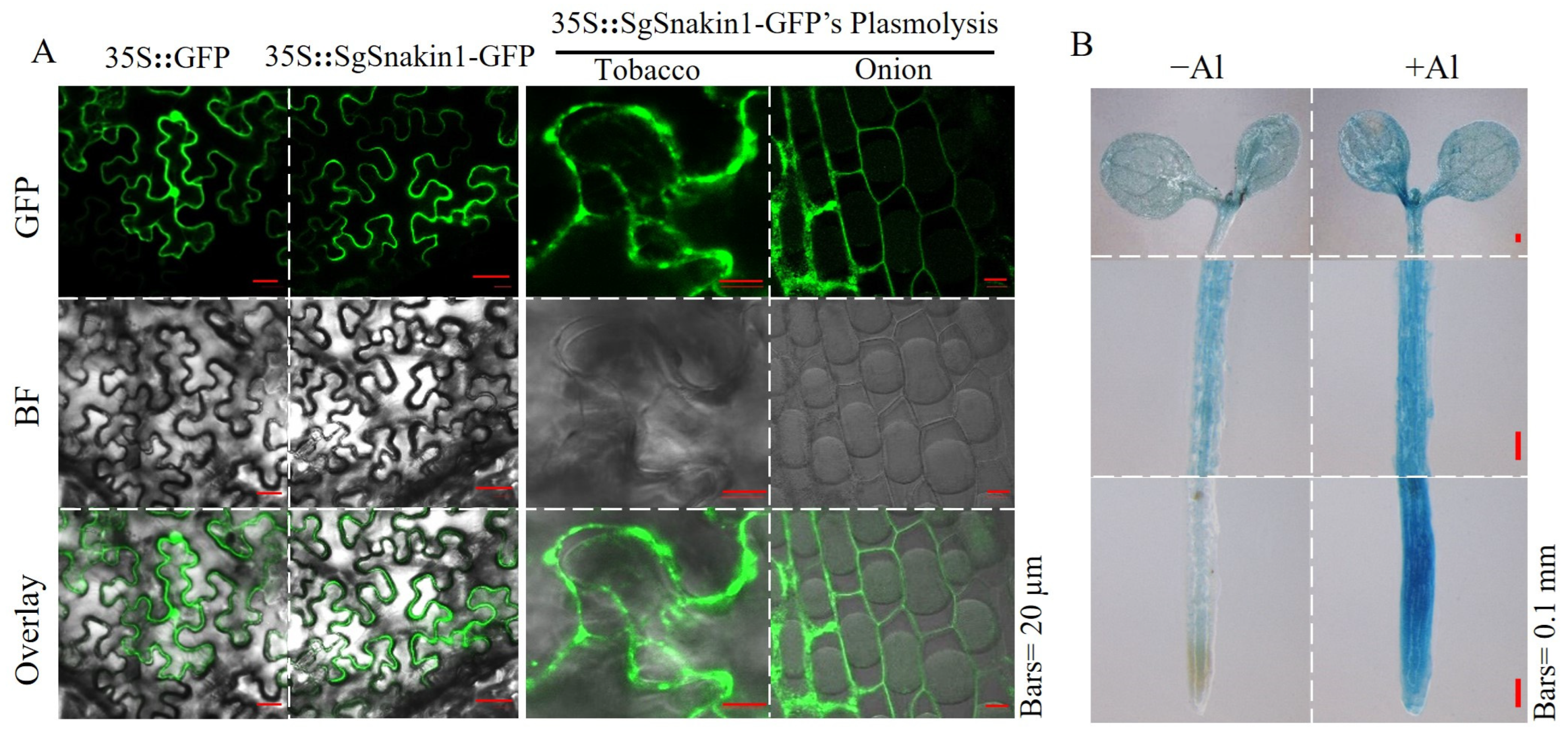
2.4. Subcellular and Histochemical Localization of SgSnakin1
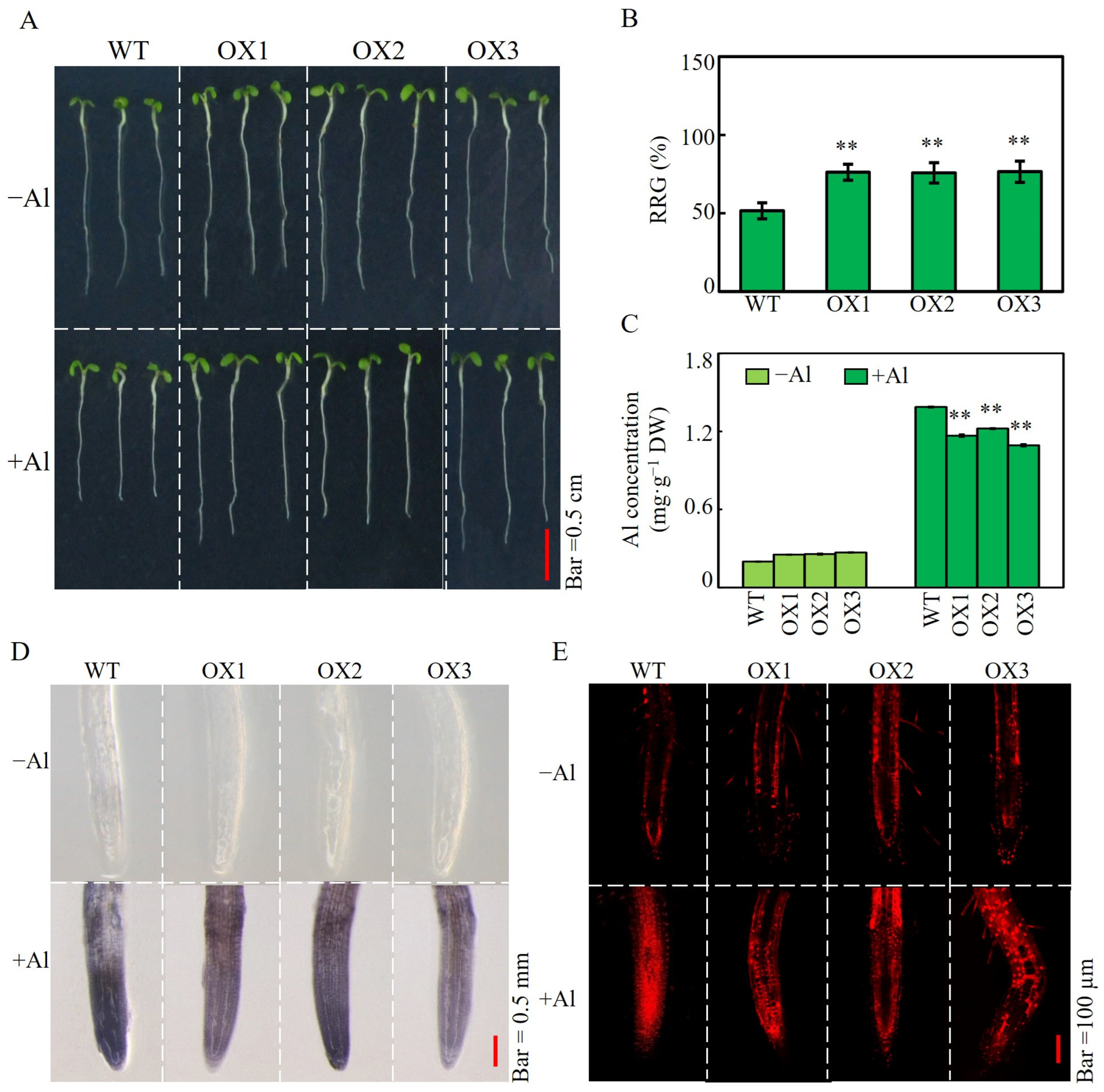
2.5. Effects of SgSnakin1 Overexpression on Al Tolerance of Transgenic Arabidopsis
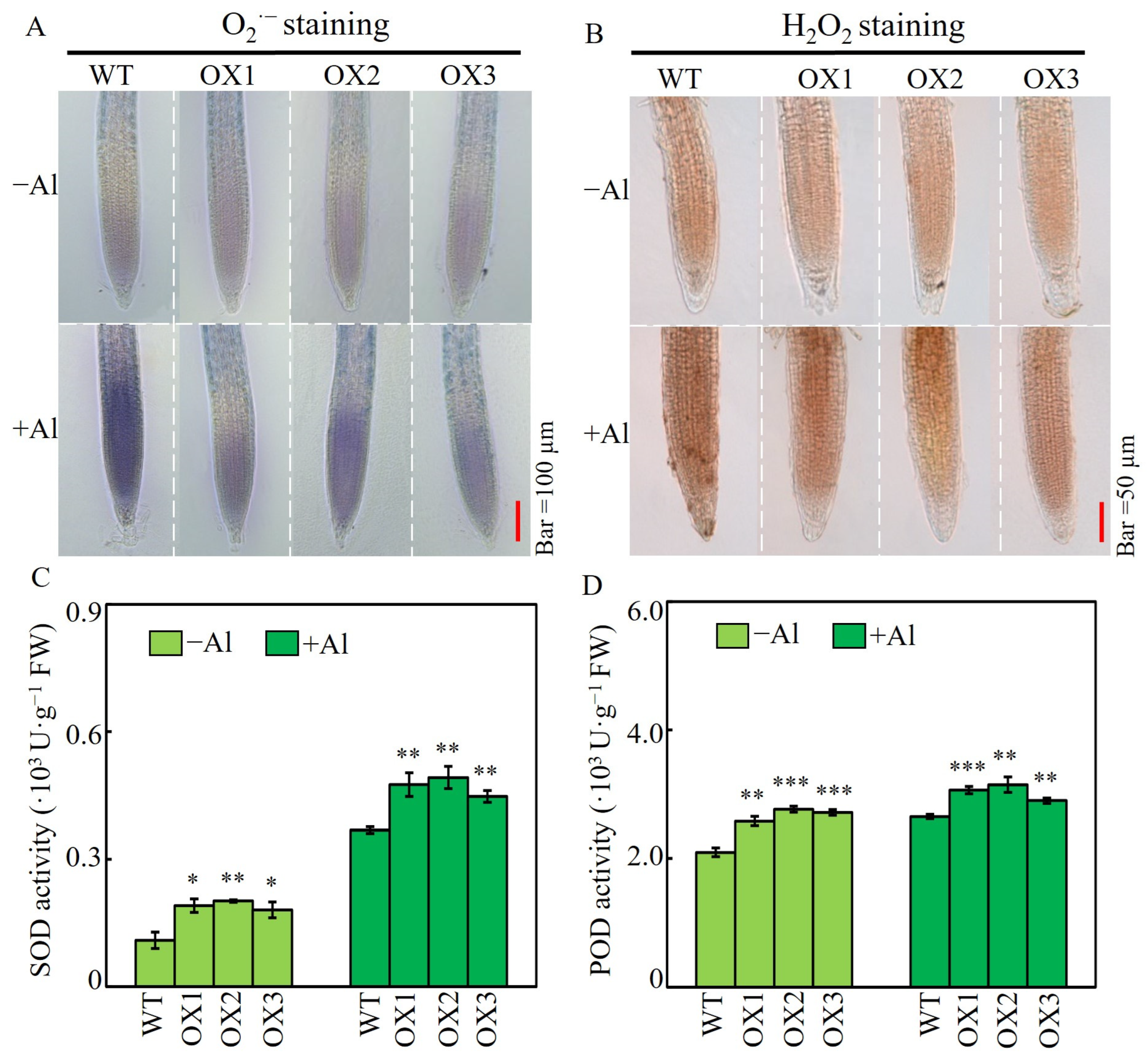
2.6. Effects of SgSnakin1 Overexpression on Root ROS Accumulation and Activities of SOD and POD in Response to Al Treatment
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Determination of Root Tips Al Accumulation
4.3. Full-Length Transcriptome Sequencing and Quantitative Real-Time PCR
4.4. Subcellular Localization of SgSnakin1
4.5. Histochemical Localization of SgSnakin1
4.6. Effects of SgSnakin1 Overexpression on Al Tolerance of Transgenic Arabidopsis
4.7. NBT and DAB Staining and Determination of SOD and POD Activities
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ranjan, A.; Sinha, R.; Sharma, T.R.; Pattanayak, A.; Singh, A.K. Alleviating aluminum toxicity in plants: Implications of reactive oxygen species signaling and crosstalk with other signaling pathways. Physiol. Plant. 2021, 173, 1765–1784. [Google Scholar] [CrossRef] [PubMed]
- Bojorquez-Quintal, E.; Escalante-Magana, C.; Echevarria-Machado, I.; Martinez-Estevez, M. Aluminum, a friend or foe of higher plants in acid soils. Front. Plant Sci. 2017, 8, 1767. [Google Scholar] [CrossRef] [PubMed]
- Kochian, L.V.; Pineros, M.A.; Liu, J.; Magalhaes, J.V. Plant adaptation to acid soils: The molecular basis for crop aluminum resistance. Annu. Rev. Plant Biol. 2015, 66, 571–598. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I.; Horst, W.J. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol. Plant. 1991, 83, 463–468. [Google Scholar] [CrossRef]
- Sivaguru, M.; Fujiwara, T.; Šamaj, J.; Baluška, F.; Yang, Z.; Osawa, H.; Maeda, T.; Mori, T.; Volkmann, D.; Matsumoto, H. Aluminum-induced 1→3-β-d-glucan inhibits cell-to-cell trafficking of molecules through plasmodesmata. A new mechanism of aluminum toxicity in plants. Plant Physiol. 2000, 124, 991–1006. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.L.; Blancaflor, E.B.; Kochian, L.V.; Gilroy, S. Spatial coordination of aluminium uptake, production of reactive oxygen species, callose production and wall rigidification in maize roots. Plant Cell Environ. 2006, 29, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Sivaguru, M.; Liu, J.; Kochian, L.V. Targeted expression of SbMATE in the root distal transition zone is responsible for sorghum aluminum resistance. Plant J. 2013, 76, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; De Paepe, R.; Foyer, C.H. Mitochondrial redox biology and homeostasis in plants. Trends Plant Sci. 2007, 12, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhu, M.; Chen, H. Aluminum-induced cell death in root-tip cells of barley. Environ. Exp. Bot. 2001, 46, 71–79. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kobayashi, Y.; Devi, S.R.; Rikiishi, S.; Matsumoto, H. Aluminum toxicity is associated with mitochondrial dysfunction and the production of reactive oxygen species in plant cells. Plant Physiol. 2002, 128, 63–72. [Google Scholar] [CrossRef]
- Li, Z.; Xing, D. Mitochondrial pathway leading to programmed cell death induced by aluminum phytotoxicity in Arabidopsis. Plant Signal. Behav. 2010, 5, 1660–1662. [Google Scholar] [CrossRef]
- Yang, Z.; Eticha, D.; Rao, I.M.; Horst, W.J. Alteration of cell-wall porosity is involved in osmotic stress-induced enhancement of aluminium resistance in common bean (Phaseolus vulgaris L.). J. Exp. Bot. 2010, 61, 3245–3258. [Google Scholar] [CrossRef] [PubMed]
- Šimonovičová, M.; Tamás, L.; Huttová, J.; Mistrík, I. Effect of aluminium on oxidative stress related enzymes activities in barley roots. Biol. Plant. 2004, 48, 261–266. [Google Scholar] [CrossRef]
- Ezaki, B.; Katsuhara, M.; Kawamura, M.; Matsumoto, H. Different mechanisms of four aluminum (Al)-resistant transgenes for Al toxicity in Arabidopsis. Plant Physiol. 2001, 127, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, Z.; How, J.; Xu, H.; Chen, L.; Li, K. Overexpression of a peroxidase gene (AtPrx64) of Arabidopsis thaliana in tobacco improves plant’s tolerance to aluminum stress. Plant Mol. Biol. 2017, 95, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Huang, Y.; Qu, M.; Li, Y.; Hu, X.; Yang, W.; Li, H.; He, W.; Ding, J.; Liu, C.; et al. A maize ZmAT6 gene confers aluminum tolerance via reactive oxygen species scavenging. Front. Plant Sci. 2020, 11, 1016. [Google Scholar] [CrossRef]
- Wang, P.; Yu, W.; Zhang, J.; Rengel, Z.; Xu, J.; Han, Q.; Chen, L.; Li, K.; Yu, Y.; Chen, Q. Auxin enhances aluminium-induced citrate exudation through upregulation of GmMATE and activation of the plasma membrane H+-ATPase in soybean roots. Ann. Bot. 2016, 118, 933–940. [Google Scholar] [CrossRef]
- Su, T.; Han, M.; Cao, D.; Xu, M. Molecular and biological properties of snakins: The foremost cysteine-rich plant host defense peptides. J. Fungi 2020, 6, 220. [Google Scholar] [CrossRef]
- Segura, A.; Moreno, M.; Madueño, F.; Molina, A.; García-Olmedo, F. Snakin-1, a peptide from potato that is active against plant pathogens. Mol. Plant. Microbe. Interact. 1999, 12, 16–23. [Google Scholar] [CrossRef]
- Zimmermann, R.; Sakai, H.; Hochholdinger, F. The gibberellic acid stimulated-like gene family in maize and its role in lateral root development. Plant Physiol. 2010, 152, 356–365. [Google Scholar] [CrossRef]
- Fan, S.; Zhang, D.; Zhang, L.; Gao, C.; Xin, M.; Tahir, M.M.; Li, Y.; Ma, J.; Han, M. Comprehensive analysis of GASA family members in the Malus domestica genome: Identification, characterization, and their expressions in response to apple flower induction. BMC Genom. 2017, 18, 827. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.Z.; Sana, A.; Jamil, A.; Nasir, J.A.; Ahmed, S.; Hameed, M.U. Abdullah A genome-wide approach to the comprehensive analysis of GASA gene family in Glycine max. Plant Mol. Biol. 2019, 100, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Wang, S.; Xu, D.; Liu, X.; Li, X.; Xiao, W.; Cao, J.; Jiang, H.; Min, X.; Wang, J.; et al. Identification and analysis of the GASR gene family in common wheat (Triticum aestivum L.) and characterization of TaGASR34, a gene associated with seed dormancy and germination. Front. Genet. 2019, 10, 980. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, C.; Peng, J.; Sun, S.; Wang, X. GASA5, a regulator of flowering time and stem growth in Arabidopsis thaliana. Plant Mol. Biol. 2009, 69, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Rubinovich, L.; Weiss, D. The Arabidopsis cysteine-rich protein GASA4 promotes GA responses and exhibits redox activity in bacteria and in planta. Plant J. 2010, 64, 1018–1027. [Google Scholar] [CrossRef]
- Sun, S.; Wang, H.; Yu, H.; Zhong, C.; Zhang, X.; Peng, J.; Wang, X. GASA14 regulates leaf expansion and abiotic stress resistance by modulating reactive oxygen species accumulation. J. Exp. Bot. 2013, 64, 1637–1647. [Google Scholar] [CrossRef]
- Zhong, C.; Xu, H.; Ye, S.; Wang, S.; Li, L.; Zhang, S.; Wang, X. Gibberellic acid-stimulated Arabidopsis6 serves as an integrator of gibberellin, abscisic acid, and glucose signaling during seed germination in Arabidopsis. Plant Physiol. 2015, 169, 2288–2303. [Google Scholar]
- Qu, J.; Kang, S.G.; Hah, C.; Jang, J. Molecular and cellular characterization of GA-Stimulated Transcripts GASA4 and GASA6 in Arabidopsis thaliana. Plant Sci. 2016, 246, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Lima, M.; Benko-Iseppon, A.M.; Neto, J.R.C.F.; Rodriguez-Decuadro, S.; Kido, E.A.; Crovella, S.; Pandolfi, V. Snakin: Structure, roles and applications of a plant antimicrobial peptide. Curr. Protein Pept. Sci. 2017, 18, 368–374. [Google Scholar] [CrossRef]
- Nahirnak, V.; Rivarola, M.; Almasia, N.I.; Barrios, B.M.; Hopp, H.E.; Vile, D.; Paniego, N.; Vazquez, R.C. Snakin-1 affects reactive oxygen species and ascorbic acid levels and hormone balance in potato. PLoS ONE 2019, 14, e0214165. [Google Scholar] [CrossRef]
- Trapalis, M.; Li, S.F.; Parish, R.W. The Arabidopsis GASA10 gene encodes a cell wall protein strongly expressed in developing anthers and seeds. Plant Sci. 2017, 260, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lyu, C.; Chen, J.; Lu, Y.; Yang, S.; Ni, S.; Zheng, S.; Yu, L.; Wang, X.; Wang, Q.; et al. Snakin-2 interacts with cytosolic glyceraldehyde-3-phosphate dehydrogenase 1 to inhibit sprout growth in potato tubers. Hortic. Res. 2022, 9, uhab060. [Google Scholar] [CrossRef]
- He, H.; Yang, X.; Xun, H.; Lou, X.; Li, S.; Zhang, Z.; Jiang, L.; Dong, Y.; Wang, S.; Pang, J.; et al. Over-expression of GmSN1 enhances virus resistance in Arabidopsis and soybean. Plant Cell Rep. 2017, 36, 1441–1455. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.B.; Woo, Y.M.; Lee, D.J.; Lee, M.C.; Kim, C.S. Enhanced tolerance to heat stress in transgenic plants expressing the GASA4 gene. Plant Physiol. Biochem. 2007, 45, 722–728. [Google Scholar] [CrossRef]
- Du, Y.M.; Tian, J.; Liao, H.; Bai, C.J.; Yan, X.L.; Liu, G.D. Aluminium tolerance and high phosphorus efficiency helps Stylosanthes better adapt to low-P acid soils. Ann. Bot. 2009, 103, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liang, C.; Chen, Z.; Liu, P.; Tian, J.; Liu, G.; Liao, H. Superior aluminium (Al) tolerance of Stylosanthes is achieved mainly by malate synthesis through an Al-enhanced malic enzyme, SgME1. New Phytol. 2014, 202, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Liu, L.; Li, X.; Han, R.; Wei, Y.; Yu, Y. Insights into aluminum-tolerance pathways in Stylosanthes as revealed by RNA-Seq analysis. Sci. Rep. 2018, 8, 6072. [Google Scholar] [CrossRef] [PubMed]
- Čiamporová, M. Morphological and structural responses of plant roots to aluminium at organ, tissue, and cellular levels. Biol. Plant. 2002, 45, 161–171. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Moore, K.L.; Lombi, E.; Gianoncelli, A.; Ferguson, B.J.; Blamey, F.P.; Menzies, N.W.; Nicholson, T.M.; McKenna, B.A.; Wang, P.; et al. Identification of the primary lesion of toxic aluminum in plant roots. Plant Physiol. 2015, 167, 1402–1411. [Google Scholar] [CrossRef]
- Siecinska, J.; Nosalewicz, A. Aluminium toxicity to plants as influenced by the properties of the root growth environment affected by other co-stressors: A review. In Reviews of Environmental Contamination and Toxicology; Springer: Cham, Switzerland, 2017; Volume 243, pp. 1–26. [Google Scholar] [CrossRef]
- Ghorbani, A.; Emamverdian, A.; Pehlivan, N.; Zargar, M.; Razavi, S.M.; Chen, M. Nano-enabled agrochemicals: Mitigating heavy metal toxicity and enhancing crop adaptability for sustainable crop production. J. Nanobiotechnol. 2024, 22, 91. [Google Scholar]
- Liu, G.D. Status of Stylosanthes development in other countries. II Stylosanthes development and utilization in China and south-east Asia. Trop. Grassl. 1997, 31, 460–466. [Google Scholar]
- Miller, C.P.; Rains, J.P.; Shaw, K.A.; Middleton, C.H. Commercial development of Stylosanthes pastures in northern Australia. II. Stylosanthes in the northern Australian beef industry. Trop. Grassl. 1997, 31, 509–514. [Google Scholar]
- Kochian, L.V.; Hoekenga, O.A.; Pineros, M.A. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu. Rev. Plant Biol. 2004, 55, 459–493. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Zuo, F.H.; Ling, G.Z.; Li, Y.Y.; Yu, Y.X.; Yang, P.Q.; Tang, X.L. Secretion of citrate from roots in response to aluminum and low phosphorus stresses in Stylosanthes. Plant Soil 2009, 325, 219–229. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, L.; Liu, P.; Liu, G.; Tian, J.; Liao, H. Malate synthesis and secretion mediated by a manganese-enhanced malate dehydrogenase confers superior manganese tolerance in Stylosanthes guianensis. Plant Physiol. 2014, 167, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Nahirnak, V.; Almasia, N.I.; Fernandez, P.V.; Hopp, H.E.; Estevez, J.M.; Carrari, F.; Vazquez-Rovere, C. Potato snakin-1 gene silencing affects cell division, primary metabolism, and cell wall composition. Plant Physiol. 2012, 158, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Herzog, M.; Dorne, A.M.; Grellet, F. GASA, a gibberellin-regulated gene family from Arabidopsis thaliana related to the tomato GAST1 gene. Plant Mol. Biol. 1995, 27, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, T.; Sakaguchi, N.; Shimada, H. Two OsGASR genes, rice GAST homologue genes that are abundant in proliferating tissues, show different expression patterns in developing panicles. Genes Genet. Syst. 2006, 81, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Almasia, N.I.; Nahirnak, V.; Hopp, H.E.; Vazquez-Rovere, C. Potato Snakin-1: An antimicrobial player of the trade-off between host defense and development. Plant Cell Rep. 2020, 39, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Peng, J.; Zhang, J.; Ran, S.; Cai, C.; Yu, L.; Ni, S.; Huang, X.; Li, L.; Wang, X. The cysteine-rich peptide snakin-2 negatively regulates tubers sprouting through modulating lignin biosynthesis and H2O2 accumulation in potato. Int. J. Mol. Sci. 2021, 22, 2287. [Google Scholar] [CrossRef]
- Shi, L.; Gast, R.T.; Gopalraj, M.; Olszewski, N.E. Characterization of a shoot-specific, GA3-and ABA-regulated gene from tomato. Plant J. 1992, 2, 153–159. [Google Scholar] [CrossRef]
- Moyano-Canete, E.; Bellido, M.L.; Garcia-Caparros, N.; Medina-Puche, L.; Amil-Ruiz, F.; González-Reyes, J.A.; Caballero, J.L.; Munoz-Blanco, J.; Blanco-Portales, R. FaGAST2, a strawberry ripening-related gene, acts together with FaGAST1 to determine cell size of the fruit receptacle. Plant Cell Physiol. 2013, 54, 218–236. [Google Scholar] [CrossRef]
- Ahmad, B.; Yao, J.; Zhang, S.; Li, X.; Zhang, X.; Yadav, V.; Wang, X. Genome-wide characterization and expression profiling of GASA genes during different stages of seed development in grapevine (Vitis vinifera L.) predict their involvement in seed development. Int. J. Mol. Sci. 2020, 21, 1088. [Google Scholar] [CrossRef] [PubMed]
- Ben-Nissan, G.; Lee, J.Y.; Borohov, A.; Weiss, D. GIP, a Petunia hybrida GA-induced cysteine-rich protein: A possible role in shoot elongation and transition to flowering. Plant. J. 2004, 37, 229–238. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, X. Expression pattern of GASA, downstream genes of DELLA, in Arabidopsis. Chin. Sci. Bull. 2008, 53, 3839–3846. [Google Scholar] [CrossRef]
- Alonso-Ramirez, A.; Rodriguez, D.; Reyes, D.; Jimenez, J.A.; Nicolas, G.; Lopez-Climent, M.; Gomez-Cadenas, A.; Nicolas, C. Evidence for a role of gibberellins in salicylic acid-modulated early plant responses to abiotic stress in Arabidopsis seeds. Plant Physiol. 2009, 150, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Rubinovich, L.; Ruthstein, S.; Weiss, D. The Arabidopsis cysteine-rich GASA5 is a redox-active metalloprotein that suppresses gibberellin responses. Mol. Plant. 2014, 7, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wei, T.; Wang, X.; Zhang, L.; Yang, M.; Chen, L.; Song, W.; Wang, C.; Chen, C. Transcriptome analyses from mutant Salvia miltiorrhiza reveals important roles for SmGASA4 during plant development. Int. J. Mol. Sci. 2018, 19, 2088. [Google Scholar] [CrossRef]
- Li, K.; Bai, X.; Li, Y.; Cai, H.; Ji, W.; Tang, L.; Wen, Y.; Zhu, Y. GsGASA1 mediated root growth inhibition in response to chronic cold stress is marked by the accumulation of DELLAs. J. Plant Physiol. 2011, 168, 2153–2160. [Google Scholar] [CrossRef]
- Nahirnak, V.; Almasia, N.I.; Hopp, H.E.; Vazquez-Rovere, C. Snakin/GASA proteins: Involvement in hormone crosstalk and redox homeostasis. Plant Signal. Behav. 2012, 7, 1004–1008. [Google Scholar] [CrossRef]
- Wu, D.; Shen, H.; Yokawa, K.; Baluška, F. Alleviation of aluminium-induced cell rigidity by overexpression of OsPIN2 in rice roots. J. Exp. Bot. 2014, 65, 5305–5315. [Google Scholar] [CrossRef] [PubMed]
- Porto, W.F.; Franco, O.L. Theoretical structural insights into the snakin/GASA family. Peptides 2013, 44, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Wigoda, N.; Ben-Nissan, G.; Granot, D.; Schwartz, A.; Weiss, D. The gibberellin-induced, cysteine-rich protein GIP2 from Petunia hybrida exhibits in planta antioxidant activity. Plant J. 2006, 48, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Han, S.; Kim, S. Salt- and ABA-inducible OsGASR1 is involved in salt tolerance. J. Plant Biol. 2015, 58, 96–101. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Zhu, S.; Xue, Y.; Lin, Y.; Mao, J.; Li, S.; Liang, C.; Lu, X.; Tian, J. The Stylo Cysteine-Rich Peptide SgSnakin1 Is Involved in Aluminum Tolerance through Enhancing Reactive Oxygen Species Scavenging. Int. J. Mol. Sci. 2024, 25, 6672. https://doi.org/10.3390/ijms25126672
Guo X, Zhu S, Xue Y, Lin Y, Mao J, Li S, Liang C, Lu X, Tian J. The Stylo Cysteine-Rich Peptide SgSnakin1 Is Involved in Aluminum Tolerance through Enhancing Reactive Oxygen Species Scavenging. International Journal of Molecular Sciences. 2024; 25(12):6672. https://doi.org/10.3390/ijms25126672
Chicago/Turabian StyleGuo, Xueqiong, Shengnan Zhu, Yingbin Xue, Yan Lin, Jingying Mao, Shuyue Li, Cuiyue Liang, Xing Lu, and Jiang Tian. 2024. "The Stylo Cysteine-Rich Peptide SgSnakin1 Is Involved in Aluminum Tolerance through Enhancing Reactive Oxygen Species Scavenging" International Journal of Molecular Sciences 25, no. 12: 6672. https://doi.org/10.3390/ijms25126672
APA StyleGuo, X., Zhu, S., Xue, Y., Lin, Y., Mao, J., Li, S., Liang, C., Lu, X., & Tian, J. (2024). The Stylo Cysteine-Rich Peptide SgSnakin1 Is Involved in Aluminum Tolerance through Enhancing Reactive Oxygen Species Scavenging. International Journal of Molecular Sciences, 25(12), 6672. https://doi.org/10.3390/ijms25126672





