Biodegradation of Photocatalytic Degradation Products of Sulfonamides: Kinetics and Identification of Intermediates
Abstract
:1. Introduction
2. Results and Discussion
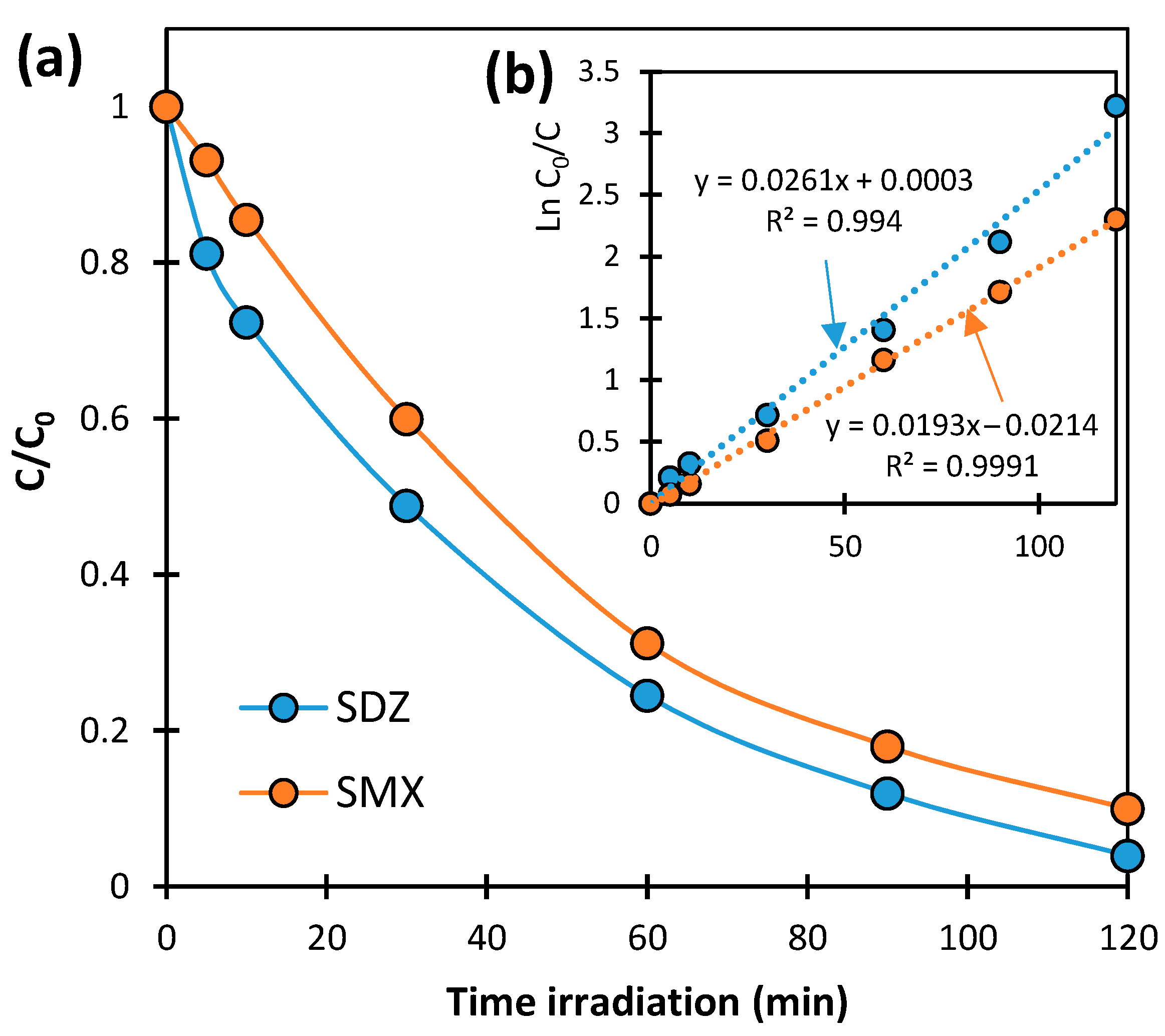
2.1. Kinetics of SN Photocatalytic Degradation
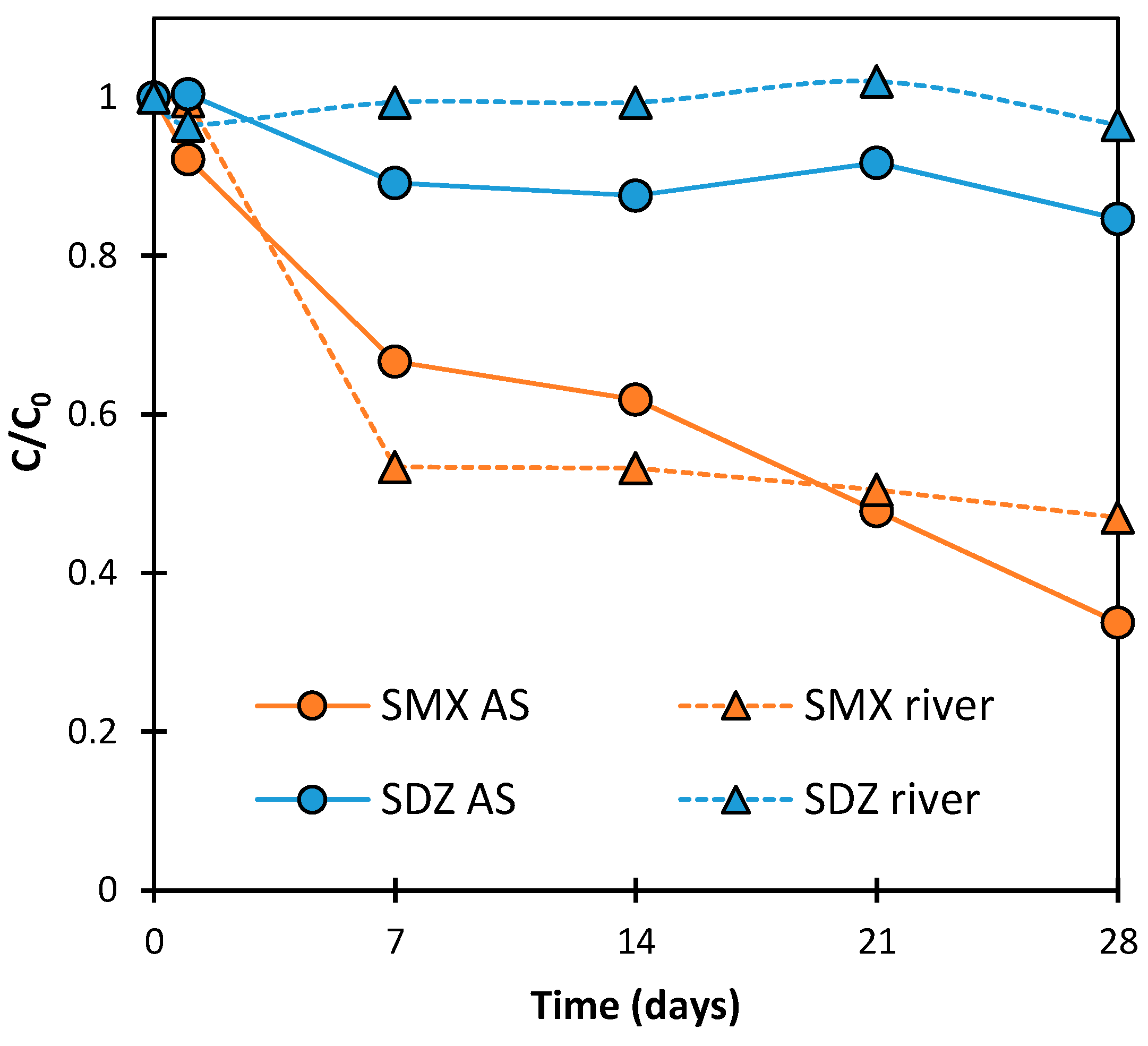
2.2. Kinetics of SN Aerobic Biodegradation
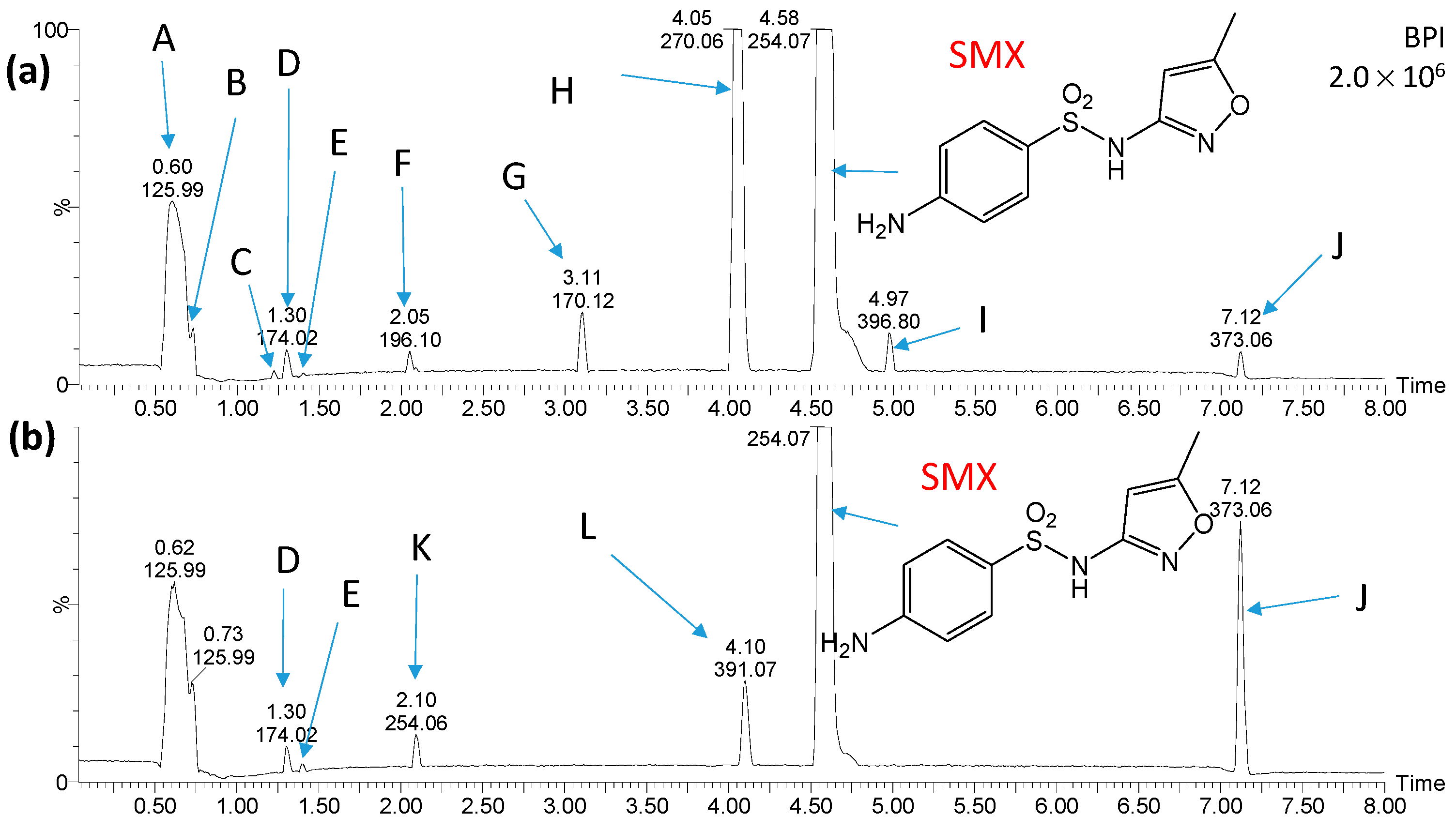
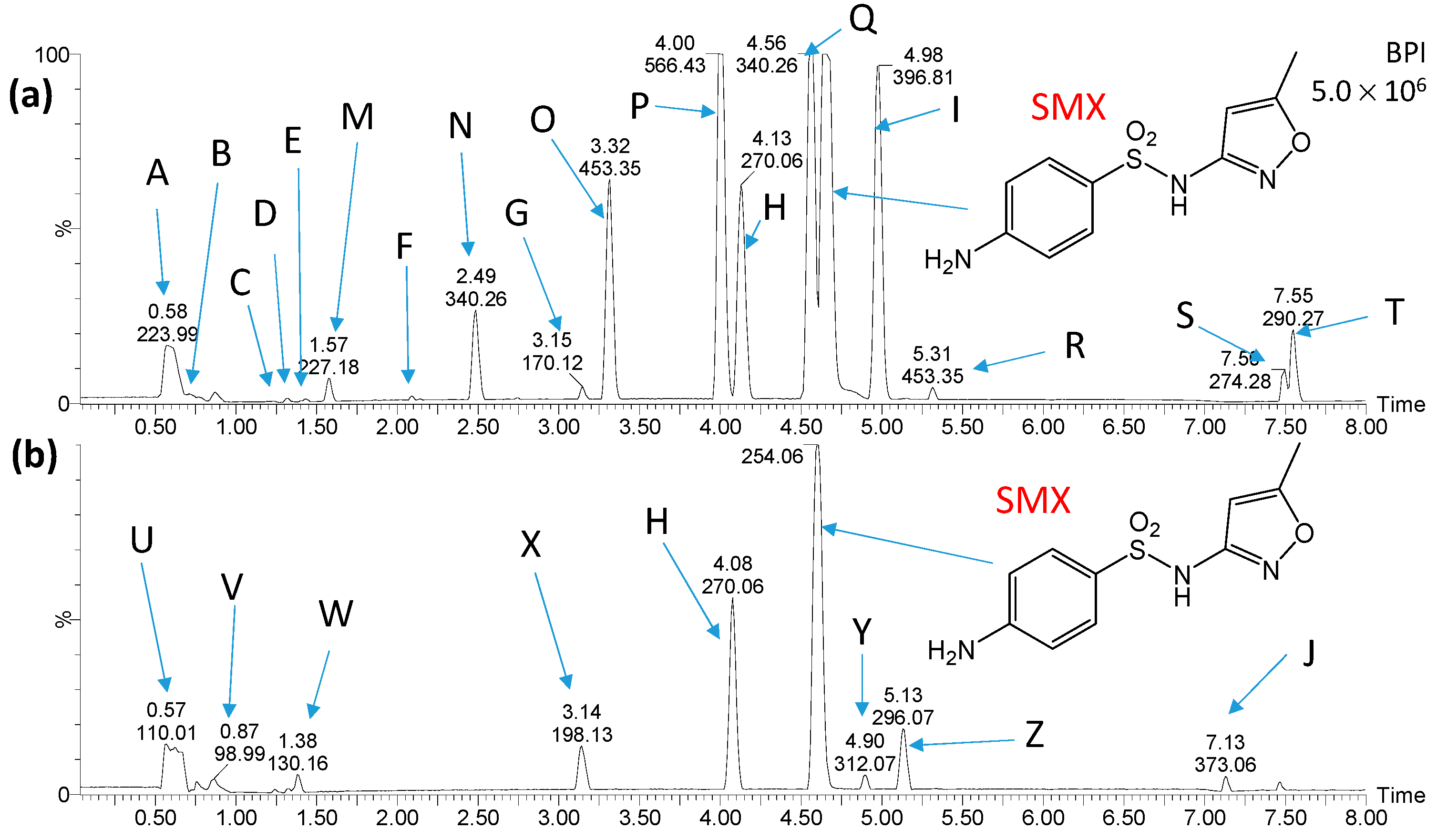
2.3. Identification and Transformations of Products of SN Photocatalytic Degradation
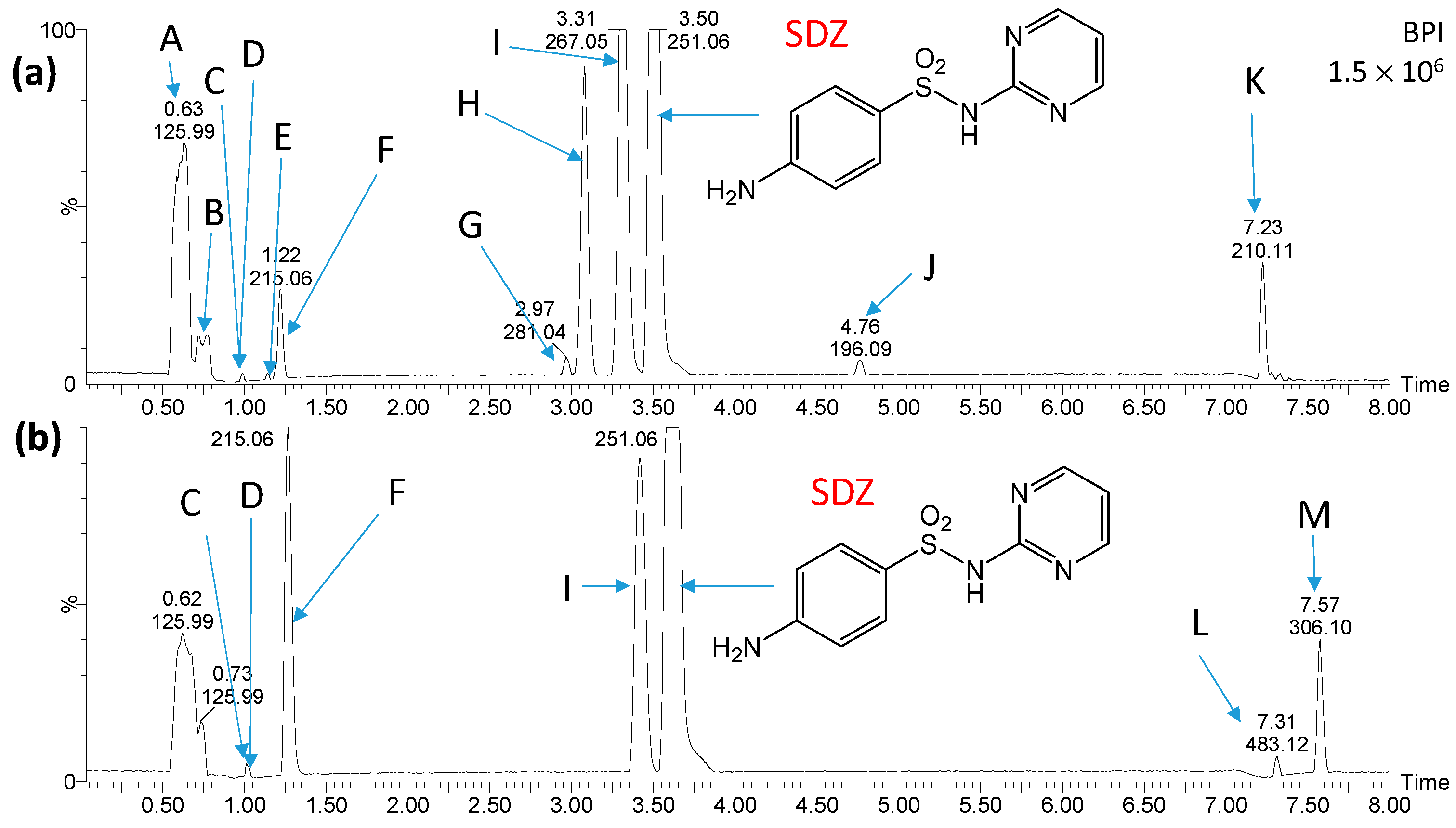

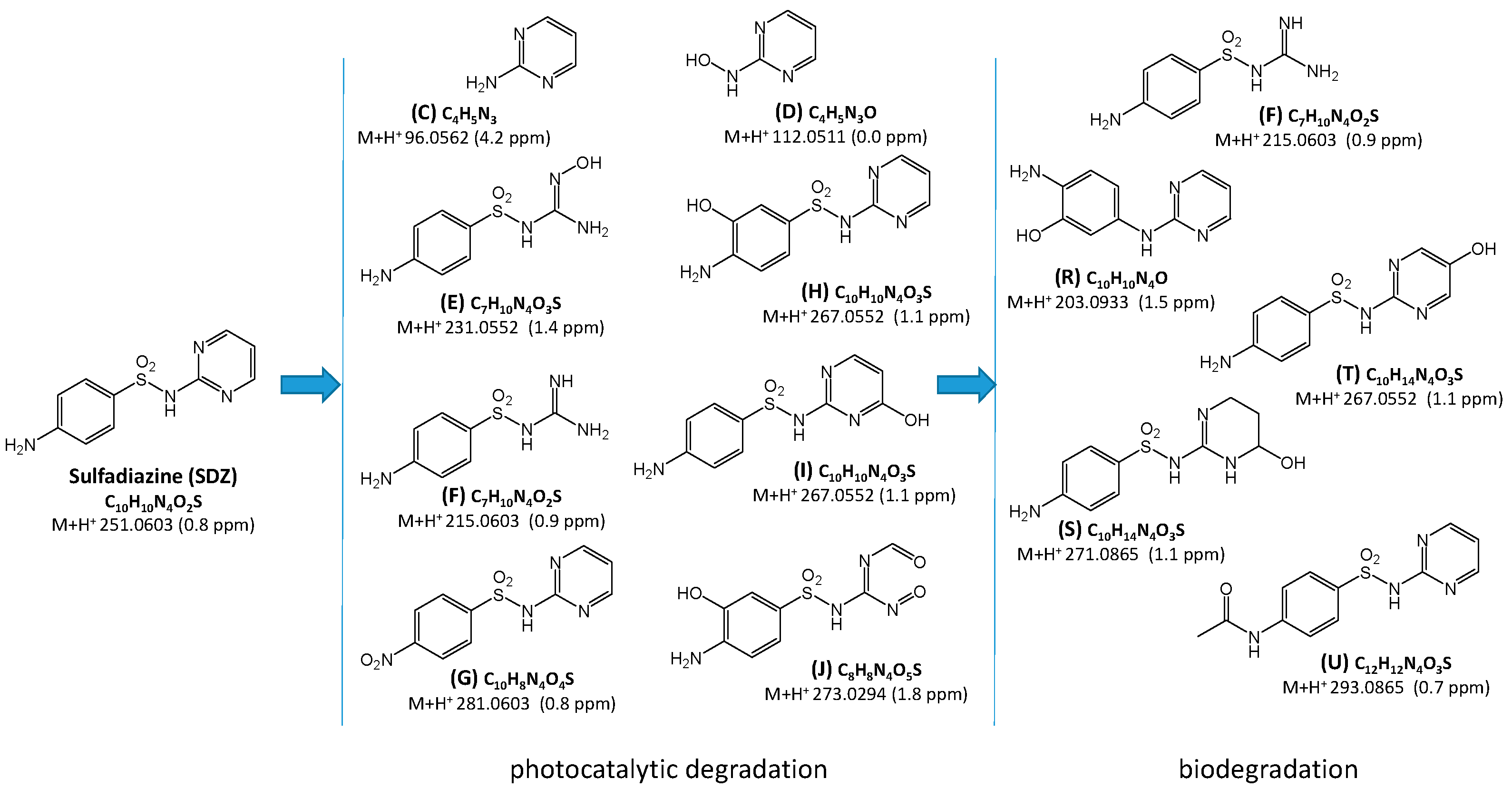
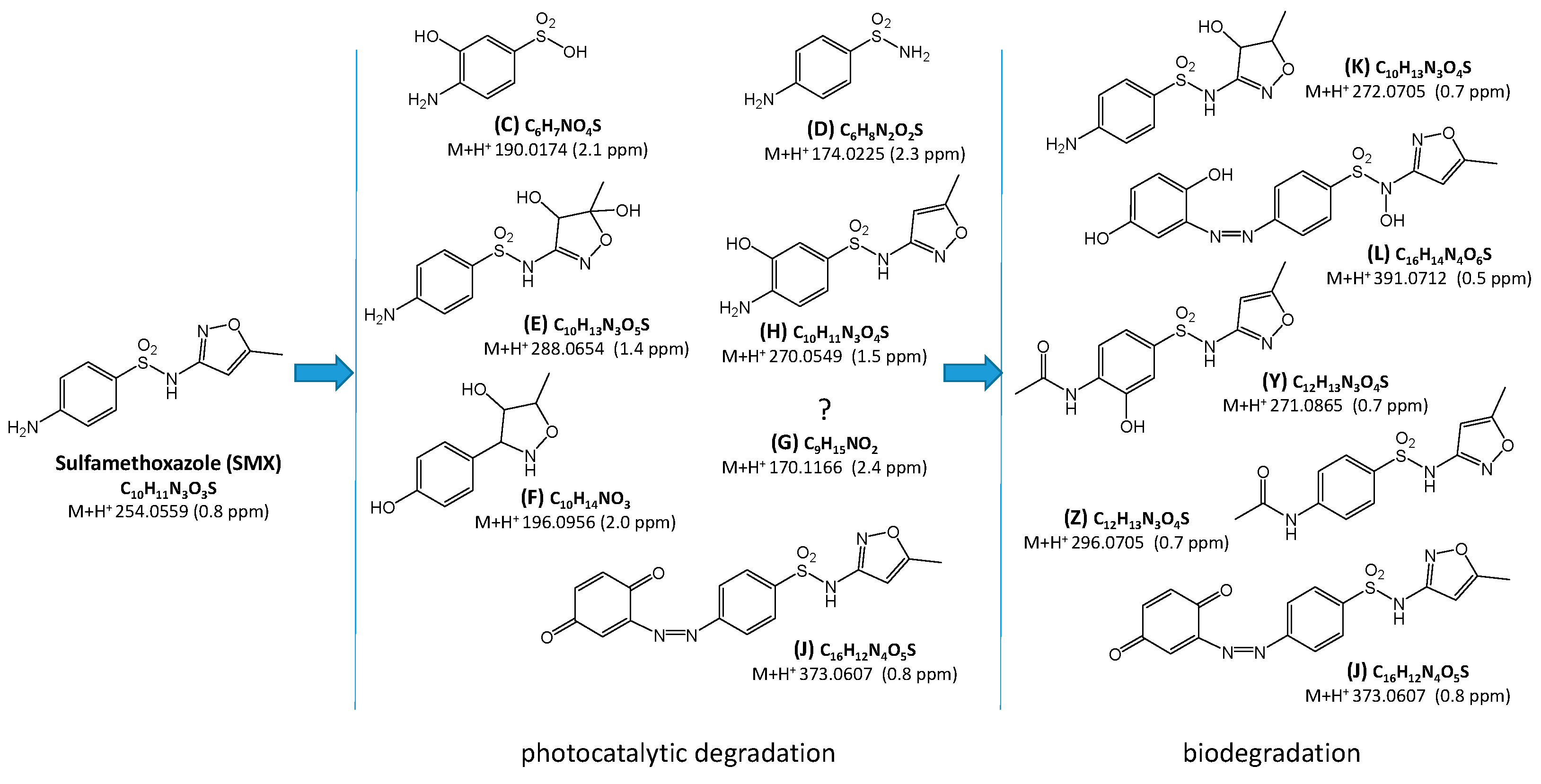
2.4. Estimation of Ecotoxicity
3. Materials and Methods
3.1. Reagents
3.2. Total Organic Carbon and Chemical Oxygen Demand Analysis
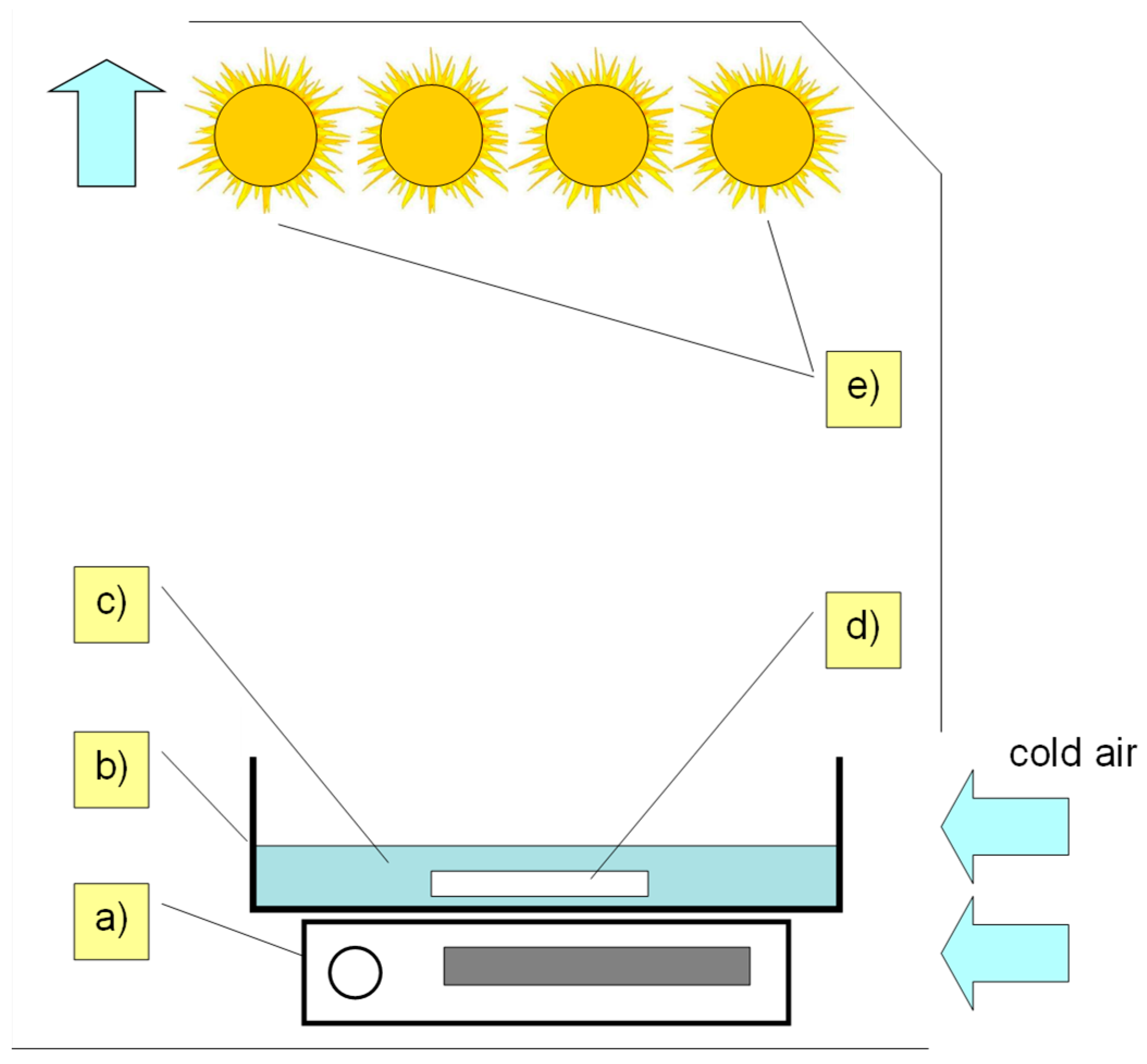
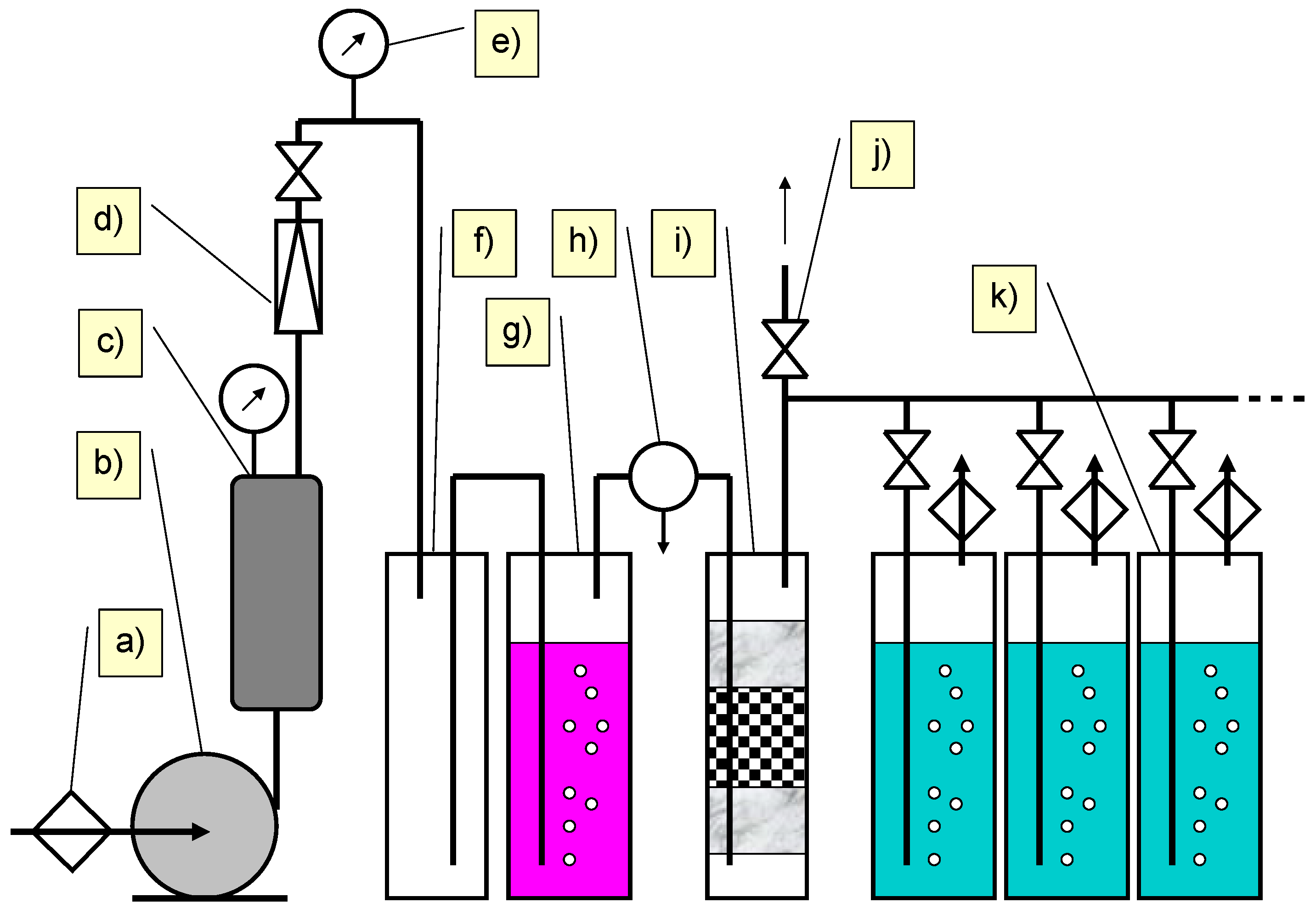
3.3. Photocatalytic Process
3.4. Biodegradation Process
3.5. Samples and Results Analysis
3.6. Prediction of Toxicity Using the In Silico Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Medicines Agency. Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2022. Available online: https://www.ema.europa.eu/en/documents/report/sales-veterinary-antimicrobial-agents-31-european-countries-2022-trends-2010-2022-thirteenth-esvac-report_en.pdf (accessed on 14 May 2024).
- Wang, H.; Chu, Y.; Fang, C. Occurrence of Veterinary Antibiotics in Swine Manure from Large-Scale Feedlots in Zhejiang Province, China. Bull. Environ. Contam. Toxicol. 2017, 98, 472–477. [Google Scholar] [CrossRef]
- An, J.; Chen, H.; Wei, S.; Gu, J. Antibiotic Contamination in Animal Manure, Soil, and Sewage Sludge in Shenyang, Northeast China. Environ. Earth Sci. 2015, 74, 5077–5086. [Google Scholar] [CrossRef]
- Thiebault, T. Sulfamethoxazole/Trimethoprim Ratio as a New Marker in Raw Wastewaters: A Critical Review. Sci. Total Environ. 2020, 715, 136916. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.Y.-C.; Tsai, Y.-T. Occurrence of Pharmaceuticals in Taiwan’s Surface Waters: Impact of Waste Streams from Hospitals and Pharmaceutical Production Facilities. Sci. Total Environ. 2009, 407, 3793–3802. [Google Scholar] [CrossRef] [PubMed]
- Baran, W.; Adamek, E.; Ziemiańska, J.; Sobczak, A. Effects of the Presence of Sulfonamides in the Environment and Their Influence on Human Health. J. Hazard. Mater. 2011, 196, 1–15. [Google Scholar] [CrossRef]
- Qin, L.-T.; Pang, X.-R.; Zeng, H.-H.; Liang, Y.-P.; Mo, L.-Y.; Wang, D.-Q.; Dai, J.-F. Ecological and Human Health Risk of Sulfonamides in Surface Water and Groundwater of Huixian Karst Wetland in Guilin, China. Sci. Total Environ. 2020, 708, 134552. [Google Scholar] [CrossRef]
- Duan, W.; Cui, H.; Jia, X.; Huang, X. Occurrence and Ecotoxicity of Sulfonamides in the Aquatic Environment: A Review. Sci. Total Environ. 2022, 820, 153178. [Google Scholar] [CrossRef]
- Zhou, J.; Yun, X.; Wang, J.; Li, Q.; Wang, Y. A Review on the Ecotoxicological Effect of Sulphonamides on Aquatic Organisms. Toxicol. Rep. 2022, 9, 534–540. [Google Scholar] [CrossRef]
- Nunes, O.C.; Manaia, C.M.; Kolvenbach, B.A.; Corvini, P.F.-X. Living with Sulfonamides: A Diverse Range of Mechanisms Observed in Bacteria. Appl. Microbiol. Biotechnol. 2020, 104, 10389–10408. [Google Scholar] [CrossRef]
- Azanu, D.; Styrishave, B.; Darko, G.; Weisser, J.J.; Abaidoo, R.C. Occurrence and Risk Assessment of Antibiotics in Water and Lettuce in Ghana. Sci. Total Environ. 2018, 622–623, 293–305. [Google Scholar] [CrossRef]
- Kovalakova, P.; Cizmas, L.; McDonald, T.J.; Marsalek, B.; Feng, M.; Sharma, V.K. Occurrence and Toxicity of Antibiotics in the Aquatic Environment: A Review. Chemosphere 2020, 251, 126351. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [PubMed]
- Adamek, E.; Baran, W.; Sobczak, A. Photocatalytic Degradation of Veterinary Antibiotics: Biodegradability and Antimicrobial Activity of Intermediates. Process Saf. Environ. Prot. 2016, 103, 1–9. [Google Scholar] [CrossRef]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Antibiotics in the Soil Environment—Degradation and Their Impact on Microbial Activity and Diversity. Front. Microbiol. 2019, 10, 338. [Google Scholar] [CrossRef]
- Kemper, N. Veterinary Antibiotics in the Aquatic and Terrestrial Environment. Ecol. Indic. 2008, 8, 1–13. [Google Scholar] [CrossRef]
- Abushaheen, M.A.; Muzaheed; Fatani, A.J.; Alosaimi, M.; Mansy, W.; George, M.; Acharya, S.; Rathod, S.; Divakar, D.D.; Jhugroo, C.; et al. Antimicrobial Resistance, Mechanisms and Its Clinical Significance. Disease-a-Month 2020, 66, 100971. [Google Scholar] [CrossRef] [PubMed]
- Turkdogan, F.I.; Yetilmezsoy, K. Appraisal of Potential Environmental Risks Associated with Human Antibiotic Consumption in Turkey. J. Hazard. Mater. 2009, 166, 297–308. [Google Scholar] [CrossRef]
- García-Galán, M.J.; Díaz-Cruz, M.S.; Barceló, D. Occurrence of Sulfonamide Residues along the Ebro River Basin: Removal in Wastewater Treatment Plants and Environmental Impact Assessment. Environ. Int. 2011, 37, 462–473. [Google Scholar] [CrossRef]
- Cui, J.; Fu, L.; Tang, B.; Bin, L.; Li, P.; Huang, S.; Fu, F. Occurrence, Ecotoxicological Risks of Sulfonamides and Their Acetylated Metabolites in the Typical Wastewater Treatment Plants and Receiving Rivers at the Pearl River Delta. Sci. Total Environ. 2020, 709, 136192. [Google Scholar] [CrossRef]
- Fu, W.; Li, B.; Yang, J.; Yi, H.; Chai, L.; Li, X. New Insights into the Chlorination of Sulfonamide: Smiles-Type Rearrangement, Desulfation, and Product Toxicity. Chem. Eng. J. 2018, 331, 785–793. [Google Scholar] [CrossRef]
- Musial, J.; Mlynarczyk, D.T.; Stanisz, B.J. Photocatalytic Degradation of Sulfamethoxazole Using TiO2-Based Materials—Perspectives for the Development of a Sustainable Water Treatment Technology. Sci. Total Environ. 2023, 856, 159122. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhuan, R. Degradation of Antibiotics by Advanced Oxidation Processes: An Overview. Sci. Total Environ. 2020, 701, 135023. [Google Scholar] [CrossRef]
- Li, J.; Zhao, L.; Feng, M.; Huang, C.-H.; Sun, P. Abiotic Transformation and Ecotoxicity Change of Sulfonamide Antibiotics in Environmental and Water Treatment Processes: A Critical Review. Water Res. 2021, 202, 117463. [Google Scholar] [CrossRef]
- Mezyk, S.P.; Neubauer, T.J.; Cooper, W.J.; Peller, J.R. Free-Radical-Induced Oxidative and Reductive Degradation of Sulfa Drugs in Water: Absolute Kinetics and Efficiencies of Hydroxyl Radical and Hydrated Electron Reactions. J. Phys. Chem. A 2007, 111, 9019–9024. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Kumar, A.; Dhiman, P.; Mola, G.T.; Sharma, G.; Lai, C.W. Recent Advances in Photocatalytic Removal of Sulfonamide Pollutants from Waste Water by Semiconductor Heterojunctions: A Review. Mater. Today Chem. 2023, 30, 101603. [Google Scholar] [CrossRef]
- Li, D.; Yuan, R.; Zhou, B.; Chen, H. Selective Photocatalytic Removal of Sulfonamide Antibiotics: The Performance Differences in Molecularly Imprinted TiO2 Synthesized Using Four Template Molecules. J. Clean. Prod. 2023, 383, 135470. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, X.; Wangjin, Y.; Ye, M.; Wu, T.; Wang, Z.; Cui, J. Degradation of Sulfamethoxazole and Antibiotic Resistance Genes from Surface Water in the Photocatalyst-Loading Bionic Ecosystems. Sci. Total Environ. 2023, 895, 165045. [Google Scholar] [CrossRef]
- Zango, Z.U.; Lawal, M.A.; Usman, F.; Sulieman, A.; Akhdar, H.; Eisa, M.H.; Aldaghri, O.; Ibnaouf, K.H.; Lim, J.W.; Khoo, K.S.; et al. Promoting the Suitability of Graphitic Carbon Nitride and Metal Oxide Nanoparticles: A Review of Sulfonamides Photocatalytic Degradation. Chemosphere 2024, 351, 141218. [Google Scholar] [CrossRef] [PubMed]
- Fukahori, S.; Fujiwara, T. Photocatalytic Decomposition Behavior and Reaction Pathway of Sulfamethazine Antibiotic Using TiO2. J. Environ. Manag. 2015, 157, 103–110. [Google Scholar] [CrossRef]
- Song, Y.; Tian, J.; Gao, S.; Shao, P.; Qi, J.; Cui, F. Photodegradation of Sulfonamides by G-C3N4 under Visible Light Irradiation: Effectiveness, Mechanism and Pathways. Appl. Catal. B Environ. 2017, 210, 88–96. [Google Scholar] [CrossRef]
- Tan, C.; Zhang, Q.; Zheng, X.; Liu, H.; Chen, P.; Zhang, W.; Liu, Y.; Lv, W.; Liu, G. Photocatalytic Degradation of Sulfonamides in 4-Phenoxyphenol-Modified g-C3N4 Composites: Performance and Mechanism. Chem. Eng. J. 2021, 421, 127864. [Google Scholar] [CrossRef]
- Yang, H.; Li, G.; An, T.; Gao, Y.; Fu, J. Photocatalytic Degradation Kinetics and Mechanism of Environmental Pharmaceuticals in Aqueous Suspension of TiO2: A Case of Sulfa Drugs. Catal. Today 2010, 153, 200–207. [Google Scholar] [CrossRef]
- Xin, X.; Liu, H.; Sun, J.; Gao, K.; Jia, R. Enhanced Photocatalytic Activity of Fe-, S- and N-Codoped TiO2 for Sulfadiazine Degradation. Int. J. Environ. Sci. Technol. 2023, 20, 11865–11876. [Google Scholar] [CrossRef]
- Sapińska, D.; Adamek, E.; Masternak, E.; Zielińska-Danch, W.; Baran, W. Influence of pH on the Kinetics and Products of Photocatalytic Degradation of Sulfonamides in Aqueous Solutions. Toxics 2022, 10, 655. [Google Scholar] [CrossRef]
- Ingerslev, F.; Halling-Sørensen, B. Biodegradability Properties of Sulfonamides in Activated Sludge. Environ. Toxicol. Chem. 2000, 19, 2467–2473. [Google Scholar] [CrossRef]
- Zhao, Q.; Guo, W.; Luo, H.; Xing, C.; Wang, H.; Liu, B.; Si, Q.; Li, D.; Sun, L.; Ren, N. Insights into Removal of Sulfonamides in Anaerobic Activated Sludge System: Mechanisms, Degradation Pathways and Stress Responses. J. Hazard. Mater. 2022, 423, 127248. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hu, Y.; Wang, J. Biodegradation of Typical Pharmaceutical Compounds by a Novel Strain Acinetobacter sp. J. Environ. Manag. 2018, 217, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Zhu, Q.; Yang, X.; Wang, C. Review of Biodegradation of Sulfonamide Antibiotics Influenced by Dissolved Organic Matter and Iron Oxides. J. Environ. Chem. Eng. 2023, 11, 111020. [Google Scholar] [CrossRef]
- Hayati, F.; Isari, A.A.; Anvaripour, B.; Fattahi, M.; Kakavandi, B. Ultrasound-Assisted Photocatalytic Degradation of Sulfadiazine Using MgO@CNT Heterojunction Composite: Effective Factors, Pathway and Biodegradability Studies. Chem. Eng. J. 2020, 381, 122636. [Google Scholar] [CrossRef]
- Liu, N.; Huang, W.; Li, Z.; Shao, H.; Wu, M.; Lei, J.; Tang, L. Radiolytic Decomposition of Sulfonamide Antibiotics: Implications to the Kinetics, Mechanisms and Toxicity. Sep. Purif. Technol. 2018, 202, 259–265. [Google Scholar] [CrossRef]
- Xiang, X.; Wu, L.; Zhu, J.; Li, J.; Liao, X.; Huang, H.; Fan, J.; Lv, K. Photocatalytic Degradation of Sulfadiazine in Suspensions of TiO2 Nanosheets with Exposed (001) Facets. Chin. Chem. Lett. 2021, 32, 3215–3220. [Google Scholar] [CrossRef]
- Yang, S.; Che, D. Degradation of Aquatic Sulfadiazine by Fe0/Persulfate: Kinetics, Mechanisms, and Degradation Pathway. RSC Adv. 2017, 7, 42233–42241. [Google Scholar] [CrossRef]
- Acosta-Rangel, A.; Sánchez-Polo, M.; Polo, A.M.S.; Rivera-Utrilla, J.; Berber-Mendoza, M.S. Sulfonamides Degradation Assisted by UV, UV/H2O2 and UV/K2S2O8: Efficiency, Mechanism and Byproducts Cytotoxicity. J. Environ. Manag. 2018, 225, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Microbial Degradation of Sulfamethoxazole in the Environment. Appl. Microbiol. Biotechnol. 2018, 102, 3573–3582. [Google Scholar] [CrossRef]
- Deng, Y.; Mao, Y.; Li, B.; Yang, C.; Zhang, T. Aerobic Degradation of Sulfadiazine by Arthrobacter spp.: Kinetics, Pathways, and Genomic Characterization. Environ. Sci. Technol. 2016, 50, 9566–9575. [Google Scholar] [CrossRef]
- Wang, L.; You, L.; Zhang, J.; Yang, T.; Zhang, W.; Zhang, Z.; Liu, P.; Wu, S.; Zhao, F.; Ma, J. Biodegradation of Sulfadiazine in Microbial Fuel Cells: Reaction Mechanism, Biotoxicity Removal and the Correlation with Reactor Microbes. J. Hazard. Mater. 2018, 360, 402–411. [Google Scholar] [CrossRef]
- Chen, J.; Xie, S. Overview of Sulfonamide Biodegradation and the Relevant Pathways and Microorganisms. Sci. Total Environ. 2018, 640–641, 1465–1477. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, R.; Chen, H.; Wang, F.; Zhou, B. Anaerobic Biodegradation of Four Sulfanilamide Antibiotics: Kinetics, Pathways and Microbiological Studies. J. Hazard. Mater. 2021, 416, 125840. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, X.; Liu, F.; Fu, W.; Lin, L.; Li, B. Comparison of Chemical and Biological Degradation of Sulfonamides: Solving the Mystery of Sulfonamide Transformation. J. Hazard. Mater. 2022, 424, 127661. [Google Scholar] [CrossRef]
- Yuan, R.; Zhu, Y.; Zhou, B.; Hu, J. Photocatalytic Oxidation of Sulfamethoxazole in the Presence of TiO2: Effect of Matrix in Aqueous Solution on Decomposition Mechanisms. Chem. Eng. J. 2019, 359, 1527–1536. [Google Scholar] [CrossRef]
- Jahdi, M.; Mishra, S.B.; Nxumalo, E.N.; Mhlanga, S.D.; Mishra, A.K. Smart Pathways for the Photocatalytic Degradation of Sulfamethoxazole Drug Using F-Pd Co-Doped TiO2 Nanocomposites. Appl. Catal. B Environ. 2020, 267, 118716. [Google Scholar] [CrossRef]
- Gao, X.; Chen, J.; Che, H.; Ao, Y.; Wang, P. Rationally Constructing of a Novel Composite Photocatalyst with Multi Charge Transfer Channels for Highly Efficient Sulfamethoxazole Elimination: Mechanism, Degradation Pathway and DFT Calculation. Chem. Eng. J. 2021, 426, 131585. [Google Scholar] [CrossRef]
- Guo, W.; Zhao, Q.; Du, J.; Wang, H.; Li, X.; Ren, N. Enhanced Removal of Sulfadiazine by Sulfidated ZVI Activated Persulfate Process: Performance, Mechanisms and Degradation Pathways. Chem. Eng. J. 2020, 388, 124303. [Google Scholar] [CrossRef]
- Li, D.; Zhang, N.; Yuan, R.; Chen, H.; Wang, F.; Zhou, B. Effect of Wavelengths on Photocatalytic Oxidation Mechanism of Sulfadiazine and Sulfamethoxazole in the Presence of TiO2. J. Environ. Chem. Eng. 2021, 9, 106243. [Google Scholar] [CrossRef]
- Zhang, T.; Cai, L.; Xu, B.; Li, X.; Qiu, W.; Fu, C.; Zheng, C. Sulfadiazine Biodegradation by Phanerochaete chrysosporium: Mechanism and Degradation Product Identification. Chemosphere 2019, 237, 124418. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Wang, M.; Zhang, C.; Xue, M.; Xie, H. Sulfadiazine Chlorination Disinfection By-Products in Constructed Wetlands: Identification of Biodegradation Products and Inference of Transformation Pathways. Environ. Pollut. 2024, 344, 123310. [Google Scholar] [CrossRef] [PubMed]
- Qin, K.; Chen, Y.; Li, J.; Xue, C.; Wei, L.; Song, X.; Ding, J.; Zhao, Q. Removal Trends of Sulfonamides and Their ARGs during Soil Aquifer Treatment and Subsequent Chlorination: Effect of Aerobic and Anaerobic Biodegradation. Environ. Sci. Wat. Res. 2020, 6, 2331–2340. [Google Scholar] [CrossRef]
- Xu, M.; Yan, S.; Liu, X.; Sun, S.; Khan, Z.U.H.; Wu, W.; Sun, J. Theoretical Investigation on the Degradation of Sulfadiazine in Water Environments: Oxidation of •OH, SO4•− and CO3•− and Reactivity of (TiO2)n Clusters (n = 1–6). J. Environ. Chem. Eng. 2023, 11, 109994. [Google Scholar] [CrossRef]
- Jebalbarezi, B.; Dehghanzadeh, R.; Sheikhi, S.; Shahmahdi, N.; Aslani, H.; Maryamabadi, A. Oxidative Degradation of Sulfamethoxazole from Secondary Treated Effluent by Ferrate(VI): Kinetics, by-Products, Degradation Pathway and Toxicity Assessment. J. Environ. Health Sci. Eng. 2022, 20, 205–218. [Google Scholar] [CrossRef]
- Baran, W.; Sochacka, J.; Wardas, W. Toxicity and Biodegradability of Sulfonamides and Products of Their Photocatalytic Degradation in Aqueous Solutions. Chemosphere 2006, 65, 1295–1299. [Google Scholar] [CrossRef]
- Gong, H.; Chu, W. Determination and Toxicity Evaluation of the Generated Products in Sulfamethoxazole Degradation by UV/CoFe2O4/TiO2. J. Hazard. Mater. 2016, 314, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, F.J.; Aguinaco, A.; García-Araya, J.F.; Oropesa, A. Ozone and Photocatalytic Processes to Remove the Antibiotic Sulfamethoxazole from Water. Water Res. 2008, 42, 3799–3808. [Google Scholar] [CrossRef] [PubMed]
- Ioannidou, E.; Frontistis, Z.; Antonopoulou, M.; Venieri, D.; Konstantinou, I.; Kondarides, D.I.; Mantzavinos, D. Solar Photocatalytic Degradation of Sulfamethoxazole over Tungsten—Modified TiO2. Chem. Eng. J. 2017, 318, 143–152. [Google Scholar] [CrossRef]
- Kim, H.Y.; Jeon, J.; Yu, S.; Lee, M.; Kim, T.-H.; Kim, S.D. Reduction of Toxicity of Antimicrobial Compounds by Degradation Processes Using Activated Sludge, Gamma Radiation, and UV. Chemosphere 2013, 93, 2480–2487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, X.; Jiang, Y.; Hu, M.; Li, S.; Zhai, Q. Combination of Enzymatic Degradation by Chloroperoxidase with Activated Sludge Treatment to Remove Sulfamethoxazole: Performance, and Eco-Toxicity Assessment. J. Chem. Technol. Biotechnol. 2016, 91, 2802–2809. [Google Scholar] [CrossRef]
- Reuschenbach, P.; Silvani, M.; Dammann, M.; Warnecke, D.; Knacker, T. ECOSAR Model Performance with a Large Test Set of Industrial Chemicals. Chemosphere 2008, 71, 1986–1995. [Google Scholar] [CrossRef]


| Compound | Equation (1) | R2 (1) | Linearity Range (1) (mg/L) | Limit of Quantification (LOQ) (1) (mg/L) | Limit of Detection (LOD) (2) (µg/L) |
|---|---|---|---|---|---|
| SDZ | y = 870.9x | 0.9991 | LOQ—125 | 0.107 | 0.075 |
| SMX | y = 940.1x | 1.0000 | LOQ—127 | 0.102 | 0.075 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madej-Knysak, D.; Adamek, E.; Baran, W. Biodegradation of Photocatalytic Degradation Products of Sulfonamides: Kinetics and Identification of Intermediates. Int. J. Mol. Sci. 2024, 25, 6688. https://doi.org/10.3390/ijms25126688
Madej-Knysak D, Adamek E, Baran W. Biodegradation of Photocatalytic Degradation Products of Sulfonamides: Kinetics and Identification of Intermediates. International Journal of Molecular Sciences. 2024; 25(12):6688. https://doi.org/10.3390/ijms25126688
Chicago/Turabian StyleMadej-Knysak, Daria, Ewa Adamek, and Wojciech Baran. 2024. "Biodegradation of Photocatalytic Degradation Products of Sulfonamides: Kinetics and Identification of Intermediates" International Journal of Molecular Sciences 25, no. 12: 6688. https://doi.org/10.3390/ijms25126688






