Adult Inception of Ketogenic Diet Therapy Increases Sleep during the Dark Cycle in C57BL/6J Wild Type and Fragile X Mice
Abstract
:1. Introduction
2. Results
2.1. Body Weight Is Reduced in Response to Ketogenic Diet
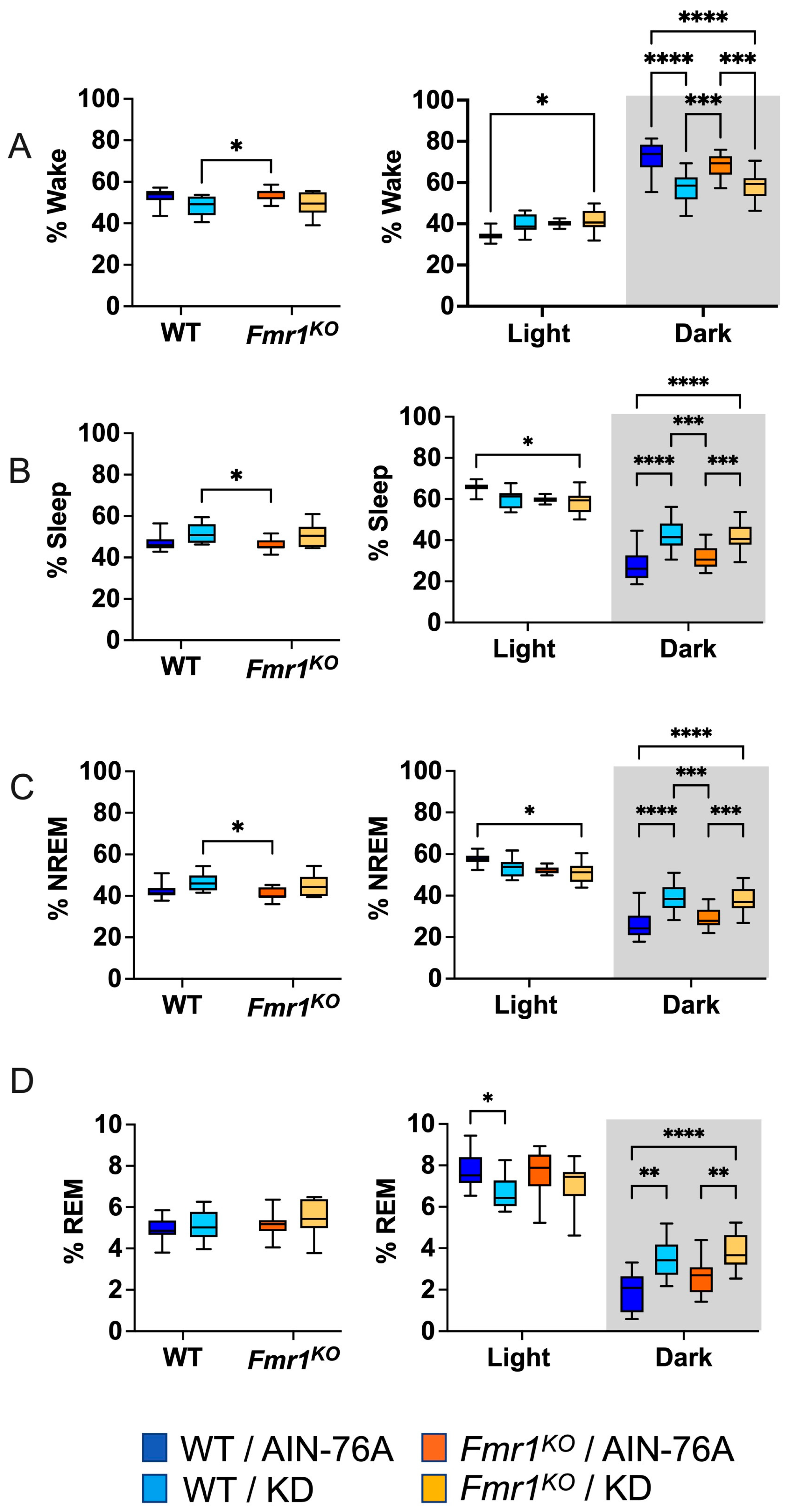
2.2. Differences in 24 h Vigilance State Distribution Are Diet-Dependent
2.3. KD Increases Time Spent in Sleep during the Dark Cycle
2.4. Sleep EEG Selectively Correlates with Actigraphy Findings during the Dark Cycle
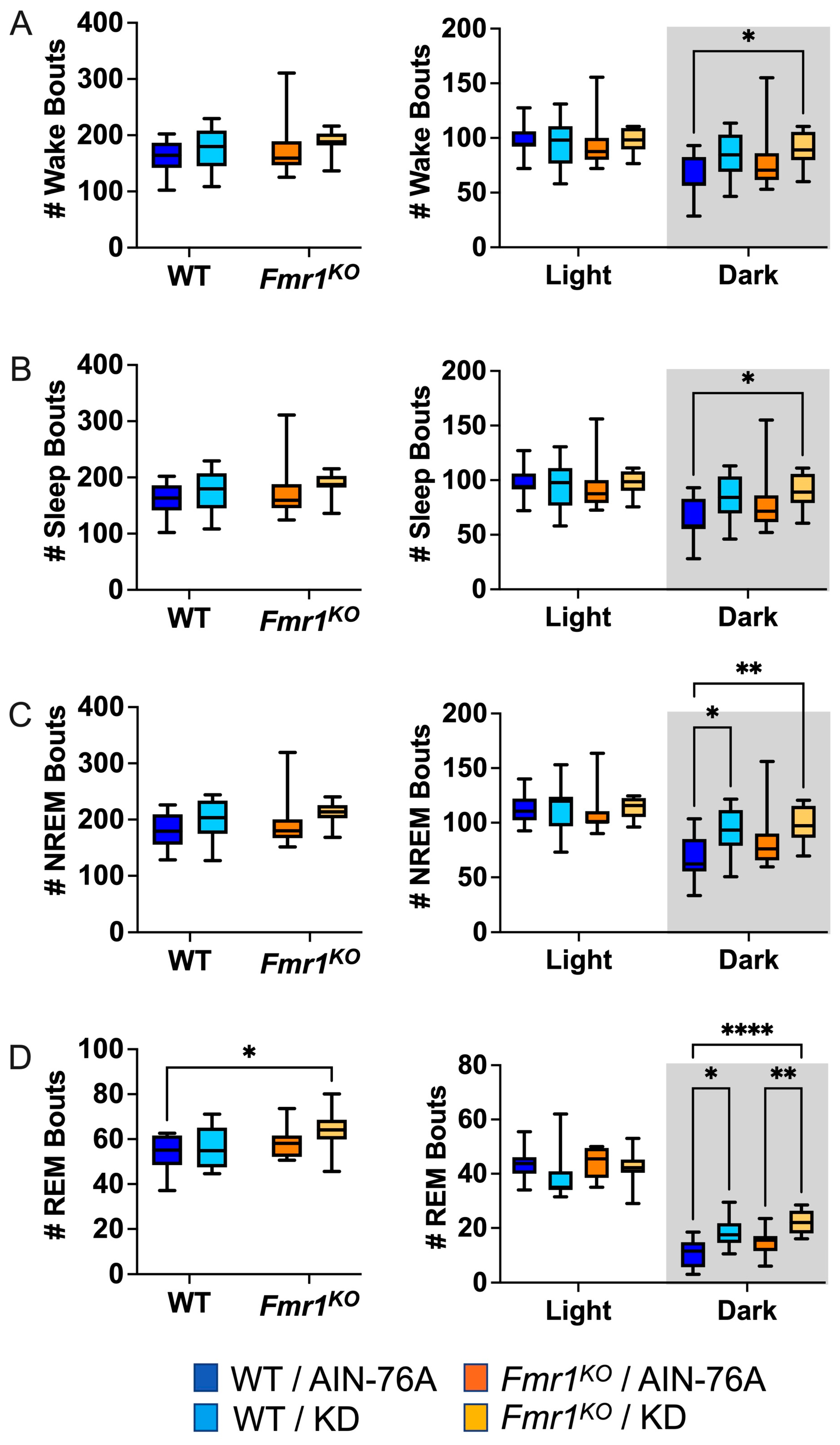
2.5. KD Increases the Number of NREM and REM Bouts during the Dark Cycle
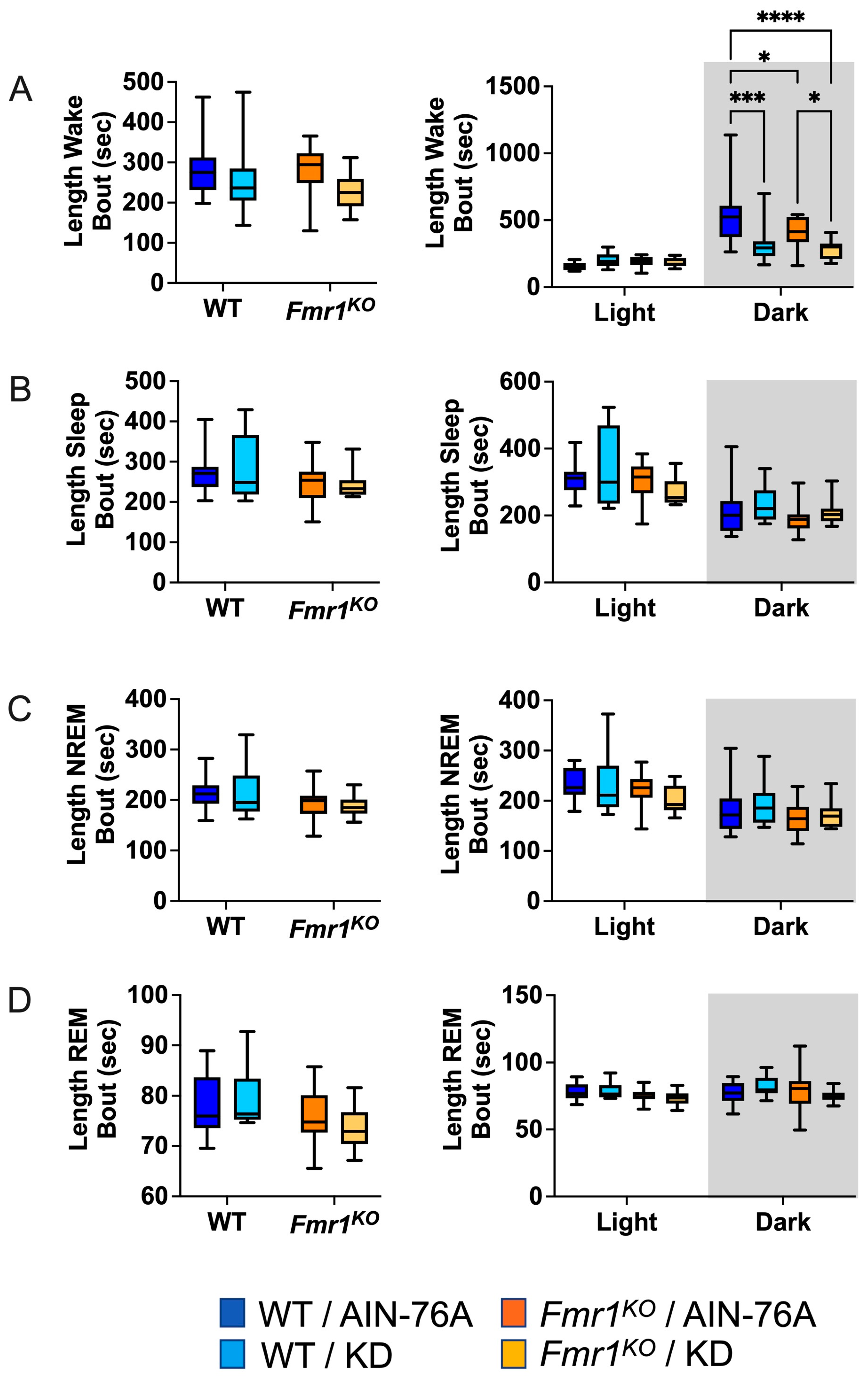
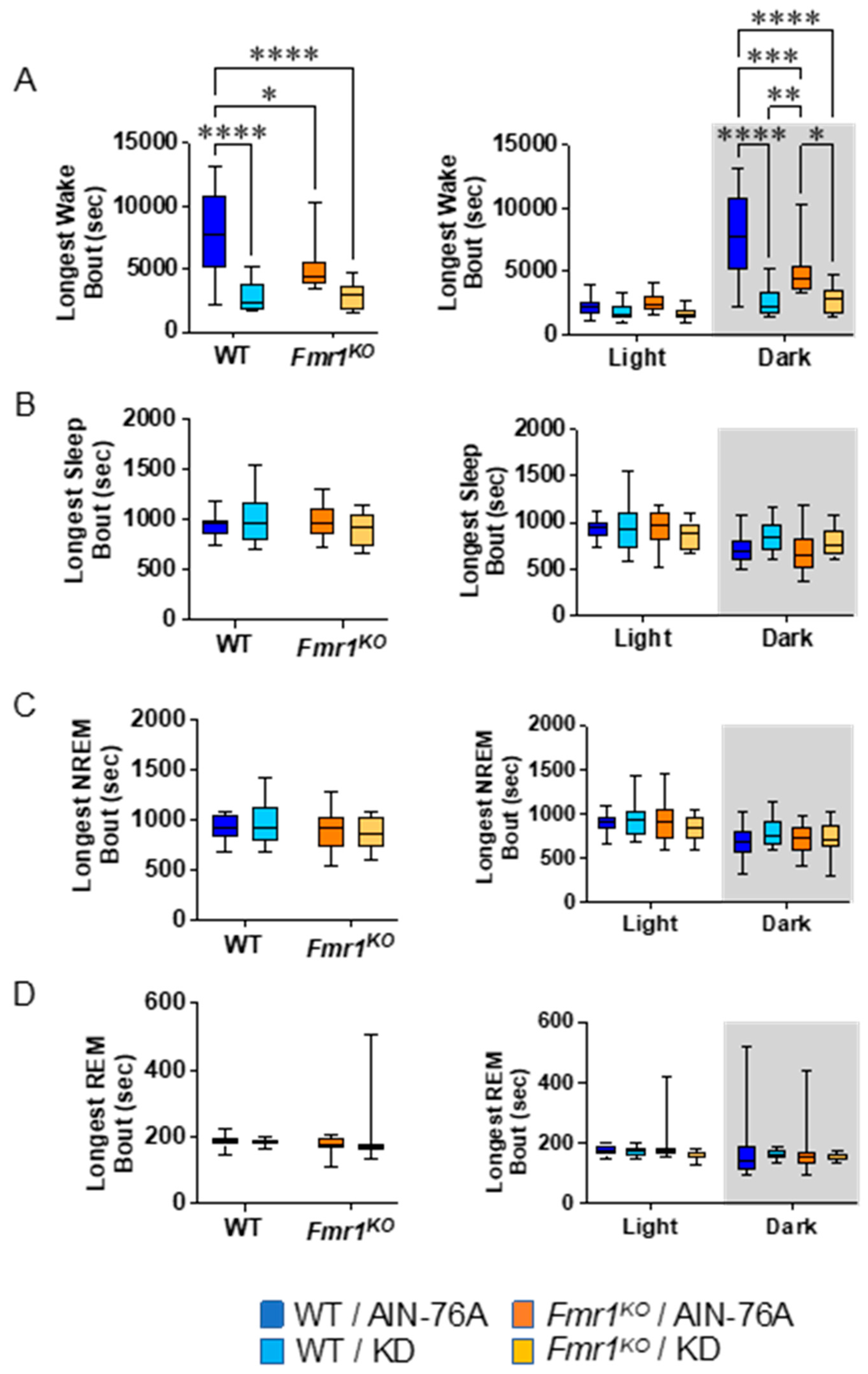
2.6. KD Decreases the Length of Wake Bouts in Mice during the Dark Cycle
2.7. Fmr1KO Mice Do Not Exhibit Epileptic Activity on Sleep EEG
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials and Mice
5.2. EEG Electrode Implantation
5.3. Biometrics
5.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hagerman, R.J.; Berry-Kravis, E.; Hazlett, H.C.; Bailey, D.B.; Moine, H.; Kooy, R.F.; Tassone, F.; Gantois, I.; Sonenberg, N.; Mandel, J.L.; et al. Fragile X syndrome. Nat. Rev. Dis. Primers 2017, 3, 17065. [Google Scholar] [CrossRef] [PubMed]
- Dutch-Belgian Fragile X Consortium. Fmr1 knockout mice: A model to study fragile X mental retardation. The Dutch-Belgian Fragile X Consortium. Cell 1994, 78, 23–33. [Google Scholar]
- Berry-Kravis, E.; Hessl, D.; Abbeduto, L.; Reiss, A.L.; Beckel-Mitchener, A.; Urv, T.K.; Groups, O.M.W. Outcome measures for clinical trials in fragile X syndrome. J. Dev. Behav. Pediatr. 2013, 34, 508–522. [Google Scholar] [CrossRef] [PubMed]
- Westmark, P.R.; Gutierrez, A.; Gholston, A.K.; Wilmer, T.M.; Westmark, C.J. Preclinical testing of the ketogenic diet in fragile X mice. Neurochem. Int. 2020, 134, 104687. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, W.E.; Kidd, S.A.; Andrews, H.F.; Budimirovic, D.B.; Esler, A.; Haas-Givler, B.; Stackhouse, T.; Riley, C.; Peacock, G.; Sherman, S.L.; et al. Autism Spectrum Disorder in Fragile X Syndrome: Cooccurring Conditions and Current Treatment. Pediatrics 2017, 139 (Suppl. S3), S194–S206. [Google Scholar] [CrossRef] [PubMed]
- Angriman, M.; Caravale, B.; Novelli, L.; Ferri, R.; Bruni, O. Sleep in children with neurodevelopmental disabilities. Neuropediatrics 2015, 46, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Kronk, R.; Bishop, E.E.; Raspa, M.; Bickel, J.O.; Mandel, D.A.; Bailey, D.B. Prevalence, nature, and correlates of sleep problems among children with fragile X syndrome based on a large scale parent survey. Sleep 2010, 33, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Kronk, R.; Dahl, R.; Noll, R. Caregiver reports of sleep problems on a convenience sample of children with fragile X syndrome. Am. J. Intellect. Dev. Disabil. 2009, 114, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Gould, E.L.; Loesch, D.Z.; Martin, M.J.; Hagerman, R.J.; Armstrong, S.M.; Huggins, R.M. Melatonin profiles and sleep characteristics in boys with fragile X syndrome: A preliminary study. Am. J. Med. Genet. 2000, 95, 307–315. [Google Scholar] [CrossRef]
- Kidd, S.A.; Lachiewicz, A.; Barbouth, D.; Blitz, R.K.; Delahunty, C.; McBrien, D.; Visootsak, J.; Berry-Kravis, E. Fragile X syndrome: A review of associated medical problems. Pediatrics 2014, 134, 995–1005. [Google Scholar] [CrossRef]
- Agar, G.; Brown, C.; Sutherland, D.; Coulborn, S.; Oliver, C.; Richards, C. Sleep disorders in rare genetic syndromes: A meta-analysis of prevalence and profile. Mol. Autism 2021, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Budimirovic, D.B.; Protic, D.D.; Delahunty, C.M.; Andrews, H.F.; Choo, T.H.; Xu, Q.; Berry-Kravis, E.; Kaufmann, W.E.; FORWARD Consortium. Sleep problems in fragile X syndrome: Cross-sectional analysis of a large clinic-based cohort. Am. J. Med. Genet. A 2022, 188, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Tirosh, E.; Borochowitz, Z. Sleep apnea in fragile X syndrome. Am. J. Med. Genet. 1992, 43, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Niemczyk, J.; von Gontard, A.; Equit, M.; Bauer, K.; Naumann, T.; Wagner, C.; Curfs, L. Detailed assessment of incontinence in boys with fragile-X-syndrome in a home setting. Eur. J. Pediatr. 2016, 175, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Carotenuto, M.; Roccella, M.; Pisani, F.; Matricardi, S.; Verrotti, A.; Farello, G.; Operto, F.F.; Bitetti, I.; Precenzano, F.; Messina, G.; et al. Polysomnographic Findings in Fragile X Syndrome Children with EEG Abnormalities. Behav. Neurol. 2019, 2019, 5202808. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, S.A.; Ferri, R.; Elia, M.; Del Gracco, S.; Scuderi, C.; Stefanini, M.C.; Castano, A.; Azan, G. Sleep Neurophysiology in Fragile X Patients. Dev. Brain Dysfunct. 1995, 8, 218–222. [Google Scholar]
- Miano, S.; Bruni, O.; Elia, M.; Scifo, L.; Smerieri, A.; Trovato, A.; Verrillo, E.; Terzano, M.G.; Ferri, R. Sleep phenotypes of intellectual disability: A polysomnographic evaluation in subjects with Down syndrome and Fragile-X syndrome. Clin. Neurophysiol. 2008, 119, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
- Balietti, M.; Casoli, T.; Di Stefano, G.; Giorgetti, B.; Aicardi, G.; Fattoretti, P. Ketogenic diets: An historical antiepileptic therapy with promising potentialities for the aging brain. Ageing Res. Rev. 2010, 9, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Stafstrom, C.E.; Rho, J.M. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front. Pharmacol. 2012, 3, 59. [Google Scholar] [CrossRef]
- El-Rashidy, O.; El-Baz, F.; El-Gendy, Y.; Khalaf, R.; Reda, D.; Saad, K. Ketogenic diet versus gluten free casein free diet in autistic children: A case-control study. Metab. Brain Dis. 2017, 32, 1935–1941. [Google Scholar] [CrossRef]
- Spilioti, M.; Evangeliou, A.E.; Tramma, D.; Theodoridou, Z.; Metaxas, S.; Michailidi, E.; Bonti, E.; Frysira, H.; Haidopoulou, A.; Asprangathou, D.; et al. Evidence for treatable inborn errors of metabolism in a cohort of 187 Greek patients with autism spectrum disorder (ASD). Front. Hum. Neurosci. 2013, 7, 858. [Google Scholar] [CrossRef] [PubMed]
- Evangeliou, A.; Vlachonikolis, I.; Mihailidou, H.; Spilioti, M.; Skarpalezou, A.; Makaronas, N.; Prokopiou, A.; Christodoulou, P.; Liapi-Adamidou, G.; Helidonis, E.; et al. Application of a ketogenic diet in children with autistic behavior: Pilot study. J. Child. Neurol. 2003, 18, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Kasprowska-Liśkiewicz, D.; Liśkiewicz, A.D.; Nowacka-Chmielewska, M.M.; Nowicka, J.; Małecki, A.; Barski, J.J. The ketogenic diet affects the social behavior of young male rats. Physiol. Behav. 2017, 179, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Ruskin, D.N.; Fortin, J.A.; Bisnauth, S.N.; Masino, S.A. Ketogenic diets improve behaviors associated with autism spectrum disorder in a sex-specific manner in the EL mouse. Physiol. Behav. 2017, 168, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Ruskin, D.N.; Murphy, M.I.; Slade, S.L.; Masino, S.A. Ketogenic diet improves behaviors in a maternal immune activation model of autism spectrum disorder. PLoS ONE 2017, 12, e0171643. [Google Scholar] [CrossRef] [PubMed]
- Ruskin, D.N.; Svedova, J.; Cote, J.L.; Sandau, U.; Rho, J.M.; Kawamura, M.; Boison, D.; Masino, S.A. Ketogenic diet improves core symptoms of autism in BTBR mice. PLoS ONE 2013, 8, e65021. [Google Scholar] [CrossRef] [PubMed]
- Verpeut, J.L.; DiCicco-Bloom, E.; Bello, N.T. Ketogenic diet exposure during the juvenile period increases social behaviors and forebrain neural activation in adult Engrailed 2 null mice. Physiol. Behav. 2016, 161, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhao, Y.; Tomi, M.; Shin, B.C.; Thamotharan, S.; Mazarati, A.; Sankar, R.; Wang, E.A.; Cepeda, C.; Levine, M.S.; et al. Sex-Specific Life Course Changes in the Neuro-Metabolic Phenotype of Glut3 Null Heterozygous Mice: Ketogenic Diet Ameliorates Electroencephalographic Seizures and Improves Sociability. Endocrinology 2017, 158, 936–949. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fang, Z.; Jud, C.; Vansteensel, M.J.; Kaasik, K.; Lee, C.C.; Albrecht, U.; Tamanini, F.; Meijer, J.H.; Oostra, B.A.; et al. Fragile X-related proteins regulate mammalian circadian behavioral rhythms. Am. J. Hum. Genet. 2008, 83, 43–52. [Google Scholar] [CrossRef]
- Allen, C.N. Circadian rhythms, diet, and neuronal excitability. Epilepsia 2008, 49 (Suppl. S8), 124–126. [Google Scholar] [CrossRef]
- Genzer, Y.; Dadon, M.; Burg, C.; Chapnik, N.; Froy, O. Effect of dietary fat and the circadian clock on the expression of brain-derived neurotrophic factor (BDNF). Mol. Cell Endocrinol. 2016, 430, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Genzer, Y.; Dadon, M.; Burg, C.; Chapnik, N.; Froy, O. Ketogenic diet delays the phase of circadian rhythms and does not affect AMP-activated protein kinase (AMPK) in mouse liver. Mol. Cell Endocrinol. 2015, 417, 124–130. [Google Scholar] [CrossRef]
- Greenhill, C. Metabolism: Ketogenic diet rewires circadian clock. Nat. Rev. Endocrinol. 2017, 13, 626. [Google Scholar] [CrossRef]
- Nakao, R.; Shimba, S.; Oishi, K. Ketogenic diet induces expression of the muscle circadian gene Slc25a25 via neural pathway that might be involved in muscle thermogenesis. Sci. Rep. 2017, 7, 2885. [Google Scholar] [CrossRef]
- Oike, H. Modulation of circadian clocks by nutrients and food factors. Biosci. Biotechnol. Biochem. 2017, 81, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Oishi, K.; Uchida, D.; Ohkura, N.; Doi, R.; Ishida, N.; Kadota, K.; Horie, S. Ketogenic diet disrupts the circadian clock and increases hypofibrinolytic risk by inducing expression of plasminogen activator inhibitor-1. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1571–1577. [Google Scholar] [CrossRef]
- Oishi, K.; Uchida, D.; Ohkura, N.; Horie, S. PPARα deficiency augments a ketogenic diet-induced circadian PAI-1 expression possibly through PPARγ activation in the liver. Biochem. Biophys. Res. Commun. 2010, 401, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Tognini, P.; Murakami, M.; Liu, Y.; Eckel-Mahan, K.L.; Newman, J.C.; Verdin, E.; Baldi, P.; Sassone-Corsi, P. Distinct Circadian Signatures in Liver and Gut Clocks Revealed by Ketogenic Diet. Cell Metab. 2017, 26, 523–538.e5. [Google Scholar] [CrossRef]
- Fenoglio-Simeone, K.A.; Wilke, J.C.; Milligan, H.L.; Allen, C.N.; Rho, J.M.; Maganti, R.K. Ketogenic diet treatment abolishes seizure periodicity and improves diurnal rhythmicity in epileptic Kcna1-null mice. Epilepsia 2009, 50, 2027–2034. [Google Scholar] [CrossRef]
- Simeone, T.A.; Samson, K.K.; Matthews, S.A.; Simeone, K.A. In vivo ketogenic diet treatment attenuates pathologic sharp waves and high frequency oscillations in in vitro hippocampal slices from epileptic Kv 1.1α knockout mice. Epilepsia 2014, 55, e44–e49. [Google Scholar] [CrossRef]
- Jain, S.V.; Glauser, T.A. Effects of epilepsy treatments on sleep architecture and daytime sleepiness: An evidence-based review of objective sleep metrics. Epilepsia 2014, 55, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Hallböök, T.; Lundgren, J.; Rosén, I. Ketogenic diet improves sleep quality in children with therapy-resistant epilepsy. Epilepsia 2007, 48, 59–65. [Google Scholar] [CrossRef]
- Westmark, P.R.; Gholston, A.K.; Swietlik, T.J.; Maganti, R.K.; Westmark, C.J. Ketogenic diet affects sleep architecture in C57BL/6J wild type and fragile X mice. Int. J. Mol. Sci. 2023, 24, 14460. [Google Scholar] [CrossRef] [PubMed]
- Campbell, I.G. EEG recording and analysis for sleep research. Curr. Protoc. Neurosci. 2009, 49, 10.2.1–10.2.19. [Google Scholar] [CrossRef] [PubMed]
- Ancoli-Israel, S.; Cole, R.; Alessi, C.; Chambers, M.; Moorcroft, W.; Pollak, C.P. The role of actigraphy in the study of sleep and circadian rhythms. Sleep 2003, 26, 342–392. [Google Scholar] [CrossRef] [PubMed]
- Berry-Kravis, E. Epilepsy in fragile X syndrome. Dev. Med. Child. Neurol. 2002, 44, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, S.A.; Colognola, R.M.; Ferri, R.; Gigli, G.L.; Petrella, M.A.; Sanfilippo, S.; Bergonzi, P.; Tassinari, C.A. Fragile-X syndrome: A particular epileptogenic EEG pattern. Epilepsia 1988, 29, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Pietropaolo, S.; Guilleminot, A.; Martin, B.; D’Amato, F.R.; Crusio, W.E. Genetic-background modulation of core and variable autistic-like symptoms in Fmr1 knock-out mice. PLoS ONE 2011, 6, e17073. [Google Scholar] [CrossRef] [PubMed]
- Berry-Kravis, E.; Knox, A.; Hervey, C. Targeted treatments for fragile X syndrome. J. Neurodev. Disord. 2011, 3, 193–210. [Google Scholar] [CrossRef]
- Pasca, L.; Quaranta, C.A.; Grumi, S.; Zanaboni, M.P.; Tagliabue, A.; Guglielmetti, M.; Vitali, H.; Capriglia, E.; Varesio, C.; Toni, F.; et al. The effects of ketogenic diet therapies on sleep: A scoping review. J. Sleep Res. 2023, e14073. [Google Scholar] [CrossRef]
- Alam, S.; Westmark, C.J.; McCullagh, E.A. Diet in treatment of autism spectrum disorders. Front. Neurosci. 2023, 16, 1031016. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.S.; Adams, J.B. Ratings of the effectiveness of 13 therapeutic diets for autism spectrum disorder: Results of a national survey. J. Pers. Med. 2023, 13, 1448. [Google Scholar] [CrossRef] [PubMed]
- Westmark, C.J. A hypothesis regarding the molecular mechanism underlying dietary soy-induced effects on seizure propensity. Front. Neurol. 2014, 5, 169. [Google Scholar] [CrossRef] [PubMed]
- Westmark, C.J.; Brower, J.; Held, P.K. Improving reproducibility to enhance to enhance scientific rigor through consideration of mouse diet. Animals 2022, 12, 3448. [Google Scholar] [CrossRef] [PubMed]
- Westmark, P.R.; Lyon, G.; Gutierrez, A.; Boeck, B.; Van Hammond, O.; Ripp, N.; Pagan-Torres, N.A.; Brower, J.; Held, P.K.; Scarlett, C.; et al. Effects of soy protein isolate on fragile X phenotypes in mice. Nutrients 2024, 16, 284. [Google Scholar] [CrossRef] [PubMed]
- Masi, D.; Spoltore, M.E.; Rossetti, R.; Watanabe, M.; Tozzi, R.; Caputi, A.; Risi, R.; Balena, A.; Gandini, O.; Mariani, S.; et al. The Influence of Ketone Bodies on Circadian Processes Regarding Appetite, Sleep and Hormone Release: A Systematic Review of the Literature. Nutrients 2022, 14, 1410. [Google Scholar] [CrossRef] [PubMed]
- Ethridge, L.E.; White, S.P.; Mosconi, M.W.; Wang, J.; Byerly, M.J.; Sweeney, J.A. Reduced habituation of auditory evoked potentials indicate cortical hyper-excitability in Fragile X Syndrome. Transl. Psychiatry 2016, 6, e787. [Google Scholar] [CrossRef] [PubMed]
- Ethridge, L.E.; White, S.P.; Mosconi, M.W.; Wang, J.; Pedapati, E.V.; Erickson, C.A.; Byerly, M.J.; Sweeney, J.A. Neural synchronization deficits linked to cortical hyper-excitability and auditory hypersensitivity in fragile X syndrome. Mol. Autism 2017, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ethridge, L.E.; Mosconi, M.W.; White, S.P.; Binder, D.K.; Pedapati, E.V.; Erickson, C.A.; Byerly, M.J.; Sweeney, J.A. A resting EEG study of neocortical hyperexcitability and altered functional connectivity in fragile X syndrome. J. Neurodev. Disord. 2017, 9, 11. [Google Scholar] [CrossRef]
- Sinclair, D.; Featherstone, R.; Naschek, M.; Nam, J.; Du, A.; Wright, S.; Pance, K.; Melnychenko, O.; Weger, R.; Akuzawa, S.; et al. GABA-B Agonist Baclofen Normalizes Auditory-Evoked Neural Oscillations and Behavioral Deficits in the Fmr1 knockout mouse model of fragile X syndrome. eNeuro 2017, 4. [Google Scholar] [CrossRef]
- Lovelace, J.W.; Ethell, I.M.; Binder, D.K.; Razak, K.A. Translation-relevant EEG phenotypes in a mouse model of Fragile X Syndrome. Neurobiol. Dis. 2018, 115, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.H.; Lovelace, J.W.; Ethell, I.M.; Binder, D.K.; Razak, K.A. Developmental Changes in EEG Phenotypes in a Mouse Model of Fragile X Syndrome. Neuroscience 2019, 398, 126–143. [Google Scholar] [CrossRef] [PubMed]
- Jonak, C.R.; Lovelace, J.W.; Ethell, I.M.; Razak, K.A.; Binder, D.K. Multielectrode array analysis of EEG biomarkers in a mouse model of Fragile X Syndrome. Neurobiol. Dis. 2020, 138, 104794. [Google Scholar] [CrossRef] [PubMed]
- Lovelace, J.W.; Rais, M.; Palacios, A.R.; Shuai, X.S.; Bishay, S.; Popa, O.; Pirbhoy, P.S.; Binder, D.K.; Nelson, D.L.; Ethell, I.M.; et al. Deletion of Fmr1 from Forebrain Excitatory Neurons Triggers Abnormal Cellular, EEG, and Behavioral Phenotypes in the Auditory Cortex of a Mouse Model of Fragile X Syndrome. Cereb. Cortex 2020, 30, 969–988. [Google Scholar] [CrossRef] [PubMed]
- Jonak, C.R.; Sandhu, M.S.; Assad, S.A.; Barbosa, J.A.; Makhija, M.; Binder, D.K. The PDE10A Inhibitor TAK-063 Reverses Sound-Evoked EEG Abnormalities in a Mouse Model of Fragile X Syndrome. Neurotherapeutics 2021, 18, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Saraf, T.S.; McGlynn, R.P.; Bhatavdekar, O.M.; Booth, R.G.; Canal, C.E. FPT, a 2-Aminotetralin, Is a Potent Serotonin 5-HT. ACS Chem. Neurosci. 2022, 13, 3629–3640. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.; Hooper, A.W.M.; Niibori, Y.; Lee, S.J.; Hategan, L.A.; Zhang, L.; Karumuthil-Melethil, S.; Till, S.M.; Kind, P.C.; Danos, O.; et al. Sexually dimorphic patterns in electroencephalography power spectrum and autism-related behaviors in a rat model of fragile X syndrome. Neurobiol. Dis. 2020, 146, 105118. [Google Scholar] [CrossRef] [PubMed]
- Kenny, A.; Wright, D.; Stanfield, A.C. EEG as a translational biomarker and outcome measure in fragile X syndrome. Transl. Psychiatry 2022, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Jonak, C.R.; Assad, S.A.; Garcia, T.A.; Sandhu, M.S.; Rumschlag, J.A.; Razak, K.A.; Binder, D.K. Phenotype analysis of multielectrode array EEG biomarkers in developing and adult male Fmr1 KO mice. Neurobiol. Dis. 2024, 195, 106496. [Google Scholar] [CrossRef]
- Van der Molen, M.J.; Van der Molen, M.W. Reduced alpha and exaggerated theta power during the resting-state EEG in fragile X syndrome. Biol. Psychol. 2013, 92, 216–219. [Google Scholar] [CrossRef]
- Radwan, B.; Dvorak, D.; Fenton, A.A. Impaired cognitive discrimination and discoordination of coupled theta-gamma oscillations in Fmr1 knockout mice. Neurobiol. Dis. 2016, 88, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Arbab, T.; Battaglia, F.P.; Pennartz, C.M.A.; Bosman, C.A. Abnormal hippocampal theta and gamma hypersynchrony produces network and spike timing disturbances in the Fmr1-KO mouse model of Fragile X syndrome. Neurobiol. Dis. 2018, 114, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Pedapati, E.V.; Schmitt, L.M.; Ethridge, L.E.; Miyakoshi, M.; Sweeney, J.A.; Liu, R.; Smith, E.; Shaffer, R.C.; Dominick, K.C.; Gilbert, D.L.; et al. Neocortical localization and thalamocortical modulation of neuronal hyperexcitability contribute to fragile X syndrome. Commun. Biol. 2022, 5, 442. [Google Scholar] [CrossRef] [PubMed]
- Boone, C.E.; Davoudi, H.; Harrold, J.B.; Foster, D.J. Abnormal Sleep Architecture and Hippocampal Circuit Dysfunction in a Mouse Model of Fragile X Syndrome. Neuroscience 2018, 384, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.G.; Pedapati, E.V.; Liu, R.; Schmitt, L.M.; Dominick, K.C.; Shaffer, R.C.; Sweeney, J.A.; Erickson, C.A. Sex differences in resting EEG power in fragile X syndrome. J. Psychiatr. Res. 2021, 138, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Takarae, Y.; Zanesco, A.; Erickson, C.A.; Pedapati, E.V. EEG microstates as markers for cognitive impairments in fragile X syndrome. Brain Topogr. 2024, 37, 432–446. [Google Scholar] [CrossRef] [PubMed]
- Kat, R.; Linkenkaer-Hansen, K.; Koopmans, M.A.; Houtman, S.J.; Bruining, H.; Kas, M.J.H. Assessment of the excitation-inhibition ratio in the Fmr1 KO2 mouse using mouse oscillation dynamics. Cereb. Cortex 2024, 34, bhae201. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.D.; Wilson, L.G.; Brancaleone, W.P.; Peterson, K.G.; Popke, D.S.; Caicedo Garzon, V.; Perez Tremble, R.E.; Donnelly, M.J.; Mendez Ortega, S.L.; Torres, D.; et al. Hypnotic treatment reverses NREM sleep disruption and EEG desynchronization in a mouse model of Fragile X syndrome to rescue memory consolidation deficits. bioRxiv 2023. [Google Scholar] [CrossRef] [PubMed]
- Abolghasemi, A.; Carullo, M.P.; Aguilera, E.C.; Laroui, A.; Plantefeve, R.; Rojas, D.; Benachenhou, S.; Ramírez, M.V.; Proteau-Lemieux, M.; Lepage, J.F.; et al. Alteration of Fatty Acid Profile in Fragile X Syndrome. Int. J. Mol. Sci. 2022, 23, 10815. [Google Scholar] [CrossRef]
- Crusio, W.E. Flanking gene and genetic background problems in genetically manipulated mice. Biol. Psychiatry 2004, 56, 381–385. [Google Scholar] [CrossRef]
- Crusio, W.E.; Goldowitz, D.; Holmes, A.; Wolfer, D. Standards for the publication of mouse mutant studies. Genes. Brain Behav. 2009, 8, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Filon, M.J.; Wallace, E.; Wright, S.; Douglas, D.J.; Steinberg, L.I.; Verkuilen, C.L.; Westmark, P.R.; Maganti, R.K.; Westmark, C.J. Sleep and diurnal rest-activity rhythm disturbances in a mouse model of Alzheimer’s disease. Sleep 2020, 43, zsaa087. [Google Scholar] [CrossRef] [PubMed]
- Wallace, E.; Kim, D.Y.; Kim, K.M.; Chen, S.; Blair Braden, B.; Williams, J.; Jasso, K.; Garcia, A.; Rho, J.M.; Bimonte-Nelson, H.; et al. Differential effects of duration of sleep fragmentation on spatial learning and synaptic plasticity in pubertal mice. Brain Res. 2015, 1615, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Wallace, E.; Wright, S.; Schoenike, B.; Roopra, A.; Rho, J.M.; Maganti, R.K. Altered circadian rhythms and oscillation of clock genes and sirtuin 1 in a model of sudden unexpected death in epilepsy. Epilepsia 2018, 59, 1527–1539. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Westmark, P.R.; Swietlik, T.J.; Runde, E.; Corsiga, B.; Nissan, R.; Boeck, B.; Granger, R.; Jennings, E.; Nebbia, M.; Thauwald, A.; et al. Adult Inception of Ketogenic Diet Therapy Increases Sleep during the Dark Cycle in C57BL/6J Wild Type and Fragile X Mice. Int. J. Mol. Sci. 2024, 25, 6679. https://doi.org/10.3390/ijms25126679
Westmark PR, Swietlik TJ, Runde E, Corsiga B, Nissan R, Boeck B, Granger R, Jennings E, Nebbia M, Thauwald A, et al. Adult Inception of Ketogenic Diet Therapy Increases Sleep during the Dark Cycle in C57BL/6J Wild Type and Fragile X Mice. International Journal of Molecular Sciences. 2024; 25(12):6679. https://doi.org/10.3390/ijms25126679
Chicago/Turabian StyleWestmark, Pamela R., Timothy J. Swietlik, Ethan Runde, Brian Corsiga, Rachel Nissan, Brynne Boeck, Ricky Granger, Erica Jennings, Maya Nebbia, Andrew Thauwald, and et al. 2024. "Adult Inception of Ketogenic Diet Therapy Increases Sleep during the Dark Cycle in C57BL/6J Wild Type and Fragile X Mice" International Journal of Molecular Sciences 25, no. 12: 6679. https://doi.org/10.3390/ijms25126679




